Abstract
To effectively improve treatment for acute myeloid leukemia (AML), new molecular targets and therapeutic approaches need to be identified. Chimeric antigen receptor (CAR)-modified T cells targeting tumor-associated antigens have shown promise in the treatment of some malignancies. However, CAR-T cell development for AML has been limited by lack of an antigen with high specificity for AML cells that is not present on normal hematopoietic stem cells, and thus will not result in myelotoxicity. Here we demonstrate that leukocyte immunoglobulin-like receptor-B4 (LILRB4) is a tumor-associated antigen highly expressed on monocytic AML cells. We generated a novel anti-LILRB4 CAR-T cell that displays high antigen affinity and specificity. These CAR-T cells display efficient effector function in vitro and in vivo against LILRB4+ AML cells. Furthermore, we demonstrate anti-LILRB4 CAR-T cells are not toxic to normal CD34+ umbilical cord blood cells in colony-forming unit assays, nor in a humanized hematopoietic-reconstituted mouse model. Our data demonstrate that anti-LILRB4 CAR-T cells specifically target monocytic AML cells with no toxicity to normal hematopoietic progenitors. This work thus offers a new treatment strategy to improve outcomes for monocytic AML, with the potential for elimination of leukemic disease while minimizing the risk for on-target off-tumor toxicity.
Keywords: CAR-T, leukemia, LILRB
John et al. demonstrate anti-LILRB4 CAR-T cells display potent anti-leukemic activity against monocytic AML, while sparing normal hematopoietic stem and progenitor cells. Utilizing the approach of targeting AML subtypes based on normal HSC-sparing restricted immunophenotype represents an effective treatment strategy that minimizes on-target off-tumor toxicity.
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia of adults and a common pediatric cancer. Currently, AML treatment involves intensive cytotoxic chemotherapy often followed by myeloablative conditioning and stem cell transplant. Despite treatment, relapsed and refractory disease remains a significant problem for both adults and children with AML, with survival rates of less than 15% in the relapsed setting.1, 2, 3 Monocytic AML (FAB M5) is a common subtype encountered in AML patients, accounting for 20% of AML cases in children and greater than half in infants with AML.4 Clinical studies also suggest that monocytic AML carries a greater risk for marrow and extramedullary relapse after stem cell transplant compared with non-monocytic subtypes.5 Novel treatment strategies are therefore needed to improve the poor outcomes of patients with relapsed or refractory monocytic AML.
Chimeric antigen receptor (CAR)-modified T cells targeting tumor-associated antigens have shown promise in the treatment of some cancers. The engineered CAR combines the specificity of antigen recognition by an antibody with co-stimulatory and activation domains of the T cell receptor complex. This receptor, when expressed on the surface of a patient’s T cells, is then able to redirect the CAR-T cell to a target antigen on tumor cells, leading to T cell activation and tumor cell death.6 Treatment with CAR-T cells directed against CD19 in patients with B cell malignancies has been demonstrated to result in sustained disease remission and prolonged survival.7, 8, 9, 10, 11, 12, 13
Ongoing efforts to develop CAR-T cells for AML are targeting antigens such as CD33, CD123, Lewis Y, folate receptor β, FLT3, and CLL-1. These CAR-T cells eliminate leukemic disease in in vitro and in vivo models;14, 15, 16 however, CAR-T cells directed against these antigens result in on-target off-tumor elimination of hematopoietic stem and progenitor cells (HSPCs), leading to severe myelosuppression or myeloablation, increased susceptibility to infection, and significant morbidity or mortality in preclinical models.14, 17, 18 Therefore, in order to effectively utilize CAR-T cells against monocytic AML, an antigen expressed on monocytic AML cells, but not on normal HSPCs, needs to be identified and targeted.
We recently showed that several members of the leukocyte immunoglobulin-like receptor-B (LILRB) family are expressed on AML cells.19, 20, 21 LILRB4 (also referred to as ILT3, CD85k) has the most restricted expression pattern among LILRBs. It is uniquely expressed on normal monocytic cells beginning at the promonocyte stage of development.22, 23 In AML, expression of LILRB4 is more specific, being able to differentiate monocytic AML (M5) from AML M1-M3 more accurately than CD14, HLA-DR, or CD11c.24 Additionally, LILRB4 is expressed on cells at all stages of AML cell maturation, including CD34+/c-kit+ cells that are likely enriched for leukemia stem cells.24
LILRB4 therefore represents an attractive target for CAR-T cell-directed therapy for monocytic AML because both leukemia blasts and associated leukemia stem cells will be eliminated by targeting this antigen. Here we demonstrate that a novel CAR-T cell directed against LILRB4 displays efficient cytotoxic ability against AML cells in vitro and reduces leukemia burden in in vivo mouse xenograft models, whereas sparing healthy HSPCs that we demonstrate do not express LILRB4. This research indicates that anti-LILRB4 CAR-T cells have the potential to improve the poor outcomes for patients with refractory or relapsed monocytic AML. Additionally, we demonstrate that targeting specific subtypes of AML based on normal HSC-sparing restricted immunophenotype represents an effective treatment strategy that may minimize off-target toxicity against vital healthy cells including HSCs and progenitors.
