Abstract
Childhood enteropathies are a group of diseases causing severe chronic (>2–3 weeks) diarrhoea often starting in the first week of life with the potential for fatal complications for the affected infant. Early identification and accurate classification of childhood enteropathies are, therefore, crucial for making treatment decisions to prevent life-threatening complications. Childhood enteropathies are classified into four groups based on the underlying pathology: (i) conditions related to defective digestion, absorption and transport of nutrients and electrolytes; (ii) disorders related to enterocyte differentiation and polarization; (iii) defects of enteroendocrine cell differentiation; and (iv) disorders associated with defective modulation of intestinal immune response. While the intestinal mucosa is usually normal in enteropathies related to congenital transport or enzyme deficiencies, the intestinal biopsy in other disorders may reveal a wide range of abnormalities varying from normal villous architecture to villous atrophy and/or inflammation, or features specific to the underlying disorder including epithelial abnormalities, lipid vacuolization in the enterocytes, absence of plasma cells, lymphangiectasia, microorganisms, and mucosal eosinophilic or histiocytic infiltration. This review intends to provide an update on small intestinal biopsy findings in childhood enteropathies, the “newcomers”, including very early onset monogenic inflammatory bowel disease (IBD), in particular, for the practicing pathologist.
Keywords: Enteropathy, Inflammatory bowel disease, Children, Histopathology
Advances in paediatric gastroenterology practice and in molecular pathology have allowed us to recognize some rare forms of childhood enteropathies where the pathologist plays an important role in defining the cause of the enteropathy. This review intends to provide an update on small intestinal biopsy findings in childhood enteropathies for the practicing pathologist. Of these, however, only the “newcomers”, including very early onset monogenic inflammatory bowel disease (IBD), will be discussed in this review, while other causes of childhood enteropathies will be briefly mentioned in the discussion of the differential diagnosis.
Childhood enteropathies are a group of diseases causing severe chronic (>2–3 weeks) diarrhoea often starting in the first week of life with the potential for fatal complications for the affected infant [1, 2]. Early identification and accurate classification of childhood enteropathies are, therefore, crucial for making treatment decisions to prevent lifethreatening complications [3–9]. Childhood enteropathies are classified into four groups based on the underlying pathology: (i) conditions related to defective digestion, absorption and transport of nutrients and electrolytes; (ii) disorders related to enterocyte differentiation and polarization; (iii) defects of enteroendocrine cell differentiation; and (iv) disorders associated with defective modulation of intestinal immune response [10]. While the intestinal mucosa is usually normal in enteropathies related to congenital transport or enzyme deficiencies, the intestinal biopsy in other disorders may reveal a wide range of abnormalities varying from normal villous architecture to villous atrophy and/or inflammation, or features specific to the underlying disorder including epithelial abnormalities, lipid vacuolization in the enterocytes, absence of plasma cells, lymphangiectasia, microorganisms, and mucosal eosinophilic or histiocytic infiltration (Table 1). However, in many disorders, the diagnosis is often delayed or incorrect as intestinal findings may be subtle or focal, and as molecular testing for enteropathy-associated mutations is not widely available in every laboratory [6–8, 11]. The following article delineates some of the recently described enteropathies of childhood and their specific histopathological features as summarized in Table 2.
Table 1.
Histopathologic spectrum of childhood enteropathies
| Normal villus morphology | Villus atrophy/inflammation | Specific or characteristic features |
|---|---|---|
| Congenital chloride diarrhoea | Autoimmune enteropathy and IPEX | Lipid-filled enterocytes • ABL • HBL • CRD |
| Carbohydrate malabsorption | Microvillus inclusion disease | Absence of plasma cells • Immunodeficiency |
| Sucrose isomaltase deficiency | Tufting enteropathy | Lymphangiectasia • Primary intestinal lymphangiectasia |
| Gluten sensitive enteropathy | Histiocytic infiltration in the lamina propria • Metabolic storage disorders |
|
| Eosinophilic gastroenteritis | Infectious agents |
Table 2.
Clinicopathologic features of childhood enteropathies
| ABL | MVID | CTE | EED | EoGE | |
| Underlying mechanism | MTP mutations | MyoVb mutations | EpCAM mutations | Neurogenin 3 mutation | Mixed T cell-IgE mediated immunity |
| Accompanying disease | Acanthocytosis Neurologic abnormalities Retinitis pigmentosa |
None | Dysmorphic features including choanal, oesophageal or rectal atresia & autoimmune diseases | Insulin-dependent diabetes | Other allergic disorders |
| Biopsy features | |||||
| Villus architecture | Normal | Variable | Variable | Variable | Villus blunting |
| Crypt architecture | Normal | Hyperplastic | Regenerative changes | Normal | Normal |
| Lamina propria inflammation | Absent | Minimal/absent | Variable | Absent | Predominantly eosinophilic |
| Intraepithelial lymphocytosis | Absent | Absent | Absent | Absent | Intraepithelial eosinophilia |
| Diagnostic histopathologic feature | Enterocyte vacuolization | Loss of brush border with apical vacuolization | Epithelial tufts | Lack of enteroendocrine cells on ChA | Extensive mucosal eosinophilia |
| Electron microscopy | Lipid vacuoles in enterocyte | Microvillus inclusion bodies | Not significant | Not significant | Not significant |
Lipid trafficking disorders are primary abnormalities involving lipid transport within the enterocyte and comprise abetalipoproteinemia (ABL), a rare autosomal recessive condition characterized by defective lipid transfer from the membrane of the endoplasmic reticulum to apolipoprotein B (ApoB) due to mutations in microsomal triglyceride transfer protein (MTP) gene located on chromosome 4q22 [12–14], hypobetalipoproteinemia (HBL), a rare autosomal dominant disorder due to mutations in ApoB gene on chromosome 2 leading to protein truncation in ApoB [14], and chylomicron retention disorder (CRD) or Anderson’s disease, a very rare autosomal recessive condition caused by mutations in SAR1B gene coding for GTPase-associated coat proteins involved in endoplasmic reticulum to Golgi transport of chylomicrons [15, 16]. Affected infants present with failure to thrive, diarrhoea and steatorrhea, and usually peripheral acanthocytosis. As lipid assembly is disorganized in these disorders, the serum lipid profile is characterized by low levels of lipid fractions accompanied by various neurologic manifestations due to malabsorption of fat-soluble vitamins, particularly vitamins E and A leading to neuromuscular impairment including poor muscle coordination, ataxia, and retinitis pigmentosa later in life [7].
