Abstract
In the past, the treatment of autosomal dominant polycystic kidney disease (ADPKD) has been limited to the management of its symptoms and complications. Recently, the US Food and Drug Administration (FDA) approved tolvaptan as the first drug treatment to slow kidney function decline in adults at risk of rapidly progressing ADPKD. Full prescribing information approved by the FDA provides helpful guidelines but does not address practical questions that are being raised by nephrologists, internists, and general practitioners taking care of patients with ADPKD, and by the patients themselves. In this review, we provide practical guidance and discuss steps that require consideration before and after prescribing tolvaptan to patients with ADPKD to ensure that this treatment is implemented safely and effectively. These steps include confirmation of diagnosis; identification of rapidly progressive disease; implementation of basic renal protective measures; counseling of patients on potential benefits and harms; exclusions to use; education of patients on aquaresis and its expected consequences; initiation, titration, and optimization of tolvaptan treatment; prevention of aquaresis-related complications; evaluation and management of liver enzyme elevations; and monitoring of treatment efficacy. Our recommendations are made on the basis of published evidence and our collective experiences during the randomized, clinical trials and open-label extension studies of tolvaptan in ADPKD.
Keywords: ADPKD, Tolvaptan, vasopressin, V2 Receptor Antagonist, polycystic kidney disease, hepatotoxicity
The treatment for autosomal dominant polycystic kidney disease (ADPKD) has been limited to the management of symptoms and complications. Two large, randomized, clinical trials recently showed that tolvaptan reduced kidney growth by 45% and eGFR decline by 26% in early ADPKD (creatinine clearance >60 ml/min) over 3 years (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 Trial [TEMPO 3:4])1 and eGFR decline by 35% in advanced ADPKD (eGFR 25–65 ml/min per 1.73 m2) over 1 year (Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD [REPRISE]).2 On the basis of these studies, the US Food and Drug Administration (FDA) approved tolvaptan to slow kidney function decline in adults at risk of rapidly progressing ADPKD. Full FDA-approved prescribing information provides helpful guidelines, but does not address practical questions raised by nephrologists, internists, general practitioners, and patients.
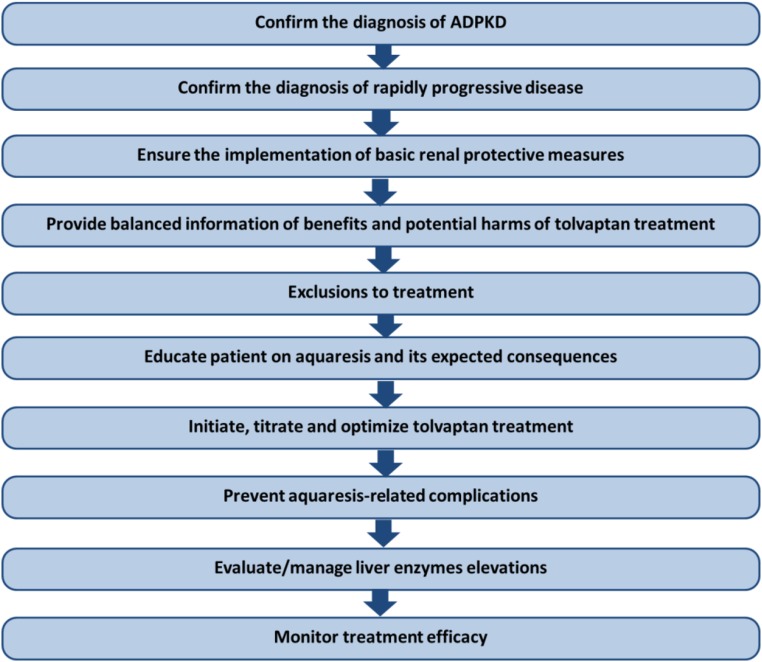
The purpose of this manuscript is to provide practical guidance and discuss steps to consider before and after prescribing tolvaptan (Figure 1). These are determined on the basis of published evidence and the authors’ collective experiences during the clinical trials and open-label extension studies of tolvaptan in ADPKD.1–4 Comprehensive descriptions of advances in the understanding of ADPKD genetics and pathophysiology can be found in excellent recent reviews.5–7
Figure 1.
A stepwise approach should be followed to evaluate patients with ADPKD for treatment eligibility and management of potential side effects.
Step 1. Confirm the Diagnosis of ADPKD
The diagnosis of ADPKD is not always obvious. When there is a family history of ADPKD, diagnosis relies primarily on imaging.8 With improving technology, ultrasound criteria to confirm or exclude the diagnosis in individuals from affected families have evolved. A recent study suggested that a total of more than ten kidney cysts by magnetic resonance imaging (MRI) in individuals younger than 30 years has 100% sensitivity and specificity, and that high-resolution ultrasonography has the potential to rival MRI.9 These criteria apply to ADPKD caused by PKD1 or PKD2 mutations, but not to polycystic disease associated with mutations in other genes.
