Abstract
The titanium cluster with the reduced band gap has been synthesized having the palladium nanoparticles over the surface, which not only binds to the atmospheric oxygen but also catalyzes the oxidation of alcohols under visible light.
Keywords: Titanium cluster, Photoactive, Heterogeneous catalysis, Visible light, Aerial oxidation, Palladium nanoparticle
Graphical Abstract
Palladium nanoparticles grafted on a titanium cluster accomplishes the selective oxidation of alcohols using aerial oxygen and visible light via band gap reduction.

1. Introduction
The increasing focus on the utilization of visible light energy is due to its abundant nature and its impeccable tolerance towards the environment.1 Harvesting visible light and performing chemical transformations with it in a concerted process is a high priority chemical research area.2 Most of the photo-catalysts, especially based on titanium oxide, are active under ultra-violet light (UV);3 visible light mediated reactions have not received similar attention.4 There are many photoactive materials available in the literature4 and a vast majority of them has received casual attention in academic publications due to low activity and/or limited applicability. Titanium-based materials have received maximum consideration as a photoactive material, the major limitation being their wider band gaps, which make it active only under UV irradiation conditions. The activity specific to UV light diminishes its role as a photoactive material, which can utilize solar energy in chemical reactions. Therefore, it becomes imperative to reduce the band gap in titanium materials in order to bring its activity into the visible spectrum of electromagnetic radiation.5
Titanium material in combination with metals and other doping elements have been used to reduce the band gap, therefore, sunlight could be utilized for the reaction.6 Nitrogen doping in titanium oxide has been known to reduce band gap to a great extent.7 However, the reaction of N-doped titanium oxide is uncontrollable in visible light due to its high activity; complete decomposition or mineralization of the organic matter is common occurrence in environmental remediation studies8 thus rendering this catalyst incongruous in organic synthesis.9 On the other hand, titanium oxide has been used as a support for the immobilization of transition metals and its application in oxidation chemistry is growing.10 Hong and coworkers have immobilized palladium and gold combination over the surface of TiO2 but they lost the ethos of TiO2 as a photoactive material when the reaction was performed under thermal heating using oxygen atmosphere.11 In this reaction, gold adsorbs the oxygen in the reaction media and transfers into the reactant via the bimetallic interaction with palladium. The use of titanium oxide becomes irreverent, as its photoactivity has not been utilized. Similarly, Li et al. utilized biosynthesized Au/Pd-TiO2 supported nanoparticle in the oxidation reaction.12 We strived to utilize the core property of titanium as photoactive material along with structural modification to create the cluster which can adsorb the atmospheric oxygen and transfer to the reactant in an oxidation process. Accordingly, we prepared nitrogenous titanium cluster, immobilized the palladium to develop photoactive gold-free catalyst, and demonstrated its application in selective oxidation of alcohols; titanium cluster performs the dual task by providing required activation energy and transmit oxygen in the oxidation step.
2. Result and Discussion
In continuation of our desire to develop benign methods for environmental remediation and green synthesis, 13 herein, we report a photoactive palladium-grafted titanium cluster and demonstrated its application in selective oxidation of alcohols to the corresponding carbonyl compounds. Generally, oxidation chemistry is conducted using transition metals along with a supporting co-oxidant.14 Often, co-oxidants comprise homogenous mix of reagents and catalysts with occasional use of oxygen. However, the utility of oxygen as co-oxidant comes with various detriments that include addition of oxygen absorbing material or metals that can bind oxygen in the reaction such as gold. The main objective here is the adsorption and transmission of the required oxygen for the catalytic process.15 However, our titanium cluster based catalyst does not require any of these additives and the reaction can be conducted under visible light using oxygen from the atmosphere. The first step in developing the active catalyst was the synthesis of titanium cluster with the significant reduction in the band gap. It has been accomplished by treating titanium (IV) isopropoxide with 4-aminobenzoic acid in isopropanol at 120 °C for 72 hours;16 shiny crystals of titanium clusters ensued which settled down at the bottom of the reaction vessel and were separated by decanting and centrifugation. The Ti-cluster was then suspended in isopropropanol and treated with palladium nitrate at 80 °C; palladium salt was reduced and deposited over the cluster. The palladium grafted titanium cluster (Pd@TiC) was separated and its application has been evaluated in the selective oxidation of alcohols (Scheme 1).
