Abstract
Cortical excitability increases during the performance of more difficult postural tasks. However, it is possible that changes in postural threat associated with more difficult tasks may in themselves lead to alterations in the neural strategies underlying postural control. Therefore, the purpose of this study was to examine whether changes in postural threat are responsible for the alterations in corticospinal excitability and short-interval intracortical inhibition (SICI) that occur with increasing postural task difficulty. Fourteen adults completed three postural tasks (supported standing, free standing, or standing on an unstable board) at two surface heights (ground level or 3 m above ground). Single- and paired-pulse magnetic stimuli were applied to the motor cortex to compare soleus (SOL) and tibialis anterior (TA) test motor-evoked potentials (MEPs) and SICI between conditions. SOL and TA test MEPs increased from 0.35 ± 0.29 to 0.82 ± 0.41 mV (SOL) and from 0.64 ± 0.51 to 1.96 ± 1.45 mV (TA), respectively, whereas SICI decreased from 52.4 ± 17.2% to 39.6 ± 15.4% (SOL) and from 71.3 ± 17.7% to 50.3 ± 19.9% (TA) with increasing task difficulty. In contrast to the effects of task difficulty, only SOL test MEPs were smaller when participants stood at high (0.49 ± 0.29 mV) compared with low height (0.61 ± 0.40 mV). Because the presence of postural threat did not lead to any additional changes in the excitability of the motor corticospinal pathway and intracortical inhibition with increasing task difficulty, it seems unlikely that alterations in perceived threat are primarily responsible for the neurophysiological changes that are observed with increasing postural task difficulty.
NEW & NOTEWORTHY We examined how task difficulty and postural threat influence the cortical control of posture. Results indicated that the motor corticospinal pathway and intracortical inhibition were modulated more by task difficulty than postural threat. Furthermore, because the presence of postural threat during the performance of various postural tasks did not lead to summative changes in motor-evoked potentials, alterations in perceived threat are not responsible for the neurophysiological changes that occur with increasing postural task difficulty.
Keywords: arousal, balance control, electromyography, postural threat, transcranial magnetic stimulation
INTRODUCTION
There is increasing evidence that motor cortical areas contribute to the maintenance of human standing posture. Studies incorporating transcranial magnetic stimulation (TMS) have noted an increase in soleus (SOL) cortical excitability when an individual is freely standing compared with sitting (Soto et al. 2006) or standing with support (Tokuno et al. 2009). As the standing postural task is made more difficult by altering the support surface (e.g., standing on foam vs. solid ground) or removing sensory information (e.g., standing with eyes closed vs. open), there is a concomitant increase in corticospinal excitability (Papegaaij et al. 2014; Solopova et al. 2003). It has been postulated that this increase in cortical excitability is facilitated through the disinhibition of intracortical inhibitory circuits, because the amount of SOL or tibialis anterior (TA) short-interval intracortical inhibition (SICI) is reduced during standing compared with sitting (Obata et al. 2014; Soto et al. 2006) or during standing on an unstable compared with a stable support surface (Papegaaij et al. 2016).
Although changes in cortical and corticospinal excitability as well as modulation of intracortical inhibition appear to be associated with postural task difficulty, it is possible that with increasing postural demands, individuals may undergo changes associated with the perceived postural threat (Hauck et al. 2008). This is problematic because physiological and psychological changes associated with the threat may in themselves lead to alterations in the neural strategies underlying postural control. For example, when individuals experience a postural threat due to standing at an elevated height, they commonly experience higher levels of fear, anxiety, and arousal, as well as decreased confidence, and consequently adopt a “stiffer” postural control (Carpenter et al. 2001). This is typically demonstrated through an increase in dorsiflexor and a decrease in plantar flexor electromyographic (EMG) activity (Brown et al. 2006; Carpenter et al. 2001; Sibley et al. 2007) as well as a reduced variability and a smaller amplitude of the anteroposterior center-of-pressure (COP) displacement (Brown et al. 2006; Carpenter et al. 2001). These biomechanical changes as a result of threat-induced anxiety may be a result of numerous neurophysiological changes, such as pathways mediated by the amygdala, vestibular system, or cerebral cortex [for detailed review, see Staab et al. (2013)]. Furthermore, Sibley et al. (2007) observed a decrease in spinal excitability, as demonstrated by a smaller SOL Hoffmann reflex (H-reflex) amplitude, when individuals stood in the presence of postural threat. This downregulation of spinal excitability is believed to be achieved via an increased contribution from cortical or supraspinal structures (Adkin et al. 2008; Llewellyn et al. 1990). Work by Tanaka et al. (2013) seems to support this possibility, where single-pulse TMS was used to demonstrate an increase in corticospinal excitability to the internal oblique muscles when individuals stood at a large compared with a narrow support base at height. In contrast, a more recent study by Johannsson et al. (2017) observed no change in TMS-evoked SOL motor-evoked potential (MEP) amplitudes when participants stood at the top or bottom of a 0.7-m staircase. Given these discrepancies, it is still unclear if and how postural threat impacts the corticospinal control of standing posture. Furthermore, because both studies only assessed corticospinal excitability during one postural task (i.e., quiet standing), it is not known whether the effects of postural task difficulty on postural control are independent or dependent on the effects of postural threat.
