Abstract
Cutaneous afferents convey exteroceptive information about the interaction of the body with the environment and proprioceptive information about body position and orientation. Four classes of low-threshold mechanoreceptor afferents innervate the foot sole and transmit feedback that facilitates the conscious and reflexive control of standing balance. Experimental manipulation of cutaneous feedback has been shown to alter the control of gait and standing balance. This has led to a growing interest in the design of intervention strategies that enhance cutaneous feedback and improve postural control. The advent of single-unit microneurography has allowed the firing and receptive field characteristics of foot sole cutaneous afferents to be investigated. In this review, we consolidate the available cutaneous afferent microneurographic recordings from the foot sole and provide an analysis of the firing threshold, and receptive field distribution and density of these cutaneous afferents. This work enhances the understanding of the foot sole as a sensory structure and provides a foundation for the continued development of sensory augmentation insoles and other tactile enhancement interventions.
Keywords: cutaneous afferents, foot sole, mechanoreceptor, microneurography, tactile feedback
INTRODUCTION
Four classes of low-threshold cutaneous mechanoreceptors innervate the glabrous skin on the sole of the foot and palm of the hand. Each class is uniquely sensitive to deformation and motion of the skin and transmits tactile and proprioceptive feedback through sensory afferents to the central nervous system (CNS) (McGlone and Reilly, 2010). The development of microneurography in the 1960s by Hagbarth and Vallbo permitted the study of single cutaneous afferents in awake human subjects (Hagbarth and Vallbo 1967; Vallbo et al. 2004). The technique was originally developed in the arm, and the understanding of cutaneous afferent firing and receptive field characteristics is largely a product of these early studies that investigated afferent recordings from the hand (Hagbarth et al. 1970; Johansson and Vallbo 1979a; Knibestöl and Vallbo 1970). The same classes of mechanoreceptor afferents as those described in the hand innervate the foot sole (Kennedy and Inglis 2002; Miller and Kasahara 1959); however, fewer studies have recorded cutaneous afferents in the lower limb. To understand the functional role of cutaneous feedback, the distribution and firing thresholds of individual cutaneous afferents across the body must first be assessed. In this review, we summarize microneurographic recordings made from several populations of foot sole cutaneous afferents. We provide an analysis of mechanoreceptor firing thresholds and receptive field characteristics, as well as provide afferent distribution and density calculations.
Why study foot sole cutaneous afferents? Cutaneous feedback from the soles of the feet plays an important role in the control of gait and standing balance (Inglis et al. 2002; Kavounoudias et al. 1998; Zehr et al. 2014). Skin stretch and pressure feedback associated with standing balance are conveyed by cutaneous afferents into the CNS, where it interacts with descending motor commands at the spinal cord and reflexively modulates motor neuron excitability (Bent and Lowrey 2013; Fallon et al. 2005; Zehr and Stein 1999). Furthermore, cutaneous feedback provides proprioceptive cues at the ankle joint (Howe et al. 2015; Lowrey et al. 2010; Mildren et al. 2017) and a sense of body movement with respect to the ground (Kavounoudias et al. 1998). In situations where this cutaneous feedback is impaired, either experimentally through cooling (Eils et al. 2004), local anesthesia (Meyer et al. 2004a), or naturally through aging (Perry 2006; Peters et al. 2016) and disease (Kars et al. 2009; Prätorius et al. 2003), the control of standing balance is compromised. To fully understand how afferent feedback can contribute to the control of standing balance, we must first establish the capabilities of foot sole cutaneous afferents to respond to tactile input.
Previous work has thoroughly presented the specialization of each mechanoreceptor ending with associated afferent firing properties in the hand (Johnson 2001; Macefield 1998). The hand and feet contain the same classes of mechanoreceptor endings and detailed descriptions of these endings can be found in previous studies (Abraira and Ginty 2013; Chambers et al. 1972; Fortman and Winkelmann 1973; Iggo and Andres 1982; Loewenstein and Skalak 1966). The objective of the current review is to provide a physiological summary of a selection of microneurographic recordings made from cutaneous afferents innervating the human foot sole.
We have compiled the published tibial nerve cutaneous afferent recordings available in the literature (Fallon et al. 2005; Kennedy and Inglis 2002; Lowrey et al. 2013; Strzalkowski et al. 2015a), in addition to 72 unpublished foot sole units. From the 401 units identified, 364 were in the plantar surface of the foot sole and form the basis of the analysis in this review. We begin with a brief description of the technique of microneurography and review how the four classes of cutaneous afferents were collected and classified. Next, we summarize the foot sole cutaneous afferent literature and provide new insights highlighting afferent firing threshold, receptive field characteristics, and distribution, as well as provide the first estimates of foot sole innervation density.
MICRONEUROGRAPHY: SINGLE-UNIT RECORDINGS
Signals provided between individual neurons represent the fundamental mechanism for information transfer in the nervous system (Parker and Newsome 1998). Microneurography is a method to record peripheral nerve activity in awake human subjects and provides a tool to link neural activity with functional outcomes. The original technique was developed in Uppsala Sweden by Karl-Erik Hagbarth and Åke Vallbo between 1965 and 1966, with the initial interest to study human muscle spindles from multiunit recordings (Vallbo et al. 2004). Since then, microneurography has been applied to the study of cutaneous mechanoreceptor, thermoreceptor, and nociceptor afferents, C-tactile afferents, Golgi tendon organs, joint receptors, muscle spindles, and cutaneous and muscle sympathetic efferents (Ackerley et al. 2014; Campero et al. 2001; Condon et al. 2014; Hagbarth 2002; Macefield 2005; Ochoa and Torebjörk 1989; Peters et al. 2017; Pruszynski and Johansson 2014; Roll and Vedel 1982; Strzalkowski et al. 2016; Wallin and Elam 1994). The technique was developed in the arm, and the majority of recordings have been made from the forearm and hand; however, there is growing interest in studying the lower limb (Aimonetti et al. 2007; Bent and Lowrey 2013; Kennedy and Inglis 2002; Lowrey et al. 2013; Ribot-Ciscar et al. 1989; Strzalkowski et al. 2015a; Trulsson 2001).
Microneurography involves the percutaneous insertion of two tungsten microelectrodes: one reference, placed a few millimeters under the skin, and one recording electrode, manually inserted into a peripheral nerve (Fig. 1). The target nerve for foot sole cutaneous afferents is the tibial nerve, and recordings are made at the level of the popliteal fossa where the tibial nerve runs several centimeters below the skin. The tibial nerve divides into three terminal branches distal to the popliteal fossa: the lateral and medial plantar nerves and the medial calcaneal branches (Davis and Schon 1995). Together these branches innervate the skin on the foot sole with the exception of the far medial arch, which is supplied by the saphaneous terminal branch of the femoral nerve. Tibial nerve microneurography therefore provides a nearly complete picture of foot sole innervation. For detailed reviews on the microneurography technique and applications, we recommend Bergenheim et al. (1999), Gandevia and Hales (1997), Hagbarth (2002), and Vallbo et al. (2004).
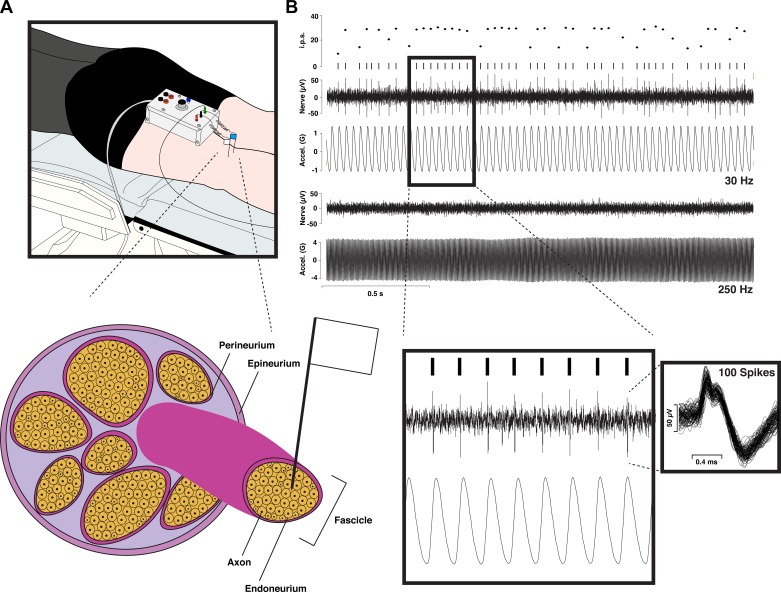
Fig. 1.
An illustration of the human microneurography technique. A, top: schematic of experimental setup for recording from the tibial nerve at the level of the knee (popliteal fossa). Two tungsten microelectrodes are inserted percutaneously, with one serving as the reference electrode inserted beneath the skin near the nerve and the other serving as the active electrode, which gets inserted into the nerve. Bottom, schematic of a peripheral nerve, showing the active electrode’s placement into an individual nerve fascicle, right up next to a single axon (i.e., intrafascicular extracellular recording). B: sample recording from a fast-adapting type I (FAI) afferent showing, from top to bottom, the instantaneous firing rate (i.p.s., impulses per second), raster plot, raw neurogram (Nerve), and vibrator acceleration (Accel.) for the case of 30- and 250-Hz vibration. As expected based on the FAI bandwidth, this unit codes precisely for the 30-Hz vibration with a phase-locked 30-Hz spike train but fails to be activated by the 250-Hz stimulation. Inset left: sample of phase-locking in the FAI response with the timescale expanded. Inset right: 100 overlaid spikes (note: the double-peaked action potential morphology indicates that the microelectrode has not caused conduction blockage; see Inglis et al. 1996).
