Abstract
Microglia are classified mainly into the M1 or M2 phenotypes, which evoke either proinflammatory or neuroprotective responses. Given the association of microglia with the pathogenesis of neuronal diseases, they are in focus as therapeutic targets for the treatment of such conditions. Stem cell factor (SCF) is a ligand for the c-kit receptor, one of the differentiation factors for bone marrow cells. In this study, characteristics of SCF-activated microglia and their effects on neurons were analyzed to investigate the therapeutic potential of SCF in neuronal diseases. SCF was found to induce proliferation, migration, and phagocytosis of microglia. In addition, SCF-derived microglia showed a neuroprotective phenotype expressing anti-inflammatory cytokines, growth factors, and M2 markers as compared to the phenotype shown by granulocyte macrophage-colony stimulating factor-derived microglia expressing inflammatory cytokines and M1 markers. Furthermore, supernatant medium from SCF-activated microglia enhanced cell proliferation and protection from cell death in NSC-34 neuronal cells. We conclude that SCF modulates microglial functions and induces activation of the neuroprotective effects of microglia, which could be used for treatment of neuronal diseases.
Keywords: Neuroscience, Cell biology
1. Introduction
Microglia are classically known as the immune cells of the central nervous system (CNS), and they show inflammatory responses like migration, chemotaxis, and phagocytosis [1, 2]. Recently, based on the development of experimental technology, microglia have been shown to play crucial roles in homeostasis of the CNS through interaction with astrocytes or neurons [3, 4, 5, 6]. Microglia are in focus in the field of neuroscience as they might be key players in elucidating unresolved neurological phenomena [7].
Microglia which mainly have proinflammatory effects, which are their classical function, are called M1 microglia [8, 9]. They express tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and cytokines and induce chemotaxis and migration of inflammatory cells [8, 10, 11]. In contrast, M2 microglia are classified as neuroprotective microglia, which express IL-4, IL-10, and growth factors. M2 microglia express insulin like growth factor (IGF)-1, transforming growth factor (TGF)-beta, and stimulate the differentiation of neuronal cells and the proliferation of satellite cells [8, 10, 12]. Microglia are reported to be induced to M1 inflammatory phenotype by granulocyte macrophage-colony stimulating factor (GM-CSF) treatment [13, 14]. GM-CSF is well recognized as one of the representative growth factors required by haematopoietic stem cells, in particular, and for monocyte/macrophage progenitors [15]. GM-CSF is produced in many kinds of haematopoietic cells such as T-cells, B-cells, macrophages, and mast cells, by inflammatory stimulation. GM-CSF induces the proliferation, differentiation, survival and activation of haematopoietic stem cells and other haematopoietic progenitor cells [13, 14, 15].
Microglia are activated, and produce proinflammatory cytokines or growth factors in neurological diseases depending on the pathological stage or the disease. This induces inflammation in some cases or protective neuronal cells in other cases [16]. As with spinal cord or brain injury, a large number of M1 microglia accumulate in lesions and express inflammatory cytokines from the acute phase of injury to the chronic phase, however, a relatively small number of M2 microglia are found in traumatic injury [12]. In amyotrophic lateral sclerosis (ALS), as one of the representative neurodegenerative diseases, M2 microglia are observed at the beginning of the pathology in the spinal cord, this phenotype switches to the M1 phenotype as the disease progresses [9, 17]. In most cases of Alzheimer's disease, microglia show mixed activation phenotypes [18]. The expression of alternative activation genes such as TNF-α and iNOS classified in M1 markers, and Arg1 and CD206 classified in M2 markers have increased at the same time in brain tissues from Alzheimer's disease [8, 19].
Stem cell factor (SCF) is the ligand for the c-kit receptor, one of the differentiation factors for bone marrow cells, which induces the differentiation of mononuclear cells [20, 21, 22]. SCF signalling modulates microglial functions and is involved in neuron-microglia interactions [4]. In a previous report, we showed that SCF-activated bone marrow-derived cells delayed the progression of motor neuron disease [23]. SCF-activated bone marrow cells were transplanted into superoxide dismutase (SOD)-1 G93A transgenic ALS model mice, which resulted in their prolonged survival, and in slowing down the progression of motor dysfunction. In the treated mice, it was observed that many numbers of bone marrow-derived cells accumulated in the degenerative spinal cord and expressed Iba1 protein known as microglia marker, which results suggested that bone marrow-derived cells differentiated to microglia. In addition, those microglia expressed the glutamate transporter (GLT)-1, one of the neuroprotective molecules. However, the detailed mechanism how SCF produced these effects was unclear. Therefore, to clarify the mechanism of SCF effects for neurodegenerative diseases, we consider that it should be elucidated how SCF directly affects to microglia. Investigation of direct effects of SCF to microglia could be supportive for us to understand the mechanism. Accordingly, we performed this study to characterize SCF-activated microglia functions and their effects on neurons comparing with those of GM-CSF as a representative control of the haematopoietic growth factors in vitro.
2. Materials and methods
2.1. Animals
Over 30 pups of C57BL/6 mice at day1 postpartum were totally purchased with nursing mother together from Jackson Laboratories (Bar Harbor, ME, USA) through Charles River Laboratories Japan (Yokohama, Japan) under genetic stability program. All mice were housed under 12-hour light and dark cycles with 40–70% relative humidity at 18–26 °C and were provided with water and mouse chow ad libitum. All animal experimental protocols were approved by the Institutional Animal Care and Usage Committee (IACUC), Shiga University of Medical Science, and were performed in accordance with the guidelines of the IACUC at the Shiga University of Medical Science.
