Abstract
Background
MADS-box genes encode transcription factors that are known to be involved in several aspects of plant growth and development, especially in floral organ specification. To date, the comprehensive analysis of potato MADS-box gene family is still lacking after the completion of potato genome sequencing. A genome-wide characterization, classification, and expression analysis of MADS-box transcription factor gene family was performed in this study.
Results
A total of 153 MADS-box genes were identified and categorized into MIKC subfamily (MIKCC and MIKC*) and M-type subfamily (Mα, Mβ, and Mγ) based on their phylogenetic relationships to the Arabidopsis and rice MADS-box genes. The potato M-type subfamily had 114 members, which is almost three times of the MIKC members (39), indicating that M-type MADS-box genes have a higher duplication rate and/or a lower loss rate during potato genome evolution. Potato MADS-box genes were present on all 12 potato chromosomes with substantial clustering that mainly contributed by the M-type members. Chromosomal localization of potato MADS-box genes revealed that MADS-box genes, mostly MIKC, were located on the duplicated segments of the potato genome whereas tandem duplications mainly contributed to the M-type gene expansion. The potato MIKC subfamily could be further classified into 11 subgroups and the TT16-like, AGL17-like, and FLC-like subgroups found in Arabidopsis were absent in potato. Moreover, the expressions of potato MADS-box genes in various tissues were analyzed by using RNA-seq data and verified by quantitative real-time PCR, revealing that the MIKCC genes were mainly expressed in flower organs and several of them were highly expressed in stolon and tubers. StMADS1 and StMADS13 were up-regulated in the StSP6A-overexpression plants and down-regulated in the StSP6A-RNAi plant, and their expression in leaves and/or young tubers were associated with high level expression of StSP6A.
Conclusion
Our study identifies the family members of potato MADS-box genes and investigate the evolution history and functional divergence of MADS-box gene family. Moreover, we analyze the MIKCC expression patterns and screen for genes involved in tuberization. Finally, the StMADS1 and StMADS13 are most likely to be downstream target of StSP6A and involved in tuber development.
Electronic supplementary material
The online version of this article (10.1186/s12864-018-5113-z) contains supplementary material, which is available to authorized users.
Keywords: Potato, MADS-box, Tuberigen, StSP6A, Tuberization
Background
The MADS-box gene family has been extensively studied for its important roles in transcriptional regulation in eukaryotes [1–3]. The word of MADS-box is an acronym for Mini chromosome maintenance 1 (MCM1) in yeast (Sacchromyces cerevisiae), AGAMOUS (AG) in Arabidopsis (Arabidopsis thaliana), DEFICIENS (DEF) in snapdragon (Antirrhinum majus) and serum response factor (SRF) in human (Homo sapiens) [4–7]. MADS-box genes are characterized by N-terminal conservative MADS-box domains that are approximately 58–60 amino acids in length, which functions in combination to DNA [7, 8]. In plants, MADS-box transcription factors are involved in almost every important process during plant growth and development [9, 10].
Based on phylogenetic relationship, MADS-box gene family has been divided into two major lineages in plants, type I and type II, which were resulting from an ancestral gene duplication [11, 12]. Type I genes are also named as M-type MADS-box genes, which contain three subgroups (Mα, Mβ, and Mγ). The classical structure of M-type MADS-box genes is an N-terminal MADS domain and a relatively less conservative domain in the C-terminal [13]. In most plants, higher frequency of segmental gene duplications and weaker purifying selection result in a faster step of birth-and-death to type I genes compared to type II genes [14]. Type II MADS-box genes are also known as MIKC-type genes, which encode MEF2-like proteins [15]. In addition to the MADS domain, type II MADS-box genes contain three other domains, including intervening (I), kertain-like (K), and C-terminal (C) domains from N-terminal to C-terminal. The intervening (I) domain consists of approximately 30 amino acids and contributes to the dimerization of MADS-box proteins [16]. The kertain-like (K) domain is about 70 amino acids and more conservative than intervening (I) domain. The coiled-coil structure is significant to regulate the dimerization of MADS-box proteins. C-terminal (C) domain is a highly variable region in MADS-box proteins related to transcriptional activation and formation of protein complexes [17]. Type II MADS-box genes can be further classified into MIKCC (the ‘C’ stands for ‘Classic’) and MIKC* based on the variable intervening (I) domain [18]. The domain compositions of these two subfamilies in type II are quite different. MIKC* subfamily exhibit a longer intervening (I) domain and less conservative kertain-like (K) domain [19]. Therefore, in early studies, MIKC* subfamily was attributed into M-type MADS-box genes named Mδ [12].
The first MADS-box gene in plants was found to be related to the differentiation of flower [3]. ABCDE model had been successfully adopted to explain the determination of floral organ identity. Recent studies have also found that MIKCC subfamily is related to photoperiod-regulated floral meristem identity, gametophyte development, sporophyte (diploid) generation, seed pigmentation, and embryo development [20–24]. Most of genes in ABCDE model belong to MIKCC subfamily [6, 25, 26]. Besides, MADS-box genes in MIKCC subfamily plays irreplaceable biological functions in the stress-responsive processes, for instance, TaMADS2 was up-regulated in response to wheat stripe rust infection [27].
The functions of MIKC* MADS-box genes are less elucidated than those in MIKCC subfamily and it is found that the heterodimers of MIKC*-type proteins are essential for the pollen maturation and pollen tube growth in Arabidopsis [28]. In potato, only three MADS-box genes have been previously reported, they are potato MADS-box 1–1 (POTM1–1), StMADS11, and StMADS16 [29, 30]. POTM1–1 gene expression is temporally and spatially regulated in both vegetative and floral organs, transcriptional suppression of POTM1–1 activates axillary meristem development by increasing the cytokinin levels [29, 31, 32]. StMADS11 is expressed in all vegetative tissues of the potato plant, mainly in the stem, but not in flower organs [33]. Ectopic expression of StMADS16 modifies the inflorescence structure by increasing both internode length and flower proliferation of the inflorescence meristems and confers vegetative features to the flower [29]. Recent study finds that FLOWERING LOCUS T in potato (StSP6A) is a mobile signal for potato tuberization. StSP6A, homologs of FT in Arabidopsis, is very likely to control tuberization through regulating the expressions of downstream MADS-box genes [34, 35]. Therefore, there is an urgent need to characterize the MADS-box gene family in potato and screen for MADS-box candidates involved in tuberization. The complete genome sequencing of the potato in 2011 enabled us to perform a genome-wide identification of MADS-box genes in potato [36].
In this study, multiple bioinformatics methods were applied to perform a comprehensive survey of MADS-box genes in potato. In addition, the gene structure, phylogenetic relationships, chromosomal locations, conserved motifs and tissue-specific expressions of MADS-box genes were investigated in potato. Our work would be useful in helping to establish the basic information of MADS-box genes in potato and in screening out several MADS-box genes related to tuberization and following tuber development.
Methods
Identification of MADS-box genes in potato
The potato genome sequence data used for the identification and annotation of StMADS genes was downloaded from Potato Genome Sequencing Consortium (PGSC, http://potato.plantbiology.msu.edu/). BLASTP, InerPro ID and keyword searches were performed to obtain the putative MADS-box genes in potato. First of all, the known Arabidopsis MADS-box protein sequences were used as query to perform BLASTP utility against the potato protein database (PGSC_DM_v3.4_pep_nonredundant.fasta) in local computer with an expected value cutoff of 1e-3. Then, InterPro ID (IPR003340) and keyword searches (MADS-box) were also applied to identify putative potato MADS-box proteins in PGSC database by online searching. All putative MADS-box sequences were collected and the redundant sequences were manually removed, the remaining candidate MADS-box sequences were submitted to NCBI Conserved Domain (CD) search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) to confirm the existence of MADS-box domain.The gene structure of MADS genes was drawn with TB tools (http://cj-chen.github.io/tbtools/) using GFF3 files downloaded from PGSC.
Chromosomal location and gene duplication
MapChart 2.2 was exploited to draw the gene location in the physical map of potato MADS-box genes [37]. Potato MADS-box genes were named based on the position information obtained from PGSC with concerning about already reported POTM1–1 (StMADS1), StMADS11, and StMADS16. According to the nomenclature method used in rice, the remaining potato MADS-box genes were named as StMADS2 to StMADS153 followed the order of MIKCC, MIKC*, Mα, Mβ, and Mγ. Potato MADS-box genes without chromosomal positions were named at the last of the list. Tandem duplicated genes were determined in PGSC with a criterion that no more than five genes between two genes with high homology (> 50%). Segmental duplicated genes of potato were obtained from the Plant Genome Duplication Database (PGDD, http://chibba.pgml.uga.edu/duplication/).
Phylogenetic and conserved motif analyses of potato MADS-box genes
Potato, rice, and Arabidopsis MADS-box protein sequences were aligned using ClustalX version 1.83. The phylogenetic trees were generated by using Neighbor joining (NJ) method in MEGA6.06 with bootstrap value 1000 replicates to evaluate the significance of the nodes. To ensure that the divergent domains could contribute to the topology of the NJ tree, pairwise gap deletion mode was used to construct the tree. Moreover, the potato MADS-box protein sequences were submitted to MEME (http://meme-suite.org/) to determine the conserved motif in these sequences.
Phylogenetic analysis of MADS-box genes between potato and tomato
To investigate the phylogenetic relationships of MADS-box genes between potato and tomato, a genome-wide search against the Solanum lycopersicum proteome (Solanum lycopersicum Annotation Release 103, ftp://ftp.ncbi.nlm.nih.gov/genomes/Solanum_lycopersicum/protein/) using blastp program in dos environment of windows. The threshold of e-value was set for 1e-3. The candidate gene was submitted to InterPro to exclude genes without MADS-box domain. A phylogenetic tree was generated to determine the relationship of MADS genes in these two species using the protein sequence aligned with Muscle program in MEGA7.0 and the same method mentioned above.
Expression analyses based on publicly available RNA-seq and microarray data
The expression patterns of potato MADS-box gene family members were determined using the data deposited in the PGSC database which derived from Illumina RNA-seq of a wide range of developmental stages [36]. The expression profiles of genes on POCI (Potato Oligo Chip Initiative) microarrays were previously performed in stolons of StSP6A-overexpression and StSP6A-RNAi plants [34]. The MADS-box genes were used as queries to search DNA probes using BLASTN and the results of MADS-box genes were used for further study with e-value set for 1e-3. The probes were selected if they were annotated as MADS-box family members by the microarray platform and were assigned to corresponding potato MADS-box genes if their identity were 100% with exceptions of single nucleotide polymorphism (SNP). The deduced FPKM value of these genes acquired from published data were normalized with Log2 to make it suitable for the further visualized in Pretty Heatmap (http://www.ehbio.com/ImageGP/index.php/Home/Index/PHeatmap.html).
Plant materials collection and qRT-PCR
The plants of potato cultivar ‘Desire’ was cultivated in greenhouse of Northwest A&F University from March to June (23 ± 2 °C,16 h light/8 h dark). The different tissues and organs were collected at different time after sprouting. Stem, leaf, and flower were sampled at flowering, whereas stolons and young tubers were collected ten days after flowering. In addition, mature tubers were taken 90 days after sprouting. All samples were immediately frozen in liquid nitrogen and stored at -80 °C until used. Total RNA was extracted using a high purity total RNA rapid extraction kit (BioTeke, RP1202, China) and first-strand cDNA was synthesized using a ReverTra Ace Kit (TOYOBO, FSK-100, Japan) following the manufacturer’s instructions.
Primer 5.0 was used to design gene-specific primers of MADS-box in potato (Additional file 1: Table S1). Real-time quantitative RT-PCR was performed by using the SYBR green mix (KAPA, KK4601, USA) in a Real-time PCR machine (BioRad, CFX96, USA). The internal reference gene was ef1α and three biological replicates were used to estimate the expression level by the method of two stand curves as described previously [38].