OVERVIEW OF CUTANEOUS AFFERENTS
Cutaneous mechanoreceptors and their associated afferents are the fundamental units for the transduction and transmission of tactile feedback to the CNS (Abraira and Ginty 2013; Johnson 2001; Zimmerman et al. 2014). Cutaneous afferents are distinguished from other sensory systems for their high sensitivity and specificity to mechanical deformations of the skin. When vibration, pressure, or stretch is applied to the skin, mechanical deformations are transmitted through the tissue to the cutaneous afferent mechanoreceptor endings. Cutaneous afferents originate in the dorsal root ganglia and project distally to specialized mechanoreceptor endings within the epidermal and dermal layers of the skin and to central targets within the dorsal horn of the spinal cord and brain stem dorsal column nuclei (Zimmerman et al. 2014). For a detailed review of cutaneous afferent projections and processing, see Abraira and Ginty (2013).
Four specialized mechanoreceptor endings have been identified that innervate the glabrous skin of the hands (Jones and Smith 2014; Knibestöl and Vallbo 1970) and feet (Kennedy and Inglis 2002). The termination depth and morphology of the different mechanoreceptors dictate the unique firing characteristics exhibited by each cutaneous afferent class (Iggo 1977; Johnson 2001; Pruszynski and Johansson 2014). It is well established that each cutaneous afferent class preferentially encodes distinct tactile stimuli (Johnson 2001). This specialization allows populations of afferents to convey a wide range of tactile feedback with high resolution. The convergence of fast and slowly adapting afferent information onto neurons in primary somatosensory cortex (Pei et al. 2009; Saal and Bensmaia 2014) suggests that ultimately groups, rather than single cutaneous afferents or classes, are responsible for encoding tactile stimuli beyond simple light touch (Strzalkowski et al. 2015a).
Classification
The combination of sensory nerve and mechanoreceptor ending makes the sensory unit, commonly referred to as the cutaneous afferent. When isolated during a microneurographic recording, cutaneous afferents are classified on the basis of their ability to respond to sustained stimuli [fast adapting (FA) or slowly adapting (SA)] as well as their receptive field characteristics (type I or type II) (Bergenheim et al. 1999; Knibestöl and Vallbo 1970; Macefield 1998).
FA afferents are sensitive to the rate of change of mechanical stimuli and typically fire throughout the dynamic (acceleration) phase of an indentation, but they cease to fire once the indentation is sustained (Iggo 1977; Knibestöl 1973). FA afferents generally fire at the onset of a sustained indentation and again once the stimulus is removed. This is referred to as an on-off response. Conversely, SA afferents continue to fire throughout sustained indentations and skin stretch (Iggo 1977). SAI afferent responses are primarily related to the magnitude of the applied stimulus (Knibestöl 1975) and encode the strain distribution within the skin, which includes information about edges (Phillips and Johnson 1981) and curvature (Goodwin et al. 1997). Type I FA afferents are more responsive to tactile events such as the motion or slippage of an object across the skin, as well as coarse vibrations (Knibestöl 1973). The specialized adaptation properties of FA and SA afferents to sustained indentations are well established and remain the primary tool for the classification of cutaneous afferents as FA or SA during single-unit recordings.
Fast- and slowly adapting cutaneous afferents are further classified as type I (FAI and SAI) or type II (FAII and SAII) based primarily on their receptive field characteristics (Johansson 1978; Vallbo and Johansson 1984). A receptive field represents the area of skin wherein stimulation (e.g., skin indentation) can elicit a response in a given afferent. First characterized in the hand, receptive fields are traditionally measured as the area over which an afferent responds to an indentation force four to five times its firing threshold (Vallbo and Johansson 1984). This convention has been widely adopted, which permits receptive fields to be compared across experiments and body location. Afferent classes display unique receptive fields that arise from the branching pattern of the distal axons and the morphology and termination location of the mechanoreceptor ending(s).
Type I afferents branch as they enter the skin and terminate in multiple, small mechanoreceptor endings located in superficial skin layers (Abraira and Ginty 2013; Miller and Kasahara 1959; Vallbo and Johansson 1978). FAI afferents terminate in Meissner corpuscles in the dermal papillae, whereas SAI afferents terminate in Merkel cells in the basal layer of the epidermis (Abraira and Ginty 2013; Macefield 1998). As a result, type I afferents typically have small receptive fields (hand palm ~12 mm2, foot sole ~78 mm2) with distinct borders and multiple hot spots (Johansson and Vallbo 1980; Kennedy and Inglis 2002). In the hand, FAI afferents typically contain 12–17 such hot spots, whereas SAI afferents contain 4–7, which are thought to correspond to the number of mechanoreceptor endings in each class (Macefield and Birznieks 2009). In contrast, type II afferents do not branch within the skin and innervate a single, relatively large mechanoreceptor in the dermis and subcutaneous tissues. FAII afferents terminate in Pacinian corpuscles, and SAII afferents terminate in Ruffini endings (Abraira and Ginty 2013; Macefield 1998). In this way type II afferents are classified by their large receptive fields (hand palm ~88 mm2, foot sole ~560 mm2), with indiscriminate borders and a single zone of maximal sensitivity (Johansson and Vallbo 1980; Kennedy and Inglis 2002). In particular, FAII afferents are exceptionally sensitive to stimuli applied within, but also remotely to, their receptive fields, highlighted by their distinct ability to respond to blowing across the skin. SAII afferents are unique among the other classes in their sensitivity to respond to skin stretch applied through their receptive fields (Hulliger et al. 1979; Kennedy and Inglis 2002; Macefield and Birznieks 2009). The receptive fields of the combined foot sole afferents summarized in this review are presented in Fig. 2.
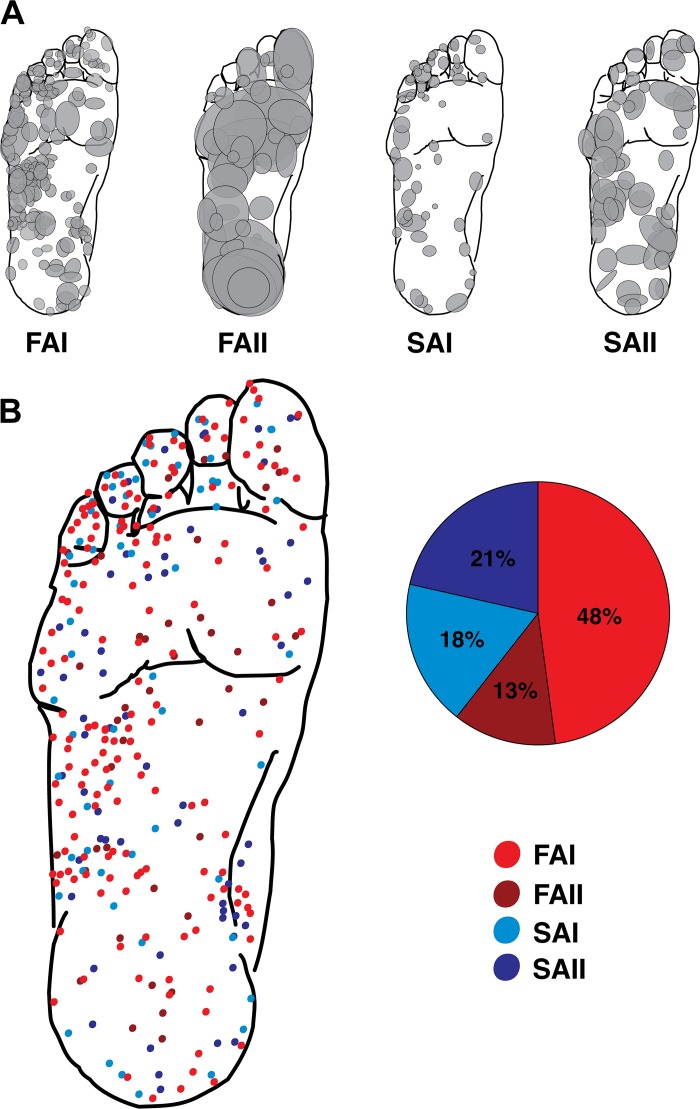
Fig. 2.
Receptive fields of the different cutaneous mechanoreceptor classes. A: foot sole maps for each afferent type showing all the receptive field locations and estimates of size in the present data set. Shaded ellipses represent individual afferent receptive fields. B: composite foot sole map showing the center of all receptive fields overlaid on the same foot template. Pie chart depicts the breakdown in terms of the percentages of each afferent type in the present data set. FAI, fast-adapting type I; FAII, fast-adapting type II; SAI, slowly adapting type I; SAII, slowly adapting type II.
CUTANEOUS AFFERENTS IN THE FOOT SOLE
Previous studies have provided an initial look at the characteristics of foot sole cutaneous afferents (Kennedy and Inglis 2002; Strzalkowski et al. 2015a, 2017); however low sample sizes have limited the ability to make clear estimates of afferent distribution and density. By combining published and unpublished microneurography recordings, this review provides a comprehensive summary of the foot sole cutaneous afferent literature and the first estimate of innervation density.
Methods Overview
We have combined published (Fallon et al. 2005; Kennedy and Inglis 2002; Lowrey et al. 2013; Strzalkowski et al. 2015a) and unpublished tibial nerve recordings to create a data set of 401 cutaneous afferents. The tibial nerve does not exclusively innervate the glabrous skin on the foot sole, and from this data set of 401 afferents, 37 were excluded from analysis because they did not have receptive fields on the sole of the foot. Of these excluded afferents, 23 afferents had receptive fields on the ankle, 4 in the nail bed, and 3 on the foot dorsum, and 7 afferents did not have locations reported. Calculations of afferent class firing threshold, receptive field size, distribution, and innervation density were made on the remaining sample of 364 foot sole cutaneous afferents (Table 1). All published and unpublished data were collected with approval from their local ethics boards and complied with the Deceleration of Helsinki.
Table 1.