Therefore, the purpose of this study was to examine whether changes in postural threat are responsible for the alterations in corticospinal excitability and SICI that may also occur with increasing postural task difficulty. This was achieved by introducing a known postural threat while individuals performed postural tasks of varying difficulty. On the basis of previous research, it was hypothesized that SOL and TA corticospinal excitability would increase but that SICI would decrease during the more difficult postural tasks. If these neurophysiological changes are a result of the perceived postural threat of performing the more difficult postural tasks, it would be expected that the addition of a secondary source of postural threat would lead to a cumulative (i.e., greater) change to both corticospinal excitability and SICI.
METHODS
Participants.
Fourteen adults (9 men) with a mean ± SD age of 28 ± 5 yr, height of 174 ± 10 cm, and mass of 68.7 ± 12.1 kg participated in this study. Seven of these participants returned ~1 wk later and completed the same six experimental conditions in the same order as the first session. The purpose of the second session was to quantify the level of physiological arousal level, via electrodermal activity recordings, during the various height and task conditions. During this second session, neither EMG nor TMS was used.
None of the participants reported any history of injuries or disorders that could affect their balance. All participants provided written informed consent before their involvement in the study. All experimental procedures were approved by the local ethics committee and were in accordance with the Declaration of Helsinki.
Experimental protocol.
Areas of skin over the SOL and TA of the right leg were shaved and cleansed with alcohol before a pair of surface electrodes (Blue sensor P; Ambu, Bad Nauheim, Germany) was placed on each of two muscles. A reference electrode was placed on the lateral aspect of the knee joint. The two EMG signals were amplified 1,000 times and sampled at 4 kHz (custom LabView-based software with National Instruments data acquisition board; Austin, TX). For participants involved in the second session, two surface electrodes (same material as above) were placed on the palm of their nondominant hand. These electrodes were connected to an electrodermal activity amplifier (EDA100C; BIOPAC Systems, Goleta, Canada) to measure the ongoing galvanic skin response at a sampling rate of 1,000 Hz (custom LabView-based software with National Instruments data acquisition board).
To elicit MEPs in the SOL and TA, the left motor cortex was stimulated using a 95-mm focal butterfly-shaped coil (D-B80) connected to a MagPro X100 with MagOption magnetic stimulator (both from MagVenture, Farum, Denmark). Each magnetic pulse was a biphasic waveform and was set to evoke posterior-anterior current flow in the interhemispheric fissure. The coil was initially positioned ~0.5 cm anterior to the vertex and was systematically moved until the largest SOL MEP could be elicited with minimal stimulator intensity. Once this ideal position was determined, the coil was mechanically fixed to a custom-designed helmet worn by the participant.
With the coil secured, the active SOL motor threshold (aMT) was determined with participants in a standing posture. Active MT was defined as the minimum intensity at which a SOL MEP, with a minimum peak-to-peak amplitude of 100 µV, could be distinguished in three of five consecutive trials (Kujirai et al. 1993). With the use of these criteria, the SOL aMT across all participants occurred at 59 ± 10% of maximal stimulator output.
Experimental conditions.
Participants completed six experimental conditions consisting of one of three standing balance tasks at one of two surface heights. The three standing tasks consisted of 1) standing with a supporting beam placed at approximately chest height (“supported”), 2) normal standing (“free”), and 3) standing on top of a 9.5-cm-high (31-cm diameter) rubber wobble balance board that had a flat surface with a small dome at the bottom (Gym Top; “unstable”). For the supported condition, a horizontal supporting beam with vertical supports was moved toward the participants’ chest until they felt that they could “rest” their torso on the beam. This condition was introduced because it involves sensory feedback from the feet and requires a similar amount of postural muscle activity compared with the free standing condition, but without the need to control for postural sway (Papegaaij et al. 2016; Tokuno et al. 2009). All tasks were completed on a force platform (AMTI OR6-7; Advanced Mechanical Technology, Watertown, MA) that was either placed at ground level (“low” surface height) or at the edge of a platform that was 3 m above ground level (“high” surface height). The purpose of the high surface height was to present a postural threat to the participant and induce a higher level of arousal. Participants completed all tasks barefoot, with their feet together, their arms to the sides, and their gaze focused toward a target that was located 7 m away at eye level. For safety reasons, participants wore a harness and were attached by a rope to an overhead support. There was enough slack in the rope to allow body movements but, if necessary, prevent a fall. The order of the three balance tasks and the two surface heights were randomized between participants, but each participant completed the three standing tasks within each surface height condition in a blocked order. Thus the three trials of a particular task were performed consecutively and the three tasks of a particular height condition were completed consecutively before being changed to the other height condition.
For each of the six experimental conditions, participants maintained their balance while they received 20 single (test)-pulse and 20 paired-pulse magnetic stimuli. For the single (test)-pulse stimulus, the stimulus intensity was set at 120% of the predetermined aMT. The paired-pulse stimuli were administered to assess the amount of SICI. SICI, and not intracortical facilitation (ICF), was examined because previous studies investigating the influence of task difficulty on the cortical control of standing have observed changes in SOL SICI (Papegaaij et al. 2014, 2016) but not ICF (Papegaaij et al. 2016). Furthermore, it was important to consider the number of TMS measures to minimize any potential habituation effects that may occur due to repeated exposure of standing at height and/or fatigue. To test for SICI, a conditioning stimulus of 80% aMT was followed 2.5 ms later by a suprathreshold test stimulus of 120% aMT (Kujirai et al. 1993; Papegaaij et al. 2014). Because SICI is dependent on the intensity of the test TMS pulse rather than the size of the test MEP (Garry and Thomson 2009), the stimulus intensities were kept constant across balance tasks and surface heights. The single- and paired-pulse stimuli were presented in an alternating order, with 5 s between stimuli. As a result, participants stood for ~3 min for each experimental condition.
