Abstract
The hepatitis C virus (HCV) is a global public health problem affecting approximately 2% of the human population. The majority of HCV infections (more than 70%) result in life-long persistence of the virus that substantially increases the risk of serious liver diseases, including cirrhosis and hepatocellular carcinoma. The remainder (less than 30%) resolves spontaneously, often resulting in long-lived protection from persistence upon reexposure to the virus. To persist, the virus must replicate and this requires effective evasion of adaptive immune responses. In this review, the role of humoral and cellular immunity in preventing HCV persistence, and the mechanisms used by the virus to subvert protective host responses, are considered.
I. INTRODUCTION
The existence of hepatitis C virus(es) was first predicted 35 years ago to explain transfusion-associated liver disease in individuals not infected with the hepatitis A or B viruses (Alter et al., 1975; Feinstone et al., 1975; Prince et al., 1974). The description in 1989 of a single hepatitis C virus (HCV) that caused most posttransfusion and community acquired non-A non-B hepatitis marked a significant turning point toward understanding the epidemiology, natural history, and pathogenesis of this disease (Choo et al., 1989; Houghton, 2009). Seroepidemiology studies indicate that HCV has infected approximately 2% of the world’s population. The virus establishes persistent, life-long viremia in about 75% of infected humans and significantly increases the risk of progressive liver diseases, including inflammation, cirrhosis, and hepatocellular carcinoma. The discovery of HCV has also facilitated the development of new small molecule inhibitors of virus replication (designated STAT-C agents) that will soon be an adjunct to, and perhaps eventually replace, current standard therapy with pegylated type I interferon and ribavirin that is toxic, expensive, and frequently ineffective (Shimakami et al., 2009).
HCV is a member of the Flaviviridae and the prototype virus in the hepacivirus genus (Moradpour et al., 2007). It has a small RNA genome of about 10,000 nucleotides encoding a single polyprotein of 3000 amino acids that is processed by host cell and viral proteases into 10 in-frame proteins (Moradpour et al., 2007). At least one small frame-shifted protein of unknown function is also produced. Structural proteins include a core or nucleocapsid and two envelope glycoproteins. Seven nonstructural proteins are important for HCV replication. There are at least six distinct genotypes that can be further classified into subtypes defined by phylo-genetic relationships (Simmonds et al., 2005). HCV circulates as a population of different but closely related genomes in infected individuals (Simmonds et al., 2005). How the virus manages to avoid immune responses and establish life-long persistence is still a mystery. It is apparent that most viral proteins important for HCV replication also participate in evasion of innate and/or adaptive immune responses. As an example, the NS3 helicase/protease is critical for HCV replication and a prime target for small-molecule STAT-C inhibitors. NS3 protease activity also disrupts induction of innate immune defenses through RIG-I (retinoic acid inducible gene 1) and toll-like receptor 3 (TLR-3) sensors by cleavage of cellular intermediates important in signal transduction (Foy et al., 2003; Gale and Foy, 2005; Li et al., 2005). Despite the tremendous efficiency of HCV in establishing persistence, spontaneous clearance of infection in some individuals provides optimism that chronic hepatitis C can be prevented by vaccination and perhaps treated by immunotherapeutic approaches.
II. PATTERNS OF HCV REPLICATION
HCV replication and adaptive immune responses have been studied in humans and chimpanzees, the only species other than man with known susceptibility to infection. Transmission of non-A non-B hepatitis from humans to chimpanzees provided an animal model for initial characterization of the agent as a small enveloped RNA virus and paved the way for molecular cloning ofthe HCV genome (Alter et al., 1978; Hollinger et al., 1978; Tabor etal., 1978). Although there havebeen few detailed studies of the issue, spontaneous resolution of HCV infection may be more common in chimpanzees than in humans (Bassett et al., 1998; Lanford et al., 2001). Moreover, these animals do not develop serious progressive liver disease, at least in the time frame described for most human infections. Despite these differences, chimpanzees have been invaluable for comparison of immunity in HCV infections that spontaneously resolve or persist. The chimpanzee model has several important advantages for the study of HCV-specific humoral and cellular immunity. Animals can be infected with genetically defined strains of HCV, including molecular clones that are sequence-matched with antigens used to probe immunity. Moreover, serial liver and blood samples can be collected from the earliest times after virus challenge for studies of immunity.
Patterns of acute phase HCV replication are well defined in both species (Abe et al., 1992; Fang et al., 2003; Larghi et al., 2002; Spada et al., 2004; Thimme et al., 2001, 2002). HCV RNA is detectable in serum within a few days of exposure to the virus and usually peaks 8–12 weeks later when serum transaminases are elevated. Three common patterns of viremia have been observed (Fig. 1). The first pattern leads to spontaneous resolution of infection. Peak viremia and serum transaminase levels are typically observed 8-12 weeks after infection and then drop sharply. This is followed by permanent clearance of HCV RNA from serum, sometimes after several weeks or months of low-level, fluctuating viremia. Spontaneous resolution appears to result in long-lived immunity that, at least for some, provides protection against HCV persistence upon reexposure to the virus (see Section IV). The second pattern is difficult to distinguish from the first because the sharp initial decline in acute phase viremia is also followed by a period of partial control so effective that HCV RNA is often intermittently undetectable in serum (Fig. 1). However, after a variable period of time (sometimes up to 1 year or more), viremia that is low and fluctuating transitions to a high, stable pattern characteristic of chronic hepatitis C (Abe et al., 1992; McGovern et al., 2009; Mosley et al., 2008; Thimme et al., 2001, 2002). In the third pattern of viremia, limited or no control of virus replication is observed before persistence is established (Fig. 1). Viremia is remarkably stable in the chronic phase of infection, although steady-state levels vary over a 2–3 log10 range among infected individuals (Arase et al., 2000; Fanning et al., 2000; Gordon et al., 1998; Nguyen et al., 1996; Thomas et al., 2000). In this review, recent evidence that adaptive immunity influences the pattern of acute phase virus replication and the outcome of infection is considered. Mechanisms of immune evasion important to persistence of this small RNA virus are also discussed.
FIGURE 1.
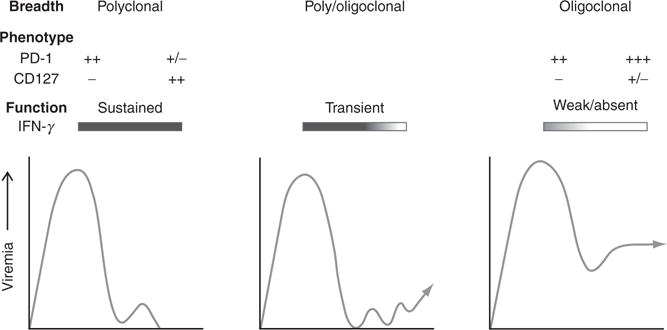
Three patterns of viremia have been described during the acute phase of HCV infection. In individuals who clear the infection (left panel), viremia peaks several weeks after infection when functional polyclonal CD4+ and CD8+ T responses targeting epitopes in most viral cell proteins are first detected in blood. CD8+ T cells lose expression of the coinhibitory molecule PD-1 and gain expression of the CD127 IL-7 receptor that is required for self-renewal of memory populations. Transient control of viremia can be observed for several months to a year after infection (middle panel). As indicated in the panel, these infections frequently persist, but resolution has also been observed after a prolonged period of low-level, fluctuating viremia. The end of transient virus control is associated with loss of CD4+ T helper cell function. Infections that persist without transient control of viremia have been described (right panel). T cell responses, if detected, are not sustained and usually target a limited number of epitopes. Most CD8+ T cells express high levels of PD-1. CD127 is low or absent. This exhausted phenotypic profile may be attenuated if the targeted viral epitope acquires an escape mutation (see Section IV, C1 and C2 for details).
III. HUMORAL IMMUNITY TO HCV
It has been known for two decades that seroconversion is substantially delayed during acute hepatitis C, with serum antibodies appearing several weeks after the initiation of virus replication regardless of infection outcome (Chen et al., 1999). Progress toward understanding the role of antibodies in HCV infection has been slow. This is due almost entirely to the technically challenging task of studying HCV attachment and entry into host cells, and whether this process is susceptible to neutralization by antibodies generated during infection. In the absence of a cell culture system supporting HCV replication, surrogate assays for virus binding, entry, and neutralization were developed using cell lines presumed to express viral receptors. As reviewed below, HCV ligands used in these cell culture models gradually evolved in sophistication from soluble recombinant envelope glycoproteins to synthetic virus-like particles (VLP) and finally retrovirus particles pseudotyped with HCV envelope glycoproteins (HCVpp). With the relatively recent advent of HCV strains that can complete the entire replication cycle in cell culture (designated HCVcc), it has been possible to validate and extend insights into the requirements for HCV entry, antibody-mediated neutralization, and evasion mechanisms.
A. Methods to study HCV entry and neutralization
Chinese hamster ovary (CHO) cell production of a soluble recombinant E2 protein that was fully glycosylated and capable of binding conformation-dependent antibodies provided the first key reagent for studying HCV attachment to cells (Rosa et al., 1996). Binding of recombinant E2 to the Molt-4 T cell line as quantified by flow cytometry represented a key breakthrough in early efforts to identify cellular receptors for HCV and develop surrogate assays for antibody neutralization (Rosa et al., 1996). Expression cloning of cDNA libraries from Molt-4 cells revealed that the tetraspanin CD81 bound soluble recombinant E2 with high affinity (Pileri et al., 1998) and this interaction could be blocked by antibodies to E2 or CD81 (Rosa et al., 1996). Noninfectious VLP produced from insect cells that expressed the HCV core, E1 and E2 proteins have also been used to assess antibody-mediated blockade of cellular entry (Baumert et al., 2000). The approaches were largely superseded by the development of pseudoparticles containing rhabdo-(Lagging et al., 2002; Meyer et al., 2000) or retro-(Bartosch et al., 2003c; Hsu et al., 2003) virus core particles bearing HCV envelope glycoproteins. HCVpp containing retrovirus core particles are now widely used in entry and neutralization assays. They offer distinct advantages for studying the virus–host cell interaction, including the ability to pseudotype retrovirus core particles with E1 and E2 glycoproteins from different HCV strains and direct visualization of target cell transduction by incorporation of a reporter gene (Bartosch et al., 2003c; Hsu et al., 2003). As detailed below, HCVpp facilitated studies of the molecular interaction between E2 and cellular receptors, and neutralization of HCV entry into hepatoma cells by antibodies from infected or vaccinated humans and animals (Bartosch et al., 2003a; Logvinoff et al., 2004). HCVpp assays were validated by correlating in vitro neutralization with antibody-mediated protection of chimpanzees from infection (Bartosch et al., 2003a). While the HCVpp model remains important, the demonstration that select genotype 1a (Yi et al., 2006), 1b (Silberstein et al., 2010), and 2a (Lindenbach et al., 2005; Wakita et al., 2005; Zhong et al., 2005) HCVcc strains productively infect cultured cells provided a major new approach to dissect virus–host cell interactions. An HCV replicon containing all structural and nonstructural genes of a genotype 2a virus from a Japanese patient with fulminant hepatitis replicated in hepatoma cells without adaptive mutations and produced particles that could initiate infection in chimpanzees. HCVcc containing complete or recombinant JFH-1 genomes are now the most widely used adjunct to HCVpp for studies of virus neutralization and receptor-mediated entry into cells (Lindenbach et al., 2005; Zhong et al., 2005).
B. HCV attachment and entry
There is a consensus that E1 and E2 are critical for cellular attachment and entry because infection fails if HCVpp lack one of these envelope glycoproteins (Bartosch et al., 2003c; Drummer et al., 2003; Hsu et al., 2003). E1 and E2 exist as heterodimers in the lipid bilayer of the virus (Dubuisson and Rice, 1996; Lavie et al., 2007) and mediate cellular entry via clathrin-dependent endocytosis, delivering the viral genome from the early endosome to the cytoplasm by a pH-dependent fusion process (Blanchard et al., 2006; Meertens et al., 2006). One recent study integrated a model of E2 tertiary structure based on the position of nine disulfide bonds with published functional data to obtain a tentative map of the CD81 binding site and identify a candidate loop involved in fusion (Krey et al., 2010). However, crystal structures of the envelope glycoproteins have not yet been solved and so there is limited information on the location of neutralizing B cell epitopes relative to the functional domains of E2.
Internalization of HCVpp and HCVcc is, with few exceptions, restricted to cultured cells of the hepatocyte lineage, suggesting that the HCV host range is defined at least in part by the distribution of cellular receptor(s) for the virus (Bartosch et al., 2003b; Flint et al., 2006; Hsu et al., 2003; Lavillette et al., 2005b). The low-density lipoprotein (LDL) receptor (Molina et al., 2007), the C-type lectins dendritic cell (DC), and liver/lymph-node (L) specific intracellular adhesion molecule-3-grabbing integrin (DC-SIGN and L-SIGN, respectively; Cormier et al., 2004; Lozach et al., 2003, 2004) and/or glycosaminoglycans (Barth et al., 2006) may facilitate virus attachment to target cells. For instance, ApoE associated with the virion can bind the LDL receptor on hepatocytes and blockade of this interaction interferes with HCV entry (Burlone and Budkowska, 2009; Owen et al., 2009). Four cellular proteins are required for virus internalization in cell culture models. These include CD81 (Bartosch et al., 2003b; Hsu et al., 2003; Lindenbach et al., 2005; Pileri et al., 1998), the scavenger receptor class member B1 (SR-B1; Bartosch et al., 2003b; Grove et al., 2007; Kapadia et al., 2007; Scarselli et al., 2002) and the tight junction proteins, claudin-1 (Evans et al., 2007) and occludin (Benedicto et al., 2009; Liu et al., 2009; Ploss et al., 2009). Claudin-6 and claudin-9 may substitute for claudin-1 in this process (Meertens et al., 2008; Zheng et al., 2007). Successful HCVpp and HCVcc infection of nonpermissive cells that coexpressed SR-B1, CD81, claudin-1, and occludin demonstrated that all four proteins were necessary for viral entry (Ploss et al., 2009). This approach involving transfection of human genes into rodent cells also facilitated mapping of cellular receptors that govern the species specificity of HCV entry. Receptor orthologues of rodent origin bind E2 with reduced efficiency and may interact less efficiently with human receptor components (Catanese et al., 2010; Haid et al., 2010; Ploss et al., 2009). How HCV is thought to interact with these cellular proteins for attachment, entry, and release of the viral genome into the cytoplasm of target cells is reviewed below.
CD81 and SR-B1 were first identified as viral receptors based on their physical association with soluble recombinant E2 proteins. The CD81 tetraspanin is widely expressed on human tissues and is important for signal transduction in a variety of cells, including B and T lymphocytes (Levy and Shoham, 2005). SR-B1 is restricted to liver cells and steroidogenic tissue and serves as a primary receptor for multiple ligands, including high-density lipoproteins (HDL), low-density lipoproteins (LDL), and very low-density lipoproteins (VLDL; Krieger, 2001). Experimental evidence implicating these proteins in virus entry is strong. Antibodies directed against CD81 or SR-B1, and siRNA-mediated silencing of the genes encoding these cellular proteins, substantially impair entry of HCVpp and/or HCVcc (Bartosch et al., 2003b; Kapadia et al., 2007; Lavillette et al., 2005b; Lindenbach et al., 2005; Zhang et al., 2004). It is likely that they cooperate in the entry process because combination of antibodies to these cellular proteins also synergistically blocks HCVcc infection of hepatoma cells (Kapadia et al., 2007; Zeisel et al., 2007). Antibodies directed against CD81 can also block HCV infection of human hepatocytes grafted into immunodeficient mice (Meuleman et al., 2008). E2 binds the large extracellular loop (LEL) of CD81 via multiple discontinuous amino acids that have been mapped using monoclonal antibodies and by mutagenesis of E2 domains (Stamataki et al., 2008), and by identification of residues required for adaptation of E2 binding to murine CD81 (Bitzegeio et al., 2010).
