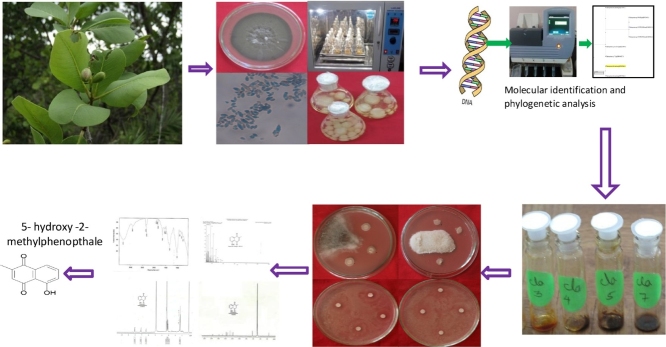
Graphical abstract
Keywords: Endophytic fungi, Cladosporium delicatulum, Structural characterization, Plumbagin, Biological activities
Highlights
-
•
Diversity of endophytic fungi from endemic medicinal plants in Eastern Ghats.
-
•
Molecular identification and anti-microbial activity of isolated endophytic cultures.
-
•
GCMS analysis and compound identification of potential crud extract.
-
•
Characterization of purified fraction using FTIR spectroscopy, MS and NMR.
-
•
Potentially inhibition of tested pathogens by identified compound 5-hydroxy-2-methylnaphthalene-1, 4-Dione.
Abstract
The rationale of the present study was to isolate and identify endophytic fungi from endemic medicinal plants in Eastern Ghats and screened for antimicrobial potential of isolated fungal crude extracts. A total of 329 endophytic strains were isolated from 600 infected leaves and stem cuttings of endemic plants. The diversity and species richness was analyzed statistically and found to be higher in leaf segments than in stem segments. From isolated fungal strains, Cladosporium delicatulum was identified using molecular identification methods and selected as the most potent plumbagin-producing endophytic strain. Further the isolation and structural characterization of endophytic fungal plumbagin (5-hydroxyl-2-methylnaptalene-1,4-dione) was purified and confirmed through spectroscopy analysis. The molecular weight was determined as m/z 188 in positive mode by ESI-MS, which confirmed to be plumbagin which potentially inhibited all tested pathogens, therefore the endophytic fungal plumbagin from the current study possesses important biological activities against pathogens.
1. Introduction
The necessity of new therapeutic and medically useful compounds continues to increase in demand to resolve complexities facing increasing antibiotic resistance and lack of new antibiotic discovery is at a great lag. Endophytes from an untapped diverse habitat like hills of Eastern Ghats are a significant source of new bioactive molecules that grabbed the attention of many investigators and may perhaps provide solutions to several unanswered questions. Endophytes are microorganisms living inside the tissues of plants without any apparent negative effects [1,2]. By definition every individual plant on earth is a host for one or more endophytes [3] and these endophytes spend all or part of their life residing asymptomatically within the host plant tissues [4]. Researchers have been studying the diversity, ecology and biotechnological applications of endophytic fungi associated with grasses and woody plants in temperate environments [5]. However, the comprehensive evidence about the diversity of endophytic fungal populations in humid forests having a rich flora biodiversity is very limited. The Eastern Ghats are isolated hill ranges in peninsular India, which harbours primary tropical deciduous vegetation, found in Andhra Pradesh lying between 13°30′- 19° 07′N and 77°28′-84°45′E and at elevations of 700–800 mt s of Eastern Ghats center at Tirumala hills Chittoor District. The Tirumala hills make a good case study as the rich biodiversity of plant species in the region is well documented and the local tradition of herbal medical practice is long associated.
The higher plants are known to harbor endophytic fungi [6]. The plant parts of Pterocarpus santalinus, Rhynchosia beddomei and Terminalia pallida three endemic plants to Eastern Ghats are used for the isolation of antimicrobial, antidiabetic and hepatoprotective compounds [[7], [8], [9]]. Plants used in traditional medicine have been a great source for the search for new bioactive metabolite producing strains of endophytic fungi as it is possible that their beneficial characteristics towards plants could be a result of the metabolites produced by the endophytic community of the host plant [10,11]. Hamada et al., reported endophytic fungal isolates were effective in suppression of the Black-pod rot disease of cacao [12]. Three endophytic fungal isolates were reported as potential biological control agents against A. panax, F. oxysporum, F.solani, Phoma herbarum and M. acerina and possibly other pathogens [13]. Endophytic fungal isolates in Brassica napus exhibited strong antagonistic activity against Sclerotinia sclerotiorum [14].
Antifungal and antibacterial properties of plant associated endophytic fungi have been reported by several groups [[15], [16], [17],11]. The mechanism involved in the biological control of endophytic fungi against plant pathogens include antibiosis [18,19], Competition for nutrients and space, induction of defense response [[20], [21], [22], [23]] and mycoparasitism [24]. In the current study, a reasonable hypothesis should govern the strategy for plant selection for bioactive metabolite prospecting of endophytic fungi; one strategy includes examination of local traditional medication or pharmacological potential of selected plant species. Hence, in this study, we have aimed to isolate plumbagin (5-hydroxy-2-methylnaphthalene-1,4-dione)-producing potential endophytic fungi Cladosporium delicatulum from endemic medicinal plants. To our knowledge, this is the first report on isolation of plumbagin-producing fungal endophytes for its valuable biomedical applications.
2. Materials and methods
2.1. Collections of plant samples for isolation of endophytes
Leaf and stem samples of three endemic medicinal plants Terminalia pallida, Rhynchosia beddomei, Pterocarpus santalinus were collected from the Tirumala hills, Eastern Ghats region (13° 10′ N 79° 04′ E), Chittoor district of Andhra Pradesh state during the monsoon season. All the plant samples were washed thoroughly under running tap water to remove the debris adhered and finally washed with double distilled deionized water. The samples were cut into small pieces and surface sterilized by sequential washes in different concentrations of sterilizing agent, finally rinsed with distilled water and allowed to air dry under sterile conditions.
The efficiency of surface sterilizations was checked by imprint method [25] and sample washed distilled water was inoculated into broth media as a control to check growth in liquid medium. Most endophytes generally sporulate after few weeks either in darkness or in daylight. In case of non-sporulating endophytes exposure to 12 h dark-light cycle induced sporulation. Endophytes are slow growing microbes (in vitro), and some isolates even require months, or a year or more, in culture before they sporulated. Endophytic fungi prevent sporulating after several subcultures [3].
2.1.1. Isolation of endophytes
The dissected tissues were inoculated on to potato dextrose agar (PDA), with 50 mg/L chloramphenicol. Endophytic fungi usually begin to produce hyphal filaments after 5–6 days of incubation at 30 °C. The hyphal tips appeared were carefully transferred to new sterile PDA plates [26,27].
2.2. Statistical analysis
The colonization frequency of fungal endophytes was analyzed using the formula given by Hata and Futai [28] and similarity of fungal endophyte collections among all samples were assayed by using the Simson’s diversity index (1D) and species richness and species evenness was evaluated by Shannon-Weaver index (Ho) [29].
2.3. Phenotypic identification of fungal endophytes
The inoculated plates were examined periodically and identified when culture isolates sporulated using morphological characteristics such as growth pattern, hyphae formations, pigmentations, and spore structures [30]. The endophytic fungal mycelium was stained in cotton blue and mounted in polyvinyl lactic acid glycerol (PVLG) for microscopic observation. The identification of the isolates was also confirmed by expert taxonomists at the Agharkar Research Institute (ARI, Pune, India). Authoritative monographs were referred for identification of endophytes [[31], [32], [33], [34]]. In addition, other taxonomic papers relating to particular genera and species of endophytes were also referred, for color differentiation of cultures ‘Methuen Handbook of Colour' [35] was also referred.