Results
LILRB4 Is a Specific Marker for Monocytic AML
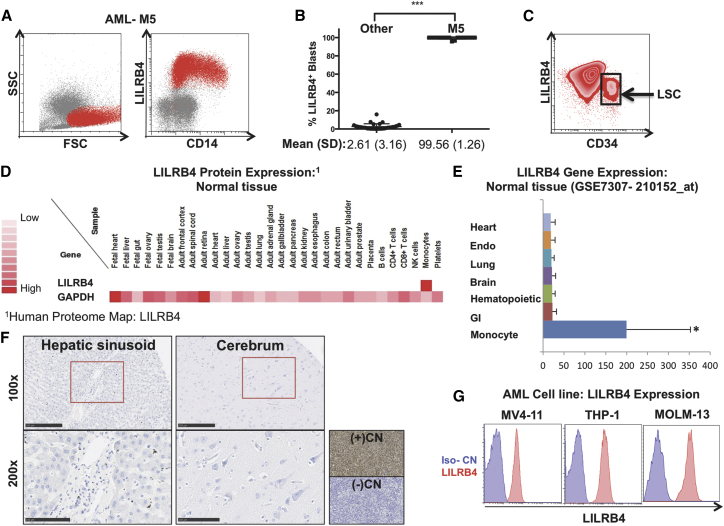
Primary patient-derived samples of AML from the University of Texas Southwestern were analyzed by flow cytometry for expression of LILRB4. Cells were gated with low to medium side scatter (SSC), medium to large forward scatter (FSC), and CD45-dim. All cases of monocytic AML (M5, n = 17) showed that the complete population (99%, 1.25) of these leukemia cells displayed surface expression of LILRB4 (Figures 1A and 1B). Myelomonocytic AML (M4) displayed expression of LILRB4 on a partial population of leukemia blasts, whereas all other subtypes were negative (Figures S1A and S1B). Of significance, we also found a sub-population of LILRB4+ monocytic leukemia cells co-express CD34, which may be enriched for leukemia stem cell activity (Figure 1C). Review of gene and protein expression from publicly available databases demonstrated that LILRB4 expression is restricted only to cells of the monocytic lineage and is not identified on normal tissue from major organ systems (Figures 1D and 1E). Additionally, we found no expression of LILRB4 by immunohistochemistry, when specifically examining liver and brain tissue where specialized tissue resident macrophages reside (Figure 1F). To identify target cells in our study, we examined the expression of LILRB4 on AML cell lines and observed positive expression on the monocytic AML cell lines MV4-11, THP-1, and MOLM-13 (Figure 1G).
Figure 1.
LILRB4 Is a Specific Marker for Monocytic AML that Displays Restricted Expression to Cells of Monocyte Lineage
(A) Flow cytometry plot of representative patient sample for monocytic AML (M5), gated from mid to large FSC, low to mid SSC and CD45-dim, demonstrating that LILRB4 is expressed in the full population of leukemia blasts. (B) Quantification of LILRB4 expression in 69 patients with AML demonstrating that LILRB4 is expressed on greater than 99% (SD = 1.25) of leukemia cells in all patients with monocytic AML. (C) Representative flow cytometry plot demonstrating that a sub-population of AML-M5 leukemia cells co-express LILRB4 and CD34. (D and E) Expression profile of LILRB4 in normal tissue at protein (D) and RNA (E) level was assessed by utilizing publicly available databases for mass-spectrometry proteomic analysis (Human Proteome Map: LILRB4; http://www.humanproteomemap.org/query.php) and gene expression (human body index-transcriptional profiling, GSE7307: 210152_at), respectively. (F) Representative images examining human LILRB4 expression by immunohistochemistry of liver (left) and brain tissue (right) (n = 8 samples/tissue). Sections of THP-1 AML tumor implants were used as positive (wild-type) and negative (LILRB4-knockout) control. Scale bars, 250 μm (top panels); 100 μm (bottom panels). (G) Flow cytometry analysis of LILRB4 expression (red) and isotype control (blue) on AML cell lines MV4-11, THP1, and MOLM13. Data are represented as mean ± SE.
Characterization of Anti-LILRB4 CAR Construct and Efficient Transduction of Primary Human T Cells
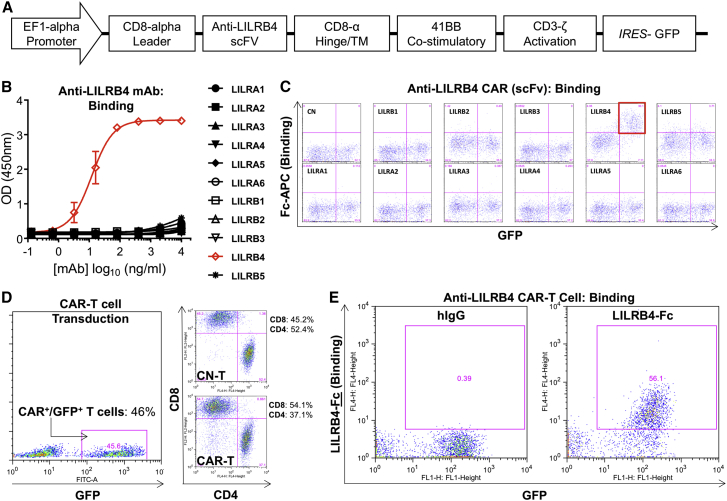
We generated a novel anti-LILRB4 CAR lentivirus vector incorporating a 41BB co-stimulatory domain and CD3ζ activation domain (Figure 2A). The single-chain variable fragment (scFv) was derived from a novel humanized rabbit anti-human antibody that has high affinity and specificity for LILRB4. Parent anti-LILRB4 monoclonal antibody (mAb) (Figure 2B) and the scFv incorporated into the anti-LILRB4 CAR derived from this mAb (Figure 2C) demonstrated specific binding to LILRB4 with no off-target binding to other members of the LILRA or LILRB family. Primary human T cells were activated and then transduced with lentivirus encoding anti-LILRB4 CAR and GFP genes, with transduction efficiencies of GFP+/CAR+ ranging from 35% to 50%. Similar CD4-to-CD8 ratios were seen between anti-LILRB4 CAR-transduced and control (untransduced) T cells (Figure 2D). GFP+ anti-LILRB4 CAR-T cells specifically bound to LILRB4-Fc fusion protein as demonstrated by flow cytometry (Figure 2E), indicating efficient transduction and surface expression of anti-LILRB4 CAR on primary human T cells. GFP+/CAR+ cells were sorted by FACS and expanded in culture for 14–21 days.