At the end of each standing task at the high surface height condition, participants rated their perceived fear, anxiety, and stability from 0 to 100% on a visual analog scale. Once all three tasks were completed, participants completed a 10-item questionnaire that assessed their level of state anxiety, specifically that related to somatic sensation and worry, while standing at height (Carpenter et al. 2006; Hauck et al. 2008). Each item was rated on a 9-point interval scale, and the total sum from all items was calculated. The questionnaire and methods of calculation were based on a modified version of the Sport Anxiety Scale (Smith et al. 1990) that was adapted to make it contextually relevant to the surface height paradigm. Hauck et al. (2008) and Carpenter et al. (2006) used this adapted version to show that state anxiety, as measured by the questionnaire, was reliable and significantly correlated with physiological measures of arousal (i.e., blood pressure).
Data analysis.
The peak-to-peak amplitude of each MEP was measured, and the average amplitudes of all test and conditioned MEPs within each condition were determined. SICI was calculated as the percentage of inhibition from the test MEP amplitude, using the formula [1 − (conditioned MEP/test MEP)] × 100%. Because MEPs can be influenced by the amount of ongoing EMG activity, the background EMG (bEMG) activity of the SOL and TA were measured as the root mean square (RMS) amplitude from the rectified and filtered (2nd-order 10- to 1,000-Hz bandpass Butterworth filter) during the 100 ms before each stimulus. From these values, an agonist-to-antagonist coactivation ratio was calculated for each task and height condition using the formula TA bEMG/SOL bEMG (Baudry and Duchateau 2012). SOL and TA bEMG values, as well as the coactivation ratio, were later compared between standing tasks and height conditions.
Statistics.
Each dependent measure was statistically analyzed using a 2 (low vs. high height) × 3 (supported vs. free vs. unstable standing task) repeated-measures ANOVA. Where appropriate, post hoc analyses were conducted using Bonferroni-corrected paired t-tests. To investigate whether alterations in bEMG or test MEP amplitudes might have influenced the size of the SICI response, Spearman’s correlations were performed. The presented correlation results were performed on the EMG data averaged between the low and high height; however, the analyses yielded similar results even when the correlations were calculated from data at each height condition separately.
Significance for all tests was set at P ≤ 0.05. All statistical analyses were performed using commercially available software (SPSS version 22). Values are means ± SD.
RESULTS
Psychological and physiological measures of arousal.
Of the three standing tasks performed at high height, participants perceived the greatest amount of fear (26 ± 29%; range 0–80%) and anxiety (23 ± 29%; range 0–90%) as well as the least amount of stability (59 ± 27%; range 10–100%) during the unstable standing task. Furthermore, participants reported a small but elevated level of state anxiety related to somatic sensation and worry (22 ± 13; range 10–57 out of 90) while performing the three tasks at the high surface height condition.
These perceived levels of psychological arousal were largely corroborated by measures of physiological arousal. The mean galvanic skin response during the standing task was influenced by main effects of height (F1,6 = 12.26; P = 0.013; = 0.67) and task (F2,22 = 9.51; P = 0.003; = 0.61). Participants demonstrated a 36 ± 31% greater skin response during the high compared with the low height conditions. Furthermore, 10 ± 7% and 15 ± 11% greater electrodermal activity was noted during the unstable compared with the free (P = 0.013) and the supported standing conditions (P = 0.046), respectively. The free and supported standing conditions did not result in a difference in skin response (5 ± 8% difference; P = 0.530).
Background EMG activity.
SOL background EMG activity was influenced by a task main effect (F2,26 = 20.32; P < 0.001; = 0.61; Table 1) but not by the height condition (F1,13 = 0.471; P = 0.505). Post hoc tests indicated that EMG activity was larger during both the unstable and free standing conditions compared with the supported standing condition (P < 0.001 and P = 0.002, respectively).
Table 1.
SOL and TA bEMG activity during postural tasks at low and high surface height conditions
| Low Surface Height |
High Surface Height |
|||||
|---|---|---|---|---|---|---|
| Supported | Free | Unstable | Supported | Free | Unstable | |
| Test trials | ||||||
| SOL EMG, mV | 0.02 ± 0.02* | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.02 ± 0.02* | 0.02 ± 0.02 | 0.03 ± 0.02 |
| TA EMG, mV | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.03 ± 0.03 | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.05 ± 0.06† |
| Coactivation ratio‡ | 0.80 ± 0.88 | 0.50 ± 0.83 | 1.77 ± 2.57 | 1.85 ± 3.38 | 0.98 ± 1.60 | 2.39 ± 3.25 |
| SICI trials | ||||||
| SOL EMG, mV | 0.02 ± 0.02* | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.02 ± 0.02* | 0.02 ± 0.02 | 0.03 ± 0.02 |
| TA EMG, mV | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.03 ± 0.03 | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.05 ± 0.06† |
| Coactivation ratio‡ | 0.68 ± 0.84 | 0.45 ± 0.69 | 1.78 ± 2.32 | 1.78 ± 3.25 | 0.94 ± 1.43 | 2.36 ± 3.06 |
Values are means ± SD.
