Abstract
Primary progressive apraxia of speech (PPAOS) is a clinical syndrome in which apraxia of speech is the initial indication of neurodegenerative disease. Prior studies of PPAOS have identified hypometabolism, grey matter atrophy, and white matter tract degeneration in the frontal gyri, precentral cortex, and supplementary motor area (SMA). Recent clinical observations suggest two distinct subtypes of PPAOS may exist. Phonetic PPAOS is characterized predominantly by distorted sound substitutions. Prosodic PPAOS is characterized predominantly by slow, segmented speech. Demographic, clinical, and neuroimaging data (MRI, DTI, and FDG-PET) were analyzed to validate these subtypes and explore anatomic correlates. The Phonetic subtype demonstrated bilateral involvement of the SMA, precentral gyrus, and cerebellar crus. The Prosodic subtype demonstrated more focal involvement in the SMA and right superior cerebellar peduncle. The findings provide converging evidence that differences in the reliably determined predominant clinical characteristics of AOS are associated with distinct imaging patterns, independent of severity.
Keywords: primary progressive apraxia of speech, primary progressive aphasia, magnetic resonance imaging, diffusion tensor imaging, positron-emission tomography
1.0. Introduction
In 1967, Darley described a motor speech disorder characterized by varying combinations of slow speaking rate, syllable segmentation, abnormal prosody, distorted sound substitutions, additions, and prolongations, sometimes accompanied by groping and trial and error articulatory movements (Darley, 1967, 1969) The disorder was termed apraxia of speech (AOS), a motor speech disorder reflecting a problem with the planning and/or programming of speech. AOS is well recognized in the context of stroke, where onset is acute and the condition improves or becomes stable and chronic (McNeil, Robin, & Schmidt, 2009). AOS that is insidious in onset and progresses over time because of neurodegeneration is less well recognized and understood, although in recent years it has been recognized that AOS can be a feature, sometime a prominent feature, of the nonfluent or agrammatic variant of primary progressive aphasia (Gorno-Tempini et al., 2011; Ogar, Dronkers, Brambati, Miller, & Gorno-Tempini, 2007; Silveri et al., 2014).
For the past decade, patients with primary progressive apraxia of speech (PPAOS) patients have been the focus of a body of research. It has been demonstrated that AOS can be the earliest and sole manifestation of an underlying neurodegenerative disease; it was recently reported that the profile of PPAOS clinical characteristics can differ among affected patients (Josephs et al., 2014). In some cases, the speech pattern is dominated by distorted sound substitutions and additions, and other features attributable to articulatory difficulty, while in other cases the pattern is dominated by slow, prosodically segmented speech. The first profile has been designated as Phonetic (Articulatory) PPAOS and the second as Prosodic PPAOS [previously referred to as type 1 and 2, respectively (Josephs et al., 2013)]. Importantly, it appears that the PPAOS pattern subtype may have prognostic implications. In a recent small longitudinal study, it was observed that, although not broken down by subtype, in some PPAOS patients the AOS remained the most prominent feature for an average of seven years. Other patients developed a severe extrapyramidal syndrome resembling progressive supranuclear palsy (PSP) within five years, causing significant morbidity. Interestingly, this more aggressive course was associated with the Prosodic subtype (Josephs et al., 2014). At present, little more is known about these subtypes, including how they initially present and if there are differences in demographic, neurologic, neuroimaging, and neuropsychological profiles.
Prior studies of PPAOS, without regard to possible subtypes, have identified involvement of frontal gyri, precentral cortex, and SMA, with hypometabolism seen on fluorodeoxyglucose positron emission tomography imaging (FDG-PET) (Josephs et al., 2012; Josephs et al., 2006), grey matter atrophy on magnetic resonance imaging (MRI) (Josephs et al., 2013), and white matter tract degeneration on diffusion tensor imaging (DTI) (Whitwell et al., 2013). In autopsy data, PPAOS is consistently associated with tau biochemistry and is thought to be a primary tauopathy. In fact, autopsy-confirmed cases of both corticobasal degeneration (Josephs et al., 2006; Josephs et al., 2016) and progressive supranuclear palsy (Josephs et al., 2005; Josephs & Duffy, 2008) have been reported in patients who presented with a progressive AOS. Currently, it is not understood whether these imaging profiles and autopsy findings are shared between the different clinical presentations of PPAOS.
The goals of the current study included 1) detailing the demographic and clinical profiles of the perceptually distinct Phonetic (Articulatory) and Prosodic subtypes of PPAOS, 2) establishing a quantitative profile of salient features (i.e. auditory and visual) of AOS, and 3) verifying the concurrent validity of the auditorily distinct subtypes through examination of neuroimaging findings (atrophy on MRI, white matter tract integrity on DTI, and hypometabolism on FDG-PET).
2.0. Methods
2.1. Participants and Diagnostic Classification
This study was approved by the Mayo Institutional Review Board. All participants consented to research. From September 2010 to January 2017, twenty-one participants diagnosed with Primary Progressive Apraxia of Speech [ten Phonetic (Articulatory) PPAOS, hereafter referred to as Phonetic PPAOS; eleven Prosodic] were seen as part of a larger study. All participants had unequivocal evidence of AOS, which was the first noted symptom, and were classified through a consensus meeting by two speech-language pathologists (JRD and HMC/EAS), a neuropsychologist (MMM), and neurologist (KAJ). These individuals did not meet clinical criteria for any other neurologic diagnosis at initial testing [including, but not limited to, progressive supranuclear palsy (Litvan et al., 1996; Respondek et al., 2013), corticobasal syndrome (Armstrong et al., 2013; Boeve, Lang, & Litvan, 2003), or Alzheimer’s disease dementia (Albert et al., 2011; Dubois et al., 2014; McKhann et al., 1984)]. Importantly, while these patients may technically meet criteria for the nonfluent/ agrammatic variant of PPA, they do not meet the requisite root criteria for PPA, as outlined in the consensus criteria (Gorno-Tempini et al., 2011), as they were not aphasic.
For a clinical diagnosis of PPAOS, a patient must present with a chief complaint of progressive impairment of speech (i.e. AOS). Evidence of aphasia precludes a diagnosis of PPAOS. Dysarthria does not preclude a diagnosis of PPAOS, as long as the AOS is greater in severity. For this study, given that the goal was to examine the neuroimaging correlates of the different characteristics of AOS, patients with evidence of dysarthria (i.e., speech features consistent with any dysarthria type that do not overlap with features of AOS, such as strained voice quality, hypophonia, hypernasality, tremor) regardless of severity, were excluded to minimize confounded results. Additionally, participants were excluded if the AOS was too severe to validly and reliably to judge the presence or severity of the specific speech characteristics. Participant inclusion and exclusion are outlined in Figure 1. All participants were monolingual, with English as their native language. Of the 21 participants in this study, 15 (71%) were included in previous studies and 6 (29%) have not before been published.
Figure 1.
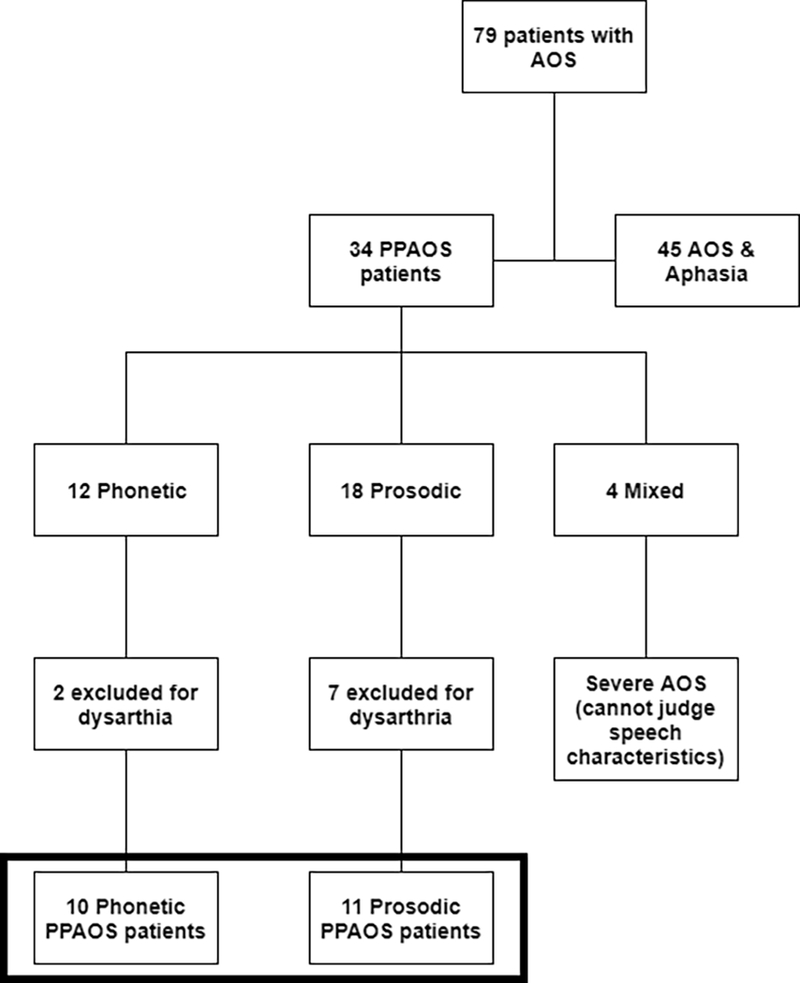
Flowchart of participant inclusion/ exclusion; patients included in the current study are noted by the shaded box.