2.4. Molecular characterization and phylogenetic analysis
Molecular identification of endophytic fungi was carried out by 18S rDNA gene sequence. Fungal isolates were cultured in potato dextrose broth (PDB) at 28 ± 2 °C. Mycelium was harvested by vacuum filtration. The genomic DNA extraction was done by the CTAB method and 18S rDNA gene was amplified with universal ITS primer pair (ITS1 forward & ITS4 reverse). The Amplified product was sequenced by Sangers dideoxy method at Genei Biosciences, Bangalore [36,37]. The ITS sequences of endophytic fungi were compared with the data in national center for biotechnology information (NCBI), USA using BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and to estimate the phylogenetic relationship, CLUSTAL X software was used to generate the phylogenetic tree alignment of fungal endophytes [38]. Phylogenetic analysis was carried out by the neighbor-joining method using MEGA software (version 6.0, USA) the bootstrap was 1000 replications to assess the reliable level to the nods of the tree [39].
2.5. Preparation of fungal crude extracts
The pure endophytic fungal culture on PDA media, normally in the log phase, was transferred into a 1 L Erlenmeyer flask containing 300 mL of PDB media. The endophytic fungal cultures were then incubated at room temperature for three weeks. Large scale cultivation (around about 10 L culture medium) was carried out using 20 Erlenmeyer flasks. After fermentation, the media and fungal mat were separated using filtration and extracellular and intracellular metabolites were extracted. Then after 250 mL ethyl acetate solvent was added into each conical flask and mixed thoroughly and kept overnight to ensure that the fungal cells have died. The mixture was then placed in ultraturrax for 10 min to disrupt the cells and then filtered using Buchner vacuum pump. The separated mycelium was discarded and the filtrate containing ethyl acetate phase was collected for further analysis. The organic phase along with the salts and other polar constituents was separated using centrifugation. The final organic phase was washed, evaporated then after dissolved in methanol and extracted with hexane to remove fatty acids and other non-polar compounds.
2.6. Screening of Antimicrobial activity of crude extracts
The crude extracts isolated were screened for anti-microbial activity against Candida albicans, Candida tropicalis Sclerotium oryzae and Fusarium moniliforme employing resazurin-based Microtitre Dilution Assay [40]. Resazurin based microtiter dilution assay was performed in 96 well microtiter plates. A volume of 100 μL of test material in 10% DMSO added into the first row of the plate. To all wells of plate, 50 μL of nutrient broth and 50 μL of normal saline were added. Serial dilutions were performed such that each well had 100 μL of the test material in serially descending concentrations. 10 μL of resazurin indicator solution was added in each well with 10 μL of fungal spore. Propiconazole (Fusarium moniliforme & Sclerotium oryzae) and Amphotericin B (Candida sp.,) were used as controls. The plates were prepared in triplicates and incubated at appropriate temperatures (27 & 37 °C) for 24–48 h. The color changes were visually assessed, any change from purple to pink or colorless was taken as a positive reaction.
2.7. Separation of bioactive compound
The potential endophytic fungi were grown on potato dextrose broth and the cell-free culture filtrate and mat were mixed with ethyl acetate under stirring and kept overnight and extracted with up to three washings with the EtOAc. The fungal mycelial mat was mixed with methanol in shaking conditions and kept overnight, later the organic phase was filtered, both ethyl acetate and methanol extracts was evaporated using rotary vacuum evaporator and yielded crude extracts were used for separation of bioactive compounds. The fungal crude extract (2.0 gm) obtained from Cladosporium delicatulum (from Terminalia pallida) was purified by column chromatography. The column was filled with 30 gm of silica gel (60–120 mesh size, S.D. fine-chem. Ltd.) and onto this 2.0 gm of endophytic fungal crude extract mixed with 2.0 gm silica powder. The column was first eluted with hexane followed by hexane: ethyl acetate mixture of increasing polarity. The fractions obtained from column chromatography were checked by Thin Layer Chromatography (TLC) using pre-coated silica gel aluminum sheets (Merck Ltd).
2.8. Structural characterization of bioactive compound
The structural identification was achieved using data generated from Fourier transform infrared spectroscopy (FTIR), Mass Spectroscopy (GC–MS & ESI-MS) and nuclear magnetic resonance (NMR) spectroscopy analysis. The extract was mixed with potassium bromide (KBr) powder, triturated and then made into a 1 mm pellets for Fourier-transform infrared (FT-IR) analysis at frequency range of 4000–400 cm−1. FT-IR analysis was performed on Perkin Elmer-100 FT-IR spectrometer. Methyl ketone derivatives formed were determined by a gas chromatography mass spectroscopy (Perkin-Elmer Clarus SQ 8 GCMS with auto sampler) using RTX-5MS 30 M, 0.32 mm column. Separation of crude extract was done by flame ionization detector (FID), and O2 free dry helium was used as carrier gas at a flow rate of 25–30 mL/min using tetramethylsilane (TMS) as an internal reference. For 1H & 13C NMR analysis, a (500 MHz) spectrum was recorded on a Bruker AV III (500 MHz NMR spectrometer using tetramethylsilane (TMS) as an internal reference. The chemical shifts are expressed in parts per million (ppm). The fractions obtained from GCMS were once again purified by column chromatography and later on checked for purity of the compound by Thin Layer Chromatography (TLC) using pre-coated silica gel aluminum sheets (Merck Ltd). Furthermore to validate, mass spectral studies of the extract were obtained by ESI-MS (Perkin-Elmer ion trap mass spectrometer). Briefly, 20 μL of the sample was injected into the ESI source through an auto sampler. The mass spectra (MS) were scanned in the range of 0–800 m/z, and the solvent was eluted as a given gradient program at 1 mL/min. The maximum ion injection time was set at 250 ns, the ion spray voltage was set at 5.3 kV, and the capillary voltage was set at 34 V. The MS positive ionization mode was ESI (±).
2.9. Anti-microbial activity
Pure fraction with a single spot on the TLC plate was assayed for anti-microbial activity by micro-plate dilution method; where the test compound was serially diluted to give a series of different concentrations (concentration range: 100 μg; 50 μg; 25 μg; 12.5 μg; 6.25 μg) of the compound and added 50 μL per well from each concentration. This method holds great potential when the compound is limited in quantity, similar to the method of extraction followed for natural bioactive compounds.
3. Results
3.1. Isolation and diversity of endophytic fungi
A total of 329 endophytic fungal isolates were obtained from 600 plant samples (segments) collected from four selected localities. All the isolated endophytic fungi were assigned to 40 species in 27 genera. The percentage of colonization density of isolated endophytes was recorded maximum for Sordariomycetes sp. (3.50%), followed by Xylaria sp. (3.16%), Cladosporium delicatulam (2.83%) and Aspergillus aculeatus (2.83%). However, rest of the endophytic fungi depicted a colonization frequency between 0.5–2.5%. The colonization density of few endophytes was recorded to be ≤0.5%, which included Penicillium crysoginum (0.5%), Neotyphodium lolii (0.5%), Sordaria fimicola (0.5%), Scopulariopsis bdrevicaulis (0.5%), Xylaria hydroxylon (0.5%), Polyancora globosa (0.5%).