SR-B1 is thought to bind hypervariable region 1 (HVR-1) that is located at the N-terminus of the E2 glycoprotein. Cellular entry of HCVpp that lack this E2 sequence cannot be blocked by SR-B1-specific antibodies (Bartosch et al., 2005). HCV and HDL appear to interact with distinct regions of SR-B1 because mutagenesis of the receptor impaired HCV infectivity without loss of HDL binding (Catanese et al., 2010). SR-B1 is involved in very early steps in virus entry, perhaps including initial virus attachment (Catanese et al., 2010), but a role in postbinding steps is also suggested by SR-B1 antibody blockade studies (Zeisel et al., 2007). Finally, infection of hepatoma cells can be enhanced or inhibited by HDL and oxidized LDL, respectively, indicating that the interplay between SR-B1, its natural ligands, and the virus is complex and not yet fully understood (Bartosch et al., 2005; Dreux et al., 2006; Voisset et al., 2006; von Hahn et al., 2006).
Soluble recombinant E2 was not used to identify claudin-1 and occludin as HCV receptors. Instead, these tight junction proteins were implicated in HCV entry by an iterative cloning strategy that depended on expression of cDNA libraries in cells that were not fully permissive for HCV infection. Physical interaction of these cellular proteins with E2, if it occurs, is poorly understood. An association between occludin and E2 in the endoplasmic reticulum has been reported (Benedicto et al., 2009), although the nature of the interaction and how it governs the entry process remains to be defined. Reduced expression of claudin-1 and occludin inhibited HCV glycoprotein-dependent cell-to-cell fusion, suggesting that both tight junction proteins act at a late stage of virus entry (Benedicto et al., 2009; Evans et al., 2007). How the virus contacts claudin-1 and occludin is unknown. The interaction of E2 and CD81 activates GTPase and this may mediate actin-dependent relocalization of the E2-CD81 complex to cell contact sites that contain tight junction proteins (Brazzoli et al., 2008). A physical interaction between claudin-1 and CD81 that is critical for HCV infection has been documented by mutagenesis (Harris et al., 2010) and antibody blockade studies (Krieger et al., 2010). Whether HCV enters cells via tight junctions is unknown, but two recent studies suggest that this is not essential at least in culture models involving hepatoma cell lines. Pools of nonjunctional claudin-1 that can complex with CD81 have been observed at the basolateral membrane of polarized hepatoma cells (Mee et al., 2009). Visualization of fluorescent-labeled HCV particles as they are internalized has provided evidence of a complex involving the virus, claudin-1, and CD81 that forms outside tight junctions (Coller et al., 2009; Harris et al., 2010; Mee et al., 2009). This model is attractive as it is consistent with virus entry into the liver through the sinusoidal blood supply and subsequent association with receptors on the basal surface of polarized hepatocytes (Stamataki et al., 2008).
C. Mapping of neutralization epitopes
E2 contains continuous (linear) and discontinuous (nonlinear) neutralizing epitopes involved in binding to CD81 and/or the SR-B1 viral receptors. Two of the best characterized regions containing linear epitope(s) are (i) the HVR-1 spanning amino acids 384–401 and (ii) an adjacent sequence spanning amino acids 413–420 of E2 (Owsianka et al., 2001, 2005; Tarr et al., 2006, 2007). Antibodies against HVR-1 are found in most if not all naturally infected humans but are isolate-specific and evolve continuously. A temporal correlation between the appearance of anti-HVR antibodies and the emergence of HCV variants provided the first presumptive evidence of an acute phase neutralizing response (Farci et al., 2000; Kato et al., 1993, 1994; Taniguchi et al., 1993; Weiner et al., 1992). Conversely, limited or no HVR-1 diversification was observed in viruses from humans with hypogamma-globulinemia or chimpanzees that failed to generate antibodies to E2 (Bassett et al., 1998; Booth et al., 1998; Penin et al., 2001; Puntoriero et al., 1998; Ray et al., 2000). Passive neutralization of HCV infectivity by anti-HVR antibodies before challenge of chimpanzees provided direct evidence that HVR-1 contains neutralizing epitope(s) (Farci et al., 1996).
The adjacent epitope spanning amino acids 413–420 that is involved in CD81 binding was first defined by monoclonal antibodies generated from rodents immunized with recombinant E2 (Owsianka et al., 2001, 2005; Tarr et al., 2006, 2007). In comparison to HVR-1, it is poorly recognized in naturally infected humans. Only 2.5% of sera from subjects with resolved or chronic hepatitis C recognized amino acids 413–420 of E2 (Tarr et al., 2007). The epitope defined by the rodent antibodies also differs from HVR-1 because it is highly conserved across most HCV genotypes (Broering et al., 2009; Owsianka et al., 2005). HCVcc with mutations in this conserved epitope can arise spontaneously (Dhillon et al., 2010) or by selection in the presence of the cognate neutralizing antibodies (Dhillon et al., 2010; Gal-Tanamy et al., 2008) during in vitro replication in hepatoma cells. Expanded use of these models of virus adaptation should provide new insights into the interaction of the virus with host cell receptors as well as mechanisms of antibody neutralization and evasion.
A number of conformation-dependent epitopes in E2 were also defined using antibodies from HCV-infected humans (Johansson et al., 2007; Keck et al., 2005, 2007; Law et al., 2008; Op De Beeck et al., 2004). Alanine-scanning mutagenesis and competitive binding assays with panels of human monoclonal antibodies, or antigen-binding fragments generated from phage display libraries, provided evidence for three conformational domains or regions (operationally designated A, B, and C) within E2 that are antigenic (Keck et al., 2004; Law et al., 2008). Antibodies targeting domain A epitopes are nonneutralizing and may be derived from isolated envelope proteins rather than virions (Keck et al., 2005; Law et al., 2008). Antibodies specific for domain B and C epitopes neutralized infectivity, probably by competitive inhibition of the CD81–E2 interaction. In support of this concept, many of the amino acids required for binding of the domain B antibodies (G530, D535, and W529; Johansson et al., 2007; Keck et al., 2008a, b, 2009; Law et al., 2008; Owsianka et al., 2008; Perotti et al., 2008) were identical to residues critical for E2 binding to CD81 (Johansson et al., 2007; Owsianka et al., 2006; Rothwangl et al., 2008). One recent study documented that a broadly cross-reactive monoclonal antibody directed against domain B provided protection from HCV infection in mice reconstituted with human hepatocytes (Law et al., 2008).
D. Antibody responses and infection outcome
Whether antibodies modify the course of HCV infection or contribute to spontaneous resolution is controversial. Direct evidence supporting involvement of the humoral immune response in control of HCV replication or resolution of infection is sparse. Spontaneous resolution without seroconversion has been observed in chimpanzees (Cooper et al., 1999) and humans (Christie et al., 1997; Post et al., 2004), including some with primary antibody deficiency (Christie et al., 1997). Although the number of subjects with hypogammaglubulinemia who permanently cleared viremia was small, this study provided support for the concept that anti-HCV antibodies are not necessarily required for a successful infection outcome (Christie et al., 1997). On the other hand, passive transfer of serum containing HCV immunoglobulins to chimpanzees substantially delayed, but did not prevent, replication of the virus upon challenge (Krawczynski et al., 1996), suggesting that antibodies can alter the trajectory of infection.
Studies conducted before validated neutralization assays were available suggested an association between the rapid onset of an antibody response to E2 and infection outcome. As an example, subjects who spontaneously resolved HCV infection were more likely to have serum antibodies against the E2 HVR within the first 6 months of infection when compared with those who developed persistent viremia, even when the timing of antibody responses to the core or nonstructural proteins was approximately the same (Dittmann et al., 1991; Zibert et al., 1997). This result was corroborated and extended by more recent studies employing the HCVpp neutralization assay. In a single-source outbreak of hepatitis C involving multiple subjects, HCV clearance was associated with rapid induction of neutralizing antibodies in the early phase of infection (Pestka et al., 2007). However, it remains uncertain if the neutralizing antibodies had a primary role in containment of infection or were simply a surrogate marker of a more effective T cell response.
A broad pattern of cross-reactivity against genetically diverse HCV strains by serum antibodies from persistently infected humans suggests continuous evolution of the virus. (Bartosch et al., 2003a; Kaplan et al., 2007; Lavillette et al., 2005b; Logvinoff et al., 2004; Meunier et al., 2005; Netski et al., 2005; Steinmann et al., 2004; von Hahn et al., 2007; Wakita et al., 2005). Few longitudinal studies have addressed this issue because construction of multiple HCVcc or HCVpp that incorporate serial envelope sequences that emerge over time is technically challenging. Nevertheless, HCVpp were used to study the evolution of neutralizing antibodies and envelope glycoprotein E2 in one subject, designated H, who provided serial serum samples over 26 years of acute and persistent HCV replication (von Hahn et al., 2007). Analysis of neutralization at the time of seroconversion revealed a response that was narrowly directed against the autologous virus (Logvinoff et al., 2004; von Hahn et al., 2007). Broadly cross-reactive antibodies were present 8 months later as persistent infection was established, but they did not neutralize HCVpp containing contemporaneous envelope glycoproteins. Emergence of the cross-reactive response was associated with diversification of E2 and loss of reactivity against the early HVR sequences encoded by the virus during the acute phase of infection (von Hahn et al., 2007). One recent detailed study of persistent viruses from subject H identified mutations in E2 that also facilitated escape from neutralizing antibodies targeting conformation-dependent domain B epitopes (Keck et al., 2009). Taken together, these data indicate that broadening of the neutralizing response was associated with loss of recognition of time-matched envelope glycoproteins, and are consistent with continuous escape of HCV under selection pressure from antibodies (von Hahn et al., 2007). Another study documented that neutralizing antibodies drive HCV envelope sequence evolution in humans that develop chronic infections (Dowd et al., 2009). Importantly, resolution of acute hepatitis C was correlated with an antibody response that effectively neutralized autologous time-matched viruses (Dowd et al., 2009).
E. Attenuation and evasion of the humoral immune response
Studies in subject H indicate that HCV evades antibody responses in part by mutational escape of conformation-dependent epitopes like those in domain B and linear epitopes like HVR-1. HVR-1 may also play a unique role in mutational escape by serving as a decoy for neutralizing antibody responses against other functionally important but less mutable epitopes (Mondelli et al., 2001; Ray et al., 1999; von Hahn et al., 2007). It may be well adapted for this role because chemicophysical properties of HVR-1 important to receptor-mediated cellular entry appear to be highly conserved (Penin et al., 2001; Puntoriero et al., 1998) despite exceptional immunogenicity and capacity for rapid mutation (Mondelli et al., 2001; Ray et al., 1999; von Hahn et al., 2007). In addition to serving as a decoy for neutralizing antibody responses, HVR-1 may physically protect or mask adjacent epitopes from B cell recognition. For instance, most humans fail to recognize the epitope spanning amino acids 413–420 defined by the rodent monoclonal antibodies (Tarr et al., 2007). It has been proposed that this CD81 binding site is concealed from immune recognition by residues 384–401 that comprise the HVR-1 domain (Bankwitz et al., 2010). At least two other mechanisms that attenuate antibody recognition of E2 neutralizing epitopes have been described. The 11 N-linked glycosylation sites of E2 are occupied and mutation of those sites located near residues important for CD81 binding increased the sensitivity of HCV to neutralization by monoclonal antibodies and patient serum (Falkowska et al., 2007; Helle et al., 2007). Finally, it appears that nonneutralizing antibodies can interfere with humoral responses against epitopes critical to virus entry. For instance, antibodies to two E2 epitopes (mapped to amino acids 412–426 and 434–446) were recently isolated from the plasma of humans with chronic hepatitis C. Peptide blockade or immunoaffinity depletion of serum antibodies against the 434–446 epitope revealed potent residual neutralizing activity against the adjacent 412–426 epitope of multiple HCV genotypes (Zhang et al., 2009).
HCV may also evade neutralization by direct cell-to-cell transfer of virus (Timpe et al., 2008; Witteveldt et al., 2009) or masking of E2 epitopes by lipids. Density and sedimentation properties of virions in human serum provided evidence for a physical association with lipoproteins (Kanto et al., 1995; Nielsen et al., 2006; Prince et al., 1996), including β-lipoprotein (Agnello et al., 1999; Prince et al., 1996; Thomssen et al., 1992; Wunschmann et al., 2000). Furthermore, involvement of the VLDL pathway in the assembly and release of HCVcc particles from cultured hepatoma cells has now been documented (Chang et al., 2007; Huang et al., 2007; Lavillette et al., 2005a). The hypothesis that virions associated with lipoproteins are less sensitive to antibody-dependent neutralization is supported by the observation that HDL facilitates HCVpp infection, and reduces the sensitivity of the virus to antibody-mediated neutralization (Bartosch et al., 2005; Dreux et al., 2006; Lavillette et al., 2005a).
IV. CELLULAR IMMUNITY TO HCV
Several observations indicate a critical role for cell-mediated immunity in spontaneous resolution of HCV infection. To summarize, early studies of infection in humans and chimpanzees established a temporal kinetic relationship between initial control of acute phase viremia and expansion of functional CD4+ helper and CD8+ cytotoxic T cells (Bowen and Walker, 2005a; Rehermann, 2009). To prevent persistence, these responses must be sustained past the point that viral genomes are eradicated from host cells. Indeed, as reviewed below, premature failure of the acute phase CD4+ T helper cell response may be the best predictor of persistence. Immunogenetic associations between the outcome of infection and expression of specific HLA class I and II alleles also support the concept that T cells influence the course of infection (Singh et al., 2007). As an example, one recent study confirmed that the DRB1*0101 class II allele and certain alleles belonging to the HLA-B*57 class I group were associated with an absence of HCV RNA in a large multiracial group of HCV seropositive women (Kuniholm et al., 2010). Animal studies have provided the most direct evidence for involvement of T cells in infection outcome. Antibody-mediated depletion of CD4+ or CD8+ prolonged T cells from immune chimpanzees resulted in or persistent infection upon rechallenge with HCV (Grakoui et al., 2003; Shoukry et al., 2003). Moreover, vaccination of chimpanzees with genes encoding nonstructural proteins substantially blunted acute phase viremia after experimental challenge with HCV (Folgori et al., 2006). Because this genetic vaccine did not encode envelope glycoproteins that elicited neutralizing antibodies, it is reasonable to conclude that T cells primed by the nonstructural proteins enhanced acute phase control of HCV replication (Folgori et al., 2006).
A. Methods to study HCV-specific T cell immunity
State of the art methods for determining the frequency, function, and phenotype of antiviral T cells have been adapted for the study of HCV infection in humans and chimpanzees (Yoon and Rehermann, 2009). Briefly, mononuclear cells are typically stimulated ex vivo with synthetic recombinant proteins or overlapping peptide sets that are usually closely matched to the polyprotein encoded by the HCV strain or genotype circulating in the infected individual(s). Virus-specific T cell responses are then measured using readouts like proliferation (for instance, incorporation of 3H-tdr or dilution of a membrane marker dye like CFSE) or cytokine production (by ELISpot or intracellular cytokine staining; Yoon and Rehermann, 2009). A key advantage of the cytokine readout is accurate quantification of functional HCV-specific T cells. If T cells lack function, as is frequently the case in chronic infections, direct visualization of virus-specific populations is preferable. Soluble tetrameric class I molecules that incorporate viral epitopes have been essential for visualization of HCV-specific T cells in persistent HCV infection (Shiina and Rehermann, 2009). Conjugation of a fluorophore to a class I “tetramer” facilitates detection of HCV-specific T cells isolated from blood or liver by flow cytometry regardless of whether they retain effector functions.