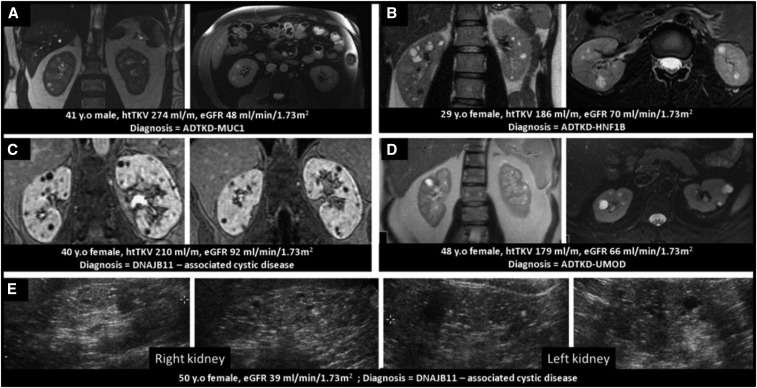
When there is no clear family history or when the appearance and function of the kidneys are not congruent or consistent with ADPKD, genetic testing is helpful to detect rare forms of ADPKD and other cystic diseases.7,10–13 For example, three patients in the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) and two patients in the Halt Progression of Polycystic Kidney Disease clinical trials were later found to have mutations in GANAB (which causes a mild form of cystic disease that does not progress to ESRD) or in DNAJB11 (which causes a type of cystic disease in which ESRD may develop without marked kidney enlargement).10,14 Patients with various forms of autosomal dominant tubulointerstitial disease often progress to ESRD without kidney enlargement and may be misdiagnosed as ADPKD (Figure 2). This underscores the importance of consistency between appearance and function of the kidneys in ADPKD.
Figure 2.
These cases illustrate the importance of an accurate diagnosis of the renal cystic disease, particularly when the renal cystic burden is not congruent with the renal function. Five cases (A–E) could have qualified as ADPKD per ultrasound/MRI imaging criteria but renal phenotype and function were inconsistent in four of them. Genetic testing revealed the presence of mutations in genes other than PKD1 or PKD2. (A) A 41 year old (y.o) man with seven and ten cysts in the right and left kidney, respectively. His htTKV was 274 ml/m. His eGFR was 48 ml/min per 1.73 m2. He had a strong family history of renal cystic disease reaching ESRD (early fifth decade). Genetic studies revealed a mutation in the MUC1 gene. (B) A 29 y.o woman with bilateral renal cysts (more than ten cysts in each kidney) with htTKV of 186 ml/m. Her eGFR was 70 ml/min per 1.73 m2. Her 66 y.o mother had 13 cysts on her CT scan. She was found to have a mutation in the HNF1B gene. (C) A 40 y.o woman with more than ten cysts in each kidney and htTKV of 210 ml/m and eGFR 92 ml/min per 1.73 m2. She was found to have a mutation in DNAJB11. (D) A 48 y.o woman with negative family history of renal disease was found to have bilateral renal cysts incidentally on her MRI scan. Her htTKV was 179 ml/m. Her eGFR was 67 ml/min per 1.73 m2. She had gout at age 44 years. She was found to have a mutation in the UMOD gene. (E) A 50 y.o woman with numerous small bilateral small cysts on ultrasound and family history of renal cystic disease and intracranial aneurysm. Her eGFR was 39 ml/min per 1.73 m2. She was enrolled in Halt Progression of Polycystic Kidney Disease study B and was later found to have a mutation in DNAJB11.
In the consensus report of the Kidney Disease: Improving Global Outcomes Controversies Conference, the potential benefits of presymptomatic diagnosis for at-risk adults were deemed to usually outweigh the risks, provided that the implications of a positive diagnosis, which vary from country to country, are discussed beforehand with the patient.5 With the approval of tolvaptan, the potential benefit of screening has increased. Because of persisting concerns with respect to health and a patient’s ability to obtain life insurance, we continue to recommend a discussion of the pros and cons of presymptomatic diagnosis along with obtaining appropriate insurance coverage before screening.
Step 2. Confirm the Diagnosis of Rapidly Progressive Disease
The FDA-approved indication for tolvaptan in ADPKD is to “slow kidney function decline in adults at risk of rapidly progressing ADPKD.”15 How to identify rapid progression has not been delineated by regulatory agencies and varies among countries.16,17 We propose the following recommendations to guide practitioners, particularly those in the United States.