Figure 2.
Anti-LILRB4 CAR-Transduced Primary Human T Cells Demonstrate Binding Specificity to Target LILRB4 Protein
(A) CAR construct with CD8α leader, humanized anti-LILRB4 scFv, CD8α hinge and transmembrane domain, intracellular 41BB co-stimulatory domain, and intracellular CD3ζ activation domain. The CAR construct was subcloned into a lentivirus expression plasmid following the EF1α promoter and utilizing GFP co-expression driven by an IRES to enable cell selection. (B) Parent anti-LILRB4 humanized monoclonal antibody (mAb) binds specifically to LILRB4, but not to other members of the LILRB and LILRA family as determined by ELISA. (C) Anti-LILRB4 CAR expressed on transfected 293T cells utilizing the scFv derived from the parent anti-LILRB4 monoclonal antibody demonstrates specific binding to LILRB4 with no off-target binding to the other members of the LILRB or LILRA family as determined by flow cytometry. (D) Efficient lentiviral transduction of primary human T cells encoding anti-LILRB4 CAR, with similar CD4/CD8 ratios in control and CAR transduced T cells. (E) Anti-LILRB4 CAR-T cells demonstrate specific binding to LILRB4-Fc fusion protein as assessed by flow cytometry. Data are represented as mean ± SE.
Anti-LILRB4 CAR-T Cells Are Cytotoxic to LILRB4+ Cells and Display Specific Cytokine Release When Stimulated by LILRB4+ AML Cells
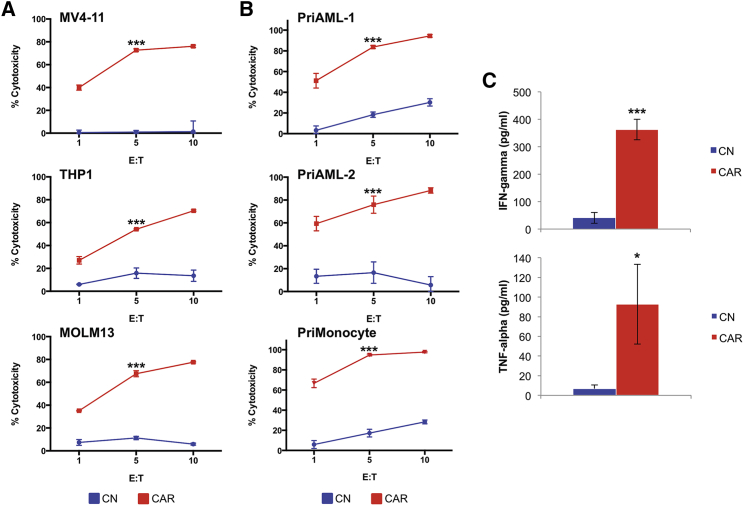
To determine whether the anti-LILRB4 CAR-T cells are cytotoxic to AML cells, we co-cultured control (untransduced) T cells or anti-LILRB4 CAR-T cells for 4 hr at various effector-to-target (E:T) ratios with LILRB4+ human AML cell lines MV4-11, THP-1, and MOLM-13. Flow-based cytotoxicity assays demonstrated strong in vitro cytotoxicity toward all LILRB4+ AML cell lines, whereas control T cells did not induce significant cell killing (Figure 3A). Anti-LILRB4 CAR-T cells were also tested against two LILRB4+ primary AML samples in addition to normal LILRB4+ primary monocytes (Figure S2) and displayed similar significant cytotoxicity against these samples (Figure 3B). Additionally, anti-LILRB4 CAR-T cells demonstrated significantly increased effector cytokine release of both IFN-γ and TNF-α when activated by MV4-11 AML cells, compared with control T cells (Figure 3C).
Figure 3.
Anti-LILRB4 CAR-T Cells Demonstrate Potent In Vitro Cytotoxicity and Specific Cytokine Release When Stimulated by LILRB4+ AML Cells
(A) Anti-LILRB4 CAR-T cells display efficient cytotoxicity against multiple LILRB4+ AML cell lines. AML cell lines were co-cultured with control T cells (blue) or anti-LILRB4 CAR-T cells (red) for 4 hr at E:T ranging from 1:1 to 10:1. Cytotoxicity was determined using a flow cytometry-based assay. (B) Anti-LILRB4 CAR-T cells display efficient cytotoxicity against LILRB4+ primary AML samples and LILRB4+ normal monocytes. (C) Supernatant was collected after 24-hr co-culture of anti-LILRB4 CAR-T cells (red) or control T cells (blue) with MV4-11 cells (E:T, 1:1) and assayed for interferon γ (IFNγ) and tumor necrosis factor alpha (TNF-α) release by ELISA. Anti-LILRB4 CAR-T cells demonstrate significantly increased cytokine release when activated by MV4-11 AML cells, compared with control T cells. *p < 0.05; ***p < 0.001. Data are represented as mean ± SE.