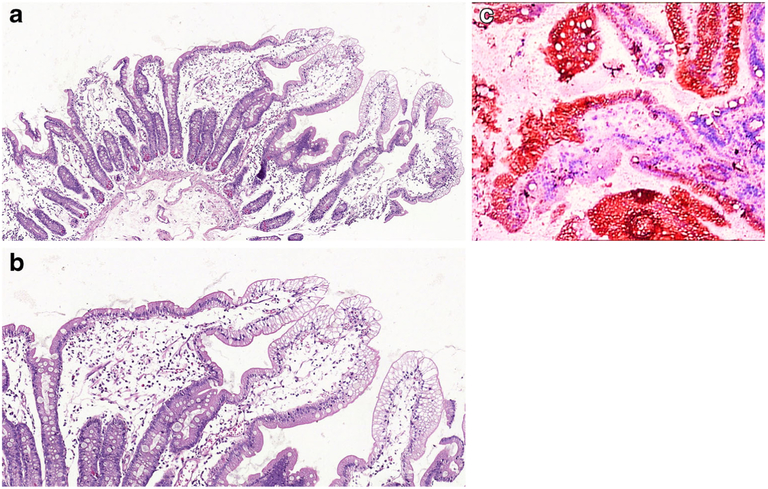
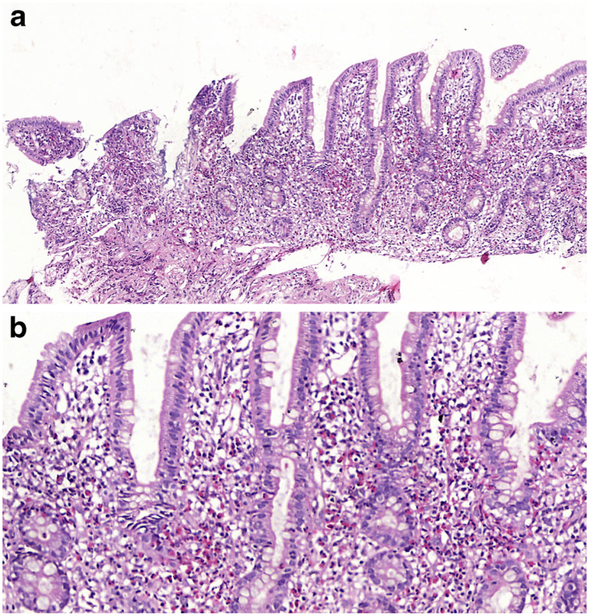
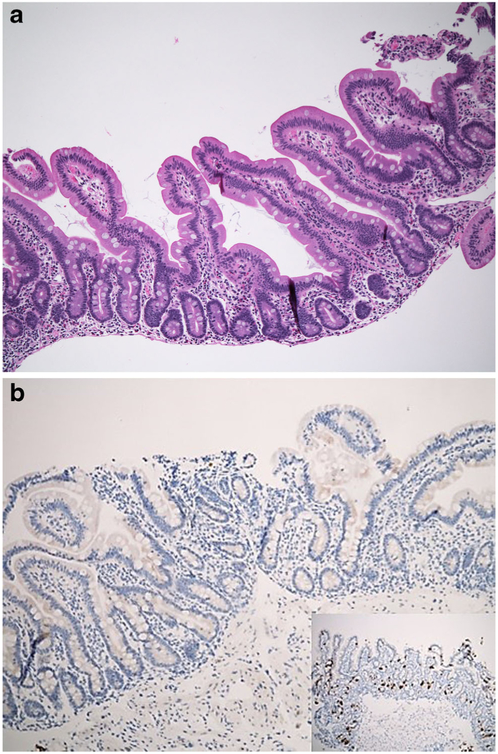
Intestinal biopsies in all three disorders show normal villus architecture without lamina propria inflammation, while surface enterocytes with intact brush border display cytoplasmic vacuolization in a heterogeneous distribution along the villi being more prominent at the villus tips [13, 16]. Though the pale vacuolated appearance of the enterocytes can be easily identified on H&E (Fig. 1a, b), lipid in the enterocytes can be demonstrated by lipid stains such as Oil Red-O (Fig. 1c) using fresh frozen tissue. Electron microscopy shows numerous vacuoles in the cytoplasm of the enterocytes and juxtaposition of intercellular membranes lacking lipid molecules. Treatment consists of a low fat diet and replacement of fat-soluble vitamins [16]. Similar but less striking findings in the small intestinal mucosa can be seen after a fatty meal, long fasting, celiac disease or megaloblastic anaemia [6–8, 17]. Despite being rarely biopsied, some infectious agents, particularly viral infections, may also cause enterocyte vacuolization and damage including rota virus which after endocytosis is uncoated and replicates in the cytoplasm of the enterocyte. The virus then acquires a temporary envelope by budding into cisternae of rough endoplasmic reticulum (RER) leading to dilatation of the RER which can be identified as vacuolization of the cytoplasm on light microscopy [18, 19].
Fig.1.
a Duodenal mucosa from a 4-year-old boy with ABL. There is surface epithelial vacuolation in otherwise normallooking duodenal mucosa with preserved villus/crypt architecture (H&E; ×40). b Vacuolated appearance of surface enterocytes (H&E; ×200). c Lipid droplets in the enterocyte cytoplasm (Oil Red O; ×200)
Congenital defects of intestinal epithelial differentiation comprising microvillus inclusion disease, tufting enteropathy and enteroendocrine cell dysgenesis [5–8] are associated with severe morbidity and mortality despite sustained TPN and/or intestinal transplantation.
Microvillus inclusion disease (MVID) is an uncommon autosomal recessive disease described by Davidson et al. in 1978 [20] as severe diarrhoea of the neonate presenting with villous atrophy in the intestinal biopsy. Recently identified mutations in MYO5B gene located on 18q21 encoding myosin Vb are the underlying genetic abnormality [21]. Myosin Vb-deficient enterocytes display disruption of cell polarity as reflected by mislocalized apical and basolateral transporter proteins including CD36, altered distribution of certain endosomal/lysosomal constituents including RabGTPases and disorganization in the distribution of actin myofilaments [22]. Although described as a disease of the small intestine, involvement of the colon, gallbladder and kidney has been reported [6, 7]. In addition to classic MVID discussed above, a variant MVID associated with mutations in Syntaxin 3 has recently been described [23]. Variant cases usually present with milder disease with a better response to TPN.
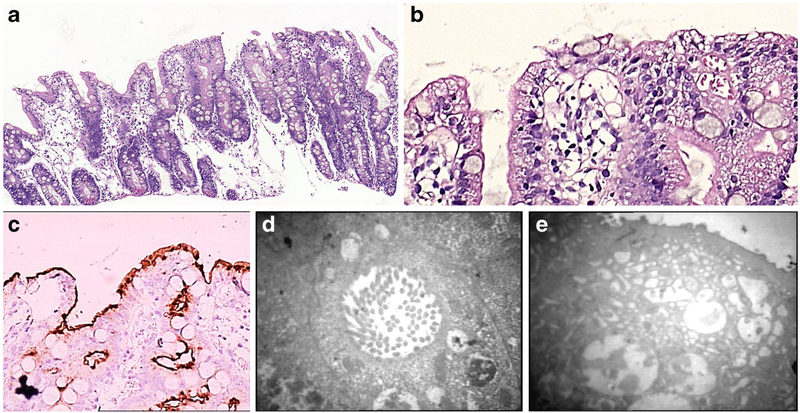
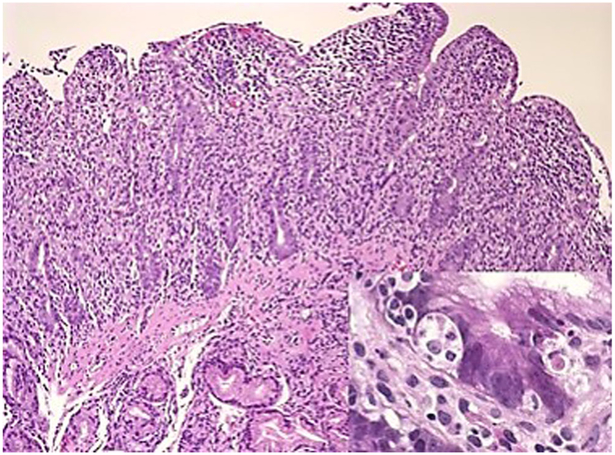
Light microscopic examination of small intestinal biopsy shows moderate to severe villus atrophy usually accompanied with crypt hyperplasia, and with no or little inflammation in the lamina propria or intraepithelial lymphocytosis (Fig. 2a). On H&E, there is irregular vacuolated appearance of the apical cytoplasm of the enterocytes (Fig. 2b) with extensive or patchy absence of the brush border [6–8, 17] which can be highlighted histochemically by PAS and immunohistochemically by CD10 (Fig. 2c), polyclonal CEA, alkaline phosphatase, villin [24, 25] or recently described anti-Rab11, a small GTPase on the surface of recycling endosomes [26], as patchy loss of the normal band-like staining of the brush border and apical cytoplasmic smudgy staining pattern corresponding to the microvillus inclusion bodies in the apical cytoplasm of the enterocytes. The ultrastructural findings of intracytoplasmic inclusions that are lined by intact microvilli are the diagnostic hallmark of the disease (Fig. 2d), with short and rudimentary or absent microvilli on the surface; unusual electron microscopic features such as intermediate structures between microvillus inclusions and lysosomes, inclusions containing few microvilli and dense apical granules in the apical cytoplasm (Fig. 2e) can also be observed [6–8, 17, 27, 28].