The CRISP trial, a longitudinal study (now in its 18th year) of patients aged 15–46 years with creatinine clearance ≥70 ml/min, characterized the relationship between total kidney volume (TKV) and measured GFR.18–20 The study showed that kidney growth precedes change in GFR; that the rate of growth is quasiexponential, unique to, and variable among patients; and that height-adjusted total kidney volume (htTKV) predicts future GFR decline. The predictive value of TKV, together with age and eGFR, was confirmed by the PKD Outcomes Consortium, a collaborative effort including the PKD Foundation, the FDA, the Critical Path Institute, academic centers, and pharma.21,22 This work led to the FDA and European Medicines Agency qualification of TKV, together with age and eGFR, as a prognostic biomarker.
Therefore, physicians prescribing tolvaptan should consider the patient’s age, htTKV, and eGFR to identify individuals at the highest risk of rapid progression. In young patients at early ADPKD stages, eGFR will likely be preserved despite significant cystic burden. In patients with more advanced disease, reduced eGFR (<60 ml/min per 1.73 m2) alone is likely to be informative. Nevertheless, imaging remains valuable and important in this setting to rule out other contributing factors. Patient’s age and cyst burden should match the level of renal function. If not, other diagnoses or contributing factors should be considered (Figure 2).
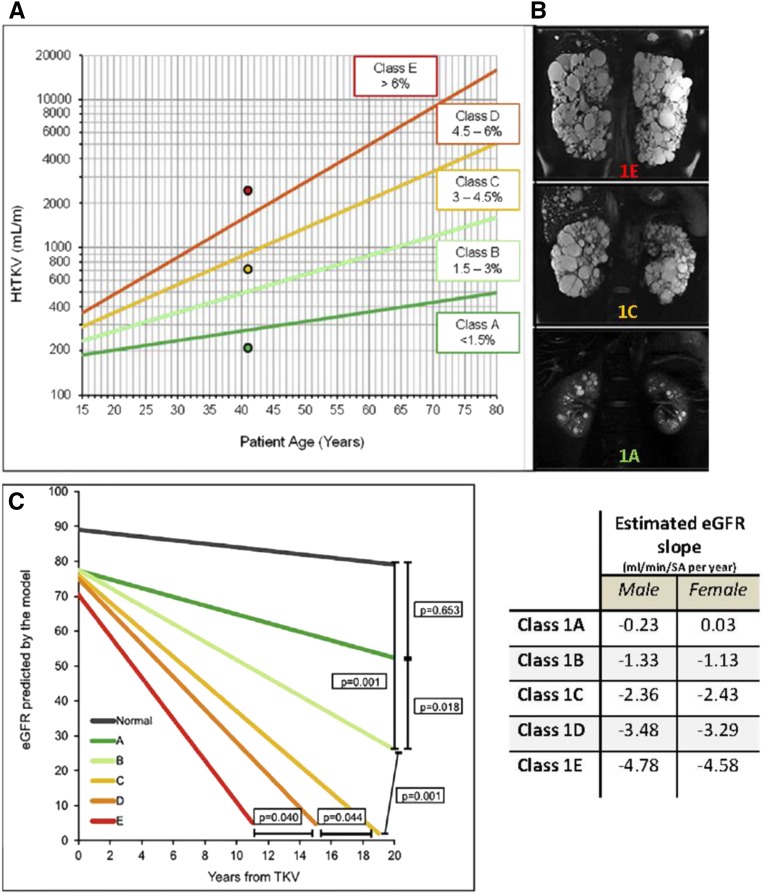
The Mayo imaging classification is a simple tool that uses htTKV and age to identify patients at the highest risk for progression independent of renal function.23,24 Most patients with ADPKD (approximately 95%) have typical disease with diffuse cystic involvement (class 1). They are stratified into five classes (A–E) on the basis of growth rates (<1.5%, 1.5%–3%, 3%–4.5%, 4.5%–6%, or >6% per year) estimated from patient age and a theoretical starting htTKV (150 ml/m) (Figure 3). A model that uses this classification plus eGFR predicts future eGFR decline with reasonable accuracy (http://www.mayo.edu/research/documents/pkd-center-adpkd-classification/doc-20094754). In the approximately 5% of patients displaying atypical renal imaging (class 2), htTKV does not predict eGFR decline. Most patients with htTKV class 2 have focal cystic disease and a few are older individuals with atrophic kidneys with cysts (Supplemental Figure 1). The Mayo imaging classification has been validated by an independent study25 and shown to be informative in post hoc analyses of several clinical trials.24,26
Figure 3.