Cutaneous afferent contribution from sources comprising the present data set
| FAI | FAII | SAI | SAII | Study Total | |
|---|---|---|---|---|---|
| Study | |||||
| Kennedy and Inglis 2002 | 59 (32%) | 10 (23%) | 15 (24%) | 14 (19%) | 98 (27%) |
| Fallon et al. 2005 | 19 (10%) | 4 (9%) | 14 (22%) | 9 (12%) | 46 (13%) |
| Bent and Lowrey 2013 | 2 (1%) | 1 (2%) | 0 (0%) | 1 (2%) | 4 (1%) |
| Lowrey et al. 2013 | 20 (11%) | 7 (17%) | 6 (10%) | 15 (20%) | 48 (13%) |
| Strzalkowski et al. 2015a | 48 (26%) | 13 (30%) | 21 (33%) | 21 (28%) | 103 (28%) |
| Bent laboratory (unpublished) | 3 (2%) | 2 (5%) | 2 (3%) | 2 (3%) | 9 (3%) |
| Inglis laboratory (unpublished) | 33 (18%) | 6 (14%) | 5 (8%) | 12 (16%) | 56 (15%) |
| Afferent total | 184 (100%) | 43 (100%) | 63 (100%) | 74 (100%) | 364 (100%) |
Values are numbers and percentages of total cutaneous afferent contributions to the present data set from published and unpublished sources. FAI, fast-adapting type I; FAII, fast-adapting type II; SAI, slowly adapting type I; SAII, slowly adapting type II.
To follow the approach of Johansson and Vallbo (1979a), who provided the first and only estimates of the afferent innervation density for the glabrous skin of the hand, we required two pieces of information: an estimate of the total number of cutaneous afferents in the plantar nerves and area measurements for the different foot sole skin regions. In lieu of cutaneous afferent counts for the plantar nerves, we approximated this value based on the value provided by Johansson and Vallbo (1979a) for the whole hand (17,023 units) and the observation that there is approximately one-tenth the myelinated fibers in the plantar nerves of the foot than in the median and ulnar nerves of the hand (Auplish and Hall 1998). This resulted in a total plantar cutaneous fiber estimate of 1,702 units. The sample of 364 foot sole units compiled in this review (Table 1) is sampled across several laboratories and multiple microneurographers and is assumed to be a random selection from this population of afferents innervating the foot sole. Although we cannot guarantee true randomness of afferent selection, we believe the sample compiled in this review provides an accurate representation of the class ratio and distribution of foot sole cutaneous afferents.
Last, to obtain area measurements for the different regions of the foot sole, we optically scanned the plantar surface of the right foot in eight adults (4 men, age 25–31 yr, US shoe size 10–12, and 4 women, age 25–28 yr, US shoe size 6–9; Scanjet 4600, Hewlett Packard), and digitally measured the various areas using ImageJ 1.42q (National Institutes for Health, Bethesda, MD). The foot sole was divided into nine distinct regions: the great toe, digits 2 to 5 (toes), the medial, middle, and lateral metatarsals, the medial, middle, and lateral arch, and the calcaneus (heel) (Fig. 3). These distinct foot regions were used to determine whether the different characteristics of interest (cutaneous afferent firing threshold, receptive field area, distribution, and density) varied by region.
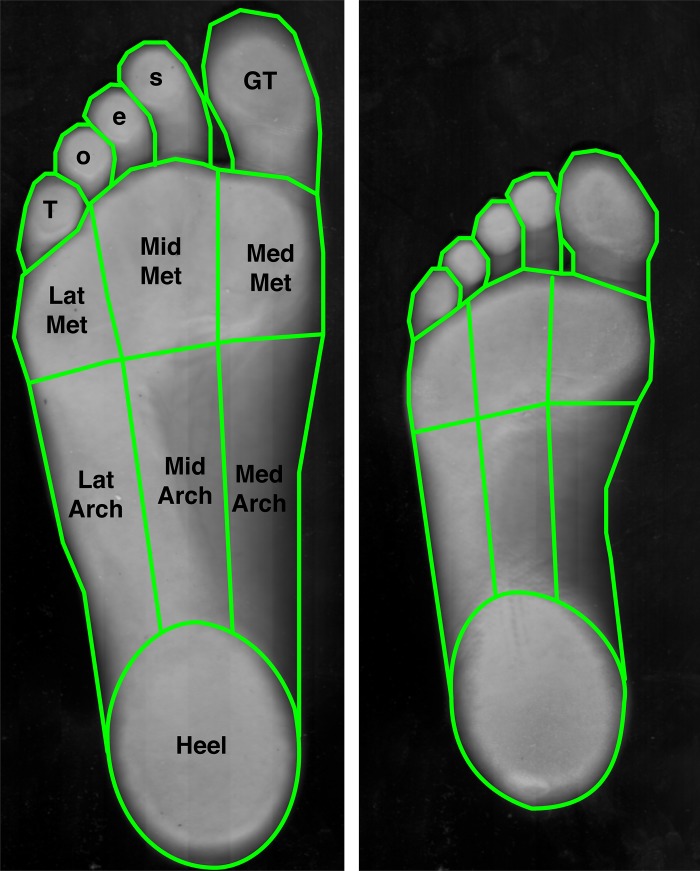
Fig. 3.
Foot sole area measurement. The surface areas of 9 different individual regions were measured on the foot soles of 4 men and 4 women. At left is the largest foot encountered (male, age 25 yr, U.S. men’s size 12 shoe), and at right is the smallest (female, age 25 yr, U.S. women’s size 6 shoe). The skin regions were traced from an optical scan of each individual’s right foot sole (light green outlines), and digital area measurements were made using ImageJ software. Toes, digits 2–5; GT, great toe; LatArch, MidArch, and MedArch, lateral, middle, and medial arch; LatMet, MidMet, and MedMet, lateral, middle, and medial metatarsals; Heel, calcaneus.
Firing Thresholds
Each class is uniquely tuned to different features of mechanical stimuli, which contributes to a comprehensive view of the tactile environment. Previous work in animals (Bensmaïa et al. 2005; Muniak et al. 2007; Phillips and Johnson 1981; Pubols et al. 1971; Werner and Mountcastle 1965) and the human hand (Condon et al. 2014; Hallin et al. 2002; Johansson and Vallbo 1979a; Johansson et al. 1982; Knibestöl and Vallbo 1970) has led to the current understanding of human cutaneous afferent firing characteristics and has formed the foundation for more recent experiments in the lower limb (Aimonetti et al. 2007; Kennedy and Inglis 2002; Strzalkowski et al. 2015a, 2017; Trulsson 2001). Below we review the firing thresholds recorded from cutaneous afferents in the foot sole (Table 2) and compare these to the hand to provide a more comprehensive look at the potential differences between the two sites.
Table 2.
Foot sole cutaneous afferent class numbers and percentages, monofilament firing thresholds, and receptive field areas
| Monofilament Threshold, g |
Receptive Field Area, mm2 |
||||
|---|---|---|---|---|---|
| Number (%) | Mean (median) | Range | Mean (median) | Range | |
| Afferent class | |||||
| FAI | 184 (51%) | 1.32 (0.69) | 0.04–8.51 | 80.6 (55.0) | 9.4–636.2 |
| FAII | 43 (12%) | 3.33 (0.50) | 0.03–60 | 872.7 (481.1) | 39.3–5222.9 |
| SAI | 63 (17%) | 4.12 (1.74) | 0.16–26 | 76.1 (66.4) | 4.7–294.5 |
| SAII | 74 (20%) | 25.16 (10.0) | 0.60–300 | 248.1 (171.6) | 7.1–1345.5 |
| Total | 364 (100%) | ||||
Values are numbers and percentages of foot sole cutaneous afferents by class, monofilament firing thresholds, and receptive field areas. FAI, fast-adapting type I; FAII, fast-adapting type II; SAI, slowly adapting type I; SAII, slowly adapting type II.
Monofilament testing is a common technique and standard measure of cutaneous afferent firing threshold. Semmes-Weinstein monofilaments (Collins et al. 2010) come in sets that include filaments of different gauges (length and diameter) that vary logarithmically in the load they apply. When applied perpendicular to the skin, each monofilament buckles and delivers a calibrated force (Collins et al. 2010). Cutaneous afferent threshold testing involves the application of monofilaments to the receptive field hotspot (most sensitive location) to determine the minimal force (threshold) that can reliably (~75%) evoke afferent discharge. Monofilaments only examine afferent light touch threshold, known to be conveyed by the FA afferents (Strzalkowski et al. 2015a), whereas other mechanical stimuli, such as stretch (Aimonetti et al. 2007) and vibration (Strzalkowski et al. 2017), have been used to further characterize the firing characteristics of lower limb cutaneous afferents. These studies have shown SAII afferents to be particularly sensitive to skin stretch and FAII afferents to be most responsive to high-frequency vibration. Despite the availability of other threshold tests, monofilaments remain the most common technique, and the literature provides a large sample of monofilament afferent firing thresholds for comparison.