Scheme 1.

Pd@TiC catalyzed oxidation of alcohols
The Pd@TiC catalyst was characterized using scanning electron microscope (SEM) and X-ray diffraction (XRD). The percentage of palladium was determined using inductive coupled plasma atomic emission spectroscopy (ICP-AES) analysis. The SEM images of Ti-cluster and Pd@TiC catalyst illustrate the immobilization of palladium (Pd) nanoparticles (Figure 1) with the apparent changes in the surface morphology. The immobilization of Pd was further established by XRD (Figure 2) and the concentration confirmed to be 4.88% by ICP-AES analysis.
Figure 1.
SEM images of Ti-Cluster (a) pyramid like structure, (b) close view of pyramid; (c) and (d) SEM images of Pd@TiC
Figure 2.
XRD of Ti-Cluster and Pd@TiC
The activity of Pd@TiC was evaluated in the aerial oxidation of alcohols under visible light irradiation with benzyl alcohol as a model substrate. The preliminary results obtained during the screening and reaction optimization are summarized in Table 1. The reaction outcome in different solvents clearly indicate that acetonitrile is the best solvent for this reaction as it gives quantitative yield of the desired product; conventionally used polar solvents (water and DMF) and non-polar solvent (toluene) were in effective in the visible light mediated aerial oxidation of alcohol.
Table 1.
Results of screening experimentsa
| Entry | Solvent | Time | Yield b |
|---|---|---|---|
| 1 | H2O | 12 h | 45 % |
| 2 | Toluene | 12 h | 48 % |
| 3 | DMF | 12 h | 16 % |
| 4 | CH3CN | 8 h | 97 % |
Reaction conditions: Benzyl alcohol (1 mmol), Pd@TiC (25 mg), Solvent (2 ml), 20 W domestic bulb, Air;
Isolated yields
After uncovering the appropriate conditions, the scope of the developed Pd@TiC catalyst was studied in the oxidation of a wide range of alcohols and the results are depicted in Table 2. They clearly demonstrate the excellent activity and selectivity towards the formation of corresponding carbonyl compounds (Table 2; entries 1–8) where electron donating and electron with drawing substituents apparently have trifling effect on the rate of the reaction (Table 2, entries 2–4). The secondary alcohols and heterocyclic alcoholic derivatives were also readily converted to corresponding carbonyl derivatives (Table 2, entries 5–8). The important feature of this reaction was the use of aerial oxygen and solar energy to accomplish the oxidation of alcohol without the formation of any by-product, which is an unprecedented finding.
Table 2.
Pd@TiC catalyzed oxidation of alcoholsa
| Entry | Substrate | Product | Yieldb |
|---|---|---|---|
| 1 |  |
 |
97 % |
| 2 |  |
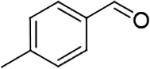 |
95 % |
| 3 |  |
 |
96 % |
| 4 |  |
 |
96 % |
| 5 |  |
 |
94 % |
| 6 |  |
 |
94 % |
| 7 |  |
 |
97 % |
| 8 |  |
 |
97 % |
Reaction conditions: Substrate (1 mmol), Pd@TiC (25 mg), CH3CN (2 ml), 20 W domestic bulb, Air, 8h;
Isolated yield
3. Conclusion
We have demonstrated the synthesis of a highly dispersed palladium nanoparticles grafted on titanium cluster. The developed Pd@TiC catalyst is found to be a highly effective in the aerial oxidation of alcohols under visible light and its activity is attributed to the reduced bandgap, which makes it active under visible light thus harnessing solar energy for the chemical reactions. The Pd@TiC is found to be highly stable and could be recycled several times without any loss in its activity. The most important feature of this catalyst is its ability to bind with the atmospheric oxygen which serves as an oxidant in the reaction cycle.