Fig. 2.
a Duodenal mucosa of a newborn showing villous atrophy, crypt hyperplasia, and surface irregularity (H&E; ×200). b Apical vacuoles within the enterocyte cytoplasm (H&E; ×400). c Smudgy staining pattern in the apical cytoplasm of the enterocyte (CD10 IHC; ×200). d Microvillus inclusion body in the apical cytoplasm of the enterocyte on TEM (×12,400). e Lack of microvilli on the surface of the enterocyte which shows intermediate structures between microvillus inclusions and lysosomes, inclusions containing few microvilli, and dense apical granules in the apical cytoplasm on TEM (×7750)
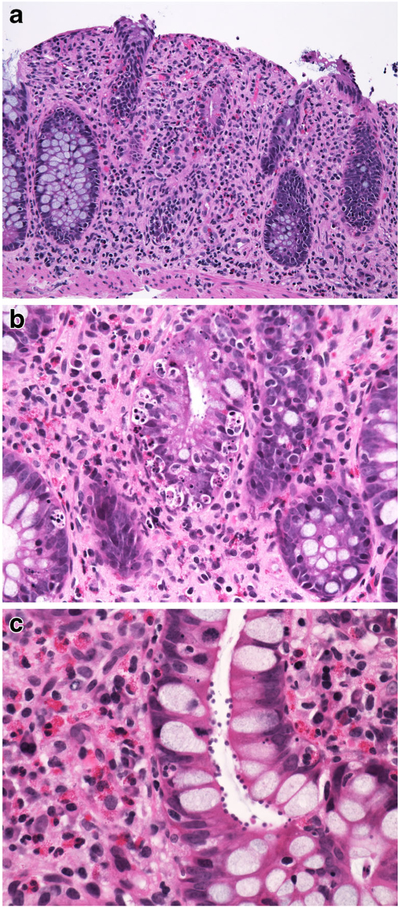
Congenital tufting enteropathy (CTE), also known as epithelial dysplasia [29], is an autosomal recessive disorder causing severe diarrhoea in the first week of life together with various dysmorphic features including choanal atresia, oesophageal/rectal atresia in some of the affected infants [6, 7, 17]. EpCAM mutations on chromosome 2p21 have recently been identified as the responsible genetic abnormality in CTE [30]. EpCAM, like most cell adhesion molecules, appears to have a vital role in cell-cell interaction by recruiting intracellular α-actinin and co-localizing with E-cadherin and claudin-7 in areas of cell-cell junctions and tight junctions, respectively [31].
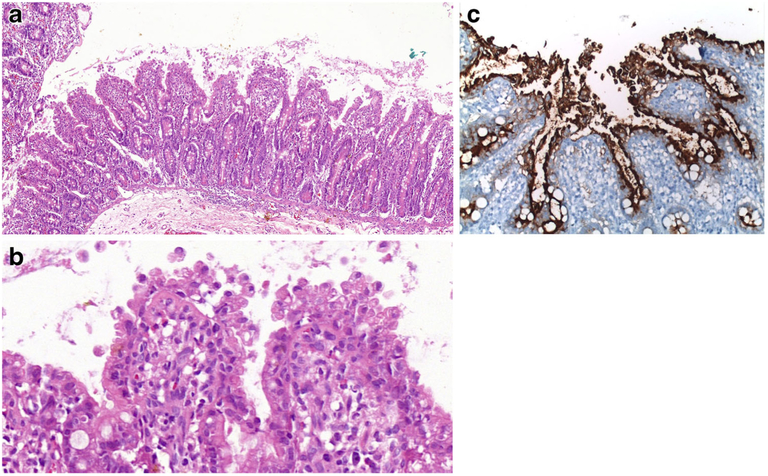
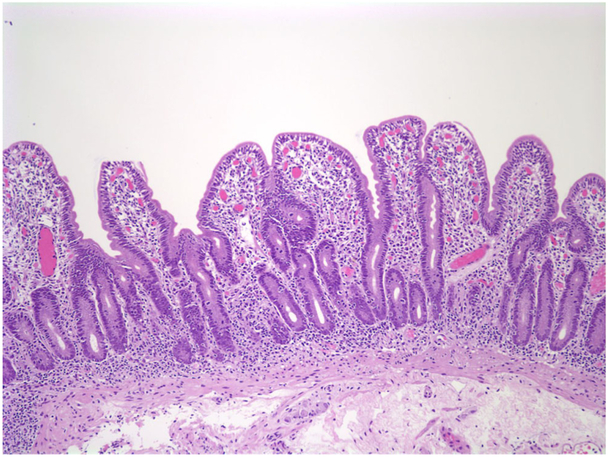
The small intestinal mucosa shows varying degrees of villous atrophy and crypt hyperplasia with low or no mononuclear cell infiltration in the lamina propria or intraepithelial lymphocytosis (Fig. 3a). Histology is in harmony with the term “tufting”, as the surface enterocytes display focal crowding forming rounded, tear-drop-shaped buds or small papillae resembling tufts some of which seem to drop off into the lumen on sections (Fig. 3b, c) [29]. These characteristic “tufts” of extruding epithelium, first described by Reifen et al. [32], are seen at the villus tips and may affect up to 70% of villi. Focal enterocyte crowding can also be observed in the crypt epithelium together with regenerative changes including crypt dilatation and branching [33, 34]. Unlike MVID, the brush border is usually intact in CTE and surrounds the tufts (Fig. 3d). Immunohistochemical loss of EpCAM staining in the enterocytes is proposed as a helpful adjunct in the diagnosis of CTE [31]. The colonic mucosa may also be involved. In some cases, diagnosis of CTE may require repeat biopsies as it takes time for the characteristic tufts to develop. Ultrastructural changes may be observed in the desmosomes, which are increased in length and number [6, 7, 17].
Fig. 3.
a Small intestinal mucosa from a 6-month-old girl. The villi are short with irregular surface (H&E; ×100). b Groups of epithelial cells forming typical epithelial tufts tufts and buds dropping off into the lumen (H&E; ×200). c Intact brush border surrounding epithelial tufts on the surface (CD10 IHC; ×400)
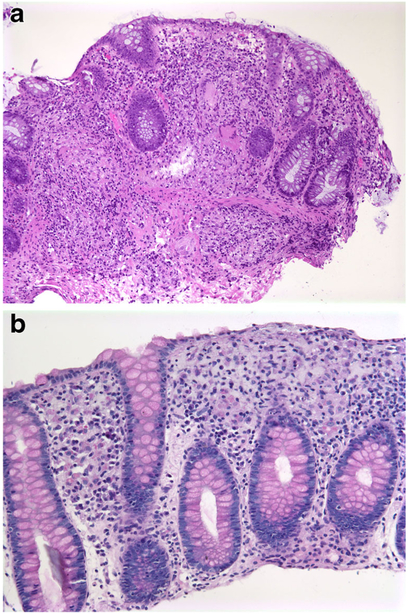
Enteroendocrine cell dysgenesis (ECD, enteric anendocrinosis) is a congenital deficiency of intestinal endocrine cells recently described by Wang et al. [35]. ECD is inherited as an autosomal recessive trait associated with mutations in Neurogenin 3 gene located on chromosome 10q21.3, which controls the developmental pathway of gut and pancreatic epithelial stem cells destined to become endocrine cells. Affected patients present with congenital diarrhoea and eventually develop type I diabetes [6, 7, 17, 35].
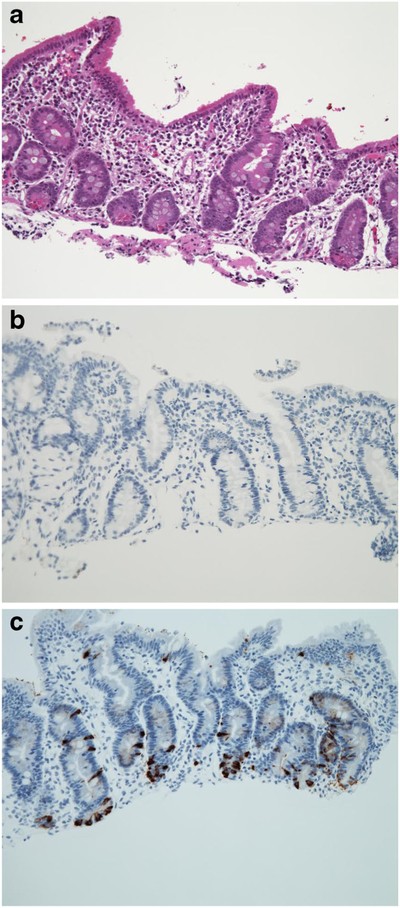
The intestinal mucosa demonstrates normal villus architecture, enterocytes with normal brush border, goblet cells and Paneth cells (Fig. 4a). Immunohistochemistry for chromogranin A reveals lack or paucity of enteroendocrine cells in the mucosa (Fig. 4b, c) with decreased intensity of staining [6, 7, 36]. A decreased number of enteroendocrine cells may also be seen in autoimmune enteropathies which show accompanying active inflammation and epithelial injury [17].