Task main effect, where the soleus (SOL) background electromyographic (bEMG) activity was smaller during the supported standing condition compared with the unstable (P < 0.001) and free standing conditions (P = 0.002).
Tibialis anterior (TA) bEMG activity was greater (P = 0.010) at high compared with low height during the unstable standing condition.
Height main effect (P = 0.044), where the coactivation ratio was greater at high compared with low height.
The TA background EMG activity was affected by a height × task interaction effect (F2,26 = 4.59; P = 0.020; = 0.26; Table 1). TA EMG activity was greater at the high compared with the low height during the unstable condition (P = 0.010), but there was no difference during the free and supported conditions.
Changes in SOL and TA bEMG between the different task and height conditions resulted in the coactivation ratio being affected by a height condition (F1,13 = 4.961 P = 0.044; = 0.28; Table 1). The 72% larger coactivation ratio at the high (1.72 ± 1.89) compared with the low height condition (1.00 ± 1.00) indicates a proportionately greater increase in the TA compared with the SOL bEMG activity when participants stood at the high height. The task main effect (P = 0.143) and all other interaction effects (P = 0.732–0.937) were not significant.
Test MEP amplitudes.
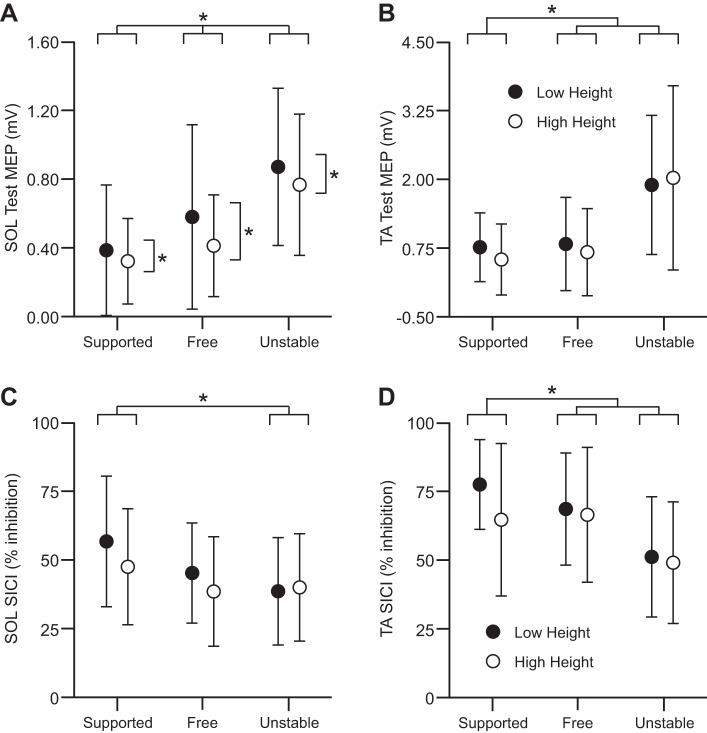
The unconditioned test SOL MEP amplitude was influenced by main effects of height (F1,13 = 5.77; P = 0.032; = 0.31) and task (F2,26 = 30.80; P < 0.001; = 0.70). Not only were SOL MEPs smaller during the high (0.49 ± 0.29 mV) compared with the low height condition (0.61 ± 0.40 mV), but post hoc tests revealed that MEPs were different between all three standing tasks. The largest test MEPs occurred during the unstable (0.82 ± 0.41 mV), followed by the free standing (0.49 ± 0.39 mV) and supported standing condition (0.35 ± 0.29 mV). The height × task interaction effect was not significant (F2,26 = 0.41; P = 0.668).
For the TA, the test MEP amplitude was affected by a task main effect (F2,26 = 14.65; P < 0.001; = 0.53). MEPs obtained during the unstable condition (1.96 ± 1.45 mV) were larger than those from the free (0.73 ± 0.80 mV) and supported standing conditions (0.64 ± 0.51 mV), but MEP amplitudes were not different between the latter two conditions. In contrast, TA test MEP amplitudes were not influenced by a surface height main effect (F1,13 = 0.61; P = 0.448) or a height × task interaction effect (F2,26 = 1.44; P = 0.255).
Short-interval intracortical inhibition.
The amount of SICI in SOL was different between tasks (F2,26 = 5.47; P = 0.010; = 0.30) but not between surface heights (F1,13 = 1.28; P = 0.278; Fig. 1). There was a 32% decrease in SICI during the unstable compared with the free standing condition (P = 0.019) and a trend toward a decrease (24%) during the free compared with the supported standing condition (P = 0.077). The height × task interaction effect was not significant (F2,26 = 0.76; P = 0.479).
Fig. 1.