2.2. Preparation of cultured microglia
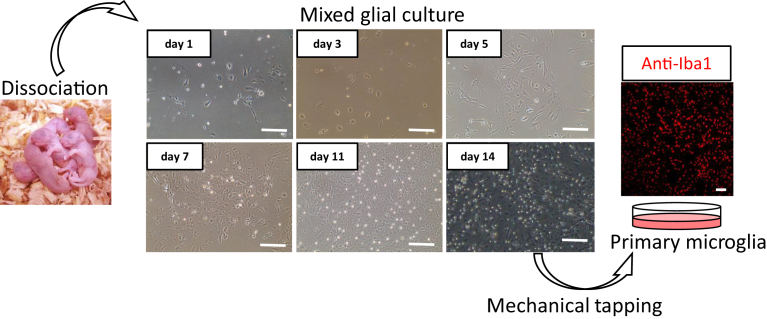
After decapitation of six C57BL/6 pups 1–2 days postpartum at the same time, mixed glial cells from brain cortex were dissociated, and primary mixed glial cell-culture was established (Fig. 1) [24, 25]. After incubation of these mixed glial cells with Dulbecco's Modified Eagle Medium (DMEM)/F12 with 10% FBS (foetal bovine serum) for 14 days at 37 °C, many number of microglia were appeared on layered astrocytes, were isolated by mechanical tapping and transferred to another culture dish. Microglia from these cultures were then used for experiments. It has been confirmed that cell viability and features of primary cultured microglia were not greatly changed even without a certain period-adaptation of pups with animal facilities.
Fig. 1.
Preparation of cultured microglia. The left panel shows the pops at the day 1–2 after birth. The pictures in the middle showed cultured mixed glial cells at each time point. Right panel showed immunocytochemistry of microglia with Iba1 antibodies (red). Scale Bar = 100 μm.
2.3. Analysis of proliferation and morphology of microglia stimulated by growth factors
Primary microglia were disseminated at a density of 104-105 cells/ml to another 24-well dish. After dissemination, these cells were cultured in DMEM/F12 medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% FBS (Thermo Fisher Scientific) including mouse SCF (100 ng/ml; R&D Systems, Minneapolis, MN, USA), mouse GM-CSF (100 ng/ml; R&D Systems), or buffer control for another three days at 37 °C. Then, the proliferation rate and morphological changes were observed using confocal laser microscopy (C1si; Nikon, Tokyo, Japan) and the EZC1 3.90 software (Nikon).
2.4. Migration analysis
After three days culture in DMEM/F12 medium with 10% FBS contained SCF (100 ng/ml), GM-CSF (100 ng/ml) or buffer control in 24-well dish at 37 °C, the monolayer of microglia were scraped in a straight line to create a “scratch” with a p200 pipet tip. We carefully scratched approximately similar area of the bottom of dishes to minimize the variation caused by the difference in the width of the scratches. Then, the debris was removed by washing the cells once with 0.5ml of the growth medium and replace with 0.5 ml of the medium with SCF, GM-CSF or buffer. The scratched area was observed and taken a photograph as the basal time point. Migration abilities were evaluated by counting the number of microglia migrating into the scratched area per 0.5 mm2 at 3 hours after scratching. The migrating cells were counted at over 5 well per each group and repeated at least three times [26].
2.5. Phagocytosis assay
Microglia were cultured for three days in DMEM/F12 medium with 10% FBS, contained SCF (100 ng/ml), GM-CSF (100 ng/ml), or buffer control, and were performed phagocytosis assay by Latex Bead-Rabbit IgG-Fluorescein isothiocyanate (FITC) complexes (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's protocol. Latex Bead-Rabbit IgG-FITC complexes (Cayman Chemical) were added to the culture medium, and microglia were incubated for 3 hours in this medium at 37 °C. After incubation, cells were washed with phosphate buffered saline (PBS) to eliminate non-phagocytic beads. The numbers of FITC-positive microglia were counted and compared among SCF, GM-CSF, and control groups [27].
2.6. Immunocytochemistry
Microglia were fixed with 4% paraformaldehyde (PFA) and were incubated with the anti-CD206 antibody (Abcam, Cambridge, UK) as a M2 microglia marker. Samples were then incubated with species-matched secondary antibodies (Alexa Fluor 555 antibody [Molecular Probes, Eugene, OR, USA]) and mounted with VECTASHIELD Antifade Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA) to enable visualization of stained cells using confocal laser microscopy (C1si; Nikon, Tokyo, Japan) and the EZC1 3.90 software (Nikon), positive cells were identified and counted.
2.7. Analysis of gene expression in microglia
Three days after incubation with growth factors, total mRNA was extracted from microglia by RNeasy Kit (QIAGEN, Valencia, CA, USA) and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed to measure the levels of transcripts for IL-1b, IL-6, iNOS, TNF, IL-4, IL-10, TGF, IGF1, CD86, and CD206 genes using a LightCycler 480 (Roche Diagnostics, Manheim, Germany) with SYBR Green method. The following primers were used: IL-1β, forward primer, 5′-CAACCAACAAGTGATATT-3′ and reverse primer, 5′-GATCCACACTCTCCAGCTG-3′; IL-6, forward primer, 5′-ACGGCCTTCCCTACTTCACA-3′ and reverse primer, 5′-CATTTCCACCATTTCCCAGA-3′; iNOS, forward primer, 5′-TTGGAGCGAGTTGTGGATTG-3′ and reverse primer, 5′-GTAGGTGAGGGCTTGGCTGA-3′; Tnfα, forward primer, 5′-CACGTCGTAGCAAACCACCAAGTGG-3′ and reverse primer, 5′-GATAGCAAATCGGCTGACGGTGTGG-3′; IL-10, forward primer, 5′-TGGACAACATACTGCTAACCGAC-3′ and reverse primer, 5′-CCTGGGGCATCACTTCTACC-3′; IL-4, forward primer, 5′-TCAACCCCCAGCTAGTTGTC-3′ and reverse primer, 5′-TGTTCTTCGTTGCTGTGAGG-3′; IGF1, forward primer, 5′-CTGAGCTGGTGGATGCTCT-3′ and reverse primer, 5′-CACTCATCCACAATGCCTGT-3′; TGF, forward primer, 5′- ATGCCGCCCTCCGGGCTGCG-3′ and reverse primer, 5′-TCAGCTGCACTTGCAGGAGC -3; CD86, forward primer, 5′-CACGAGCTTTGACAGGAACA-3′ and reverse primer, 5′-TTAGGTTTCGGGTGACCTTG-3′; CD206, forward primer, 5′-CTATGCAGGCCACTGCTACA-3′ and reverse primer, 5′-GTTCTCATGGCTTGGCTCTC-3′; β-actin, forward primer, 5′-CGTGCGTGACATCAAAGAGAA-3′ and reverse primer, 5′-TGGATGCCACAGGATTCCAT-3′. The results were analyzed by LightCycler 480 software, version 1.5 (Roche Diagnostics Manheim, Germany). All data were normalized to β-actin expression level.