Additionally, a cohort of 63 healthy, cognitively normal controls, without any neurologic deficits, was selected from the Mayo Clinic Study of Aging (MCSA) as a control cohort for the imaging analyses. The MCSA is an epidemiological study of cognitive aging in Olmsted County, Minnesota (Petersen et al., 2010; Roberts et al., 2008). The PPAOS cohort was matched on age and gender with MCSA participants (3 controls: 1 patient), who were all amyloid-negative (SUVR < 1.42) as determined by Pittsburgh Compound B (PiB) PET (Jack et al., 2013). All control and patient participants had 3-T volumetric MRI, DTI, FDG-PET, and PiB PET scans using identical acquisition parameters described in detail below (section 2.4).
2.2. Clinical Data
Clinical and demographic information of the patients were obtained. Neurologic testing was conducted by a behavioral neurologist (KAJ) and included assessments to characterize general cognitive ability [Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005), with a cut-off score of 26 or lower to indicate impairment], frontal lobe function [Frontal Assessment Battery (FAB) (Dubois, Slachevsky, Litvan, & Pillon, 2000)], neuropsychiatric features, [Neuropsychiatric Inventory Questionnaire (NPI-Q) (Kaufer et al., 2000)], and motor impairments [the Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale motor subsection (UPDRS III) (Goetz et al., 2008), and the apraxia subtest of the Western Aphasia Battery (WAB) (Kertesz, 2007)]. Non-verbal oral apraxia (NVOA) was assessed with an 8-item measure consisting of 4 gestures (“cough,” “click your tongue,” “blow,” “smack your lips”), each repeated twice. Each item received 0 to 4 points, with a score of 4 awarded for an immediate, accurate response; a score of 32 indicates no errors. Consistent with past research, a cutoff of 29 was used to establish the presence of NVOA (Botha et al., 2014).
A clinical neuropsychologist (MMM) oversaw test administration, scoring accuracy, and quality control of neuropsychological assessment. Tests were selected to assess different domains of cognitive function and to complement the speech and language battery. Domains tested include motor speed [Trail Making Test A (Spreen & Strauss, 1998)], executive function (Trail Making Test B), visuospatial and visuoperceptual function [Cube Analysis and Incomplete Letters of the Visual Object and Space Perception Battery (VOSP), respectively (Warrington & James, 1991)], and visual constructional skills [Rey-Osterrieth Complex Figure test (Rey-O) (Osterrieth, 1944; Rey, 1964)].
2.3. Speech and Language Data
Several speech and language measures were administered, as previously reported (Duffy et al., 2017; Duffy et al., 2015; Josephs et al., 2014; Josephs et al., 2013; Josephs et al., 2012). Judgments of speech included: (1) a 0–4 rating of AOS severity (1 = mild; 4 = severe), as an index of AOS severity regardless of its specific features, (2) a 1–10 rating (10 = normal) of motor speech disorder severity [MSD severity, adapted from (Yorkston, Strand, Miller, & Hillel, 1993)], which indexed the degree to which speech is understandable (i.e. functional impairment), (3) the Apraxia of Speech Rating Scale 3 (ASRS-3), detailed below (section 2.3.2), and (4) an Articulation Error Score (AES), also described below (section 2.3.3). Complete evaluations of oral structure and function were conducted. Strength, tone, and range of motion were normal, with no evidence of any associated dysarthria.
Language measures included the Western Aphasia Battery Aphasia Quotient (WAB-AQ) (Kertesz, 2007), which served as a composite measure of global language ability; it includes measures of repetition, naming, spontaneous speech fluency, word finding, grammatical competence, verbal and reading comprehension, and writing. A WAB-AQ score of 93.8 or above was considered normal, consistent with standard test guidelines. Grammar was assessed by review of conversational speech and verbal and written picture description tasks. The patients included in this study showed no evidence of agrammatism (e.g. omission of articles/ function words or syntax errors) in conversational speech, narrative picture description, and writing. The 15-item Boston Naming Test (BNT) (Lansing, Ivnik, Cullum, & Randolph, 1999) served as a sensitive measure of confrontation naming ability; a score of 13 and above was considered normal. As some of the WAB subtests can be influenced by non-aphasic deficits, including, but not limited to, apraxia of speech, participants were required to perform below normal on at least two measures of language, including demonstration of agrammatism in spoken language or writing, poor scores on the BNT, or reduced WAB-AQ. No patients in this study met criteria for aphasia.
2.3.1. PPAOS Subtype Assessment
Previous study of reliability for PPAOS subtype designation demonstrated 95% independent inter-rater agreement, with consensus achieved by the two SLPs after discussion on the remaining patients (Josephs et al., 2012). Additionally, a second independent consensus rating was achieved one-to-five years following initial consensus diagnosis. All patients included in this study were reliably rated as representative of each subtype at both consensus meetings. Specifically, a designation of Phonetic PPAOS was made if distorted sound substitutions or additions (often increasing in prominence with increased utterance length or syllable or word complexity) were judged to clearly dominate the speech pattern (i.e. were more prominent than the characteristics that dominate Prosodic PPAOS). A designation of Prosodic PPAOS was made if syllable segmentation or lengthened intersegment durations between syllables, words, or phrases were judged to clearly dominate the speech pattern (i.e. were more prominent than the characteristics that dominate Phonetic PPAOS). See Supplementary Videos for representative samples of each subtype. Importantly, the subtype designation is reflective of the pattern that predominates; it does not imply that it is the sole disruption of speech output. If there was no clear predominance of characteristics for Phonetic PPAOS or Prosodic PPAOS, the patient received a diagnosis of Mixed PPAOS (four patients were classified as Mixed and subsequently excluded from the current study). The presence, type, and severity of dysarthria were also assessed. To ensure any differences between groups were attributable to AOS, and not confounded by dysarthria, patients with any evidence of dysarthria were excluded from the study (See Figure 1).
Judgments of PPAOS subtype were based on spoken responses to the Spontaneous Speech subtests of the WAB (answers to basic questions and a narrative picture description) and several supplementary motor speech tasks that included vowel prolongation, speech alternating motion rates (e.g. rapid repetition of ‘puhpuhpuh’), speech sequential motion rates (e.g. rapid repetition of ‘puhtuhkuh’), word and sentence repetition tasks, and a conversational speech sample. The tasks most important to the classification of PPAOS subtype include narrative speech (picture description) and repetition of sentences and words of increasing length and phonetic complexity. The combination of these sources of speech responses allows for reliable judgments of PPAOS subtype (Josephs et al., 2013). The consensus classification of PPAOS subtype was made by the perceptual judgment of speech characteristics only; the primary rater who conducted the speech and language examination presented video recordings of the speech exam and the second rater was blinded to the examiner’s judgments.
2.3.2. Apraxia of Speech Rating Scale-3 (ASRS-3)
To provide a quantified description of the characteristics of AOS, the ASRS-3 was used to rate the presence or absence, relative prominence, and severity of associated characteristics (see Appendix). An earlier version of the ASRS-3 was used in previous studies of progressive AOS (Duffy et al., 2017; Duffy et al., 2015; Josephs et al., 2013; Josephs et al., 2012). The total ASRS-3 score can range from 0 to 52 (where 0 = no abnormal speech characteristics); it captures the severity of AOS but does not capture the specific quality of the AOS. For this study, those items that capture the salient features (i.e. auditory and visual) associated with each subtype were of particular interest. Toward that end, the sum of scores for the four items that best capture phonetic (articulatory) problems (items 1–4) minus the sum of scores for the four items that best capture prosodic abnormalities (items 5–8) was used to quantify the degree to which articulatory and prosodic abnormalities differed, and to further assess the reliability and validity of the non-quantified judgment of PPAOS subtype. In addition to showing the wide range of variability, this composite score minimizes the need for multiple comparisons and allows us to interpret the relative predominance of characteristics, independent of severity (i.e. value of the individual phonetic and prosodic variables).