Lack of a cell culture model supporting virus replication hindered studies of the interaction between HCV-specific T cells and infected hepatocytes. This situation has improved with the development of genomic and subgenomic HCV replicons and HCVcc strains that replicate in hepatoma cells (Jo et al., 2009; Soderholm et al., 2006; Uebelhoer et al., 2008). For instance, these models can be used to assess activation or inhibition of T lymphocytes by infected cells, susceptibility of virus replication to T cell cytotoxic activity or cytokine production, and the impact of immune escape mutations on viral fitness for replication (Jo et al., 2009; Soderholm et al., 2006; Uebelhoer et al., 2008).
Finally, there are some important limitations and caveats in the study of HCV-specific T cell immunity, particularly in persistently infected individuals. First and foremost, blood is the most accessible tissue but HCV-specific T cells are generally absent or present at low frequency in circulation (Bowen and Walker, 2005a). Whether circulating T cells are representative of intrahepatic populations is controversial. Most HCV-specific T cells are highly localized to the liver where effector functions and phenotype might differ. With the exception of explanted liver obtained during transplant, the tissue is commonly accessed only by percutaneous biopsy. From these small tissue samples, the number of recovered mononuclear cells is usually too low for direct assessment of T cell frequency, phenotype, or function. Moreover, serial liver biopsy of humans is uncommon, particularly during the acute phase of infection, and so evolution of the cellular immune response at the site of infection is difficult to study.
B. Acute phase T cell responses
T cells are present in blood and liver of most humans and chimpanzees with acute hepatitis C but the response is remarkably delayed when compared with many other viral infections. Although the kinetic is highly variable, expansion of T cells in blood is often not evident until 8–12 weeks after infection, coincident with the peak in serum transaminases and initial control of viremia (Fig. 1; Bowen and Walker, 2005a; Rehermann, 2009). This exceptionally long delay in generation of primary T cell immunity is unexplained, but is observed in most infections regardless of outcome. Available data strongly suggest that successful control of infection requires cooperation between CD8+ cytotoxic and CD4+ helper T cells.
1. CD8+ T cells
Acute phase CD8+ T cell responses in chimpanzees and humans that clear the infection are remarkably broad, targeting multiple unique class I restricted epitopes in all viral proteins (Cooper et al., 1999; Lechner et al., 2000b; Thimme et al., 2001, 2002), with the possible exception of the alternative reading frame protein designated F (Drouin et al., 2010). In blood CD8+ T cells frequencies against individual epitopes can exceed 3–4% when measured in functional assays or by tetramer analysis (Lechner et al., 2000b). Frequencies are almost certainly higher in the liver, the site of virus replication. During the acute phase of infection CD8+ T cells appear to expand in blood several days or weeks before they acquire effector functions (Lechner et al., 2000b; Shoukry et al., 2003; Thimme et al., 2001). The significance of this phenomenon is uncertain because effector activity (most notably the ability to produce IFN-γ) eventually recovers and is evident regardless of whether infections ultimately resolve or persist. CD8+ T cells that expand during the acute phase of infection transiently express the coinhibitory molecule programmed cell death 1 (PD-1; Kasprowicz et al., 2008; Radziewicz et al., 2008; Urbani et al., 2006b) and activation markers including HLA class II, CD38, and CD69 (Appay et al., 2002; Lechner et al., 2000a; Thimme et al., 2001; Urbani et al., 2006b). Resolution of HCV infection results in contraction of the CD8+ T cell response and enhanced expression of molecules associated with long-term memory including BcL-2 that inhibits apoptosis and CD127, a component of the IL-7 receptor that is required for self-renewal of memory populations (Badr et al., 2008; Golden-Mason et al., 2006; Urbani et al., 2006a). PD-1 expression is also lost or substantially reduced as virus load declines and the infection is terminated (Bowen et al., 2008; Kasprowicz et al., 2008; Radziewicz et al., 2007).
There is considerable heterogeneity in the strength of acute phase CD8+ T cell responses in those who follow a chronic course. Responses can be transiently detected in the blood of many subjects during acute hepatitis C and sometimes target multiple epitopes (Cox et al., 2005a; Kaplan et al., 2007; Lauer et al., 2005; Lechner et al., 2000a). Differences in the vigor of acute phase CD8+ T cell activity are illustrated by studies in chimpanzees experimentally infected with the virus (Gottwein et al., 2010; Thimme et al., 2002). Two animals in a recent study developed a persistent infection after HCV challenge, but one had CD8+ T cell activity against all HCV proteins and transient control of replication. CD8+ T cells from the other animal targeted one HCV protein and exerted limited if any antiviral activity (Gottwein et al., 2010). Where CD8+ T cell populations have been tracked from the acute to chronic phase of infection, responses that initially target several epitopes narrow dramatically as persistence is established (Cox et al., 2005a; Lauer et al., 2005; Lechner et al., 2000a). Even though responses are often more narrowly focused in the chronic phase of infection, there is no apparent preference for epitopes in the structural, nonstructural, and alternative reading frame proteins (Bain et al., 2004; Ward et al., 2002). It is likely that effector functions are lost sequentially during the transition from the acute to chronic phase of infection, as is the case in LCMV infection of mice where cytotoxicity and production of IL-2 fail early in infection, followed by IFN-γ production just before exhaustion is fully established (Shin and Wherry, 2007).
Intrahepatic CD8+ T cells that are a remnant of an earlier acute phase response can be recovered from the liver of humans and chimpanzees several years after persistence is established (Grabowska et al., 2001; He et al., 1999; Koziel et al., 1992; Meyer-Olson et al., 2004; Nelson et al., 1997; Wong et al., 1998). Many of these populations target class I epitopes that acquired escape mutations years earlier. However, some recognize intact epitopes but provide no apparent control of HCV replication (Erickson et al., 2001; He et al., 1999; Koziel et al., 1992; Nakamoto et al., 2008; Nelson et al., 1997; Neumann-Haefelin et al., 2008; Spangenberg et al., 2005; Wong et al., 1998), probably because they lack effector functions due to exhaustion (Golden-Mason et al., 2007a; Gruener et al., 2001; Kasprowicz et al., 2008; Nakamoto et al., 2008; Radziewicz et al., 2007; Spangenberg et al., 2005). Exhausted HCV-specific CD8+ T cells are prone to apoptosis unless rescued in cell culture by cytokines like IL-2 (Radziewicz et al., 2007). Unlike CD8+ T cells from those who resolve infection, CD127 is usually reduced or undetectable and PD-1 is constitutively expressed (Golden-Mason et al., 2007a; Kasprowicz et al., 2008; Nakamoto et al., 2008; Radziewicz et al., 2007; Urbani et al., 2006b). How T cells targeting intact and mutated epitopes survive in the persistently infected liver without any apparent mechanism for self-renewal is uncertain but probably depends on constant stimulation with HCV antigens. One intriguing possibility is that lymph nodes draining the liver are a site of CD8+ T cell renewal in chronic hepatitis C. Perihepatic lymph nodes from individuals with advanced hepatitis C were recently shown to harbor HCV-specific CD8+ T cells that appeared to retain effector functions when compared with those in the liver and blood (Moonka et al., 2008).
While most HCV-specific CD8+ T cells are exhausted, there is evidence that some populations have alternate functions that could facilitate persistence or modulate the course of infection. For instance, HCV-specific CD8+ T cells that produce IL-10 (Abel et al., 2006; Accapezzato et al., 2004; Kaplan et al., 2008; Rowan et al., 2008) and/or TGF-β (Alatrakchi et al., 2007; Rowan et al., 2008) have been observed in the blood and liver of persistently infected subjects. These cytokines are considered anti-inflammatory and have the potential to block effective antiviral immunity in acute or chronic phases of infection. Why CD8+ T cells would produce this set of suppressive cytokines is unclear, but one recent study has shown that ligation of the viral receptor CD81 drives naïve CD4+ and CD8+ T cells to produce IL-13 (Serra et al., 2008). Antigen-specific stimulation of PBMC from individuals with chronic hepatitis C can also elicit IL-17, a proinflammatory mediator (Billerbeck et al., 2010; Rowan et al., 2008). Production of this cytokine appears to be associated with an expansion of CD8+ T cells that express CD161, a type C lectin also known as NKRP1A (Billerbeck et al., 2010; Northfield et al., 2008). Interestingly, CD8+ T cells with intense cell surface expression of CD161 had a unique phenotypic profile that included expression of the transcription factor retinoic acid-related orphan receptor γ-t, cytokine receptors (IL-23R and IL-18R) and chemokine receptors including CXCR6 that is important for liver homing (Billerbeck et al., 2010). CD8+ T cells that expressed CD161 were markedly enriched in liver and have the potential to modulate HCV replication or liver disease by production of IL-17 and IL-22 (Billerbeck et al., 2010).
2. CD4+ T cells
HCV-specific CD4+ T cells are particularly important to the outcome of infection, at least in part because they provide support for effector CD8+ T cells in acute hepatitis C. Loss of helper activity has been associated with poor CD8+ T cell function (Francavilla et al., 2004; Kaplan et al., 2007, 2008; Urbani et al., 2006a). Moreover, antibody-mediated depletion of CD4+ T cells from immune chimpanzees resulted in failure of CD8+ T cell-mediated control of HCV replication and emergence of viruses with escape mutations in class I restricted epitopes (Grakoui et al., 2003). A key role for CD4+ T cells is also indicated by the striking temporal relationship between control of acute viremia and detection of a functional, multispecific CD4+ T cell response in blood as first documented using proliferation assays (Diepolder et al., 1995; Gerlach et al., 1999; Missale et al., 1996). Acute phase CD4+ T cells are generally present at lower frequency and target fewer epitopes in infections that persist, even though transient responses that are indistinguishable from acute resolving infections have been described (Thimme et al., 2001). More recently, HLA class II tetramers were used to visualize HCV-specific CD4+ T cells in the blood of all HCV-infected subjects regardless of infection outcome (Lucas et al., 2007). CD4+ T cell populations remain detectable in the circulation of subjects who resolved the infection but frequencies dropped below the level of detection in those who followed a persistent course (Day et al., 2003; Lucas et al., 2007). This is consistent with comprehensive mapping of CD4+ T cell responses in individuals with chronic versus resolved infections (Day et al., 2002; Schulze zur Wiesch et al., 2005). CD4+ T cells targeting multiple epitopes were detected in the blood of subjects with resolved but not chronic infections (Day et al., 2002; Schulze zur Wiesch et al., 2005). Studies in humans subjects have demonstrated that transient control of HCV replication is lost if CD4+ T cells lose the ability to proliferate or produce antiviral (proinflammatory) cytokines like IFN-γ or IL-2 during the acute phase of infection (Gerlach et al., 1999; Ulsenheimer et al., 2003, 2006). HCV-specific CD4+ T cells can be recovered from the blood and liver of humans with chronic hepatitis C after stimulation with antigens and cytokines (Penna et al., 2002; Schirren et al., 2000) but whether they are functional in situ is unknown. An analysis of CD4+ T cells in peripheral blood demonstrated a selective loss in the ability to produce IL-2 even though IFN-γ production was retained (Semmo et al., 2005). Explanations for spontaneous CD4+ T cell failure in some infections remain poorly developed even though it is perhaps the most reliable predictor of HCV persistence.
C. Mechanisms for evading and silencing T cell responses
As noted above, CD4+ and CD8+that T cells are primed in most if not all HCV infections persist. Deletion of primed HCV-specific T cells from the repertoire has not been formally demonstrated, although the virus can exploit holes in the TcR repertoire in some individuals who develop a persistent infection (Wolfl et al., 2008). Multiple mechanisms have been proposed to explain the failure of intrahepatic T cells to control HCV infection. Inadequate priming by dysfunctional professional antigen presenting cells and/or an absence of innate signals may impair generation of an adaptive immune response (Szabo and Dolganiuc, 2008), but these features of HCV infection have not yet been precisely correlated with suboptimal T cell differentiation, survival, or effector function. Recombinant viral proteins can modulate T cell function in cell culture models by binding surface receptors. For instance, the HCV core protein can bind the gC1qR complement receptor on T cells, impairing proliferation and interferon-γ production by inhibiting Stat phosphorylation of SOCS signaling molecules (Cummings et al., 2009). Similarly, ligation of CD81 on naive CD4+ and CD8+ recently T cells by the E2 glycoprotein was reported to drive production of IL-13 (Serra et al., 2008). It is important to emphasize that the relevance of these mechanisms identified in cell culture models to HCV persistence has not yet been established in humans or chimpanzees. How binding of an HCV protein to a receptor that is widely expressed on resting or activated lymphocytes leads to a very specific lesion in HCV-specific T cell immunity, without imposing a more global suppressive effect on all T cells, remains to be explained.
Three mechanisms contributing to the evasion and silencing of T cell immunity against HCV will be considered in detail. They include (i) mutational escape of epitopes, (ii) expression of coinhibitory molecules that deliver negative signals to T cells, and (iii) regulatory T cell activity. Emerging data suggest that these mechanisms are interconnected and together may contribute to a profound, virus-specific defect in adaptive cellular immunity. Most published studies describe mechanisms of CD8+ T cell failure, but the fate of CD4+ T cells is also considered where data are available.
1. Mutational escape
The positive-stranded RNA genome of HCV is replicated by the error-prone RNA-dependent RNA polymerase encoded by the viral NS5b gene. The lack of a proofreading mechanism imparts on the virus a remarkable capacity for adaptive mutation. Mutations in class II epitopes have been reported to skew patterns of cytokine production (Wang et al., 2003), and antagonize (Frasca et al., 1999) or abrogate recognition (von Hahn et al., 2007; Wang and Eckels, 1999) by HCV-specific CD4+ T cells. Nevertheless, a pervasive pattern of class I epitope escape has not been observed in naturally infected humans (Fleming et al., 2010) or chimpanzees (Fuller et al., 2010), except perhaps in animals vaccinated before virus challenge (Puig et al., 2006). It is possible that CD4+ T cells do not consistently exert selection pressure against the virus, perhaps because they fail very early in acute infection. These results reinforce the view that helper activity is silenced by other as yet unidentified mechanisms. CD8+ T cells, on the other hand, are a potent selective force that act on HCV genomes to enrich for immune escape variants (Bowen and Walker, 2005b). This was first demonstrated in chimpanzees experimentally infected with a genetically defined HCV inoculum (Weiner et al., 1995). Identification of class I MHC epitopes and sequencing of viruses that emerged during the acute and chronic phases of infection provided the first statistical evidence that CD8+ T cells exert Darwinian selection pressure against some (but not all) class I epitopes (Erickson et al., 2001). This mechanism is also operational in humans where a persistent outcome of infection has been correlated with emergence of HCV escape variants (Cox et al., 2005b; Ray et al., 2005; Tester et al., 2005; Timm et al., 2004). Escape mutations that arise after several years of chronic infection have been described (Erickson et al., 2001; von Hahn et al., 2007). For instance in subject H an escape mutation in a class I epitope was observed more than two decades after persistent infection was established (von Hahn et al., 2007). Nevertheless, late escape may not be a common occurrence. Studies in chimpanzees (Fernandez et al., 2004) and humans (Kuntzen et al., 2007) indicate that the rate of nonsynonymous mutation is highest in the first few weeks or months of persistent infection, consistent with the view that CD8+ T cells can exert selection pressure for a limited period of time before they lose function (Cox et al., 2005a; Urbani et al., 2005).