The Mayo imaging classification provides a simple tool for the identification of patients with rapidly progressive ADPKD. This imaging classification predicts the change in eGFR over time in patients with typical, bilateral, and diffuse distribution of cysts. (A) The A–E classification is on the basis of htTKV and age at the time of imaging, assuming kidney growth rates of <1.5%, 1.5%–3%, 3%–3.5%, 4.5%–6%, or >6% per year and a theoretical initial htTKV of 150 ml/m; the dots correspond to the patients in (B). (B) MRI studies corresponding to three 41-year-old patients in classes A (bottom), C (middle), and E (top). (C) eGFR slopes in cohort of 376 patients stratified by imaging class (−0.23, −1.33, −2.63, −3.48, and −4.78 ml/min per 1.73 m2 per year for classes A–E, respectively). Average eGFR at baseline (75 ml/min per 1.73 m2) and average age at baseline (44 years) for all patients were used for the model; values for normal slope were obtained from a population of healthy kidney donors; eGFR slopes were significantly different among the classes, and all but class A were significantly different from the control population of healthy kidney donors. The table shows the estimated eGFR slopes for each class by sex. Reprinted from reference 22, with permission.
In most patients, the ellipsoid equation using coronal, sagittal, and transverse diameters (obtained by various imaging modalities) provides a fairly accurate estimation of TKV, image class, and eligibility for treatment. We prefer a computed tomography (CT) scan (including contrast enhancement in patients with eGFR>60 ml/min per 1.73 m2) or MRI scan without contrast (in patients with reduced eGFR).27 These images allow the physician to directly confirm the typical classification and to measure TKV by the ellipsoid equation using a web-based calculator (http://www.mayo.edu/research/documents/pkd-center-adpkd-classification/doc-20094754). The cost of imaging is justified when long-term treatment with tolvaptan is considered. Although ultrasound measurements of kidney length have been found to be good predictors of GFR decline in cohorts of patients in a research setting,28 the imprecision of these measurements in routine clinical practice limits their utility for making treatment decisions in individual patients. More accurate and time-consuming methods, such as planimetry or stereology, have been utilized when using TKV as an end point in clinical trials. They may also be more reliable for prognostication in young individuals (<25 years) where small differences in htTKV may affect the image classification (Figure 3). Variants of these methods have been automated and validated and we expect that they will become increasingly accessible and available for disease prognostication.29,30
Once a patient is determined to have typical ADPKD, the Mayo class should be ascertained (Figure 4). Patients in class 1A progress slowly and should not be treated. Patients in class 1B should be reassessed and their TKV measured after 2–3 years to confirm a slow rate of progression. Patients in class 1C, 1D or 1E have rapidly progressing disease and are the most likely to benefit from treatment. Predicted benefit is greater for young patients with rapid progression who start treatment at early CKD stages (3A or earlier) (Figure 5, Table 1). In the REPRISE trial, patients aged >55 years did not benefit from tolvaptan.2 This might have been because of slow disease progression, as suggested by their lower rate of eGFR decline on placebo (−2.34 ml/min per 1.73 m2) compared with those aged ≤55 years (−4.60 ml/min per 1.73 m2). Therefore, we recommend confirming the diagnosis of rapid progression by the Mayo classification if patients aged >55 years are considered for treatment, even in the presence of a reduced eGFR.
Figure 4.
The algorithm depicted in the Figure summarizes a practical approach to identify the patients more likely to benefit from treatment with tolvaptan and optimize their overall management. After confirming ADPKD diagnosis, the patients are classified into typical and atypical ADPKD on the basis of their kidney imaging features. This is followed by measurement of htTKV, which allows the stratification of the typical patients into slowly (Mayo class 1A or 1B) or rapidly progressing (Mayo class 1C, 1D, and 1E). Treatment with tolvaptan to slow down the disease progression should be considered for 18- to 55-year-old patients at risk for rapid progression (Mayo class 1C, 1D, or 1E) with eGFR>25 ml/min per 1.73 m2. The reduced benefit of treatment in the patients with advanced CKD should be pondered in the decision. The Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD trial did not show benefit from tolvaptan in patients older than 55 years probably because many of them had slowly progressive disease. General measures that may improve the outcome of ADPKD should be implemented in all patients. BMI, body mass index.
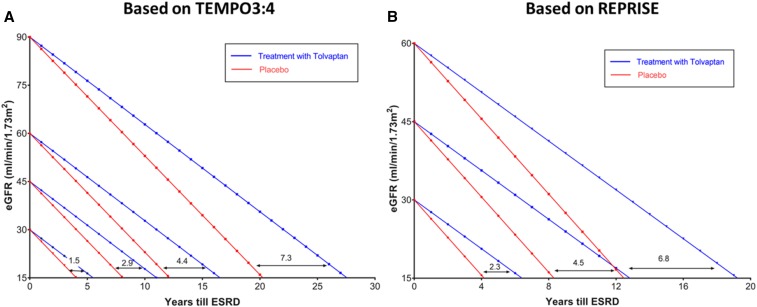
Figure 5.