2.3.3. Articulatory Error Score (AES)
An Articulatory Error Score (AES) was derived from the proportion of incorrectly produced words on the supplementary speech tasks (three repetitions of 13 words plus one repetition of three sentences for a total of 56 words of increasing length and phonetic complexity). Productions were counted as incorrect if any of the following characteristics were noted: distorted or undistorted sound substitutions, additions, or repetitions; sound omissions; sound prolongations (beyond those consistent with overall speech rate); false starts; and successful or unsuccessful attempts to correct sound errors. This error score may underestimate total abnormalities within and across words because a word scored as incorrect might contain more than one error, and because distortions that did not cross phonemic boundaries were not scored as incorrect. In general, it is expected that the AES score, on average, will be higher in the Phonetic group, capturing the predominance of their articulatory errors.
2.3.4. Reliability of Perceptual measures
Two raters (RLU and JRD) independently reviewed each patient’s examination, completed the ASRS-3, and calculated the AES. Krippendorff’s alpha coefficient for the ratings was calculated, where 1 suggests perfect reliability. Coefficients were 0.932 for the AES and 0.968 for the ASRS-3. For ratings on the ASRS-3, all discrepancies were resolved by reviewing the recordings and coming to a consensus score. For the AES, the average of the two ratings was utilized; all scores were within 5% of each other (capturing different ratings on 2/56 items). For the global AOS severity rating, there was 95% agreement, with all scores falling within a 0.5 rating on a 0–4 scale.
2.4. Imaging Data
2.4.1. Magnetic Resonance Imaging (MRI)
All participants underwent a standardized imaging protocol on 3T GE scanners. A 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) T1-weighted sequence was acquired (TR/TE/T1, 2300/3/900 ms; flip angle 8°, 26-cm field of view (FOV); 256 × 256 in-plane matrix with a phase FOV of 0.94, slice thickness of 1.2 mm, in-plane resolution 1.0 mm). Voxel-wise differences in gray matter atrophy patterns between participants with each PPAOS subtype and their respective sets of matched-controls were assessed using Voxel-Based Morphometry (VBM) with the Statistical Parametric Mapping (SPM) 12 package (Ashburner & Friston, 2000; Senjem, Gunter, Shiung, Petersen, & Jack, 2005). Briefly, SPM12 Unified Segmentation (Ashburner & Friston, 2005) was used to perform bias correction, segmentation, and spatial normalization of each MRI using the Mayo Clinic Adult Lifespan Template (MCALT), a publicly available population-matched template (www.nitrc.org/projects/mcalt/) (Schwarz et al., 2017). Gray matter probability images were transformed (with modulation) to template-space and smoothed (8mm FWHM Gaussian kernel) prior to performing voxel-based comparisons using SPM12.
2.4.2. Diffusion Tensor Imaging (DTI)
DTI acquisition consisted of a single-shot echo-planar pulse sequence in the axial plane, with TR ≈ 10 ms; in-plane matrix 128/128; FOV 35 cm; phase FOV 0.66 or 1.00; 2.7mm isotropic resolution, with 41 diffusion-encoding steps with weighting (b) = 1000 s/mm2 and four non-diffusion-weighted (b=0) T2 images. Parallel imaging with a SENSE factor of two was used. Scans were corrected for participant motion and residual eddy current distortion by affine registration of each volume to the first b=0 image. Weighted linear least squares optimization was used to fit diffusion tensors and fractional anisotropy (FA) images were calculated from the tensor eigenvalues using Dipy (Garyfallidis et al., 2014). A previously-published pipeline for voxel-wise comparisons across groups (Schwarz et al., 2014) was then utilized. Briefly, DTI-FA images were spatially normalized to a study-specific template using the ANTs package (Avants et al., 2010), masked to include only white matter voxels, and smoothed (8mm FWHM Gaussian kernel). Voxel-level comparisons between participants with each PPAOS subtype and their respective matched controls were then performed using SPM12 (Ashburner & Friston, 2000).
2.4.3. Fluorodeoxyglucose positron emission tomography (FDG-PET)
All participants underwent 18F-FDG-PET performed using a PET/CT scanner (GE Healthcare) operating in 3-dimensional mode. Participants were injected with 18F-FDG and after injection were allowed to wait in a comfortable chair for the 30-min uptake period, in a dimly lit room, without talking, moving, or sleeping. After the 30-min uptake period, an 8-min 18F-FDG scan was obtained consisting of four 2-min dynamic frames after a low-dose CT scan for attenuation correction. Individual frames of the dynamic series where excess motion was detected were omitted, and then a mean (summed) image was created. Corresponding MRI scans were co-registered using spm_coreg in SPM12 and used to automatically localize the pons region via the MCALT template (Schwarz et al., 2017). Intensity values of the FDG-PET images were normalized by the intensity in the pons to produce standardized uptake value ratio (SUVR) images. These FDG-PET SUVR images were spatially transformed to the MCALT template using normalization parameters previously computed from the co-registered MRI. Voxel-level comparisons of FDG-PET SUVR values between participants with each PPAOS subtype and their respective matched controls were then performed using SPM12 (Ashburner & Friston, 2000).
2.5. Statistical Analyses
Tests of differences in behavioral measures were performed using JMP (version 10.0.0; SAS Institute Inc.). Significance was assessed at p ≤ 0.05. Wilcoxon signed-rank tests were used to compare continuous data. Chi-Squared tests were used to compare categorical data.
3.0. Results
3.1. Demographic Data
Demographic information, including age, handedness, and disease duration is detailed in Table 1. All patients self-identified as Caucasian. Patients with Prosodic PPAOS were, on average, older (median age of 73 years) than Phonetic PPAOS patients (median age of 56.5 years) (p = .0066). There was a relatively even distribution of male and female patients in each subgroup. There was no statistically significant difference in disease duration (median= 2.8 years for Phonetic; median = 3 years for Prosodic). Only two patients were left-handed; both were Phonetic PPAOS. Median education was 16 years for Phonetic and 17 years for Prosodic PPAOS patients. Median amyloid burden, measured by global PiB ratio, was 1.2 in Phonetic and 1.3 in Prosodic patients (no statistically significantly difference between groups). Two Phonetic and one Prosodic patient had an overall global PiB ratio deemed positive for amyloid burden (with a cortical-to-cerebellar ratio cut-point of 1.5).
Table 1.
Demographic Data
| Subject | Subtype | Gender | Handedness | Education | Age at Onset* |
Disease Duration | PiB ratio |
|---|---|---|---|---|---|---|---|
| 1 | Phonetic | Male | Right | 10 | 60 | 2.5 | 1.23 |
| 2 | Phonetic | Female | Left | 18 | 58 | 1 | 1.12 |
| 3 | Phonetic | Female | Right | 18 | 51 | 10 | 1.79+ |
| 4 | Phonetic | Female | Right | 13 | 65 | 5 | 1.88+ |
| 5 | Phonetic | Male | Right | 18 | 54 | 2 | 1.18 |
| 6 | Phonetic | Male | Left | 20 | 62 | 3 | 1.12 |
| 7 | Phonetic | Male | Right | 16 | 55 | 2 | 1.39 |
| 8 | Phonetic | Male | Right | 12 | 49 | 4.75 | 1.24 |
| 9 | Phonetic | Female | Right | 12 | 68 | 2 | 1.38 |
| 10 | Phonetic | Female | Right | 16 | 49 | 7 | DNT |
| Median | 16 | 56.5 | 2.8 | 1.2 | |||
| St. Dev. | 3.0 | 6.6 | 2.8 | 0.3 | |||
| 11 | Prosodic | Female | Right | 13 | 77 | 4 | 1.23 |
| 12 | Prosodic | Female | Right | 15 | 74 | 1.5 | 1.35 |
| 13 | Prosodic | Female | Right | 12 | 78 | 1.5 | 1.45 |
| 14 | Prosodic | Male | Right | 17 | 56 | 5 | 1.31 |
| 15 | Prosodic | Female | Right | 15 | 76 | 3 | 1.97+ |
| 16 | Prosodic | Male | Right | 18 | 55 | 5 | 1.13 |
| 17 | Prosodic | Male | Right | 20 | 68 | 2 | 1.45 |
| 18 | Prosodic | Female | Right | 17 | 70 | 2.5 | 1.2 |
| 19 | Prosodic | Female | Right | 17.5 | 55 | 3 | 1.17 |
| 20 | Prosodic | Male | Right | 18 | 73 | 2 | 1.12 |
| 21 | Prosodic | Female | Right | 12 | 75 | 3.5 | 1.3 |
| Median | 17 | 73 | 3 | 1.3 | |||
| St. Dev. | 2.7 | 9.1 | 1.3 | 0.2 | |||
(Notes:
indicates significant difference between groups on Wilcoxon test;
PiB = Pittsburgh Compound B (PiB)
PET; global cortical PiB is noted as positive (+), with a cortical-to-cerebellar ratio cut-point of 1.5;
DNT = did not test).