Results
Identification and comparative analysis of MADS genes in potato
Three bioinformatics methods were used to identify the MADS-box genes in potato. A local BLASTP search was performed with a cutoff e-value of 1e-3 by using the Arabidopsis MADS-box proteins as query, which resulted in 169 MADS-box candidates. The keyword and InterPro ID (IPR003340) searches against the PGSC website resulted in 145 and 156 MADS-box candidates, respectively. These candidates were submitted to NCBI CDD to confirm the existence of MADS-box domain. After removing the redundant sequences, 153 total MADS-box genes were found in potato. The names of StMADS1, StMADS11, and StMADS16 were introduced by previous reports and remaining genes were named from StMADS2 to 153 (except for StMADS11 and StMADS16) according to their chromosomal locations and subfamily affiliation (Table 1). Based on phylogenetic relationships of Arabidopsis, rice, and potato MADS-box proteins (Fig. 1), 153 potato MADS-box proteins were classified into two subfamily MIKC and M-type. In potato, the number of MADS-box genes in MIKC subfamily was 39 and this subfamily was mainly comprised of two subgroups, 30 MADS-box genes in MIKCC and 9 MADS-box genes in MIKC*. The number of MADS-box genes in M-type is 114 and this subfamily contained three subgroups, 70 MADS-box genes in Mα, 28 MADS-box genes in Mβ, and 16 MADS-box genes in Mγ.
Table 1.
The detailed information of potato MADS-box gene family. Genes with an asterisk tail means identified in previous studies
| Name | Locus | Chromosomal locations | Protein (aa) | Exon | Subfamily |
|---|---|---|---|---|---|
| StMADS1* | PGSC0003DMG400004081 | chr06 51191112–51198207 | 250 | 8 | MIKCC |
| StMADS2 | PGSC0003DMG401006771 | chr01 64818874–64826057 | 287 | 8 | MIKCC |
| StMADS3 | PGSC0003DMG400000008 | chr01 71388491–71392334 | 224 | 8 | MIKCC |
| StMADS4 | PGSC0003DMG400000136 | chr01 71421222–71426490 | 255 | 8 | MIKCC |
| StMADS5 | PGSC0003DMG400026563 | chr02 24953367–24959857 | 234 | 8 | MIKCC |
| StMADS6 | PGSC0003DMG400028442 | chr02 31027789–31034809 | 248 | 9 | MIKCC |
| StMADS7 | PGSC0003DMG400003541 | chr02 39864994–39869063 | 225 | 7 | MIKCC |
| StMADS8 | PGSC0003DMG400001378 | chr02 45341112–45345702 | 247 | 8 | MIKCC |
| StMADS9 | PGSC0003DMG400001377 | chr02 45353007–45357495 | 246 | 8 | MIKCC |
| StMADS10 | PGSC0003DMG401024252 | chr03 41578383–41583244 | 119 | 3 | MIKCC |
| StMADS11* | PGSC0003DMG400033570 | chr01 82400439–82408299 | 221 | 9 | MIKCC |
| StMADS12 | PGSC0003DMG401015205 | chr03 50457307–50460580 | 200 | 8 | MIKCC |
| StMADS13 | PGSC0003DMG400024625 | chr03 54879384–54886641 | 248 | 8 | MIKCC |
| StMADS14 | PGSC0003DMG400024626 | chr03 54896515–54901975 | 246 | 8 | MIKCC |
| StMADS15 | PGSC0003DMG400003709 | chr04 70409561–70413264 | 228 | 7 | MIKCC |
| StMADS16* | PGSC0003DMG400009363 | chr04 64959661–64967188 | 235 | 11 | MIKCC |
| StMADS17 | PGSC0003DMG400028359 | chr05 757295–763364 | 242 | 8 | MIKCC |
| StMADS18 | PGSC0003DMG400028358 | chr05 766418–772401 | 244 | 8 | MIKCC |
| StMADS19 | PGSC0003DMG400021899 | chr05 14664987–14671873 | 241 | 8 | MIKCC |
| StMADS20 | PGSC0003DMG400025279 | chr06 36656761–36660368 | 111 | 3 | MIKCC |
| StMADS21 | PGSC0003DMG400005176 | chr06 47282868–47291175 | 232 | 9 | MIKCC |
| StMADS22 | PGSC0003DMG400017295 | chr07 51027282–51036001 | 254 | 6 | MIKCC |
| StMADS23 | PGSC0003DMG401007392 | chr08 40704456–40708461 | 210 | 7 | MIKCC |
| StMADS24 | PGSC0003DMG400022748 | chr08 53768699–53776641 | 224 | 7 | MIKCC |
| StMADS25 | PGSC0003DMG400010263 | chr10 38698247–38706939 | 214 | 6 | MIKCC |
| StMADS26 | PGSC0003DMG400023729 | chr10 57811655–57814557 | 229 | 7 | MIKCC |
| StMADS27 | PGSC0003DMG400016203 | chr11 2317625–2323465 | 238 | 8 | MIKCC |
| StMADS28 | PGSC0003DMG400025525 | chr11 16127866–16137240 | 232 | 7 | MIKCC |
| StMADS29 | PGSC0003DMG401018787 | chr11 16991875–16995488 | 116 | 4 | MIKCC |
| StMADS30 | PGSC0003DMG400001938 | chr00 12593321–12596548 | 188 | 2 | MIKCC |
| StMADS31 | PGSC0003DMG400019525 | chr03 55865548–55867778 | 142 | 3 | MIKC* |
| StMADS32 | PGSC0003DMG400038617 | chr04 31598124–31598495 | 84 | 2 | MIKC* |
| StMADS33 | PGSC0003DMG400036414 | chr04 52111872–52112228 | 82 | 2 | MIKC* |
| StMADS34 | PGSC0003DMG400044568 | chr04 52327361–52331720 | 158 | 3 | MIKC* |
| StMADS35 | PGSC0003DMG400045842 | chr06 35336837–35338244 | 151 | 5 | MIKC* |
| StMADS36 | PGSC0003DMG401026197 | chr07 47364697–47367540 | 155 | 6 | MIKC* |
| StMADS37 | PGSC0003DMG403026197 | chr07 47437818–47445958 | 94 | 9 | MIKC* |
| StMADS38 | PGSC0003DMG400017759 | chr12 55492383–55493047 | 108 | 2 | MIKC* |
| StMADS39 | PGSC0003DMG400017760 | chr12 55554242–55562194 | 225 | 3 | MIKC* |
| StMADS40 | PGSC0003DMG400038539 | chr01 14769002–14769334 | 110 | 1 | Mα |
| StMADS41 | PGSC0003DMG400045528 | chr01 40043124–40043513 | 129 | 1 | Mα |
| StMADS42 | PGSC0003DMG400011328 | chr01 57376759–57377670 | 303 | 1 | Mα |
| StMADS43 | PGSC0003DMG400011316 | chr01 57795443–57796789 | 421 | 1 | Mα |
| StMADS44 | PGSC0003DMG400006290 | chr01 60247610–60248203 | 197 | 1 | Mα |
| StMADS45 | PGSC0003DMG400041956 | chr01 60265275–60265868 | 197 | 1 | Mα |
| StMADS46 | PGSC0003DMG400046706 | chr01 60293060–60293646 | 189 | 2 | Mα |
| StMADS47 | PGSC0003DMG400000062 | chr01 72596658–72597790 | 143 | 1 | Mα |
| StMADS48 | PGSC0003DMG400040999 | chr01 75519222–75519740 | 172 | 1 | Mα |
| StMADS49 | PGSC0003DMG400022465 | chr01 75665917–75666411 | 495 | 1 | Mα |
| StMADS50 | PGSC0003DMG400022464 | chr01 75669936–75670403 | 155 | 1 | Mα |
| StMADS51 | PGSC0003DMG400037739 | chr01 75677724–75678191 | 155 | 1 | Mα |
| StMADS52 | PGSC0003DMG400022462 | chr01 75682108–75682572 | 154 | 1 | Mα |
| StMADS53 | PGSC0003DMG400022460 | chr01 75693397–75693888 | 163 | 1 | Mα |
| StMADS54 | PGSC0003DMG400044662 | chr01 75699473–75699940 | 155 | 1 | Mα |
| StMADS55 | PGSC0003DMG400042000 | chr01 75715667–75716134 | 155 | 1 | Mα |
| StMADS56 | PGSC0003DMG400035140 | chr01 75719828–75720178 | 116 | 1 | Mα |
| StMADS57 | PGSC0003DMG400005119 | chr01 83295937–83296952 | 213 | 1 | Mα |
| StMADS58 | PGSC0003DMG400044387 | chr01 83297295–83297960 | 221 | 1 | Mα |
| StMADS59 | PGSC0003DMG400005131 | chr01 83300154–83300747 | 197 | 1 | Mα |
| StMADS60 | PGSC0003DMG400046652 | chr02 14861994–14862299 | 101 | 1 | Mα |
| StMADS61 | PGSC0003DMG400038730 | chr02 15687958–15688452 | 164 | 1 | Mα |
| StMADS62 | PGSC0003DMG400013012 | chr02 22892178–22892726 | 182 | 1 | Mα |
| StMADS63 | PGSC0003DMG400039595 | chr02 25493501–25494016 | 171 | 1 | Mα |
| StMADS64 | PGSC0003DMG400042442 | chr03 3618726–3619148 | 140 | 1 | Mα |
| StMADS65 | PGSC0003DMG400037384 | chr03 3660635–3661183 | 182 | 1 | Mα |
| StMADS66 | PGSC0003DMG400045551 | chr03 4972791–4973312 | 173 | 1 | Mα |