Extrapolations from the results of the TEMPO 3:4 and REPRISE trials allow estimations of the potential benefit of tolvaptan treatment in delaying the need of renal replacement therapy depending on the eGFR at the initiation of treatment. The effect of tolvaptan is predicted to be sustained and cumulative on the basis of tolvaptan trial extension and single-center experiences.4 (A) According to baseline GFR at time of treatment initiation, tolvaptan might delay reaching stage 5 CKD by 7.3, 4.4, 2.9, or 1.5 years if baseline eGFR was 90, 60, 45, or 30 ml/min, respectively. These extrapolations are made using the average decline in eGFR between placebo (−3.7 ml/min per year) and tolvaptan (−2.72 ml/min per year) groups in the TEMPO3:4 trial. (B) According to baseline GFR at time of treatment initiation, tolvaptan might delay reaching stage 5 CKD by 6.8, 4.5, or 2.3 years if baseline eGFR was 60, 45, or 30 ml/min, respectively. These extrapolations are made using the average decline in eGFR between placebo (−3.61 ml/min per year) and tolvaptan (−2.34 ml/min per year) groups in the REPRISE trial. Although this prediction model is simplistic as it assumes that all patients progress at the same slope to ESRD, it allows for visualizing of the benefit gained by patients if treated with tolvaptan early in their disease state. Predictions in (A) may underestimate the long-term benefit because the treatment effect observed in the TEMPO 3:4 trial in patients with earlier disease was less than that observed in the REPRISE trial in patients with more advanced disease. REPRISE, Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD; TEMPO 3:4, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes.
Table 1.
Potential long-term benefit on kidney function on the basis of the rates of eGFR decline in tolvaptan-treated patients and controls observed in the TEMPO 3:4 and REPRISE clinical trials
| Study | eGFR Decline, ml/min per 1.73 m2 (No. of Patients) | Estimated Delay to CKD 5 in Years | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Tolvaptan | Difference (Treatment Effect, %) | P Value | From eGFR 90 | From eGFR 60 | From eGFR 45 | From eGFR 30 | |
| TEMPO 3:4 (randomized, parallel-arm, controlled trial, eCrCl>60 ml/min) | ||||||||
| All | −3.70 | −2.72 | −0.98 | <0.001 | 7.3 | 4.4 | 2.9 | 1.5 |
| CKD stage 2 | −3.90 (216) | −2.76 (411) | −1.14 (29.2) | <0.001 | 7.9 | 4.8 | — | — |
| CKD stage 3 | −5.36 (84) | −3.70 (151) | −1.66 (30.9) | <0.001 | — | 3.8 | 2.5 | — |
| REPRISE (Randomized, withdrawal, controlled trial, eGFR 25–65 ml/min per 1.73 m2) | ||||||||
| All | −3.61 (663) | −2.34 (668) | −1.27 (35.1) | <0.001 | — | 6.8 | 4.5 | 2.3 |
| Age ≤55 yr | −4.60 (569) | −3.07 (572) | −1.53 (33.2) | <0.001 | — | 4.9 | 3.3 | 1.6 |
| Age >55 yr | −2.34 (94) | −2.54 (96) | 0.2 (-8.5) | 0.65 | — | −1.5 | −1.0 | −0.5 |
| CKD stage 2 | −4.65 (38) | −2.81 (31) | −1.84 (39.5) | 0.14 | 10.6 | 6.3 | — | — |
| CKD stage 3a | −4.49 (196) | −2.13 (206) | −2.36 (52.5) | <0.001 | — | 11.1 | 7.4 | — |
| CKD stage 3b | −3.99 (304) | −3.20 (194) | −0.79 (19.7) | 0.008 | — | — | 1.9 | — |
| CKD stage 4 | −4.60 (125) | −3.80 (137) | −0.8 (17.3) | 0.02 | — | — | — | 0.7 |
Average eGFR decline is listed in placebo and tolvaptan columns. Number of patients in each group is listed in parentheses. Estimated delay to CKD stage 5 in years was calculated using the following formula: delay =([initial eGFR−15]/rate of yearly eGFR decline of tolvaptan-treated patients)−([initial eGFR−15]/rate of yearly eGFR decline of placebo patients), e.g., from eGFR 90: delay=([90−15]/2.72)−([90−15]/3.7)=27.57–20.27=7.3 year delay. TEMO 3:4, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes; REPRISE, Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD; eCrCl, estimated Creatinine Clearance; —, eGFR unit is ml/min per 1.73 m2.