4. Experimental Section
a). Synthesis of Pd@TiC catalyst
Titanium (IV) isopropoxide (0.518 mL) was added to a solution of 4-aminobenzoic acid (0.96 g, 7.0 mmol) in 2-propanol (30.0 mL). The reaction mixture was stirred at room temperature for 30 min; an orange-colored slurry was obtained. The slurry was heated at 120 °C for 72 hours in a pressure reactor when bright yellow crystals of titanium cluster ensued. The catalyst was isolated by decanting and centrifugation, washed with 2-propanol and dried under vacuum for 2 hours. The Ti cluster (100 mg) was then suspended in isopropropanol (10 ml) and treated with palladium nitrate (5 mol %) at 80 °C. 2-propanol reduced the Pd metal into Pd-nanoparticle which were deposited over the titanium cluster. The palladium nanoparticle grafted titanium cluster (Pd@TiC) was isolated and characterized using SEM, XRD and ICP-AES.
b). General procedure for the oxidation of alcohols
A 10 mL side-armed round bottomed flask equipped with a magnetic stirring bar and a balloon filled with air was charged with alcohol (1 mmol), catalyst Pd@TiC (25 mg) and acetonitrile (2 mL). The reaction mixture was exposed to visible light irradiation using 20 watt domestic bulb (Figure 3) and the progress of reaction was monitored using TLC. After the completion of the reaction, the Pd@TiC catalyst was separated using a centrifuge and the product was isolated by extracting with ethyl acetate, dried over sodium sulfate, concentrated under reduced pressure and characterized.
Figure 3.
Pictorial representation of the reaction set-up
c). Recycling of Pd@TiC
A set of experiments were performed using benzyl alcohol as a model substrate in acetonitrile solvent. After the completion of each reaction, the Pd@TiC catalyst was recovered using a centrifuge, washed with methanol and reused for the oxidation of fresh batch of benzyl alcohol. The Pd@TiC catalyst could be recycled and reused up to six times without any loss in its activity (Table 3). The metal leaching in reaction with Pd@TiC was examined by ICP-AES analysis before and after the completion of reaction; concentration of palladium was found to be 4.88% before the reaction and 4.87% after the 6th cycle. The ICP-AES of the mother liquor did not show the presence of palladium confirming the fact that titanium cluster holds the Pd nanoparticles tightly.
Table 3.
Recycling of Pd@TiC catalyst in the oxidation of benzyl alcohola
| Run | 1 | 2 | 3 | 4 | 5 | 6 |
| Isolated Yield | 97% | 97% | 97% | 97% | 96% | 96% |
Reaction conditions: Benzyl alcohol (1 mmol), Pd@TiC (25 mg), CH3CN (2 ml), 20 W domestic bulb, Air
The SEM image of the Pd@TiC before and after the reaction confirms that there is no significant change in the morphology of the catalyst, which signifies high stability of Pd@TiC during the course of the reaction (Figure 4).
Figure 4.
SEM image of the recycled Pd@TiC
Supplementary Material
Acknowledgements
SV and RBNB were supported by the Postgraduate Research Program at the National Risk Management Research Laboratory administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Environmental Protection Agency.