2.8. Cell proliferation and protection assay of neuronal cells stimulated with supernatant medium from cultured microglia
NSC-34 cells were purchased from CELLutions BIOSYSTEMS (Ontario, Canada). These cells were cultured in DMEM high glucose medium with 10% FBS at 37 °C. For the cell proliferation assay, NSC-34 cells were cultured under mixture of DMEM high glucose medium with 1% FBS, and supernatant media from cultured microglia after stimulation of SCF or GM-CSF for three days. To exclude the direct effects of SCF or GM-CSF to NSC-34 cells, NSC-34 cells were cultured under mixture of DMEM high glucose medium with 1% FBS, and supernatant media from cultured media without microglia after stimulation of SCF or GM-CSF for three days. Forty-eight or seventy-two hours later, the absorbance at 450nm for each group was measured as the indicators reflecting proliferation levels of NSC-34 cells using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer's protocol. The absorbance ratio for each group was calculated by standardization with absorbance of the control group after 48 hour treatment. The ratios were compared to each group, and evaluated as proliferation levels.
As neuronal cell protection assay, neuronal cell death was induced by 1-Hydroxy-2-oxo-3,3-bis(2-aminoethyl)-1-triazene (NOC18) stimulation in NSC-34 cells after addition of supernatant medium from cultured microglia incubated with none, GM-CSF or SCF for three days to DMEM/F12 with 1% medium at the 1:1 ratio. Twenty-four hours later, the absorbance at 450nm for each group was measured using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) as above. The absorbance ratio for each group was calculated by standardization with absorbance of no NOC18 stimulation group. The ratios were compared to each group, and evaluated as cell protection levels.
2.9. Statistical analysis
Data are expressed as means + SD. All experiments were performed in triplicates with at least three independent experiments. For multiple data-sets, one-way analysis of variance (ANOVA) and Scheffe's tests were used. P-value less than 0.05 was considered significant.
3. Results
3.1. Effects of SCF treatment to microglial viability and function
Mixed glial cells were isolated from mouse brain cortex and were cultured for 14 days (Fig. 1). At first, astrocytes grew up and spread in sheet form from day 7 to day 14. After 2 weeks-mixed glial cell culture, microglia are observed on astrocytes sheet as small shining cells (Fig. 1, day 14 picture). Then, primary microglia were isolated by mechanical tapping and disseminated to fresh culture dishes, which were confirmed with immunocytochemistry of anti-Iba1 antibody (Fig. 1, most right panel). After that, microglia were used for the following experiments.
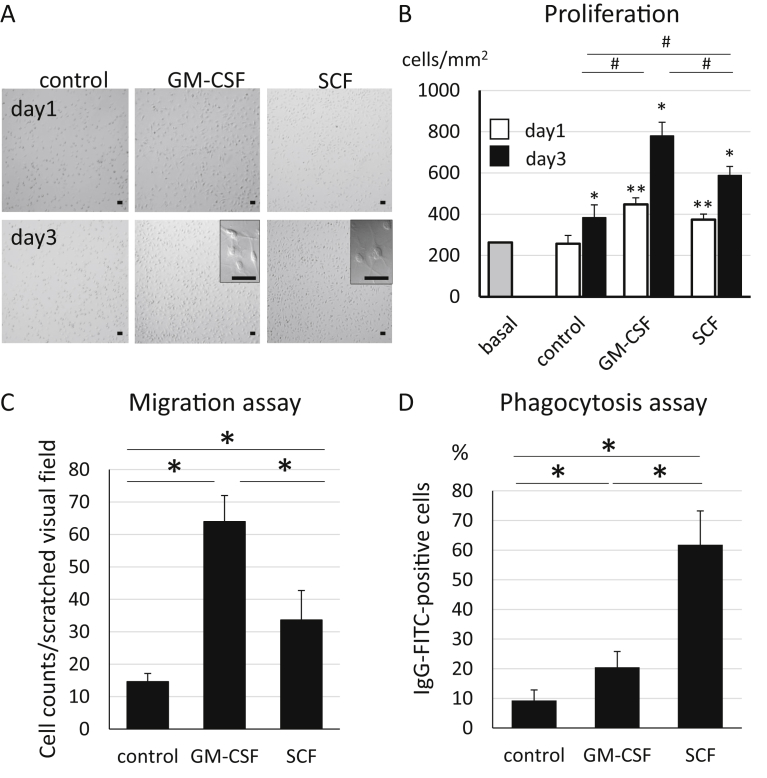
To evaluate the effects of SCF stimulation to microglia, we analysed the proliferation, the migration and the phagocytosis in cultured microglia with GM-CSF or SCF (Fig. 2). At first, the cell numbers of microglia were counted on day 1 and day 3 after stimulation by GM-CSF or SCF, and was compared to that of control group containing no growth factors. In all groups (control, GM-CSF, and SCF), microglia sequentially proliferated in complete medium (Fig. 2A and B). Especially, the level of proliferation of the GM-CSF group was highest among the three groups (Fig. 2B). The rate of proliferation was approximately twice as compared to that of the control group. The speed of proliferation after SCF stimulation was slower than GM-CSF group but significantly faster than the control group (Fig. 2B). The rate was about 1.5 times than that of the control group (Fig. 2B). As the morphological observation, a part of cultured microglia were activated and differentiated to ramified or amoeboid microglia by both SCF and GM-CSF stimulation (Fig. 2A). Next, the scratch assay was performed to investigate the influence of growth factors on the migration of microglia. After incubating for three days in culture medium containing no growth factors, GM-CSF, and SCF, microglia were scratched. Migration of microglia was evaluated by counting the cell number twelve hours later in the scratched area (Fig. 2C). In the GM-CSF group, microglia were observed most among three groups (Fig. 2C). A significantly larger number of microglia from the SCF group had migrated as compared to those in the control group, and their migration level was approximately half of that in the GM-CSF group (Fig. 2C). The effect of SCF on the phagocytosis abilities of microglia was evaluated by performing the phagocytosis assay using latex Beads-Rabbit IgG-FITC complexes (Fig. 2D). After three days of incubation in the culture medium with no growth factors, GM-CSF or SCF, microglia were incubated for six hours with the complexes. A majority of the complexes were observed in microglia treated with SCF among the three groups (Fig. 2D). These results suggested that SCF stimulation markedly induced proliferation, migration and phagocytosis in microglia.