| StMADS67 | PGSC0003DMG400046651 | chr03 13264973–13265449 | 158 | 1 | Mα |
| StMADS68 | PGSC0003DMG400037338 | chr04 750699–751226 | 175 | 1 | Mα |
| StMADS69 | PGSC0003DMG400019742 | chr04 9343623–9346031 | 90 | 6 | Mα |
| StMADS70 | PGSC0003DMG400040226 | chr04 21299801–21300325 | 174 | 1 | Mα |
| StMADS71 | PGSC0003DMG400041035 | chr04 27499096–27499626 | 176 | 1 | Mα |
| StMADS72 | PGSC0003DMG400036160 | chr04 27559777–27560277 | 166 | 1 | Mα |
| StMADS73 | PGSC0003DMG400044513 | chr04 27753241–27753636 | 131 | 1 | Mα |
| StMADS74 | PGSC0003DMG400040625 | chr04 27761388–27761732 | 114 | 1 | Mα |
| StMADS75 | PGSC0003DMG400035221 | chr04 27826362–27826868 | 168 | 1 | Mα |
| StMADS76 | PGSC0003DMG400036006 | chr04 28232462–28232779 | 105 | 1 | Mα |
| StMADS77 | PGSC0003DMG400034832 | chr04 28463661–28464235 | 164 | 2 | Mα |
| StMADS78 | PGSC0003DMG400040127 | chr04 28566215–28566718 | 167 | 1 | Mα |
| StMADS79 | PGSC0003DMG400045239 | chr04 29232278–29232646 | 122 | 1 | Mα |
| StMADS80 | PGSC0003DMG400042688 | chr04 49390684–49391226 | 180 | 1 | Mα |
| StMADS81 | PGSC0003DMG400044761 | chr04 49734932–49735474 | 180 | 1 | Mα |
| StMADS82 | PGSC0003DMG400039153 | chr04 54460837–54461322 | 161 | 1 | Mα |
| StMADS83 | PGSC0003DMG400037581 | chr04 54575543–54576028 | 161 | 1 | Mα |
| StMADS84 | PGSC0003DMG400024799 | chr04 58551552–58552247 | 231 | 1 | Mα |
| StMADS85 | PGSC0003DMG400040743 | chr06 10343031–10343519 | 162 | 1 | Mα |
| StMADS86 | PGSC0003DMG400040848 | chr06 45773429–45773971 | 180 | 1 | Mα |
| StMADS87 | PGSC0003DMG400043595 | chr07 15176598–15177149 | 183 | 1 | Mα |
| StMADS88 | PGSC0003DMG400004482 | chr08 38320573–38321088 | 171 | 1 | Mα |
| StMADS89 | PGSC0003DMG400029147 | chr08 38625243–38625695 | 150 | 1 | Mα |
| StMADS90 | PGSC0003DMG400039537 | chr09 4843300–4843812 | 170 | 1 | Mα |
| StMADS91 | PGSC0003DMG400036125 | chr09 6381512–6381946 | 144 | 1 | Mα |
| StMADS92 | PGSC0003DMG400043218 | chr09 8426313–8426747 | 144 | 1 | Mα |
| StMADS93 | PGSC0003DMG400038182 | chr09 8460217–8460714 | 165 | 1 | Mα |
| StMADS94 | PGSC0003DMG400043614 | chr09 28872055–28872590 | 149 | 2 | Mα |
| StMADS95 | PGSC0003DMG400040285 | chr09 45285005–45285310 | 101 | 1 | Mα |
| StMADS96 | PGSC0003DMG400037372 | chr09 45311443–45311877 | 144 | 1 | Mα |
| StMADS97 | PGSC0003DMG400019393 | chr10 6655038–6655631 | 173 | 1 | Mα |
| StMADS98 | PGSC0003DMG400043633 | chr10 13335280–13335894 | 204 | 1 | Mα |
| StMADS99 | PGSC0003DMG400035501 | chr10 16298995–16299315 | 106 | 1 | Mα |
| StMADS100 | PGSC0003DMG400042890 | chr10 43539248–43539784 | 178 | 1 | Mα |
| StMADS101 | PGSC0003DMG400039674 | chr10 43571982–43572518 | 178 | 1 | Mα |
| StMADS102 | PGSC0003DMG400036190 | chr11 36057399–36057959 | 186 | 1 | Mα |
| StMADS103 | PGSC0003DMG400047285 | chr11 36128116–36128568 | 150 | 1 | Mα |
| StMADS104 | PGSC0003DMG400039835 | chr11 36737512–36737970 | 152 | 1 | Mα |
| StMADS105 | PGSC0003DMG400043978 | chr11 41767865–41768500 | 211 | 1 | Mα |
| StMADS106 | PGSC0003DMG400038207 | chr11 41778980–41779333 | 117 | 1 | Mα |
| StMADS107 | PGSC0003DMG400001143 | chr11 41867026–41867556 | 176 | 1 | Mα |
| StMADS108 | PGSC0003DMG400044457 | chr12 52437112–52437654 | 180 | 1 | Mα |
| StMADS109 | PGSC0003DMG400046733 | chr00 38424953–38425450 | 165 | 1 | Mα |
| StMADS110 | PGSC0003DMG400046736 | chr01 44165290–44165835 | 181 | 1 | Mβ |
| StMADS111 | PGSC0003DMG400037342 | chr01 60364849–60365394 | 181 | 1 | Mβ |
| StMADS112 | PGSC0003DMG400024916 | chr01 80241931–80242893 | 320 | 1 | Mβ |
| StMADS113 | PGSC0003DMG400024902 | chr01 80529617–80530232 | 139 | 1 | Mβ |
| StMADS114 | PGSC0003DMG400005968 | chr04 1911128–1911634 | 168 | 1 | Mβ |
| StMADS115 | PGSC0003DMG400035463 | chr05 9324497–9325042 | 181 | 1 | Mβ |
| StMADS116 | PGSC0003DMG400007682 | chr05 9990538–9991511 | 157 | 1 | Mβ |
| StMADS117 | PGSC0003DMG400018623 | chr05 10026168–10026958 | 219 | 1 | Mβ |
| StMADS118 | PGSC0003DMG400041411 | chr05 51418059–51418661 | 200 | 1 | Mβ |
| StMADS119 | PGSC0003DMG400047062 | chr06 8761350–8762057 | 235 | 1 | Mβ |
| StMADS120 | PGSC0003DMG400026582 | chr06 44289139–44289651 | 131 | 1 | Mβ |
| StMADS121 | PGSC0003DMG400033092 | chr06 52148889–52149384 | 131 | 1 | Mβ |
| StMADS122 | PGSC0003DMG400027886 | chr07 39107047–39108380 | 299 | 2 | Mβ |
| StMADS123 | PGSC0003DMG400027613 | chr11 12890859–12892025 | 388 | 1 | Mβ |
| StMADS124 | PGSC0003DMG400035247 | chr11 13686758–13687444 | 228 | 1 | Mβ |
| StMADS125 | PGSC0003DMG400026881 | chr11 17707080–17707472 | 130 | 1 | Mβ |
| StMADS126 | PGSC0003DMG400036234 | chr11 29461097–29461483 | 128 | 1 | Mβ |
| StMADS127 | PGSC0003DMG400044576 | chr11 40025123–40025641 | 172 | 1 | Mβ |
| StMADS128 | PGSC0003DMG400044231 | chr12 10345869–10346366 | 165 | 1 | Mβ |
| StMADS129 | PGSC0003DMG400035476 | chr12 10440142–10440801 | 219 | 1 | Mβ |
| StMADS130 | PGSC0003DMG400034608 | chr12 10457590–10458198 | 202 | 1 | Mβ |
| StMADS131 | PGSC0003DMG400045649 | chr12 10473059–10473718 | 219 | 1 | Mβ |
| StMADS132 | PGSC0003DMG400044285 | chr12 11631459–11632118 | 219 | 1 | Mβ |
| StMADS133 | PGSC0003DMG400016835 | chr00 15615908–15616303 | 131 | 1 | Mβ |
| StMADS134 | PGSC0003DMG400016838 | chr00 15735051–15735446 | 131 | 1 | Mβ |
| StMADS135 | PGSC0003DMG400005533 | chr00 27564359–27564892 | 177 | 1 | Mβ |
| StMADS136 | PGSC0003DMG400039981 | chr00 27565967–27566461 | 164 | 1 | Mβ |
| StMADS137 | PGSC0003DMG400005534 | chr00 27590544–27591083 | 179 | 1 | Mβ |
| StMADS138 | PGSC0003DMG400036527 | chr01 45487714–45488157 | 147 | 1 | Mγ |
| StMADS139 | PGSC0003DMG400024023 | chr01 45617221–45618372 | 383 | 1 | Mγ |
| StMADS140 | PGSC0003DMG400024024 | chr01 45621657–45622640 | 327 | 1 | Mγ |
| StMADS141 | PGSC0003DMG400039025 | chr03 5616516–5617253 | 245 | 1 | Mγ |
| StMADS142 | PGSC0003DMG400030053 | chr04 20856443–20857615 | 390 | 1 | Mγ |
| StMADS143 | PGSC0003DMG400034827 | chr04 23826227–23827363 | 378 | 1 | Mγ |
| StMADS144 | PGSC0003DMG400038060 | chr05 38909497–38910506 | 213 | 2 | Mγ |
| StMADS145 | PGSC0003DMG400040524 | chr05 39047174–39048184 | 336 | 1 | Mγ |
| StMADS146 | PGSC0003DMG400045326 | chr05 39101622–39102656 | 344 | 1 | Mγ |
| StMADS147 | PGSC0003DMG400044997 | chr05 39139534–39142691 | 349 | 2 | Mγ |
| StMADS148 | PGSC0003DMG400044633 | chr05 41389552–41390400 | 282 | 1 | Mγ |
| StMADS149 | PGSC0003DMG400039358 | chr05 43411054–43411578 | 174 | 1 | Mγ |
| StMADS150 | PGSC0003DMG400041221 | chr05 43419172–43421058 | 259 | 2 | Mγ |
| StMADS151 | PGSC0003DMG400038225 | chr05 43446588–43447571 | 327 | 1 | Mγ |
| StMADS152 | PGSC0003DMG400040157 | chr07 41201874–41202581 | 235 | 1 | Mγ |
| StMADS153 | PGSC0003DMG400028638 | chr12 50083239–50084024 | 261 | 1 | Mγ |
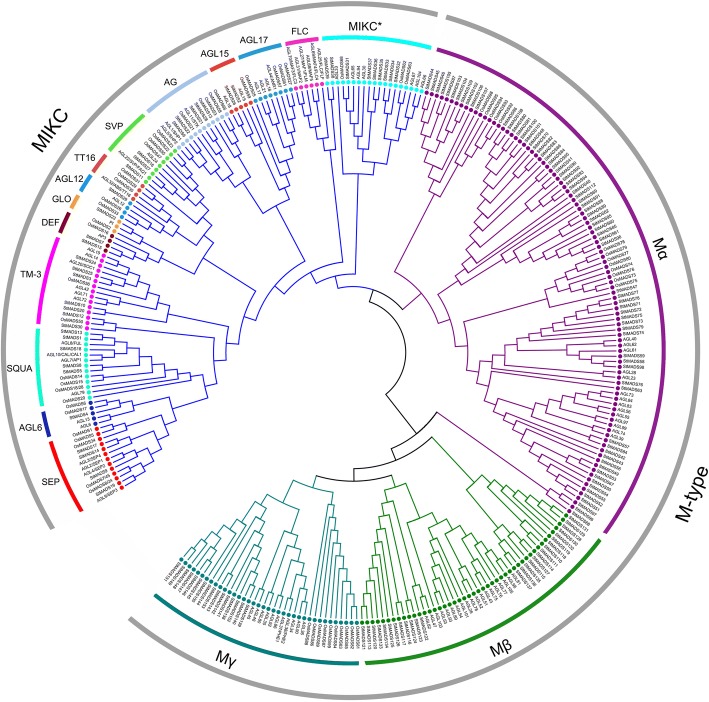
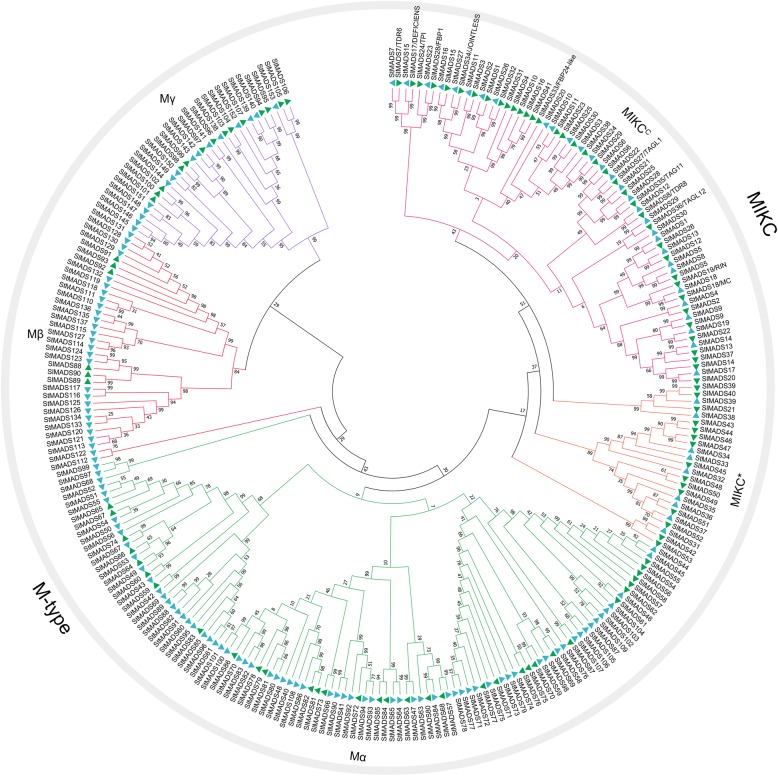
Fig. 1.