Large populations of HCV sequences derived from HLA-defined humans have provided phylogenetic evidence of class I mutational escape (Neumann-Haefelin et al., 2008; Poon et al., 2007; Rauch et al., 2009; Timm et al., 2007). While these studies have yielded strong evidence for divergent evolution caused by CD8+ T cell selection pressure, many changes are often in the direction of an ancestral HCV sequence (Ray et al., 2005; Salloum et al., 2008; Timm et al., 2004). This pattern has been interpreted as evidence for reversion of escape variants that impair replicative fitness of the virus. Cell culture models of HCV replication support this view (Ray et al., 2005; Salloum et al., 2008; Timm et al., 2004). Growth of replicons or HCVcc engineered to contain some class I escape mutations is substantially impaired in cultured cells (Dazert et al., 2009; Soderholm et al., 2006; Uebelhoer et al., 2008). One of these studies examined a series of sequential escape mutations that appeared in one epitope of a genotype 1a virus as persistence was established in a chimpanzee (Erickson et al., 2001). Mutations that appeared in the epitope at the earliest time points after infection of the chimpanzee were least fit for replication in the cell culture model (Uebelhoer et al., 2008). It remains to be determined if these escape mutations also impair replication of the virus after it is transmitted to a new host, and the extent to which compensating adaptive mutations increase stability of amino acid substitutions that facilitate escape from antiviral CD8+ T cells.
The issue of whether mutational escape in class I epitopes is a cause or consequence of persistent infection remains unsettled. As noted above, not all class I epitopes targeted by CD8+ T cells escape and so it may not be a requirement for persistence. The percentage of epitopes that undergo mutation is highly variable, ranging from less than 20% of epitopes in some studies (Komatsu et al., 2006; Kuntzen et al., 2007) to greater than 60% in others (Cox et al., 2005b). The importance of escape mutations in some key epitopes is favored by studies in chimpanzees, where there was a statistically significant increase in the rate of nonsynonymous mutation in class I epitopes of viruses that persist when compared with those that are cleared (Erickson et al., 2001). In support of this statistical analysis, an association between escape mutation and loss of immune control early in infection has been documented in one study involving a limited number of subjects (Guglietta et al., 2009). Finally, immune selection pressure driven by CD8+ T cells might be altered by vaccination (Zubkova et al., 2009) or new antiviral therapies that target nonstructural HCV proteins important for virus replication. As an example, known drug resistance sites in the HCV protease and polymerase may overlap with class I epitopes, raising the possibility of HLA-associated immune resistance to the drugs (Gaudieri et al., 2009; Salloum et al., 2010).
2. Inhibitory receptor signaling
As noted above, some HCV class I epitopes remain intact during chronic infection even though cognate CD8+ T cells persist in the liver, sometimes at high frequency (Erickson et al., 2001; Spangenberg et al., 2005). This indicates that mechanism(s) other than mutational escape silence CD8+ T cells in persistent HCV infection. Functional exhaustion of HCV-specific CD8+ T cells targeting intact epitopes is almost certainly critical for persistence and may be mediated or reinforced by signaling through PD-1 that has an intracellular domain containing an immunoreceptor tyrosine-based inhibitory (ITIM) as well as an immunoreceptor tyrosine-based switch (ITSM) motif. PD-1 ligation by its receptors, PD-L1 or PD-L2, impairs induction of the cell survival factor Bcl-xL and expression of transcription factors associated with effector cell function, including GATA-3, Tbet, and Eomes (Keir et al., 2008).
Establishing an association between PD-1 expression on T cells during acute hepatitis and the outcome of infection is complicated because this receptor is naturally upregulated on activated CD8+ T cells. PD-1 expression is elevated on HCV-specific CD8+ T cells during acute hepatitis C, but has not been consistently associated with resolution or persistence of infection (Kasprowicz et al., 2008; Radziewicz et al., 2008; Urbani et al., 2008). Very high levels of PD-1 expression are associated with caspase-9 dependent apoptosis of CD8+ T cells from individuals that ultimately develop a chronic infection (Radziewicz et al., 2008). Regulation of T cell function and survival during the acute phase of infection is probably determined by other positive and negative signals in addition to those delivered by PD-1. As an example, the costimulatory B7 ligand CD86 that is normally present on antigen presenting cells was coexpressed with PD-1 on HCV-specific CD8+ T cells and served as a unique marker of effective IL-2 signaling as measured by the phosphorylation state of STAT-5 (Radziewicz et al., 2010). Loss of CD86 and sustained expression of PD-1 on the T cell surface was observed as HCV persistence was established. Whether expression of molecules like CD86 transiently tempers or counter-balances inhibitory PD-1 signals during acute hepatitis C remains to be determined.
As noted above strong circumstantial evidence implicates PD-1 in maintenance of the persistent state once it has been established. HCV-specific CD8+ T cells in the blood and liver of persistently infected humans display high levels of PD-1 (Golden-Mason et al., 2007b; Penna et al., 2007; Urbani et al., 2006b, 2008) and the intrahepatic populations are prone to apoptosis (Radziewicz et al., 2007). Importantly, antibody-mediated block-ade of PD-1 signaling in cell culture models restores proliferation if not function to these exhausted CD8+ T cells (Golden-Mason et al., 2007b; Nakamoto et al., 2008; Radziewicz et al., 2007; Urbani et al., 2008). High, sustained expression of PD-1 on HCV-specific T cells, combined with restoration of function in the cell culture model by receptor blockade, suggests that anti-PD-1 antibodies could provide a therapeutic effect in patients with chronic infection. Early phase clinical trials of PD-1 blockade have been initiated to test in humans the safety of a strategy that could provide an important adjunct to direct suppression of HCV replication by antiviral agents (see ClinicalTrials.gov identifier NCT00703469 for details).
Studies in the LCMV model of persistent infection predict that multiple inhibitory receptors coregulate CD8+ T cell exhaustion (Blackburn et al., 2009). PD-1 is not the only inhibitory molecule expressed by HCV-specific CD8+ T cells. Inhibitory receptors such as PD1, 2B4 (a SLAM-receptor that can deliver activating or inhibitory signals), CD160 (a coinhibitory glycoylphosphatidylinositol-anchored receptor), and KLRG1 (killer cell-lectin-like receptor G1) were coexpressed by many HCV-specific CD8+ T cells in one study (Bengsch et al., 2010). Collectively, they impaired proliferative capacity and effector functions in persistent infection. The inhibitory receptor cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) was also preferentially upregulated on PD-1-positive T cells from the liver but not blood of chronically HCV-infected patients (Nakamoto et al., 2009). Coexpression of PD-1 and CTLA-4 inhibitory receptors on intrahepatic T cells was associated with a profound loss of virus-specific effector functions that could be reversed in cell culture by simultaneous blockade of the PD-1 and CTLA-4 signaling pathways (Nakamoto et al., 2009). In this study antibody-mediated inhibition of either receptor alone did not restore T cell function. Other receptors may also contribute to HCV-specific T cell exhaustion. Recently, coexpression of PD-1 and Tim-3 (the T cell immunoglobulin and mucin domain-containing molecule 3) was described on CD4+ and CD8+ T cells in chronic hepatitis C, including some intrahepatic populations that were HCV-specific (Golden-Mason et al., 2009). Interference with TIM-3 signaling also restored antigen-stimulated proliferation of T cells and increased IFN-γ production while decreasing IL-10 output (Golden-Mason et al., 2009).
Finally, it is very likely that mutational escape of epitopes and exhaustion mediated by coinhibitory receptor signaling are interdependent. As an example, CD8+ T cells targeting escaped epitopes tend to express higher levels of CD127 and lower levels of PD-1 than those targeting intact epitopes in the acute (Rutebemberwa et al., 2008) and chronic (Bengsch et al., 2010; Kasprowicz et al., 2010) phases of infection. These studies suggest that continuous stimulation with viral antigens reinforces the exhausted phenotype. Expression of CD127, even at low levels, could explain persistence of CD8+ T cells targeting epitopes that have escaped. Whether these T cells truly retain some effector functions or a capacity for self-renewal that distinguishes them from those targeting intact epitopes remains unknown.
3. Regulatory CD8+ T cell activity
Two types of regulatory T cells (Treg) have been defined. They include natural Treg that develop in the specialized environment of the thymus and adaptive Treg that mature extrathymically (Sakaguchi et al., 2010). Phenotypic markers that uniquely define Treg are controversial, but most studies operationally use CD4, the transcription factor forkhead box P3 (FoxP3), and the IL-2Rα chain (CD25) to identify and manipulate these populations (Sakaguchi et al., 2010). Treg are thought to act by production of suppressive cytokines like IL-10 or TGF-β, or by receptor-mediated sequestration of IL-2 that is required for effector T cell activity (Bluestone and Abbas, 2003; Sakaguchi et al., 2010). These functional activities are important for maintenance of immune tolerance but also have the potential to modulate the course of HCV infection (Bluestone and Abbas, 2003; Sakaguchi et al., 2010). It has been proposed that they dampen HCV-specific effector T cell activity during the acute or chronic phases of infection, simultaneously contributing to a persistent state and tempering immunopathological damage to the liver.
At present, there is no direct experimental evidence that Treg activity during acute hepatitis C results in persistent infection. HCV infection of chimpanzees did provoke proliferation of Treg because T cell receptor excision circles in FoxP3-positive cells were diluted when compared to uninfected animals (Manigold et al., 2006). Functional FoxP3-positive CD4+ T cells have been expanded from blood of human subjects during the acute phase of HCV infection, but frequencies did not differ in subjects with acute resolving versus persistent outcomes (Smyk-Pearson et al., 2008). Similar results were obtained when HCV-specific Treg that expressed FoxP3 were visualized with MHC class II tetramers (Heeg et al., 2009). Only transient expansion of adaptive Treg was observed in blood and did not correlate with development of persistent viremia (Heeg et al., 2009). While it is possible that analysis of intrahepatic Treg function would reveal an influence on the outcome of acute hepatitis C, it is perhaps more likely that changes in the frequency and function of these regulatory T cells represent a response to the inflammatory environment in the liver of most infected individuals.
Treg may be active in the chronic phase of infection to reinforce exhaustion of effector T cells. Production of anti-inflammatory mediators like TGF-β and IL-10 by natural and adaptive CD4+ Treg was documented in chronically infected subjects (Ebinuma et al., 2008; Kaplan et al., 2008; Langhans et al., 2010; MacDonald et al., 2002; Ulsenheimer et al., 2003). Virus-responsive Treg from the blood of individuals with chronic hepatitis C have a stable FoxP3 phenotype and function, and may be genetically programmed for survival (Li et al., 2009). Others have documented that depletion of CD25-positive mononuclear cells from PBMC of subjects with chronic hepatitis C increased the frequency of T cells responding to HCV antigen stimulation in proliferation or IFN-γ ELISpot assays (Boettler et al., 2005; Cabrera et al., 2004; Rushbrook et al., 2005; Sugimoto et al., 2003). While this ex vivo experimental approach provided some preliminary circumstantial evidence for enhanced Treg activity in chronic hepatitis C, their importance in maintaining persistent HCV replication in the liver of infected humans has remained unresolved. Immunohistochemical staining has revealed that a high proportion of CD4+ T cells in the liver express the transcriptional regulator FoxP3 (Ward et al., 2007). One recent study confirmed that the liver harbors high frequencies of Foxp3 positive-Treg, and that they suppress effector T cell activity via a contact-dependent mechanism (Franceschini et al., 2009). Impressively, the frequency of intrahepatic Foxp3 positive-Treg correlated directly with serum HCV virus load and inversely with hepatocellular injury. These Treg also expressed exceptionally high levels of PD-1 that modulated suppressive activity upon ligation of PD-L1 that is expressed on liver parenchymal cells. Antibody-mediated inhibition of PD-1 signaling relieved a block on IL-2 driven STAT-5 phosphorylation, resulting in enhanced proliferation and suppressive activity by Treg (Franceschini et al., 2009). Finally, while some of the intrahepatic Treg proliferated in response to HCV antigens, the proportion that are natural versus adaptive (i.e., antigen-specific) remains to be determined.
V. IMMUNITY ACQUIRED BY NATURAL INFECTION CAN PROTECT AGAINST HCV PERSISTENCE: IMPLICATIONS FOR VACCINATION
HCV-specific T cells are detectable in blood for at least two decades after resolution of infection even in humans who no longer have detectable antibody responses to the virus (Takaki et al., 2000). There is evidence that memory T cells primed naturally by successful resolution of infection protect against persistence upon reexposure to the virus. Studies involving humans serially exposed to the virus through intravenous drug use have provided valuable insight into this issue. Virus replication following reinfection of humans who spontaneously cleared a prior HCV infection is well documented so sterilizing immunity is probably uncommon if it occurs at all (Aberle et al., 2006; Aitken et al., 2008a; Grebely et al., 2006; Mehta et al., 2002; Micallef et al., 2007; Mizukoshi et al., 2008; Osburn et al., 2009; Page et al., 2009; van de Laar et al., 2009). However, the magnitude and duration of viremia is substantially decreased in secondary versus primary infection and is associated with HCV-specific T cell immunity (Aberle et al., 2006; Bharadwaj et al., 2009; Mehta et al., 2002; Mizukoshi et al., 2008; Osburn et al., 2009). Critical support for the role of memory T cells in HCV reinfection was generated using the animal model. Rechallenge of immune chimpanzees often resulted in low-level transient virus replication (Bassett et al., 2001; Lanford et al., 2004; Major et al., 2002) that was associated with recall of CD4+ and CD8+ T cell responses (Grakoui et al., 2003; Nascimbeni et al., 2003; Shoukry et al., 2003). Antibody-mediated depletion of CD4+ helper (Grakoui et al., 2003) or CD8+ cytotoxic (Shoukry et al., 2003) T cells from immune chimpanzees caused prolonged or even persistent infection. It is important to note that protection afforded by successful resolution of one HCV infection is not absolute in humans or chimpanzees. This is perhaps best illustrated by an animal study where transient virus replication was observed after multiple sequential infections before persistence was finally established (Bukh et al., 2008). Moreover, some humans with chronic hepatitis C harbor T cells that target HCV strains or genotypes unrelated to the persistent virus (Schulze Zur Wiesch et al., 2007; Sugimoto et al., 2005). It is likely that these T cells are a marker of an earlier resolved infection that ultimately failed to protect against persistence. Documented persistent infection in humans who had spontaneously cleared earlier infection(s) supports this interpretation (Mehta et al., 2002; van de Laar et al., 2009). It is possible that immunity is less effective against heterologous HCV genotypes (Prince et al., 2005), although sequential infection and clearance of unrelated HCV genotypes has been documented in animals (Bukh et al., 2008; Lanford et al., 2004) and humans (Aitken et al., 2008b). A key unanswered question is whether immunity primed by natural infection can be recapitulated by vaccination. As noted above, vaccination of chimpanzees with nonstructural HCV proteins does not prevent infection with HCV (Folgori et al., 2006). However, the course of infection was altered because acute phase viremia was substantially reduced when compared to unvaccinated control animals (Folgori et al., 2006). These preliminary studies suggest that priming of T cells alone could be sufficient to shift the balance of most HCV infections away from persistence and toward resolution. However, the ability of the virus to undermine the response even when replication is substantially controlled cannot be underestimated and a comprehensive approach that involves priming of humoral and cellular immunity may be desirable (Frey et al., 2010; Houghton and Abrignani, 2005).