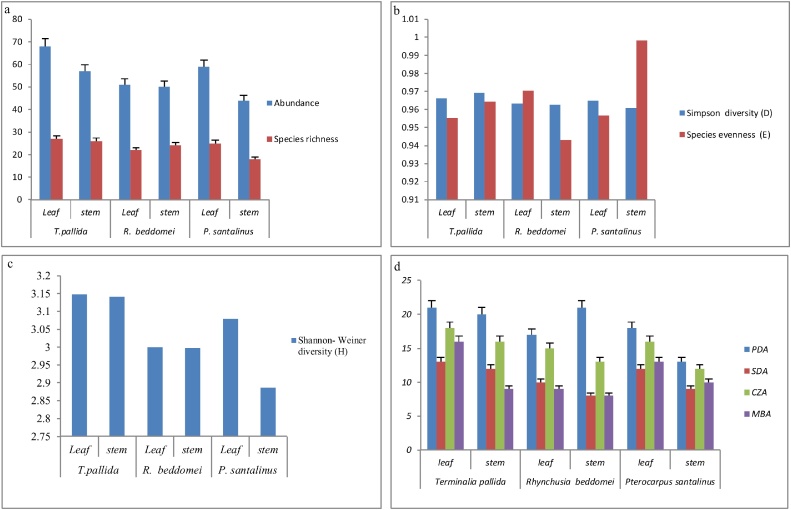
Notably, Terminalia pallida leaf exhibited the highest endophytic fungi species diversity 68, Species richness 27 Shannon-Wiener index (3.14), Simpson diversity index (0.96) and Evenness (0.95) compared to the other species followed by Terminalia pallida stem, Pterocarpus santalinus leaf, Rhynchosia beddomei stem and Pterocarpus santalinus stem.(Table 2, Fig. 1a–c).
Table 2.
Statistical analysis of endophytic fungal occurrence in selected endemic plants.
| Samples | Abundance | Species richness | Shannon- Weiner diversity (H) | Simpson diversity (D) |
Species evenness (E) |
|
|---|---|---|---|---|---|---|
| Terminalia pallida | Leaf | 68 | 27 | 3.148253 | 0.966198 | 0.95522113 |
| stem | 57 | 26 | 3.141486 | 0.969298 | 0.964209 | |
| Rhynchusia beddomei | Leaf | 51 | 22 | 2.999078 | 0.963137 | 0.97024808 |
| stem | 50 | 24 | 2.997427 | 0.962449 | 0.94316433 | |
| Pterocarpus santalinus | Leaf | 59 | 25 | 3.079009 | 0.964933 | 0.95654793 |
| stem | 44 | 18 | 2.885941 | 0.960888 | 0.99846706 | |
Fig. 1.
(a): Abundance and species richness of endophytic fungi, (b): Simpson diversity and species evenness of endophytic fungi, (c): Shannon- Weiner diversity index (H) of endophytic fungi, (d): Isolation of endophytic fungi in different nutrient medias.
Some of the endophytic fungi isolated from the three endemic medicinal plants showed a typical host-specificity (Table 1). Alternaria alternata, Scopulariopsis bdrevicaulis, Xylaria hydroxylon, Polyancora globosa were isolated from Terminalia pallida, and Aspergillus niger, Aspergillus japonicas, Microsporum gypseum were isolated from the segments of Rhynchosia beddomei and Cladosporium cf. tenuissimum, Penicillium crysoginum were isolated from Pterocarpus santalinus, Rhizopus sp., Paecilomyces variotii were isolated in both Terminalia pallida and Rhynchosia beddomei plant segments, Pestalotiopsis sp., Cunninghamella sp., Paraphaeosphaeria sp., isolated in Terminalia pallida and Pterocarpus santalinus. Remaining 24 fungal isolates were isolated in three plant segments. It is noteworthy that, endophytic fungal isolation different media yeilded different number of isolates and the highest number of isolates were isolated in PDA media followed by Czapek dox agar, Martins Bengal agar and Sabouraud dextrose agar (CDA, MBA and SDA) (Fig. 1d).
Table 1.
Isolation and colonization frequency of endophytic fungi from selected plants.
| Isolates | Name Endophytic fungi |
Terminalia pallida |
Rhychosia beddomei |
Pterocarpus Santalinus |
No. of isolates | CF% | |||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | stem | Leaf | stem | Leaf | Stem | ||||
| EF1 | Colletotrichum gloesporioides | 4 | 1 | 2 | – | 3 | – | 10 | 1.66 |
| EF2 | Trichoderma harzianum | 2 | – | – | 1 | 2 | – | 5 | 0.83 |
| EF3 | Aspergillus versicolor | 3 | – | 1 | 1 | 1 | 1 | 7 | 1.16 |
| EF4 | Chaetomium globosum | – | 2 | 2 | – | 1 | 3 | 8 | 1.33 |
| EF5 | Microsporum ferrugineum | 2 | – | – | – | – | 2 | 4 | 0.66 |
| EF6 | Penicillium sp. | 4 | 1 | 1 | 2 | 1 | – | 9 | 1.50 |
| EF7 | Acremonium chrysogenum | 1 | 3 | 2 | 2 | 1 | – | 9 | 1.50 |
| EF8 | Aspergillus flavus | – | 2 | 3 | 2 | 2 | 1 | 10 | 1.66 |
| EF9 | Cladosporium sp., | 1 | 2 | 2 | 1 | 3 | – | 9 | 1.50 |
| EF10 | Beauveria sp. | – | 1 | 1 | – | 1 | 2 | 5 | 0.833 |
| EF11 | Alternaria alternata | 3 | 2 | – | – | – | – | 5 | 0.833 |
| EF12 | Penicillium crysoginum | – | – | – | – | 2 | 1 | 3 | 0.50 |
| EF13 | Phyllosticta elongata | 4 | 2 | 3 | 1 | – | – | 10 | 1.66 |
| EF14 | Verticilium sp., | 2 | – | – | 2 | – | 3 | 7 | 1.16 |
| EF15 | Pestalotiopsis sp. | 1 | – | – | – | 5 | 2 | 8 | 1.33 |
| EF16 | Neotyphodium lolii | – | 2 | 1 | – | – | – | 3 | 0.50 |
| EF17 | Pithomyces chartarum | – | 3 | 2 | – | 1 | – | 6 | 1.00 |
| EF18 | Helminthosporium sp. | 1 | – | 5 | – | 2 | 4 | 12 | 2.00 |
| EF19 | Xylaria hydroxylon | 2 | 1 | – | – | – | – | 3 | 0.50 |
| EF20 | Aspergillus niger | – | – | 2 | 2 | – | – | 4 | 0.66 |
| EF21 | Phoma glomerata | 2 | – | 1 | 2 | 3 | – | 8 | 1.33 |
| EF22 | Mortriella alpina | – | 2 | 1 | 1 | – | 1 | 5 | 0.83 |
| EF23 | Rhizopus sp., | 2 | 5 | – | 2 | – | – | 9 | 1.50 |
| EF24 | Paecilomyces variotii | 1 | 4 | – | 1 | – | – | 6 | 1.00 |
| EF25 | Scopulariopsis bdrevicaulis | 1 | 2 | – | – | – | – | 3 | 0.50 |
| EF26 | Microsporum gypseum | – | – | 2 | 4 | – | – | 6 | 1.00 |
| EF27 | Cunninghamella sp. | – | 3 | – | – | 1 | – | 4 | 0.66 |
| EF28 | Phomopsis sp. | 1 | – | 4 | 2 | 1 | – | 8 | 1.33 |
| EF29 | Fusarium sp., | 4 | 3 | 1 | 2 | 4 | 1 | 15 | 2.50 |
| EF30 | Polyancora globosa | 2 | 1 | – | – | – | – | 3 | 0.50 |
| EF31 | Cochliobolus sp. | 5 | 3 | – | – | 2 | 1 | 11 | 1.83 |
| EF32 | Fusarium equiseti | 4 | 1 | – | 2 | – | 3 | 10 | 1.66 |
| EF33 | Cladosporium delicatulum | 2 | 1 | 4 | 3 | 5 | 2 | 17 | 2.83 |
| EF34 | Aspergillus aculeatus | 3 | 3 | 1 | 2 | 4 | 4 | 17 | 2.83 |
| EF35 | Aspergillus japonicus | – | – | 4 | 5 | – | – | 9 | 1.50 |
| EF36 | Xylaria sp. | 5 | 2 | 3 | 4 | 2 | 3 | 19 | 3.16 |
| EF37 | Sordariomycetes sp. | 5 | 4 | 3 | 5 | 2 | 2 | 21 | 3.50 |
| EF38 | Paraphaeosphaeria sp., | – | 1 | – | – | 3 | 4 | 8 | 1.33 |
| EF39 | Hypocreals sp., | 1 | – | – | 1 | 3 | 2 | 7 | 1.16 |
| EF40 | Cladosporium cf. tenuissimum | – | – | – | – | 4 | 2 | 6 | 1.00 |
| Total isolates | 68 | 57 | 51 | 50 | 59 | 44 | 329 | ||
CF% colonization frequency.