Phylogenetic tree of Arabidopsis, rice and potato MADS-box proteins. A total of 153 protein sequences of potato MADS-box genes, 89 of rice and 60 of Arabidopsis were pre-aligned by ClustalX (1.83) and used for constructing a NJ-tree in Mega 7 with 1000 replicates in bootstrap values. As is shown above, all clades are colored and arced to make it clear
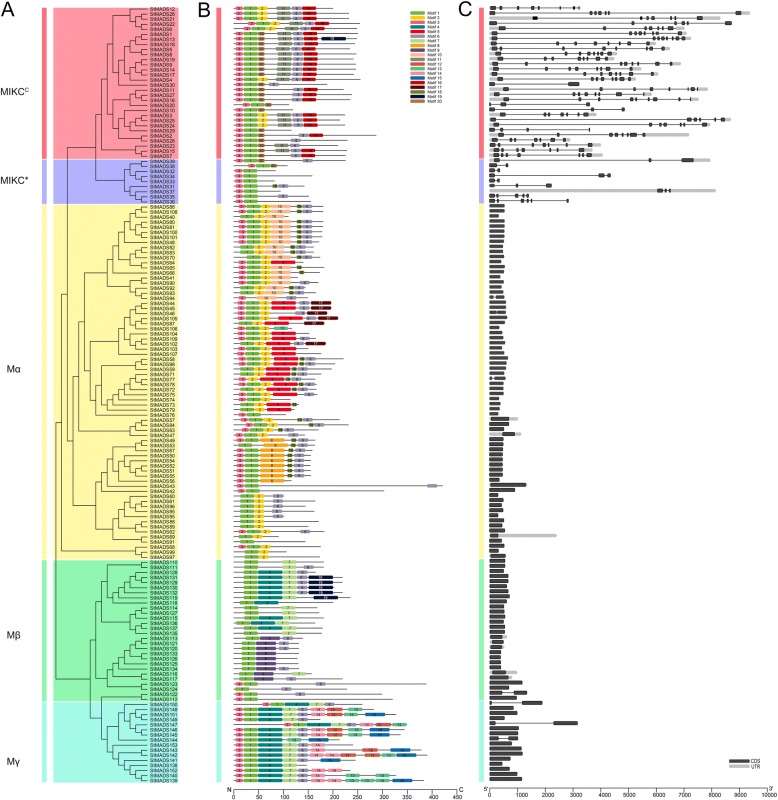
MIKC subfamily members are about 200 amino acid in length and contain more exons than those of M-type subfamily (Table 1). The MIKC family members have an average of 6.4 exon number, and 86.7% of them contain more than 5. But for the M-type, most of them (106 of 114) have only one exon (Table 1). These results about exon number in MADS-box genes are similar to those have been reported in Arabidopsis, rice, cucumber, and apple. The exon-intron structures of MIKC members are more complex than those of M-type.
The total number of MADS-box genes in 10 species that had been previously reported was quite different (Table 2) [12, 39–49]. The reported numbers of MADS-box genes were from 43 to 167, which were positively correlated to corresponding genome size except for that of Arabidopsis (Table 2). Generally, MIKC subfamily consisted of more members than M-Type subfamily as reported in previous studies, but we found that the number of M-type MADS-box genes (114) is approximately three times to that of MIKC MADS-box genes (39) in potato (Table 2). In the other nine species, the number of MIKC subfamily members was close to or more than that of M-type subfamily members.
Table 2.
The number of MADS genes in thirteen plant species
| Species | Genome size (Mb) | Total MADS genes | Total MIKC | MIKCC | MIKC* | Total M-type | Mα | Mβ | Mγ |
|---|---|---|---|---|---|---|---|---|---|
| Cucumis sativus | 350 | 43 | 33 | 30 | 3 | 10 | 5 | 2 | 3 |
| Brachypodium distachyon | 260 | 57 | 39 | 32 | 7 | 18 | 9 | 7 | 2 |
| Sesamum indicum | 340 | 57 | 33 | 28 | 5 | 24 | 14 | 0 | 10 |
| Oryza sativa | 466 | 75 | 44 | 38 | 6 | 32 | 13 | 9 | 10 |
| Prunus mume | 280 | 80 | 37 | 32 | 5 | 43 | 20 | 14 | 9 |
| Vitis vinifera | 490 | 90 | 48 | 42 | 6 | 42 | 23 | 0 | 19 |
| Populus trichocarpa | 480 | 105 | 64 | 55 | 9 | 41 | 23 | 12 | 6 |
| Arabidopsis thaliana | 125 | 106 | 45 | 39 | 6 | 61 | 25 | 20 | 16 |
| Solanum lycopersicum | 900 | 107 | 53 | 41 | 12 | 54 | 34 | 6 | 14 |
| Raphanus raphanistrum | 253 | 144 | 76 | 70 | 6 | 68 | 31 | 12 | 25 |
| Malus domestica | 1874 | 146 | 92 | 75 | 17 | 54 | 22 | 8 | 24 |
| Solanum tuberosum | 844 | 153 | 39 | 30 | 9 | 114 | 70 | 28 | 16 |
| Brassica rapa | 485 | 167 | 100 | 89 | 11 | 67 | 29 | 16 | 22 |
Chromosomal distribution and duplication events of StMADS genes
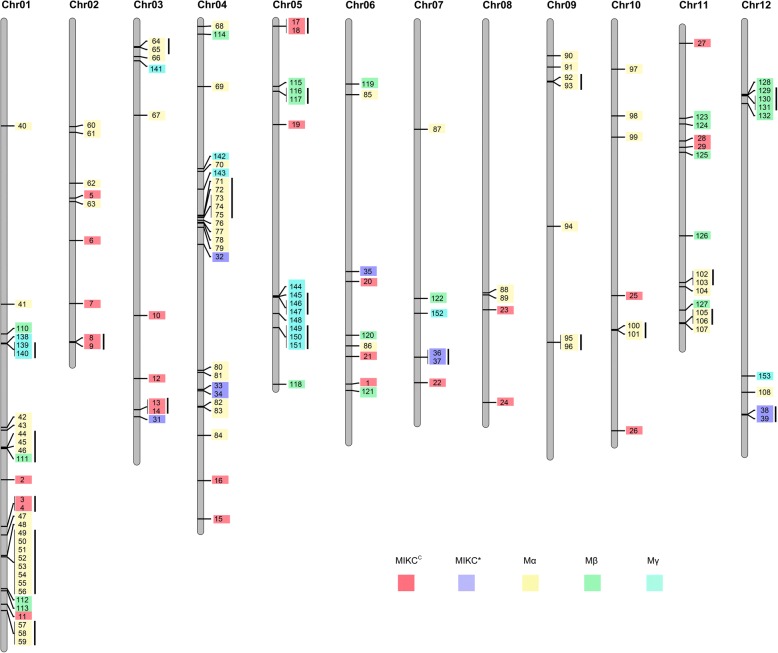
The MapChart software was used to map the physical position of MADS-box genes on 12 chromosomes of potato, which would be helpful for us to perform further study of function of MADS-box genes in potato (Fig. 2). Based on the information of chromosomal locations of potato MADS-box genes, it was found that seven genes were not localized to the chromosomes of potato, five of which belonged to the M-type subfamily (Table 1). The rest MADS-box genes (146) were distributed on the 12 chromosomes and the top five chromosomes with more MADS-box genes are Chr01 (31 genes), Chr04 (25 genes), Chr05 (15 genes), Chr11 (14 genes), and Chr03 (10) (Fig. 2).
Fig. 2.
Physical map of 153 MADS-box genes at 12 potato chromosomes. Different subfamilies are shown in different colors. Genes covered with a single line means a tandem duplication gene group
To further explore the distribution patterns of MADS-box genes, a radar map was exhibited to show the distributions of each subfamily in 12 chromosomes. It was found that substantial clustering was detected in each of at least four chromosomes which was mainly contributed by the gene number of M-type subfamily rather than MIKC subfamily, implying there may be a selective expansion pattern mainly happened in the M-type subfamily (Fig. 3). The MADS-box genes belong to MIKC subfamily distributed on all chromosomes except on Chr09 (Fig. 2). For the M-type MADS-box genes, 52.9% MADS-box genes of the Mα subgroup was clustered on Chr01 and Chr04.
Fig. 3.
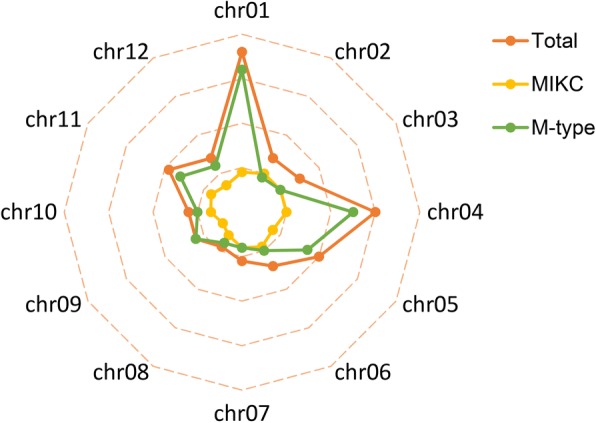
Number of MAD-box genes at 12 potato chromosomes. Different color represents different group
Moreover, the gene duplication events in the MADS-box gene family were analyzed and it was found that 47.7% (73 of 153) MADS-box genes derived from gene duplications (Figs. 2 and 4a). Tandem duplicated genes were mainly located on chromosome 1 and chromosome 5, accounting for about 51.9% of tandem duplicated genes. 78.9% (45 of 57) tandem duplicated genes of belonged to the M-type, indicating that tandem duplications played an important role in the expansion of M-type family genes. 31.5% (12 of 38) genes of the MIKC subfamily were resulted from tandem duplications. Interestingly, it was found that tandem duplications could occur between different subgroups (e.g. StMADS44–46 belonged to Mα and StMADS111 belonged to Mβ), indicating that gene duplication not only contributed to the expansion of MADS-box gene family but also lead to functional diversifications.
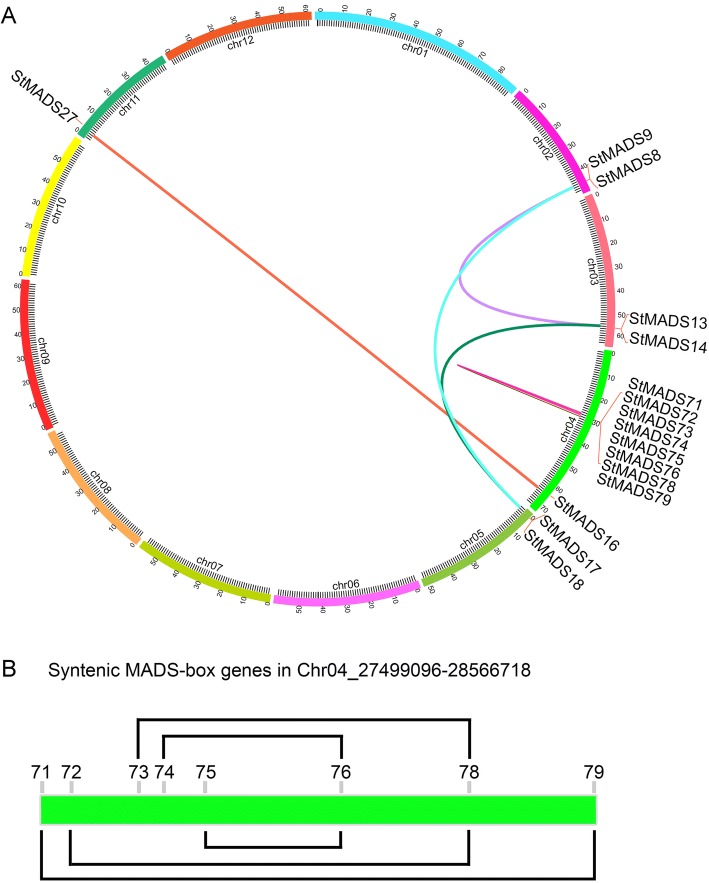
Fig. 4.