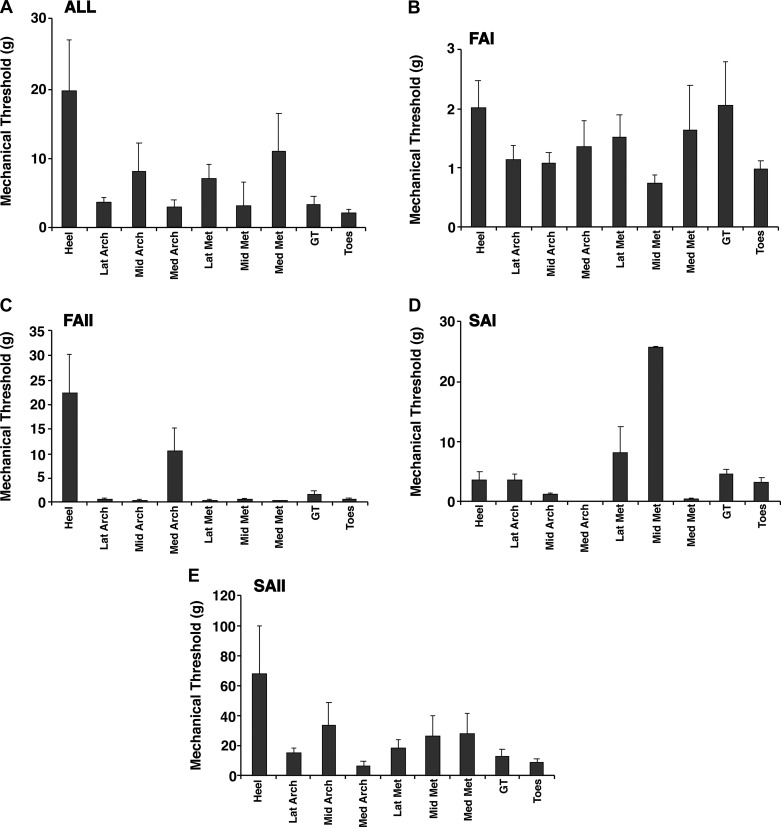
In the present review, we compiled the afferent monofilament firing thresholds across 1) classes and 2) foot sole region (Fig. 4). Afferents with firing thresholds outside ±3 SD of the class mean were excluded (4 units excluded). To determine if differences in mechanical thresholds between afferent classes and skin regions were significant, we performed a 4 (classes) × 9 (regions) factorial ANOVA on the observed threshold values. We observed significant effects of afferent class (F3,311 = 11.254, P < 0.001) and skin region (F8,311 = 2.329, P = 0.02); however, there was no class × region interaction (F24,311 = 1.547, P = 0.055). For afferent class, Tukey’s post hoc tests revealed that SAII afferents had higher mechanical thresholds than the other three classes (P < 0.001). For the different skin regions, Tukey’s post hoc tests additionally revealed that the heel has higher thresholds than the lateral arch and the toes (P < 0.05). Regional variation in afferent firing thresholds correspond well with previously reported monofilament (light touch) perceptual thresholds that are consistently found to be highest in the heel (Hennig and Sterzing 2009; Kekoni et al. 1989; Nurse and Nigg 1999; Strzalkowski et al. 2015a, 2015b). Across the foot sole, FA afferents consistently have lower firing thresholds than SA afferents. Median FAI and FAII afferent thresholds are 0.69 and 0.5 g, respectively, whereas SAI and SAII afferent thresholds are 1.74 and 10.0 g, respectively. Cutaneous afferent classes in the hand are similarly segregated by firing threshold, but at much lower thresholds (~10-fold) than those in the foot sole (hand median: FAI 0.06 g, FAII 0.05 g, SAI 0.13 g, SAII 0.76 g; Johansson and Vallbo 1980). Differences in firing threshold between hands and feet likely reflect an adaptation to the different functional demands of each region. Low firing thresholds in the hands are advantageous for manipulating objects, whereas high-threshold afferents from the foot sole may better serve the high forces of standing balance. The mechanical properties of the skin can partially explain some differences in firing thresholds between the hands and feet (Strzalkowski et al. 2015a); however, it is unclear if regional differences exist between the mechanoreceptor endings themselves. Future studies are needed to explore the firing patterns of cutaneous afferents under natural loaded and/or dynamic conditions.
Fig. 4.
Mechanical thresholds for the different cutaneous mechanoreceptor classes. Mean (SE) thresholds for evoking an action potential in the 9 different skin regions are given for all afferent types (A), fast-adapting type I (FAI; B), fast-adapting type II (FAII; C), slowly adapting type I (SAI; D), and slowly adapting type II (SAII; E). Heel, calcaneus; LatArch, MidArch, and MedArch, lateral, middle, and medial arch; LatMet, MidMet, and MedMet, lateral, middle, and medial metatarsals; GT, great toe; Toes, digits 2–5.
Receptive Field Characteristics
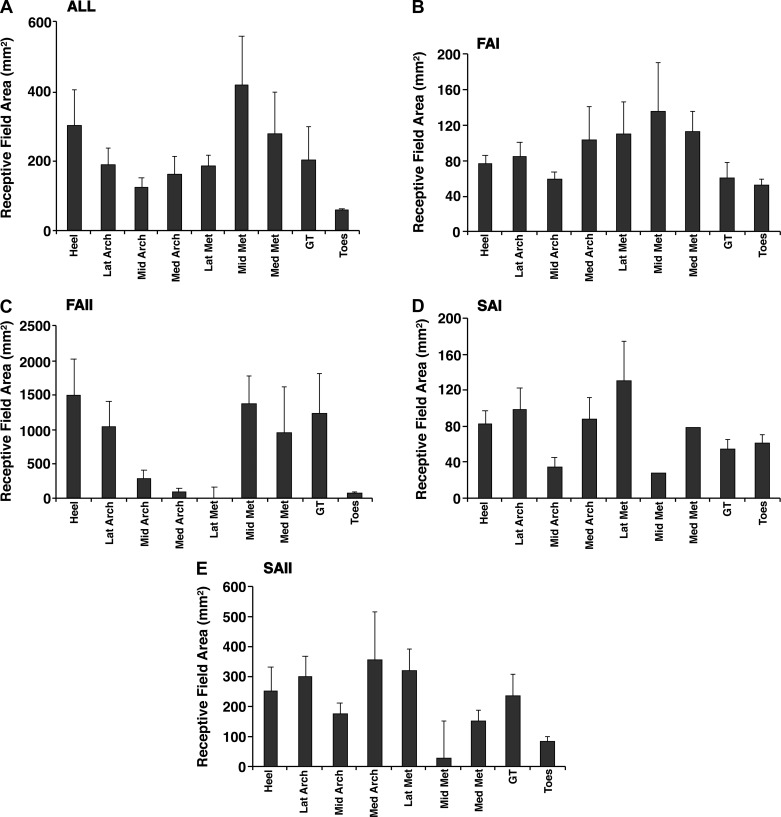
Receptive fields are traditionally mapped onto the skin surface using a monofilament that delivers a force four to five times greater than the afferent firing threshold (Johansson and Vallbo 1980; Vallbo and Johansson 1978). Receptive field borders are then drawn onto the foot sole by connecting the furthest points from the receptive field hotspot at which an afferent discharge can be evoked. These methods were used for all afferents in the present review (Figs. 2 and 5). To determine if differences in receptive field area between afferent classes and skin regions are significant, we performed a 4 (classes) × 9 (regions) factorial ANOVA on the observed receptive field area values. We observed significant effects of afferent class (F3,315 = 23.510, P < 0.001) and skin region (F8,315 = 3.643, P < 0.001), as well as a class × region interaction (F24,311 = 2.397, P < 0.001). For afferent class, Tukey’s post hoc tests revealed that FAII afferents have larger receptive fields than the other three classes (P < 0.001). SAII afferents also have larger receptive fields than FAI afferents (P < 0.05). For the different skin regions, Tukey’s post hoc tests additionally revealed that the toes have smaller receptive fields than the heel and middle metatarsal regions (P < 0.05).
Fig. 5.
Receptive field sizes for the different cutaneous mechanoreceptor classes. Mean (SE) areas of receptive fields in the 9 different skin regions are given for all afferent types (A), fast-adapting type I (FAI; B), fast-adapting type II (FAII; C), slowly adapting type I (SAI; D), and slowly adapting type II (SAII; E). Heel, calcaneus; LatArch, MidArch, and MedArch, lateral, middle, and medial arch; LatMet, MidMet, and MedMet, lateral, middle, and medial metatarsals; GT, great toe; Toes, digits 2–5.
The relationships between receptive field size, afferent class, and foot sole location are similar to those reported in the hand, although hand receptive fields are smaller than those in the foot sole (Johansson and Vallbo 1980; Knibestöl 1973, 1975). Type II afferents in the foot sole and hand have larger receptive fields (median foot sole: FAII 481.1 mm2, SAII 171.6 mm2; median hand: FAII 101.3 mm2, SAII 58.9 mm2) compared with type I afferents (median foot sole: FAI 55.0 mm2, SAI 66.4 mm2; median hand: FAI 12.6 mm2, SAI 11.0 mm2; Johansson and Vallbo 1980) (Table 2, Figs. 2 and 5). The toes and fingers have smaller receptive fields compared with the foot sole and hand palm, which is thought to reflect the physical boundaries of these regions. In the hand, FAI receptive fields have been shown to be 52% and SAI receptive fields 23% smaller in the fingers than the palm (Knibestöl 1973, 1975). Knibestöl used a glass probe to measure receptive fields, and direct area comparisons with the present data are not possible; however, toe receptive fields (median: FAI 42.4 mm2, FAII 71.1mm2, SAI 51.8 mm2, SAII 137.4 mm2) are smaller compared with the rest of the foot sole. Receptive field sizes reflect mechanoreceptor size and termination depth, and further work is needed to investigate the functional significance of receptive field differences between regions in the foot sole.
In summary, receptive field data provide a valuable way to understand the relative responsive areas between cutaneous afferent classes and regions. Smaller receptive fields enables the potential for greater resolution of tactile feedback. Foot sole receptive fields are found to be larger than those reported in the hands, with type II afferents displaying the largest receptive fields in both regions. Receptive field characteristics are thought to reflect class-specific mechanoreceptor morphology and termination depths. It is important to note that the method using four to five times threshold to calculate receptive fields in the hands and feet is arbitrary; however, it is a consistent method that has been used to quantify activation areas across body regions and afferent classes.
Receptive Field Distribution
The distribution of cutaneous afferents across the foot sole could indicate areas of relative tactile importance (concentration of afferents). In the hand, the high concentration of type I afferents in the finger tips relative to the palm is thought to reflect the functional significance of tactile feedback from the fingers (Johansson and Vallbo 1979b). To analyze the cutaneous afferent distribution in the foot sole, we began with a χ2 test across nine foot sole regions (Fig. 2). Based on the relative size of each plantar skin region, this test indicated that the observed proportion of units in each area was highly nonuniformly distributed (χ2 = 31.999, P < 0.001). We calculated the likelihood ratio of randomly sampling a cutaneous receptor in general, and for each class by dividing the proportion of the total units sampled in each region by the proportion of the total foot sole area for each region (Table 3). Following Johansson and Vallbo (1979a), we used binomial tests to examine pairwise differences between different plantar skin regions. The hypothesis tested by these binomial tests is given by the equation
where PA is the proportion of units sampled from region A of the total number of units sampled from regions A and B, and a and b are the areas of the two corresponding skin regions. Previous work reports an even distribution of cutaneous afferents across the foot sole (Kennedy and Inglis 2002); however, the present data demonstrate regional variation. Notably, the present data reveal a higher proportion of cutaneous afferents to innervate the toes (digits 2–5), as well as the lateral metatarsals and lateral arch, than expected if an even distribution was present (Table 3). To simplify the interpretation of this analysis, we chose to perform pairwise binomial tests for three distinct comparisons: proximal-distal over the whole foot sole, and medial to lateral for two regions, metatarsal and arch (see Fig. 6).