3.2. Speech Data
Results of speech testing are detailed in Table 2. The ASRS-3 total score did not significantly differ between subgroups. This is consistent with the lack of differences in severity between subgroups in the overall AOS severity rating and functional impairment (as measured by the MSD severity rating). ASRS-3 phonetic and prosody sub-scores were calculated post-hoc to validate clinical subtyping judgments; consistent with our hypotheses, the ASRS-3 sub-scores for Phonetic (p = .0014; Cohen’s d = .612) and Prosody (p = .0008; Cohen’s d = .475) were statistically significant between groups in the expected direction. Not surprisingly, the Phonetic -Prosody composite score was also statistically significant between groups (p < .001; Cohen’s d = 1.76); the wide range of variability afforded by the composite score maximizes the power of these comparisons. When examining the ASRS items related to supplementary speech tasks and non-speech symptoms (items 9–13), there is a statistically, and likely clinically, significant difference between the groups on one item; a higher frequency of visible/ audible groping in the Phonetic group (ASRS item 12). The AES was statistically significant between groups (p = .0092; Cohen’s d = 1.08), with the Phonetic group having higher scores, reflective of more prominent difficulties with articulation.
Table 2.
Speech and Language Data
| Subject | Subtype | Phonetic Total (Items 1–4)* |
Prosody
Total (Items 5–8)* |
Phonetic – Prosody* |
ASRS- 3: Total |
AOS Severity (/4) |
AES* (% error) |
MSD Severity (/10) |
WAB AQ (/100) |
BNT (/15) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Phonetic | 3 | 2 | 1 | 6 | 0.5 | 5.4 | 8 | 99.8 | 15 |
| 2 | Phonetic | 3 | 0 | 3 | 4 | 1 | 25 | 9 | 96.2 | 14 |
| 3 | Phonetic | 13 | 11 | 2 | 29.5 | 3 | 71.5 | 6 | 94.6 | 14 |
| 4 | Phonetic | 14 | 7 | 7 | 25 | 3 | 82.9 | 4 | 95.2 | DNT |
| 5 | Phonetic | 6 | 3 | 3 | 10 | 1 | 21.4 | 8 | 99.4 | 14 |
| 6 | Phonetic | 5 | 2 | 3 | 9 | 1 | 7.1 | 8 | 97.4 | 15 |
| 7 | Phonetic | 6 | 0 | 6 | 8 | 1.5 | 37.5 | 6 | 97.2 | 14 |
| 8 | Phonetic | 12 | 6 | 6 | 22 | 2 | 51.8 | 6 | 96.6 | 12 |
| 9 | Phonetic | 3 | 5 | −2 | 11 | 1 | 12.5 | 8 | 99 | 15 |
| 10 | Phonetic | 15 | 5 | 10 | 18 | 3 | 33 | 5 | 98.8 | 15 |
| Median | 6 | 4 | 3 | 10.5 | 1.3 | 29 | 7 | 97.3 | 14 | |
| St. Dev. | 4.9 | 3.4 | 3.4 | 8.8 | 1.0 | 26.6 | 1.6 | 1.8 | 1.0 | |
| 11 | Prosodic | 2 | 10 | −8 | 14 | 1.5 | 8.05 | 7 | 100 | 13 |
| 12 | Prosodic | 4 | 8 | −4 | 14 | 1.5 | 13.4 | 7 | 98.7 | 14 |
| 13 | Prosodic | 3 | 9 | −6 | 14 | 1.5 | 26.8 | 7 | 97 | 14 |
| 14 | Prosodic | 4 | 10 | −6 | 15 | 1 | 9.8 | 8 | 100 | 15 |
| 15 | Prosodic | 0 | 7 | −7 | 11 | 1 | 0 | 8 | 100 | 15 |
| 16 | Prosodic | 2 | 7 | −5 | 12 | 1.5 | 17.9 | 7 | 97.6 | 15 |
| 17 | Prosodic | 3 | 9 | −6 | 16 | 1.5 | 9.8 | 7 | 98.8 | 15 |
| 18 | Prosodic | 1 | 8 | −7 | 12 | 1 | 7.1 | 7 | 96.3 | 15 |
| 19 | Prosodic | 2 | 8 | −6 | 11 | 1 | 8.9 | 8 | 95.8 | 13 |
| 20 | Prosodic | 2 | 7 | −5 | 11 | 1 | 8.9 | 8 | 99.8 | 15 |
| 21 | Prosodic | 2 | 11 | −9 | 15 | 1.5 | 8 | 7 | 96.4 | 15 |
| Median | 2 | 8 | −6 | 14 | 1.5 | 8.9 | 7 | 98.7 | 15 | |
| St. Dev. | 1.2 | 1.4 | 1.4 | 1.8 | 0.3 | 6.8 | 0.5 | 1.7 | 0.8 | |
(Notes:
indicates significant difference between groups on Wilcoxon test;
DNT = did not test;
ASRS = Apraxia of Speech Rating Scale;
AOS = Apraxia of Speech;
AES = Articulatory Error Score;
MSD = Motor Speech Disorder Severity Rating Scale;
WAB-AQ = Western Aphasia Battery- Aphasia Quotient;
BNT = Boston Naming Test, short form)
3.3. Language Data
Results of language testing are detailed in Table 2. Consistent with the overall consensus that aphasia was absent in all patients, there were no differences between groups on any language measures, including the WAB-AQ, and BNT. On the WAB-AQ, scores for all participants fell within the normal range. Scores for all WAB-AQ subtests are reported in Supplementary Table 1. On the BNT, one patient in the Phonetic PPAOS group scored a 12, while all other patients scores within the normal range; importantly, this patient reported a tenth grade education. No frank evidence of agrammatism was detected in any patient from either group.
3.4. Neurologic Data
Results of neurologic testing are detailed in Table 3. On the MOCA, the Phonetic group’s median score was 27.5, while the Prosodic group’s median score was 29 (out of a possible 30). Despite this statistically significant difference (p = .031), most patients in both groups scored within the normal range (seven of ten and ten of eleven Prosodic patients). There was no difference in group performance on the FAB, a test of frontal lobe function. There were no differences in limb apraxia, motor impairment, or behavioral changes; however, there was an overall higher presence of impaired motor function in the Prosodic group (noted by higher UPDRS III scores) and more evident behavioral/psychological symptoms in the Phonetic group (noted by higher NPI-Q scores). On the measure of non-verbal oral apraxia, no statistically significant difference was found between groups; four Phonetic patients and three Prosodic patients met criteria for a NVOA.
Table 3.