Segmental duplication genes in 12 potato chromosomes. Genes linked with a line shows a pair of segmental duplicated genes. a Segmental gene pairs between 12 chromosomes. b Micro-syntenic map in Chr04_27,499,096–28,566,718
Compared with tandem duplications, segmental duplications only accounted for 10.5% of the total MADS-box genes in potato (Fig. 4a). 26.7% (8 of 30) of MIKCC MADS-box genes were resulted from segmental duplications. Those genes were located in Chr02 (two genes), Chr03 (two genes), Chr04 (one genes), Chr05 (two genes), and Chr11 (one genes). We found, interestingly, three copies of the segmental duplicated gene pair (StMADS17 and 18, StMADS13 and 14, StMADS8 and 9), among which StMADS9, 14 and 17 are from SEP group, while StMADS8, 13 and 18 from SQUA group. Moreover, the Mα subfamily members StMADS71–79 located in chr04 were segmental duplicated genes, which shows a cluster in the physical map (Fig. 4b). It clearly shows that there probably a chromosome doubling event in chr04 in the process of potato evolution, which contribute greatly to the expansion of Mα type MADS-box genes.
Phylogenetic relationships and conversed motifs of StMADS proteins
An unrooted tree was built based on the full-length amino acid sequences of 153 potato, 89 Arabidopsis, and 60 rice MADS-box proteins using MEGA6.0 software (Fig. 1). StMADS proteins can be classified into two major subfamilies, MIKC (also known as type II, 39 genes) and M-type (also known as type I, 114 genes), based on the phylogenetic tree. MIKC subfamily can be further divided into MIKCC (30 genes) and MIKC* (9 genes), whereas M-type contains Mα (70), Mβ (28) and Mγ (16). According to the classification method defined in Malus domestica, Oryza sativa, and Brassica rapa, MIKCC subgroup was organized into 13 clades. Interestingly, potato MADS-box genes were absent in the FLC-like, AGL15-like, and TT16-like clades. Subsequently, potato MIKCC subgroup consisted of ten clades. TM3-like clade was the largest clade containing seven StMADS proteins. The orthologous and paralogous relationships of MADS-box proteins are analyzed in potato, rice, and Arabidopsis, it was found that most of the M-type subfamily members were concentrated in a cluster, namely all of these homologous MADS-box proteins are paralogous genes. These results indicated that the MADS-box gene family was formed in an ancestral species before the divergence of monocotyledonous and dicotyledonous plants, which was consistent with the results of previous studies [12, 39–52]. Moreover, orthologous pairs come from the MIKC family were with relatively high homology, indicating that the functions of MIKC family genes were relatively conservative in the evolutionary process.
Similarly, an unrooted tree was also built based on the full-length amino acid sequences of 153 potato MADS-box proteins, which could be partitioned into MIKCC, MIKC*, Mα, Mβ, and Mγ with good supporting values (Fig. 5a). To further analyze the motif compositions of potato MADS-box proteins, MEME online software was used to analyze the conserved motifs (Fig. 5b). The number of conserved motif was set to 20, where motifs 1, 2, 3, and 20 were located at MADS-box domains and motifs 6, 11 and 16 were located at K domain. Moreover, motifs 4, 5, and 7–10 represented coil regions and low complexity regions. Besides, the rest motifs were less conservative and only appeared in several MADS-box proteins. As shown in Fig. 4, MIKCC subgroup contains seven conservative motifs 1, 2, 3, 6, 11, 16, and 20 and motifs 11 and 16 belonging to K domain only existed in this subgroup. The MIKC* subgroup contains fewer motif varieties, mainly motifs 1 and 3, and some of them had motifs 6 and 20 similar to MIKC. Specifically, StMADS31 had motif 18 adjacent to motif6 which was similar to the members belonging to subgroup Mα. These results showed that the MADS-box proteins included in the same clade in the phylogenetic tree have almost identical motif distribution types. Moreover, the structure of MIKC* had both characteristics of M-type and MIKCC, but in Arabidopsis the subgroup MIKC* was attributed to Mδ, which were attributed to MIKC group later in the analyses of MADS-box families of other species [12, 39–52].
Fig. 5.
Phylogenetic relationships, conserved motif and gene structure of potato MADS genes. a The NJ-tree of 153 potato MADS-box genes, constructed with the same method mentioned above. b Conserved motif analysis of 153 MADS-box genes. Rounded rectangle with different colors represent different motif. c Gene structure of MADS-box genes. Exon and UTR are box colored with black and grey respectively, among which the black line represent introns
Phylogenetic relationship of MADS-box genes of potato and tomato
Tomato is the most studied model plant in Solanaceae family. Therefore, a comparison with tomato MADS genes could provide more clues on the function differentiation of potato MADS genes (Fig. 6 and Additional file 2: Table S2). There was a total of 107 MADS-box genes in tomato, including 53 MIKC type and 54 M-type MADS-box genes. Tomato compromised more MIKC genes (53) compared with those in potato (39) even if the total number of potato MADS-box genes (153) is more than those in tomato (107). On the contrary, there were more M-type MADS-box genes in potato compared with those in tomato, especially the number of Mα (70) and Mβ (28) in higher than those in tomato (Mα, 34; Mβ, 6), respectively (Fig. 6 and Table 2). These evidences suggested that the expansion of MADS-box in Solanaceae might be quite different.
Fig. 6.
Phylogenetic analyses of MADS-box genes between Solanum tuberosum and Solanum lycopersicum. The NJ-tree was generated using the method mentioned above
To speculate the functions of potato MADS-box genes, we compare the MIKCC MADS-box genes with their closely related homologs. The orthologs of MIKCC potato MADS-box genes was screened by following criteria, which were BLASTP e-Value was less than 10e-10) with more than 80% coverage in length and the ortholog was the best-matching homolog than other candidate in tomato. The orthologs of most potato MIKCC MADS-box genes could be found in tomato except StMADS3, 5, and 20 (Additional file 3: Table S3). The Orthologs in different species have evolved from a common ancestral gene via speciation, which often retain the same functions during evolution.
Tissue specific expression patterns of MIKCCStMADS genes
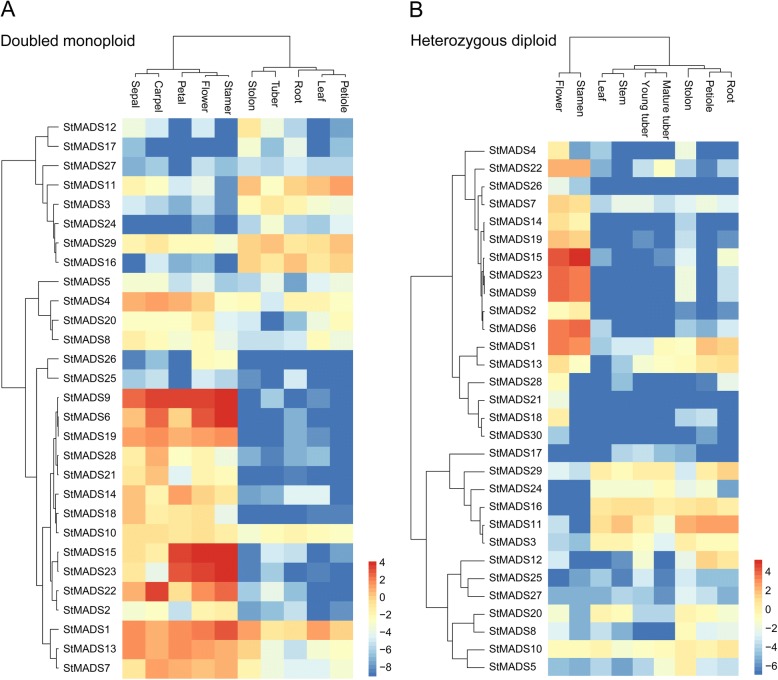
Illumina RNA-Seq transcriptome data of DM and RH was retrieved to explore the expression patterns of StMADS genes, including vegetative organs (including root, stem, petiole, and leaf), floral organs (including flower, stamen, sepal, petal, and carpel) and storage organs (including stolon and tuber) [36]. The expression levels of StMADS genes were estimated by using FKPM (fragments per kilobase of transcript per million mapped fragment) method. A gene was designated as expressed if its FKPM value in any tissue or organ was greater than 1. According to this standard, 37 of 39 MIKC-type StMADS genes were expressed but only 16 of 123 M-type StMADS genes were expressed, indicating that MIKC-type StMADS genes were actively expressed and most of M-type StMADS genes were not expressed even if there were many M-type StMADS genes presented on the potato genome.
The MIKCC StMADS genes were selected for further expression analysis because they were probable downstream targets of tuberigen StSP6A based on previous studies about its homologue−FLOWERING LOCUS T in Arabidopsis and rice [34]. Hierachical clustering of MIKCC StMADS genes was performed by using the transcriptome data of DM and RH, respectively (Fig. 7). The MIKCC StMADS genes in both DM and RH were similarly divided into two major clusters. The first group of MIKCC StMADS genes was mainly expressed in floral organs and the other group was expressed in vegetative and storage organs. More specifically for their expressions in DM, 21 genes, five genes, and four genes were with highest expression in floral organs, vegetative organs, and storage organs, respectively (Fig. 7a). And for RH, 16 genes, five genes, and nine genes were with highest expression in floral organs, vegetative organs, and storage organs, respectively (Fig. 7b). It was found that most MIKCC StMADS genes were expressed in floral organs, indicating their possible roles in controlling floral organ development. Whereas, there were more MIKCC StMADS genes expressed in storage organs of RH compared with DM.
Fig. 7.
Expression profiles of MADS-box genes in double monoploid and heterozygous. The RNA-seq data is retrieved from PGSC. FPKM values of MADS genes are normalized and the heatmap is drawn with Pretty Heatmap at ImageGP. a Expression profiles of the StMADS genes in DM (doubled monoploid S. tuberosum Group PhurejaDM1-3). b Expression profiles of the StMADS genes in RH (heterozygous diploid S. tuberosum Group Tuberosum RH89-039-16)
Moreover, the expressions of MIKCC StMADS genes in storage organs were further analyzed. It was found that 17 and 26 MIKCC StMADS genes were expressed (FKPM > 1) in storage organs of DM and RH, respectively. And nine and eight and 12 MIKCC StMADS genes were highly expressed (FKPM > 10) in storage organs of DM and RH, respectively. Taken together, six genes (StMADS1, 3, 11, 13, 16, and 29) were consistently with high expression levels in storage organs of both DM and RH, which may be involved in tuberization and following tuber development. Based on the phylogenic relationship, it was found that StMADS1 and StMADS13 were homologous genes of AGL8/FUL and OsMADS14/15, StMADS3 was homologous gene of SOC1 and OsMADS56, StMADS11 and StMADS16 were homologous genes of AGL22/SVP, and StMADS29 was homologous gene of AGL12. Among these genes expressed in potato stolon and tubers, StMADS1, StMADS3, and StMADS13 were the most likely downstream genes of tuberigen StSP6A because their homologous genes AGL8/FUL, OsMADS14/15, and SOC1 were proved to be downstream targets of Arabidopsis and rice FLOWERING LOCUS T [53–57].