3.2. Identification of fungal endophytes
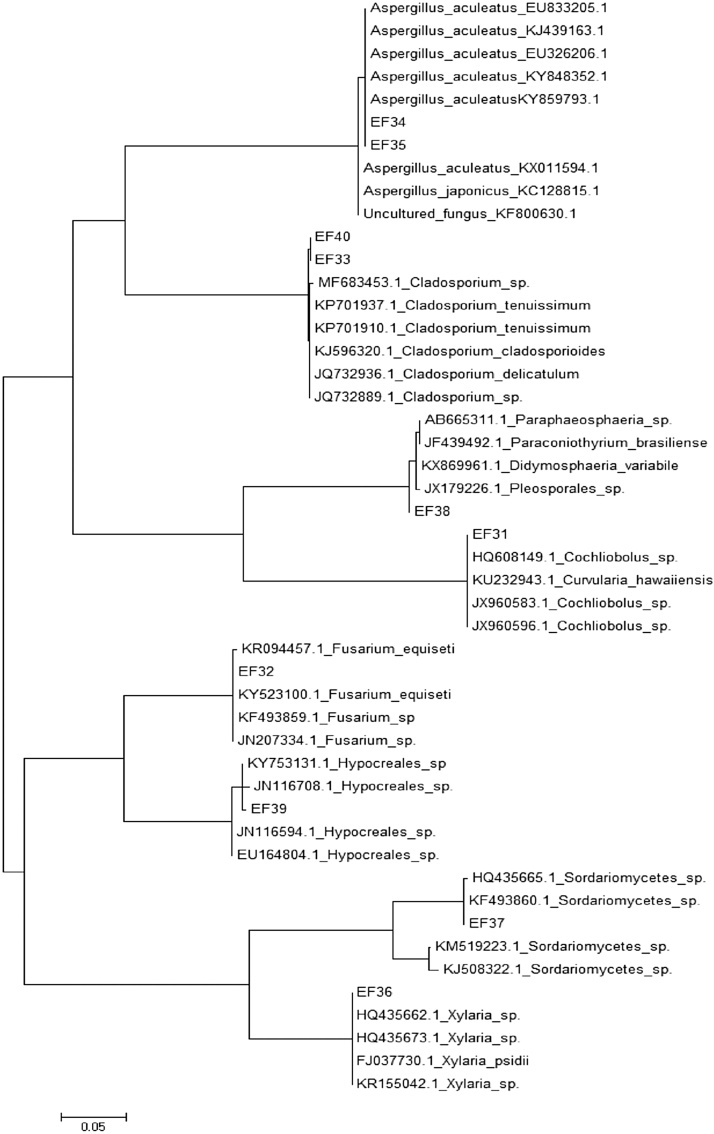
All the isolated endophytic fungi were assigned to 40 species of these, 27 sporulating strains (designated as EF1 to EF27) (Supplementary Table 1) were identified based on their morphological characteristics and non sporulating isolates were grouped into thirteen morphotypes (designated as EF28 to EF40). Nonetheless, these thirteen non-sporulating isolates were identified using partial 18S gene and ITS rDNA sequence analysis; the sequences were analyzed with BLAST in order to identify their taxonomic characteristics from the partial sequence of 18S rDNA gene, the similarity analysis of the sequences showed a high conserved region between each isolate. It was complex to make the genus-level identification by using only a partial 18S rDNA sequence since the sequence was highly conserved. The ITS rDNA sequences contained partial 18S rDNA, ITS1 (internal transcribed spacer1), 5.8S rDNA ITS2 sequence. The sequence lengths were approximately 500-600bp. The conserved sequences from rDNA of the 18S, 5.8S and 28S regions could be observed while a variation in the ITS region was also clearly noted. Based on the BLAST analysis of ITS sequence some endophytes were identified up to genera only and few isolates could be identified up to species level depending on the taxonomic history of each fungus. Notably, 13 endophytic fungi were identified by ITS rDNA sequences and the obtained sequences were submitted to NCBI and the accession numbers are mentioned in (Table 3, Fig. 2).
Table 3.
List of 18 s rDNA based identified fungal endophytes with accession numbers.
| S.No | Isolates | Host plant | Name Endophytic fungi | Accession No. |
|---|---|---|---|---|
| 1. | EF28 | Pterocarpus santalinus | Phomopsis sp. | KF493857 |
| 2. | EF29 | ״ | Fusarium sp. | KF493859 |
| 3. | EF30 | Terminalia pallida | Polyancora globosa | KJ638719 |
| 4. | EF31 | ״ | Cochliobolus sp. | KJ638720 |
| 5. | EF32 | ״ | Fusarium equiseti | KF493867 |
| 6. | EF33 | ״ | Cladosporium delicatulum | KF493866 |
| 7. | EF34 | Rhynchosia beddomei | Aspergillus aculeatus | KF493861 |
| 8. | EF35 | ״ | Aspergillus japonicas | KF493862 |
| 9. | EF36 | Pterocarpus santalinus | Xylaria sp. | KF493856 |
| 10. | EF37 | Terminalia pallida | Sordariomycetes sp. | KF493865 |
| 11. | EF38 | Pterocarpus santalinus | Paraphaeosphaeria sp. | KF493855 |
| 12. | EF39 | ״ | Hypocreales sp. | KF493854 |
| 13. | EF40 | Terminalia pallida | Cladosporium cf. tenuissimum | KF493858 |
Fig. 2.
Phylogenetic analysis of the sequences of endophytic fungi (highlight) associated with endemic plants compared with reference sequences of the closest species following BLAST analysis, deposited in the GenBank database. The trees were constructed on the basis of the ITS region sequences using the maximum composite likelihood method.
