Abstract
Direction selectivity is a fundamental computation in the visual system and is first computed by the direction-selective circuit in the mammalian retina. Although landmark discoveries on the neural basis of direction selectivity have been made in the rabbit, many technological advances designed for the mouse have emerged, making this organism a favored model for investigating the direction-selective circuit at the molecular, synaptic, and network levels. Studies using diverse motion stimuli in the mouse retina demonstrate that retinal direction selectivity is implemented by multilayered mechanisms. This review begins with a set of central mechanisms that are engaged under a wide range of visual conditions and then focuses on additional layers of mechanisms that are dynamically recruited under different visual stimulus conditions. Together, recent findings allude to an emerging theme: robust motion detection in the natural environment requires flexible neural mechanisms.
Keywords: direction selectivity, motion detection, retina, synaptic circuit, visual motion
INTRODUCTION
Direction selectivity, a classic model of neural computation, has inspired scientists in both experimental and computational disciplines for decades (Barlow et al. 1964; Borst 2000; Ferster 1998; Hassenstein and Reichardt 1956; Hubel and Wiesel 1959; Poggio and Reichardt 1973). In mammals, direction-selective ganglion cells (DSGCs) were first discovered in the rabbit retina (Barlow and Hill 1963; Barlow et al. 1964). These cells fire maximally to images moving in their preferred direction and are silenced by movement in the opposite (null) direction. Based on the activity of DSGCs during sequential presentation of a pair of stationary light flashes in the preferred and null directions, Barlow and Levick proposed the “null-direction inhibition” model that attributes direction selectivity of DSGCs to a stronger inhibitory mechanism during motion in the null direction (Barlow and Levick 1965). Since then, uncovering neural mechanisms underlying null-direction inhibition has been a major goal in visual neuroscience.
During the quest to identify the neural mechanisms of retinal direction selectivity, the rabbit has been the prevalent model organism for a series of remarkable discoveries at the cellular and synaptic levels. In the past 10 years, an increasing repertoire of cell type-specific genetic markers and molecular tools available for the mouse retina has prompted the field to gradually transition to the mouse as a model for probing the developmental and mature mechanisms of retinal direction selectivity. The ability to target specific DSGC subtypes and their key presynaptic partner, the starburst amacrine cell (SAC), has accelerated the analysis of this circuit (Dhande et al. 2013; Huberman et al. 2009; Kay et al. 2011; Kim et al. 2008; Rivlin-Etzion et al. 2011; Trenholm et al. 2011; Watanabe et al. 1998; Yonehara et al. 2008). Based on the results from both rabbit and mouse studies, the neural substrate of the null-direction inhibition model originally proposed by Barlow and Levick (1965) has been pinpointed at the GABAergic connections between SACs and DSGCs (Fig. 1A).
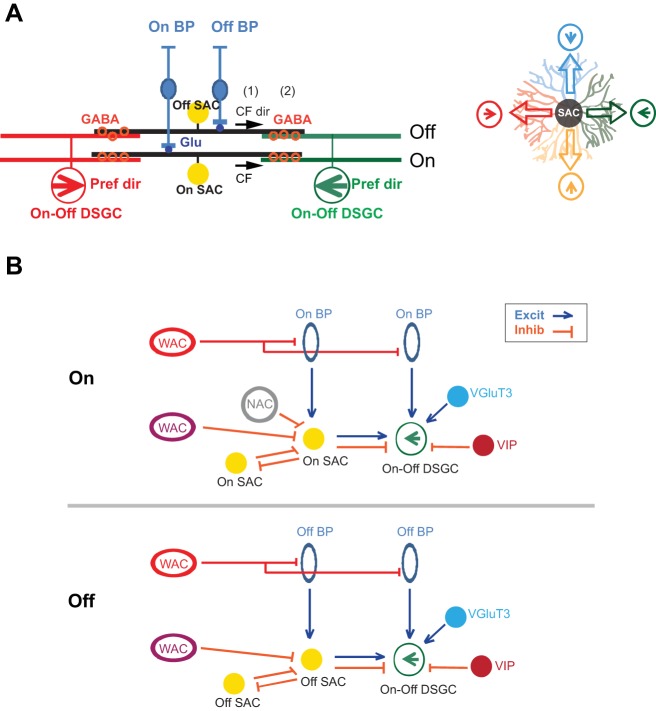
Fig. 1.
Wiring diagrams of retinal direction selectivity. A: model of null-direction inhibition. Left: schematic shows side view of glutamatergic bipolar cell (BP) inputs to the proximal dendrites of ON and OFF starburst amacrine cells (SACs) and GABA release (orange circles) from SAC distal dendrites to two ON-OFF direction-selective ganglion cells (DSGCs) of opposite preferred directions (pref dir). The preferred directions of ON-OFF DSGCs are represented by the green and red arrows. Directional inhibition from SACs to the DSGC depends on two properties: 1) centrifugal (CF) direction selectivity of SAC dendrites (CF direction (CF dir) of SAC dendrites is represented by black arrows) and 2) asymmetric wiring between SACs and DSGCs. SAC dendrites oriented to the right form GABAergic synapses with the DSGC whose preferred direction is to the left. Right: Top view shows the asymmetric inhibition from a SAC (center) to four DSGCs that prefer four different cardinal directions. Each SAC dendritic quadrant preferentially forms GABAergic synapses with the DSGC with the same color. B: schematic shows anatomically and/or functionally identified connections in the inner plexiform layer that participate in the direction-selective circuit. Top: ON pathway. Bottom: OFF pathway. Excit, excitatory; Inhib, inhibitory; VGluT3, vesicular glutamate transporter 3; VIP, vasoactive intestinal polypeptide; WAC, wide-field amacrine cell.
The triumph of understanding the null-direction inhibition at the level of SAC-DSGC interactions marks the beginning, rather than the end, of our search for the neural basis of direction selectivity. SACs and DSGCs, the cell types that have been in the spotlight, are embedded in the extensive retinal network consisting of ~100 types of neurons (Baden et al. 2016; Masland 2012; Sanes and Masland 2015). Immediately connected to the SAC-DSGC microcircuit are the diverse sets of synapses from multiple types of bipolar cells and amacrine cells (Fig. 1B). These connections profoundly shape the light responses of both SACs and DSGCs, making them sensitive or resilient to various features of visual input. A comprehensive understanding of motion detection therefore requires an integrated circuit model that encompasses all relevant neurons and their radiating connections, with the ultimate goal of a complete functional wiring diagram for motion detection in the natural environment.