Fig. 2.
Morphological changes, proliferation, migration, and phagocytosis of microglia after stimulation by growth factors. (A) Cultured primary microglia on day 1 and day 3 after activation by GM-CSF or SCF. Scale Bar = 50 μm. (B) Cell number of microglia (cells/mm2) on day 1 and day 3 after activation by GM-CSF or SCF. “Basal” showed cell counts of microglia at the time of dissemination. Bars showed means + SD. *p < 0.05 (as compared to) day 1 in each group. **p < 0.05 as compared to day 1 control. #p < 0.05. (C) Migration assay after stimulation of microglia by growth factors. Numbers of microglia were counted 12 hours after the scratching test, after 3 days pre-incubation with GM-CSF or SCF. Bars show means + SD. *p < 0.05. (D) Phagocytosis assay after stimulation of microglia by growth factors. Percentages of FITC-positive microglia were calculated for each group. Bars showed means + SD. *p < 0.05.
3.2. Immunocytochemistry of microglia after SCF treatment
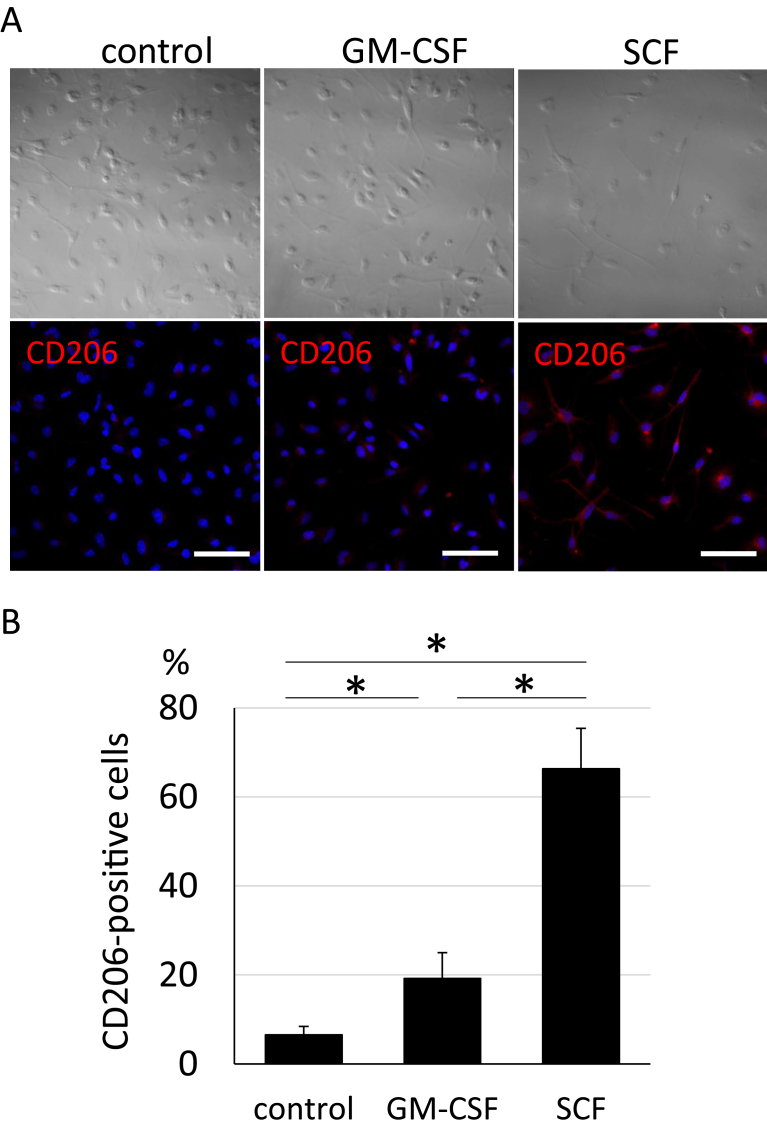
To clarify whether SCF has a high potential to induce the neuroprotective response in microglia, immunocytochemistry was performed using the anti-CD206 antibody which is a marker of neuroprotective microglia. Immunostaining using CD206 was performed and over 60% microglia from the SCF-activated group showed positive immunostaining (Fig. 3A and B). The percentage of CD206 positive cells was much higher in the SCF-activated group than that of microglia in the control (6.5%) and GM-CSF (19.2%) groups (Fig. 3B). This result suggested that SCF has high potential to polarise microglia to the neuroprotective phenotype.
Fig. 3.
Immunocytochemistry of microglia after stimulation by growth factors. (A) Immunocytochemistry using the CD206 antibody (lower panels; red: CD206 staining, blue: DAPI stained nuclei) and bright fields (upper panels) in microglia after incubation with GM-CSF or SCF for 3 days. Scale Bar = 50 μm. (B) Percentage of CD206 positive microglia in (A). Bars show means + SD. *p < 0.05.