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
5. References
- 1.Bita CE; Gerats T Front Plant Sci 2013, 4, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L; Zhang S; Xu L; Wang J; Shi L-X; Chen Z-N; Honga M; Luo J. Chem. Sci 2014, 5, 3808. [Google Scholar]
- 3.Kumar SG; Devi LG J. Phys. Chem. A 2011, 115, 13211. [DOI] [PubMed] [Google Scholar]
- 4.a) Prier CK; Rankic DA; MacMillan DWC Chem. Rev, 2013, 113, 5322; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kanhere P; Chen Z Molecules 2014, 19, 19995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Schneider J; Matsuok M; Takeuchi M; Zhang J; Horiuchi Y; Anpo M; Bahnemann DW Chem. Rev 2014, 114, 9919; [DOI] [PubMed] [Google Scholar]; b) Colmenares JC, Lisowski P, Łomot D, Chernyayeva O and Lisovytskiy D ChemSusChem, 2015, 8, 1676. [DOI] [PubMed] [Google Scholar]
- 6.Zaleska A Recent Patents on Engineering 2008, 2, 157. [Google Scholar]
- 7.Cong Y; Zhang J; Chen F; Anpo MJ Phys. Chem. C 2007, 111, 6976. [Google Scholar]
- 8.Asahi R; Morikawa T; Irie H; Ohwaki T Chem. Rev 2014, 114, 9824. [DOI] [PubMed] [Google Scholar]
- 9.Yang G; Jiang Z; Shi H; Xiao T; Yan ZJ Mater. Chem 2010, 20, 5301. [Google Scholar]
- 10.Bagheri S; Julkapli NM; Bee S; Hamid A Scientific World Journal 2014, 2014, 727496.25383380 [Google Scholar]
- 11.Chang JB; Liu CH; Liu J; Zhou YY; Gao X; Wang SD Nano-Micro Lett. 2015, 7, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong Y; Jing X; Huang J; Sun D; Odoom-Wubah T; Yang F; Du M; Li Q ACS Sustainable Chem. Eng 2014, 2, 1752. [Google Scholar]
- 13.a) Varma RS Green Chem. 2014, 16, 2027; [Google Scholar]; b) Verma S; Mungse HP; Kumar N; Choudhary S; Jain SL; Sain B; Khatri OP Chem. Commun 2011, 47, 12673; [DOI] [PubMed] [Google Scholar]; c) Nasir Baig RB; Varma RS Green Chem. 2013, 15, 1839; [Google Scholar]; d) Verma S; Singh R; Tripathi D; Gupta P; Bahuguna GM; Jain SL RSC Adv. 2013, 3, 4184; [Google Scholar]; e) Nasir Baig RB; Varma RS ACS Sustain. Chem. Eng. 2014, 2, 2155; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Nasir Baig RB; Nadagouda MN; Varma RS Green Chem. 2014, 16, 4333. [Google Scholar]
- 14.a) Verma S; Nasir Baig RB; Nadagouda MN; Varma RS ACS Sustain. Chem. Eng 2016, 4, 2333; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Verma S; Baig RBN; Han C; Nadagouda MN; Varma RS Green Chemistry 2015, 18, 251; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Verma S; Baig RBN; Nadagouda MN; Varma RS ACS Sustain. Chem. Eng 2016, 4, 1094; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Verma S; Verma D; Sinha AK; Jain SL Appl. Catal. A: Gen 2015, 489, 17; [Google Scholar]; e) Verma S; Aila M; Kaul S; Jain SL RSC Adv. 2014, 4, 30598; [Google Scholar]; f) Verma S; Le Bras, J., Jain SL; Muzart J Appl. Catal. A: Gen 2013, 468, 334; [Google Scholar]; g) Mungse HP; Verma S; Kumar N; Sain B; Khatri OP J. Mat. Chem 2012, 22, 5427. [Google Scholar]
- 15.a) Pina CD; Falletta E Catal. Sci. Technol 2011, 1, 1564; [Google Scholar]; b) Biella S; Prati L; Rossi M J. Mol. Catal. A: Chem 2003, 197, 207. [Google Scholar]
- 16.a) Yuan S; Liu TF; Feng D; Tian J; Wang K; Qin J; Zhang Q; Chen YP; Bosch M; Zou L; Teat SJ; Dalgarno SJ; Zhou HC Chem. Sci. 2015, 6, 3926; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Feng D; Wang K; Wei Z; Chen Y-P; Simon CM; Arvapally RK; Martin RL; Bosch M; Liu T-F; Fordham S; Yuan D; Omary MA; Haranczyk M; Smit B; Zhou H-C Nat. Commun 2014, 5, 5723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