Anti-LILRB4 CAR-T Cells Significantly Reduce Leukemia Burden in the MV4-11 AML Xenograft Mouse Model
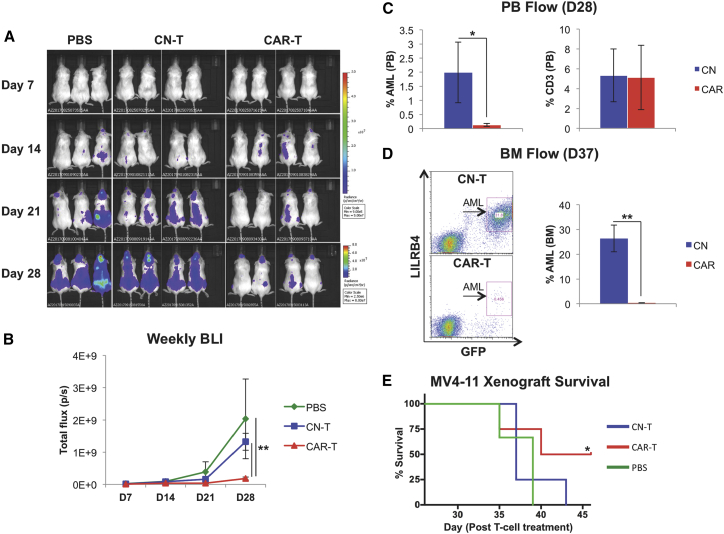
Next, we evaluated the efficacy of anti-LILRB4 CAR-T cells in a human AML mouse xenograft model. Immunocompromised NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were irradiated and on the following day (day 0) were injected with 1 × 106 MV4-11 luciferase-expressing AML cells. On day 5, following AML cell engraftment (Figure S3), mice were treated with PBS, control (untransduced) T cells (2 × 106 cells in 200 μL of PBS), or anti-LILRB4 CAR-T cells (2 × 106 cells in 200 μL of PBS) and monitored weekly using bioluminescence imaging (BLI) for leukemia development. Mice treated with anti-LILRB4 CAR-T cells displayed significantly decreased leukemia burden as demonstrated by BLI (Figures 4A and 4B) and by flow cytometry analysis of circulating leukemia cells in peripheral blood (Figure 4C) as compared with mice in control-treated conditions. Anti-LILRB4 CAR-T cells could be found circulating in peripheral blood at day 28 as well (Figure 4C). Furthermore, bone marrow obtained from mice sacrificed 28–37 days following control or anti-LILRB4 CAR-T cell treatment demonstrated near-complete elimination of leukemic disease in anti-LILRB4 CAR-T cell-treated mice (0.34% AML) compared with mice treated with control-T cells (26% AML) (Figure 4D). Additionally, CAR-T cell treatment prolonged survival compared with control mice treated with PBS (Figure 4E). These results demonstrate anti-LILRB4 CAR-T cells are efficacious to treat LILRB4+ AML in vivo.
Figure 4.
Anti-LILRB4 CAR-T Cells Significantly Reduce Leukemia Burden in the MV4-11 AML Xenograft Mouse Model
NSG mice were irradiated on day −1, injected with MV4-11 AML cells on day 0, and treated with PBS, control (untransduced) T cells, or anti-LILRB4 CAR-T cells on day 5. (A) Weekly BLI of mice treated with PBS, control T cells, and anti-LILRB4 CAR-T cells. (B) Plot of total flux (p/s) as a function of time demonstrates that anti-LILRB4 CAR-T cell-treated mice had significantly decreased leukemia burden as compared with PBS and control T cell-treated mice (PBS: n = 3, control T cell [CN-T]: n = 9, CAR-T: n = 9; **p < 0.01). (C) Percent human leukemia blasts in peripheral blood on day 28. Anti-LILRB4 CAR-T cell-treated mice show significantly decreased circulating leukemia blasts in peripheral blood compared with control-T cell-treated mice (*p < 0.05) (left). Anti-LILRB4 CAR-T cells found to be persistent in PB at 28 days (right). (D) Left: percent human leukemia blasts in bone marrow on day 37, representative mice flow cytometry plot. Right: bone marrow harvested and analyzed for presence of LILRB4+ AML cells from mice sacrificed on days 28–37 demonstrated significantly reduced marrow leukemia burden in anti-LILRB4 CAR-T cell-treated mice compared with control (n = 3 for each group; **p < 0.01). (E) Survival analysis of anti-LILRB4 CAR-T cell-treated mice and PBS-treated mice (PBS: n = 6, CN-T: n = 8, CAR-T: n = 8; *p < 0.05). Data are represented as mean ± SE.
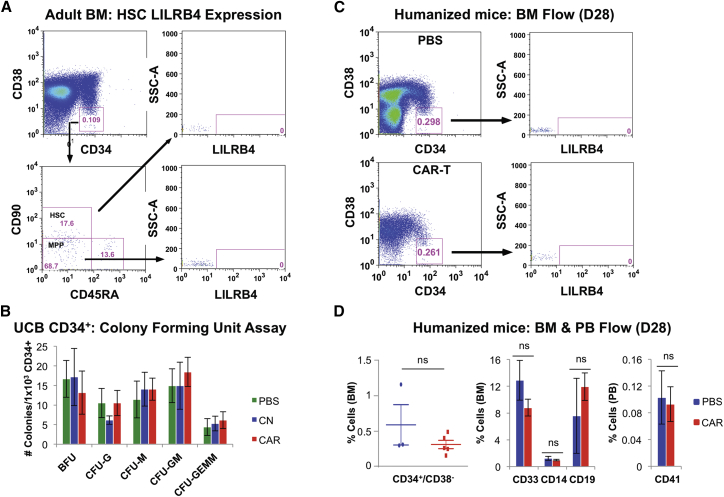
Anti-LILRB4 CAR-T Cells Have No Toxicity against CD34+ Hematopoietic Stem Cells
Because previous CAR-T cells directed against AML have shown cytotoxicity to HSPCs, we first systematically analyzed expression of LILRB4 on HSCs and multipotent progenitors (MPPs) from both normal adult bone marrow and umbilical cord blood. HSCs were defined by multi-color flow cytometry as previously reported25 as low SSC, low FSC, CD45-dim, CD34+/CD38−/CD90+/CD45RA−. When analyzed for LILRB4, both HSCs and MPPs showed no expression (Figure 5A; Figures S4A–S4C). We next evaluated anti-LILRB4 CAR-T cells for potential on-target off-tumor toxicity against CD34+ umbilical cord blood cells (UCB-CD34) using a colony-forming unit (CFU) assay. Anti-LILRB4 CAR-T cells or control (untransduced) T cells were co-cultured with UCB-CD34 cells for 4 hr; then CFU assay was performed. There were no differences in BFU-erythroid (E), CFU-granulocyte (G), CFU-monocyte (M), CFU-granulocyte-monocyte (GM), or CFU-granulocyte-erythroid-monocyte-megakaryocyte (GEMM) colony numbers when cells treated with PBS, control T cells, and anti-LILRB4 CAR-T cells were compared (Figure 5B). Next, anti-LILRB4 CAR-T cells were evaluated in a human UCB-CD34 hematopoietic reconstituted mouse model. Mice were treated with PBS versus anti-LILRB4 CAR-T cells after human cell engraftment (>1% human CD45+ cells in peripheral blood [PB]), and bone marrow was collected on day 28 following treatment. There was no difference between PBS and anti-LILRB4 CAR-T cell-treated mice in all cell populations examined including CD34+/CD38− HSCs (Figures 5C and 5D), CD33+ myeloid cells, CD14+ monocytes, CD19+ B cells, or peripheral blood CD41+ platelets (Figure 5D). These results demonstrate that anti-LILRB4 CAR-T cells are able to specifically and efficiently kill LILRB4+ AML cells in vitro and in vivo, while, importantly, sparing normal HSPCs.