3.3. Analysis of gene expression in microglia upon SCF treatment
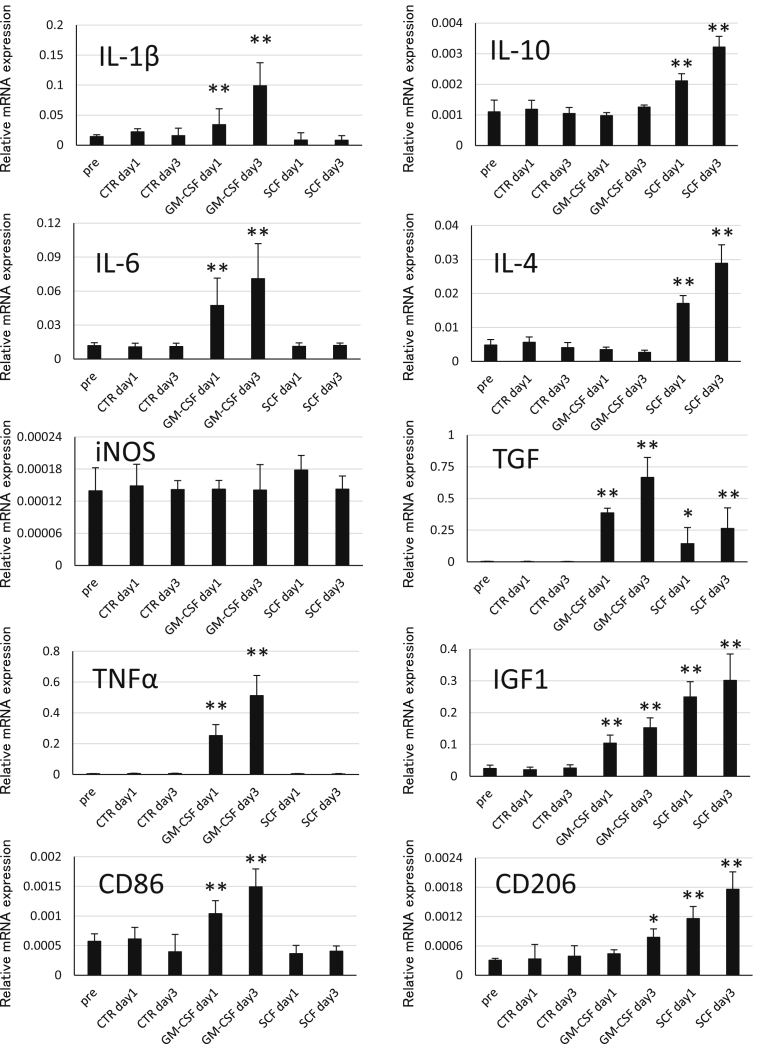
To investigate which genes are up-regulated or down-regulated in SCF-activated microglia, qRT-PCR was performed to determine mRNA expression of cytokines, growth factors, and CD markers (Fig. 4). IL-10 and IL-4 known as anti-inflammatory cytokines, were significantly up-regulated in the SCF group on day 1 and day 3. In contrast, the levels of IL-1β, IL-6 and TNFα known as inflammatory cytokines were elevated in the GM-CSF group and were much higher than those in the SCF group on day 1 and day 3. mRNA expression of TGF and IGF1, growth factors, gradually increased both in the GM-CSF and SCF groups from day 1 to day 3. In addition, IGF1 expression levels were higher in the SCF group than those in the GM-CSF group, and TGF expression levels were higher in the GM-CSF group than those in the SCF group. Next, we performed qRT-PCR analysis of CD86 and CD206, surface antigens, in microglia from the GM-CSF and SCF groups. mRNA expression of CD86, an inflammatory microglia marker, was elevated only in GM-CSF-activated microglia on both, day 1 and day 3. mRNA expression of CD206, a neuroprotective microglia marker, significantly increased on day 1 and day 3 in the SCF group, and on day 3 in the GM-CSF group. Moreover, its expression levels were much higher in the SCF group than those in the GM-CSF group. Over all, gene expression analysis showed that the levels of, anti-inflammatory genes and neuroprotective surface marker genes were elevated in SCF-activated microglia. On the other hand, levels of inflammatory genes and inflammatory surface marker genes increased in GM-CSF-activated microglia. Expression levels of genes of growth factors were elevated in both, GM-CSF and SCF groups.
Fig. 4.
Analysis of gene expression in microglia after stimulation by growth factors. Relative expression of mRNA of cytokines, growth factors and CD markers associated with inflammation and neuroprotection in microglia after treatment with GM-CSF or SCF for one or three days. mRNA expression was normalised to that of β-actin. CTR, control mice. *p < 0.05 as compared to pre-condition. **p < 0.01 as compared to pre-condition.