Table 3.
Distribution and innervation density estimate of cutaneous afferents across the foot sole
| Foot Sole Regions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| GT | Toes | MedMet | MidMet | LatMet | MedArch | MidArch | LatArch | Heel | |
| Mean area, cm2 | 13.2 | 16.9 | 17.2 | 16.6 | 14.2 | 19.3 | 20.4 | 21.6 | 38.8 |
| Total area, % | 7.4 | 9.5 | 9.7 | 9.3 | 8.0 | 10.8 | 11.4 | 12.1 | 21.8 |
| All afferents, % | 6.6 | 23.1 | 4.9 | 6.3 | 9.3 | 7.7 | 7.4 | 19.5 | 15.1 |
| No. of afferents | |||||||||
| All afferents | 24 | 84 | 18 | 23 | 34 | 28 | 27 | 71 | 55 |
| FAI | 13 | 44 | 7 | 12 | 17 | 15 | 12 | 40 | 24 |
| FAII | 4 | 5 | 3 | 5 | 2 | 2 | 5 | 7 | 10 |
| SAI | 3 | 25 | 1 | 1 | 5 | 3 | 3 | 13 | 9 |
| SAII | 4 | 10 | 7 | 5 | 10 | 8 | 7 | 11 | 12 |
| Likelihood ratios | |||||||||
| All afferents | 0.89 | 2.44 | 0.51 | 0.68 | 1.17 | 0.71 | 0.65 | 1.61 | 0.69 |
| FAI | 0.96 | 2.52 | 0.39 | 0.70 | 1.16 | 0.75 | 0.57 | 1.80 | 0.60 |
| FAII | 1.26 | 1.23 | 0.72 | 1.25 | 0.58 | 0.43 | 1.02 | 1.34 | 1.07 |
| SAI | 0.65 | 4.19 | 0.16 | 0.17 | 0.99 | 0.44 | 0.42 | 1.70 | 0.66 |
| SAII | 0.73 | 1.43 | 0.98 | 0.72 | 1.69 | 1.00 | 0.83 | 1.23 | 0.74 |
| Innervation density per cm2 | |||||||||
| All afferents | 8.53 | 23.25 | 4.88 | 6.48 | 11.16 | 6.78 | 6.21 | 15.39 | 6.62 |
| FAI | 4.62 | 12.18 | 1.90 | 3.38 | 5.58 | 3.63 | 2.76 | 8.67 | 2.89 |
| FAII | 1.42 | 1.38 | 0.81 | 1.41 | 0.66 | 0.48 | 1.15 | 1.52 | 1.20 |
| SAI | 1.07 | 6.92 | 0.27 | 0.28 | 1.64 | 0.73 | 0.69 | 2.82 | 1.08 |
| SAII | 1.42 | 2.77 | 1.90 | 1.41 | 3.28 | 1.94 | 1.61 | 2.38 | 1.45 |
GT, great toe; MedMet, MidMet, and LatMet, medial, middle, and lateral metatarsals; MedArch, MidArch, and LatArch, medial, middle, and lateral arch; FAI, fast-adapting type I; FAII, fast-adapting type II; SAI, slowly adapting type I; SAII, slowly adapting type II.
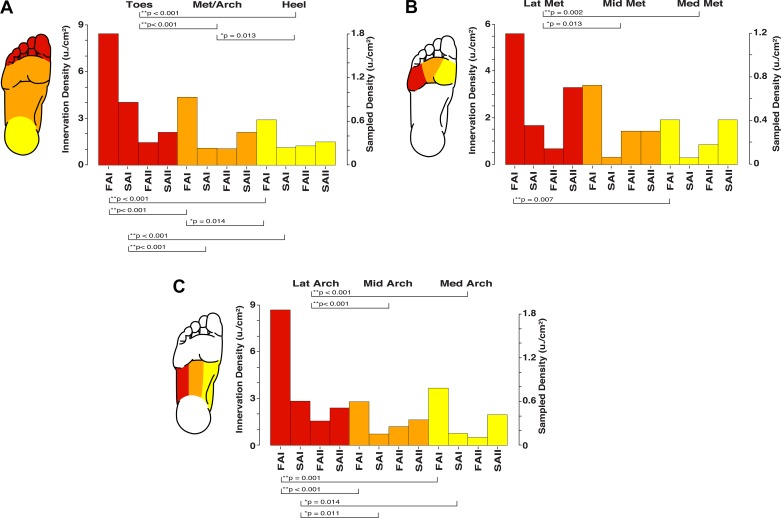
Fig. 6.
Estimates of the relative and absolute density for the different cutaneous mechanoreceptor classes across the foot sole. Significant differences are indicated by brackets and P values in A–C. A: depiction of the proximal-distal gradient in receptive field density, with greater innervation density in the toes (red) than in the metatarsals/arch (Met/Arch; orange) and heel (yellow). B: depiction of the medial-lateral gradient in receptive field density across the metatarsals, with greater innervation density in the lateral region (LatMet; red) than in the middle (MidMet; orange), and medial (MedMet; yellow) regions. C: depiction of the medial-lateral gradient in receptive field density across the arch, with greater innervation density in the lateral region (LatArch; red) than in the middle (MidArch; orange) and medial (MedArch; yellow) regions. FAI, fast-adapting type I; FAII, fast-adapting type II; SAI, slowly adapting type I; SAII, slowly adapting type II.
To investigate the potential for any proximal-distal distribution gradient, we compared the toes (collapsing over the great toe and digits 2–5), metatarsals/arch (collapsing over medial, middle, and lateral portions), and the heel. For all units, binomial tests revealed that the toes had significantly more sampled afferents than the metatarsals/arch (P < 0.001) and heel (P < 0.001), and the metatarsals/arch had significantly more sampled afferents than the heel (P = 0.013; see Fig. 6A). For FAI afferents, binomial tests revealed that the toes had significantly more sampled afferents than the metatarsals/arch (P < 0.001) and heel (P < 0.001), and the metatarsals/arch had significantly more sampled afferents than the heel (P = 0.014); for SAI afferents, binomial tests revealed that the toes had significantly more sampled afferents than the metatarsals/arch (P < 0.001) and heel (P < 0.001; Fig. 6A). For type II afferents (FAII and SAII), there were no significant differences in afferent distribution across the three skin regions. Thus we observed that the distribution of foot sole cutaneous afferents increases from the heel to the toes, driven primarily by type I afferents, with little evidence of a gradient for FAII and SAII afferents. This mirrors previous observations for the hand, where an abrupt increase in type I afferent density is observed in the fingertips compared with the middle phalanges and the palm (Johansson and Vallbo 1979a).
We additionally investigated the potential for a medial-lateral sampled distribution gradient. To accomplish this, we compared the medial, middle, and lateral portions of both the metatarsals and of the arch. In the metatarsals, for all units, binomial tests revealed that the lateral portion had a significantly greater number of sampled afferents than the middle (P = 0.013) and medial (P = 0.002) portions (see Fig. 6B). For FAI afferents, binomial tests revealed that the lateral portion of the metatarsals had significantly more sampled afferents than the medial portion (P = 0.007); SAI, FAII, and SAII afferents were uniformly distributed across the metatarsals (P > 0.05; Fig. 6B). Similarly, in the arch, for all units, binomial tests revealed that the lateral portion had significantly more sampled afferents than the middle (P < 0.001) and medial (P < 0.001) portions (see Fig. 6C). For FAI afferents, binomial tests revealed that the lateral portion of the arch had significantly more sampled afferents than the middle (P < 0.001) and medial portions (P = 0.001); similarly, for SAI afferents, binomial tests revealed that the lateral portion of the arch had significantly more sampled afferents than the middle (P = 0.011) and medial portions (P = 0.014), and FAII and SAII afferents were uniformly distributed across the arches (P > 0.05; Fig. 6C). These observations support the presence of a medial-to-lateral distribution gradient across both the metatarsals and arch, with a greater proportion of receptors residing in more lateral regions. A similar medial-lateral afferent distribution gradient is not observed in median nerve recordings of hand cutaneous afferents (Johansson and Vallbo 1979a).
The proximal-distal and medial-lateral distribution gradients of type I cutaneous afferents across the foot sole have not been reported previously. The smaller sample of cutaneous afferents analyzed by Kennedy and Inglis (2002) revealed an even distribution of cutaneous afferents across the foot sole. The present larger data set demonstrates that the foot sole displays regions of relatively high (toes, lateral border) and low (heel and medial border) afferent innervation, which is similar to the density gradients in the proximal-distal increase of cutaneous afferent innervation long understood in the hand (Johansson and Vallbo 1979a). The functional implication of these afferent distribution gradients is discussed below.