Figure 5.
LILRB4 Is Not Expressed on Human HSCs, and Anti-LILRB4 CAR-T Cells Have No Toxicity against Human HSPCs In Vitro or In Vivo
(A) Flow cytometry analysis of LILRB4 expression on human HSCs and MPPs obtained from normal- healthy adult bone marrow. Cells were gated from low SSC/low FSC/CD45-Dim. (B) UCB-CD34 cells were co-cultured with control T cells or anti-LILRB4 CAR-T cells at 10:1 E:T ratio for 4 hr. Total cell culture was resuspended in MethoCult Classic (STEMCELL) and plated, and colonies were counted after 10 days. No significant difference was found in erythroid burst-forming units (BFU-E), granulocyte colony-forming unit (CFU-G), monocyte CFU (M), CFU-GM, or CFU-GEMM colony numbers in cells treated with PBS, control (untransduced) T cells, or anti-LILRB4 CAR-T cells. (C and D) 8 × 104 umbilical cord blood CD34+ (UCB-CD34) cells were transplanted into NSG mice to generate a humanized hematopoietic reconstituted mouse model. Mice were treated with PBS (n = 3) or anti-LILRB4 CAR-T cells (n = 5) following engraftment and analyzed for human (C) CD34+/C38− HSC population in BM (representative mice flow cytometry plot) and (D) quantified for HSC (CD34+/C38−), myeloid (CD33), monocyte (CD14), and B cell (CD19) populations in bone marrow, and platelet (CD41) population in peripheral blood. No difference was observed in any cell population between mice treated with anti-LILRB4 CAR-T cells and those in PBS-treated conditions. Data are represented as mean ± SE.
Discussion
Relapsed and refractory AML carries a poor prognosis with few effective treatment options. Novel therapeutics being developed for AML include several epigenetic modifier agents (IDH1, IDH2, and histone deacetylase inhibitors), antibody-drug conjugates (anti-CD33: gemtuzumab, vadastuximab), and CAR-T cells targeting various surface-expressed antigens.14, 26, 27 Because many of the antigens targeted by CAR-T cells are co-expressed on normal HSPCs, myelotoxicity has been encountered in preclinical models.
In this work, we show in preclinical in vitro and in vivo models that targeting of LILRB4, which is highly expressed on the monocytic subset of AML, by a novel anti-LILRB4 CAR-T cell leads to significant leukemia cell killing and reduction in leukemia burden, whereas sparing normal HSPCs. As we demonstrate in this study, anti-LILRB4 CAR-T cells hold several key advantages when compared with other treatment strategies in development. First, anti-LILRB4 CAR-T cells display potent and specific cytotoxicity against LILRB4+ AML cell lines and primary AML patient samples. These anti-LILRB4 CAR-T cells have no off-target binding toward other members of the LILRB and LILRA families. Furthermore, many CAR constructs in use currently utilize a mouse anti-human-derived scFv. In this work, we have generated our CAR construct with a humanized scFv in an effort to minimize potential immunogenicity in humans. This may minimize undesirable sequelae including CAR-T rejection or serious allergic or anaphylactic reactions.28
Second, targeting of LILRB4 by CAR-T cells does not have off-target toxicity toward hematopoietic stem or progenitor cells. Previous efforts to target AML-associated antigens such as CD123 and CD33 have led to severe myelotoxicity because these antigens are also expressed on early stem and progenitor cells.18, 29 With the incorporation of “suicide switches” or by utilizing transient CAR-T cells, some of these CAR-T cells have translated to early clinical trials. Here we demonstrated that LILRB4 is not expressed on immunophenotypically defined HSCs or MPPs obtained from multiple sources including healthy adult bone marrow (BM), umbilical cord blood, and PB G-CSF mobilized stem cells. When tested in various in vitro and in vivo models, anti-LILRB4 CAR-T cells spare normal HSPCs and do not ablate normal human hematopoiesis, which we believe will have significantly less myelotoxicity when applied in humans.
This strategy of targeting a subclass of AML based on HSC-sparing restricted immunophenotype rather than targeting an antigen expressed on all types of AML may be advantageous for several reasons. First, because significant myelotoxicity is not expected as seen when targeting other AML antigens, anti-LILB4 CAR-T cells may be allowed to persist longer in patients, which may better protect from disease relapse. Importantly, hematopoietic reconstitution by stem cell transplant or rescue may not be necessary, because HSPC toxicity is not observed with anti-LILRB4 CAR-T cells. Additionally, because myelosuppression is not expected, we would expect there to be fewer bacterial and fungal infectious complications due to prolonged neutropenia and also less transfusion dependence.