VI. SUMMARY
HCV is somewhat unique amongst human viruses in its ability to establish either persistent life-long infection or durable immunity that can protect against persistence after reexposure to the virus. This has provided a unique opportunity to define mechanisms of protective immunity and evasion by a small human RNA virus. Mutational escape from humoral and cellular immune responses is a common finding in humans and chimpanzees with a persistent outcome of infection. However, this mechanism alone cannot explain the remarkable ability of HCV to persist. Failure of the CD4+ T helper cell activity early in infection is likely a central event in subversion of B and CD8+ T lymphocyte responses and establishment of persistence. Mechanisms underpinning an HCV-specific defect in CD4+ T cell immunity remain very poorly understood. Closing this gap in knowledge would likely accelerate development of safe and effective vaccines.
HCV can also rapidly acquire resistance to STAT-C inhibitors targeting the protease and polymerase enzymes and so it is likely that they will be used in combination with type I interferon and ribavirin for the foreseeable future. How interferon and ribavirin inhibit the virus is not known, but could involve direct interference with replication, modulation of immunity, or both. Replacement of interferon and ribavirin is desirable because of toxicity and lack of a predictable therapeutic effect in humans. Further studies of adaptive immunity may suggest immunotherapeutic approaches to chronic hepatitis C that could be used in combination with small molecule inhibitors of HCV replication may provide substitutes. For instance, a detailed picture of how the virus utilizes CD81, SR-B1, and the tight junction proteins to initiate infection should facilitate development of attachment and entry inhibitors that may be useful in combination with STAT-C inhibitors. Finally, modulation of cellular immunity by interference with inhibitory signaling pathways like PD-1 and CTLA-4 could conceivably provide a well-defined approach to restoration of host responses capable of eradicating the infection.
References
- Abe K, Inchauspe G, Shikata T, Prince AM. Three different patterns of hepatitis C virus infection in chimpanzees. Hepatology. 1992;15:690. doi: 10.1002/hep.1840150423. [DOI] [PubMed] [Google Scholar]
- Abel M, Sene D, Pol S, Bourliere M, Poynard T, Charlotte F, Cacoub P, Caillat-Zucman S. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology. 2006;44:1607. doi: 10.1002/hep.21438. [DOI] [PubMed] [Google Scholar]
- Aberle JH, Formann E, Steindl-Munda P, Weseslindtner L, Gurguta C, Perstinger G, Grilnberger E, Laferl H, Dienes HP, Popow-Kraupp T, Ferenci P, Holzmann H. Prospective study of viral clearance and CD4(+) T-cell response in acute hepatitis C primary infection and reinfection. J Clin Virol. 2006;36:24. doi: 10.1016/j.jcv.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96:12766. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken CK, Lewis J, Tracy SL, Spelman T, Bowden DS, Bharadwaj M, Drummer H, Hellard M. High incidence of hepatitis C virus reinfection in a cohort of injecting drug users. Hepatology. 2008a;48:1746. doi: 10.1002/hep.22534. [DOI] [PubMed] [Google Scholar]
- Aitken CK, Tracy SL, Revill P, Bharadwaj M, Bowden DS, Winter RJ, Hellard ME. Consecutive infections and clearances of different hepatitis C virus genotypes in an injecting drug user. J Clin Virol. 2008b;41:293. doi: 10.1016/j.jcv.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol. 2007;81:5882. doi: 10.1128/JVI.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter HJ, Holland PV, Morrow AG, Purcell RH, Feinstone SM, Moritsugu Y. Clinical and serological analysis of transfusion-associated hepatitis. Lancet. 1975;2:838. doi: 10.1016/s0140-6736(75)90234-2. [DOI] [PubMed] [Google Scholar]
- Alter HJ, Purcell RH, Holland PV, Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978;1:459. doi: 10.1016/s0140-6736(78)90131-9. [DOI] [PubMed] [Google Scholar]
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, et al. Memory CD8 T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Arase Y, Ikeda K, Chayama K, Murashima N, Tsubota A, Suzuki Y, Saitoh S, Kobayashi M, Suzuki F, Kumada H. Fluctuation patterns of HCV-RNA serum level in patients with chronic hepatitis C. J Gastroenterol. 2000;35:221. doi: 10.1007/s005350050334. [DOI] [PubMed] [Google Scholar]
- Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sekaly RP, Bruneau J, Shoukry NH. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008;82:10017. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain C, Parroche P, Lavergne JP, Duverger B, Vieux C, Dubois V, Komurian-Pradel F, Trepo C, Gebuhrer L, Paranhos-Baccala G, Penin F, Inchauspe G. Memory T-cell-mediated immune responses specific to an alternative core protein in hepatitis C virus infection. J Virol. 2004;78:10460. doi: 10.1128/JVI.78.19.10460-10469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankwitz D, Steinmann E, Bitzegeio J, Ciesek S, Friesland M, Herrmann E, Zeisel MB, Baumert TF, Keck ZY, Foung SK, Pecheur EI, Pietschmann T. Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J Virol. 2010;84:5751. doi: 10.1128/JVI.02200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth H, Schnober EK, Zhang F, Linhardt RJ, Depla E, Boson B, Cosset FL, Patel AH, Blum HE, Baumert TF. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J Virol. 2006;80:10579. doi: 10.1128/JVI.00941-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosch B, Bukh J, Meunier JC, Granier C, Engle RE, Blackwelder WC, Emerson SU, Cosset FL, Purcell RH. In vitro assay for neutralizing antibody to hepatitis C virus: Evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci USA. 2003a;100:14199. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003b;197:633. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S, Scarselli E, Cortese R, Nicosia A, Cosset FL. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003c;278:41624. doi: 10.1074/jbc.M305289200. [DOI] [PubMed] [Google Scholar]
- Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, Pawlotsky JM, Lavillette D, Cosset FL. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SE, Brasky KM, Lanford RE. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR, Lanford RE. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- Baumert TF, Wellnitz S, Aono S, Satoi J, Herion D, Tilman Gerlach J, Pape GR, Lau JY, Hoofnagle JH, Blum HE, Liang TJ. Antibodies against hepatitis C virus-like particles and viral clearance in acute and chronic hepatitis C. Hepatology. 2000;32:610. doi: 10.1053/jhep.2000.9876. [DOI] [PubMed] [Google Scholar]
- Benedicto I, Molina-Jimenez F, Bartosch B, Cosset FL, Lavillette D, Prieto J, Moreno-Otero R, Valenzuela-Fernandez A, Aldabe R, Lopez-Cabrera M, Majano PL. The tight junction-associated protein occludin is required for a postbinding step in hepatitis C virus entry and infection. J Virol. 2009;83:8012. doi: 10.1128/JVI.00038-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj M, Thammanichanond D, Aitken CK, Moneer S, Drummer HE, Tracy S, Holdsworth R, Bowden S, Jackson D, Hellard M, Torresi J, McCluskey J. TCD8 response in diverse outcomes of recurrent exposure to hepatitis C virus. Immunol Cell Biol. 2009;87:464. doi: 10.1038/icb.2009.24. [DOI] [PubMed] [Google Scholar]
- Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, Ramamurthy N, Zitzmann N, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA. 2010;107:3006. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzegeio J, Bankwitz D, Hueging K, Haid S, Brohm C, Zeisel MB, Herrmann E, Iken M, Ott M, Baumert TF, Pietschmann T. Adaptation of hepatitis C virus to mouse CD81 permits infection of mouse cells in the absence of human entry factors. PLoS Pathog. 2010;6:e1000978. doi: 10.1371/journal.ppat.1000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouille Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsacker F, Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JC, Kumar U, Webster D, Monjardino J, Thomas HC. Comparison of the rate of sequence variation in the hypervariable region of E2/NS1 region of hepatitis C virus in normal and hypogammaglobulinemic patients. Hepatology. 1998;27:223. doi: 10.1002/hep.510270134. [DOI] [PubMed] [Google Scholar]
- Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005a;436:946. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med. 2005b;201:1709. doi: 10.1084/jem.20050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen DG, Shoukry NH, Grakoui A, Fuller MJ, Cawthon AG, Dong C, Hasselschwert DL, Brasky KM, Freeman GJ, Seth NP, Wucherpfennig KW, Houghton M, et al. Variable patterns of programmed death-1 expression on fully functional memory T cells after spontaneous resolution of hepatitis C virus infection. J Virol. 2008;82:5109. doi: 10.1128/JVI.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazzoli M, Bianchi A, Filippini S, Weiner A, Zhu Q, Pizza M, Crotta S. CD81 is a central regulator of cellular events required for hepatitis C virus infection of human hepatocytes. J Virol. 2008;82:8316. doi: 10.1128/JVI.00665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering TJ, Garrity KA, Boatright NK, Sloan SE, Sandor F, Thomas WD, Jr, Szabo G, Finberg RW, Ambrosino DM, Babcock GJ. Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. J Virol. 2009;83:12473. doi: 10.1128/JVI.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J, Thimme R, Meunier JC, Faulk K, Spangenberg HC, Chang KM, Satterfield W, Chisari FV, Purcell RH. Previously infected chimpanzees are not consistently protected against reinfection or persistent infection after reexposure to the identical hepatitis C virus strain. J Virol. 2008;82:8183. doi: 10.1128/JVI.00142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlone ME, Budkowska A. Hepatitis C virus cell entry: role of lipoproteins and cellular receptors. J Gen Virol. 2009;90:1055. doi: 10.1099/vir.0.008300-0. [DOI] [PubMed] [Google Scholar]
- Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis virus infection. Hepatology. 2004;40:1062. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- Catanese MT, Ansuini H, Graziani R, Huby T, Moreau M, Ball JK, Paonessa G, Rice CM, Cortese R, Vitelli A, Nicosia A. Role of scavenger receptor class B type I in hepatitis C virus entry: Kinetics and molecular determinants. J Virol. 2010;84:34. doi: 10.1128/JVI.02199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KS, Jiang J, Cai Z, Luo G. Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J Virol. 2007;81:13783. doi: 10.1128/JVI.01091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Sallberg M, Sonnerborg A, Weiland O, Mattsson L, Jin L, Birkett A, Peterson D, Milich DR. Limited humoral immunity in hepatitis C virus infection. Gastroenterology. 1999;116:135. doi: 10.1016/s0016-5085(99)70237-4. [DOI] [PubMed] [Google Scholar]
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Christie JM, Healey CJ, Watson J, Wong VS, Duddridge M, Snowden N, Rosenberg WM, Fleming KA, Chapel H, Chapman RW. Clinical outcome of hypogammaglobulinaemic patients following outbreak of acute hepatitis C: 2 year follow up. Clin Exp Immunol. 1997;110:4. doi: 10.1046/j.1365-2249.1997.5081412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller KE, Berger KL, Heaton NS, Cooper JD, Yoon R, Randall G. RNA interference and single particle tracking analysis of hepatitis C virus endocytosis. PLoS Pathog. 2009;5:e1000702. doi: 10.1371/journal.ppat.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, Houghton M, Parham P, Walker CM. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- Cormier EG, Durso RJ, Tsamis F, Boussemart L, Manix C, Olson WC, Gardner JP, Dragic T. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc Natl Acad Sci USA. 2004;101:14067. doi: 10.1073/pnas.0405695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005a;42:104. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, Sidney J, Sette A, Pardoll D, Thomas DL, Ray SC. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005b;201:1741. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KL, Rosen HR, Hahn YS. Frequency of gC1qR(+)CD4(+) T cells increases during acute hepatitis C virus infection and remains elevated in patients with chronic infection. Clin Immunol. 2009;132:401. doi: 10.1016/j.clim.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Lauer GM, Robbins GK, McGovern B, Wurcel AG, Gandhi RT, Chung RT, Walker BD. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J Virol. 2002;76:12584. doi: 10.1128/JVI.76.24.12584-12595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Seth NP, Lucas M, Appel H, Gauthier L, Lauer GM, Robbins GK, Szczepiorkowski ZM, Casson DR, Chung RT, Bell S, Harcourt G, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112:831. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazert E, Neumann-Haefelin C, Bressanelli S, Fitzmaurice K, Kort J, Timm J, McKiernan S, Kelleher D, Gruener N, Tavis JE, Rosen HR, Shaw J, et al. Loss of viral fitness and cross-recognition by CD8+ T cells limit HCV escape from a protective HLA-B27-restricted human immune response. J Clin Invest. 2009;119:376. doi: 10.1172/JCI36587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon S, Witteveldt J, Gatherer D, Owsianka AM, Zeisel MB, Zahid MN, Rychlowska M, Foung SK, Baumert TF, Angus AG, Patel AH. Mutations within a conserved region of the hepatitis C virus E2 glycoprotein that influence virus-receptor interactions and sensitivity to neutralizing antibodies. J Virol. 2010;84:5494. doi: 10.1128/JVI.02153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, Eichenlaub D, Pape GR. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- Dittmann S, Roggendorf M, Durkop J, Wiese M, Lorbeer B, Deinhardt F. Long-term persistence of hepatitis C virus antibodies in a single source outbreak. J Hepatol. 1991;13:323. doi: 10.1016/0168-8278(91)90076-n. [DOI] [PubMed] [Google Scholar]
- Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M, Pietschmann T, Granier C, Voisset C, Ricard-Blum S, Mangeot PE, Keck Z, Foung S, Vu-Dac N, Dubuisson J, Bartenschlager R, Lavillette D, et al. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J Biol Chem. 2006;281:18285. doi: 10.1074/jbc.M602706200. [DOI] [PubMed] [Google Scholar]
- Drouin C, Lamarche S, Bruneau J, Soudeyns H, Shoukry NH. Cell-mediated immune responses directed against hepatitis C virus (HCV) alternate reading frame protein (ARFP) are undetectable during acute infection. J Clin Virol. 2010;47:102. doi: 10.1016/j.jcv.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummer HE, Maerz A, Poumbourios P. Cell surface expression of functional hepatitis C virus E1 and E2 glycoproteins. FEBS Lett. 2003;546:385. doi: 10.1016/s0014-5793(03)00635-5. [DOI] [PubMed] [Google Scholar]
- Dubuisson J, Rice CM. Hepatitis C virus glycoprotein folding: Disulfide bond formation and association with calnexin. J Virol. 1996;70:778. doi: 10.1128/jvi.70.2.778-786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, Tobias J, Kwok WW, Chang KM. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J Virol. 2008;82:5043. doi: 10.1128/JVI.01548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson AL, Kimura Y, Igarashi S, Eichelberger J, Houghton M, Sidney J, McKinney D, Sette A, Hughes AL, Walker CM. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity. 2001;15:883. doi: 10.1016/s1074-7613(01)00245-x. [DOI] [PubMed] [Google Scholar]
- Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- Falkowska E, Kajumo F, Garcia E, Reinus J, Dragic T. Hepatitis C virus envelope glycoprotein E2 glycans modulate entry, CD81 binding, and neutralization. J Virol. 2007;81:8072. doi: 10.1128/JVI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CT, Tobler LH, Haesche C, Busch MP, Phelps B, Leparc G. Fluctuation of HCV viral load before seroconversion in a healthy volunteer blood donor. Transfusion. 2003;43:541. doi: 10.1046/j.1537-2995.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- Fanning L, Kenny-Walsh E, Levis J, Choudhury KR, Cannon B, Sheehan M, Whelton M, Shanahan F. Natural fluctuations of hepatitis C viral load in a homogeneous patient population: A prospective study. Hepatology. 2000;31:225. doi: 10.1002/hep.510310133. [DOI] [PubMed] [Google Scholar]
- Farci P, Shimoda A, Wong D, Cabezon T, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter HJ, Purcell RH. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci USA. 1996;93:15394. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A, Purcell RH, Alter HJ. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med. 1975;292:767. doi: 10.1056/NEJM197504102921502. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Taylor D, Morhardt DR, Mihalik K, Puig M, Rice CM, Feinstone SM, Major ME. Long-term persistence of infection in chimpanzees inoculated with an infectious hepatitis C virus clone is associated with a decrease in the viral amino acid substitution rate and low levels of heterogeneity. J Virol. 2004;78:9782. doi: 10.1128/JVI.78.18.9782-9789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming VM, Harcourt G, Barnes E, Klenerman P. Virological footprint of CD4+ T-cell responses during chronic hepatitis C virus infection. J Gen Virol. 2010;91:1396. doi: 10.1099/vir.0.017699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint M, von Hahn T, Zhang J, Farquhar M, Jones CT, Balfe P, Rice CM, McKeating JA. Diverse CD81 proteins support hepatitis C virus infection. J Virol. 2006;80:11331. doi: 10.1128/JVI.00104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, Lahm A, Luzzago A, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- Foy E, Li K, Wang C, Sumpter R, Jr, Ikeda M, Lemon SM, Gale M., Jr Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300:1145. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- Francavilla V, Accapezzato D, De Salvo M, Rawson P, Cosimi O, Lipp M, Cerino A, Cividini A, Mondelli MU, Barnaba V. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: Exploring the immunological mechanisms. Eur J Immunol. 2004;34:427. doi: 10.1002/eji.200324539. [DOI] [PubMed] [Google Scholar]
- Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca L, Del Porto P, Tuosto L, Marinari B, Scotta C, Carbonari M, Nicosia A, Piccolella E. Hypervariable region 1 variants act as TCR antagonists for hepatitis C virus-specific CD4+ T cells. J Immunol. 1999;163:650. [PubMed] [Google Scholar]
- Frey SE, Houghton M, Coates S, Abrignani S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie A, Rinella P, Hill H, Wolff MC, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28:6367. doi: 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MJ, Shoukry NH, Gushima T, Bowen DG, Callendret B, Campbell KJ, Hasselschwert DL, Hughes AL, Walker CM. Selection-driven immune escape is not a significant factor in the failure of CD4 T cell responses in persistent hepatitis C virus infection. Hepatology. 2010;51:378. doi: 10.1002/hep.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- Gal-Tanamy M, Keck ZY, Yi M, McKeating JA, Patel AH, Foung SK, Lemon SM. In vitro selection of a neutralization-resistant hepatitis C virus escape mutant. Proc Natl Acad Sci USA. 2008;105:19450. doi: 10.1073/pnas.0809879105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudieri S, Rauch A, Pfafferott K, Barnes E, Cheng W, McCaughan G, Shackel N, Jeffrey GP, Mollison L, Baker R, Furrer H, Gunthard HF, et al. Hepatitis C virus drug resistance and immune-driven adaptations: Relevance to new antiviral therapy. Hepatology. 2009;49:1069. doi: 10.1002/hep.22773. [DOI] [PubMed] [Google Scholar]
- Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- Golden-Mason L, Burton JR, Jr, Castelblanco N, Klarquist J, Benlloch S, Wang C, Rosen HR. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44:1098. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic HCV-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007a;81:9249. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007b;81:9249. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon B, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 Is overexpressed in hepatitis C infection and its blockade rescues dysfunctional Cd4+ and Cd8+ T cells. J Virol. 2009;83:9122. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SC, Dailey PJ, Silverman AL, Khan BA, Kodali VP, Wilber JC. Sequential serum hepatitis C viral RNA levels longitudinally assessed by branched DNA signal amplification. Hepatology. 1998;28:1702. doi: 10.1002/hep.510280634. [DOI] [PubMed] [Google Scholar]
- Gottwein JM, Scheel TK, Callendret B, Li YP, Eccleston HB, Engle RE, Govindarajan S, Satterfield W, Purcell RH, Walker CM, Bukh J. Novel infectious cDNA clones of hepatitis C virus genotype 3a (strain S52) and 4a (strain ED43): Genetic analyses and in vivo pathogenesis studies. J Virol. 2010;84:5277. doi: 10.1128/JVI.02667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska AM, Lechner F, Klenerman P, Tighe PJ, Ryder S, Ball JK, Thomson BJ, Irving WL, Robins RA. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur J Immunol. 2001;31:2388. doi: 10.1002/1521-4141(200108)31:8<2388::aid-immu2388>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139. doi: 10.1002/hep.21376. [DOI] [PubMed] [Google Scholar]
- Grove J, Huby T, Stamataki Z, Vanwolleghem T, Meuleman P, Farquhar M, Schwarz A, Moreau M, Owen JS, Leroux-Roels G, Balfe P, McKeating JA. Scavenger receptor BI and BII expression levels modulate hepatitis C virus infectivity. J Virol. 2007;81:3162. doi: 10.1128/JVI.02356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, Klenerman P. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglietta S, Garbuglia AR, Salichos L, Ruggeri L, Folgori A, Perrone MP, Camperio C, Mellace V, Maio G, Maio P, Capobianchi MR, Spada E, et al. Impact of viral selected mutations on T cell mediated immunity in chronically evolving and self limiting acute HCV infection. Virology. 2009;386:398. doi: 10.1016/j.virol.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Haid S, Windisch MP, Bartenschlager R, Pietschmann T. Mouse-specific residues of claudin-1 limit hepatitis C virus genotype 2a infection in a human hepatocyte cell line. J Virol. 2010;84:964. doi: 10.1128/JVI.01504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HJ, Davis C, Mullins JG, Hu K, Goodall M, Farquhar MJ, Mee CJ, McCaffrey K, Young S, Drummer H, Balfe P, McKeating JA. Claudin association with CD81 defines hepatitis C virus entry. J Biol Chem. 2010;285:21092. doi: 10.1074/jbc.M110.104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XS, Rehermann B, Lopez-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright TL, Davis MM, Greenberg HB. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg MH, Ulsenheimer A, Gruner NH, Jung MC, Gerlach JT, Raziorrouh B, Schraut W, Horster S, Kauke T, Spannagl M, Diepolder HM. FOXP3+ expression in hepatitis C virus specific CD4+ T cells during acute hepatitis C. Gastroenterology. 2009;137:1280. doi: 10.1053/j.gastro.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Helle F, Goffard A, Morel V, Duverlie G, McKeating J, Keck ZY, Foung S, Penin F, Dubuisson J, Voisset C. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol. 2007;81:8101. doi: 10.1128/JVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger FB, Gitnick GL, Aach RD, Szmuness W, Mosley JW, Stevens CE, Peters RL, Weiner JM, Werch JB, Lander JJ. Non-A, non-B hepatitis transmission in chimpanzees: A project of the transfusion-transmitted viruses study group. Intervirology. 1978;10:60. doi: 10.1159/000148969. [DOI] [PubMed] [Google Scholar]
- Houghton M. Discovery of the hepatitis C virus. Liver Int. 2009;29(Suppl 1):82. doi: 10.1111/j.1478-3231.2008.01925.x. [DOI] [PubMed] [Google Scholar]
- Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA. 2003;100:7271. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M, Jr, Ye J. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Aichele U, Kersting N, Klein R, Aichele P, Bisse E, Sewell AK, Blum HE, Bartenschlager R, Lohmann V, Thimme R. Analysis of CD8+ T-cell-mediated inhibition of hepatitis C virus replication using a novel immunological model. Gastroenterology. 2009;136:1391. doi: 10.1053/j.gastro.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Johansson DX, Voisset C, Tarr AW, Aung M, Ball JK, Dubuisson J, Persson MA. Human combinatorial libraries yield rare antibodies that broadly neutralize hepatitis C virus. Proc Natl Acad Sci USA. 2007;104:16269. doi: 10.1073/pnas.0705522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanto T, Hayashi N, Takehara T, Hagiwara H, Mita E, Naito M, Kasahara A, Fusamoto H, Kamada T. Density analysis of hepatitis C virus particle population in the circulation of infected hosts: Implications for virus neutralization or persistence. J Hepatol. 1995;22:440. doi: 10.1016/0168-8278(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81:374. doi: 10.1128/JVI.01134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH, Reddy KR, McKeating JA, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Kaplan DE, Ikeda F, Li Y, Nakamoto N, Ganesan S, Valiga ME, Nunes FA, Rajender Reddy K, Chang KM. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J Hepatol. 2008;48:903. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N, Kwok WW, McGovern BG, et al. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz V, Kang YH, Lucas M, Schulze zur Wiesch J, Kuntzen T, Fleming V, Nolan BE, Longworth S, Berical A, Bengsch B, Thimme R, Lewis-Ximenez L, et al. Hepatitis C virus (HCV) sequence variation induces an HCV-specific T-cell phenotype analogous to spontaneous resolution. J Virol. 2010;84:1656. doi: 10.1128/JVI.01499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, Shimotohno K. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, Ootsuyama Y, Sekiya H, Ohkoshi S, Nakazawa T, Hijikata M, Shimotohno K. Genetic drift in hypervariable region 1 of the viral genome in persistent hepatitis C virus infection. J Virol. 1994;68:4776. doi: 10.1128/jvi.68.8.4776-4784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ZY, Op De Beeck A, Hadlock KG, Xia J, Li TK, Dubuisson J, Foung SK. Hepatitis C virus E2 has three immunogenic domains containing conformational epitopes with distinct properties and biological functions. J Virol. 2004;78:9224. doi: 10.1128/JVI.78.17.9224-9232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ZY, Li TK, Xia J, Bartosch B, Cosset FL, Dubuisson J, Foung SK. Analysis of a highly flexible conformational immunogenic domain a in hepatitis C virus E2. J Virol. 2005;79:13199. doi: 10.1128/JVI.79.21.13199-13208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ZY, Xia J, Cai Z, Li TK, Owsianka AM, Patel AH, Luo G, Foung SK. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J Virol. 2007;81:1043. doi: 10.1128/JVI.01710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ZY, Li TK, Xia J, Gal-Tanamy M, Olson O, Li SH, Patel AH, Ball JK, Lemon SM, Foung SK. Definition of a conserved immunodominant domain on hepatitis C virus E2 glycoprotein by neutralizing human monoclonal antibodies. J Virol. 2008a;82:6061. doi: 10.1128/JVI.02475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ZY, Olson O, Gal-Tanamy M, Xia J, Patel AH, Dreux M, Cosset FL, Lemon SM, Foung SK. A point mutation leading to hepatitis C virus escape from neutralization by a monoclonal antibody to a conserved conformational epitope. J Virol. 2008b;82:6067. doi: 10.1128/JVI.00252-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ZY, Li SH, Xia J, von Hahn T, Balfe P, McKeating JA, Witteveldt J, Patel AH, Alter H, Rice CM, Foung SK. Mutations in hepatitis C virus E2 located outside the CD81 binding sites lead to escape from broadly neutralizing antibodies but compromise virus infectivity. J Virol. 2009;83:6149. doi: 10.1128/JVI.00248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Lauer G, Pybus OG, Ouchi K, Wong D, Ward S, Walker B, Klenerman P. Do antiviral CD8+ T cells select hepatitis C virus escape mutants? Analysis in diverse epitopes targeted by human intrahepatic CD8+ T lymphocytes. J Viral Hepat. 2006;13:121. doi: 10.1111/j.1365-2893.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- Koziel MJ, Dudley D, Wong JT, Dienstag J, Houghton M, Ralston R, Walker BD. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992;149:3339. [PubMed] [Google Scholar]
- Krawczynski K, Alter MJ, Tankersley DL, Beach M, Robertson BH, Lambert S, Kuo G, Spelbring JE, Meeks E, Sinha S, Carson DA. Effect of immune globulin on the prevention of experimental hepatitis C virus infection. J Infect Dis. 1996;173:822. doi: 10.1093/infdis/173.4.822. [DOI] [PubMed] [Google Scholar]
- Krey T, d’Alayer J, Kikuti CM, Saulnier A, Damier-Piolle L, Petitpas I, Johansson DX, Tawar RG, Baron B, Robert B, England P, Persson MA, et al. The disulfide bonds in glycoprotein E2 of hepatitis C virus reveal the tertiary organization of the molecule. PLoS Pathog. 2010;6:e1000762. doi: 10.1371/journal.ppat.1000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger SE, Zeisel MB, Davis C, Thumann C, Harris HJ, Schnober EK, Mee C, Soulier E, Royer C, Lambotin M, Grunert F, Dao Thi VL, et al. Inhibition of hepatitis C virus infection by anti-claudin-1 antibodies is mediated by neutralization of E2-CD81-claudin-1 associations. Hepatology. 2010;51:1144. doi: 10.1002/hep.23445. [DOI] [PubMed] [Google Scholar]
- Kuniholm MH, Kovacs A, Gao X, Xue X, Marti D, Thio CL, Peters MG, Terrault NA, Greenblatt RM, Goedert JJ, Cohen MH, Minkoff H, et al. Specific human leukocyte antigen class I and II alleles associated with hepatitis C virus viremia. Hepatology. 2010;51:1514. doi: 10.1002/hep.23515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntzen T, Timm J, Berical A, Lewis-Ximenez LL, Jones A, Nolan B, Schulze zur Wiesch J, Li B, Schneidewind A, Kim AY, Chung RT, Lauer GM, et al. Viral sequence evolution in acute hepatitis C virus infection. J Virol. 2007;81:11658. doi: 10.1128/JVI.00995-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagging LM, Meyer K, Westin J, Wejstal R, Norkrans G, Lindh M, Ray R. Neutralization of pseudotyped vesicular stomatitis virus expressing hepatitis C virus envelope glycoprotein 1 or 2 by serum from patients. J Infect Dis. 2002;185:1165. doi: 10.1086/339679. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Bigger C, Bassett S, Klimpel G. The chimpanzee model of hepatitis C virus infections. ILAR J. 2001;42:117. doi: 10.1093/ilar.42.2.117. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang XH, Ray SC, Thomas DL. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans B, Braunschweiger I, Arndt S, Schulte W, Satoguina J, Layland LE, Vidovic N, Hoerauf A, Oldenburg J, Sauerbruch T, Spengler U. Corespecific adaptive regulatory T-cells in different outcomes of hepatitis C. Clin Sci (Lond) 2010;119:97. doi: 10.1042/CS20090661. [DOI] [PubMed] [Google Scholar]
- Larghi A, Zuin M, Crosignani A, Ribero ML, Pipia C, Battezzati PM, Binelli G, Donato F, Zanetti AR, Podda M, Tagger A. Outcome of an outbreak of acute hepatitis C among healthy volunteers participating in pharmacokinetics studies. Hepatology. 2002;36:993. doi: 10.1053/jhep.2002.36129. [DOI] [PubMed] [Google Scholar]
- Lauer GM, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Schulze Zur Wiesch J, Paranhos-Baccala G, Sheridan I, Casson DR, Reiser M, Gandhi RT, et al. Full-breadth analysis of CD8+ T-cell responses in acute hepatitis C virus infection and early therapy. J Virol. 2005;79:12979. doi: 10.1128/JVI.79.20.12979-12988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie M, Goffard A, Dubuisson J. Assembly of a functional HCV glycoprotein heterodimer. Curr Issues Mol Biol. 2007;9:71. [PubMed] [Google Scholar]