Although our preferred method to identify rapid progression uses the Mayo classification, the European Renal Association–European Dialysis and Transplant Association Working Groups on Inherited Kidney Disorders and European Renal Best Practice puts emphasis first on eGFR indexed for age.17 This group proposed a hierarchical decision algorithm encompassing a sequence of risk-factor assessments. It uses the premise that in the majority of patients, eGFR indexed for age will distinguish rapid from slowly progressive disease. Patients aged 40–50 years with eGFR>60 ml/min per 1.73 m2 (CKD stages 1 and 2) or patients 30–40 years with eGFR>90 ml/min per 1.73 m2 (CKD stage 1) are considered slow progressors and not appropriate for treatment. In the remaining patients, at least one of the following additional criteria is required to diagnose rapid progression and thus indication for treatment: (1) a confirmed eGFR decline of ≥5 ml/min per 1.73 m2 in 1 year or ≥2.5 ml/min per 1.73 m2 per year over a period of 5 years; (2) a TKV increase of >5% per year by repeated measurements (preferably three or more, each at least 6 months apart and by MRI); (3) Mayo image class 1C, 1D, or 1E; (4) kidney length assessed by ultrasound of >16.5 cm in patients aged <45 years; and/or (5) having a truncating PKD1 mutation in conjunction with early onset of clinical symptoms consistent with a Predicting Renal Outcome in Polycystic Kidney Disease (PROPKD) score >6.
In our opinion, the European Renal Association–European Dialysis and Transplant Association algorithm is complicated and not entirely justified by available evidence (Table 2). The premise that eGFR indexed for age can distinguish rapidly from slowly progressive disease in the majority of patients with ADPKD is not accurate, nor is it helpful in patients aged 18–30 years, and those with rapid progression in this age group are likely to benefit the most from tolvaptan. We do not agree that most patients aged 30–40 years with an eGFR >90 ml/min per 1.73 m2 and most patients aged 40–50 years with an eGFR >60 ml/min per 1.73 m2 have slow progression; many have rapidly progressive disease. For example, in the CRISP study, 30 patients aged 30–39 years had a baseline eGFR >90 ml/min per 1.73 m2; 15 had class 1A or 1B and 15 had class 1C–E ADPKD. After a median follow-up of 13.5 years, eGFR had declined 26.7±21.9 in class 1A and 1B patients, 34.9±15.4 ml/min per 1.73 m2 in class 1C–E patients, and one patient (class 1D) had reached ESRD. Forty-five CRISP patients aged 40–50 years had a baseline eGFR >60 ml/min per 1.73 m2; 26 had class 1A, 1B, or 2A and 19 had class 1C or 1D. After a median follow-up of 13.5 years, eGFR had declined 16.6±23.1 ml/min per 1.73 m2 in class 1A, 1B, and 2A patients, 37.2±18.3 ml/min per 1.73 m2 in class 1C and 1D patients, and six patients (one class B, three class C, and two class D) had reached ESRD (V.E. Torres, C. Shen, D.P. Landsittel, A.S.L. Yu, A.B. Chapman, K.T. Bae, M. Mrug, P.C. Harris, F.F. Rahbari-Oskoui, W.M. Bennett, unpublished results). Historical evidence of eGFR decline16,17 is unreliable in many cases because of the high variability of eGFR values >60 ml/min per 1.73 m2 and because information on a multitude of factors affecting the values is often unavailable.
Table 2.
European Renal Association–European Dialysis and Transplant Association algorithm to identify rapidly progressive ADPKD
| Steps | Criteria | Limitations |
|---|---|---|
| 1 | Exclusion of slow progressors by eGFR indexed for age above high cut-off values | Not helpful in 18- to 30-yr-old patients. Incorrect in many 30- to 40-yr-old patients with CKD stage 1 and 40- to 50-yr-old patients with CKD stage 1 or 2 |
| 1 | Inclusion of patients with eGFR indexed for age compatible with rapid progression | Does not exclude factors other than rapid ADPKD progression contributing to the reduced eGFR |
| 2 | eGFR decline ≥5 ml/min per 1.73 m2 in 1 yr or ≥2.5 ml/min per 1.73 m2 per yr over 5 yr | High variability of eGFR values >60 ml/min per 1.73 m2; historical factors affecting historical values often unavailable |
| 3 | TKV increase >5% per year by repeated measurements (preferably three or more, each at least 6 mo apart) | Very few patients will have three or more MRIs or CTs; does not exclude atypical cases; requires precise measurements (planimetry or stereology); rates of TKV increase in patients with PKD1 and PKD2 mutations are similar |
| 4 | Mayo image class 1C, 1D, or 1E | Cost, but this is minor compared with the cost of tolvaptan and safety laboratory testing |
| 4 | Kidney length by ultrasound >16.5 cm in patients aged <45 yr | Operator-dependent measurements; young patients with lengths <16.5 cm may have rapidly progressive disease; atypical patients with slow progression may have lengths >16.5 cm because of large cysts |
| 4 | PROPKD score >6 | Not helpful in patients aged <35 yr unless already hypertensive and have experienced urologic complications |
In addition, very few patients will have three or more CT or MRI scans for historical determinations of TKV growth17 unless repeated scans were obtained for indications that often might have affected TKV. For measurements of TKV growth to be reliably predictive, atypical cases must be excluded and laborious planimetry or stereology with intraobserver variabilities of 0.8%–1.8% are required.30 Furthermore, these errors become magnified when the change in TKV between measurements over an interval of only a few months is extrapolated to 1 year.31 Additionally ultrasound measurements are operator dependent, young patients with lengths <16.5 cm may have rapidly progressive disease, and atypical patients with slow progression may have lengths >16.5 cm because of large cysts. The PROPKD score incorporates genetics, early onset of urological complications and hypertension, and sex into a model predicting disease progression.32 This scoring system cannot be used in patients aged <35 years unless they were hypertensive or experienced urologic complications. In patients aged <35 years without complications or in patients with missing clinical information, genetic information alone could be used for prognosis because truncating PKD1 mutations, nontruncating PKD1 mutations, and PKD2 mutations are associated with most severe, intermediate, and least severe disease, respectively.32,33 Nevertheless, imaging is still desirable in these cases because ADPKD progression is highly variable for individuals within these three mutation classes,20 even among affected individuals with the same mutation or of the same family.34,35 Given these limitations, the Mayo ADPKD classification, in our opinion, is simpler and easier to implement in clinical practice (Table 3).