The two major DSGC types, ON and ON-OFF DSGCs, are both dependent on SACs for their direction selectivity but exhibit interesting differences in other receptive field properties and visual functions [reviewed by Vaney et al. (2012)]. Most of the literature focuses on ON-OFF DSGCs, because of their higher density compared with that of ON DSGCs in the retina and the availability of multiple subtype-specific markers. The preferred directions of ON-OFF DSGCs are grouped into four clusters (Oyster and Barlow 1967) that correspond to the forward/backward and upward/downward directions of translational motion (Sabbah et al. 2017). ON-OFF DSGCs project to two major visual nuclei, the dorsal lateral geniculate nucleus (dLGN) and superior colliculus (Cruz-Martín et al. 2014; Huberman et al. 2009; Kay et al. 2011; Rivlin-Etzion et al. 2011). In the superior colliculus, direction-selective retinal inputs give rise to direction selectivity in collicular neurons (Shi et al. 2017). In the dLGN, thalamic neurons in the region innervated by axons of ON-OFF DSGCs exhibit direction and orientation selectivity (Cruz-Martín et al. 2014; Marshel et al. 2012; Piscopo et al. 2013), suggesting an instructive role of direction-selective retinal inputs in feature selectivity in the dLGN. Furthermore, perturbing retinal direction selectivity has been shown to alter the receptive field properties of direction-selective neurons in the mouse primary visual cortex (Hillier et al. 2017).
In this review, we first summarize the recent literature on the “core mechanisms” underlying direction selectivity in ON-OFF DSGCs that are invariant in a wide range of visual stimulus conditions. For a more comprehensive summary of the rich literature on this topic and the developmental aspects of retinal direction selectivity, readers are encouraged to consult other excellent reviews (Mauss et al. 2017; Morrie and Feller 2016; Vaney et al. 2012). The rest of this review focuses on the recently discovered neural mechanisms in the mouse retina that are selectively recruited or modulated by specific features of motion stimuli. These findings indicate that direction selectivity in the retina is not implemented by a rigid set of mechanisms. Rather, dynamic and flexible sets of mechanisms are engaged and tailored to the specific visual conditions to ensure reliable and robust motion detection as the animal navigates in the versatile natural environment.
CORE MECHANISMS
Null-Direction Inhibition of DSGCs
As a key mechanism of direction selectivity, the inhibitory inputs onto DSGCs are strongly tuned to motion in the null direction. This directional inhibition is provided by ON and OFF SACs, which are axonless, monostratified amacrine cells releasing GABA and acetylcholine (ACh) onto the ON and OFF dendritic layers of the bistratified ON-OFF DSGCs (Fig. 1A). When GABAergic synapses between SACs and DSGCs are genetically disrupted by knocking out vesicular GABA transporter in SACs, the direction selectivity of DSGCs is severely reduced (Pei et al. 2015).
Two properties of SAC-DSGC GABAergic synapses are required to generate directional inhibition of DSGCs.
1) SAC dendrites are direction selective and prefer motion in the centrifugal direction (away from the soma, Fig. 1A; Euler et al. 2002). The centrifugal direction selectivity of SAC dendrites has been attributed to multiple mechanisms. In the mouse retina, spatially segregated synaptic inputs and outputs along the dendrites have been implicated in generating this centrifugal preference. Glutamatergic inputs from bipolar cells and inhibitory inputs from neighboring amacrine cells are enriched in the proximal dendrites of mouse SACs (Ding et al. 2016; Vlasits et al. 2016). This proximal distribution of synaptic inputs in the mouse is in contrast to the more uniform distribution of inputs along the SAC dendrites in the rabbit (Famiglietti 1991). On the other hand, neurotransmitters GABA and ACh are released from varicosities in the outer third of SAC dendritic arbors in both rabbits and rodents (Brecha et al. 1988; Famiglietti 1991; Kosaka et al. 1988; O’Malley and Masland 1989; Vaney and Young 1988). Computational modeling indicates that the proximal distribution of synaptic inputs and distal distribution of synaptic outputs in the mouse SAC contribute to the centrifugal direction selectivity of SACs (Ding et al. 2016; Vlasits et al. 2016).
In addition to the spatial segregation of inputs and outputs, other mechanisms have also been reported, which include dendritic morphology (Miller and Bloomfield 1983; Morrie and Feller 2018; Tukker et al. 2004; Velte and Miller 1997), lateral inhibition (Chen et al. 2016; Ding et al. 2016; Kostadinov and Sanes 2015; Lee and Zhou 2006), patterned distribution of dendritic voltage-gated channels (Euler et al. 2002; Hausselt et al. 2007; Oesch and Taylor 2010) and chloride cotransporters (Gavrikov et al. 2003, 2006), and spatially segregated excitatory inputs of different kinetics from distinct bipolar cell types (Fransen and Borghuis 2017; Kim et al. 2014; but see Stincic et al. 2016; Vlasits et al. 2016).
Although the SAC dendritic branches are tuned to different linear motion directions, motion-evoked dendritic activation of individual sectors is not completely isolated from other sectors. Computational modeling suggests that global signal integration across the dendritic field contributes to the centrifugal response of SAC distal dendrites (Tukker et al. 2004). A role of global dendritic signal integration is experimentally demonstrated by a multiphoton calcium-imaging experiment, in which the centrifugal activation of SAC distal dendrites during full-field linear motion stimuli is stronger and starts earlier compared with the centrifugal response locally generated within a dendritic sector (Koren et al. 2017).