Innervation Density
The density of mechanoreceptor afferents in the skin influences tactile sensitivity (ability to detect small changes in stimulus amplitude) and acuity (ability to distinguish spatially distributed points on the skin surface). To provide estimates of the innervation density of the four afferent classes for each plantar skin region, we derived a scaling factor based on the approximate total number of cutaneous afferents in the plantar nerves. To obtain this scaling factor, we divided the estimated total number of cutaneous afferents (1,702 units) by the total number of sampled units (364 units), giving the value 4.676. By multiplying this scaling factor by the sampled densities (i.e., the number of units sampled divided by the size of the skin region), we arrive at estimates for the absolute innervation density in each region. The estimated total innervation densities, as well as the innervation densities of the four different receptor classes, are presented in Fig. 6 and listed in Table 3. In accordance with the distribution results, the highest innervation density was in the toes (23.3 units/cm2), followed by the lateral arch (15.4 units/cm2) and the lateral metatarsals (11.2 units/cm2). The lowest innervation density was in the medial metatarsals (4.9 units/cm2). Type I afferents most densely innervate the toes (FAI: 12.2 units/cm2; SAI: 6.9 units/cm2), followed by the lateral arch (FAI: 8.7 units/cm2; SAI: 2.8 units/cm2) and the lateral metatarsals (FAI: 5.6 units/cm2; SAI: 1.6 units/cm2). FAII afferents most densely innervate the lateral arch (1.5 units/cm2), followed by the great toe (1.4 units/cm2) and the middle metatarsals (1.4 units/cm2). SAII afferents most densely innervate the lateral metatarsals (3.3 units/cm2), followed by the toes (2.8 units/cm2) and the lateral arch (2.4 units/cm2).
FUNCTIONAL INTERPRETATION: A ROLE IN STANDING BALANCE AND GAIT
The control of balance, whether in standing or during gait, is a complex sensorimotor task that is facilitated by the integration of sensory feedback from multiple sources including the vestibular, visual, and somatosensory systems (Horak et al. 1990; Thomas et al. 2003; Winter 1995). Although it is difficult to equate behavior at a systems level to the firing of individual neurons, it is through neuronal interactions that functional outcomes emerge. There is mounting evidence that plantar cutaneous input is crucial for the control of standing balance and gait (Kavounoudias et al. 1998; Meyer et al. 2004a; Nurse and Nigg 1999; Zehr et al. 2014). Evidence suggests that standing posture is sensed in part by the tactile and pressure feedback transmitted by cutaneous afferents in the feet. The functional importance of this feedback has been highlighted through different experimental designs, including the experimental reduction (Eils et al. 2004; Howe et al. 2015; McKeon and Hertel 2007; Perry et al. 2000) or enhancement (Kavounoudias et al. 1999; Lipsitz et al. 2015; Perry et al. 2008; Priplata et al. 2006) of skin feedback, as well as through the study of naturally reduced cutaneous feedback that can occur with age (Perry, 2006; Peters et al. 2016) and disease (Deshpande et al. 2008; Patel et al. 2009). In cases where foot sole cutaneous feedback is reduced, measures of balance and gait performance are altered (Meyer et al. 2004a; Nurse and Nigg 1999; Perry et al. 2000). Conversely, measures of standing balance and gait performance have been improved through different interventions that increase foot sole cutaneous feedback (Lipsitz et al. 2015; Perry et al. 2008; Priplata et al. 2006). Together, these studies support a role of cutaneous feedback in the control of balance and gait; however, more work is necessary to link neural firing to balance control.
In both standing balance and gait, posture is controlled through the manipulation of the center of mass (COM) location relative to the base of support (BOS) (Winter 1995). In other words, if our body mass falls forward or backward, we need cues that will tell us to step as we have lost our balance. For bipeds, the soles of the feet are the only interface with the ground. Forces from the ground on the foot and the foot on the ground are perceived through the foot sole skin and are manipulated to control body equilibrium and orientation. In healthy people, small adjustments of ankle torque are sufficient to control the COM body position during standing balance. This ankle strategy, however, may not work in populations where tactile feedback is impaired, such as in older adults (Manchester et al. 1989; Perry, 2006; Peters et al. 2016), because the feedback from the foot sole is not sufficient to give cues as to how far forward or backward the body is leaning. Indeed, it has been suggested that the CNS uses cutaneous feedback from the soles of the feet to deduce body orientation (verticality) and to help control the forces applied by the feet to manipulate the body COM (Kavounoudias et al. 1998; Meyer et al. 2004b). Although cutaneous afferent firing has not been measured during standing balance, we speculate that foot sole cutaneous afferent firing corresponds to foot sole ground reaction forces and provides feedback about the movement and position of the COM over the feet.
Our findings on the distribution and density of foot sole cutaneous afferents presented in this review contribute new information about how these receptors might modulate balance outcomes. With high receptor populations in the toes and lateral border of the foot, these regions are identified as important sensory locations with populations able to delineate the physical limits of the BOS and evoke appropriate postural responses. The toes dictate the anterior limit of the BOS. Through plantar and dorsiflexor muscles activation, we can control the posterior and anterior movement of the COM within the confines of the BOS, which is identified by these toe mechanoreceptors. Naturally, we stand with our COM further toward the front of our foot lever (Winter 1995); specifically, over 60% of the load during stance is applied to the metatarsals and toes (Fernández-Seguín et al. 2014), supporting the need for a density of receptors in the toes to define the contact limits. Similarly, the heel provides the initial contact site during gait and dictates the posterior boundary of the BOS; however, unlike the toes, the heel is not a segment that can be independently manipulated to control the COM. The increased distribution of cutaneous afferents in the toes compared with the heel may reflect the postural significance of feedback from the toes in the control of standing balance. In the frontal plane, the lateral border of the right and left feet defines the boundary of the BOS. If the COM moves beyond the lateral BOS, a stepping reaction is required to prevent a fall (McIlroy and Maki 1996). In contrast, a medial movement of the COM is relatively less threatening to balance due to the support of two legs. FAI afferents have been shown to have strong synaptic coupling to lower limb motor neurons (Fallon et al. 2005), and the relatively large population of FAI afferents in the toes and lateral foot sole border may help facilitate reflexive loops important in balance control. In fact, increasing cutaneous feedback from the foot sole border has been shown to increase the COM-lateral BOS stability margin in older adults (Perry et al. 2008). Furthermore, activation of location specific skin regions on the sole of the foot has been shown to modulate muscles of the lower limb to facilitate gait (Zehr et al. 2014). This very direct evidence supports the notion that the individual mechanoreceptors have a significant role in spinal reflexes to control the magnitude of muscle activation for successful ambulation. With pressure distribution across the foot during walking that travels from heel to the great toe while favoring greater pressure on the lateral border (Buldt et al. 2018), the density and distribution of receptors in these regions makes inherent sense for this dynamic control of movement.
FUTURE CONSIDERATIONS
Collectively, the studies and data highlighted in this review enhance the understanding of foot sole cutaneous afferent firing thresholds and receptive field distribution and density that, together, help shape how the foot sole is viewed as a sensory structure. Continued investigations into the foot sole skin are needed to understand the contribution of class-specific and integrated foot sole cutaneous feedback in balance control. Some directions for future steps include the histological study of cutaneous afferent innervation of the foot sole and structure of the mechanoreceptor endings. How do they compare with hand mechanoreceptors? Measurements of the number of Aβ-fibers innervating the foot sole would provide more accurate estimates of the mechanoreceptor innervation density. How accurate is the estimated innervation ratio of 10 times fewer foot sole afferents compared with the hand? Foot sole mechanoreceptor morphology may adapt in response to the larger forces associated with standing balance and gait. Understanding how foot sole cutaneous afferents respond under loaded conditions is critical to assign functionality to cutaneous feedback in postural control. Vibration perception thresholds were recently shown to be elevated in a standing compared with sitting posture (Mildren et al. 2016); however the behavior of the underlying mechanoreceptors in different loading conditions is unknown. Therefore, future work is needed to investigate firing characteristics of foot sole afferents under loaded and more functionally relevant conditions.
SUMMARY AND CONCLUSIONS
The foot sole is a critical sensory structure, often our only contact with the environment during upright stance. In this review, we combined data sets with unpublished recordings to provide a collated and detailed view of the cutaneous innervation of the foot sole. By combining data sets, we are able to highlight significant functional differences in the skin of the foot as compared with the hand. Our principal novel finding was the observation that there is unequal distribution of afferents across the foot sole. Similar to the hand (Johansson and Vallbo 1980), a proximal (heel)-to-distal (toes) increase in afferent density was found. In addition, the data support a higher density of afferents on the lateral border of the foot sole compared with the midline or medial border. Afferent firing thresholds did not show the same proximal-distal or medial-lateral distribution pattern, although the heel was the least sensitive location as well as the least densely populated area. It is well established that in situations where cutaneous feedback is impaired experimentally (Meyer et al. 2004b) or naturally with age (Peters et al. 2016) and disease (Prätorius et al. 2003), balance impairment are prevalent (Kars et al. 2009). Advances have been made in the development of sensory augmentation devices as a strategy to improve standing balance. These developmental intervention strategies have attempted to improve the quality of foot sole cutaneous feedback through specialized shoe insoles (Lipsitz et al. 2015; Perry et al. 2008). However, optimizing these interventions requires an understanding of the underlying cutaneous mechanoreceptor afferents, notably, their capacity to provide functionally relevant feedback (Parker and Newsome 1998). The toes and lateral boards of the feet are important regions for balance control because they delineate the borders of the base of support. The observed afferent distribution and firing thresholds are thought to reflect the functional role of the foot sole, where tactile feedback from the toes and lateral border may be more meaningful for the control of standing balance. These data significantly advance how the foot sole is viewed as a sensory structure; however, future work is needed to investigate the firing characteristics of cutaneous afferents under loaded and more natural conditions.
GRANTS
This work was funded in part by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants (to J. T. Inglis and L. R. Bent), as well as an NSERC Research Tools and Instruments Grant (to J. T. Inglis). R. M. Peters received salary support from the NSERC Discovery Grant of J. T. Inglis. N. D. J. Strzalkowski was supported through an NSERC Postgraduate Doctoral Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.D.S., R.M.P., J.T.I., and L.R.B. conceived and designed research; N.D.S., R.M.P., J.T.I., and L.R.B. performed experiments; N.D.S., R.M.P., J.T.I., and L.R.B. analyzed data; N.D.S., R.M.P., J.T.I., and L.R.B. interpreted results of experiments; N.D.S. and R.M.P. prepared figures; N.D.S. and R.M.P. drafted manuscript; N.D.S., R.M.P., J.T.I., and L.R.B. edited and revised manuscript; N.D.S., R.M.P., J.T.I., and L.R.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Catherine Lowrey, Robyn Mildren, Paul Kennedy, James Fallon, and Vaughan Macefield for work that contributed many of the single-unit recordings compiled and presented in this review.