- Lavillette D, Morice Y, Germanidis G, Donot P, Soulier A, Pagkalos E, Sakellariou G, Intrator L, Bartosch B, Pawlotsky JM, Cosset FL. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005a;79:6023. doi: 10.1128/JVI.79.10.6023-6034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavillette D, Tarr AW, Voisset C, Donot P, Bartosch B, Bain C, Patel AH, Dubuisson J, Ball JK, Cosset FL. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology. 2005b;41:265. doi: 10.1002/hep.20542. [DOI] [PubMed] [Google Scholar]
- Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, Ball JK, McKeating JA, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, Cerny A, Phillips R, Ferrari C, Pape GR, Klenerman P. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000a;30:2479. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000b;191:1499. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M, Jr, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Floess S, Hamann A, Gaudieri S, Lucas A, Hellard M, Roberts S, Paukovic G, Plebanski M, Loveland BE, Aitken C, Barry S, et al. Analysis of FOXP3+ regulatory T cells that display apparent viral antigen specificity during chronic hepatitis C virus infection. PLoS Pathog. 2009;5:e1000707. doi: 10.1371/journal.ppat.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- Liu S, Yang W, Shen L, Turner JR, Coyne CB, Wang T. Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent superinfection. J Virol. 2009;83:2011. doi: 10.1128/JVI.01888-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci USA. 2004;101:10149. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozach PY, Lortat-Jacob H, de Lacroix de Lavalette A, Staropoli I, Foung S, Amara A, Houles C, Fieschi F, Schwartz O, Virelizier JL, Arenzana-Seisdedos F, Altmeyer R. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J Biol Chem. 2003;278:20358. doi: 10.1074/jbc.M301284200. [DOI] [PubMed] [Google Scholar]
- Lozach PY, Amara A, Bartosch B, Virelizier JL, Arenzana-Seisdedos F, Cosset FL, Altmeyer R. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J Biol Chem. 2004;279:32035. doi: 10.1074/jbc.M402296200. [DOI] [PubMed] [Google Scholar]
- Lucas M, Ulsenheimer A, Pfafferot K, Heeg MH, Gaudieri S, Gruner N, Rauch A, Gerlach JT, Jung MC, Zachoval R, Pape GR, Schraut W, et al. Tracking virus-specific CD4+ T cells during and after acute hepatitis C virus infection. PLoS ONE. 2007;2:e649. doi: 10.1371/journal.pone.0000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AJ, Duffy M, Brady MT, McKiernan S, Hall W, Hegarty J, Curry M, Mills KH. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J Infect Dis. 2002;185:720. doi: 10.1086/339340. [DOI] [PubMed] [Google Scholar]
- Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM, Piccirillo CA, Rehermann B. Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424. doi: 10.1182/blood-2005-09-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern BH, Birch CE, Bowen MJ, Reyor LL, Nagami EH, Chung RT, Kim AY. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis. 2009;49:1051. doi: 10.1086/605561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee CJ, Harris HJ, Farquhar MJ, Wilson G, Reynolds G, Davis C, van ISC, Balfe P, McKeating JA, McKeating JA. Polarization restricts hepatitis C virus entry into HepG2 hepatoma cells. J Virol. 2009;83:6211. doi: 10.1128/JVI.00246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L, Bertaux C, Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol. 2006;80:11571. doi: 10.1128/JVI.01717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L, Bertaux C, Cukierman L, Cormier E, Lavillette D, Cosset FL, Dragic T. The tight junction proteins claudin-1, -6, and -9 are entry cofactors for hepatitis C virus. J Virol. 2008;82:3555. doi: 10.1128/JVI.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Cox A, Hoover DR, Wang XH, Mao Q, Ray S, Strathdee SA, Vlahov D, Thomas DL. Protection against persistence of hepatitis C. Lancet. 2002;359:1478. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- Meuleman P, Hesselgesser J, Paulson M, Vanwolleghem T, Desombere I, Reiser H, Leroux-Roels G. Anti-CD81 antibodies can prevent a hepatitis C virus infection in vivo. Hepatology. 2008;48:1761. doi: 10.1002/hep.22547. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Engle RE, Faulk K, Zhao M, Bartosch B, Alter H, Emerson SU, Cosset FL, Purcell RH, Bukh J. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc Natl Acad Sci USA. 2005;102:4560. doi: 10.1073/pnas.0501275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Basu A, Ray R. Functional features of hepatitis C virus glycoproteins for pseudotype virus entry into mammalian cells. Virology. 2000;276:214. doi: 10.1006/viro.2000.0547. [DOI] [PubMed] [Google Scholar]
- Meyer-Olson D, Shoukry NH, Brady KW, Kim H, Olson DP, Hartman K, Shintani AK, Walker CM, Kalams SA. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J Exp Med. 2004;200:307. doi: 10.1084/jem.20040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef JM, Macdonald V, Jauncey M, Amin J, Rawlinson W, van Beek I, Kaldor JM, White PA, Dore GJ. High incidence of hepatitis C virus reinfection within a cohort of injecting drug users. J Viral Hepat. 2007;14:413. doi: 10.1111/j.1365-2893.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi MG, Houghton M, Fiaccadori F, Ferrari C. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Investig. 1996;98:706. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi E, Eisenbach C, Edlin BR, Newton KP, Raghuraman S, Weiler-Normann C, Tobler LH, Busch MP, Carrington M, McKeating JA, O’Brien TR, Rehermann B. Hepatitis C virus (HCV)-specific immune responses of long-term injection drug users frequently exposed to HCV. J Infect Dis. 2008;198:203. doi: 10.1086/589510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina S, Castet V, Fournier-Wirth C, Pichard-Garcia L, Avner R, Harats D, Roitelman J, Barbaras R, Graber P, Ghersa P, Smolarsky M, Funaro A, et al. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J Hepatol. 2007;46:411. doi: 10.1016/j.jhep.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Mondelli MU, Cerino A, Segagni L, Meola A, Cividini A, Silini E, Nicosia A. Hypervariable region 1 of hepatitis C virus: Immunological decoy or biologically relevant domain? Antiviral Res. 2001;52:153. doi: 10.1016/s0166-3542(01)00180-2. [DOI] [PubMed] [Google Scholar]
- Moonka D, Milkovich KA, Rodriguez B, Abouljoud M, Lederman MM, Anthony DD. Hepatitis C virus-specific T-cell gamma interferon and proliferative responses are more common in perihepatic lymph nodes than in peripheral blood or liver. J Virol. 2008;82:11742. doi: 10.1128/JVI.01130-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Mosley JW, Operskalski EA, Tobler LH, Buskell ZJ, Andrews WW, Phelps B, Dockter J, Giachetti C, Seeff LB, Busch MP. Transfusion-transmitted Viruses Study, Retrovirus Epidemiology Donor Study Group. The course of hepatitis C viraemia in transfusion recipients prior to availability of antiviral therapy. J Viral Hepat. 2008;15:120. doi: 10.1111/j.1365-2893.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, Rehermann B. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Marousis CG, Davis GL, Rice CM, Wong J, Houghton M, Lau JY. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J Immunol. 1997;158:1473. [PubMed] [Google Scholar]
- Netski DM, Mosbruger T, Depla E, Maertens G, Ray SC, Hamilton RG, Roundtree S, Thomas DL, McKeating J, Cox A. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis. 2005;41:667. doi: 10.1086/432478. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Timm J, Spangenberg HC, Wischniowski N, Nazarova N, Kersting N, Roggendorf M, Allen TM, Blum HE, Thimme R. Virological and immunological determinants of intrahepatic virus-specific CD8+ T-cell failure in chronic hepatitis C virus infection. Hepatology. 2008;47:1824. doi: 10.1002/hep.22242. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Sedghi-Vaziri A, Wilkes LB, Mondala T, Pockros PJ, Lindsay KL, McHutchison JG. Fluctuations in viral load (HCV RNA) are relatively insignificant in untreated patients with chronic HCV infection. J Viral Hepat. 1996;3:75. doi: 10.1111/j.1365-2893.1996.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Nielsen SU, Bassendine MF, Burt AD, Martin C, Pumeechockchai W, Toms GL. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol. 2006;80:2418. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northfield JW, Kasprowicz V, Lucas M, Kersting N, Bengsch B, Kim A, Phillips RE, Walker BD, Thimme R, Lauer G, Klenerman P. CD161 expression on hepatitis C virus-specific CD8+ T cells suggests a distinct pathway of T cell differentiation. Hepatology. 2008;47:396. doi: 10.1002/hep.22040. [DOI] [PubMed] [Google Scholar]
- Op De Beeck A, Voisset C, Bartosch B, Ciczora Y, Cocquerel L, Keck Z, Foung S, Cosset FL, Dubuisson J. Characterization of functional hepatitis C virus envelope glycoproteins. J Virol. 2004;78:2994. doi: 10.1128/JVI.78.6.2994-3002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2009;138:315. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DM, Huang H, Gale M. Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology. 2009;394:99. doi: 10.1016/j.virol.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianka A, Clayton RF, Loomis-Price LD, McKeating JA, Patel AH. Functional analysis of hepatitis C virus E2 glycoproteins and virus-like particles reveals structural dissimilarities between different forms of E2. J Gen Virol. 2001;82:1877. doi: 10.1099/0022-1317-82-8-1877. [DOI] [PubMed] [Google Scholar]
- Owsianka A, Tarr AW, Juttla VS, Lavillette D, Bartosch B, Cosset FL, Ball JK, Patel AH. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol. 2005;79:11095. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianka AM, Timms JM, Tarr AW, Brown RJ, Hickling TP, Szwejk A, Bienkowska-Szewczyk K, Thomson BJ, Patel AH, Ball JK. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J Virol. 2006;80:8695. doi: 10.1128/JVI.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianka AM, Tarr AW, Keck ZY, Li TK, Witteveldt J, Adair R, Foung SK, Ball JK, Patel AH. Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J Gen Virol. 2008;89:653. doi: 10.1099/vir.0.83386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, Andrews W, Avanesyan L, Cooper S, Busch MP. Acute hepatitis C virus infection in young adult injection drug users: A prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penin F, Combet C, Germanidis G, Frainais PO, Deleage G, Pawlotsky JM. Conservation of the conformation and positive charges of hepatitis C virus E2 envelope glycoprotein hypervariable region 1 points to a role in cell attachment. J Virol. 2001;75:5703. doi: 10.1128/JVI.75.12.5703-5710.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A, Missale G, Lamonaca V, Pilli M, Mori C, Zanelli P, Cavalli A, Elia G, Ferrari C. Intrahepatic and circulating HLA class II-restricted, hepatitis C virus-specific T cells: Functional characterization in patients with chronic hepatitis C. Hepatology. 2002;35:1225. doi: 10.1053/jhep.2002.33153. [DOI] [PubMed] [Google Scholar]
- Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- Perotti M, Mancini N, Diotti RA, Tarr AW, Ball JK, Owsianka A, Adair R, Patel AH, Clementi M, Burioni R. Identification of a broadly cross-reacting and neutralizing human monoclonal antibody directed against the hepatitis C virus E2 protein. J Virol. 2008;82:1047. doi: 10.1128/JVI.01986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, Rispeter K, Blum HE, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon AF, Kosakovsky Pond SL, Bennett P, Richman DD, Leigh Brown AJ, Frost SD. Adaptation to human populations is revealed by within-host polymorphisms in HIV-1 and hepatitis C virus. PLoS Pathog. 2007;3:e45. doi: 10.1371/journal.ppat.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post JJ, Pan Y, Freeman AJ, Harvey CE, White PA, Palladinetti P, Haber PS, Marinos G, Levy MH, Kaldor JM, Dolan KA, Ffrench RA, et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J Infect Dis. 2004;189:1846. doi: 10.1086/383279. [DOI] [PubMed] [Google Scholar]
- Prince AM, Brotman B, Grady GF, Kuhns WJ, Hazzi C, Levine RW, Millian SJ. Long-incubation post-transfusion hepatitis without serological evidence of exposure to hepatitis-B virus. Lancet. 1974;2:241. doi: 10.1016/s0140-6736(74)91412-3. [DOI] [PubMed] [Google Scholar]
- Prince AM, Huima-Byron T, Parker TS, Levine DM. Visualization of hepatitis C virions and putative defective interfering particles isolated from low-density lipoproteins. J Viral Hepat. 1996;3:11. doi: 10.1111/j.1365-2893.1996.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Prince AM, Brotman B, Lee DH, Pfahler W, Tricoche N, Andrus L, Shata MT. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J Infect Dis. 2005;192:1701. doi: 10.1086/496889. [DOI] [PubMed] [Google Scholar]
- Puig M, Mihalik K, Tilton JC, Williams O, Merchlinsky M, Connors M, Feinstone SM, Major ME. CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology. 2006;44:736. doi: 10.1002/hep.21319. [DOI] [PubMed] [Google Scholar]
- Puntoriero G, Meola A, Lahm A, Zucchelli S, Ercole BB, Tafi R, Pezzanera M, Mondelli MU, Cortese R, Tramontano A, Galfre G, Nicosia A. Towards a solution for hepatitis C virus hypervariability: Mimotopes of the hypervariable region 1 can induce antibodies cross-reacting with a large number of viral variants. EMBO J. 1998;17:3521. doi: 10.1093/emboj/17.13.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, Freeman GJ, Lennox JL, Workowski KA, Hanson HL, Grakoui A. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808. doi: 10.1128/JVI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziewicz H, Ibegbu CC, Hon H, Bedard N, Bruneau J, Workowski KA, Knechtle SJ, Kirk AD, Larsen CP, Shoukry NH, Grakoui A. Transient CD86 expression on hepatitis C virus-specific CD8+ T cells in acute infection is linked to sufficient IL-2 signaling. J Immunol. 2010;184:2410. doi: 10.4049/jimmunol.0902994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, James I, Pfafferott K, Nolan D, Klenerman P, Cheng W, Mollison L, McCaughan G, Shackel N, Jeffrey GP, Baker R, Freitas E, et al. Divergent adaptation of hepatitis C virus genotypes 1 and 3 to human leukocyte antigen-restricted immune pressure. Hepatology. 2009;50:1017. doi: 10.1002/hep.23101. [DOI] [PubMed] [Google Scholar]
- Ray SC, Wang YM, Laeyendecker O, Ticehurst JR, Villano SA, Thomas DL. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: Hypervariable region 1 as a decoy. J Virol. 1999;73:2938. doi: 10.1128/jvi.73.4.2938-2946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SC, Mao Q, Lanford RE, Bassett S, Laeyendecker O, Wang YM, Thomas DL. Hypervariable region 1 sequence stability during hepatitis C virus replication in chimpanzees. J Virol. 2000;74:3058. doi: 10.1128/jvi.74.7.3058-3066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SC, Fanning L, Wang XH, Netski DM, Kenny-Walsh E, Thomas DL. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J Exp Med. 2005;201:1753. doi: 10.1084/jem.20050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: A tale of coevolution and coexistence. J Clin Invest. 2009;119:1745. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa D, Campagnoli S, Moretto C, Guenzi E, Cousens L, Chin M, Dong C, Weiner AJ, Lau JY, Choo QL, Chien D, Pileri P, et al. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: Cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwangl KB, Manicassamy B, Uprichard SL, Rong L. Dissecting the role of putative CD81 binding regions of E2 in mediating HCV entry: Putative CD81 binding region 1 is not involved in CD81 binding. Virol J. 2008;5:46. doi: 10.1186/1743-422X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan AG, Fletcher JM, Ryan EJ, Moran B, Hegarty JE, O’Farrelly C, Mills KH. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J Immunol. 2008;181:4485. doi: 10.4049/jimmunol.181.7.4485. [DOI] [PubMed] [Google Scholar]
- Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A, Sidney J, Pardoll DM, et al. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Salloum S, Oniangue-Ndza C, Neumann-Haefelin C, Hudson L, Giugliano S, aus dem Siepen M, Nattermann J, Spengler U, Lauer GM, Wiese M, Klenerman P, Bright H, et al. Escape from HLA-B*08-restricted CD8 T cells by hepatitis C virus is associated with fitness costs. J Virol. 2008;82(1):1803. doi: 10.1128/JVI.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum S, Kluge SF, Kim AY, Roggendorf M, Timm J. The resistance mutation R155K in the NS3/4A protease of hepatitis C virus also leads the virus to escape from HLA-A*68-restricted CD8 T cells. Antiviral Res. 2010;87:272. doi: 10.1016/j.antiviral.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirren CA, Jung MC, Gerlach JT, Worzfeld T, Baretton G, Mamin M, Hubert Gruener N, Houghton M, Pape GR. Liver-derived hepatitis C virus (HCV)-specific CD4(+) T cells recognize multiple HCV epitopes and produce interferon gamma. Hepatology. 2000;32:597. doi: 10.1053/jhep.2000.9635. [DOI] [PubMed] [Google Scholar]
- Schulze zur Wiesch J, Lauer GM, Day CL, Kim AY, Ouchi K, Duncan JE, Wurcel AG, Timm J, Jones AM, Mothe B, Allen TM, McGovern B, et al. Broad repertoire of the CD4 Th cell response in spontaneously controlled hepatitis C virus infection includes dominant and highly promiscuous epitopes. J Immunol. 2005;175:3603. doi: 10.4049/jimmunol.175.6.3603. [DOI] [PubMed] [Google Scholar]
- Schulze Zur Wiesch J, Lauer GM, Timm J, Kuntzen T, Neukamm M, Berical A, Jones AM, Nolan BE, Longworth SA, Kasprowicz V, McMahon C, Wurcel A, et al. Immunologic evidence for lack of heterologous protection following resolution of HCV in patients with non-genotype 1 infection. Blood. 2007;110:1559. doi: 10.1182/blood-2007-01-069583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmo N, Day CL, Ward SM, Lucas M, Harcourt G, Loughry A, Klenerman P. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology. 2005;41:1019. doi: 10.1002/hep.20669. [DOI] [PubMed] [Google Scholar]
- Serra A, Nuti S, Tavarini S, Sammicheli C, Rosa D, Saletti G, Soldaini E, Abrignani S, Wack A. Coligation of the hepatitis C virus receptor CD81 with CD28 primes naive T lymphocytes to acquire type 2 effector function. J Immunol. 2008;181:174. doi: 10.4049/jimmunol.181.1.174. [DOI] [PubMed] [Google Scholar]
- Shiina M, Rehermann B. Analysis of HCV-specific T cells by flow cytometry. Methods Mol Biol. 2009;510:415. doi: 10.1007/978-1-59745-394-3_31. [DOI] [PubMed] [Google Scholar]
- Shimakami T, Lanford RE, Lemon SM. Hepatitis C: Recent successes and continuing challenges in the development of improved treatment modalities. Curr Opin Pharmacol. 2009;9:537. doi: 10.1016/j.coph.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein E, Mihalik K, Ulitzky L, Plant EP, Puig M, Gagneten S, Yu MY, Kaushik-Basu N, Feinstone SM, Taylor DR. Persistent growth of a human plasma-derived hepatitis C virus genotype 1b isolate in cell culture. PLoS Pathog. 2010;6:e1000910. doi: 10.1371/journal.ppat.1000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, Halfon P, Inchauspe G, Kuiken C, Maertens G, Mizokami M, Murphy DG, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- Singh R, Kaul R, Kaul A, Khan K. A comparative review of HLA associations with hepatitis B and C viral infections across global populations. World J Gastroenterol. 2007;13:1770. doi: 10.3748/wjg.v13.i12.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyk-Pearson S, Golden-Mason L, Klarquist J, Burton JR, Jr, Tester IA, Wang CC, Culbertson N, Vandenbark AA, Rosen HR. Functional suppression by FoxP3+CD4+CD25(high) regulatory T cells during acute hepatitis C virus infection. J Infect Dis. 2008;197:46. doi: 10.1086/523651. [DOI] [PubMed] [Google Scholar]
- Soderholm J, Ahlen G, Kaul A, Frelin L, Alheim M, Barnfield C, Liljestrom P, Weiland O, Milich DR, Bartenschlager R, Sallberg M. Relation between viral fitness and immune escape within the hepatitis C virus protease. Gut. 2006;55:266. doi: 10.1136/gut.2005.072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada E, Mele A, Berton A, Ruggeri L, Ferrigno L, Garbuglia AR, Perrone MP, Girelli G, Del Porto P, Piccolella E, Mondelli MU, Amoroso P, et al. Multi-specific T cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut. 2004;53:1673. doi: 10.1136/gut.2003.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, von Weizsacker F, Blum HE, Thimme R. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828. doi: 10.1002/hep.20856. [DOI] [PubMed] [Google Scholar]
- Stamataki Z, Grove J, Balfe P, McKeating JA. Hepatitis C virus entry and neutralization. Clin Liver Dis. 2008;12:693. doi: 10.1016/j.cld.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Steinmann D, Barth H, Gissler B, Schurmann P, Adah MI, Gerlach JT, Pape GR, Depla E, Jacobs D, Maertens G, Patel AH, Inchauspe G, et al. Inhibition of hepatitis C virus-like particle binding to target cells by antiviral antibodies in acute and chronic hepatitis C. J Virol. 2004;78:9030. doi: 10.1128/JVI.78.17.9030-9040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Kaplan DE, Ikeda F, Ding J, Schwartz J, Nunes FA, Alter HJ, Chang KM. Strain-specific T-cell suppression and protective immunity in patients with chronic hepatitis C virus infection. J Virol. 2005;79:6976. doi: 10.1128/JVI.79.11.6976-6983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A. Hepatitis C and innate immunity: Recent advances. Clin Liver Dis. 2008;12:675. doi: 10.1016/j.cld.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor E, Gerety RJ, Drucker JA, Seeff LB, Hoofnagle JH, Jackson DR, April M, Barker LF, Pineda-Tamondong G. Transmission of non-A, non-B hepatitis from man to chimpanzee. Lancet. 1978;1:463. doi: 10.1016/s0140-6736(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Okamoto H, Sakamoto M, Kojima M, Tsuda F, Tanaka T, Munekata E, Muchmore EE, Peterson DA, Mishiro S. A structurally flexible and antigenically variable N-terminal domain of the hepatitis C virus E2/NS1 protein: Implication for an escape from antibody. Virology. 1993;195:297. doi: 10.1006/viro.1993.1378. [DOI] [PubMed] [Google Scholar]
- Tarr AW, Owsianka AM, Timms JM, McClure CP, Brown RJ, Hickling TP, Pietschmann T, Bartenschlager R, Patel AH, Ball JK. Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology. 2006;43:592. doi: 10.1002/hep.21088. [DOI] [PubMed] [Google Scholar]
- Tarr AW, Owsianka AM, Jayaraj D, Brown RJ, Hickling TP, Irving WL, Patel AH, Ball JK. Determination of the human antibody response to the epitope defined by the hepatitis C virus-neutralizing monoclonal antibody AP33. J Gen Virol. 2007;88:2991. doi: 10.1099/vir.0.83065-0. [DOI] [PubMed] [Google Scholar]
- Tester I, Smyk-Pearson S, Wang P, Wertheimer A, Yao E, Lewinsohn DM, Tavis JE, Rosen HR. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J Exp Med. 2005;201:1725. doi: 10.1084/jem.20042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99:15661. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL, Astemborski J, Vlahov D, Strathdee SA, Ray SC, Nelson KE, Galai N, Nolt KR, Laeyendecker O, Todd JA. Determinants of the quantity of hepatitis C virus RNA. J Infect Dis. 2000;181:844. doi: 10.1086/315314. [DOI] [PubMed] [Google Scholar]
- Thomssen R, Bonk S, Propfe C, Heermann KH, Kochel HG, Uy A. Association of hepatitis C virus in human sera with beta-lipoprotein. Med Microbiol Immunol. 1992;181:293. doi: 10.1007/BF00198849. [DOI] [PubMed] [Google Scholar]
- Timm J, Lauer GM, Kavanagh DG, Sheridan I, Kim AY, Lucas M, Pillay T, Ouchi K, Reyor LL, Zur Wiesch JS, Gandhi RT, Chung RT, et al. CD8 epitope escape and reversion in acute HCV infection. J Exp Med. 2004;200:1593. doi: 10.1084/jem.20041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm J, Li B, Daniels MG, Bhattacharya T, Reyor LL, Allgaier R, Kuntzen T, Fischer W, Nolan BE, Duncan J, Schulze zur Wiesch J, Kim AY, et al. Human leukocyte antigen-associated sequence polymorphisms in hepatitis C virus reveal reproducible immune responses and constraints on viral evolution. Hepatology. 2007;46:339. doi: 10.1002/hep.21702. [DOI] [PubMed] [Google Scholar]
- Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, Schwarz A, Desombere I, Roels GL, Balfe P, McKeating JA. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47:17. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- Uebelhoer L, Han JH, Callendret B, Mateu G, Shoukry NH, Hanson HL, Rice CM, Walker CM, Grakoui A. Stable cytotoxic T cell escape mutation in hepatitis C virus is linked to maintenance of viral fitness. PLoS Pathog. 2008;4:e1000143. doi: 10.1371/journal.ppat.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulsenheimer A, Gerlach JT, Gruener NH, Jung MC, Schirren CA, Schraut W, Zachoval R, Pape GR, Diepolder HM. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology. 2003;37:1189. doi: 10.1053/jhep.2003.50194. [DOI] [PubMed] [Google Scholar]
- Ulsenheimer A, Lucas M, Seth NP, Tilman Gerlach J, Gruener NH, Loughry A, Pape GR, Wucherpfennig KW, Diepolder HM, Klenerman P. Transient immunological control during acute hepatitis C virus infection: Ex vivo analysis of helper T-cell responses. J Viral Hepat. 2006;13:708. doi: 10.1111/j.1365-2893.2006.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Cariani E, Fisicaro P, Orlandini A, Missale G, Ferrari C. The impairment of CD8 responses limits the selection of escape mutations in acute hepatitis C virus infection. J Immunol. 2005;175:7519. doi: 10.4049/jimmunol.175.11.7519. [DOI] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006a;44:126. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006b;80:11398. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Pedrazzi G, Sacchelli L, Cavallo MC, Orlandini A, Missale G, Ferrari C. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: Effect of viremia levels and antiviral treatment. J Hepatol. 2008;48:548. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- van de Laar TJ, Molenkamp R, van den Berg C, Schinkel J, Beld MG, Prins M, Coutinho RA, Bruisten SM. Frequent HCV reinfection and superinfection in a cohort of injecting drug users in Amsterdam. J Hepatol. 2009;51:667. doi: 10.1016/j.jhep.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Voisset C, Op de Beeck A, Horellou P, Dreux M, Gustot T, Duverlie G, Cosset FL, Vu-Dac N, Dubuisson J. High-density lipoproteins reduce the neutralizing effect of hepatitis C virus (HCV)-infected patient antibodies by promoting HCV entry. J Gen Virol. 2006;87:2577. doi: 10.1099/vir.0.81932-0. [DOI] [PubMed] [Google Scholar]
- von Hahn T, Lindenbach BD, Boullier A, Quehenberger O, Paulson M, Rice CM, McKeating JA. Oxidized low-density lipoprotein inhibits hepatitis C virus cell entry in human hepatoma cells. Hepatology. 2006;43:932. doi: 10.1002/hep.21139. [DOI] [PubMed] [Google Scholar]
- von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Eckels DD. Mutations in immunodominant T cell epitopes derived from the nonstructural 3 protein of hepatitis C virus have the potential for generating escape variants that may have important consequences for T cell recognition. J Immunol. 1999;162:4177. [PubMed] [Google Scholar]
- Wang JH, Layden TJ, Eckels DD. Modulation of the peripheral T-Cell response by CD4 mutants of hepatitis C virus: Transition from a Th1 to a Th2 response. Hum Immunol. 2003;64:662. doi: 10.1016/s0198-8859(03)00070-3. [DOI] [PubMed] [Google Scholar]
- Ward S, Lauer G, Isba R, Walker B, Klenerman P. Cellular immune responses against hepatitis C virus: The evidence base 2002. Clin Exp Immunol. 2002;128:195. doi: 10.1046/j.1365-2249.2002.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, Fleming KA, Banham AH, Klenerman P. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316. doi: 10.1016/j.jhep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Weiner AJ, Geysen HM, Christopherson C, Hall JE, Mason TJ, Saracco G, Bonino F, Crawford K, Marion CD, Crawford KA, et al. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: Potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89:3468. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A, Erickson AL, Kansopon J, Crawford K, Muchmore E, Hughes AL, Houghton M, Walker CM. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci USA. 1995;92:2755. doi: 10.1073/pnas.92.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteveldt J, Evans MJ, Bitzegeio J, Koutsoudakis G, Owsianka AM, Angus AG, Keck ZY, Foung SK, Pietschmann T, Rice CM, Patel AH. CD81 is dispensable for hepatitis C virus cell-to-cell transmission in hepatoma cells. J Gen Virol. 2009;90:48. doi: 10.1099/vir.0.006700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfl M, Rutebemberwa A, Mosbruger T, Mao Q, Li HM, Netski D, Ray SC, Pardoll D, Sidney J, Sette A, Allen T, Kuntzen T, et al. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. J Immunol. 2008;181:6435. doi: 10.4049/jimmunol.181.9.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DK, Dudley DD, Afdhal NH, Dienstag J, Rice CM, Wang L, Houghton M, Walker BD, Koziel MJ. Liver-derived CTL in hepatitis C virus infection: Breadth and specificity of responses in a cohort of persons with chronic infection. J Immunol. 1998;160:1479. [PubMed] [Google Scholar]
- Wunschmann S, Medh JD, Klinzmann D, Schmidt WN, Stapleton JT. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J Virol. 2000;74:10055. doi: 10.1128/jvi.74.21.10055-10062.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc Natl Acad Sci USA. 2006;103:2310. doi: 10.1073/pnas.0510727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JC, Rehermann B. Determination of HCV-specific T-cell activity. Methods Mol Biol. 2009;510:403. doi: 10.1007/978-1-59745-394-3_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel MB, Koutsoudakis G, Schnober EK, Haberstroh A, Blum HE, Cosset FL, Wakita T, Jaeck D, Doffoel M, Royer C, Soulier E, Schvoerer E, et al. Scavenger receptor class B type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology. 2007;46:1722. doi: 10.1002/hep.21994. [DOI] [PubMed] [Google Scholar]
- Zhang J, Randall G, Higginbottom A, Monk P, Rice CM, McKeating JA. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J Virol. 2004;78:1448. doi: 10.1128/JVI.78.3.1448-1455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zhong L, Struble EB, Watanabe H, Kachko A, Mihalik K, Virata-Theimer ML, Alter HJ, Feinstone S, Major M. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc Natl Acad Sci USA. 2009;106:7537. doi: 10.1073/pnas.0902749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng A, Yuan F, Li Y, Zhu F, Hou P, Li J, Song X, Ding M, Deng H. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J Virol. 2007;81:12465. doi: 10.1128/JVI.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibert A, Meisel H, Kraas W, Schulz A, Jung G, Roggendorf M. Early antibody response against hypervariable region 1 is associated with acute self-limiting infections of hepatitis C virus. Hepatology. 1997;25:1245. doi: 10.1002/hep.510250530. [DOI] [PubMed] [Google Scholar]
- Zubkova I, Choi YH, Chang E, Pirollo K, Uren T, Watanabe H, Wells F, Kachko A, Krawczynski K, Major ME. T-cell vaccines that elicit effective immune responses against HCV in chimpanzees may create greater immune pressure for viral mutation. Vaccine. 2009;27:2594. doi: 10.1016/j.vaccine.2009.02.045. [DOI] [PubMed] [Google Scholar]