The balance between signal integration and compartmentalization in SAC dendrites is maintained by intricate interactions between passive and active membrane properties (Koren et al. 2017; Ozaita et al. 2004; Tukker et al. 2004) and regulated by metabotropic glutamate receptor 2 (mGluR2) signaling (Koren et al. 2017). mGluR2 blockade increases voltage-gated calcium channel activity and thereby reduces the electrotonic isolation between SAC sectors. This leads to enhanced propagation of centrifugal response from one dendritic sector to the opposite, centrifugally activated sector. As a result, centrifugal direction selectivity of SAC dendrites is impaired during mGluR2 blockade (Fig. 2; Koren et al. 2017). The molecular targets of mGluR2 activation include N- and P/Q-type voltage-gated calcium channels in SACs, which are inhibited shortly after bath application of an mGluR2 agonist. However, mGluR2 blockade specifically promotes cross-sector signal propagation but does not affect signal processing within a sector. Therefore, the site of endogenous mGluR2 action is likely the perisomatic/proximal dendritic compartment, without directly inhibiting the presynaptic calcium channels at the distal dendritic tips. Although the subcellular distribution of mGluR2 along SAC dendrites is not yet known, glutamate release onto the SAC from bipolar cells is skewed to SAC proximal dendrites in the mouse retina (Ding et al. 2016; Vlasits et al. 2016). Concomitant activation of ionotropic and metabotropic glutamate receptors in the proximal dendrites may serve as a homeostatic mechanism to prevent aberrant backpropagation of local depolarization of SAC dendrites when the glutamatergic drive to the SAC is strong. Future studies that investigate mGluR2 signaling under different visual stimulus conditions will elucidate the intriguing link between visually evoked activity and the flexible dendritic computation algorithm modulated by mGluR2 signaling. Interestingly, voltage-gated KV3 potassium channels (Ozaita et al. 2004) and GABAergic inputs (Ding et al. 2016) are also concentrated in the proximal dendrites of SACs and contribute to local dendritic processing (Ozaita et al. 2004; Poleg-Polsky et al. 2018), making the proximal dendritic region well equipped for regulation of compartmentalized signaling of SAC dendrites.
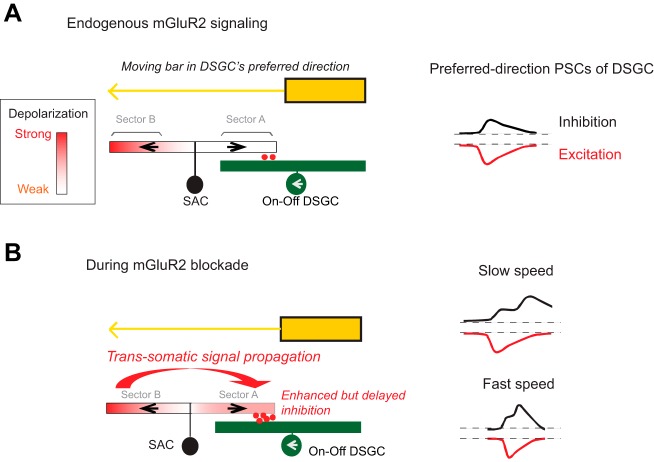
Fig. 2.
Metabotropic glutamate receptor 2 (mGluR2)-mediated electrotonic isolation of starburst amacrine cell (SAC) dendritic sectors is required for broad speed tuning of ON-OFF direction-selective ganglion cells (DSGCs). A: model of mGluR2-dependent dendritic compartmentalization in SACs. Left: endogenous mGluR2 signaling promotes the electrotonic isolation between SAC dendritic branches. A bar moving in the preferred direction of the DSGC (leftward) triggers minimal GABA release (red dots) from the centripetally activated sector A of the SAC. Subsequent activation of sector B in the centrifugal direction does not propagate efficiently to sector A to trigger GABA release. Right: weak centripetal activation of SAC dendrites leads to weak inhibitory postsynaptic current (IPSC) of the DSGC in the preferred direction. B, left: during mGluR2 blockade, electrotonic isolation between SAC branches is reduced. Therefore, the strong centrifugal response of sector B propagates more efficiently to sector A. This leads to enhanced dendritic activation of sector A and more GABA release onto the DSGC during motion in the DSGC’s preferred direction. Right: since the relative timing of sector B and A activation depends on the speed of the moving bar, the enhanced component of IPSC during mGluR2 blockade is more delayed and shows less overlap with excitatory postsynaptic current (EPSC) at slower speed. Therefore, preferred-direction spiking of DSGC is less affected. At higher speed, enhanced IPSC shows more overlap with EPSC, leading to reduced firing of DSGC. [Adapted from Koren et al. (2017).]
2) The GABAergic connections between SACs and DSGCs are highly asymmetric along the preferred null axis. Each DSGC is selectively inhibited by SAC dendrites that are oriented in the DSGC’s null direction (Briggman et al. 2011; Fried et al. 2002; Lee et al. 2010; Wei et al. 2011; Yonehara et al. 2011; Fig. 1A). In the mouse, this asymmetry emerges during the second postnatal week before eye opening (Wei et al. 2011; Yonehara et al. 2011) because of increased number of synapses between null direction-oriented SAC dendrites and DSGCs (Morrie and Feller 2015). The establishment of direction selectivity is not affected by pharmacological blockade of neural activity (Sun et al. 2011; Wei et al. 2011), whereas the clustering of the four preferred directions is refined by visual activity after eye opening (Bos et al. 2016).
Together, the centrifugal direction selectivity of SAC dendrites and the asymmetric wiring of SAC-DSGC GABAergic synapses result in directionally tuned inhibition of DSGCs. Motion in the null direction of the DSGC activates its presynaptic SAC dendrites in the centrifugal direction, causing strong GABAergic inputs from SACs to DSGCs (Fig. 1A).
Directional Excitation onto DSGCs
Stronger preferred-direction excitation of DSGCs has been consistently observed in multiple studies using whole cell voltage-clamp recordings, although the tuning of excitatory postsynaptic currents (EPSCs) appears weaker compared with that of inhibitory postsynaptic currents (Fried et al. 2005; Lee et al. 2010; Park et al. 2014; Pei et al. 2015; Taylor and Vaney 2002). EPSCs of DSGCs consist of the glutamatergic component and the SAC-mediated cholinergic component. When the glutamatergic component is examined with alternative methods such as calcium imaging in bipolar cell terminals and glutamate imaging in DSGCs with a fluorescent glutamate sensor, intensity-based glutamate-sensing fluorescent reporter (iGluSnFR), it does not appear to be directional (Chen et al. 2014; Park et al. 2014; Yonehara et al. 2013). One complication of voltage-clamp recording is that EPSCs in the null direction may be distorted by the concomitant strong inhibitory inputs due to imperfect space clamp, leading to an underestimation of EPSCs in the null direction (Poleg-Polsky and Diamond 2011). However, recent studies indicate that directional excitation is not simply a voltage-clamp artifact. First, the tuning strength of excitation is not well correlated with the tuning strength or absolute amplitude of inhibition in wild-type rabbits (Percival et al. 2017) or in mutant mice that lack SAC-DSGC inhibition (Pei et al. 2015), suggesting that directional excitation is not solely due to voltage-clamp errors. Second, abolishing directional inhibition by genetically removing GABA release from SACs does not fully eliminate directional excitation or directional spiking activity of DSGCs (Pei et al. 2015). Although direction selectivity of DSGCs in mutant mice is significantly reduced, a subset of the ON-OFF DSGCs still receive directional cholinergic excitation and exhibit residual, albeit weakened, directional tuning of their spiking activity (Pei et al. 2015). These results indicate that directional excitation is a physiological mechanism that contributes to the direction selectivity of DSGC spiking activity, together with the more universal and prominent null-direction inhibition. The contribution of cholinergic excitation to direction selectivity may depend on visual stimuli, since earlier studies report different effects of nicotinic receptor antagonists on direction selectivity of DSGCs during moving-bar and drifting-grating stimuli (Grzywacz et al. 1998a, 1998b).