REFERENCES
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 79: 618–639, 2013. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley R, Backlund Wasling H, Liljencrantz J, Olausson H, Johnson RD, Wessberg J. Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J Neurosci 34: 2879–2883, 2014. doi: 10.1523/JNEUROSCI.2847-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol 580: 649–658, 2007. doi: 10.1113/jphysiol.2006.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auplish S, Hall S. An immunohistochemical study of palmar and plantar digital nerves. J Hand Surg [Br] 23: 6–11, 1998. doi: 10.1016/S0266-7681(98)80208-8. [DOI] [PubMed] [Google Scholar]
- Bensmaïa SJ, Leung YY, Hsiao SS, Johnson KO. Vibratory adaptation of cutaneous mechanoreceptive afferents. J Neurophysiol 94: 3023–3036, 2005. doi: 10.1152/jn.00002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent LR, Lowrey CR. Single low-threshold afferents innervating the skin of the human foot modulate ongoing muscle activity in the upper limbs. J Neurophysiol 109: 1614–1625, 2013. doi: 10.1152/jn.00608.2012. [DOI] [PubMed] [Google Scholar]
- Bergenheim M, Roll JP, Ribot-Ciscar E. Microneurography in humans. In: Modern Techniques in Neuroscience Research, edited by Windhorst U, Johansson H. Berlin: Springer, 1999, p. 803–819. [Google Scholar]
- Buldt AK, Forghany S, Landorf KB, Murley GS, Levinger P, Menz HB. Centre of pressure characteristics in normal, planus and cavus feet. J Foot Ankle Res 11: 3, 2018. doi: 10.1186/s13047-018-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol 535: 855–865, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MR, Andres KH, von Duering M, Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci 57: 417–445, 1972. [DOI] [PubMed] [Google Scholar]
- Collins S, Visscher P, De Vet HC, Zuurmond WW, Perez RS. Reliability of the Semmes Weinstein Monofilaments to measure coetaneous sensibility in the feet of healthy subjects. Disabil Rehabil 32: 2019–2027, 2010. doi: 10.3109/09638281003797406. [DOI] [PubMed] [Google Scholar]
- Condon M, Birznieks I, Hudson K, Chelvanayagam DK, Mahns D, Olausson H, Macefield VG. Differential sensitivity to surface compliance by tactile afferents in the human finger pad. J Neurophysiol 111: 1308–1317, 2014. doi: 10.1152/jn.00589.2013. [DOI] [PubMed] [Google Scholar]
- Davis TJ, Schon LC. Branches of the tibial nerve: anatomic variations. Foot Ankle Int 16: 21–29, 1995. doi: 10.1177/107110079501600105. [DOI] [PubMed] [Google Scholar]
- Deshpande N, Ferrucci L, Metter J, Faulkner KA, Strotmeyer E, Satterfield S, Schwartz A, Simonsick E. Association of lower limb cutaneous sensitivity with gait speed in the elderly: the health ABC study. Am J Phys Med Rehabil 87: 921–928, 2008. doi: 10.1097/PHM.0b013e31818a5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eils E, Behrens S, Mers O, Thorwesten L, Völker K, Rosenbaum D. Reduced plantar sensation causes a cautious walking pattern. Gait Posture 20: 54–60, 2004. doi: 10.1016/S0966-6362(03)00095-X. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Bent LR, McNulty PA, Macefield VG. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J Neurophysiol 94: 3795–3804, 2005. doi: 10.1152/jn.00359.2005. [DOI] [PubMed] [Google Scholar]
- Fernández-Seguín LM, Diaz Mancha JA, Sánchez Rodríguez R, Escamilla Martínez E, Gómez Martín B, Ramos Ortega J. Comparison of plantar pressures and contact area between normal and cavus foot. Gait Posture 39: 789–792, 2014. doi: 10.1016/j.gaitpost.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Fortman GJ, Winkelmann RK. A Merkel cell nuclear inclusion. J Invest Dermatol 61: 334–338, 1973. doi: 10.1111/1523-1747.ep12676616. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Hales JP. The methodology and scope of human microneurography. J Neurosci Methods 74: 123–136, 1997. doi: 10.1016/S0165-0270(97)02243-7. [DOI] [PubMed] [Google Scholar]
- Goodwin AW, Macefield VG, Bisley JW. Encoding of object curvature by tactile afferents from human fingers. J Neurophysiol 78: 2881–2888, 1997. doi: 10.1152/jn.1997.78.6.2881. [DOI] [PubMed] [Google Scholar]
- Hagbarth K-E. Microelectrode recordings from human peripheral nerves (microneurography). Muscle Nerve Suppl 11: S28–S35, 2002. doi: 10.1002/mus.10144. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hongell A, Hallin RG, Torebjörk HE. Afferent impulses in median nerve fascicles evoked by tactile stimuli of the human hand. Brain Res 24: 423–442, 1970. doi: 10.1016/0006-8993(70)90183-6. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo ÅB. Mechanoreceptor activity recorded percutaneously with semi-microelectrodes in human peripheral nerves. Acta Physiol Scand 69: 121–122, 1967. doi: 10.1111/j.1748-1716.1967.tb03498.x. [DOI] [PubMed] [Google Scholar]
- Hallin RG, Carlstedt T, Wu G. Population behaviour of human cutaneous mechanoreceptive units. Behav Brain Res 135: 19–26, 2002. doi: 10.1016/S0166-4328(02)00150-X. [DOI] [PubMed] [Google Scholar]
- Hennig EM, Sterzing T. Sensitivity mapping of the human foot: thresholds at 30 skin locations. Foot Ankle Int 30: 986–991, 2009. doi: 10.3113/FAI.2009.0986. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res 82: 167–177, 1990. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- Howe EE, Toth AJ, Vallis LA, Bent LR. Baseline skin information from the foot dorsum is used to control lower limb kinematics during level walking. Exp Brain Res 233: 2477–2487, 2015. doi: 10.1007/s00221-015-4318-5. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Thelin AE, Vallbo ÅB. The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J Physiol 291: 233–249, 1979. doi: 10.1113/jphysiol.1979.sp012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Cutaneous and subcutaneous sense organs. Br Med Bull 33: 97–102, 1977. doi: 10.1093/oxfordjournals.bmb.a071432. [DOI] [PubMed] [Google Scholar]
- Iggo A, Andres KH. Morphology of cutaneous receptors. Annu Rev Neurosci 5: 1–31, 1982. doi: 10.1146/annurev.ne.05.030182.000245. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Kennedy PM, Wells C, Chua R. The role of cutaneous receptors in the foot. In: Sensorimotor Control of Movement and Posture, edited by Gandevia SC, Proske U, Stuart DG. Boston, MA: Springer, 2002, p. 111–117. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Leeper JB, Burke D, Gandevia SC. Morphology of action potentials recorded from human nerves using microneurography. Exp Brain Res 110: 308–314, 1996. doi: 10.1007/BF00228561. [DOI] [PubMed] [Google Scholar]
- Johansson RS. Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. J Physiol 281: 101–125, 1978. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Landström U, Lundström R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244: 17–25, 1982. doi: 10.1016/0006-8993(82)90899-X. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo ÅB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol 286: 283–300, 1979a. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo ÅB. Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. J Physiol 297: 405–422, 1979b. doi: 10.1113/jphysiol.1979.sp013048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo ÅB. Spatial properties of the population of mechanoreceptive units in the glabrous skin of the human hand. Brain Res 184: 353–366, 1980. doi: 10.1016/0006-8993(80)90804-5. [DOI] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11: 455–461, 2001. doi: 10.1016/S0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Jones LA, Smith AM. Tactile sensory system: encoding from the periphery to the cortex. Wiley Interdiscip Rev Syst Biol Med 6: 279–287, 2014. doi: 10.1002/wsbm.1267. [DOI] [PubMed] [Google Scholar]
- Kars HJ, Hijmans JM, Geertzen JH, Zijlstra W. The effect of reduced somatosensation on standing balance: a systematic review. J Diabetes Sci Technol 3: 931–943, 2009. doi: 10.1177/193229680900300441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. The plantar sole is a ‘dynamometric map’ for human balance control. Neuroreport 9: 3247–3252, 1998. doi: 10.1097/00001756-199810050-00021. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll J-P. Specific whole-body shifts induced by frequency-modulated vibrations of human plantar soles. Neurosci Lett 266: 181–184, 1999. doi: 10.1016/S0304-3940(99)00302-X. [DOI] [PubMed] [Google Scholar]
- Kekoni J, Hämäläinen H, Rautio J, Tukeva T. Mechanical sensibility of the sole of the foot determined with vibratory stimuli of varying frequency. Exp Brain Res 78: 419–424, 1989. doi: 10.1007/BF00228915. [DOI] [PubMed] [Google Scholar]
- Kennedy PM, Inglis JT. Distribution and behaviour of glabrous cutaneous receptors in the human foot sole. J Physiol 538: 995–1002, 2002. doi: 10.1113/jphysiol.2001.013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of rapidly adapting mechanoreceptors in human glabrous skin area. J Physiol 232: 427–452, 1973. doi: 10.1113/jphysiol.1973.sp010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of slowly adapting mechanoreceptors in the human glabrous skin area. J Physiol 245: 63–80, 1975. doi: 10.1113/jphysiol.1975.sp010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestöl M, Vallbo ÅB. Single unit analysis of mechanoreceptor activity from the human glabrous skin. Acta Physiol Scand 80: 178–195, 1970. doi: 10.1111/j.1748-1716.1970.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Lough M, Niemi J, Travison T, Howlett H, Manor B. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch Phys Med Rehabil 96: 432–439, 2015. doi: 10.1016/j.apmr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein WR, Skalak R. Mechanical transmission in a Pacinian corpuscle. An analysis and a theory. J Physiol 182: 346–378, 1966. doi: 10.1113/jphysiol.1966.sp007827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey CR, Strzalkowski NDJ, Bent LR. Skin sensory information from the dorsum of the foot and ankle is necessary for kinesthesia at the ankle joint. Neurosci Lett 485: 6–10, 2010. doi: 10.1016/j.neulet.2010.08.033. [DOI] [PubMed] [Google Scholar]