QRT-PCR verifications of tissue specific MIKCCStMADS genes
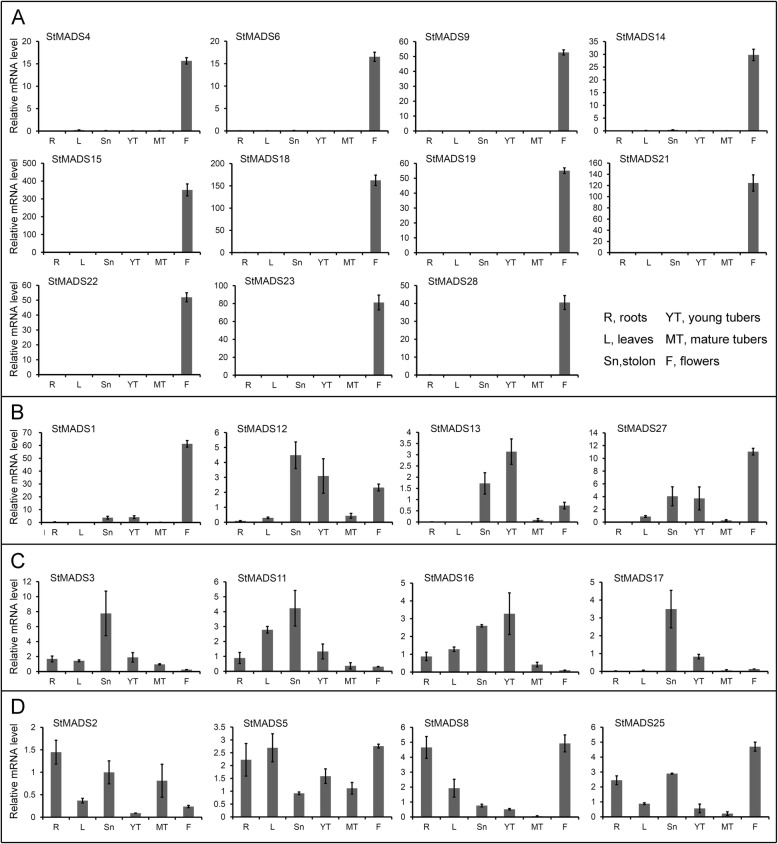
To validate the results of RNA-seq analysis, real-time PCR analysis was performed for 29 MIKCC StMADS genes. Our tests showed that the real-time PCR experiments of 25 MIKCC StMADS genes (except StMADS7, 10, 20, and 26) were successfully conducted in tissues including roots, leaves, stolons, young tubers, mature tubers, and flowers. The results of real-time PCR showed that the expression patterns of most were in general agreement with the data of RNA-seq analysis. For example, eleven StMADS genes (StMADS4, 6, 9, 14, 15, 18, 19, 21, 22, 23, and 28) were overwhelmingly expressed in flowers compared with any other tissues (Fig. 8a), which were perfectly consistent with their expression patterns in flowers of both DM and RH (Fig. 7). The StMADS genes specifically expressed in potato flowers were most likely to control floral organ formation like their homologues in ABCDE model of other species [58, 59].
Fig. 8.
QRT-PCR verifications of representative StMADS genes in various potato tissues. a Genes are mainly expressed in flowers. b Genes preferentially show expression in flowers, stolons, and young tubers. c Genes are abundantly expressed in stolons. d Genes are expressed in nearly all examined tissues
StMADS1, 12, 13, and 27 were not only expressed in flowers but also expressed in stolons and young tubers (Fig. 8b), indicating that they might control the formations of both flower organs and tuberization. StMADS3, 11, 16, and 17 were highly expressed in stolons and/or young tubers but their expressions in flower were relatively low (Fig. 8c). Besides, we found six StMADS genes were expressed in almost all examined tissues without obvious tissue specific patterns (Fig. 8d, the expressions of StMADS24 and StNADS29 were not showed.
Screening for downstream targets of tuberigen StSP6A
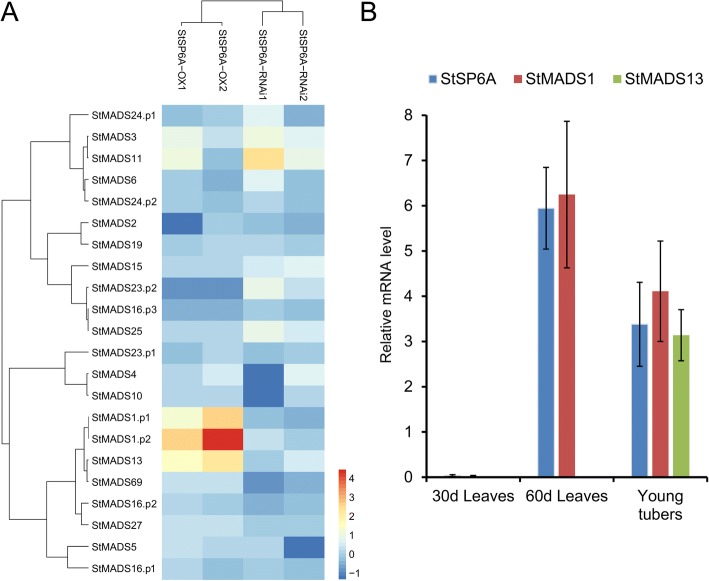
StSP6A, a FLOWERING LOCUS T homologue in potato, have been reported to be a mobile signal in controlling not only flowering but also tuberization, while its homologue StSP3D is mainly involved in floral transition [34]. According to previous studies about flowering, MADS-box genes encoding proteins involved in flowering identity determination are major targets of FLOWERING LOCUS T and it was speculated that StMADS genes were downstream targets of tuberigen StSP6A. Therefore, the whole-genome microarray data from stolon tissue of StSP6A-overexpression (StSP6A-OX) and StSP6A-RNAi plants was used to screen for downstream StMADS genes. Firstly, the DNA probe sequences on POCI (Potato Oligo Chip Initiative) [34] microarrays were used as queries to perform BLASTN searches against the transcript sequences of StMADS genes. It was found that 22 probes corresponded to 17 StMADS genes (16 genes were belonged to MIKCC type) were presented on the POCI microarray chip and four genes had two or three probes. Hierachical clustering of the expressions of StMADS genes in StSP6A-OX and StSP6A-RNAi plants showed that StMADS1 and StMADS13 were the most possible downstream targets of StSP6A because the expressions of StMADS1 and StMADS13 were activated in both biological replicates of StSP6A-OX plants and was repressed in both biological replicates of StSP6A-RNAi plants. The expressions of StMADS3 and StMADS11 were only activated in one biological replicate of StSP6A-OX plants and not repressed in StSP6A-RNAi plants. The expressions of other StMADS genes were not obviously changed in either StSP6A-OX or StSP6A-RNAi plants.
Moreover, to verify the whether the expressions of StAMDS1 and StMADS13 were associated with the expression of StSP6A, we investigate the expressions of StSP6A, StMADS1, and StMADS13 in leaves of 30 days after sprouting, leaves of 60 days after sprouting, and young tubers, respectively. It was found that these three genes were not expressed in potato leaves at juvenile stage (30 days after sprouting), whereas StMADS1 and StSP6A were highly expressed in potato leaves at early flowering stage (60 days after sprouting) and young tubers (Fig. 9b). The expression of StMADS13 was not detected in 60d leaves but was observed in young tubers (Fig. 9b). These results indicated that StMADS1 expressions were associated with StSP6A in both 60d leaves and young tubers, whereas StMADS13 was only associated with StSP6A in young tubers. Though both StMADS1 and StMADS13 were putative downstream genes of StSP6A, their regulatory mechanism might be different depending on tissue types.
Fig. 9.
Expression relationships between MADS-box genes and StSP6A. a Heatmap of the expression of 17 MADS-box genes in StSP6A-OX and StSP6A-RNAi plants. b Expressions of StSP6A, StMADS1, and StMADS13 in leaves of 30 days after sprouting, leaves of 60 days after sprouting, and young tubers, respectively
Discussion
Potato is one of the major food crops, which feeds millions of people all over the world [60]. As an important tuber crop, the improvement of yield is a key issue to potato breeder in china, owing to its low production far fewer than the global average. Thus, the investigation of molecular mechanism of tuberization and tuber development remains unclear. It will be helpful to identify candidate genes related to tuberization and tuber development, which are the key resources to promote the improvement of yield for both genetic modified crop and traditional breeding. Previous study had shown that FT protein StSP6A functioned as a mobile signal in controlling tuberization under short-day condition [34], and its paralogue StSP3D was involved in day-neutral flowering control. FT was a classical upstream regulator of MADS-box genes in the conservative ABC model in flower organ identity [59]. Based on clues that we mentioned above, it was reasonable to believe that some potato MADS-box genes were related to tuberization and tuber development.
In this study, a total of 153 members of MADS-box gene family were characterized in potato (Table 1). To confirm the gain and loss of MADS-box genes in potato, a phylogenetic tree was produced using ammo acid sequence of 153 potato MADS-box genes and representative MADS genes of Arabidopsis and rice (Fig. 1). It was found that most of MADS-box genes had their orthologs in potato, except for TT16-like, AGL17-like, and FLC-like subgroups. Besides, it was found that members of M-type, specifically Mα, were much more than any other species ever studied (Table 2), implying that potato MADS-box genes might have a different evolution pattern [39–52]. Apparently, the large potato MADS-box family might be due to its larger genome, which was produced by differently genomic duplication events in different species during the course of plant evolution [61, 62]. To explore what behind the extremely different composition of MADS-box family in potato, the effects of gene duplication events on expansion of MADS-box genes were investigated (Fig. 2). It was suggested that segmental duplications mainly contributed to expansion of MIKC subfamily, whereas the boom of M-type was mostly derived from tandem duplications (Figs. 2 and 3). It was believed that this phenomenon was due to the M-type genes mainly derived from the site-specific duplications within the same chromosome, while MIKC mainly came from the whole genome duplication events [61, 63–65]. Birth-and-death rates of MADS-box genes after gene duplications in different species were different which resulted in variable number of MADS-box genes in the same subfamily of different species [61, 62]. These evidences could be one explanation for the abnormality of number of Mα in potato.
Besides gene duplication events, gene mutation and loss of certain domain, might also play important role in generating a part of Mα in potato. It had been reported that MIKC* was the intermediate form between MIKCC and M-type in the course of plant evolution [58, 59, 62]. Based on intron-exon structures of potato MADS-box genes, it was found that the intron number of MIKCC was the most and MIKC* had less intron than MIKCC, whereas most of M-type MADS-box genes was intronless. It could be speculated that the exon-intron loss mutations mainly happened in the K-box domain of MIKCC in the process of plant evolution, thus a new group, MIKC*, was born. MIKC* further lost several exons and introns corresponding to K-box domain and then produced M-type, which was also found in previous studies [49]. These evidences could be the other explanation for the abnormality of number of Mα in potato.