Neurologic Data
| Subject | Subtype | MoCA* (/30) |
FAB (/18) |
Apraxia (/60) |
UPDRS III (/120) |
NPI-Q Total (/10) |
NVOA (/32) |
|---|---|---|---|---|---|---|---|
| 1 | Phonetic | 28 | 18 | 60 | 5 | 3 | 32 |
| 2 | Phonetic | 22 | 15 | 58 | 11 | 5 | 21 |
| 3 | Phonetic | 27 | 18 | 57 | 5 | 5 | 14 |
| 4 | Phonetic | 26 | 17 | 57 | 16 | 6 | 19 |
| 5 | Phonetic | 30 | 17 | 58 | 5 | 0 | 31 |
| 6 | Phonetic | 27 | 18 | 60 | 8 | 0 | 32 |
| 7 | Phonetic | 28 | 18 | 60 | 9 | 4 | 31 |
| 8 | Phonetic | 25 | 16 | 59 | 5 | 9 | 24 |
| 9 | Phonetic | 28 | 17 | 59 | 3 | 1 | 31 |
| 10 | Phonetic | 29 | 18 | 59 | 20 | 3 | 30 |
| Median | 27.5 | 17.5 | 59 | 6.5 | 3.5 | 30.5 | |
| St. Dev. | 2.3 | 1.03 | 1.2 | 5.5 | 2.8 | 6.3 | |
| 11 | Prosodic | 28 | 16 | 58 | 22 | 0 | 32 |
| 12 | Prosodic | 28 | 17 | 57 | 6 | 1 | 32 |
| 13 | Prosodic | 29 | 18 | 49 | 5 | 0 | 32 |
| 14 | Prosodic | 30 | 16 | 60 | 1 | 3 | 32 |
| 15 | Prosodic | 30 | 17 | 59 | 24 | 2 | 29 |
| 16 | Prosodic | 30 | 18 | 60 | 8 | 0 | 28 |
| 17 | Prosodic | 29 | 17 | 57 | 14 | 2 | 25 |
| 18 | Prosodic | 23 | 16 | 40 | 22 | 1 | 14 |
| 19 | Prosodic | 29 | 18 | 55 | 33 | 6 | 32 |
| 20 | Prosodic | 29 | 16 | 59 | 4 | 4 | 31 |
| 21 | Prosodic | 29 | 18 | 59 | 13 | 1 | 30 |
| Median | 29 | 17 | 58 | 13 | 1 | 31 | |
| St. Dev. | 2.0 | 0.9 | 6.1 | 10.2 | 1.9 | 5.2 | |
(Note:
indicates significant difference between groups on Wilcoxon test;
MoCA = Montreal Cognitive Assessment;
FAB = Frontal Assessment Battery;
Apraxia = apraxia subtest of the Western Aphasia Battery;
UPDRS III = the Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale motor subsection;
NPI-Q = Neuropsychiatric Inventory Questionnaire;
NVOA = Non-verbal oral apraxia)
3.5. Neuropsychological Data
Results of neuropsychological testing are detailed in Table 4. Statistical comparisons revealed a statistically significant difference in performance on Trails A (p = .031), with the Prosodic PPAOS patients, on average, taking longer to complete the task. This was possibly mediated by the Prosodic group’s more prominent motor symptoms. Although there was no significant difference in group performance on Trails B, the Prosodic PPAOS patients also, on average, took longer to complete that task. There were also no differences between groups on the Rey-Osterrieth Complex Figure Copy task, VOSP letters, or VOSP cube tasks.
Table 4.
Neuropsychological Data
| Subject | Subtype | Trails A* | Trails B | Rey-O | VOSP Letters | VOSP Cube |
|---|---|---|---|---|---|---|
| 1 | Phonetic | 47 | 90 | 33 | 20 | 10 |
| 2 | Phonetic | 48 | 210 | 15 | 19 | 6 |
| 3 | Phonetic | 28 | 63 | 36 | 20 | 10 |
| 4 | Phonetic | 44 | 186 | 24.5 | 19 | 8 |
| 5 | Phonetic | 26 | 84 | 35 | 20 | 10 |
| 6 | Phonetic | 20 | 52 | 29 | 20 | 10 |
| 7 | Phonetic | 27 | 115 | 25 | 20 | 10 |
| 8 | Phonetic | 19 | 60 | 29.5 | 20 | 8 |
| 9 | Phonetic | 33 | 74 | 24 | 20 | 10 |
| 10 | Phonetic | 47 | 89 | 25 | 20 | 10 |
| Median | 30.5 | 86.5 | 27 | 20 | 10 | |
| St. Dev. | 11.6 | 53.9 | 6.3 | 0.4 | 1.4 | |
| 11 | Prosodic | 49 | 124 | 25.5 | 20 | 10 |
| 12 | Prosodic | 43 | 104 | 31 | 20 | 10 |
| 13 | Prosodic | 52 | 122 | 32 | 20 | 10 |
| 14 | Prosodic | 26 | 68 | 35 | 20 | 10 |
| 15 | Prosodic | 56 | 119 | 21 | 20 | 10 |
| 16 | Prosodic | 29 | 65 | 31 | 19 | 9 |
| 17 | Prosodic | 64 | 154 | 31 | 20 | 10 |
| 18 | Prosodic | 55 | 111 | 23 | 20 | 8 |
| 19 | Prosodic | 46 | 85 | 20 | 20 | 10 |
| 20 | Prosodic | 33 | 78 | 34 | 20 | 10 |
| 21 | Prosodic | 53 | 154 | 36 | 18 | 10 |
| Median | 49 | 111 | 31 | 20 | 10 | |
| St. Dev. | 12.1 | 31.2 | 5.7 | 0.7 | 0.7 | |
(Notes: Raw scores;
indicates significant difference between groups on Wilcoxon test;
Trails A = Trail Making Test A;
Trails B = Trail Making Test B;
Rey-O = Rey-Osterrieth Complex Figure test;
VOSP = Visual Object and Space Perception Battery subtest)
3.6. Imaging Data
3.6.1. MRI
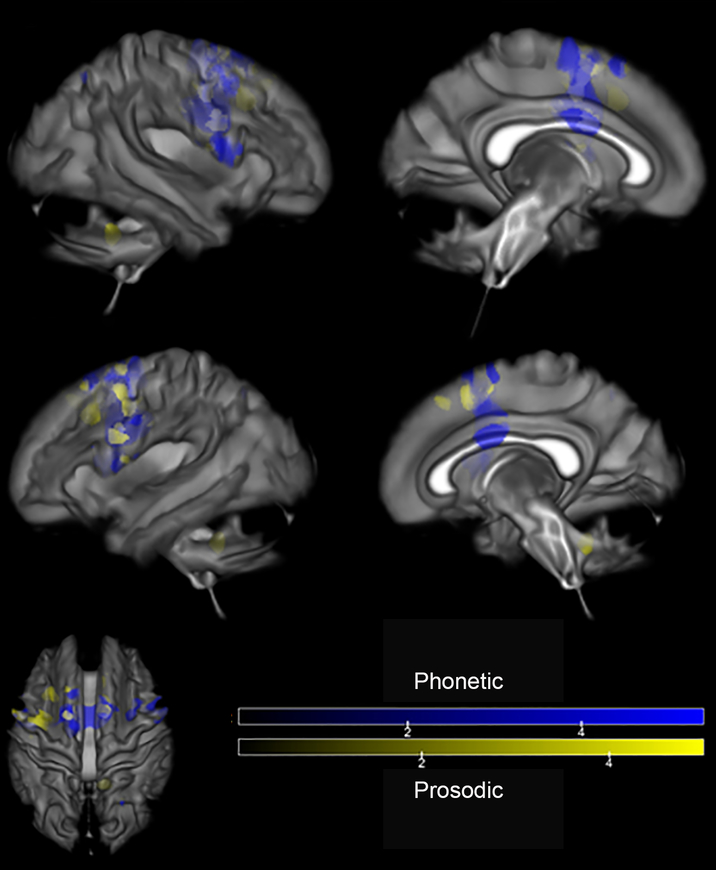
From the MRI analysis (uncorrected, p < .001), areas of atrophy in the PPAOS groups compared to their respective controls are shown in Figure 2. In both groups, there was involvement of the left supplementary motor area (SMA). While the Prosodic group demonstrated focal atrophy of the SMA, the atrophy in the Phonetic group was also seen in the prefrontal cortex. Additionally, there was atrophy in the cerebellum (AAL atlas region VIIB) and cerebellar crus (AAL atlas region Crus 2) in the Phonetic group. SPM values, t-values, and coordinates of peaks for Phonetic and Prosodic group comparisons are provided in Supplementary Tables 2 and 3, respectively. A direct comparison of Phonetic and Prosodic groups requires including age as a covariate; with this, the analysis did not yield any significant results.
Figure 2.
Areas of greater atrophy in the PPAOS groups compared to their respective controls, resulting from MRI analyses (uncorrected, p < .001). Results are displayed on a semitransparent 3D brain render, made using MRICroGL.