It is important to note that normal healthy monocytes may be removed by anti-LILRB4 CAR-T cells as demonstrated in this work. Because normal monocytes infiltrate tissue to sites of infection and mature into macrophages, some phagocytic and antigen-presenting ability may be diminished; however, neutrophils may compensate some of this function. Prolonged monocytopenia may leave certain groups of patients susceptible to specific infections including atypical mycobacterium as in MonoMAC syndrome; however, patients with this disease often have additional B and natural killer (NK) cell cytopenias due to germline GATA2 mutations.30 Conversely, we have also demonstrated LILRB4 is not expressed in normal liver and brain, organs that contain tissue resident macrophages (Kupffer cells, microglia), and therefore believe anti-LILRB4 CAR-T cells will not target and spare these specialized cells.
A further unique advantage of targeting LILRB4 on AML cells by CAR-T cells is due to the expression pattern of this antigen on both leukemia stem cells and mature circulating blasts, as we and others have demonstrated.22, 23, 24 Leukemia stem cells represent a minute sub-population of total leukemic blasts; however, they are most likely responsible for causing relapsed or refractory disease in AML.31, 32 Because LILRB4 is seen on CD34+/c-Kit+ AML stem cells, but not on normal hematopoietic stem cells, anti-LILRB4 CAR-T cells may effectively eliminate this leukemia cell population and possibly prevent disease relapse. Therefore, targeting LILRB4 may be effective in both de novo and relapsed or refractory disease settings.
Recent work has demonstrated interleukin-6 (IL-6), one of the main cytokines that contributes to the pathology of cytokine release syndrome (CRS) encountered in CAR-T cell therapy, is secreted from monocytic lineage cells.33 CRS can lead to critical illness including cardiorespiratory compromise and multi-organ failure. By targeting normal monocytes with anti-LILRB4 CAR-T cells, these cells may be eliminated along with monocytic AML cells, which may possibly result in less severe CRS, because this cell source of IL-6 will have been removed.
In addition to monocytic AML, LILRB4 has also been reported to be expressed in other hematologic malignancies, including 50% of chronic lymphocytic leukemia cases, where expression of LILRB4 may be predictive of prognosis.34 Our study and data analysis also suggests that LILRB4 is expressed on some cases of multiple myeloma and pre-B acute lymphoblastic leukemia (ALL), including the high-risk subtype carrying the mixed lineage leukemia (MLL) gene mutation35 (and data not shown). Anti-LILRB4 CAR-T cells may also be applied in the treatment of these malignancies.
Lastly, LILRB4 expression on certain myeloid cell populations in cancer patients may allow for tumor cell evasion of immune response. Previous studies have demonstrated that LILRB4 is expressed on myeloid-derived suppressor cells,36 tolerogenic dendritic cells,37 and tumor-associated macrophages.22, 38 These cells create a tolerogenic immune suppressive niche whereby cancer cells are protected from immune system discovery and destruction.39, 40, 41 Targeting these tumor-protective cells in the tumor microenvironment may allow for improved disease control.42 LILRB4 therefore represents an attractive target for CAR-T cell-directed therapy for treatment of various types of malignancies, including solid tumors, where CAR-T cells may be used in combination with other targeted therapies to eliminate either the malignant tumor cell or its surrounding LILRB4+ immune-suppressive cells.
Future work will entail scaling CAR-T production methods and transitioning to produce good manufacturing practice (GMP) grade anti-LILRB4 CAR-T cells in order to test these cells in clinical trials. This study holds potential to improve the poor outcomes for patients with monocytic AML, and potentially other malignancies, by offering a new therapeutic strategy utilizing novel anti-LILRB4 CAR-T cells.
Materials and Methods
Construction of Anti-LILRB4 CAR
The gene encoding an scFv derived from a humanized anti-LILRB4 antibody was generated by splicing the variable region of the heavy chain to the variable region of the light chain via a (Gly4Ser)3 linker. This was cloned in-frame to the CD8α hinge and transmembrane domain. In this construct, the transmembrane domain is followed by a 41BB intracellular domain that serves as the co-stimulatory domain of the CAR, terminating with the CD3ζ intracellular activation domain. The CAR construct was codon optimized and synthesized for expression in human cells (Genscript, Piscataway, NJ, USA). The complete CAR construct was sub-cloned into the lentiviral expression plasmid, pLVX-ZsGreen (Clontech, Mountain View, CA, USA) driven by an EF1α promoter and containing the internal ribosome entry site (IRES)-GFP signal for cell selection.
Primary Human T Cell Lentivirus Transduction and CAR-T Cell Expansion
On day 0, 1 × 106 T cells were thawed in 1.5 mL of T cell medium (Immunocult with 100 U/mL human interleukin-2 [hIL-2]) and were stimulated with anti-CD3/CD28 Dynabeads (Life Technologies) according to the manufacturer’s directions. After 24 hr, concentrated lentivirus at MOI 50 was added to activated T cells. Cell culture was monitored daily during transduction, and additional media were added to maintain a cell concentration of 0.5–1 × 106 cells/mL. On day 4 following transduction, virus-containing medium and Dynabeads were removed, and cells were washed and resuspended at 1 × 106 cells/mL in fresh T cell medium supplemented with 100 U/mL hIL-2. In some experiments, GFP+-CAR-T cells were sorted on day 7 on a FACSAria and re-stimulated with anti-CD3/CD28 antibody for 3 days. Sorted CAR-T cells were then washed and resuspended in T cell medium supplemented with 100 U/mL hIL-2. Cells were allowed to expand in culture until day 14 or 21. Cell culture was monitored daily during expansion, and additional media were added to maintain a cell concentration of 1 × 106 cells/mL.
For all experiments using anti-LILRB4 CAR-T cells, paired (from same donor) untransduced T cells, activated and cultured for equivalent time, served as control T cells.