To compare the composition of MADS-box genes of potato and tomato, phylogenetic analysis was performed. Since these two species are genetically close species, it was surprising that tomato contain much more MIKC genes, while potato included more M-type genes. This suggested that these species might undergo a different evolution history of MADS-box. The candidate genes StMADS1 and StMADS13 that may be related to tuberization, the functions of their orthologous genes in tomato, SlMADS26 and SlMADS12, were still undiscovered. Though potato and tomato are both Solanaceae plants, the stolons and tubers are only found in potato. Thus, the functions of StMADS1 and StMADS13 need to be investigated in potato. Nevertheless, there was a homolog of StMADS1 and StMADS13, RIN found in tomato, which is proved to play important roles in induction of tomato ripening [66, 67]. Besides, compared with potato, most genes that had been studied in tomato have few homologs, indicating that a different history of gain and loss functions of MADS-box genes [68–70].To investigate the possible roles of StMADS genes in tuberization, we used RNA-seq data of DH and RM available in PGSC. Most of the StMADS genes showed tissue-specific expression patterns. Among these genes, StMADS1, 3, 11, 13, 16, and 29 were highly expressed in storage organs of both DM and RH (Fig. 7). Consistent with RNA-seq data, the results of QRT-PCR showed that StMADS1, 3, 11 and 16 were overwhelmingly expressed in stolons and/or young tubers (Fig. 8), indicating that these genes were probably involved in tuberization and/or tuber development. Previous studies had shown us a fine regulation map of MADS-box genes and its significant roles in flower organ differentiation in several model plants. In Arabidopsis, the expression of FLOWERING LOCUS T (FT), a core flower development regulator, was suppressed by the FLOWERING LOCUS C (FLC), a typical MADS-box genes, bound in its CArG site between first intron and promoter [71–73]. Interestingly, the expressions of MADS-box genes including APETALA1 (AP1) and SUPPRESSOR OF OVEREXPRESSION OF CO 1(SOC1) were related to flowering promotion that was controlled by two interacted flowering-related proteins FT and FD (FLOWERING LOCUS D) [74]. In monocot plants, orthologs and paralogs of FT and MADS-box presented nearly the same transcriptional regulation, for instance, a pair of FT genes Heading-date 3a (Hd3a) and RICE FLOWERING LOCUS T (RFT1) upregulated the expression of OsMADS15, which is crucial for floral initiation [75–78]. Given the highly conservative model of FT and MADS-box genes, it was reasonable to believe that this model would probably work in potato. Recent study showed that there was a functional diversification of FT proteins in potato. StSP3D was mainly involved in floral transition, and StSP6A was involved in tuberization transition. [34]. Therefore, these StMADS genes (StMADS1, 3, 11–13, 17, and 27) mainly expressed in stolons and/or young tubers were possible downstream targets of StSP6A. Interestingly, it was found StMADS1 and 13 were strongly correlated with the expression of StSP6A in leaves and/or young tubers. More evidences were obtained through analyzing the microarray data from stolon tissue of StSP6A-overexpression (StSP6A-OX) and StSP6A-RNAi plants, the expression of StMADS1 and 13 were upregulated in StSP6A-OX plants and downregulated in StSP6A-RNAi plants. Given the evidence that discussed above, StSP6A and several MADS-box genes are probably share the same regulation map with their homologs in the model plant. However, the truth of how StSP6A regulate StMADS1 and 13, in a directly interaction way or in the promotor region, remain unclear. As a master of transcription, tracing the target of MADS-box gene would also be a valuable subject in the future study.
Conclusion
This study present genomic annotation and expression profiling of potato MADS-box genes. Comprehensive analyses about the evolution and functional differentiation of potato MADS-box were also performed, which would provide solid basis for further functional studies about this gene family. Potato MADS-box genes were putative downstream targets of the potato FT homolog tuberigen StSP6A, which is a mobile signal in controlling tuberization. Furthermore, StMADS1 and StMADS13 were believed to be candidate genes in the downstream of StSP6A. Thus, the utilization of these MADS-box genes for both genetically modified crop and traditional breeding practice in genetic improvement would be possible.
Additional files
Table S1. QRT-PCR primers corresponding to potato MADS-box genes. (DOCX 18 kb)
Table S2. MADS-box genes in tomato. (DOCX 25 kb)
Table S3. Orthologs of potato MIKCC MADS-box genes in tomato. (DOCX 18 kb)
Acknowledgements
The authors acknowledge the members of Dr. Chen’s lab in State Key Laboratory of Crop Stress Biology in Arid Areas, China for technical laboratory advices.
Funding
This research was supported by the National Natural Science Foundation of China (31500159), the Natural Science Foundation of Shaanxi Province (2015JQ3090). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All of the datasets supporting the results of this article are included within the article and its Additional files. The raw data generated in this study can be obtained by reasonable request to the corresponding authors.
Abbreviations
- AA
Amino acid
- AP1
APETALA1
- BLASTP
Basic local alignment search tool-protein
- FLC
FLOWERING LOCUS C
- FT
FLOWERING LOCUS T
- Hd3a
Heading-date 3a
- ORF
Open reading frame
- PGDD
Plant genome duplication database
- PGSC
Potato genome sequencing consortium
- QRT-PCR
Quantitative real-time polymerase chain reaction
- RFT1
RICE FLOWERING LOCUS T
- SOC1
SUPPRESSOR OF OVEREXPRESSION OF CO 1
Authors’ contributions
HM secured most funding to support this study. HZ and HM conceived of and designed the research plans. HG, SL, MH, YZ (Yao Zhou), and YZ (Yaqi Zhao) isolated RNA from potato tissues, conducted the qRT-PCR analysis, and completed statistical analysis of the qRT-PCR analysis dataset. HG, ZW, and GL completed the data processing, normalization, and bioinformatics analyses. HG and HZ wrote the article with contributions from all the authors. HM supervised and supported the writing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huhu Gao, Email: tiger0829@nwsuaf.edu.cn.
Ziming Wang, Email: 2014302220084@whu.edu.cn.
Silu Li, Email: krysii@163.com.
Menglu Hou, Email: houmenglu@nwafu.edu.cn.
Yao Zhou, Email: yaozhou@nwafu.edu.cn.
Yaqi Zhao, Email: zhaoyaqi@nwafu.edu.cn.
Guojun Li, Email: guojunli@nwsuaf.edu.cn.
Hua Zhao, Email: zhaohua362@nwsuaf.edu.cn.
Haoli Ma, Email: mahaoli@nwsuaf.edu.cn.
References
- 1.Riechmann JL, Meyerowitz EM. MADS domain proteins in plant development. Biol Chem. 1997;378:1079–1101. [PubMed] [Google Scholar]
- 2.de Folter S, Angenent GC. trans meets cis in MADS science. Trends Plant Sci. 2006;11:224–231. doi: 10.1016/j.tplants.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Gramzow L, Ritz MS, Theißen G. On the origin of MADS-domain transcription factors. Trends Genet. 2010;26:149–153. doi: 10.1016/j.tig.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Passmore S, Maine GT, Elble R, Christ C, Tye BK. Saccharomyces cerevisiae protein involved in plasmid maintenance is necessary for mating of MATα cells. J Mol Biol. 1988;204:593–606. doi: 10.1016/0022-2836(88)90358-0. [DOI] [PubMed] [Google Scholar]
- 5.Norman C, Runswick M, Pollock R, Treisman R, Almendral JM, Sommer D, et al. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 6.Sommer H, Beltrán JP, Huijser P, Pape H, Lönnig WE, Saedler H, et al. Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J. 1990;9:605–613. doi: 10.1002/j.1460-2075.1990.tb08152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990;346:35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- 8.West AG, Shore P, Sharrocks AD. DNA binding by MADS-box transcription factors: a molecular mechanism for differential DNA bending. Mol Cell Biol. 1997;17:2876–2887. doi: 10.1128/MCB.17.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rounsley SD, Ditta GS, Yanofsky MF. Diverse roles for MADS box genes in Arabidopsis development. Plant Cell. 1995;7:1259–1269. doi: 10.1105/tpc.7.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez-Buylla ER, Liljegren SJ, Pelaz S, Gold SE, Burgeff C, Ditta GS, et al. MADS-box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. Plant J. 2000;24:457–466. doi: 10.1046/j.1365-313x.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Castilla LP, Alvarez-Buylla ER. Adaptive evolution in the Arabidopsis MADS-box gene family inferred from its complete resolved phylogeny. Proc Natl Acad Sci. 2003;100:13407–13412. doi: 10.1073/pnas.1835864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parenicova L. Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell. 2003;15:1538–1551. doi: 10.1105/tpc.011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masiero S, Colombo L, Grini PE, Schnittger A, Kater MM. The emerging importance of type I MADS box transcription factors for plant reproduction. Plant Cell. 2011;23:865–872. doi: 10.1105/tpc.110.081737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam J, Kim J, Lee S, An G, Ma H, Nei M. Type I MADS-box genes have experienced faster birth-and-death evolution than type II MADS-box genes in angiosperms. Proc Natl Acad Sci. 2004;101:1910–1915. doi: 10.1073/pnas.0308430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez-Buylla ER, Pelaz S, Liljegren SJ, Gold SE, Burgeff C, Ditta GS, et al. An ancestral MADS-box gene duplication occurred before the divergence of plants and animals. Proc Natl Acad Sci. 2000;97:5328–5333. doi: 10.1073/pnas.97.10.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masiero S, Imbriano C, Ravasio F, Favaro R, Pelucchi N, Gorla MS, et al. Ternary complex formation between MADS-box transcription factors and the histone fold protein NF-YB. J Biol Chem. 2002;277:26429–26435. doi: 10.1074/jbc.M202546200. [DOI] [PubMed] [Google Scholar]
- 17.Kramer EM, Su HJ, Wu CC, Hu JM. A simplified explanation for the frameshift mutation that created a novel C-terminal motif in the APETALA3 gene lineage. BMC Evol Biol. 2006;6:30. doi: 10.1186/1471-2148-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henschel K, Kofuji R, Hasebe M, Saedler H, Münster T, Theißen G. Two ancient classes of MIKC-type MADS-box genes are present in the moss Physcomitrella patens. Mol Biol Evol. 2002;19:801–814. doi: 10.1093/oxfordjournals.molbev.a004137. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann K, Melzer R, Theißen G. MIKC-type MADS-domain proteins: structural modularity, protein interactions and network evolution in land plants. Gene. 2005;347:183–198. doi: 10.1016/j.gene.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Becker A, Theißen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol Phylogenet Evol. 2003;29:464–489. doi: 10.1016/S1055-7903(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 21.Colombo M, Masiero S, Vanzulli S, Lardelli P, Kater MM, Colombo L. AGL23, a type I MADS-box gene that controls female gametophyte and embryo development in Arabidopsis. Plant J. 2008;54:1037–1048. doi: 10.1111/j.1365-313X.2008.03485.x. [DOI] [PubMed] [Google Scholar]
- 22.Kwantes M, Liebsch D, Verelst W. How MIKC* MADS-box genes originated and evidence for their conserved function throughout the evolution of vascular plant gametophytes. Mol Biol Evol. 2012;29:293–302. doi: 10.1093/molbev/msr200. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Cui S, Wu F, Yan S, Lin X, Du X, et al. Functional conservation of MIKC*-type MADS box genes in Arabidopsis and Rice pollen maturation. Plant Cell. 2013;25:1288–1303. doi: 10.1105/tpc.113.110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sang X, Li Y, Luo Z, Ren D, Fang L, Wang N, et al. CHIMERIC FLORAL ORGANS1, encoding a monocot-specific MADS box protein, regulates floral organ identity in Rice. Plant Physiol. 2012;160:788–807. doi: 10.1104/pp.112.200980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Causier B, Schwarz-Sommer Z, Davies B. Floral organ identity: 20 years of ABCs. Semin Cell Dev Biol. 2010;21:73–79. doi: 10.1016/j.semcdb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Causier B, Castillo R, Xue Y, Schwarz-Sommer Z, Davies B. Tracing the evolution of the floral homeotic B-and C-function genes through genome synteny. Mol Biol Evol. 2010;27:2651–2664. doi: 10.1093/molbev/msq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo J, Shi XX, Zhang JS, Duan YH, Bai PF, Guan XN, et al. A type I MADS-box gene is differentially expressed in wheat in response to infection by the stripe rust fungus. Biol Plantarum. 2013;57:540–546. doi: 10.1007/s10535-012-0297-6. [DOI] [Google Scholar]