3.6.2. DTI
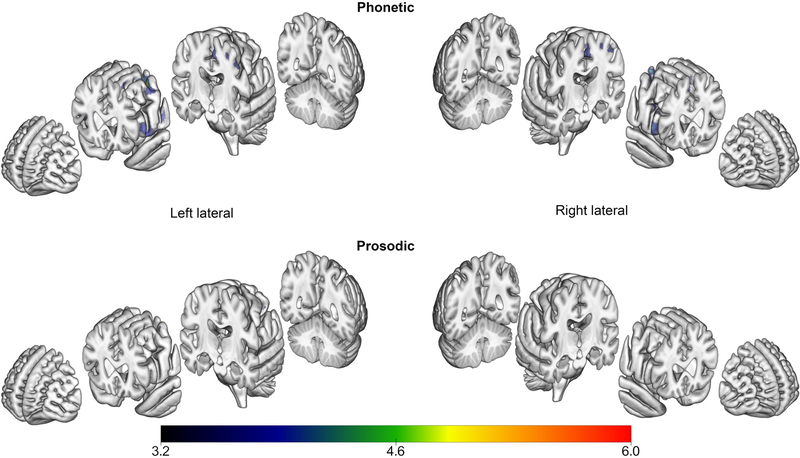
Reduced fractional anisotropy was observed in patients, compared to their respective controls (FWE corrected, p < .05), in the white matter underlying the left SMA in both groups, with more prominent and bilateral changes in the Phonetic group (see Figure 3). Uncorrected (p < .001) results are shown in Supplementary Figure 1. The effect in the Phonetic group extends into the superior frontal region and to the body of the corpus callosum and cingulum bilaterally. The Prosodic group showed reduced FA in the region of the right superior cerebellar peduncle, not seen in the Phonetic group. There were no areas of reduced fractional anisotropy in controls when compared to patients. SPM values, t-values, and coordinates of peaks for Phonetic and Prosodic group comparisons are provided in Supplementary Tables 4 and 5, respectively. A direct comparison of Phonetic and Prosodic groups requires including age as a covariate; with this, the analysis did not yield any significant results.
Figure 3.
Regions of reduced white matter tract integrity (measured by DTI fractional anisotropy) in PPAOS subgroups, compared to their respective controls (FWE corrected, p < .05). Results are displayed on a semitransparent 3D brain render, made using MRICroGL. Affected areas are shown in blue for Phonetic and yellow for Prosodic.
3.6.3. FDG-PET
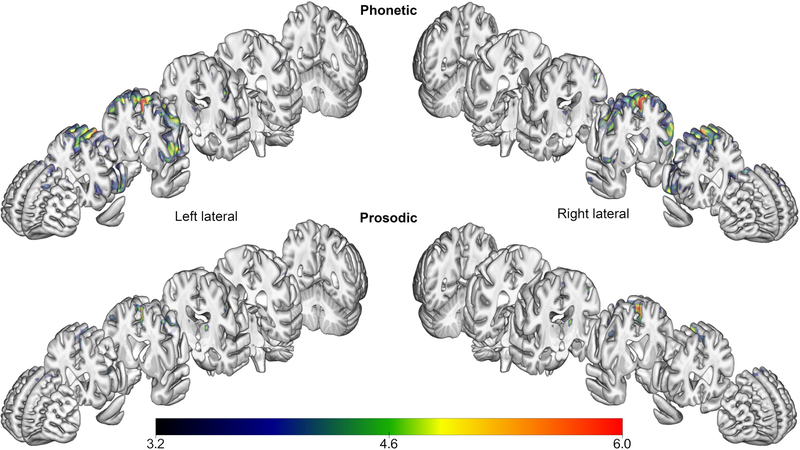
Areas of hypometabolism seen in PPAOS subgroups, compared to their respective controls, can be seen in Figure 4 (FDR, p < .05). In both PPAOS groups, there was hypometabolism in the left SMA. Hypometabolism in the Phonetic group extends to the superior frontal regions bilaterally, insular, and additionally shows involvement in the right cerebellum (AAL atlas region Crus 2). There were no areas of reduced hypometabolism in controls when compared to PPAOS. SPM values, t-values, and coordinates of peaks for Phonetic and Prosodic group comparisons are provided in Supplementary Tables 6 and 7, respectively. A direct comparison of Phonetic and Prosodic groups requires including age as a covariate; with this, the analysis did not yield any significant results.
Figure 4.
Areas of hypometabolism on FDG-PET in PPAOS subgroups, compared to their respective controls (FDR, p < .05). Results are displayed on a semitransparent 3D brain render, made using MRICroGL.
4.0. Discussion
The study supports previous work establishing that neurodegenerative AOS can exist in the absence of aphasia, dysarthria, or any neurological abnormalities that meet criteria for other neurodegenerative disease (e.g., CBS, PSP). While there are no population prevalence estimates for PPAOS, a recent review suggests that 20% of patients diagnosed with the nonfluent variant of primary progressive aphasia (PPA), progressive nonfluent aphasia, or PPA- not otherwise specified, had little to no evidence of aphasia, but rather an isolated apraxia of speech (Duffy, Strand, & Josephs, 2014). These patients would be more accurately classified as PPAOS. The abnormal speech characteristics identified in this study are consistent with features commonly associated with AOS due to stroke and other non-neurodegenerative causes (Ballard et al., 2015; Duffy, 2013; Duffy & Josephs, 2014; McNeil et al., 2009). Although previous work has raised the possibility of subtypes of stroke-induced AOS (Kent & Rosenbek, 1983) and neurodegenerative AOS (Josephs et al., 2013), the current study is the first to formally examine and validate two clinically distinguishable subtypes of PPAOS. That is, the two primary problems reflected in broad characterizations of AOS - articulation and prosody - can be perceptually dissociated to varying degrees in PPAOS, as demonstrated by the differences in ratings of a number of phonetic and prosodic features on the ASRS-3. Difference in overall severity between the phonetic and prosodic subtypes is an unlikely explanation for the subtype differences because neither global AOS severity ratings nor ratings of functional impairment differed between the two subtype groups. It is possible that the two subtypes may be most easily appreciated at mild-moderate levels of severity, as seen in this cohort; the distinction may blur (as it does with variants of primary progressive aphasia) as PPAOS becomes more severe.
4.1. Clinical speech, language, neuropsychological, and non-speech motor features
The different manifestations of AOS in this cohort were reliably detected by independent raters and during consensus meetings; this reliability is critical because the clinical identification of the salient features (i.e. auditory and visual) of AOS served as the gold standard for this study. The sub-scores of the ASRS-3, designed to quantify the presence and severity of different aspects of AOS, support the gestalt diagnosis, such that all patients designated as Prosodic had higher Prosody sub-scores and nine of ten Phonetic patients had higher Phonetic sub-scores. The data supports the notion that the extent of Prosodic and Phonetic differences can vary among patients, reflected in the wide range of Phonetic-Prosody composite score on the ASRS-3. It is hypothesized that as severity of AOS increases, the distinction between the subtypes may blur, but this must be assessed through longitudinal study. The notion of subtypes described here serves as a starting point; it is not proposed that the predominant perceptual features occur in isolation, but rather that their predominance early in the disease course may herald the condition to follow. That is, the subtypes may predict the nature or rate of disease evolution and the type of additional deficits that may eventually emerge (Josephs et al., 2014).
The Prosodic patients, on average, were significantly older than the Phonetic patients. Research on healthy aging suggests that different areas in the speech-language network may vary in their vulnerability to natural senescence (notably white and gray matter changes in the inferior frontal cortex and to a lesser extent the premotor cortex) (Yang et al., 2014). Such an interaction between age and disease pathophysiology is seen in Alzheimer’s disease, where the same pathology results in different clinical presentations, strongly associated with age (Barnes et al., 2015; Dickerson, Brickhouse, McGinnis, & Wolk, 2016). By extension, one hypothesis for the PPAOS subgroup differences might be that they reflect an interaction between similar pathophysiology (e.g., tau pathology within the speech planning/programming network) and age-related vulnerability in somewhat different portions of that network.
It is important to note that at initial presentation, there were no differences in language function between the two subgroups; no member in either group had aphasia. This supports considering PPAOS as a distinct entity of motor planning and programming and not a disorder of language (Josephs et al., 2012). While the Phonetic group had lower scores on the MOCA, a measure of general cognition; the mechanism for this difference was largely from the subsection of the MOCA that requires repetition of phrases (a total of 2 points for this section). To receive points, the patient must provide an exact repetition of two lengthy sentences. No points are awarded if there are any errors, including unintelligible responses. While this was intended to reflect cognitive and language abilities, it is possible this is biased against the Phonetic patients whose sound level (articulatory) errors were responsible for lower scores on this task. Prosodic patients, whose rate and rhythm are abnormal, while articulation is relatively preserved, carry an advantage to complete this task free of error. However, while the MOCA score differences may have been contaminated by the specific characteristics of speech difficulties, such difficulties are unlikely to account for the trend toward differences in the neuropsychiatric inventory- questionnaire, designed to index behavioral or other psychiatric symptoms. While none of the PPAOS patients had a formal diagnosis of behavioral or other psychiatric problems, two of the eleven Prosodic patients and five of the ten Phonetic patients had scores ranging from four to nine (ten or above reflects the presence neuropsychiatric deficits). This trend may portend development of other behavioral or cognitive changes among patients with Phonetic PPAOS, such as anxiety and depression. Finally, the data support that NVOA occurs frequently, but not invariably, with PPAOS (Botha et al., 2014; Josephs et al., 2012), but without differences between the Phonetic and Prosodic subgroups.