- Lowrey CR, Strzalkowski NDJ, Bent LR. Cooling reduces the cutaneous afferent firing response to vibratory stimuli in glabrous skin of the human foot sole. J Neurophysiol 109: 839–850, 2013. doi: 10.1152/jn.00381.2012. [DOI] [PubMed] [Google Scholar]
- Macefield VG. The signalling of touch, finger movements and manipulation forces by mechanoreceptors in human skin. In: Neural Aspects in Tactile Sensation, edited by Morley JW. Amsterdam: Elsevier, 1998, p. 89–130. [Google Scholar]
- Macefield VG. Physiological characteristics of low-threshold mechanoreceptors in joints, muscle and skin in human subjects. Clin Exp Pharmacol Physiol 32: 135–144, 2005. doi: 10.1111/j.1440-1681.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Birznieks I. Cutaneous mechanoreceptors, functional behavior. In: Encyclopedia of Neuroscience, edited by Binder MD, Hirokawa N, Windhorst U. Berlin: Springer, 2009, p. 914–922. [Google Scholar]
- Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol 44: M118–M127, 1989. doi: 10.1093/geronj/44.4.M118. [DOI] [PubMed] [Google Scholar]
- McGlone F, Reilly D. The cutaneous sensory system. Neurosci Biobehav Rev 34: 148–159, 2010. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. Age-related changes in compensatory stepping in response to unpredictable perturbations. J Gerontol A Biol Sci Med Sci 51A: M289–M296, 1996. doi: 10.1093/gerona/51A.6.M289. [DOI] [PubMed] [Google Scholar]
- McKeon PO, Hertel J. Diminished plantar cutaneous sensation and postural control. Percept Mot Skills 104: 56–66, 2007. doi: 10.2466/pms.104.1.56-66. [DOI] [PubMed] [Google Scholar]
- Meyer PF, Oddsson LI, De Luca CJ. Reduced plantar sensitivity alters postural responses to lateral perturbations of balance. Exp Brain Res 157: 526–536, 2004a. doi: 10.1007/s00221-004-1868-3. [DOI] [PubMed] [Google Scholar]
- Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res 156: 505–512, 2004b. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- Mildren RL, Hare CM, Bent LR. Cutaneous afferent feedback from the posterior ankle contributes to proprioception. Neurosci Lett 636: 145–150, 2017. doi: 10.1016/j.neulet.2016.10.058. [DOI] [PubMed] [Google Scholar]
- Mildren RL, Strzalkowski ND, Bent LR. Foot sole skin vibration perceptual thresholds are elevated in a standing posture compared to sitting. Gait Posture 43: 87–92, 2016. doi: 10.1016/j.gaitpost.2015.10.027. [DOI] [PubMed] [Google Scholar]
- Miller MR, Kasahara M. The pattern of cutaneous innervation of the human foot. Am J Anat 105: 233–255, 1959. doi: 10.1002/aja.1001050205. [DOI] [PubMed] [Google Scholar]
- Muniak MA, Ray S, Hsiao SS, Dammann JF, Bensmaia SJ. The neural coding of stimulus intensity: linking the population response of mechanoreceptive afferents with psychophysical behavior. J Neurosci 27: 11687–11699, 2007. doi: 10.1523/JNEUROSCI.1486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse MA, Nigg BM. Quantifying a relationship between tactile and vibration sensitivity of the human foot with plantar pressure distributions during gait. Clin Biomech (Bristol, Avon) 14: 667–672, 1999. doi: 10.1016/S0268-0033(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Torebjörk E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol 415: 583–599, 1989. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci 21: 227–277, 1998. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- Patel M, Magnusson M, Kristinsdottir E, Fransson PA. The contribution of mechanoreceptive sensation on stability and adaptation in the young and elderly. Eur J Appl Physiol 105: 167–173, 2009. doi: 10.1007/s00421-008-0886-4. [DOI] [PubMed] [Google Scholar]
- Pei YC, Denchev PV, Hsiao SS, Craig JC, Bensmaia SJ. Convergence of submodality-specific input onto neurons in primary somatosensory cortex. J Neurophysiol 102: 1843–1853, 2009. doi: 10.1152/jn.00235.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SD. Evaluation of age-related plantar-surface insensitivity and onset age of advanced insensitivity in older adults using vibratory and touch sensation tests. Neurosci Lett 392: 62–67, 2006. doi: 10.1016/j.neulet.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res 877: 401–406, 2000. doi: 10.1016/S0006-8993(00)02712-8. [DOI] [PubMed] [Google Scholar]
- Perry SD, Radtke A, McIlroy WE, Fernie GR, Maki BE. Efficacy and effectiveness of a balance-enhancing insole. J Gerontol A Biol Sci Med Sci 63: 595–602, 2008. doi: 10.1093/gerona/63.6.595. [DOI] [PubMed] [Google Scholar]
- Peters RM, Dalton BH, Blouin JS, Inglis JT. Precise coding of ankle angle and velocity by human calf muscle spindles. Neuroscience 349: 98–105, 2017. doi: 10.1016/j.neuroscience.2017.02.034. [DOI] [PubMed] [Google Scholar]
- Peters RM, McKeown MD, Carpenter MG, Inglis JT. Losing touch: age-related changes in plantar skin sensitivity, lower limb cutaneous reflex strength, and postural stability in older adults. J Neurophysiol 116: 1848–1858, 2016. doi: 10.1152/jn.00339.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JR, Johnson KO. Tactile spatial resolution. II. Neural representation of Bars, edges, and gratings in monkey primary afferents. J Neurophysiol 46: 1192–1203, 1981. doi: 10.1152/jn.1981.46.6.1192. [DOI] [PubMed] [Google Scholar]
- Prätorius B, Kimmeskamp S, Milani TL. The sensitivity of the sole of the foot in patients with Morbus Parkinson. Neurosci Lett 346: 173–176, 2003. doi: 10.1016/S0304-3940(03)00582-2. [DOI] [PubMed] [Google Scholar]
- Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol 59: 4–12, 2006. doi: 10.1002/ana.20670. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Johansson RS. Edge-orientation processing in first-order tactile neurons. Nat Neurosci 17: 1404–1409, 2014. doi: 10.1038/nn.3804. [DOI] [PubMed] [Google Scholar]
- Pubols LM, Pubols BH Jr, Munger BL. Functional properties of mechanoreceptors in glabrous skin of the raccoon’s forepaw. Exp Neurol 31: 165–182, 1971. doi: 10.1016/0014-4886(71)90185-3. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Vedel JP, Roll JP. Vibration sensitivity of slowly and rapidly adapting cutaneous mechanoreceptors in the human foot and leg. Neurosci Lett 104: 130–135, 1989. doi: 10.1016/0304-3940(89)90342-X. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47: 177–190, 1982. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Saal HP, Bensmaia SJ. Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci 37: 689–697, 2014. doi: 10.1016/j.tins.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Strzalkowski ND, Ali RA, Bent LR. The firing characteristics of foot sole cutaneous mechanoreceptor afferents in response to vibration stimuli. J Neurophysiol 118: 1931–1942, 2017. doi: 10.1152/jn.00647.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalkowski ND, Incognito AV, Bent LR, Millar PJ. Cutaneous mechanoreceptor feedback from the hand and foot can modulate muscle sympathetic nerve activity. Front Neurosci 10: 568, 2016. doi: 10.3389/fnins.2016.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalkowski ND, Mildren RL, Bent LR. Thresholds of cutaneous afferents related to perceptual threshold across the human foot sole. J Neurophysiol 114: 2144–2151, 2015a. doi: 10.1152/jn.00524.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzalkowski ND, Triano JJ, Lam CK, Templeton CA, Bent LR. Thresholds of skin sensitivity are partially influenced by mechanical properties of the skin on the foot sole. Physiol Rep 3: e12425, 2015b. doi: 10.14814/phy2.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas VJ, Patil KM, Radhakrishnan S, Narayanamurthy VB, Parivalavan R. The role of skin hardness, thickness, and sensory loss on standing foot power in the development of plantar ulcers in patients with diabetes mellitus—a preliminary study. Int J Low Extrem Wounds 2: 132–139, 2003. doi: 10.1177/1534734603258601. [DOI] [PubMed] [Google Scholar]
- Trulsson M. Mechanoreceptive afferents in the human sural nerve. Exp Brain Res 137: 111–116, 2001. doi: 10.1007/s002210000649. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth K-E, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol (1985) 96: 1262–1269, 2004. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Vallbo ÅB, Johansson RS. The tactile sensory innervation of the glabrous skin of the human hand. In: Active Touch, edited by Gorden G. Oxford: Pergamon, 1978. [Google Scholar]
- Vallbo ÅB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol 3: 3–14, 1984. [PubMed] [Google Scholar]
- Wallin BG, Elam M. Insights from intraneural recordings of sympathetic nerve traffic in humans. Physiology (Bethesda) 9: 203–207, 1994. doi: 10.1152/physiologyonline.1994.9.5.203. [DOI] [Google Scholar]
- Werner G, Mountcastle VB. Neural activity in mechanoreceptive cutaneous afferents: Stimulus-response relation, weber functions, and information transmission. J Neurophysiol 28: 359–397, 1965. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]
- Winter DA. Human balance and posture control during standing and walking. Gait Posture 3: 193–214, 1995. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]
- Zehr EP, Nakajima T, Barss T, Klarner T, Miklosovic S, Mezzarane RA, Nurse M, Komiyama T. Cutaneous stimulation of discrete regions of the sole during locomotion produces “sensory steering” of the foot. BMC Sports Sci Med Rehabil 6: 33, 2014. doi: 10.1186/2052-1847-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999. doi: 10.1016/S0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science 346: 950–954, 2014. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]