Fig. 4.
a Intestinal mucosa demonstrating normal villus architecture, enterocytes with normal brush border, goblet cells and paneth cells (H&E; ×200). b Lack of enteroendocrine cells in the small intestinal mucosa of a patient with EED (ChA IHC; 200). c Normal distribution of enteroendocrine cells in a healthy individual (ChA IHC; 200)
Eosinophilic gastroenteritis (EoGE) is a spectrum of diseases characterized by eosinophilic infiltration of various segments of the gastrointestinal tract together with peripheral eosinophilia and co-existing allergies to foreign antigens including cow’s milk, egg and soy proteins [37]. Symptoms vary depending on the location of the infiltrate within the bowel wall and include gastrointestinal bleeding, nausea, vomiting, abdominal pain and diarrhoea, while peripheral eosinophilia, hypoalbuminemia, anaemia and immunoglobulin E (IgE) elevation are often found in laboratory analysis [38, 39]. The underlying immune mechanism involves Th2 lymphocytes leading to an increased production of proallergenic interleukins, IL-4, IL-5, and IL13 which promote maturation of eosinophils and migration from the bone marrow into the circulation through upregulation of Eotaxin 3 by the epithelium, a chemokine responsible for attracting the eosinophils into the mucosa. As a result, mature eosinophils accumulate in the intestine, are activated and degranulated to release multiple cytotoxic agents leading to defective peristaltism and malabsorption in this condition. The disease may be patchy and the eosinophilic infiltrate may be mucosal, mural or serosal [40], mucosal involvement being the most commonwhereas mural form is more frequent in children with a predominance of antral involvement leading to gastric outlet obstruction [6, 7].
The intestinal biopsy shows varying degrees of villus blunting due to expansion of the lamina propria by eosinophilic infiltration (Fig. 5a). Since eosinophils are normal residents of the lamina propria, except for the oesophagus, as part of the innate immune system, it is difficult to determine the increase in eosinophils with confidence. The cut-off value for pathological eosinophilia has been variously defined between 20 and 80 per high-power field depending on the site involved within the gastrointestinal tract, the stomach having a slightly lower eosinophilic distribution than other sites, whereas higher densities (up to 30 eosinophils per high-power field) are commonly found in the appendix, terminal ileum, cecum and proximal colon. Thus, it is equally important to evaluate accompanying features such as villous atrophy due to epithelial damage [41, 42], intraepithelial eosinophils (Fig. 5b) forming eosinophilic microabscesses (Fig. 5c), and eosinophilic infiltration of the muscularis mucosae (Fig. 5d), and large numbers of degranulating mast cells. Other causes of eosinophilia including parasitic infections, immunodeficiencies, drug reactions, Crohn’s disease, connective tissue disorders and hypereosinophilic syndrome have to be carefully ruled out before the diagnosis of EoGE is made [6, 7]. Elimination or elemental diets and corticosteroid therapy are the treatments of choice, though some patients may show spontaneous remission [37, 43].
Fig. 5.
a Duodenal biopsy from a 10-year-old girl with a family history of allergy/atopy. Small intestinal mucosa shows villus blunting due to expansion of the lamina propria by heavy eosinophilic infiltration (H&E; ×100). b Eosinophilic infiltration of the crypt epithelium forming microabscesses (H&E; ×200)
Childhood enteropathies associated with immune dysregulation comprise of autoimmune enteropathies that include the immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and APS1 syndromes, and very early onset inflammatory bowel disease.
Autoimune enteropathy (AIE) is probably the most frequent disorder leading to infantile intractable diarrhoea [44]. Most cases occur in infancy or the first year of life, manifesting primarily with a secretory diarrhoea. There is a strong male preponderance, family history of other affected siblings, frequent extra-intestinal involvement and various circulating autoantibodies [45]. However, this entity has also been reported in older children, girls and adults [46].
The most consistently recognized association is with the X-linked syndrome of immunodysregulation, polyendocrinopathy and enteropathy (IPEX syndrome) initially described by Powell et al. [47]. Extra-intestinal disease in these patients includes insulin-dependent diabetes, thyroiditis, membranous glomerulopathy and interstitial nephritis in association with a number of autoantibodies including antinuclear antibody, anti-smooth muscle antibodies and antibodies against parietal cells, pancreatic islets, insulin, thyroglobulin and thyroid microsomes [44, 48]. This syndrome has been related to mutations in the FOXP3 gene (Xp11.23) [49], and humans bearing this mutation share a very similar phenotype to that of the scurfy mouse, with the absence of naturally occurring CD4+ CD25+ regulatory T cells (Tregs). Mutations in the FOXP3 gene or in its promoter region result in impaired suppression of lymphocyte proliferation by regulatory T cells following an initial inflammatory trigger, eventuating in an extensive autoimmune disorder [50, 51]. More recently, an expanding spectrum of autosomal gene defects that also affect Treg function, including loss of function mutations in CD25, STAT5b and ITCH and gain of function mutations in STAT1, has been described in patients with an IPEX-like phenotype [52].
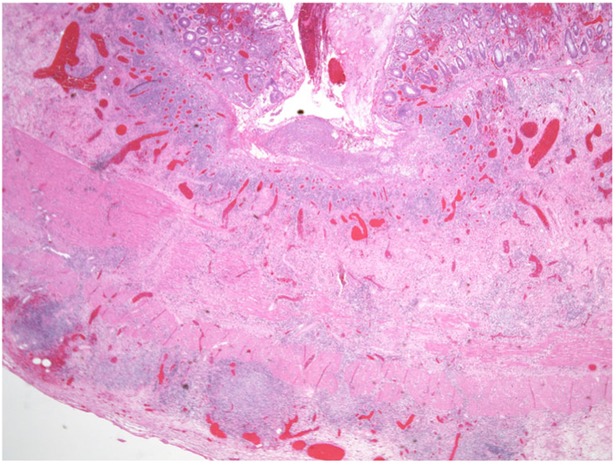
The entire GI tract is often involved in AIE, though the histopathological findings are most severe in the duodenum and small bowel where biopsies are usually characterized by marked villous atrophy, crypt hyperplasia and a mixed inflammatory infiltrate of the lamina propria. Inflammatory destruction of intestinal crypts with extensive apoptosis is a feature noted in many of the cases, similar to those noted in intestinal graft-versus-host disease, and confirms an immune-mediated attack against intestinal epithelium [48] (Fig. 6). A characteristic feature not routinely observed in other inflammatory intestinal diseases is depletion of goblet and Paneth cells. However, there can be marked variation in the histological features, with some cases, especially in older children or adults, showing milder degrees of intestinal damage (Fig. 7).In contrast to cases of gluten-sensitive enteropathy with flat villi, intraepithelial lymphocytes tend to be relatively few in number. A concomitant crypt-destructive colitis and gastritis are present in the majority of cases [53].
Fig. 6.
Duodenal biopsy from a 3-month-old boy with IPEX. There is a marked mucosal crypt-destructive inflammatory infiltrate with villous atrophy and absence of goblet and Paneth cells. The inset shows extensive basal crypt apoptosis
Fig. 7.