3.3. Screening of crude extract against pathogens
Antimicrobial activities of 40 endophytic isolates belonging to different taxa isolated from three different host plants were tested. The crude extracts obtained were assayed against the selected plant and human pathogens by resazurin micro dilution plate method. The anti-microbial compounds from endophytic fungi were well distributed among the different groups. Out of forty endophytic isolates 10 cultures effectively inhibited all four pathogenic cultures. Seventeen cultures were moderately inhibiting to the pathogenic cultures. Thirteen cultures were non inhibiting to the pathogenic culture (Supplementary Fig. 1).
3.4. GC–MS analysis
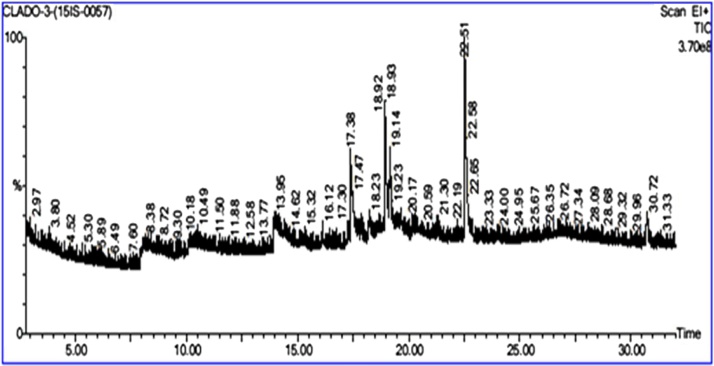
To identify the volatile matter, long chain hydrocarbons, alcohols and ketones etc., the active fungal crude extracts of Cladosporium delicatulum, the most potent in anti-microbial activity was used in this analysis; five major chemical components were identified in the crude extract viz. Pentadecanoic acid, 14-methyl-, methyl ketone, 13,16-Octadecadienoic acid, methyl ketone, Heptacosanoic acid, methyl ketone, Bis (2-ethylhexyl) phthalate. The chromatograms and compounds identified were listed in Table 4, Fig. 4 with a clear depiction of individual retention time,molecular weights, and peak areas respectively. Decanoic acid, Heptacosanoic acid, trisiloxane were present in the extracts which are known to exhibit major role in the anti-pathogenic activity.
Table 4.
List of chemical components detected in GC–MS analysis.
| Name of compounds | Formula | Retention time | M.W | Peak area% |
|---|---|---|---|---|
| Pentadecanoic acid, 14-methyl-, methyl ketone | C17H34O2 | 17.384 | 270 | 19.52 |
| 13,16-Octadecadienoic acid, methyl ketone | C19H34O2 | 18.935 | 294 | 22.72 |
| Heptacosanoic acid, methyl ketone | C28H56O2 | 19.170 | 424 | 10.35 |
| Bis (2-ethylhexyl) phthalate | C24H38O4 | 22.516 | 390 | 42.16 |
| 3-butoxy-1,1,1,5,5,5-hexamethyl-3-(trimethylsiloxy) trisiloxane | C13H36O4Si4 | 30.725 | 368 | 5.23 |
Fig. 4.
GC–MS Cromatogram of Cladosporium delicatulum.
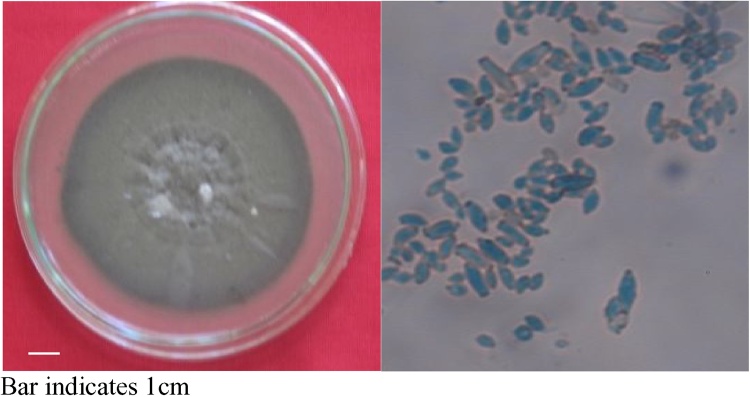
3.5. Identification of bioactive compound plumbagin
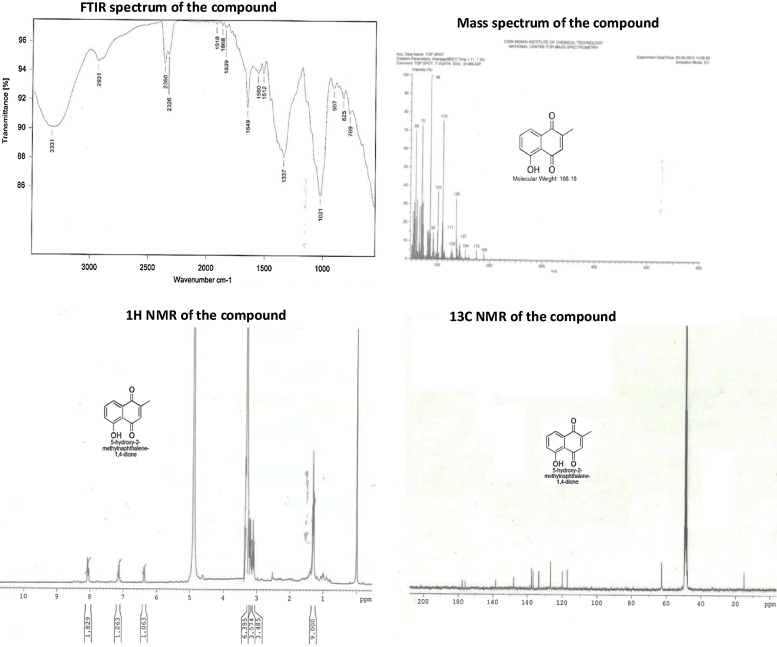
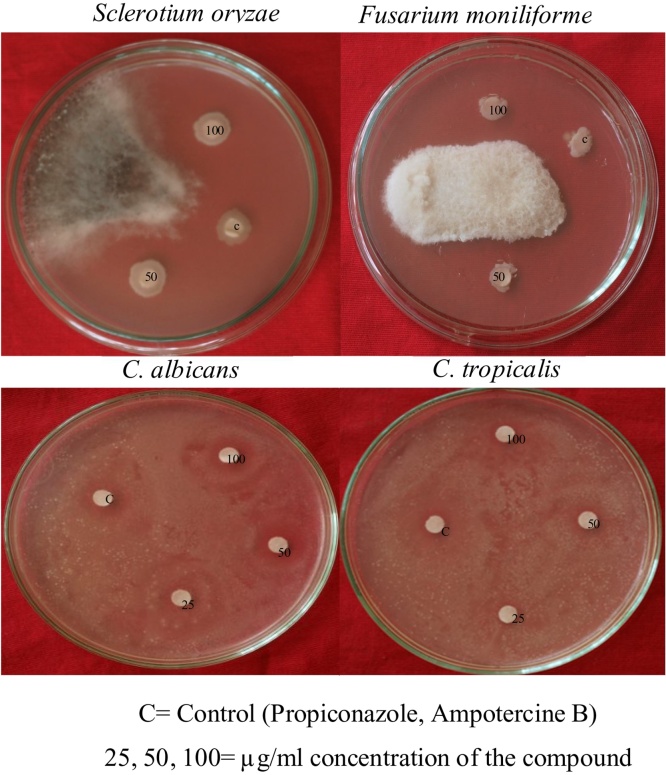
The Potential endophytic fungi Cladosporium delicatulum (Fig. 3) extract was purified further and pure fraction from the column chromatography (4:6 methanol and ethyl acetate) was analyzed further. The compound was obtained as a brown color solid upon drying, the IR spectrum showed (Fig. 5) absorption bands, an intense band in the region of 3500–3000 cm−1 appeared, which can be attributed to the symmetrical and asymmetrical stretching modes of O—H. An aromatic stretch at (1560 and 1512 cm−1) and a strong vibrational stretch of carbonyl group (C O) was noted at 1649 cm−1. All of the C—H stretching frequencies were in the range of 2930 cm−1 functional groups. In the 1H NMR spectrum (Fig. 5), two protons appeared as a multiplate at 8.07-8.03 ppm, one proton appeared as a singlet at 7.16 ppm and one proton appeared as a doublet at 6.38 ppm, which corresponds to four aromatic protons. The 13C NMR spectrum (Fig. 5) shows eleven carbon signals, eight signals for the aromatic ring (158.4, 147.8, 138.6, 137.9, 134.5, 126.8, 119.7 and 117.2 ppm), two signals at 178.3 and 176.6 ppm indicates the presence of carbonyl group and a peak at 115.5 ppm corresponds to methyl group. An additional chromatographic technique was employed to confirm the plumbagin (5-hydroxy-2-methylnaphthalene-1,4-dione), based on its ESI-MS values. The exact molecular weight was determined as m/z 188 in positive mode by ESI-MS (Fig. 5), which clearly confirmed the separated constituent to be plumbagin which potentially inhibited all tested pathogens, and MIC was calculated as 12.5 mg/mL (Table 5, Fig. 6).