A and B: soleus (SOL) and tibialis anterior (TA) test motor-evoked potential (MEP) amplitudes, respectively, for each of the 3 standing tasks at the 2 surface height conditions. C and D: SOL and TA short-interval intracortical inhibition (SICI), respectively, for each of the 3 standing tasks at the 2 surface height conditions. Values are means ± SD. For all plots, closed and open circles represent the low and high surface height condition, respectively. *P < 0.05, statistically significant differences.
Similar effects were observed with regards to the amount of SICI in TA. There was no difference between the low and high height conditions (F1,13 = 1.50; P = 0.243), but the amount of inhibition was influenced by a task main effect (F2,26 = 13.23; P < 0.001; = 0.50). Post hoc analyses indicated a lesser amount of SICI during the unstable compared with both the free (35% decrease; P = 0.005) and supported standing conditions (42% decrease; P = 0.003). The height × task interaction effect was not significant (F2,26 = 1.07; P = 0.359).
Correlational analyses.
Correlational analyses were performed to examine whether relationships between the various background EMG and TMS-related measures existed. The following correlations were based on data averaged between the low and high height conditions, but analyses from each height condition, including the corresponding R2 values, are reported in Table 2. First, it was found the change in test MEP amplitude was not significantly correlated with the change in SICI between the easiest (i.e., supported standing) and the hardest (i.e., unstable standing) standing conditions. Pearson’s r values were −0.20 for the SOL (P = 0.498) and −0.21 for the TA (P = 0.480). Second, the change in background EMG activity was not correlated with the change in test MEP amplitudes between the supported and unstable standing conditions. Pearson’s r values of 0.36 and 0.44 were observed for the SOL (P = 0.201) and TA (P = 0.114), respectively. Similarly, the change in background EMG activity was not correlated with the change in SICI between the supported and unstable standing conditions, with r values of −0.23 and −0.24 for the SOL (P = 0.437) and TA (P = 0.409), respectively. Taken together, it is unlikely that the observed changes in test MEP or SICI could be attributed to variations in background EMG or motor threshold between the different postural task conditions.
Table 2.
Pearson’s correlation coefficient values for relationship between various TMS and EMG measures
| Low Height | High Height | Both Heights | |
|---|---|---|---|
| bEMG and test MEP | |||
| SOL | 0.13 (0.18) | 0.53 (0.28) | 0.36 (0.13) |
| TA | 0.82 (0.67)* | 0.91 (0.83)* | 0.44 (0.20) |
| bEMG and SICI | |||
| SOL | −0.48 (0.23) | 0.04 (0.00) | −0.23 (0.05) |
| TA | 0.01 (0.00) | −0.21 (0.04) | −0.24 (0.06) |
| Test MEP and SICI | |||
| SOL | 0.45 (0.20) | 0.23 (0.05) | −0.20 (0.04) |
| TA | 0.23 (0.05) | −0.13 (0.02) | −0.21 (0.04) |
Values are Pearson’s correlation coefficient (r) used to examine the relationship between various transcranial magnetic stimulation (TMS) and electromyographic (EMG) measures in soleus (SOL) and tibialis anterior (TA) muscles. bEMG, background EMG; SICI, short-interval intracranial inhibition. Values in parentheses indicate R2 for each correlation.
Significant correlation.
DISCUSSION
Numerous studies have examined how the cortical contributions to postural control are altered with postural task difficulty. However, these studies have not considered the possibility that a change in postural threat, caused by an increase in postural task difficulty, may impact the neural mechanisms underlying postural control. The potential for such a confounding effect was evidenced in this study, where an increase in physiological arousal (i.e., galvanic skin response), perceived fear, and perceived anxiety were observed during the unstable compared with the free and supported postural tasks. This is similar to the findings of Hauck et al. (2008), who reported increases in both physiological and state anxiety as young adults completed postural tasks of increasing difficulty (i.e., quiet standing, maximum reach, and one-legged stance tests). Thus, to examine the effect of postural threat on the neurophysiological control of posture, participants of this study performed three postural tasks at low and high surface heights. Data from this study indicate that the excitability of the motor corticospinal pathway was increased and intracortical inhibition decreased with increasing postural task difficulty. However, when postural threat, in the form of standing at an elevated surface height, was introduced to further increase levels of arousal and anxiety, a summative effect of our neurophysiological measures was not observed. Because there also were no significant height × task difficulty interaction effects, it is unlikely that neurophysiological changes that are associated with increasing postural task difficulty can primarily be attributed to alterations in postural threat.
Effects of postural task difficulty.
The three postural tasks examined in this study resulted in a systematic increase in SOL and TA corticospinal excitability, as reflected by the test MEP amplitude, as well as a decrease in SOL and TA intracortical inhibition (i.e., SICI). The observed magnitude of MEP and SICI change supports previously reported findings, particularly when the level of postural task difficulty is considered. For example, in this study we observed a 40% increase in SOL MEP amplitude from the supported to the free standing conditions, and a further 67% increase from the free to the unstable standing conditions, respectively. This compares to the 15–35% increase in SOL MEP amplitude as individuals were tested from supported to unsupported standing (Papegaaij et al. 2016; Tokuno et al. 2009) or the ~50% increase when tested from a sitting to a standing posture (Obata et al. 2009). Likewise, the observed 24–42% smaller SICI between the three postural tasks is similar to the 14% change from rest to postural contraction (Soto et al. 2006), the 38% change from sitting to standing (Obata et al. 2009), and the 30% decrease during unsupported compared with supported standing (Papegaaij et al. 2016). One exception to this task-dependent modulation of SICI was reported by Papegaaij et al. (2014), who observed no change in SICI when young adults stood with their eyes closed instead of open or when they stood on foam instead of a rigid surface. However, this contrasting result could be attributed to the muscle under investigation (i.e., the TA may be less responsive to changes in postural task) or to the fact that the differences in the difficulty and sensory conditions of their chosen postural tasks were quite small compared with other studies.