Duodenal biopsy from an 18-year-old female with a diffuse autoimmune disorder with thyroiditis, a protein-losing enteropathy and positive anti-enterocyte antibodies. There is partial villous atrophy and a milder inflammatory infiltrate as compared to the previous figure. This biopsy was initially interpreted as consistent with celiac disease. The patient had negative celiac serology and did not respond to a gluten-free diet. There is mild intraepithelial lymphocytosis; however, the absence of goblet and Paneth cells noted in this case is not a feature of celiac enteropathy
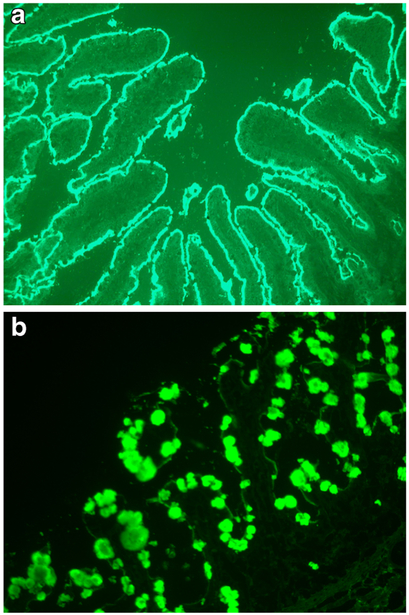
One of the hallmarks of this entity, noted since the first reports of AIE, is the presence of anti-enterocyte antibodies, detected by indirect immunofluorescence using the patient’s serum on frozen sections of normal bowel. Positive fluorescent staining results in a linear pattern along the apex and baso-lateral border of the enterocyte [1, 45, 54–56] (Fig. 8a). The antibodies are predominantly IgG and have been described as complement-fixing [55], though IgM and IgA have also been described [55, 57]. Enterocyte antigens harmonin (75 kDa USH1C protein) and villin (actin-binding 95 kDa protein) have been identified as targets of these autoantibodies, and the presence of these autoantibodies appears to differentiate IPEX from other childhood enteropathies [58]. However, the mechanisms responsible for autoimmunization and the role of these autoantibodies in IPEX are unknown, as the inflammatory mucosal injury is mainly T cell mediated. Antibodies reacting against mucus or goblet cells have also been described, and intestinal biopsies in these cases have shown a marked depletion of goblet cells [59, 60] (Fig. 8b). Of note, anti-enterocyte antibodies have also been described in adult AIDS patients without digestive symptoms [61], whereas anti-goblet cell antibodies have been detected in patients with chronic inflammatory bowel disease (Crohn’s and ulcerative colitis) and their first-degree relatives [62], and in a series of treated and untreated patients with celiac disease and controls [63]. The usefulness of these antibodies in the diagnosis of AIE in young infants is also questionable, given that there is little IgG production in the first 3 months of life and that IgG detected in the infant is likely maternal in origin [64].
Fig. 8.
a Indirect immunofluorescence using serum from a 4-month-old boy with IPEX syndrome on frozen sections of normal human small bowel. There is diffuse linear staining along the apical border of the enterocytes all along the villi. b Indirect immunofluorescence from another patient’s serum showing staining in goblet cells
The clinico-pathologic findings in AIE overlap extensively with other primary immunodeficiencies such as common variable immunodeficiency (CVID), where features such as increased basal apoptosis and depletion of goblet cells have also been described [65–67]. CVID may be distinguished in most cases by the relative paucity of inflammatory cells and the history of repeated infections. In the older patients, celiac disease is the most important consideration; a high index of suspicion for AIE should be present when there is a severe inflammatory pan-enteric crypt destructive process with villous atrophy without significantly increased intraepithelial lymphocytes, with increased apoptosis in the base of the crypts and loss of goblet and Paneth cells. In these patients, serologic testing for celiac disease is usually negative and the patients do not respond to a gluten-free diet. Testing for circulating anti-enterocyte antibodies may be useful in the older child and adult but is likely of more use limited in the young infant.
Mortality in cases of AIE has been high, occurring in up to one third of reported cases but more frequent in the older descriptions. Diet changes and steroids alone have been largely ineffective, and immunosuppression is required in children as well as adults [46]. A good response to sirolimus [68] and budenoside has been reported [69]. Bone marrow transplantation may be effective in some cases.
Autoimmune Polyendocrine syndrome Autoimmune polyendocrinopathy, candidiasis, ectodermal dystrophy (APECED) is an autosomal recessive disorder with heterogeneous clinical manifestations. It is caused by mutations in the AIRE gene, which codes for a transcription factor primarily expressed in medullary thymic epithelial cells where negative selection is thought to occur [70]. The type I form is characterized by various endocrinopathies of an autoimmune nature, often beginning in childhood or early teenage years, by chronic mucocutaneous candidiasis due to a T cell defect, starting soon after birth, and by dystrophy of ectodermal tissues [71]. Malabsorption can occur in up to 25% of patients and appears to result from destruction of intestinal endocrine cells [72] (Fig. 9). A specific deficiency of cholecystokinin-producing enteroendocrine cells has been reported in one patient, with seeming reappearance of the cells when the diarrhoea abated [73]. Small bowel biopsies show mild changes or can even be normal, in contrast with the crypt-destructive inflammation usually seen in autoimmune enteropathy.
Fig. 9.
a Duodenal biopsy from an 18-year-old patient with autoimmune polyendocrinopathy, candidiasis and ectodermal dystrophy (APECED) syndrome with malabsorption. H&E is unremarkable. b Immunostain with chromogranin shows loss of enteroendocrine cells (chromogranin stain on normal control (inset))
Very early onset inflammatory bowel disease Approximately 25% of incident cases of inflammatory bowel disease occur during childhood, most commonly diagnosed during the young teenage years. In about 15% of patients, the diagnosis is established prior to 6 years of age, with up to 6% diagnosed at less than 3 years of age [74]. This subgroup of IBD patients is referred to as “very early onset IBD (VEO-IBD)” and presents significant differences from IBD occurring in older children and in adults, including more severe clinical disease unresponsive to conventional IBD therapy and a greater proportion of cases featuring an underlying monogenic disorder [75]. Individuals with monogenic disorders may have features not typically associated with IBD, such as nail and hair anomalies, epidermolysis bullosa, and autoimmune hemolytic anaemia [76]. They may develop significant problems such as immunodeficiency, impacting treatment options, and a greater potential for receiving escalated treatment regimens involving extensive surgery and more intensive medical therapies rather than specific therapy directed toward the underlying defect [76]. It is also well-known that patients with primary immunodeficiencies such as common variable immunodeficiency (CVID), Wiskott-Aldrich syndrome (WAS) and chronic granulomatous disease may present with VEO-IBD [77, 78].
Recent advances in molecular technology, such as whole exome sequencing (WES), have allowed the discovery of genes and pathways associated with VEO-IBD [79]. These genes are for the most part different from the genetic variants found in genome-wide association studies of IBD in older children and adults [80]. The defects that are associated with VEO-IBD include genes involved in the intestinal epithelial barrier function, phagocyte bacterial killing, hyper or autoimmune inflammatory disorders, and function of the adaptive immune system [81] (Table 3). The landmark discovery of mutations involving the anti-inflammatory IL-10 cytokine and its receptors IL-10RA and IL10RB, resulting in severe infantile enterocolitis and perianal disease, was the first to demonstrate causal genetic defects in patients with VEO-IBD [82]. The essential role for IL-10 in limiting intestinal inflammation had already been demonstrated by the spontaneous development of severe colitis in IL-10-deficient mice [83]. From a pathologist’s perspective, intestinal histology in these patients may overlap with conventional IBD but may also present unique features, such as the acute inflammation and mucosal ulceration observed in patients with NEMO (IKBKG mutations) [84]. Chronic granulomatous disease (CGD), which results from defective bacterial clearing due impaired phagocytosis by intestinal granulocytes, may present with intestinal inflammation mimicking Crohn’s disease in as many as 40% of patients [85]. In contrast with typical Crohn’s disease, the histologic features in CGD frequently include multiple granulomas, sometimes necrotizing, with or without associated active colitis, and mucosal accumulation of pigmented macrophages during quiescent phases (Fig. 10). The therapies used to treat such patients differ from conventional IBD therapy. For example, anti-TNFα is contraindicated and can be fatal in these patients. Other therapies, including allogeneic stem cell transplantation, have demonstrated some success [86]. Defects that affect development or function of B and T cells, as occur with mutations in recombination activating genes (RAG1 or RAG2), in IL-7R (Omenn syndrome) and with the multiple gene defects associated with severe combined immunodeficiency (SCID), may be associated with severe intestinal disease. Patients with DOCK 8 (dedicator of cytokinesis 8 protein) deficiency, a combined T and B cell immunodeficiency syndrome, present with susceptibility to viral, fungal and bacterial infections in association with increased serum levels of IgE [87]. Some patients may present with chronic diarrhoea and severe intestinal disease (Fig. 11). Several autoinflammatory diseases have also been linked to VEO-IBD. These include mevalonate kinase deficiency [88], familial Mediterranean fever [89], Hermansky-Pudlak syndrome [90] and X-linked inhibitor of apoptosis protein (XIAP) [91]. XIAP is involved in NOD2-mediated NFKB signalling, and these children may have impaired ability to sense bacteria. In addition, as an inhibitor of apoptosis, it prevents apoptosis of activated T cells, allowing for expansion of T cells in response to pathogens [92] (Fig. 12).
Table 3.