Fig. 3.
Culturaland spore morphology of cladosporium delicatulum.
Fig. 5.
Structural characterization of bioactive compound.
Table 5.
Antimicrobial activity of purified compound.
| Pathogenic fungi/compound | Zone of inhibition (mm) |
MIC of compound(mg/mL) |
||||||
|---|---|---|---|---|---|---|---|---|
| F.moniliforme | S.oryzae | C.albicans | C.tropicalis | F.moniliforme | S.oryzae | C.albicans | C.tropicalis | |
| Compound (50 mg) | 12.5 ± 0.18 | 13.8 ± 0.15 | 11.5 ± 0.15 | 12.4 ± 0.14 | 12.5 | 12.5 | 6.25 | 6.25 |
| Compound (100 mg) | 14.5 ± 0.22 | 16.6 ± 0.14 | 14.8 ± 0.20 | 15.6 ± 0.22 | – | – | – | – |
| Amphotericin- B | – | – | 18.0 | 18.0 | – | – | – | – |
| Propiconazole | 17.5 | 17.5 | – | – | – | – | – | – |
Fig. 6.
Anti-fungal activity Cladosporium delicatulum isolated bioactive compound 5- hydroxyl- 2- methylnaptalene-1,4-dione.
4. Discussion
Medicinal plants around the biosphere appear to harbor a vast diversity of endophytic microorganisms, including many fungal organisms which are potential producers of bioactive metabolites [5]. Some of these metabolites may serve as potential precursors for developing new drugs against plant and human pathogens. With this rationale in mind we investigated the phylogenetic diversity of endophytic fungi associated with Terminalia pallida, Rhyncosia beddomei, Pterocarpus santalinus, endemic medicinal plants as well as the ability of endophytes to produce antimicrobial metabolites.
The diversity of fungal endophytes found associated with the above endemic medicinal plants has been reported to exhibit antimicrobial and anticancer activity [41,42]. Endophytes may also be significant in developing ecological adaptability by enhancing tolerance to environmental stress conditions [43,44]. Among the benefits that endophytes confer to their host plant includes higher antioxidant level, plant growth hormone production and anti-herbivore alkaloids in addition to enriched photosynthesis, which is likely to develop the fitness of the host plant [45,46].
Therefore in this study we isolated endophytic fungi using four different nutrient Media and statistically analyzed the abundance, species richness, Shannon-Weaver diversity (H), Simpson diversity (D), Species evenness (E) of individual leaf and stem samples from three well established endemic medicinal plants and also calculated colonization frequency of all endophytic fungal isolates.
In this study, we identified five major compounds by GC–MS analysis and found Decanoic acid as a major compound. Previously Senthil kumar et al., reported tetradecanoic acid, dodecanoic acid and n-hexadecanoic acid in the extracts of conidia bearing heads of Aspergillus versicolor [47]. Similarly, Griffith et al., and Wang et al., reported Dodecanoic acid and tetradecanoic acid from P. chrysogenum [48,49]. However, Pentadecanoic acid and myristic acid isolation and their biological activities were reported by Theantana et al., [50]. Interestingly, Hexadecanoic acid and octadecanoic acid methyl ketones were isolated in fungal endophytes obtained from medicinal plants which are used in traditional medicines. Hexadecene was isolated from an extract of Monochaetia kansensis, Dodecene was isolated from an endophytic fungi Fusarium solani associated with Taxus baccata [51]. Dodecanoic acid ethyl ketones, Phthalic acid, octyl 2-pentyl ketones might be the reason for antimicrobial activity of endophytic fungi [52]. Neodin 18, Naphthalene is used as an antimicrobial, insecticide, insect repellent, anthelminthic and vermicide [53]. This study clearly demonstrates that the endophytic fungus is an efficient source for the commercial production of plumbagin. Furthermore, the commercialization of plumbagin needs additional work to optimize the yield of plumbagin from milligrams to grams by studying its biosynthetic and genetic pathway details of Cladosporium delicatulum.
Many endophytic microorganisms produce secondary metabolites that are useful to host plant in defense against pathogens and pests [55,54]. However, our target is not to limit the fungal endophytes for the exploration of their host metabolites but to explore potential applications and characterization of their novel and potential bioactive compounds. Previously crude extracts of Fusarium tricinctum, Gibberella avenaceae and Alternaria sp., were effective against fungal pathogens and Candida sp., [56]. Endophytes are also source of novel bioactive metabolites that are produced by endophytic fungi in the Cassia spectabilis, Isolation and antimicrobial, anticancer activity of (E)-3-(2, 3-dihydroxyphenyl) acrylic acid from Fusarium equseti from endemic medicinal plants in the Eastern Ghats [57]. Nithya & Muthumary verified that the genus Phomopsis possesses strong antibacterial activity [58]. Natural compound isolated from C. cladosporioides showed effective inhibition of several pathogenic bacteria [59]. In vitro antimicrobial and insecticidal activity of plumbagin isolated from plumbago species is reported by Rischer et al., and Paiva et al., [60,61]. In the current study we prepared crude extracts from 40 endophytic fungal isolates and screened for antimicrobial activities against 4 plant and human pathogenic organisms. This crude extract was fractionated by using column chromatography technique and the pure fraction was characterized and identified as plumbagin (5- hydroxyl- 2- methylnaptalene-1,4-dione) as analyzed by GC–MS and ESI-MS analysis.
5. Conclusion
In conclusion, endophytic fungi have been considered as a promising source for the development of biological control agents (BCAs) against human and phytopathogens, as they exhibit the beneficial functions towards host plants. From isolated fungal strains, Cladosporium delicatulum was identified and selected as the most potent plumbagin-producing strain. Further the isolation and structural characterization of Endophytic fungal plumbagin was confirmed through TLC, FT-IR, GC–MS, ESI-MS and NMR spectroscopy analysis. In addition, the molecular weight plumbagin (5- hydroxyl- 2- methylnaptalene-1,4-dione) was determined as m/z 188 in positive mode by ESI-MS, which clearly confirmed the separated constituent to be plumbagin which potentially inhibited all tested pathogens, with an MIC values of 12.5 mg/mL. Investigations on the interactions of endemic plants and its endophyte would be the next direction for the future research.