The synaptic circuitry underlying directional excitation remains poorly understood. The cholinergic excitation from SACs to DSGCs is isotropic when the SACs are stimulated by depolarizing voltage steps in dual voltage-clamp recordings. However, these cholinergic synapses are activated in a directional manner during the presentation of moving stimuli (Fried et al. 2005; Lee et al. 2010; Pei et al. 2015). Bath application of GABAA receptor antagonists abolishes directional excitation (Fried et al. 2005; Lee et al. 2010; Pei et al. 2015), suggesting that an upstream inhibitory circuit mediates the directional ACh release from SACs. The neural substrate of this inhibitory mechanism is not yet identified.
Postsynaptic Mechanisms at DSGC Dendrites
After the arrival of the patterned excitatory and inhibitory inputs at DSGC dendrites, synaptic inputs are further integrated and processed by postsynaptic mechanisms to amplify the direction selectivity of DSGC spiking response through N-methyl-d-aspartate (NMDA) receptor signaling (Poleg-Polsky and Diamond 2016a) and local dendritic spike generation (Brombas et al. 2017; Oesch et al. 2005; Schachter et al. 2010; Sivyer and Williams 2013). Furthermore, the fluctuations of excitatory and inhibitory inputs (also termed “synaptic noise”) during motion stimuli show direction-dependent covariation. Synaptic noise covariation during null-direction motion is stronger compared with that during the preferred-direction motion, leading to more efficient cancellation of excitatory and inhibitory inputs onto DSGC dendrites during motion in the null direction (Cafaro and Rieke 2010).
STIMULUS-DEPENDENT MECHANISMS
Contrast
Direction selectivity of DSGCs is robust over a wide range of stimulus contrasts (Grzywacz et al. 1998b; Poleg-Polsky and Diamond 2016b). In the mouse retina, multiple mechanisms have been reported to maintain robust direction selectivity at different contrasts. Inhibitory circuits have been shown to play a role at high-contrast conditions since bath application of GABAA receptor antagonist SR95531 leads to a more severe reduction of the centrifugal direction selectivity of ON SAC dendrites at higher contrast compared with low contrast (Ding et al. 2016). However, selective removal of GABAA receptors from ON SACs does not affect the centrifugal direction selectivity of SAC dendrites over a range of contrast levels (Chen et al. 2016), suggesting that the locus of inhibition is at the bipolar terminals presynaptic to SACs (refer to Fig. 1B for the sources of inhibition of SACs).
Excitatory inputs to DSGCs are also modulated by contrast. The bipolar cells that drive SACs exhibit higher contrast sensitivity compared with those that drive DSGCs (Poleg-Polsky and Diamond 2016b). However, the glutamatergic inputs undergo nonlinear transformation by SAC dendrites, likely caused by the nonlinear activation of synaptic calcium channels, which results in a reduced contrast sensitivity of ACh and GABA release from SAC presynaptic terminals (Poleg-Polsky and Diamond 2016b). As a result, the contrast sensitivity of GABAergic and cholinergic inputs matches that of bipolar cell-mediated glutamatergic inputs onto DSGCs. This balanced excitation-to-inhibition ratio in DSGCs ensures reliable direction selectivity independent of contrast (Poleg-Polsky and Diamond 2016b). In an alternative model, the contrast sensitivity of SAC-mediated inputs to DSGCs does not match that of bipolar cell-mediated glutamatergic inputs. Different contrast sensitivities of SACs and DSGCs arise from different compositions of their glutamate receptors (Sethuramanujam et al. 2016, 2017). Cholinergic inputs dominate DSGC excitation at low contrast and work in conjunction with GABAergic inputs to implement direction selectivity (Sethuramanujam et al. 2016). By comparison, a different strategy has been reported in guinea pigs and rabbits. In these animals, excitatory inputs onto DSGCs vary more linearly with contrast to encode contrast information in preferred-direction spiking activity, whereas strong null-direction inhibitory inputs exhibit highly nonlinear contrast sensitivity and saturate at low contrast (Lipin et al. 2015). Saturated inhibition ensures sufficient suppression of null-direction spiking across different contrast levels and therefore safeguards direction selectivity. It is noteworthy that the background light intensity and the contrast range differ across studies. Therefore, differences in results reported from different studies may be explained by differences in experimental or stimulus conditions.
ON-OFF Contrast Polarity
Segregation of the ON and OFF pathways is a general feature of the synaptic organization in the inner retina. For ON-OFF DSGCs, direction selectivity for bright and dark contours is processed in the ON and OFF layers of DSGC dendrites that receive synaptic inputs from the ON and OFF SACs, respectively. Although the asymmetric wiring between SACs and DSGCs is mirrored in the ON and OFF pathways (Briggman et al. 2011), recent studies demonstrate notable differences in the neural mechanisms underlying the direction selectivity of ON and OFF SAC dendrites.
First, inhibitory inputs to ON and OFF SACs show functional and anatomical divergence. In the ON pathway, inhibitory inputs onto ON SACs are not required for direction selectivity of ON SACs and the ON response of DSGCs during a simple moving-bar stimulus against a uniform background (Chen et al. 2016). However, feed-forward inhibition from non-SAC amacrine cells to OFF SACs contributes to direction selectivity in the OFF pathway under the same stimulus condition (Chen et al. 2016). Interestingly, connectomic analysis shows that OFF SACs receive a higher fraction of wide-field amacrine cell inputs than ON SACs (Ding et al. 2016), consistent with a more prominent role of inhibition from non-SAC amacrine cells to OFF SACs. On the other hand, ON SACs, but not OFF SACs, receive inputs from a class of narrow-field amacrine cells (Ding et al. 2016). These results highlight that the inhibitory circuitries impinging on the ON and OFF SACs are not identical. Additionally, NMDA receptor function has been reported to differ between the two SAC populations, since NMDA receptor antagonist affects the temporal response of OFF, but not ON, SACs (Fransen and Borghuis 2017).