Table 3.
Mayo imaging classification to identify rapidly progressive ADPKD: advantages and limitations
| Criteria | Advantages | Limitations |
|---|---|---|
| Class 1C, 1D, or 1E | One-time measurement of htTKV | Lack of validation in nonwhite ethnic or racial populations |
| Most helpful in patients with eGFR>60 ml/min per 1.73 m2 | If MRI is contraindicated or not tolerated, it can be substituted by CT | |
| Confirmatory in patients with eGFR<60 ml/min per 1.73 m2 (if discordant, consider other disease process contributing to reduced eGFR) | Cost, but this is minor compared with the cost of tolvaptan and safety laboratory testing | |
| Most commonly in patients with a truncating PKD1 mutations (if discordant, it may be a clue to other factors contributing to disease severity) |
Step 3. Ensure that Basic Renal Protective Measures Are Implemented
Physicians prescribing tolvaptan should not overlook other simpler interventions that, in combination, can have a substantial effect on the long-term outcome of ADPKD (Figure 4). These are discussed in a recent review36 and consist of specific BP target goals, treatment with preferred antihypertensive agents (i.e., angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers), and lifestyle modifications. Their inclusion in the tolvaptan protocol presents an opportunity to optimize the management of ADPKD.
Step 4. Provide Balanced Information of Benefits and Potential Harms
The potential benefits and harms of tolvaptan treatment (Table 4) should be discussed in an individualized manner, on the basis of the patient’s age, current eGFR, and ability to tolerate the medication.
Table 4.
Potential benefits and harms from tolvaptan treatment in ADPKD
| Benefits | Harms |
|---|---|
| Slows kidney growth | Polyuria, pollakiuria, and nocturia |
| Slows eGFR decline | Thirst and fatigue |
| May delay need for renal replacement | Uric acid elevations (rarely gout) |
| Reduces pain, hematuria, stone, and urinary tract infection events | Transaminase elevations and risk of severe hepatocellular toxicity |
| Slight reduction in BP | Need for frequent monitoring of liver function |
| Possible drug interaction (CYP3A inhibitors) | |
| Financial burden |
Tolvaptan slows the rate of cyst growth and the rate of eGFR decline. The major expected but still unproven benefit is delaying the need for RRT. The TEMPO 3:4 and REPRISE trials showed its effectiveness over a broad range of disease stages (Figure 5, Table 1).1,2,4 An open-label study (TEMPO 4:4) and a small single-center retrospective analysis suggest that tolvaptan’s slowing of the rate of eGFR decline is sustained and cumulative (approximately 1 ml/min per 1.73 m2 per year of treatment) over time (Figure 5, Table 1).3,4 Other benefits of tolvaptan treatment include a reduction in the frequency of events of kidney pain, nephrolithiasis, hematuria, and urinary tract infection,1 and a slight reduction in mean arterial pressure and systolic BP.37
The most common side effects associated with tolvaptan are related to its aquaretic effect (polyuria, increased urinary frequency, nocturia, thirst, and in some cases, fatigue).1,2 These are more disruptive during the initial weeks of treatment. The aquaretic effect is less marked in patients with reduced GFR versus those with normal GFR.38 In the TEMPO 3:4 trial, researchers found that moderate elevations in serum uric acid are common (change from baseline, 0.8±1.0 mg/dl in tolvaptan-treated patients compared with 0.2±0.86 mg/dl in placebo-treated patients at month 12), but gout occurred rarely (in 2.9% of tolvaptan-treated patients versus 1.4% of placebo-treated patients, respectively).