Conflict of interest
We declare we have no conflict of interest.
Acknowledgment
The present study was supported by the Department of Biotechnology, New Delhi, Govt. of India under major research project (BT/PR5194/MED/29/449/2012) The authors are grateful to the University Grants Commission (UGC), New Delhi, India for their financial support (F.4-1/2016(BSR) 7-359/2012.BSR).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2018.e00282.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Tran H.B.O., McRae J.M., Lynch F., Palombo E.A. In: Current Research, Technology and Education Topics in Applied Microbiology and Biotechnology. Mendez-Vilas A., editor. 2010. Identification and bioactive properties of endophytic fungi isolated from phyllodes of Acacia species. pp. 377–382. [Google Scholar]
- 2.Thompson M. University of Southern Queensland; 2014. An Investigation of the Ecology and Bioactive Compounds of Pittosporum angustifolium Endophytes. Doctoral dissertation. [Google Scholar]
- 3.Strobel G.A., Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debbab A., Aly A.H., Proksch P. Endophytes and associated marine derived fungi-ecological and chemical perspectives. Fungal Divers. 2012;57:45–83. [Google Scholar]
- 5.Rosa L.H., Vieira M.L.A., Santiago I.F., Rosa C.A. Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antartica. FEMS Microbiol. Ecol. 2010;73:178–189. doi: 10.1111/j.1574-6941.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj A., Sharma D., Jodan N., Agrawal P.K. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus rouxburghii. Arch. Clin. Microbiol. 2015;6(3):1. [Google Scholar]
- 7.Reddy K.N., Reddy C.S. First red list of medicinal plants of Andhra Pradesh, India-conservation assessment and management planning. Ethnobot. Leaflets. 2008;12:103–107. [Google Scholar]
- 8.Pullaiah T., Kuruppusamy S. vol. 5. Scientific Publishers; New Delhi: 2008. (Flora of Andhra Pradesh). (5) 83. [Google Scholar]
- 9.Madhava Chetty K., Sivaji K., Tulasi Rao K. Scientific Publishers; Jodhpur India: 2008. Flowering plants of Chittoor district Andhra Pradesh India; pp. 330–333. [Google Scholar]
- 10.Kaul S., Gupta S., Ahmed M., Dhar M.K. Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites. Phytochem. Rev. 2012;11:487–505. doi: 10.1007/s11101-012-9260-62. [DOI] [Google Scholar]
- 11.Kusari S., Pandey S.P., Spiteller M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Photochemistry. 2013;91:81–87. doi: 10.1016/j.phytochem.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Hanada R.E., Pomella A.W.V., Costa H.S., Bezerra J.L., Loguercio L.L., Pereira J.O. Endophytic fungal diversity in Theobroma cacao (cacao) and T. Grandiflorum (cupuaçu) trees and their potential for growth promotion and biocontrol of black-pod disease. Fungal Biol. 2010;114(11):901–910. doi: 10.1016/j.funbio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Zheng You-Kun, Miao Cui-Ping, Chen Hua-Hong, Huang Fang-Fang, Xia Yu-Mei, Chen You-Wei, Zhao Li-Xing. Endophytic fungi harbored in Panax notoginseng: diversity and potential as biological control agents against host plant pathogens of root-rot disease. J. Ginseng Res. 2017;41(3):353–360. doi: 10.1016/j.jgr.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q., Zhang J., Yang L., Zhang L., Jiang D., Chen W., Li G. Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol. Control. 2014;72:98–108. [Google Scholar]
- 15.Liang H., Xing Y., Chen J., Zhang D., Guo S., Wang C. Antimicrobial activities of endophytic fungi isolated from Ophiopogon japonicus (Liliaceae) BMC Complement. Altern. Med. 2012;12(238):1–6. doi: 10.1186/1472-6882-12-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Idris A., Ietidal A., Idris M. Antibacterial activity of endophytic fungi extracts from the medicinal plant Kigelia africana. Egypt Acad. J. Biol. Sci. 2013;5(1):1–9. [Google Scholar]
- 17.Gherbawy Y., Gashgari R. Molecular characterization of endophytic fungi from Calotropisprocera plants in Taif region (Saudi Arabia) and their antifungal activities. Plant Biosyst. 2014;148(6):1085–1092. doi: 10.1080/11263504.2013.819043. [DOI] [Google Scholar]
- 18.Morath S.U., Hung R., Bennett J.W. Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012;26:73–83. [Google Scholar]
- 19.Kumar S., Kaushik N. Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narisawa K., Usuki F., Hashiba T. Control of verticillium yellows in Chinese cabbage by the dark septate endophytic fungus LtVB3. Phytopathology. 2004;94:412–418. doi: 10.1094/PHYTO.2004.94.5.412. [DOI] [PubMed] [Google Scholar]
- 21.Waller F., Achatz B., Baltruschat H., Fodor J., Becker K., Fischer M., Heier T., Huckelhoven R., Neumann C., von Wettstein D., Franken P., Kogel K.H. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13386–13391. doi: 10.1073/pnas.0504423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke B.B., White J.F.J., Hurley R.H., Torres M.S., Sun S., Huff D.R. Endophyte-mediated suppression of dollar spot disease in fine fescues. Plant Dis. 2006;90:994–998. doi: 10.1094/PD-90-0994. [DOI] [PubMed] [Google Scholar]
- 23.Varma A., Bakshi M., Lou B.G., Hartmann A., Oelmueller R. Piriformosporaindica: a novel plant growth-promoting mycorrhizal fungus. Agric. Res. 2012;1:117–131. [Google Scholar]
- 24.Gao K., Liu X., Kang Z., Mendgen K. Mycoparasitism of Rhizoctoniasolani by endophytic chaetomiumspirale ND35: ultrastructure and cytochemistry of the interaction. J. Phytopathol. 2005;153:280–290. [Google Scholar]
- 25.Schulz B., Wanke U., Draeger S., Aust H.J. Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilisation methods. Mycol. Res. 1993;97:1447–1450. [Google Scholar]
- 26.Strobel G., Daisy B., Castillo U., Harper J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004;67:257–268. doi: 10.1021/np030397v. [DOI] [PubMed] [Google Scholar]
- 27.Kharwar R.N., Verma V.C., Strobel G., Ezra D. The endophytic fungal complex of Catharanthus roseus (L.) G. Don. Curr. Sci. 2008;95:228–233. [Google Scholar]
- 28.Hata K., Futai K. Endophytic fungi associated healthy pine needles infested by the pine needle gall midge, Thecodiplosis japonensis. Can. J. Bot. 1995;73:384–390. [Google Scholar]
- 29.Gond S.K., Mishra A., Sharma V.K., Verma S.K., Kumar J., Kharwar R.N., Kumar A. Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis, a well-known medicinal plant of India. Mycoscience. 2012;53(2):113–121. [Google Scholar]
- 30.Barnett H.L., Hunter B.B. 4th ed. APS Press; St. Paul, Minnesota: 1998. Illustrated Genera of Imperfect Fungi; pp. 1–218. [Google Scholar]