Monogenic disorders associated with IBD-like pathology
| Epithelial barrier defects |
| Dystrophic EB |
| Kindler syndrome |
| X-linked ectodermal dysplasia |
| ADAM 17 deficiency |
| Familial diarrhoea |
| Neutropenia and phagocyte defects |
| CGD |
| CSD type 1 |
| Congenital neutropenia |
| Leukocyte adhesion deficiency |
| Hyper and autoinflammatory disorders |
| Mevalonate kinase deficiency |
| Phospholipase C2 defects |
| Familial Mediterranean fever |
| Familial macrophage activation syndrome |
| X-linked lymphoproliferative syndrome |
| Hermansky-Pudlak syndrome |
| Complex defects in T and B cell function (WAS, CVID, SCID) |
| Defects in regulatory T cells and IL-10 signalling |
Fig. 10.
a Colon biopsy from a 5-year-old boy with CGD with numerous granulomas. b Accumulation of PAS-positive diastase-resistant macrophages in the lamina propria during a quiescent phase
Fig. 11.
a Five-year-old boy with DOCK 8 deficiency and eosinophilic colitis. b Extensive apoptosis is noted in the crypts. c Cryptosporidium infection is also present
Fig. 12.
Patient with XIAP deficiency. One of multiple intestinal resections shows deep transmural inflammation with ulceration suggestive of Crohn’s disease
VEO-IBD, thus, represents a subset of intestinal inflammatory disorders, typically occurring in a younger age group, and enriched in rare monogenic disorders. Patients with VEO-IBD typically do not respond to conventional IBD therapy and require treatment ideally guided toward the specific defect and monitoring of their complications. The diagnosis of these unique disorders rests on the use of next-generation sequencing, and their identification not only offers these patients the possibility of “personalized medicine” but also deepens our insight into the function of the immune response.
The entities discussed in the above text, besides involving mainly small and large intestines, have one other feature in common: They occur mostly in the early days of life, thereby making the diagnosis extremely difficult for the clinicians as well as the pathologists. Gastrointestinal biopsies are more difficult to obtain in paediatric age group, particularly in newborn babies compared to adult patients. However, the improvements in endoscopic devices allow the paediatric gastroenterologists to obtain sufficient amounts of tissue during upper and lower GI endoscopy. Duodenal biopsy including the first (duodenal bulb) and second parts of the duodenum and multiple colonic biopsies from different sites including terminal ileum should be taken and sent for pathologic examination in separately coded vials containing formalin. In the case of suspicion of congenital enterocyte abnormalities or enzyme deficiencies, one piece should be sent for electron microscopy, while for lipid transport abnormalities, an additional fresh piece of biopsy should be sent to pathology for lipid stains. More importantly, however, a good clinicopathologic collaboration is crucial for the differential diagnosis of childhood enteropathies and very early onset IBD.
Acknowledgments
Funding No funding was received for the work presented in the manuscript.
References
- 1.Cutz E, Sherman PM, Davidson GP (1997) Enteropathies associated with protracted diarrhoea of infancy: clinicopathological features, cellular and molecular mechanisms. Pediatr Radiol 27(6): 523–527 [PubMed] [Google Scholar]
- 2.Avery GB, Villavicencio O, Lilly JR, Randolph JG (1968) Intractable diarrhoea in early infancy. Pediatrics 41(4):712–722 [PubMed] [Google Scholar]
- 3.Gambarara M, Diamanti A, Ferretti F, Papadatou B, Knafelz D, Pietrobattista A, Castro M (2003) Intractable diarrhoea of infancy with congenital intestinal mucosa abnormalities: outcome of four cases. Transplant Proc 35(8):3052–3053 [DOI] [PubMed] [Google Scholar]
- 4.Sherman PH, Mitchell DJ, Cutz E (2004) Neonatal enteropathies: defining the causes of protracted diarrhoea of infancy. J Pediatr Gastroenterol Nutr 38:16–26 [DOI] [PubMed] [Google Scholar]
- 5.Terrin G, Tomaiuolo R, Passariello A, Elce A, Amato F, Di Costanzo M, Castaldo G, Berni Canani R (2012) Congenital diarrheal disorders: an updated diagnostic approach. Int J Mol Sci 13: 4168–4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo P (2013) Enteropathies of infancy and childhood. Adv Pediatr Infect Dis 60:217–261 [DOI] [PubMed] [Google Scholar]
- 7.Russo P (2014) Enteropathies associated with chronic diarrhea and malabsorption in childhood In: Russo Pierre, Ruchelli Eduardo D., Piccoli David a (Eds.) pathology of pediatric gastrointestinal and liver disease 2nd edn Springer, berlin, pp 99–154 [Google Scholar]
- 8.Ensari A (2014) The malabsorption syndrome: its causes and consequences In: McManus LM, Mitchell RN (eds) Patholobiology of human disease. Elsevier, San Diego, pp 1266–1287 [Google Scholar]
- 9.Eğritaş O, Sari S, Dalgiç B, Poyraz A, Ensari A (2011) The diagnosis and outcomes of persistent diarrhea in infants aged 0-24 months—a Turkish cohort study. Turk J Gastroenterol 22(3):260–269 [PubMed] [Google Scholar]
- 10.Berni Canani R, Terrin G, Cardillo G, Tomaiuolo R, Castaldo G (2010) Congenital diarrheal disorders: improved understanding of gene defects is leading to advances in intestinal physiology and clinical management. J Pediatr Gastroenterol Nutr 50:360–366 [DOI] [PubMed] [Google Scholar]
- 11.Murch SH (2002) Toward a molecular understanding of complex childhood enteropathies. J Pediatr Gastroenterol Nutr 34(Suppl. 1):S4–S10 [DOI] [PubMed] [Google Scholar]
- 12.Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR (2000) The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr 20:663–697 [DOI] [PubMed] [Google Scholar]
- 13.Pautler D, Easley D, Pohl JF (2008) Abetalipoproteinemia. J Pediatr Gastroenterol Nutr 46:355–360 [DOI] [PubMed] [Google Scholar]
- 14.Khatun I, Walsh MT, Hussain MM (2013) Loss of both phospholipid and triglyceride transfer activities of microsomal triglyceride transfer protein in abetalipoproteinemia. J Lipid Res 54:1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, Meadows N, Quattrone A, Islam SA, Naoumova RP, Angelin B, Infante R, Levy E, Roy CC, Freemont PS, Scott J, Shoulders CC (2003) Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet 34(1):29–31 [DOI] [PubMed] [Google Scholar]
- 16.Peretti N, Sassolas A, Roy CC, Deslandres C, Charcosset M, Castagnetti J, Pugnet-Chardon L, Moulin P, Labarge S, Bouthillier L, Lachaux A, Levy E (2010) Guidelines for the diagnosis and management of chylomicron retention disease based on a review of literature and the experience of two centers. Orphanet J Rare Dis 5:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yee EU, Goldsmith GD (2013) Diarrheal illness in the pediatric population. A review of neonatal enteropathies and childhood idiopathic inflammatory bowel disease. Surg Pathol 6:523–543 [DOI] [PubMed] [Google Scholar]
- 18.Davidson GP, Barnes GL (1979) Structural and functional abnormalities of the small intestine in infants and young children with rotavirus enteritis. Acta Paediatr Scand 68:181–186 [DOI] [PubMed] [Google Scholar]
- 19.Uhnoo IS, Freihorst J, Riepenhofftalty M, Fisher JE, Ogra PL (1990) Effect of rotavirus infection and malnutrition on uptake of a dietary antigen in the intestine. Pediatr Res 27:153–160 [DOI] [PubMed] [Google Scholar]
- 20.Davidson GP, Cutz E, Hamilton JR, Gall DG (1978) Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology 75(5): 783–790 [PubMed] [Google Scholar]