Correlational analyses suggested that the observed changes in SOL or TA SICI were not related to changes in background EMG activity or different motor thresholds between postural tasks. Accordingly, our results provide further support of a postural task-dependent modulation of corticospinal excitability and intracortical inhibition, with an increased reliance of motor cortical pathways during more challenging postural tasks (Papegaaij et al. 2016). Reducing intracortical inhibition when a more challenging postural task is experienced could facilitate the excitability of the motor cortical areas so that it is more easily activated when a loss of balance is unexpectedly experienced (Papegaaij et al. 2016).
Effects of postural threat.
Despite a threefold increase in physiological arousal during the high compared with the low height condition, a summative effect on corticospinal excitability or SICI with postural task difficulty was not observed. Only one dependent measure, the SOL test MEP amplitude (i.e., corticospinal excitability), was different between the low and high surface height conditions, but this change occurred in the opposite direction to the effect of task difficulty (i.e., a decrease vs. an increase in MEP amplitude at height and with increasing task difficulty, respectively). No other changes in the TA corticospinal excitability, SOL SICI, and TA SICI were observed between the two surface height conditions. These results suggest two things. First, because the additional emotional response induced via postural threat generally did not lead to any further changes in our neurophysiological measures, arousal alone is unlikely to be the main responsibility for the changes in neural excitability that are associated with postural task difficulty. Second, changes in postural strategies that occur due to the presence of postural threat are not primarily controlled by altering intracortical inhibitory control. Rather than changes in responsiveness at the level of the motor cortex being responsible for the previously observed reductions in spinal excitability (Sibley et al. 2007), other mediators of spinal excitability, such as the vestibulospinal or reticulospinal pathways, may be more strongly influenced by the elevated levels of anxiety and arousal. This would be supported by the work of Naranjo et al. (2016), who observed increased vestibular response gains in the presence of postural threat, and that of Marker et al. (2017), who found alterations in reticulospinal effects during periods of increased psychosocial stress. Furthermore, indirect pathways arising via the basal ganglia or cerebellum are also known to be affected by threat (Staab et al. 2013).
Although it appears that postural threat had a smaller effect than expected on the corticospinal control of posture, it is important to consider that the effects of postural threat on corticospinal excitability have varied greatly between studies. For example, whereas this study observed a decrease in SOL corticospinal excitability (test MEP amplitude) in the presence of postural threat, Tanaka et al. (2013) found larger internal oblique MEPs when individuals stood at an elevated height with minimal surround surface, whereas Johannsson et al. (2017) reported no change in SOL MEPs in individuals standing at the top or bottom of a staircase. This contrasts with the more consistent findings regarding the effects of postural threat on the cortical inhibitory mechanisms during standing. Both Tanaka et al. (2013) and Johannsson et al. (2017) noted no change in the duration of the cortical silent period, which is believed to be partially a result of the amount of GABAB-mediated intracortical inhibition (Werhahn et al. 1999) and is similar to the lack of change in SOL and TA SICI, which is attributed to GABAA receptor-mediated intracortical inhibition (Kujirai et al. 1993; Ziemann et al. 1996), observed in this study.
Several reasons may account for why there is a large between-study variation in how the motor cortex responds to postural threat. Perhaps the most important consideration is the size of postural threat. For example, standing on a narrow surround at a 60-cm height (Tanaka et al. 2013) or looking down a 70-cm staircase (Johannsson et al. 2017) is likely to present a much lesser or even an insufficient amount of postural threat than standing at the edge of a 3-m platform (Brown and Frank 1997). Similarly, how the presented threat is perceived by the participant may also influence the postural control strategy and, consequently, the cortical involvement in postural control. In the data presented by Johannsson et al. (2017), none of the participants reported a fear of falling while standing at the top of a staircase, suggesting that the postural threat may have been perceived as minimal by the participants. This contrasts with the current study, where 5 of 14 participants reported at least a 50% fear of falling when completing the unstable postural task at the high surface height. Because individuals adopt different postural control strategies depending on whether they are fearful or not fearful to the presented threat (Davis et al. 2009), this may partially explain the large differences in postural control strategies that have been observed between studies. In fact, when individuals are presented fearful images to induce psychosocial stress, the amount of motor cortical excitability and intracortical facilitation is associated with the individual’s likelihood to experience fear and anxiety (Borgomaneri et al. 2017). Exploratory analyses were conducted to examine whether a similar relationship between the perceived level of postural threat and cortical response could be observed in the current data. However, perhaps due to the small sample sizes (n = 4 or 5 per group), no systematic differences in test MEP amplitudes or SICI were found between individuals reporting no fear and those reporting a high level of fear (>50%), or between individuals reporting a low vs. high level of somatic sensation at height. Similarly, there were no noticeable differences in the effect of postural threat between individuals who completed the high height condition first compared with those who experienced the low height condition first, though the presentation order of the threat condition is known to influence an individual’s perception of threat and, consequently, alter measures of postural control (Adkin et al. 2000). Clearly, further investigation into whether these factors truly affect the neurophysiological measures of standing posture is warranted.