3.4. Neuroprotective effects of microglia induced by SCF treatment
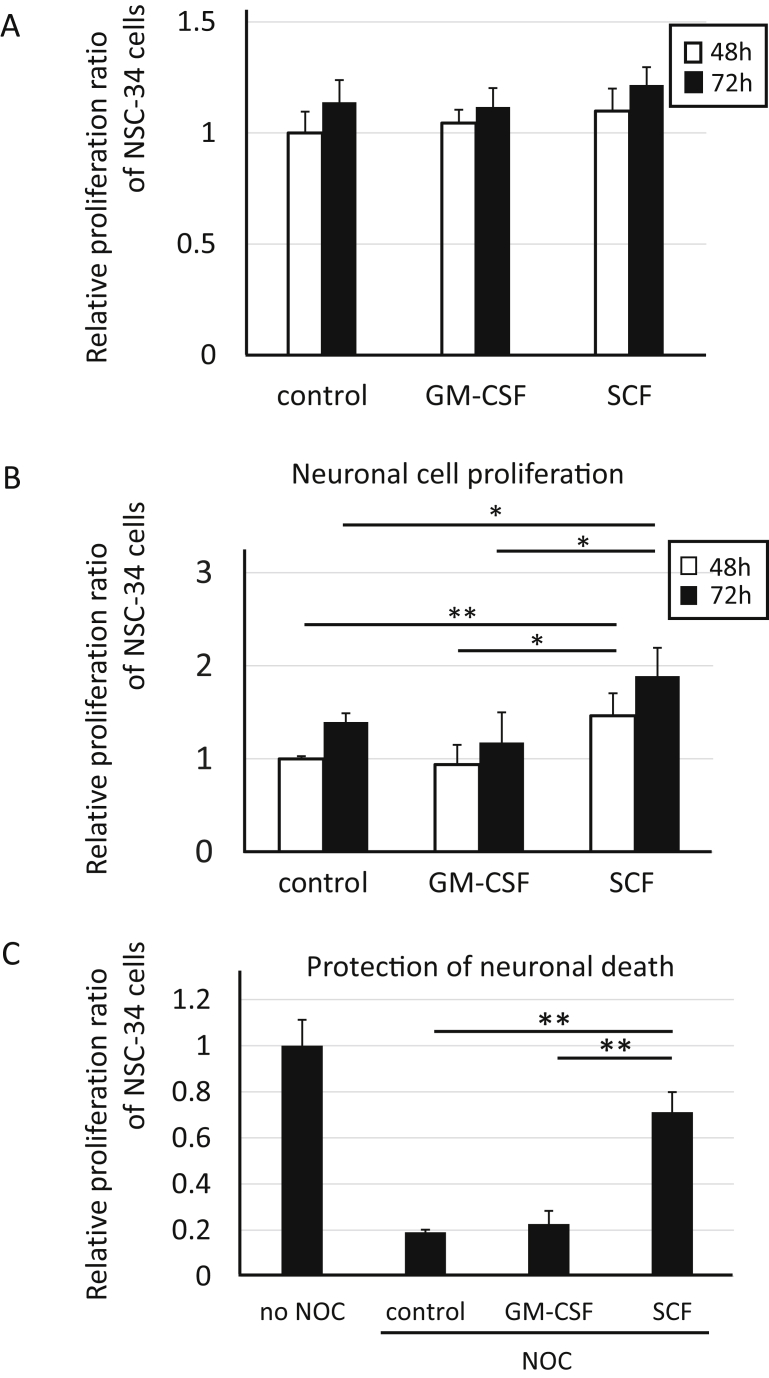
To investigate the effects of neuroprotection by microglia, cell proliferation assays were performed in NSC-34 motor neuron cells. At first, as the basic analysis, NSC-34 cells were incubated for 48–72 hours with medium after incubation with none, GM-CSF or SCF for three days without microglia (only exposure at 37 °C for three days in incubator) to clarify whether GM-CSF or SCF have direct growth effects to NSC-34 cells or not (Fig. 5A). Both GM-CSF and SCF didn't show the direct growing effects to NSC-34 (Fig. 5A). Based on the above results, next, NSC-34 cells were incubated for 48–72 hours with supernatant medium from cultures of untreated microglia (as a control), GM-CSF-treated or SCF-treated microglia to evaluate their neuroprotection effects (Fig. 5B). Comparison of neuronal cell proliferation between 48 and 72 hours incubation showed a trend of increasing proliferation in each group (Fig. 5B). In the SCF group, neuronal proliferation was significantly higher than that in the control and GM-CSF groups at both 48 and 72 hours (Fig. 5B). In addition, the experiments for the effects of GM-CSF or SCF to protect NSC-34 cells were performed under oxidative stress stimulation as cell death condition (Fig. 5C). NSC-34 were incubated with NOC18 to be induced neuronal cell death, and the protective effects for their cells were evaluated by incubation together with the supernatant medium from GM-CSF- or SCF-stimulated microglia. The proliferation ratio of NSC-34 cells in the SCF group was conserved at the 70% level of no cell death-inducible (no NOC) group (Fig. 5C). The neuronal protective effect in SCF group was significantly higher than those in control and in GM-CSF group. These results suggested that supernatant medium from microglia stimulated by SCF treatment caused neuronal cell proliferation and protection, which suggested the possibility that microglia might be responsible for exocrine secretion of growth factors for neuronal cells.
Fig. 5.
Neuronal cell proliferation and protection with supernatant medium from SCF-treated microglia. (A) Relative proliferation ratio against 48 hours control group in NSC-34 neuronal cells at 48 or 72 hours after treatment with supernatant media from cultured dishes without microglia and with none (control), GM-CSF or SCF. (B) Relative proliferation ratio against 48 hours control group in NSC-34 neuronal cells at 48 or 72 hours after treatment with supernatant media from cultured microglia after incubation with none (control), GM-CSF or SCF. (C) Relative proliferation ratio against 24 hours control group (no NOC) in NSC-34 neuronal cells under oxidative stress (NOC) at 24 hours after treatment with supernatant media from microglia after incubation with none (control), GM-CSF or SCF.*p < 0.05. **p < 0.01.
4. Discussion
Microglia came into focus as therapeutic targets for neuronal diseases after the theory of “non-cell-autonomous neuronal death” became well known [28, 29, 30]. Gradually, it has been elucidated that the environment surrounding the neurons is very important for the maintenance of physiological condition in neuronal tissues [3, 28, 31]. This environment is formed by satellite cells such as astrocytes, oligodendrocytes, microglia and endothelial cells of the central nervous system [3, 31]. Microglia are considered to be unique because they can exhibit two opposing features such as proinflammatory (M1) or neuroprotective (M2) responses [11]. Considering the development of the environment around neurons, the crucial aspects are the timing and levels of M1 and M2 microglia migration. M1 microglia are necessary for phagocytosis and accurate Wallerian degeneration, however, over-accumulation of M1 microglia induces excessive inflammation in neuronal tissues [8, 11]. Taking into consideration the regenerative state of neuronal tissues, M2 microglia play a pivotal role in neuronal growth or differentiation by production of growth factors such as IGF-1 and TGF, but cannot induce clearance of dead cells and injured tissues [8, 11]. Therefore, situation dependent control of the controversial phenotypes of microglia might be a remarkable advantage for the treatment of neuronal diseases. In this study, it has been proven that SCF induced polarisation of microglia to the M2 phenotype. This finding is significant because it can be used to establish and develop novel treatment strategies for neuronal diseases. Our results showed that both, GM-CSF and SCF promoted proliferation, migration, and phagocytosis in microglia. However, the nature of the course of induction of differentiation is completely different between GM-CSF and SCF treatments. At the time-points of cytokine, growth factor and CD marker secretion, GM-CSF induced polarisation of microglia to the M1 phenotype, and SCF induced polarisation of microglia to the M2 phenotype.