In addition to the distinct mechanisms underlying direction selectivity of ON and OFF SACs, interactions between ON and OFF pathways have been implicated under specific visual stimulation conditions. Crossover excitation of OFF SACs from the ON pathway that originates from rod and M-cone signaling has been reported in the mouse retina [Rosa et al. 2016; but see Kittila and Massey (1995) in rabbit]. In another study, the polarity of excitatory inputs to ON and OFF SACs can be reversed by a short period of repetitive drifting-grating stimulation (Vlasits et al. 2014), which may underlie the reversal of the preferred direction of DSGCs under the same condition (Rivlin-Etzion et al. 2012).
Together, these recent studies indicate that motion of bright and dark contours is not processed by completely mirror-symmetric mechanisms in the ON and OFF pathways in the mouse retina. Instead, divergent circuit motifs and synaptic properties as well as the interactions between the two pathways collectively shape the directionally tuned ON and OFF responses of DSGCs.
Noise
A moving-bar stimulus over a uniform background has been pivotal to uncovering many key mechanisms of direction selectivity. However, visual motion in the natural environment is usually accompanied by other nonmotion features that put extra strains on the direction-selective circuit. To maintain robust direction selectivity in the presence of these competing features or “noise,” the retina recruits additional layers of neural mechanisms that are selectively engaged when visual noise is present in motion stimuli. One example is the NMDA receptor signaling in DSGCs that amplifies correlated excitation and improves the fidelity of direction selectivity in noisy conditions (Poleg-Polsky and Diamond 2016a). When the background and bar intensities vary independently and randomly, direction selectivity of DSGCs deteriorates upon NMDA receptor blockade (Poleg-Polsky and Diamond 2016a). By contrast, NMDA receptor blockade has no effect on direction selectivity during noise-free moving-bar stimuli (Kittila and Massey 1997; Poleg-Polsky and Diamond 2016a).
Visual noise also recruits specific microcircuit motifs to the direction-selective circuit. This is exemplified by selective activation of lateral inhibition onto ON SACs (Chen et al. 2016). In the absence of background noise, the direction selectivity of the ON SACs and ON component of DSGCs does not require lateral inhibition onto ON SACs. However, when a randomly flickering checkerboard is introduced in the background, the lack of GABAergic inputs onto ON SACs significantly impairs direction selectivity in the ON pathway, highlighting the importance of lateral inhibition in preserving feature selectivity under complex visual conditions.
Speed
Direction-selective firing of ON-OFF DSGCs exhibits broad speed tuning. In the null direction, to ensure reliable suppression of DSGC spiking, strong inhibitory inputs need to be generated in time to sufficiently coincide with excitation at all speeds. Steep saturation of inhibitory inputs onto DSGCs has been shown in rabbits and guinea pigs to render the strong null-direction inhibition speed invariant (Lipin et al. 2015). In the temporal domain, cholinergic and GABAergic inputs onto DSGCs are well correlated in the null direction across a range of speeds because of the corelease of ACh and GABA from the centrifugally activated SAC dendrites. The fast onset of GABAergic inhibition onto DSGCs in the null direction also ensures a timely cancellation of glutamatergic excitation from bipolar cells. This fast onset of strong null-direction inhibition depends on global signal integration across the entire SAC dendritic field along the motion trajectory (Koren et al. 2017).
In the preferred direction, mGluR2-dependent electrotonic isolation of SAC dendrites is involved in maintaining the broad speed tuning of DSGC spiking (Koren et al. 2017). When mGluR2 is blocked, the strong centrifugal activation of one sector propagates more readily into the opposite, centripetally activated sector and thereby enhances the preferred-direction inhibition of DSGCs (Fig. 2). This timing of the enhanced centripetal response during mGluR2 blockade depends on the speed of linear motion across SAC dendrites. At faster motion speed, backpropagating signals arrive faster and enhance preferred-direction GABAergic inputs onto DSGCs during concomitant excitation. Therefore, blockade of endogenous mGluR2 signaling significantly reduces the preferred-direction firing of DSGCs at faster speeds. The impact of mGluR2 blockade on DSGC preferred-direction spiking is less prominent at lower speed because of the reduced overlap between excitation and aberrant but more delayed inhibition of DSGCs (Fig. 2).
ANATOMICAL SUBSTRATES FOR AN EXTENDED NEURAL NETWORK OF DIRECTION SELECTIVITY
The stimulus-dependent neural mechanisms discussed above indicate that the receptive field properties of SACs and DSGCs are intricately influenced by the extended neural circuitry under different stimulus conditions. Recently, physiological studies revealed two new types of amacrine cells that provide synaptic inputs onto DSGCs. The vesicular glutamate transporter 3 amacrine cells provide glutamatergic excitation to ON-OFF and ON DSGCs (Kim et al. 2015; Lee et al. 2014, 2016), whereas the vasoactive intestinal polypeptide-positive amacrine cells send GABAergic inhibition to ON-OFF DSGCs (Park et al. 2015).
Connectomic analysis reveals a separate set of narrow and wide amacrine cell types that are connected to SACs and bipolar cells (Ding et al. 2016; Hoggarth et al. 2015). Reconstruction of the direction-selective circuit at the electron microscopic level also delineates partially overlapping sets of bipolar cell types that innervate the ON-OFF DSGCs, ON and OFF SACs (Ding et al. 2016; Helmstaedter et al. 2013; Kim et al. 2014). In the ON layer, both DSGC and ON SAC receive inputs from cone bipolar cell CBC5 subtypes, but only ON SAC receives additional CBC7 inputs at proximal dendrites. In the OFF layer, both DSGC and OFF SAC receive inputs from CBC3 and CBC4, but OFF SAC receives additional inputs from CBC1 and CBC2 at proximal dendrites. These findings will inspire future studies that unite anatomy with function for these new cellular players to generate a more comprehensive model of direction selectivity under diverse visual conditions.