AML Mouse Xenograft
All vertebrate animal experiments were conducted under Institutional Animal Care and Use Committee (IACUC)-reviewed and -approved protocols. Mice were maintained in pathogen-free conditions in the animal resource core facility at the University of Texas Southwestern Medical Center (UTSW). Six- to 8-week-old NSG mice (Jackson Laboratory, Sacramento, CA, USA) were sublethally irradiated (200 cGy) on day −1. On day 0, each mouse was injected via tail vein, with 1 × 106 human leukemia cells resuspended in 200 μL of PBS. On day 5, 2 × 106 control (untransduced) T cells or anti-LILRB4 CAR-T cells resuspended in 200 μL of PBS were injected into each mouse via tail vein. Mice were weighed, peripheral blood obtained via retro-orbital bleeding, and BLI was conducted weekly. Death was recorded when moribund animals were euthanized per IACUC protocol requirements. Bone marrow was harvested for analysis of leukemia disease burden after mice were euthanized per protocol requirements.
Author Contributions
S.J. designed and conducted experiments and wrote the manuscript. H.C. and M.D. designed and conducted experiments. X.G., G.W., W.C., and Z.L. conducted experiments. N.Z. and Z.A. supervised. C.C.Z. supervised and wrote the manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgments
We thank the NIH (grants 1R01CA172268 and 5K12HD-068369-08), the St. Baldrick's Foundation (Fellowship grant 552535), the Leukemia & Lymphoma Society (grant 1024-14), the March of Dimes Foundation (grant 1-FY14-201), the Cancer Prevention and Research Institute of Texas (grants DP150056, RP180435, and PR150551), the Robert A. Welch Foundation (grants I-1834 and AU-0042-20030616), the Children’s Cancer Fund, and Micaela’s Army Foundation for generous support.
Footnotes
Supplemental Information includes Supplemental Materials and Methods and four figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.08.001.
Contributor Information
Zhiqiang An, Email: zhiqiang.an@uth.tmc.edu.
Cheng Cheng Zhang, Email: alec.zhang@utsouthwestern.edu.
Supplemental Information
References
- 1.Döhner H., Weisdorf D.J., Bloomfield C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.Rowe J.M., Tallman M.S. How I treat acute myeloid leukemia. Blood. 2010;116:3147–3156. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]
- 3.Rubnitz J.E. How I treat pediatric acute myeloid leukemia. Blood. 2012;119:5980–5988. doi: 10.1182/blood-2012-02-392506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verschuur, A.C. (2004). Acute monocytic leukemia. Orphanet Encyclopedia. https://www.orpha.net/data/patho/GB/uk-AMLM5.pdf.
- 5.Harris A.C., Kitko C.L., Couriel D.R., Braun T.M., Choi S.W., Magenau J., Mineishi S., Pawarode A., Yanik G., Levine J.E. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica. 2013;98:179–184. doi: 10.3324/haematol.2012.073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheadle E.J., Sheard V., Hombach A.A., Chmielewski M., Riet T., Berrevoets C., Schooten E., Lamers C., Abken H., Debets R., Gilham D.E. Chimeric antigen receptors for T-cell based therapy. Methods Mol. Biol. 2012;907:645–666. doi: 10.1007/978-1-61779-974-7_36. [DOI] [PubMed] [Google Scholar]
- 7.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruella M., June C.H. Chimeric antigen receptor T cells for B cell neoplasms: choose the right CAR for you. Curr. Hematol. Malig. Rep. 2016;11:368–384. doi: 10.1007/s11899-016-0336-z. [DOI] [PubMed] [Google Scholar]
- 10.Perales M.A., Kebriaei P., Kean L.S., Sadelain M. Building a safer and faster CAR: seatbelts, airbags, and CRISPR. Biol. Blood Marrow Transplant. 2017;24:27–31. doi: 10.1016/j.bbmt.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brudno J.N., Kochenderfer J.N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat. Rev. Clin. Oncol. 2018;15:31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PubMed] [Google Scholar]
- 12.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J.H., Rivière I., Gonen M., Wang X., Sénéchal B., Curran K.J., Sauter C., Wang Y., Santomasso B., Mead E. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalabi H., Angiolillo A., Fry T.J. Beyond CD19: opportunities for future development of targeted immunotherapy in pediatric relapsed-refractory acute leukemia. Front Pediatr. 2015;3:80. doi: 10.3389/fped.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Mao H., Zhang J., Chu J., Devine S., Caligiuri M.A., Yu J. Targeting FLT3 by chimeric antigen receptor T cells for the treatment of acute myeloid leukemia. Leukemia. 2017;31:1830–1834. doi: 10.1038/leu.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tashiro H., Sauer T., Shum T., Parikh K., Mamonkin M., Omer B., Rouce R.H., Lulla P., Rooney C.M., Gottschalk S., Brenner M.K. Treatment of acute myeloid leukemia with T cells expressing chimeric antigen receptors directed to C-type lectin-like molecule 1. Mol. Ther. 2017;25:2202–2213. doi: 10.1016/j.ymthe.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mardiros A., Forman S.J., Budde L.E. T cells expressing CD123 chimeric antigen receptors for treatment of acute myeloid leukemia. Curr. Opin. Hematol. 2015;22:484–488. doi: 10.1097/MOH.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill S., Tasian S.K., Ruella M., Shestova O., Li Y., Porter D.L., Carroll M., Danet-Desnoyers G., Scholler J., Grupp S.A. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J., Umikawa M., Cui C., Li J., Chen X., Zhang C., Huynh H., Kang X., Silvany R., Wan X. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature. 2012;485:656–660. doi: 10.1038/nature11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng M., Lu Z., Zheng J., Wan X., Chen X., Hirayasu K., Sun H., Lam Y., Chen L., Wang Q. A motif in LILRB2 critical for Angptl2 binding and activation. Blood. 2014;124:924–935. doi: 10.1182/blood-2014-01-549162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang X., Lu Z., Cui C., Deng M., Fan Y., Dong B., Han X., Xie F., Tyner J.W., Coligan J.E. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat. Cell Biol. 2015;17:665–677. doi: 10.1038/ncb3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang X., Kim J., Deng M., John S., Chen H., Wu G., Phan H., Zhang C.C. Inhibitory leukocyte immunoglobulin-like receptors: immune checkpoint proteins and tumor sustaining factors. Cell Cycle. 2016;15:25–40. doi: 10.1080/15384101.2015.1121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Expression of inhibitory receptor ILT3 on normal hematopoietic stem cells and leukemic progenitors. Dobrowolska H., Vlad G., Suciu-Foca N., editors. J Cell Sci Ther. 2013;4:98. [Google Scholar]