Although not statistically different between the subgroups, there was an overall higher presence of impaired motor function in the Prosodic group, as reflected in their higher UPDRS III scores. These higher scores are primarily attributable to slowing and reduced amplitude of extremity AMRs (finger, hand, pronation, toe tap, and leg). A final observation is a difference in inclusion and exclusion features that yielded the sample for the study. There were a larger number of patients with Prosodic PPAOS who were excluded because of co-occurring dysarthria (seven of eighteen patients with Prosodic PPAOS, without aphasia) compared to Phonetic PPAOS (two of twelve patients with Phonetic AOS, without aphasia). This is a possible consequence of the aforementioned decline in motor function that appears more prominent in the Prosodic group. Perhaps this reflects the finding of a longitudinal study that suggests a subset of PPAOS patients evolve to a syndrome reminiscent of progressive supranuclear palsy (Josephs et al., 2014). This likely will be clarified through longitudinal study of the evolution of deficits within and between the subgroups.
4.2. Imaging Findings
The literature has documented consistent patterns of hypometabolism on FDG-PET (Josephs et al., 2012; Josephs et al., 2006), atrophy on MRI, and white matter tract degeneration on diffusion tensor imaging (Whitwell et al., 2013) in patients with PPAOS compared to controls. The current study supports this converging evidence while at the same time suggesting that these global imaging patterns may differ depending on the specific profile of the speech deficits (i.e., PPAOS subtypes).
A consistently shared imaging finding between the PPAOS subtypes is the involvement of premotor regions, in particular the SMA. The SMA has a known role in the preparation and initiation of voluntary movements, including speech (Duffy, 2013). Specifically, the SMA has been implicated in the transition between the conceptualization of a word to different aspects of speech production, including articulation and prosody (Hertrich, Dietrich, & Ackermann, 2016). In addition to experimental findings, theories of speech motor control also recognize the SMA as influencing the initiation of speech (Tourville & Guenther, 2011). It is unclear if further parcellation of the SMA to the pre-SMA (anterior portion) and SMA proper would provide further insight; some data suggest the pre-SMA is differentially involved in the earlier, higher levels of motor planning (Alario, Chainay, Lehericy, & Cohen, 2006). In the future, larger sample sizes, and finer-grained imaging analyses, will allow us to make more confident estimations of relative contributions of different portions of the SMA to disrupted aspects of speech production in PPAOS.
The results seen on MRI, FDG, and DTI in the Phonetic group suggest bilateral involvement, spanning the frontal gyri, precentral cortex, and SMA. This relatively widespread involvement is a possible harbinger for behavior, language, and cognitive changes in the Phonetic group (discussed above). This is unlikely to purely reflect clinical severity, as there were no significant differences between the two subgroups on the AOS severity rating and ASRS totals, intended to capture the severity of the apraxia of speech, nor on the MSD severity rating, intended to capture the severity of the associated functional impairment. In fact, the Prosodic group demonstrated worse overall ASRS totals. This supports the notion that the more diffuse anatomical involvement in the Phonetic group yields a difference in the quality of impairment and not the severity of the impairment. Further, the FDG analyses showed hypometabolism in the insula in the Phonetic group that was not seen in the Prosodic group. In the stroke literature, insular damage has been linked to AOS (Ogar et al., 2006). While prosodic versus articulatory differences have not been explicitly addressed in other studies of AOS, general descriptions suggest that stroke-induced AOS may be predominated by articulatory errors versus prosodic errors, or that the AOS is mixed in the distribution of phonetic and prosodic features (Dronkers, 1996). At this time, descriptions of the quality of AOS associated with stroke is somewhat lacking in the literature; formal comparison of the speech features of stroke-induced and degenerative AOS warrants continued study.
There are some limitations to this current study. The inherent age difference in the cohorts suggests comparing each clinical subtype to their respective age-sex matched controls is the best means to ameliorate any potential age effects. For this reason, as well as the underpowered sample, direct imaging comparisons between the PPAOS types were not significant. Also given the sample size, and the large number of regions of interest, correlations between the behavioral measures and imaging are deferred until we have a larger cohort with which to explore potential relationships. While the sample size was small, it is likely the largest sample yet reported, and it allows for some insight into the anatomical and pathological correlates of different clinical subtypes of PPAOS. These patients will be followed longitudinally to allow for documentation of how their presentations evolve. Ultimately, autopsy confirmation will reveal the underlying pathology in these patients.
5.0. Conclusions
In this study, it is demonstrated that PPAOS has two differing clinical presentations - one predominated by prosodic abnormalities and the other predominated by phonetic (articulatory) disturbances - with converging supportive imaging evidence. The clinical methods developed to quantify these distinctions (ASRS-3 and AES) will permit more reliable and refined diagnosis of PPAOS. The findings support the need for continued study of these differing clinical presentations as it relates to the evolution of the clinical syndrome, including whether there is differential emergence of language, cognitive, or motor difficulties, with subsequent implications for patient counseling and care planning. Ultimately, autopsy data will confirm the distribution and type of pathologies underpinning these different clinical presentations and determine the degree to which PPAOS subtypes predict underlying pathology.
Supplementary Material
6.0. Acknowledgments
We would like to acknowledge the patients who participated in this study and their family members who made their participation possible. We express gratitude to Sarah Boland, for her work as study psychometrist and study coordinator.
7.0 Funding
The study was funded by NIH grants R01 DC010367 (PI: Josephs), R21 NS94684 (PI: Josephs), R01 DC12519 (PI: Whitwell), R01 NS89757 (PIs: Josephs and Whitwell), U01 AG006786 (PI: Petersen), The Elsie and Marvin Dekelboum Family Foundation, and a grant from the Department of Radiology, Mayo Clinic.
Appendix
Apraxia of Speech Rating Scale 3.0
| 0 | 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|---|
| Not observed in any task | Infrequent | Frequent but not pervasive | Nearly always evident but not marked in severity | Nearly always evident and marked in severity | ||
| No more than one occurrence | Noted more than once (but less than about 20%) | Noted 20–50% of all utterances, but not on most tasks or utterances | Noted on many utterances on most tasks but not enough to decrease overall intelligibility | Noted on most utterances
on most tasks and severe enough to impact
intelligibility or Noted on all utterances on all tasks |
||
| Score no higher than “2” if present only during repetition | ||||||
| Performance on AMRs and SMRs considered only for items 9–11 | ||||||
| Phonetic Features | ||||||
| 1 | Sound distortions (excluding distorted substitutions or distorted additions) | |||||
| 2 | Distorted sound substitutions | |||||
| 3 | Distorted sound additions (including intrusive schwa) | |||||
| 4 | Increased sound distortions or distorted sound substitutions with increased utterance length or increased syllable/word articulatory complexity | |||||
| Prosodic Features | ||||||
| 5 | Syllable segmentation
within words > 1 syllable (Brief silent interval between syllables and/or inappropriate equalized stress across syllables) |
|||||
| 6 | Syllable segmentation
across words in phrases/sentences (Increased inter-word intervals and/or inappropriate equalized stress across words) |
|||||
| 7 | Slow overall speech rate (apart from pauses for word retrieval and/or verbal formulation) | |||||
| 8 | Lengthened vowel &/or consonant segments independent of overall slow speaking rate | |||||
| Other | ||||||
| 9 | RATE ONLY FOR AMRs (alternating motion rates, as in rapid repetition of “puh puh puh”): Off-target (in place, manner, and/or voicing) speech AMR’s (includes distortions and distorted substitutions). Score on severity only: 0= AMRs normal; 1= rare and mild distortions, 2= frequent but mild distortions; 3 = moderate distortions, 4 = severe distortions | |||||
| 10 |
RATE ONLY FOR
SMRs (sequential motion rates, as in rapid repetition
of “puh tuh kuh”): Deliberate, slowly sequenced,
segmented (gaps between sequences), and/or distorted (including
distorted substitutions) speech SMRs in comparison to AMRs.