SCF and its receptor, c-kit were reported to be expressed in the central nervous system [32, 33]. SCF was mainly expressed in neurons, whereas c-kit was expressed largely by glial cells and weakly by neurons [34]. After brain injury, c-kit expression is up-regulated in microglia, and SCF expression is elevated in neuron and astrocyte [33]. Other reports showed that SCF/c-kit signalling stimulates neurogenesis and survival for neuronal stem cells [32, 35]. Therefore, these findings suggest that SCF/c-kit signalling is involved in neuron-neuron and neuron-glia interactions. Our results could be consistent with neuron-glia interaction. Moreover, some effects of SCF on microglia have been reported in another literature, which shows that SCF down-regulates the expression of inflammation-associated cytokines; IL-1β, and TNF-α, and up-regulates the expression levels of growth factors; nerve growth factor, and brain-derived neurotrophic factor in microglia [36]. However, this study is the first report to demonstrate the neuroprotective effects of SCF-activated microglia, and to clearly distinguish SCF-activated microglia as those showing the M2 phenotype from the microglia showing the M1 phenotype which is induced by GM-CSF, in a comparative analysis.
Other cytokines, IL-4, IL-10 were reported to induce polarisation of microglia to the anti-inflammatory phenotype [37]. IL-4 and IL-10 are classically known as immunomodulatory cytokines, which suppress or limit immune-inflammation [38]. In LPS-stimulated microglia, it was previously shown that IL-4 and IL-10 suppressed the expression of cytokines such as IL-6 and TNF-α, and IL-1β expression was suppressed by IL-10, and not IL-4 [39]. Similarly, our experiments showed that mRNA expression of IL-1β, IL-6 and TNF-α was not up-regulated in SCF-stimulated microglia. Moreover, it has been reported that IL-4 partly modulated IGF-1 expression [37, 40], and the expression of TGF was up-regulated by IL-10 [41, 42]. An increase in expression of IGF-1 and TGF was also observed in SCF-stimulated microglia in this study. Similarly, the expression of, IL-4 and IL-10 were up-regulated during the regulation of inflammatory cytokines and growth factors in SCF-stimulated microglia, which is consistent with the stable expression levels of these cytokines and the elevation of growth factors. Although these might be secondary effects of IL-4 and IL-10, it has been elucidated that SCF contributes to the suppression of inflammation and to the neuroprotective effects of microglia along with growth factors.
As described above, microglia are deeply associated with neuroinflammation in various neurodegenerative diseases, the concept of “non-cell-autonomous neuronal death” has been recently established, which indicates that glial cells contribute to the pathogenesis of neuronal cell death [16]. M1 microglia showed notable infiltration in nervous tissues of patients with Alzheimer's disease [18, 43], Parkinson's disease [44, 45] and ALS who showed pathological changes [46, 47]. In SOD1-G93A ALS model mice, we reported that the migration of microglia to the spinal cord was observed gradually accompanying the advance of the disease [23]. It revealed that, transplanted SCF-activated bone marrow cells showed greater migration to the spinal cord than the non-stimulated bone marrow cells, which showed the neuroprotective phenotype with the expression of GLT-1 [23]. We demonstrated the direct effect of SCF treatment on microglia, which polarised to the neuroprotective phenotype in vitro. These findings in this study are supportive and not contradictory with the therapeutic effects of SCF in neurodegenerative diseases in vivo.
Declarations
Author contribution statement
Tomoya Terashima: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yuki Nakae, Miwako Katagi: Performed the experiments.
Junko Okano, Yoshihisa Suzuki, Hideto Kojima: Analyzed and interpreted the data.