- 28.Adamczyk BJ, Fernandez DE. MIKC* MADS domain heterodimers are required for pollen maturation and tube growth in Arabidopsis. Plant Physiol. 2009;149:1713–1723. doi: 10.1104/pp.109.135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Maroto F, Ortega N, Lozano R, Carmona MJ. Characterization of the potato MADS-box gene STMADS16 and expression analysis in tobacco transgenic plants. Plant Mol Biol. 2000;42:499–513. doi: 10.1023/A:1006397427894. [DOI] [PubMed] [Google Scholar]
- 30.Kang S-G, Hannapel DJ, Suh S-G. Potato MADS-box gene POTM1-1 transcripts are temporally and spatially distributed in floral organs and vegetative meristems. Mol Cells. 2003;15:48–54. [PubMed] [Google Scholar]
- 31.Kan S, Kan H. Characterization of potato vegetative MADS . Box gene , POTMI-1 , in response to hormone applications. J Plant Biol. 2002;45:196–200. [Google Scholar]
- 32.Rosin FM. Suppression of a vegetative MADS box gene of potato activates axillary meristem development. Plant Physiol. 2003;131:1613–1622. doi: 10.1104/pp.102.012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmona MJ, Ortega N, Garcia-Maroto F. Isolation and molecular characterization of a new vegetative MADS-box gene from Solanum tuberosum L. Planta. 1998;207:181–188. doi: 10.1007/s004250050471. [DOI] [PubMed] [Google Scholar]
- 34.Navarro C, Abelenda JA, Cruz-Oró E, Cuéllar CA, Tamaki S, Silva J, et al. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature. 2011;478:119–122. doi: 10.1038/nature10431. [DOI] [PubMed] [Google Scholar]
- 35.González-Schain ND, Díaz-Mendoza M, Zurczak M, Suárez-López P. Potato CONSTANS is involved in photoperiodic tuberization in a graft-transmissible manner. Plant J. 2012;70:678–690. doi: 10.1111/j.1365-313X.2012.04909.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Pan S, Cheng S, Zhang B, Mu D, Ni P, et al. Genome sequence and analysis of the tuber crop potato. Nature. 2011;475:189–195. doi: 10.1038/nature10158. [DOI] [PubMed] [Google Scholar]
- 37.Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- 38.Tang X, Zhang N, Si H, Calderón-Urrea A. Selection and validation of reference genes for RT-qPCR analysis in potato under abiotic stress. Plant Methods. 2017;13:85. doi: 10.1186/s13007-017-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leseberg CH, Li A, Kang H, Duvall M, Mao L. Genome-wide analysis of the MADS-box gene family in Populus trichocarpa. Gene. 2006;378:84–94. doi: 10.1016/j.gene.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Arora R, Agarwal P, Ray S, Singh AK, Singh VP, Tyagi AK, et al. MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genomics. 2007;8:242. doi: 10.1186/1471-2164-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu L, Liu S, Somers DJ. Genome-wide analysis of the MADS-box gene family in cucumber. Genome. 2012;55:245–256. doi: 10.1139/g2012-009. [DOI] [PubMed] [Google Scholar]
- 42.Cao J, Han X, Zhang T, Yang Y, Huang J, Hu X. Genome-wide and molecular evolution analysis of the subtilase gene family in Vitis vinifera. BMC Genomics. 2014;15:1116. doi: 10.1186/1471-2164-15-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei B, Zhang RZ, Guo JJ, Liu DM, Li AL, Fan RC, et al. Genome-wide analysis of the MADS-box gene family in Brachypodium distachyon. PLoS One. 2014;9:e84781. doi: 10.1371/journal.pone.0084781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saha G, Park J-I, Jung H-J, Ahmed NU, Kayum MA, Chung M-Y, et al. Genome-wide identification and characterization of MADS-box family genes related to organ development and stress resistance in Brassica rapa. BMC Genomics. 2015;16:178. doi: 10.1186/s12864-015-1349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Wang Y, Xu L, Nie S, Chen Y, Liang D, et al. Genome-wide characterization of the MADS-box gene family in radish (Raphanus sativus L.) and assessment of its roles in flowering and floral organogenesis. Front Plant Sci. 2016;7:1390. doi: 10.3389/fpls.2016.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan W, Song X, Liu T, Huang Z, Ren J, Hou X, et al. Genome-wide analysis of the MADS-box gene family in Brassica rapa (Chinese cabbage) Mol Gen Genomics. 2014;290:239–255. doi: 10.1007/s00438-014-0912-7. [DOI] [PubMed] [Google Scholar]
- 47.Xu Z, Zhang Q, Sun L, Du D, Cheng T, Pan H, et al. Genome-wide identification, characterisation and expression analysis of the MADS-box gene family in Prunus mume. Mol Gen Genomics. 2014;289:903–920. doi: 10.1007/s00438-014-0863-z. [DOI] [PubMed] [Google Scholar]
- 48.Tian Y, Dong Q, Ji Z, Chi F, Cong P, Zhou Z. Genome-wide identification and analysis of the MADS-box gene family in apple. Gene. 2015;555:277–290. doi: 10.1016/j.gene.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Wei X, Wang L, Yu J, Zhang Y, Li D, Zhang X. Genome-wide identification and analysis of the MADS-box gene family in sesame. Gene. 2015;569:66–76. doi: 10.1016/j.gene.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Fan CM, Wang X, Wang YW, Hu RB, Zhang XM, Chen JX, et al. Genome-wide expression analysis of soybean MADS genes showing potential function in the seed development. PLoS One. 2013;8:e62288. doi: 10.1371/journal.pone.0062288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shu Y, Yu D, Wang D, Guo D, Guo C. Genome-wide survey and expression analysis of the MADS-box gene family in soybean. Mol Biol Rep. 2013;40:3901–3911. doi: 10.1007/s11033-012-2438-6. [DOI] [PubMed] [Google Scholar]
- 52.Grimplet J, Martínez-Zapater JM, Carmona MJ. Structural and functional annotation of the MADS-box transcription factor family in grapevine. BMC Genomics. 2016;17:80. doi: 10.1186/s12864-016-2398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrándiz C, Liljegren SJ, Yanofsky MF. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science (80- ) 2000;289:436–438. doi: 10.1126/science.289.5478.436. [DOI] [PubMed] [Google Scholar]
- 54.Lim J, Moon YH, An G, Jang SK. Two rice MADS domain proteins interact with OsMADS1. Plant Mol Biol. 2000;44:513–527. doi: 10.1023/A:1026517111843. [DOI] [PubMed] [Google Scholar]
- 55.Yoo SK. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 2005;139:770–778. doi: 10.1104/pp.105.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim SL, Lee S, Kim HJ, Nam HG, An G. OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol. 2007;145:1484–1494. doi: 10.1104/pp.107.103291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C, Chen H, Er HL, Soo HM, Kumar PP, Han J-H, et al. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development. 2008;135:1481–1491. doi: 10.1242/dev.020255. [DOI] [PubMed] [Google Scholar]
- 58.Kanno A, Saeki H, Kameya T, Saedler H, Theissen G. Heterotopic expression of class B floral homeotic genes supports a modified ABC model for tulip (Tulipa gesneriana) Plant Mol Biol. 2003;52:831–841. doi: 10.1023/A:1025070827979. [DOI] [PubMed] [Google Scholar]
- 59.Litt A, Kramer EM. The ABC model and the diversification of floral organ identity. Semin Cell Dev Biol. 2010;21:129–137. doi: 10.1016/j.semcdb.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Birch PRJ, Bryan G, Fenton B, Gilroy EM, Hein I, Jones JT, et al. Crops that feed the world 8: potato: are the trends of increased global production sustainable? Food Secur. 2012;4:477–508. doi: 10.1007/s12571-012-0220-1. [DOI] [Google Scholar]
- 61.Airoldi CA, Davies B. Gene duplication and the evolution of plant MADS-box transcription factors. J Genet Genomics. 2012;39:157–165. doi: 10.1016/j.jgg.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Nam J, DePamphilis CW, Ma H, Nei M. Antiquity and evolution of the MADS-box gene family controlling flower development in plants. Mol Biol Evol. 2003;20:1435–1447. doi: 10.1093/molbev/msg152. [DOI] [PubMed] [Google Scholar]
- 63.Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, et al. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003;424:85–88. doi: 10.1038/nature01741. [DOI] [PubMed] [Google Scholar]
- 64.Edger PP, Pires JC. Gene and genome duplications: the impact of dosage-sensitivity on the fate of nuclear genes. Chromosom Res. 2009;17:699–717. doi: 10.1007/s10577-009-9055-9. [DOI] [PubMed] [Google Scholar]
- 65.Gramzow L, Weilandt L, Theißen G. MADS goes genomic in conifers: towards determining the ancestral set of MADS-box genes in seed plants. Ann Bot. 2014;114:1407–1429. doi: 10.1093/aob/mcu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito Y, Nishizawa-Yokoi A, Endo M, Mikami M, Shima Y, Nakamura N, Kotake-Nara E, Kawasaki S, Toki S. Re-evaluation of the rin mutation and the role of RIN in the induction of tomato ripening. Nat Plants. 2017;3:866–874. doi: 10.1038/s41477-017-0041-5. [DOI] [PubMed] [Google Scholar]
- 67.Vrebalov J, Ruezinsky D, Padmanabhan V, et al. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (Rin) Locus. Science. 2002;296:343–346. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- 68.Quinet M, Bataille G, Dobrev PI, Capel C, Gomez P, Capel J, Lutts S, Motyka V, Angosto T, Lozano R. Transcriptional and hormonal regulation of petal and stamen development by STAMENLESS, the tomato (Solanum lycopersicum L.) orthologue to the B-class APETALA3 gene. J Exp Bot. 2014;65:2243–2256. doi: 10.1093/jxb/eru089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S, Lu G, Hou Z, Luo Z, Wang T, Li H, Zhang J, Ye Z. Members of the tomato FRUITFULL MADS-box family regulate style abscission and fruit ripening. J Exp Bot. 2014;65:3005–3014. doi: 10.1093/jxb/eru137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakano T, Kimbara J, Fujisawa M, Kitagawa M, Ihashi N, Maeda H, Kasumi T, Ito Y. MACROCALYX and JOINTLESS interact in the transcriptional regulation of tomato fruit abscission zone development. Plant Physiol. 2012;158:439–450. doi: 10.1104/pp.111.183731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006;46:183–192. doi: 10.1111/j.1365-313X.2006.02686.x. [DOI] [PubMed] [Google Scholar]
- 72.Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, et al. The AGAMOUS-lIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 2000;14:2366–2376. doi: 10.1101/gad.813600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michaels SD. Flowering time regulation produces much fruit. Curr Opin Plant Biol. 2009;12:75–80. doi: 10.1016/j.pbi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srikanth A, Schmid M. Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci. 2011;68:2013–2037. doi: 10.1007/s00018-011-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen M, Ni M. RFI2, a RING-domain zinc finger protein, negatively regulates CONSTANS expression and photoperiodic flowering. Plant J. 2006;46:823–833. doi: 10.1111/j.1365-313X.2006.02740.x. [DOI] [PubMed] [Google Scholar]
- 76.Schmitz RJ, Tamada Y, Doyle MR, Zhang X, Amasino RM. Histone H2B Deubiquitination is required for transcriptional activation of FLOWERING LOCUS C and for proper control of flowering in Arabidopsis. Plant Physiol. 2008;149:1196–1204. doi: 10.1104/pp.108.131508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuji H, Taoka KI, Shimamoto K. Regulation of flowering in rice: two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol. 2011;14:45–52. doi: 10.1016/j.pbi.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Hecht V, Laurie R, Vander Schoor J, Ridge S, Knowles C, Liew L, et al. The pea GIGAS gene is a FLOWERING LOCUS T homolog necessary for graft-transmissible specification of flowering but not for responsiveness to photoperiod. Plant Cell. 2011;23:147–161. doi: 10.1105/tpc.110.081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. QRT-PCR primers corresponding to potato MADS-box genes. (DOCX 18 kb)
Table S2. MADS-box genes in tomato. (DOCX 25 kb)
Table S3. Orthologs of potato MIKCC MADS-box genes in tomato. (DOCX 18 kb)
Data Availability Statement
All of the datasets supporting the results of this article are included within the article and its Additional files. The raw data generated in this study can be obtained by reasonable request to the corresponding authors.