Another consideration that may contribute to the divergent findings between studies is the muscle under examination. It is conceivable that participants in the study of Tanaka et al. (2013) increased corticospinal drive to the trunk muscles to stabilize posture when standing in the presence of postural threat. Although a similar strategy could be employed for the SOL, it is also possible that participants perceived the TMS pulse over the triceps surae as an externally induced, destabilizing perturbation. By lowering the corticospinal excitability of the triceps surae, this would serve as an efficient countermeasure to cope with TMS such that the magnetic stimulus would result in a smaller evoked muscle response and, consequently, less postural sway.
Although the variation of postural control strategies and levels of emotion observed between studies may be responsible for some of the inconsistencies in cortical responses obtained during standing, it is important to note that the variable effects of emotional state on cortical excitability are not limited to studies involving postural threat. For example, when individuals are tested under psychosocial stress, MEP amplitudes have been found to be greater (Marker et al. 2014) or not different (Borgomaneri et al. 2015; Tanaka et al. 2011) between the high and low stress conditions. Similarly, Tanaka et al. (2011) observed a decrease in SICI between nonpressure and pressure conditions, whereas Borgomaneri et al. (2015) found individuals to show no change in SICI or MEP when seeing fearful body language. Although the emotional responses to psychosocial stress will certainly differ from those observed under postural threat, the fact that there is a large range of cortical responses in situations of elevated emotion across a variety of studies and experimental paradigms involving stress or anxiety suggests the need for future studies to include much larger sample sizes to better establish whether a relationship between the size of emotional response (e.g., perceived fear and anxiety), specific postural control strategies, and changes in the cortical contribution to postural control exist.
In conclusion, the results of this study support the notion that the motor control of differently demanding postural tasks rely greatly on cortical mechanisms, as reflected by the increased SOL and TA corticospinal excitability as well as the reduced SOL and TA intracortical inhibition in the more challenging postural tasks. However, when the tasks were performed in the presence of postural threat, a decrease in SOL corticospinal excitability and no effects on intracortical inhibition were observed. Because summative effects on test MEP amplitude and SICI were not observed when the postural tasks were performed in the presence of postural threat, these results would suggest that the neurophysiological changes that occur with increasing postural task difficulty are unlikely the result of threat-related changes in emotional state.
GRANTS
This work was supported by funding from the Natural Sciences and Engineering Research Council of Canada and the Swiss National Science Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.D.T., M.G.C., and W.T. conceived and designed research; C.D.T., M.K., G.M., and W.T. performed experiments; C.D.T. and M.K. analyzed data; C.D.T., M.G.C., and W.T. interpreted results of experiments; C.D.T. prepared figures; C.D.T. drafted manuscript; C.D.T., M.K., M.G.C., G.M., and W.T. edited and revised manuscript; C.D.T., M.K., M.G.C., G.M., and W.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Audrey Mouthon, Jan Ruffieux, and Michael Wälchli for help during pilot testing.
REFERENCES
- Adkin AL, Campbell AD, Chua R, Carpenter MG. The influence of postural threat on the cortical response to unpredictable and predictable postural perturbations. Neurosci Lett 435: 120–125, 2008. doi: 10.1016/j.neulet.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Adkin AL, Frank JS, Carpenter MG, Peysar GW. Postural control is scaled to level of postural threat. Gait Posture 12: 87–93, 2000. doi: 10.1016/S0966-6362(00)00057-6. [DOI] [PubMed] [Google Scholar]
- Baudry S, Duchateau J. Age-related influence of vision and proprioception on Ia presynaptic inhibition in soleus muscle during upright stance. J Physiol 590: 5541–5554, 2012. doi: 10.1113/jphysiol.2012.228932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgomaneri S, Vitale F, Avenanti A. Behavioral inhibition system sensitivity enhances motor cortex suppression when watching fearful body expressions. Brain Struct Funct 222: 3267–3282, 2017. doi: 10.1007/s00429-017-1403-5. [DOI] [PubMed] [Google Scholar]
- Borgomaneri S, Vitale F, Gazzola V, Avenanti A. Seeing fearful body language rapidly freezes the observer’s motor cortex. Cortex 65: 232–245, 2015. doi: 10.1016/j.cortex.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Brown LA, Frank JS. Postural compensations to the potential consequences of instability: kinematics. Gait Posture 6: 89–97, 1997. doi: 10.1016/S0966-6362(96)01106-X. [DOI] [Google Scholar]