Rate the best
effort Score on severity only: 0= not noted, SMRs normal; 1= slow, 2= mildly segmented and/or distorted; 3 = moderately segmented and/or distorted, 4 = severely segmented and/or distorted. |
|||||
| 11 | One or both of the following: Consistently Reduced words per breath group during phrase/sentence production relative to maximum vowel duration; reduced # of AMR repetitions per breath group in the absence of decreased respiratory capacity. Score on maximum number of syllables/repetitions per breath group: 0 = more than 7; 1= 6–7; 2 = 4–5; 3 = 3–4; 4 = 2 or less | |||||
| 12 | Visible or silent articulatory groping | |||||
| 13 | Audible false starts/restarts including sound repetitions (excluding fillers and unambiguous language-related false starts (e.g., sp…fork) | |||||
References
- Alario FX, Chainay H, Lehericy S, & Cohen L (2006). The role of the supplementary motor area (SMA) in word production. Brain Research, 1076(1), 129–143. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, &al e. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, & al e. (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2000). Voxel-Based Morphometry—The Methods. Neuroimage, 11(6), 805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2005). Unified segmentation. Neuroimage, 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczkowski M, Detre J, & Gee JC (2010). The optimal template effect in hippocampus studies of diseased populations. Neuroimage, 49(3), 2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard KJ, Wambaugh JL, Duffy JR, Layfield C, Maas E, Mauszycki S, & McNeil MR (2015). Treatment for Acquired Apraxia of Speech: A Systematic Review of Intervention Research Between 2004 and 2012. Am J Speech Lang Pathol, 24(2), 316–337. doi: 10.1044/2015_ajslp-14-0118 [DOI] [PubMed] [Google Scholar]
- Barnes J, Dickerson BC, Frost C, Jiskoot LC, Wolk D, & van der Flier WM (2015). Alzheimer’s disease first symptoms are age dependent: Evidence from the NACC dataset. Alzheimer’s & Dementia, 11(11), 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, & Litvan I (2003). Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Annals of Neurology, 54(Suppl 5), S15–19. [DOI] [PubMed] [Google Scholar]
- Botha HA, Duffy JR, Strand EA, Machulda MM, Whitwell JL, & Josephs KJ (2014). Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology, 82(19), 1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley FL (1967). Lacunae and research approaches to them Brain mechanisms underlying (pp. 236–240). New York: Grune & Stratton. [Google Scholar]
- Darley FL (1969). Aphasia: Input and output disturbances in speech and language processing. Paper presented at the American Speech and Hearing Association, Chicago. [Google Scholar]
- Dickerson BC, Brickhouse M, McGinnis S, & Wolk DA (2016). Alzheimer’s disease: The influence of age on clinical heterogeneity through the human brain connectome. Alzheimer’s & Dementia, 6, 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF (1996). A new brain region for coordinating speech articulation. Nature, 384(6605), 159–161. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, & al e. (2014). Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurology, 13, 614–629. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, & Pillon B (2000). The FAB: a Frontal Assessment Battery at bedside. Neurology, 55, 1621–1626. [DOI] [PubMed] [Google Scholar]
- Duffy JR (2013). Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 3e. St. Louis: Mosby. [Google Scholar]
- Duffy JR, Hanley H, Utianski RL, Clark HM, Strand EA, Josephs KA, & Whitwell JL (2017). Temporal acoustic measures distinguish primary progressive apraxia of speech from primary progressive aphasia. Brain and Language, 168, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, & Josephs KA (2014). The Diagnosis and Understanding of Apraxia of Speech: Why Including Neurodegenerative Etiologies May Be Important. Journal of Speech, Language, and Hearing Research, 55(5), S1518–S1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JR, Strand EA, Clark HM, Machulda MM, Whitwell JL, & Josephs KA (2015). Primary progressive apraxia of speech: Clinical features and acoustic and neurologic correlates. American journal of speech-language pathology, 24(2), 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, & Nimmo-Smith I (2014). Dipy, a library for the analysis of diffusion MRI data. Neuroinformatics, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz C, Tilley B, Shaftman S, Stebbins G, Fahn S, Martinex-Martin P, . . . LaPelle N. (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement Disorders, 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SE, & Manes F (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrich I, Dietrich S, & Ackermann H (2016). The role of the supplementary motor area for speech and language processing. Neuroscience & Biobehavioral Reviews, 68, 602–610. [DOI] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, . . . Petersen RC. (2013). Brain β-amyloid load approaches a plateau. Neurology, 80(10), 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Boeve B, Duffy J, Smith G, Knopman D, Parisi J, . . . Dickson D. (2005). Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase, 283–296. [DOI] [PubMed] [Google Scholar]
- Josephs KA, & Duffy JR (2008). Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Current Opinions in Neurology, 21, 688–692. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, . . . Whitwell JL. (2014). The evolution of primary progressive apraxia of speech. Brain, 137(10), 2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, . . . Whitwell JL. (2013). Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 81(4), 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, . . . Whitwell JL. (2012). Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain, 135(5), 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, . . . Petersen RC. (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell J, Tacik P, Duffy J, Senjem M, Tosakulwong N, . . .Murray M. (2016). [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol, 931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer D, Cummings J, Ketchel P, Smith V, MacMillan A, Shelley T, . . . DeKosky S. (2000). Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences, 12, 233–239. [DOI] [PubMed] [Google Scholar]
- Kent RD, & Rosenbek JC (1983). Acoustic Patterns of Apraxia of Speech. Journal of Speech and Hearing Research, 26, 231–249. [DOI] [PubMed] [Google Scholar]
- Kertesz A (2007). Western Aphasia Battery (Revised). San Antonio, TX: PsychCorp. [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, & Randolph C (1999). An empirically derived short form of the Boston Naming Test. Archives of Clinical Neuropsychology, 14, 481–487. [PubMed] [Google Scholar]
- Litvan I, Agin Y, Calne D, Campbell G, Dubois B, Duvoisin RC, & al e. (1996). Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology, 47, 1–9. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, & Stadlan EM (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34, 939–944. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Robin DA, & Schmidt RA (2009). Apraxia of speech: definition and differential diagnosis Clinical management of sensorimotor speech disorders (pp. 249–268). New York: Thieme. [Google Scholar]
- Nasreddine Z, Phillips N, Bedirian V, Charbonneau S, Whitehead V, Collin I, . . . Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–669. [DOI] [PubMed] [Google Scholar]
- Ogar J, Dronkers NF, Brambati SM, Miller BL, & Gorno-Tempini ML (2007). Progressive Nonfluent Aphasia and Its Characteristic Motor Speech Deficits. Alzheimer Disease & Associated Disorders, 21(4), S23–S30. doi: 10.1097/WAD.0b013e31815d19fe [DOI] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, & Dronkers N (2006). Clinical and anatomical correlates of apraxia of speech. Brain and Language, 97(3), 343–350. [DOI] [PubMed] [Google Scholar]
- Osterrieth P (1944). Le test de copie d’une figure complex: Coontribution a l’etude de la perception et de la memoire. Archives de Psychologie, 30, 286–356. [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, . . . Petersen RC. (2010). The Mayo Clinic Study of Aging: prevalence of mild cognitive impairment is higher in men. Neurology, 75, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, & al e. (2013). Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Movement Disorders, 28, 504–509. [DOI] [PubMed] [Google Scholar]
- Rey A (1964). L’examen clinique en psychologie. Paris: Presses Universitaires de France. [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, . . . Rocca WA. (2008). The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology, 30, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Ward CP, Vemuri P, Senjem ML, Wiste HJ, . . . Jack CR. (2017). The Mayo Clinic Adult Lifespan Template (MCALT): Better Quantification across the Lifespan. Paper presented at the Alzheimer’s Association International Conference. [Google Scholar]
- Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, . . . Jack CR. (2014). Improved DTI registration allows voxel-based analysis that outperforms Tract-Based Spatial Statistics. Neuroimage, 94, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senjem ML, Gunter JL, Shiung MM, Petersen RC, & Jack CR (2005). Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage, 26(2), 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MC, Pravatà E, Brita AC, Improta E, Ciccarelli N, Rossi P, & Colosimo C (2014). Primary progressive aphasia: Linguistic patterns and clinical variants. Brain and Language, 135(Supplement C), 57–65. doi: 10.1016/j.bandl.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Spreen O, & Strauss E (1998). Compendium of Neuropsychological tests, second edition: administration, norms and commentary. New York: Oxford University Press. [Google Scholar]
- Tourville JA, & Guenther FH (2011). The DIVA model: A neural theory of speech acquisition and production. Language and Cognitive Processes, 26(7), 952–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E, & James M (1991). The visual object and space perception battery. Bury St Edmonds, UK: Thames Valley Test Company. [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, . . . Josephs KA (2013). Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. European Journal of Neurology, 20(4), 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Dai B, Howell P, Wang X, Li K, & Lu C (2014). White and Grey Matter Changes in the Language Network during Healthy Aging. PLoS ONE, 19(9), e108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston K, Strand EA, Miller R, & Hillel A (1993). Speech deterioration in amyotrophic lateral sclerosis: Implications for timing of intervention. Journal of Medical Speech-Language Pathology, 1, 35–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.