SUMMARY
An intuitive assumption of visual processing in the retina is that computations need to be robust so that visual features can be faithfully reported to the higher visual centers in the brain. This is in contrast to many cortical circuits that feature flexible input-output relationships, such as circuits involved in learning and memory (Holtmaat and Caroni 2016). However, robust computation does not necessarily imply a rigid circuit. Instead, the direction-selective circuit uses a highly dynamic set of mechanisms both at the circuit and dendritic levels. This flexibility is necessary because visual motion in the natural environment is complex, dynamic, and often obscured by other visual features. A rigid set of mechanisms may quickly fail in the large parameter space of natural stimuli. By acute modulation and selective engagement of multilayered mechanisms, retinal circuits are well equipped to meet the challenges of feature extraction in the ever-changing natural environment and reliably convey visual information to downstream visual areas.
GRANTS
This work was supported by NIH Grant R01-EY-024016 (W. Wei).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.W. prepared figures; Q.C. and W.W. drafted manuscript; Q.C. and W.W. edited and revised manuscript; Q.C. and W.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank laboratory members Hector Acaron Ledesma, Xiaolin Huang, and Jennifer Ding for helpful comments on the manuscript.
REFERENCES
- Baden T, Berens P, Franke K, Román Rosón M, Bethge M, Euler T. The functional diversity of retinal ganglion cells in the mouse. Nature 529: 345–350, 2016. doi: 10.1038/nature16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science 139: 412–414, 1963. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Hill RM, Levick WR. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J Physiol 173: 377–407, 1964. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit’s retina. J Physiol 178: 477–504, 1965. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A. Models of motion detection. Nat Neurosci 3, Suppl: 1168, 2000. doi: 10.1038/81435. [DOI] [PubMed] [Google Scholar]
- Bos R, Gainer C, Feller MB. Role for visual experience in the development of direction-selective circuits. Curr Biol 26: 1367–1375, 2016. [Erratum in Curr Biol 27: 927, 2017.] 10.1016/j.cub.2016.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha N, Johnson D, Peichl L, Wässle H. Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and gamma-aminobutyrate immunoreactivity. Proc Natl Acad Sci USA 85: 6187–6191, 1988. doi: 10.1073/pnas.85.16.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471: 183–188, 2011. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Brombas A, Kalita-de Croft S, Cooper-Williams EJ, Williams SR. Dendro-dendritic cholinergic excitation controls dendritic spike initiation in retinal ganglion cells. Nat Commun 8: 15683, 2017. doi: 10.1038/ncomms15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro J, Rieke F. Noise correlations improve response fidelity and stimulus encoding. Nature 468: 964–967, 2010. doi: 10.1038/nature09570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lee S, Park SJ, Looger LL, Zhou ZJ. Receptive field properties of bipolar cell axon terminals in direction-selective sublaminas of the mouse retina. J Neurophysiol 112: 1950–1962, 2014. doi: 10.1152/jn.00283.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Pei Z, Koren D, Wei W. Stimulus-dependent recruitment of lateral inhibition underlies retinal direction selectivity. eLife 5: e21053, 2016. doi: 10.7554/eLife.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, Huberman AD. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507: 358–361, 2014. doi: 10.1038/nature12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Estevez ME, Quattrochi LE, El-Danaf RN, Nguyen PL, Berson DM, Huberman AD. Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization. J Neurosci 33: 17797–17813, 2013. doi: 10.1523/JNEUROSCI.2778-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Smith RG, Poleg-Polsky A, Diamond JS, Briggman KL. Species-specific wiring for direction selectivity in the mammalian retina. Nature 535: 105–110, 2016. doi: 10.1038/nature18609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418: 845–852, 2002. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J Comp Neurol 309: 40–70, 1991. doi: 10.1002/cne.903090105. [DOI] [PubMed] [Google Scholar]
- Ferster D. A sense of direction. Nature 392: 433–434, 1998. doi: 10.1038/33005. [DOI] [PubMed] [Google Scholar]
- Fransen JW, Borghuis BG. Temporally diverse excitation generates direction-selective responses in ON- and OFF-type retinal starburst amacrine cells. Cell Reports 18: 1356–1365, 2017. doi: 10.1016/j.celrep.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Fried SI, Münch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature 420: 411–414, 2002. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- Fried SI, Münch TA, Werblin FS. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron 46: 117–127, 2005. doi: 10.1016/j.neuron.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Gavrikov KE, Dmitriev AV, Keyser KT, Mangel SC. Cation–chloride cotransporters mediate neural computation in the retina. Proc Natl Acad Sci USA 100: 16047–16052, 2003. doi: 10.1073/pnas.2637041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proc Natl Acad Sci USA 103: 18793–18798, 2006. doi: 10.1073/pnas.0604551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz NM, Amthor FR, Merwine DK. Necessity of acetylcholine for retinal directionally selective responses to drifting gratings in rabbit. J Physiol 512: 575–581, 1998a. doi: 10.1111/j.1469-7793.1998.575be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz NM, Merwine DK, Amthor FR. Complementary roles of two excitatory pathways in retinal directional selectivity Vis Neurosci 15: 1119–1127, 1998b. doi: 10.1017/S0952523898156109. [DOI] [PubMed] [Google Scholar]
- Hassenstein B, Reichardt WE. Systemtheoretische Analyse der Zeit, Reihenfolgen, und Vorzeichenauswertung bei der Bewegungsperzeption des Russelkäfers, Chlorophanus. Z Naturforsch B 11b: 513–524, 1956. doi: 10.1515/znb-1956-9-1004. [DOI] [Google Scholar]
- Hausselt SE, Euler T, Detwiler PB, Denk W. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol 5: e185, 2007. doi: 10.1371/journal.pbio.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500: 168–174, 2013. doi: 10.1038/nature12346. [DOI] [PubMed] [Google Scholar]