Funding statement
This work was supported by a Grant-in-Aid (#23790988 to T. Terashima) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by research funding from Takeda Science Foundation.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Graeber M.B. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 2.Graeber M.B., Streit W.J. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 3.Cerbai F., Lana D., Nosi D., Petkova-Kirova P., Zecchi S., Brothers H.M., Wenk G.L., Giovannini M.G. The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat Hippocampus. PloS One. 2012;7 doi: 10.1371/journal.pone.0045250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S.C., Fedoroff S. Neuron-microglia interactions in vitro. Acta Neuropathol. 1996;91:385–395. doi: 10.1007/s004010050440. [DOI] [PubMed] [Google Scholar]
- 5.Suzumura A. Neuron-microglia interaction in neuroinflammation. Curr. Protein Pept. Sci. 2013;14:16–20. doi: 10.2174/1389203711314010004. [DOI] [PubMed] [Google Scholar]
- 6.Pascual O., Ben Achour S., Rostaing P., Triller A., Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. 2012;109:E197–E205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 8.Crain J.M., Nikodemova M., Watters J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y., Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 10.Ransohoff R.M. A polarizing question: Do M1 and M2 microglia exist. Nat. Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 11.Cherry J.D., Olschowka J.A., O’Banion M.K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation. 2014;11 doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegyi B., Környei Z., Ferenczi S., Fekete R., Kudlik G., Kovács K.J., Madarász E., Uher F. Regulation of mouse microglia activation and effector functions by bone marrow-derived mesenchymal stem cells. Stem Cell. Dev. 2014;23:2600–2612. doi: 10.1089/scd.2014.0088. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.C., Liu W., Brosnan C.F., Dickson D.W. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994;12:309–318. doi: 10.1002/glia.440120407. [DOI] [PubMed] [Google Scholar]
- 14.Boche D., Perry V.H., Nicoll J.A.R. Review: activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton J.A. GM-CSF in Inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 16.Perry V.H., Nicoll J.A.R., Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 17.Lewis K.E., Rasmussen A.L., Bennett W., King A., West A.K., Chung R.S., Chuah M. Microglia and motor neurons during disease progression in the SOD1G93A mouse model of amyotrophic lateral sclerosis: changes in arginase1 and inducible nitric oxide synthase. J. Neuroinflammation. 2014;11:55. doi: 10.1186/1742-2094-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 19.Cherry J.D., Olschowka J.A., O’Banion M.K. Arginase 1+ microglia reduce Aβ plaque deposition during IL-1β-dependent neuroinflammation. J. Neuroinflammation. 2015;12 doi: 10.1186/s12974-015-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyle-Cezar F., Echevarria-Lima J., Goldenberg R.C.D.S., Rumjanek V.M. Expression of c-kit and Sca-1 and their relationship with multidrug resistance protein 1 in mouse bone marrow mononuclear cells. Immunology. 2007;121:122–128. doi: 10.1111/j.1365-2567.2007.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashman L.K. The biology of stem cell factor and its receptor C-kit. Int. J. Biochem. Cell Biol. 1999;31:1037–1051. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 22.Edling C.E., Hallberg B. c-Kit-A hematopoietic cell essential receptor tyrosine kinase. Int. J. Biochem. Cell Biol. 2007;39:1995–1998. doi: 10.1016/j.biocel.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Terashima T., Kojima H., Urabe H., Yamakawa I., Ogawa N., Kawai H., Chan L., Maegawa H. Stem cell factor-activated bone marrow ameliorates amyotrophic lateral sclerosis by promoting protective microglial migration. J. Neurosci. Res. 2014;92:856–869. doi: 10.1002/jnr.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lian H., Roy E., Zheng H. Protocol for primary microglial culture preparation. BIO-PROTOCOL. 2016;6 doi: 10.21769/BioProtoc.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terashima T., Ogawa N., Nakae Y., Sato T., Katagi M., Okano J., Maegawa H., Kojima H. Gene therapy for neuropathic pain through siRNA-IRF5 gene delivery with homing peptides to microglia. Mol. Ther. Nucleic Acids. 2018;11:203–215. doi: 10.1016/j.omtn.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang C.-C., Park A.Y., Guan J.-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 27.Park M.J., Park H.S., You M.J., Yoo J., Kim S.H., Kwon M.S. Dexamethasone induces a specific form of ramified dysfunctional microglia. Mol. Neurobiol. 2018:1–16. doi: 10.1007/s12035-018-1156-z. [DOI] [PubMed] [Google Scholar]
- 28.Shinozaki Y., Nomura M., Iwatsuki K., Moriyama Y., Gachet C., Koizumi S. Microglia trigger astrocyte-mediated neuroprotection via purinergic gliotransmission. Sci. Rep. 2014;4 doi: 10.1038/srep04329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilieva H., Polymenidou M., Cleveland D.W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobsiger C.S., Cleveland D.W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biber K., Vinet J., Boddeke H.W.G.M. Neuron-microglia signaling: chemokines as versatile messengers. J. Neuroimmunol. 2008;198:69–74. doi: 10.1016/j.jneuroim.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Erlandsson A., Larsson J., Forsberg-Nilsson K. Stem cell factor is a chemoattractant and a survival factor for CNS stem cells. Exp. Cell Res. 2004;301:201–210. doi: 10.1016/j.yexcr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S.C., Fedoroff S. Expression of stem cell factor and c-kit receptor in neural cells after brain injury. Acta Neuropathol. 1999;97:393–398. doi: 10.1007/s004010051003. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S.C., Fedoroff S. Cellular localization of stem cell factor and c-kit receptor in the mouse nervous system. J. Neurosci. Res. 1997;47:1–15. [PubMed] [Google Scholar]
- 35.Jin K., Mao X.O., Sun Y., Xie L., Greenberg D.A. Stem cell factor stimulates neurogenesis in vitro and in vivo. J. Clin. Invest. 2002;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S.C., Fedoroff S. Modulation of microglia by stem cell factor. J. Neurosci. Res. 1998;53:29–37. doi: 10.1002/(SICI)1097-4547(19980701)53:1<29::AID-JNR4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Zhao W., Xie W., Xiao Q., Beers D.R., Appel S.H. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J. Neurochem. 2006;99:1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- 38.Murray P.J. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Ledeboer A., Brevé J.J.P., Poole S., Tilders F.J.H., Dam A.V.A.N. Transforming growth factor-  differentially regulate production of pro-inflammatory cytokines and nitric oxide in Co-cultures of rat astroglial and microglial cells. Glia. 2000;142:134–142. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Butovsky O., Ziv Y., Schwartz A., Landa G., Talpalar A.E., Pluchino S., Martino G., Schwartz M. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Norden D.M., Fenn A.M., Dugan A., Godbout J.P. TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saijo K., Glass C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 43.Varnum M.M., Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in alzheimer's disease brain. Arch. Immunol. Ther. Exp. (Warsz). 2012;60:251–266. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spittau B. Interleukin 4-induced neuroprotection and regulation of microglia activation as a therapeutic approach in the MPTP model of Parkinson's disease. Neural Regen. Res. 2017;12:1433–1434. doi: 10.4103/1673-5374.215250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moehle M.S., West A.B. M1 and M2 immune activation in Parkinson's Disease: foe and ally? Neuroscience. 2015;302:59–73. doi: 10.1016/j.neuroscience.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hooten K.G., Beers D.R., Zhao W., Appel S.H. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12:364–375. doi: 10.1007/s13311-014-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao W., Beers D.R., Appel S.H. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 2013;8:888–899. doi: 10.1007/s11481-013-9489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]