- Brown LA, Polych MA, Doan JB. The effect of anxiety on the regulation of upright standing among younger and older adults. Gait Posture 24: 397–405, 2006. doi: 10.1016/j.gaitpost.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Adkin AL, Brawley LR, Frank JS. Postural, physiological and psychological reactions to challenging balance: does age make a difference? Age Ageing 35: 298–303, 2006. doi: 10.1093/ageing/afl002. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP, Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res 138: 210–218, 2001. doi: 10.1007/s002210100681. [DOI] [PubMed] [Google Scholar]
- Davis JR, Campbell AD, Adkin AL, Carpenter MG. The relationship between fear of falling and human postural control. Gait Posture 29: 275–279, 2009. doi: 10.1016/j.gaitpost.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Garry MI, Thomson RH. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp Brain Res 193: 267–274, 2009. doi: 10.1007/s00221-008-1620-5. [DOI] [PubMed] [Google Scholar]
- Hauck LJ, Carpenter MG, Frank JS. Task-specific measures of balance efficacy, anxiety, and stability and their relationship to clinical balance performance. Gait Posture 27: 676–682, 2008. doi: 10.1016/j.gaitpost.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Johannsson J, Duchateau J, Baudry S. Spinal and corticospinal pathways are differently modulated when standing at the bottom and the top of a three-step staircase in young and older adults. Eur J Appl Physiol 117: 1165–1174, 2017. doi: 10.1007/s00421-017-3603-3. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn M, Yang JF, Prochazka A. Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83: 22–28, 1990. doi: 10.1007/BF00232189. [DOI] [PubMed] [Google Scholar]
- Marker RJ, Campeau S, Maluf KS. Psychosocial stress alters the strength of reticulospinal input to the human upper trapezius. J Neurophysiol 117: 457–466, 2017. doi: 10.1152/jn.00448.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker RJ, Stephenson JL, Kluger BM, Curran-Everett D, Maluf KS. Modulation of intracortical inhibition in response to acute psychosocial stress is impaired among individuals with chronic neck pain. J Psychosom Res 76: 249–256, 2014. doi: 10.1016/j.jpsychores.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo EN, Cleworth TW, Allum JH, Inglis JT, Lea J, Westerberg BD, Carpenter MG. Vestibulo-spinal and vestibulo-ocular reflexes are modulated when standing with increased postural threat. J Neurophysiol 115: 833–842, 2016. doi: 10.1152/jn.00626.2015. [DOI] [PubMed] [Google Scholar]
- Obata H, Sekiguchi H, Nakazawa K, Ohtsuki T. Enhanced excitability of the corticospinal pathway of the ankle extensor and flexor muscles during standing in humans. Exp Brain Res 197: 207–213, 2009. doi: 10.1007/s00221-009-1874-6. [DOI] [PubMed] [Google Scholar]
- Obata H, Sekiguchi H, Ohtsuki T, Nakazawa K. Posture-related modulation of cortical excitability in the tibialis anterior muscle in humans. Brain Res 1577: 29–35, 2014. doi: 10.1016/j.brainres.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Papegaaij S, Baudry S, Négyesi J, Taube W, Hortobágyi T. Intracortical inhibition in the soleus muscle is reduced during the control of upright standing in both young and old adults. Eur J Appl Physiol 116: 959–967, 2016. doi: 10.1007/s00421-016-3354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papegaaij S, Taube W, Hogenhout M, Baudry S, Hortobágyi T. Age-related decrease in motor cortical inhibition during standing under different sensory conditions. Front Aging Neurosci 6: 126, 2014. doi: 10.3389/fnagi.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley KM, Carpenter MG, Perry JC, Frank JS. Effects of postural anxiety on the soleus H-reflex. Hum Mov Sci 26: 103–112, 2007. doi: 10.1016/j.humov.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Smith RE, Smoll FL, Schutz RW. Measurement and correlates of sport-specific cognitive and somatic trait anxiety: the sport anxiety scale. Anxiety Res 2: 263–280, 1990. doi: 10.1080/08917779008248733. [DOI] [Google Scholar]
- Solopova IA, Kazennikov OV, Deniskina NB, Levik YS, Ivanenko YP. Postural instability enhances motor responses to transcranial magnetic stimulation in humans. Neurosci Lett 337: 25–28, 2003. doi: 10.1016/S0304-3940(02)01297-1. [DOI] [PubMed] [Google Scholar]
- Soto O, Valls-Solé J, Shanahan P, Rothwell J. Reduction of intracortical inhibition in soleus muscle during postural activity. J Neurophysiol 96: 1711–1717, 2006. doi: 10.1152/jn.00133.2006. [DOI] [PubMed] [Google Scholar]
- Staab JP, Balaban CD, Furman JM. Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol 33: 297–306, 2013. doi: 10.1055/s-0033-1356462. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Matsugi A, Kamata N, Hiraoka K. Postural threat increases corticospinal excitability in the trunk flexor muscles in the upright stance. J Psychophysiol 27: 165–172, 2013. doi: 10.1027/0269-8803/a000101. [DOI] [Google Scholar]
- Tanaka Y, Funase K, Sekiya H, Sasaki J, Takemoto T. Multiple EMG activity and intracortical inhibition and facilitation during a fine finger movement under pressure. J Mot Behav 43: 73–81, 2011. doi: 10.1080/00222895.2010.542508. [DOI] [PubMed] [Google Scholar]
- Tokuno CD, Taube W, Cresswell AG. An enhanced level of motor cortical excitability during the control of human standing. Acta Physiol (Oxf) 195: 385–395, 2009. doi: 10.1111/j.1748-1716.2008.01898.x. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 517: 591–597, 1999. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40: 367–378, 1996. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]