- 21.Szperl AM, Golachowska MR, Bruinenberg M, Prekeris R, Thunnissen AM, Karrenbeld A, Dijkstra G, Hoekstra D, Mercer D, Ksiazyk J, Wijmenga C, Wapenaar MC, Rings EH, SC VIJ (2011) Functional characterization of mutations in the myosin Vb gene associated with microvillus inclusion disease. J Pediatr Gastroenterol Nutr 52:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller T, Hess MW, Schiefermeier N, Pfaller K, Ebner HL, Heinz-Erian P, Ponstingl H, Partsch J, Röllinghoff B, Köhler H, Berger T, Lenhartz H, Schlenck B, Houwen RJ, Taylor CJ, Zoller H, Lechner S, Goulet O, Utermann G, Ruemmele FM, Huber LA, Janecke AR (2008) MYO5B mutations cause microvillus inclusiondisease and disrupt epithelial cell polarity. Nat Genet 40(10):1163–1165 [DOI] [PubMed] [Google Scholar]
- 23.Wiegerinck CL, Janecke AR, Schneeberger K, Vogel GF, Van Haaften-Visser DY, Escher JC, Adam R, Thöni AC, Pfaller K, Jordan AJ, Weis C-A, Nijman IJ, Monroe GR, van Hasselt PM, Cutz E, Klumperman J, Clevers H, EES N, RHJ H, van Haaften G, Hess MW, Huber LA, Stapelbroek JM, Müller T, Middendorp S (2014) Loss of Syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology 147:65–68 [DOI] [PubMed] [Google Scholar]
- 24.Groisman GM, Amar M, Livne E (2002) CD10, a valuable tool for the light microscopic diagnosis of microvillous inclusion disease (familial microvillous atrophy). Am J Surg Pathol 26(7):902–907 [DOI] [PubMed] [Google Scholar]
- 25.Martin BA, Kerner JA, Hazard FK, Longacre TA (2014) Evaluation of intestinal biopsies for pediatric enteropathy. A proposed Immunohistochemical panel approach. Am J Surg Pathol 38: 1387–1395 [DOI] [PubMed] [Google Scholar]
- 26.Talmon G, Holzapfel M, Dimaio DJ, Muirhead D (2012) Rab11 is a useful tool for the diagnosis of microvillous inclusion disease. Int J Surg Pathol 20(3):252–256 [DOI] [PubMed] [Google Scholar]
- 27.Khubchandani SR, Vohra P, Chitale AR, Sidana P (2011) Microvillous inclusion disease-an ultrastructural diagnosis: with a review of the literature. Ultrastruct Pathol 35:87–91 [DOI] [PubMed] [Google Scholar]
- 28.Iancu TC, Mahajnah M, Manov I, Shaoul R (2007) Microvillous inclusion disease: ultrastructural variability. Ultrastruct Pathol 31(3):173–188 [DOI] [PubMed] [Google Scholar]
- 29.Goulet O, Salomon J, Ruemmele F, de Serres NP, Brousse N (2007) Intestinal epithelial dysplasia (tufting enteropathy). Orphanet J Rare Dis 20: 2:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sivagnanam M, Mueller JL, Lee H, Chen Z, Nelson SF, Turner D, Zlotkin SH, Pencharz PB, Ngan BY, Libiger O, Schork NJ, Lavine JE, Taylor S, Newbury RO, Kolodner RD, Hoffman HM (2008) Identification of EpCAM as the gene for congenital tufting enter-opathy. Gastroenterology 135(2):429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnell U, Kuipers J, Mueller JL, Veenstra-Algra A, Sivagnanam M, Giepmans BN (2013) Absence of cell-surface EpCAM in congenital tuftingenteropathy. Hum Mol Genet 22:2566–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reifen RM, Cutz E, Griffiths AM, Ngan BY, Sherman PM (1994) Tufting enteropathy: a newly recognized clinicopathological entity associated with refractory diarrhea in infants. J Pediatr Gastroenterol Nutr 18:379–385 [PubMed] [Google Scholar]
- 33.Lemale J, Coulomb A, Dubern B, Boudjemaa S, Viola S, Josset P, Tounian P, Girardet JP (2011) Intractable diarrhea with tufting enteropathy: a favorable outcome is possible. J Pediatr Gastroenterol Nutr 52:734–739 [DOI] [PubMed] [Google Scholar]
- 34.Kahvecioğlu D, Yildiz D, Kiliç A, Ince-Alkan B, Erdeve O, Kuloğlu Z, Atasay B, Ensari A, Yilmaz R, Arsan S (2014) A rare cause of congenital diarrhea in a Turkish newborn: tufting enteropathy. Turk J Pediatr 56(4):440–443 [PubMed] [Google Scholar]
- 35.Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, Hill ID, Vargas JH, Gershman G, Farmer DG, Reyen L, Martin MG (2006) Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med 355(3):270–280 [DOI] [PubMed] [Google Scholar]
- 36.Cortina G, Smart CN, Farmer DG, Bhuta S, Treem WR, Hill ID, Martin MG (2007) Enteroendocrine cell dysgenesis and malabsorption, a histopathologic and immunohistochemical characterization. Hum Pathol 38(4):570–580 [DOI] [PubMed] [Google Scholar]
- 37.Justinich CJ (2000) Update in gastrointestinal allergic diseases. Curr Opin Ped 12:456–459 [DOI] [PubMed] [Google Scholar]
- 38.Rothenberg ME (2004) Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol 113:11–28 [DOI] [PubMed] [Google Scholar]
- 39.Shaker M, Woodmansee D (2009) An update on food allergy. Curr Opin Pediatr 21(5):667–674 [DOI] [PubMed] [Google Scholar]
- 40.Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH (1970) Eosinophilic gastroenteritis. Medicine 49(4):299–319 [DOI] [PubMed] [Google Scholar]
- 41.Collins MH (2009) Histopathology associated with eosinophilic gastrointestinal diseases. Immunol Allergy Clin N Am 29(1): 109–117 [DOI] [PubMed] [Google Scholar]
- 42.Hurrell JM, Genta RM, Melton SD (2011) Histopathologic diagnosis of eosinophilic conditions inthe gastrointestinal tract. Adv Anat Pathol 18(5):335–348 [DOI] [PubMed] [Google Scholar]
- 43.Khan S, Orenstein SR (2008) Eosinophilic gastroenteritis. Gastroenterol Clin N Am 37(2):333–348 [DOI] [PubMed] [Google Scholar]
- 44.Montalto M, D’Onofrio F, Santoro L, Gallo A, Gasbarrini A, Gasbarrini G (2009) Autoimmune enteropathy in children and adults. Scand J Gastroenterol 44(9):1029–1036 [DOI] [PubMed] [Google Scholar]
- 45.Russo PA, Brochu P, Seidman EG, Roy CC (1999a) Autoimmune enteropathy. Pediatr Dev Pathol 2(1):65–71 [DOI] [PubMed] [Google Scholar]
- 46.Akram S, Murray JA, Pardi DS, Alexander GL, Schaffner JA, Russo PA, Abraham SC (2007) Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol 5(11):1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powell BR, Buist NR, Stenzel P (1982) An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr 100(5):731–737 [DOI] [PubMed] [Google Scholar]
- 48.Patey-Mariaud de Serre N, Canioni D, Ganousse S, Rieux-Laucat F, Goulet O, Ruemmele F, Brousse N (2009) Digestive histopathological presentation of IPEX syndrome. Mod Pathol 22(1):95–102 [DOI] [PubMed] [Google Scholar]
- 49.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27(1):20–21 [DOI] [PubMed] [Google Scholar]
- 50.Ochs HD, Gambineri E, Torgerson TR (2007) IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res 38(1— 3):112–121 [DOI] [PubMed] [Google Scholar]
- 51.Ochs HD, Torgerson TR (2007) Immune dysregulation, polyendocrinopathy, enteropathy, X-linked inheritance: model for autoaggression. Adv Exp Med Biol 601:27–36 [DOI] [PubMed] [Google Scholar]
- 52.Verbsky JW, Chatila TA (2013) Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr 25(6):708–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masia R, Peyton S, Lauwers GY, Brown I (2014) Gastrointestinal biopsy findings of autoimmune enteropathy: a review of 25 cases. Am J Surg Pathol 38(10):1319–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirakian R, Richardson A, Milla PJ, Walker-Smith JA, Unsworth J, Savage MO, Bottazzo GF (1986) Protracted diarrhoea of infancy: evidence in support of an autoimmune variant. Br Med J (Clin Res Ed) 293(6555):1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savage MO, Mirakian R, Wozniak ER, Jenkins HR, Malone M, Phillips AD, Milla PJ, Bottazzo GF, Harries JT (1985) Specific autoantibodies to gut epithelium in two infants with severe protracted diarrhoea. J Pediatr Gastroenterol Nutr 4(2):187–195 [DOI] [PubMed] [Google Scholar]
- 56.Unsworth J, Hutchins P, Mitchell J, Phillips A, Hindocha P, Holborow J, Walker-Smith J (1982) Flat small intestinal mucosa and autoantibodies against the gut epithelium. J Pediatr Gastroenterol Nutr 1 (4):503–513 [DOI] [PubMed] [Google Scholar]