- Hillier D, Fiscella M, Drinnenberg A, Trenholm S, Rompani SB, Raics Z, Katona G, Juettner J, Hierlemann A, Rozsa B, Roska B. Causal evidence for retina-dependent and -independent visual motion computations in mouse cortex. Nat Neurosci 20: 960–968, 2017. doi: 10.1038/nn.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggarth A, McLaughlin AJ, Ronellenfitch K, Trenholm S, Vasandani R, Sethuramanujam S, Schwab D, Briggman KL, Awatramani GB. Specific wiring of distinct amacrine cells in the directionally selective retinal circuit permits independent coding of direction and size. Neuron 86: 276–291, 2015. doi: 10.1016/j.neuron.2015.02.035. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Caroni P. Functional and structural underpinnings of neuronal assembly formation in learning. Nat Neurosci 19: 1553–1562, 2016. doi: 10.1038/nn.4418. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J Physiol 148: 574–591, 1959. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 62: 327–334, 2009. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci 31: 7753–7762, 2011. doi: 10.1523/JNEUROSCI.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature 452: 478–482, 2008. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Kim JS, Greene MJ, Zlateski A, Lee K, Richardson M, Turaga SC, Purcaro M, Balkam M, Robinson A, Behabadi BF, Campos M, Denk W, Seung HS; EyeWirers . Space-time wiring specificity supports direction selectivity in the retina. Nature 509: 331–336, 2014. doi: 10.1038/nature13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Soto F, Kerschensteiner D. An excitatory amacrine cell detects object motion and provides feature-selective input to ganglion cells in the mouse retina. eLife 4: e08025, 2015. doi: 10.7554/eLife.08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittila CA, Massey SC. Effect of ON pathway blockade on directional selectivity in the rabbit retina. J Neurophysiol 73: 703–712, 1995. doi: 10.1152/jn.1995.73.2.703. [DOI] [PubMed] [Google Scholar]
- Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol 77: 675–689, 1997. doi: 10.1152/jn.1997.77.2.675. [DOI] [PubMed] [Google Scholar]
- Koren D, Grove JCR, Wei W. Cross-compartmental modulation of dendritic signals for retinal direction selectivity. Neuron 95: 914–927.e4, 2017. doi: 10.1016/j.neuron.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Tauchi M, Dahl JL. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res 70: 605–617, 1988. doi: 10.1007/BF00247609. [DOI] [PubMed] [Google Scholar]
- Kostadinov D, Sanes JR. Protocadherin-dependent dendritic self-avoidance regulates neural connectivity and circuit function. eLife 4: e08964, 2015. doi: 10.7554/eLife.08964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chen L, Chen M, Ye M, Seal RP, Zhou ZJ. An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron 84: 708–715, 2014. doi: 10.1016/j.neuron.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 68: 1159–1172, 2010. doi: 10.1016/j.neuron.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhang Y, Chen M, Zhou ZJ. Segregated glycine-glutamate co-transmission from vGluT3 amacrine cells to contrast-suppressed and contrast-enhanced retinal circuits. Neuron 90: 27–34, 2016. doi: 10.1016/j.neuron.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhou ZJ. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron 51: 787–799, 2006. doi: 10.1016/j.neuron.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipin MY, Taylor WR, Smith RG. Inhibitory input to the direction-selective ganglion cell is saturated at low contrast. J Neurophysiol 114: 927–941, 2015. doi: 10.1152/jn.00413.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Kaye AP, Nauhaus I, Callaway EM. Anterior-posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron 76: 713–720, 2012. doi: 10.1016/j.neuron.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The tasks of amacrine cells. Vis Neurosci 29: 3–9, 2012. doi: 10.1017/S0952523811000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss AS, Vlasits A, Borst A, Feller M. Visual circuits for direction selectivity. Annu Rev Neurosci 40: 211–230, 2017. doi: 10.1146/annurev-neuro-072116-031335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Bloomfield SA. Electroanatomy of a unique amacrine cell in the rabbit retina. Proc Natl Acad Sci USA 80: 3069–3073, 1983. doi: 10.1073/pnas.80.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrie RD, Feller MB. An asymmetric increase in inhibitory synapse number underlies the development of a direction selective circuit in the retina. J Neurosci 35: 9281–9286, 2015. doi: 10.1523/JNEUROSCI.0670-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrie RD, Feller MB. Development of synaptic connectivity in the retinal direction selective circuit. Curr Opin Neurobiol 40: 45–52, 2016. doi: 10.1016/j.conb.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrie RD, Feller MB. A dense starburst plexus is critical for generating direction selectivity. Curr Biol 28: 1204–1212.e5, 2018. doi: 10.1016/j.cub.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch N, Euler T, Taylor WR. Direction-selective dendritic action potentials in rabbit retina. Neuron 47: 739–750, 2005. doi: 10.1016/j.neuron.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Taylor WR. Tetrodotoxin-resistant sodium channels contribute to directional responses in starburst amacrine cells. PLoS One 5: e12447, 2010. doi: 10.1371/journal.pone.0012447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley DM, Masland RH. Co-release of acetylcholine and gamma-aminobutyric acid by a retinal neuron. Proc Natl Acad Sci USA 86: 3414–3418, 1989. doi: 10.1073/pnas.86.9.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyster CW, Barlow HB, Direction-selective units in rabbit retina: distribution of preferred directions. Science 155: 841–842, 1967. doi: 10.1126/science.155.3764.841. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Petit-Jacques J, Völgyi B, Ho CS, Joho RH, Bloomfield SA, Rudy B. A unique role for Kv3 voltage-gated potassium channels in starburst amacrine cell signaling in mouse retina. J Neurosci 24: 7335–7343, 2004. doi: 10.1523/JNEUROSCI.1275-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Borghuis BG, Rahmani P, Zeng Q, Kim IJ, Demb JB. Function and circuitry of VIP+ interneurons in the mouse retina. J Neurosci 35: 10685–10700, 2015. doi: 10.1523/JNEUROSCI.0222-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Kim IJ, Looger LL, Demb JB, Borghuis BG. Excitatory synaptic inputs to mouse On-Off direction-selective retinal ganglion cells lack direction tuning. J Neurosci 34: 3976–3981, 2014. doi: 10.1523/JNEUROSCI.5017-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Chen Q, Koren D, Giammarinaro B, Acaron Ledesma H, Wei W. Conditional knock-out of vesicular GABA transporter gene from starburst amacrine cells reveals the contributions of multiple synaptic mechanisms underlying direction selectivity in the retina. J Neurosci 35: 13219–13232, 2015. doi: 10.1523/JNEUROSCI.0933-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival KA, Venkataramani S, Smith RG, Taylor WR. Directional excitatory input to direction-selective ganglion cells in the rabbit retina. J Comp Neurol. 2017. doi: 10.1002/cne.24207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. Diverse visual features encoded in mouse lateral geniculate nucleus. J Neurosci 33: 4642–4656, 2013. doi: 10.1523/JNEUROSCI.5187-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio T, Reichardt W. Considerations on models of movement detection. Kybernetik 13: 223–227, 1973. doi: 10.1007/BF00274887. [DOI] [PubMed] [Google Scholar]