- 31.Bhat M.I., Rashid A., Rasool F., Mahdi S.S., Haq S.A., Bhat R.A. Effect of Rhizobium and VA-mycorrhizae on green gram under temperate conditions. Res. J. Agric. Sci. 2010;1(2):113–116. [Google Scholar]
- 32.Hanlin R.T. APS Press, The American Phytopathological Society; St. Paul, Minnesota: 1990. Illustrated Genera of Ascomycetes. pp. 247–255. [Google Scholar]
- 33.Manohara chary C., Sridhar K., Singh R., Adholeya A., Suryanarayanan T.S., Rawat S., Johri B.N. Fungal biodiversity: distribution, conservation and prospecting of fungi from India. Curr. Sci. 2005;89(1):58–71. [Google Scholar]
- 34.Leslie J.F., Summerell B.A. Blackwell Publishing Ltd; Iowa: 2006. The Fusarium Laboratory Manual. [Google Scholar]
- 35.Kornerup A., Wanscher J.H. 3rd english edition. Methuen & Co.; London: 1978. Methuen Handbook of Colour; p. 3. revised. [Google Scholar]
- 36.Bellemain E., Carlsen T., Brochmann C. ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol. 2010;10:189–195. doi: 10.1186/1471-2180-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsson R.H., Ryberg M., Sjökvist E., Abarenkov K. Rethinking taxon sampling in the light of environmental sequencing. Cladistics. 2011;27:197–203. doi: 10.1111/j.1096-0031.2010.00336.x. [DOI] [PubMed] [Google Scholar]
- 38.Larkin M.A., Blackshields G., Brown N.P., Chenna R., Mc Gettigan P.A., Mc William H., Thompson J.D. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 39.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 40.Gahlaut A., Chhillar A.K. Evaluation of antibacterial potential of plant extracts using resazurin based microtiter dilution assay. Int. J. Pharm. Pharm. Sci. 2013;5(2):372–376. [Google Scholar]
- 41.Carvalho C.R., Gonçalves V.N., Pereira C.B., Johann S., Galliza I.V., Alves T.M.A. The diversity, antimicrobial and anticancer activity of endophytic fungi associated with the medicinal plant Stryphnodendron adstringens (Mart.) Coville (Fabaceae) from the Brazilian savannah. Symbiosis. 2012;57:95–107. [Google Scholar]
- 42.Vieira M.L.A., Hughes A.F.S., Gil V.B., Vaz A.B.M., Alves T.M.A., Zani C.L. Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuumVell. (Solanaceae) Can. J. Microbiol. 2012;58:54–66. doi: 10.1139/w11-105. [DOI] [PubMed] [Google Scholar]
- 43.Khan A.L., Hamayun M., Kim Y.H., Kang S.M., Lee J.H., Lee I.J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, plant growth and isoflavone biosynthesis in soybean under salt stress. Process Biochem. 2011;46:440–447. [Google Scholar]
- 44.Stępniewska Z., Kuźniar A., Pytlak A., Szymczycha J. Detection of methanotrophic endosymbionts in Sphagnum sp. originating from Moszne peat bog (East Poland) Afr. J. Microbiol. Res. 2013;7:1319–1325. [Google Scholar]
- 45.Sanchez-Azofeifa A., Oki Y., Wilson fernandes G., Ball R.A., Gamon J. Relationships between endophyte diversity and leaf optical properties. Trees. 2011;26:291–299. [Google Scholar]
- 46.Gundel P.E., Helander M., Casas C., Hamilton C.E., Faeth S.H., Saikkonen K. Neotyphodium fungal endophyte in tall fescue (Schedonorus phoenix): a comparison of three Northern European wild populations and the cultivar Kentucky- 31. Fungal Divers. 2012;60:15–24. [Google Scholar]
- 47.Senthilkumar G., Madhanraj P., Panneerselvam S. A study on the compounds and its antifungal potentiality of Fungi isolated from paddy field soils of Jenbagapuram Village, Thanjavur District, and South India. Asian J. Pharm. Res. 2011;1:19–21. [Google Scholar]
- 48.Griffith R.T., Jayachandran K., Shetty K., Whitstine W., Futron K.G. Differential of toxic molds via headspace SPME-GC/Ms and canine detection. Sensors. 2007;7:1496–1508. [Google Scholar]
- 49.Wang X., Yao J., Yu Z. GC–MS determination of fatty acids in arachidonic acid high-yield strain induced by low energy ion implantation. Chem. Pap. 2005;59:240–243. [Google Scholar]
- 50.Theantana T., Kanjanapothi D., Lumyong S. In vitro inhibition of lipid peroxidation and the antioxidant system of endophytic Fungi from Thai medicinal plants. Chiang Mai. J Sci. 2002;39:429–444. [Google Scholar]
- 51.Tayung K., Barik B.P., Jha D.K., Deka D.C. Identification and characterization of antimicrobial metabolite from an endophytic fungus, Fusarium solani isolated from bark of Himalayan yew. Mycosphere. 2001;2:203–213. [Google Scholar]
- 52.Aoyagi A., Ito-Kobayashi M., Ono Y., Furukawa Y., Takahashi M., Muramatsu Y., Umetani M., Takatsu T. Colletoic acid, a novel β -hydroxysteroid dehydrogenase Type 1 inhibitor from Colletotrichum gloeosporioides SANK 21404. J. Antibiot. 2008;61:136–141. doi: 10.1038/ja.2008.122. [DOI] [PubMed] [Google Scholar]
- 53.Demain A.L. Biodiversity: New Leads for Pharmaceutical and Agrochemical Industries. The Royal Society of Chemistry; Cambridge, United Kingdom: 2000. Microbial natural products: a past with a future. pp. 3–16. [Google Scholar]
- 54.Aly A.H., Debbab A., Kjer J., Proksch P. Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010;41(1):1–16. [Google Scholar]
- 55.Taechowisan T., Lu C., Shen Y., Lumyong S. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology. 2005;151:1691–1695. doi: 10.1099/mic.0.27758-0. [DOI] [PubMed] [Google Scholar]
- 56.Meca G., Soriano J.M., Gaspari A., Ritieni A., Moretti A., Mañes J. Antifungal effects of the bioactive compounds enniatins A, A(1), B, B(1) Toxicon. 2010;56(3):480–485. doi: 10.1016/j.toxicon.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 57.Venkateswarulu N., Chari P.V.B., Nagaraju C., Vijaya T. Isolation and purification of (E)-3-(2, 3-dihydroxyphenyl) acrylic acid from endophytic fungi Fusarium equseti EF-32 and its anti-candidal and anticancer activities. Biocatal. Agric. Biotechnol. 2017;11:294–301. [Google Scholar]
- 58.Nithya K., Muthumary J. Secondary metabolite from Phomopsis sp. isolated from Plumeria acutifolia Poiret. Recent Res. Sci. Technol. 2010;4:99–103. [Google Scholar]
- 59.Zhang P., Zhou P.P., Yu L.J. An endophytic taxol producing fungus from Taxus media, Cladosporium cladosporioides MD2. Curr. Microbiol. 2009;59:227–232. doi: 10.1007/s00284-008-9270-1. [DOI] [PubMed] [Google Scholar]
- 60.Rischer Heiko, Hamm Andreas, Bringmann Gerhard. Nepenthes insignis uses a C2-portion of the carbon skeleton of L-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry. 2002;59(6):603–609. doi: 10.1016/s0031-9422(02)00003-1. [DOI] [PubMed] [Google Scholar]
- 61.Paiva Selma Ribeirode, Figueiredo Maria Raquel, Aragão TâniaVerônica, Coelho Kaplan Maria Auxiliadora. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Memorias do Instituto Oswaldo Cruz. 2003;98(7):959–961. doi: 10.1590/s0074-02762003000700017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.