- 57.Charritat JL, Polonovski C (1987) Pediatric autoimmune enteropathies with anti-cytoplasmic enterocytic auto-antibodies. Ann Pediatr (Paris) 34(3):195–203 [PubMed] [Google Scholar]
- 58.Lampasona V, Passerini L, Barzaghi F, Lombardoni C, Bazzigaluppi E, Brigatti C, Bacchetta R, Bosi E (2013) Autoantibodies to harmonin and villin are diagnostic markers in children with IPEX syndrome. PLoS One 8(11):e78664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore L, Xu X, Davidson G, Moore D, Carli M, Ferrante A (1995) Autoimmune enteropathy with anti-goblet cell antibodies. Hum Pathol 26(10):1162–1168 [DOI] [PubMed] [Google Scholar]
- 60.Rogahn D, Smith CP, Thomas A (1999) Autoimmune enteropathy with goblet-cell antibodies. J R Soc Med 92(6):311–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martín-Villa JM, Camblor S, Costa R, Arnaiz-Villena A (1993) Gut epithelial cell autoantibodies in AIDS pathogenesis. Lancet 342(8867):380. [DOI] [PubMed] [Google Scholar]
- 62.Folwaczny C, Noehl N, Tschöp K, Endres SP, Heldwein W, Loeschke K, Fricke H (1997) Goblet cell autoantibodies in patients with inflammatory bowel disease and their first-degree relatives. Gastroenterology 113(1): 101–106 [DOI] [PubMed] [Google Scholar]
- 63.Biagi F, Bianchi PI, Trotta L, Corazza GR (2009) Anti-goblet cell antibodies for the diagnosis of autoimmune enteropathy? Am J Gastroenterol 104(12):3112. [DOI] [PubMed] [Google Scholar]
- 64.Morell A, Skvaril F, Hitzig WH, Barandun S (1972) IgG subclasses: development of the serum concentrations in “normal” infants and children. J Pediatr 80(6):960–964 [DOI] [PubMed] [Google Scholar]
- 65.Daniels JA, Lederman HM, Maitra A, Montgomery EA (2007) Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol 31(12): 1800–12 [DOI] [PubMed] [Google Scholar]
- 66.Guerrerio AL, Frischmeyer-Guerrerio PA, Lederman HM, Oliva-Hemker M (2010) Recognizing gastrointestinal and hepatic manifestations of primary immunodeficiency diseases. J Pediatr Gastroenterol Nutr 51 (5):548–555 [DOI] [PubMed] [Google Scholar]
- 67.Singhi AD, Goyal A, Davison JM, Regueiro MD, Roche RL, Ranganathan S (2014) Pediatric autoimmune enteropathy: an entity frequently associated with immunodeficiency disorders. Mod Pathol 27(4):543–553 [DOI] [PubMed] [Google Scholar]
- 68.Yong PL, Russo P, Sullivan KE (2008) Use of sirolimus in IPEX and IPEX-like children. J Clin Immunol 28(5):581–587 [DOI] [PubMed] [Google Scholar]
- 69.Gentile NM, Murray JA, Pardi DS (2012) Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep 14(5):380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kampe O (2009) Introduction: autoimmune polyendocrine syndrome type 1 (APS-1): a rare monogenic disorder as a model to improve understanding of tolerance and autoimmunity. J Intern Med 265(5):511–513 [DOI] [PubMed] [Google Scholar]
- 71.Betterle C, Greggio NA, Volpato M (1998) Clinical review 93: autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab 83(4):1049–1055 [DOI] [PubMed] [Google Scholar]
- 72.Posovszky C, Lahr G, von Schnurbein J, Buderus S, Findeisen A, Schröder C, Schütz C, Schulz A, Debatin KM, Wabitsch M, Barth TF (2012) Loss of enteroendocrine cells in autoimmune-polyendocrine-candidiasis-ectodermal-dystrophy (APECED) syndrome with gastrointestinal dysfunction. J Clin Endocrinol Metab 97(2):E292–E300 [DOI] [PubMed] [Google Scholar]
- 73.Högenauer C, Meyer RL, Netto GJ, Bell D, Little KH, Ferries L, Santa Ana CA, Porter JL, Fordtran JS (2001) Malabsorption due to cholecystokinin deficiency in a patient with autoimmune polyglandular syndrome type I. N Engl J Med 344(4):270–274 [DOI] [PubMed] [Google Scholar]
- 74.Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, Winter HS, Fain P, King C, Smith T, El-Serag HB (2005) Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr 146(1):35–40 [DOI] [PubMed] [Google Scholar]
- 75.Uhlig HH (2013) Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut 62(12):1795–1805 [DOI] [PubMed] [Google Scholar]
- 76.Ashton JJ, Andreoletti G, Coelho T, Haggarty R, Batra A, Afzal NA, Beattie RM, Ennis S (2016) Identification of variants in genes associated with single-gene inflammatory bowel disease by whole-exome sequencing. Inflamm Bowel Dis 22(10):2317–2327 [DOI] [PubMed] [Google Scholar]
- 77.Glocker E, Grimbacher B (2012) Inflammatory bowel disease: is it a primary immunodeficiency? Cell Mol Life Sci 69(1):41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cannioto Z, Berti I, Martelossi S, Bruno I, Giurici N, Crovella S, Ventura A (2009) IBD and IBD mimicking enterocolitis in children younger than 2 years of age. Eur J Pediatr 168(2):149–155 [DOI] [PubMed] [Google Scholar]
- 79.Kammermeier J, Drury S, James CT, Dziubak R, Ocaka L, Elawad M, Beales P, Lench N, Uhlig HH, Bacchelli C, Shah N (2014) Targeted gene panel sequencing in children with very early onset inflammatory bowel disease—evaluation and prospective analysis. J Med Genet 51(11):748–755 [DOI] [PubMed] [Google Scholar]
- 80.Jostins L, Ripke S, Weersma RK et al. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491(7422):119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelsen JR, Baldassano RN, Artis D, Sonnenberg GF (2015) Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 1(5):462–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glocker EO et al. (2009) Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361(21):2033–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kühn R, Löhler J, Rennick D, Rajewsky K, Muller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75(2):263–274 [DOI] [PubMed] [Google Scholar]
- 84.Cheng LE, Kanwar B, Tcheurekdjian H, Grenert JP, Muskat M, Heyman MB, McCune JM, Wara DW (2009) Persistent systemic inflammation and atypical enterocolitis in patients with NEMO syndrome. Clin Immunol 132(1):24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenzweig SD (2008) Inflammatory manifestations in chronic granulomatous disease (CGD). J Clin Immunol 28(Suppl 1): S67–S72 [DOI] [PubMed] [Google Scholar]
- 86.Kato K, Kojima Y, Kobayashi C, Mitsui K, Nakajima-Yamaguchi R, Kudo K, Yanai T, Yoshimi A, Nakao T, Morio T, Kasahara M, Koike K, Tsuchida M (2011) Successful allogeneic hematopoietic stem cell transplantation for chronic granulomatous disease with inflammatory complications and severe infection. Int J Hematol 94(5):479–482 [DOI] [PubMed] [Google Scholar]
- 87.Aydin SE et al. (2015) DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol 35(2):189–198 [DOI] [PubMed] [Google Scholar]
- 88.Bianco AM, Girardelli M, Vozzi D, Crovella S, Kleiner G, Marcuzzi A. Mevalonate kinase deficiency and IBD: shared genetic background. Gut, 2014. 63(8) 1367–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuloğlu Z, Kansu A, Ustündağ G, Birsin Özçakar Z, Ensari A, Ekim M (2012) An infant with severe refractory Crohn’s disease and homozygous MEFV mutation who dramatically responded to colchicine. Rheumatol Int 32(3):783–785 [DOI] [PubMed] [Google Scholar]
- 90.Mora AJ, Wolfsohn DM (2011) The management of gastrointestinal disease in Hermansky-Pudlak syndrome. J Clin Gastroenterol 45(8):700–702 [DOI] [PubMed] [Google Scholar]
- 91.Worthey EA et al. (2011) Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med 13(3):255–262 [DOI] [PubMed] [Google Scholar]
- 92.Pedersen J, LaCasse EC, Seidelin JB, Coskun M, Nielsen OH (2014) Inhibitors of apoptosis (IAPs) regulate intestinal immunity and inflammatory bowel disease (IBD) inflammation. Trends Mol Med 20(11):652–665 [DOI] [PubMed] [Google Scholar]