- Poleg-Polsky A, Diamond JS. Imperfect space clamp permits electrotonic interactions between inhibitory and excitatory synaptic conductances, distorting voltage clamp recordings. PLoS One 6: e19463, 2011. doi: 10.1371/journal.pone.0019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg-Polsky A, Diamond JS. NMDA receptors multiplicatively scale visual signals and enhance directional motion discrimination in retinal ganglion cells. Neuron 89: 1277–1290, 2016a. doi: 10.1016/j.neuron.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg-Polsky A, Diamond JS. Retinal circuitry balances contrast tuning of excitation and inhibition to enable reliable computation of direction selectivity. J Neurosci 36: 5861–5876, 2016b. doi: 10.1523/JNEUROSCI.4013-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleg-Polsky A, Ding H, Diamond JS. Functional compartmentalization within starburst amacrine cell dendrites in the retina. Cell Reports 22: 2898–2908, 2018. doi: 10.1016/j.celrep.2018.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Wei W, Feller MB. Visual stimulation reverses the directional preference of direction-selective retinal ganglion cells. Neuron 76: 518–525, 2012. doi: 10.1016/j.neuron.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD, Feller MB. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci 31: 8760–8769, 2011. doi: 10.1523/JNEUROSCI.0564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa JM, Morrie RD, Baertsch HC, Feller MB. Contributions of rod and cone pathways to retinal direction selectivity through development. J Neurosci 36: 9683–9695, 2016. doi: 10.1523/JNEUROSCI.3824-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah S, Gemmer JA, Bhatia-Lin A, Manoff G, Castro G, Siegel JK, Jeffery N, Berson DM. A retinal code for motion along the gravitational and body axes. Nature 546: 492–497, 2017. doi: 10.1038/nature22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu Rev Neurosci 38: 221–246, 2015. doi: 10.1146/annurev-neuro-071714-034120. [DOI] [PubMed] [Google Scholar]
- Schachter MJ, Oesch N, Smith RG, Taylor WR. Dendritic spikes amplify the synaptic signal to enhance detection of motion in a simulation of the direction-selective ganglion cell. PLoS Comput Biol 6: e1000899, 2010. doi: 10.1371/journal.pcbi.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuramanujam S, McLaughlin AJ, deRosenroll G, Hoggarth A, Schwab DJ, Awatramani GB. A central role for mixed acetylcholine/GABA transmission in direction coding in the retina. Neuron 90: 1243–1256, 2016. doi: 10.1016/j.neuron.2016.04.041. [DOI] [PubMed] [Google Scholar]
- Sethuramanujam S, Yao X, deRosenroll G, Briggman KL, Field GD, Awatramani GB. “Silent” NMDA synapses enhance motion sensitivity in a mature retinal circuit. Neuron 96: 1099–1111.e3, 2017. doi: 10.1016/j.neuron.2017.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Barchini J, Ledesma HA, Koren D, Jin Y, Liu X, Wei W, Cang J. Retinal origin of direction selectivity in the superior colliculus. Nat Neurosci 20: 550–558, 2017. doi: 10.1038/nn.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivyer B, Williams SR. Direction selectivity is computed by active dendritic integration in retinal ganglion cells. Nat Neurosci 16: 1848–1856, 2013. doi: 10.1038/nn.3565. [DOI] [PubMed] [Google Scholar]
- Stincic T, Smith RG, Taylor WR. Time course of EPSCs in ON-type starburst amacrine cells is independent of dendritic location. J Physiol 594: 5685–5694, 2016. doi: 10.1113/JP272384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Han X, He S. Direction-selective circuitry in rat retina develops independently of GABAergic, cholinergic and action potential activity. PLoS One 6: e19477, 2011. doi: 10.1371/journal.pone.0019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J Neurosci 22: 7712–7720, 2002. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, Johnson K, Li X, Smith RG, Awatramani GB. Parallel mechanisms encode direction in the retina. Neuron 71: 683–694, 2011. doi: 10.1016/j.neuron.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukker JJ, Taylor WR, Smith RG. Direction selectivity in a model of the starburst amacrine cell. Vis Neurosci 21: 611–625, 2004. doi: 10.1017/S0952523804214109. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Sivyer B, Taylor WR. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat Rev Neurosci 13: 194–208, 2012. doi: 10.1038/nrn3165. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Young HM. GABA-like immunoreactivity in cholinergic amacrine cells of the rabbit retina. Brain Res 438: 369–373, 1988. doi: 10.1016/0006-8993(88)91366-2. [DOI] [PubMed] [Google Scholar]
- Velte TJ, Miller RF. Spiking and nonspiking models of starburst amacrine cells in the rabbit retina. Vis Neurosci 14: 1073–1088, 1997. doi: 10.1017/S0952523800011780. [DOI] [PubMed] [Google Scholar]
- Vlasits AL, Bos R, Morrie RD, Fortuny C, Flannery JG, Feller MB, Rivlin-Etzion M. Visual stimulation switches the polarity of excitatory input to starburst amacrine cells. Neuron 83: 1172–1184, 2014. doi: 10.1016/j.neuron.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasits AL, Morrie RD, Tran-Van-Minh A, Bleckert A, Gainer CF, DiGregorio DA, Feller MB. A role for synaptic input distribution in a dendritic computation of motion direction in the retina. Neuron 89: 1317–1330, 2016. doi: 10.1016/j.neuron.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Kobayashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell 95: 17–27, 1998. doi: 10.1016/S0092-8674(00)81779-1. [DOI] [PubMed] [Google Scholar]
- Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature 469: 402–406, 2011. doi: 10.1038/nature09600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Balint K, Noda M, Nagel G, Bamberg E, Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature 469: 407–410, 2011. doi: 10.1038/nature09711. [DOI] [PubMed] [Google Scholar]
- Yonehara K, Farrow K, Ghanem A, Hillier D, Balint K, Teixeira M, Jüttner J, Noda M, Neve RLL, Conzelmann K-K, Roska B. The first stage of cardinal direction selectivity is localized to the dendrites of retinal ganglion cells. Neuron 79: 1078–1085, 2013. doi: 10.1016/j.neuron.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Yonehara K, Shintani T, Suzuki R, Sakuta H, Takeuchi Y, Nakamura-Yonehara K, Noda M. Expression of SPIG1 reveals development of a retinal ganglion cell subtype projecting to the medial terminal nucleus in the mouse. PLoS One 3: e1533, 2008. doi: 10.1371/journal.pone.0001533. [DOI] [PMC free article] [PubMed] [Google Scholar]