- 24.Dobrowolska H., Gill K.Z., Serban G., Ivan E., Li Q., Qiao P., Suciu-Foca N., Savage D., Alobeid B., Bhagat G., Colovai A.I. Expression of immune inhibitory receptor ILT3 in acute myeloid leukemia with monocytic differentiation. Cytometry B Clin. Cytom. 2013;84:21–29. doi: 10.1002/cyto.b.21050. [DOI] [PubMed] [Google Scholar]
- 25.Majeti R., Park C.Y., Weissman I.L. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saygin C., Carraway H.E. Emerging therapies for acute myeloid leukemia. J. Hematol. Oncol. 2017;10:93. doi: 10.1186/s13045-017-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenegger F.S., Krupka C., Haubner S., Köhnke T., Subklewe M. Recent developments in immunotherapy of acute myeloid leukemia. J. Hematol. Oncol. 2017;10:142. doi: 10.1186/s13045-017-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonifant C.L., Jackson H.J., Brentjens R.J., Curran K.J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenderian S.S., Ruella M., Shestova O., Klichinsky M., Aikawa V., Morrissette J.J., Scholler J., Song D., Porter D.L., Carroll M. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29:1637–1647. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camargo J.F., Lobo S.A., Hsu A.P., Zerbe C.S., Wormser G.P., Holland S.M. MonoMAC syndrome in a patient with a GATA2 mutation: case report and review of the literature. Clin. Infect. Dis. 2013;57:697–699. doi: 10.1093/cid/cit368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlush L.I., Mitchell A., Heisler L., Abelson S., Ng S.W.K., Trotman-Grant A., Medeiros J.J.F., Rao-Bhatia A., Jaciw-Zurakowsky I., Marke R. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature. 2017;547:104–108. doi: 10.1038/nature22993. [DOI] [PubMed] [Google Scholar]
- 32.Pollyea D.A., Gutman J.A., Gore L., Smith C.A., Jordan C.T. Targeting acute myeloid leukemia stem cells: a review and principles for the development of clinical trials. Haematologica. 2014;99:1277–1284. doi: 10.3324/haematol.2013.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh N., Hofmann T.J., Gershenson Z., Levine B.L., Grupp S.A., Teachey D.T., Barrett D.M. Monocyte lineage-derived IL-6 does not affect chimeric antigen receptor T-cell function. Cytotherapy. 2017;19:867–880. doi: 10.1016/j.jcyt.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colovai A.I., Tsao L., Wang S., Lin H., Wang C., Seki T., Fisher J.G., Menes M., Bhagat G., Alobeid B., Suciu-Foca N. Expression of inhibitory receptor ILT3 on neoplastic B cells is associated with lymphoid tissue involvement in chronic lymphocytic leukemia. Cytometry B Clin. Cytom. 2007;72:354–362. doi: 10.1002/cyto.b.20164. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong S.A., Staunton J.E., Silverman L.B., Pieters R., den Boer M.L., Minden M.D., Sallan S.E., Lander E.S., Golub T.R., Korsmeyer S.J. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat. Genet. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 36.de Goeje P.L., Bezemer K., Heuvers M.E., Dingemans A.C., Groen H.J., Smit E.F., Hoogsteden H.C., Hendriks R.W., Aerts J.G., Hegmans J.P. Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. OncoImmunology. 2015;4:e1014242. doi: 10.1080/2162402X.2015.1014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang C.C., Ciubotariu R., Manavalan J.S., Yuan J., Colovai A.I., Piazza F., Lederman S., Colonna M., Cortesini R., Dalla-Favera R., Suciu-Foca N. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat. Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 38.Suciu-Foca N., Feirt N., Zhang Q.Y., Vlad G., Liu Z., Lin H., Chang C.C., Ho E.K., Colovai A.I., Kaufman H. Soluble Ig-like transcript 3 inhibits tumor allograft rejection in humanized SCID mice and T cell responses in cancer patients. J. Immunol. 2007;178:7432–7441. doi: 10.4049/jimmunol.178.11.7432. [DOI] [PubMed] [Google Scholar]
- 39.Brenk M., Scheler M., Koch S., Neumann J., Takikawa O., Häcker G., Bieber T., von Bubnoff D. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J. Immunol. 2009;183:145–154. doi: 10.4049/jimmunol.0803277. [DOI] [PubMed] [Google Scholar]
- 40.Ge G., Tian P., Liu H., Zheng J., Fan X., Ding C., Jin Z., Luo X., Xue W. Induction of CD4+ CD25+ Foxp3+ T regulatory cells by dendritic cells derived from ILT3 lentivirus-transduced human CD34+ cells. Transpl. Immunol. 2012;26:19–26. doi: 10.1016/j.trim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Andersen M.H. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia. 2014;28:1784–1792. doi: 10.1038/leu.2014.108. [DOI] [PubMed] [Google Scholar]
- 42.Ruella M., Klichinsky M., Kenderian S.S., Shestova O., Ziober A., Kraft D.O., Feldman M., Wasik M.A., June C.H., Gill S. Overcoming the immunosuppressive tumor microenvironment of Hodgkin lymphoma using chimeric antigen receptor T cells. Cancer Discov. 2017;7:1154–1167. doi: 10.1158/2159-8290.CD-16-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.