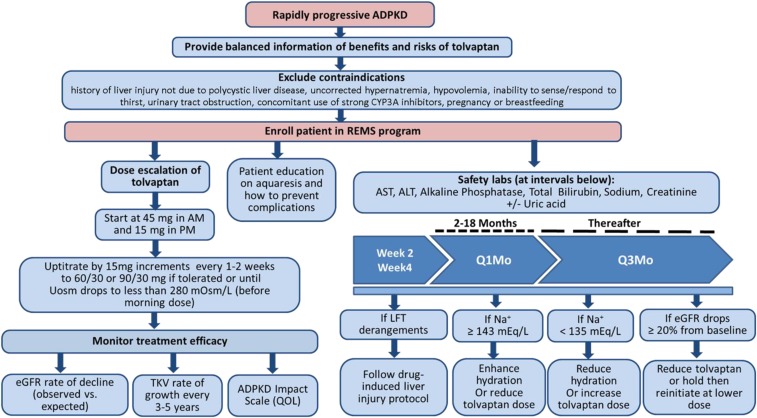
An important adverse event associated with tolvaptan is idiosyncratic hepatocellular injury.39 Alterations in bile acid disposition and inhibition of mitochondrial respiration have been identified in in vitro assays as potential mechanisms.40 In the TEMPO 3:4 and TEMPO 4:4 studies, monitoring every 3–4 months revealed transaminase elevations more than three times the upper limit of normal (ULN) occurred at least once in 4.4% of patients receiving tolvaptan compared with 1% of patients receiving placebo.1,3 Three of 1271 tolvaptan-treated patients in these studies met the Hy law criteria, i.e., serum alanine aminotransferase (ALT) more than three times the ULN and bilirubin more than two times the ULN, which denote a 10% risk of progression to acute and irreversible hepatic failure. The elevations of hepatic transaminases occurred mostly during the first 18 months, suggesting a window of susceptibility, and resolved within 1–4 months after discontinuation of tolvaptan.39 In the REPRISE trial, monthly monitoring revealed the occurrence of transaminase elevations more than three times the ULN in 5.6% of tolvaptan-treated patients and 1.2% of placebo-treated patients; no cases met the Hy law criteria, likely because of more frequent monitoring and earlier discontinuation of tolvaptan.2 Acute liver failure requiring liver transplantation has occurred in one patient in the postmarketing ADPKD experience. Because of the potential hepatocellular toxicity, a risk evaluation and mitigation strategy (REMS) program, including liver function testing before initiation and at specific intervals (after 2 and 4 weeks, then monthly for 18 months, and every 3 months thereafter), is a required component of tolvaptan treatment in all patients with ADPKD.
Step 5. Exclusions to Treatment
Pregnancy, lactation, uncorrected hypernatremia, history of significant liver injury not due to polycystic liver disease, hypovolemia, inability to sense or respond to thirst, and urinary tract obstruction are contraindications. As there are insufficient data to determine tolvaptan’s risk to fetal development, females of reproductive potential should be educated to discontinue the medication before a planned pregnancy and to inform their prescriber of a known or suspected pregnancy. Breastfeeding during treatment with tolvaptan is not advised.
Drug interactions should be considered. Concomitant use of strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, lopinavir, ritonavir, and indinavir) is contraindicated. Moderate CYP3A inhibitors (e.g., amiodarone, erythromycin, fluconazole, diltiazem, verapamil, grapefruit, imatinib, and fosamprenavir) can increase tolvaptan exposure and lowering tolvaptan dosing may be necessary. Tolvaptan could raise the levels of OATP1B1/3 and OAT3 transporter substrates (e.g., statins, furosemide, glyburide, repaglinide, and methotrexate) and BCRP transporter substrates (e.g., rosuvastatin), so concomitant use of tolvaptan with such drugs generally should be avoided. When the need for treatment with such agents outweighs potential risks, monitoring of drug-related adverse effects and dose adjustment may be needed. Approximately 14% of the patients randomized to tolvaptan in the TEMPO 3:4 trial were treated with statins; no association with liver toxicity was detected.41 We recommend using statins with caution and only when clearly indicated.
Concomitant use of diuretics and tolvaptan is likely to further decrease eGFR, elevate circulating vasopressin, and increase the risk for gout. Nevertheless, a case report suggested that a thiazide may increase the tolerability to tolvaptan by reducing the polyuria.42 At present, we recommend avoiding the concomitant use of these drugs.
Step 6. Patient Education on Aquaresis and its Expected Consequences
Tolvaptan blocks the actions of vasopressin on V2 receptors in the distal nephron and collecting duct, including urinary concentration, inhibition of tubuloglomerular feedback, and promotion of sodium reabsorption. As a result, tolvaptan promotes aquaresis and stimulates tubuloglomerular feedback, increasing afferent arteriolar constriction and lowering intraglomerular pressure and GFR.38,43 It also reduces sodium reabsorption in the distal nephron and collecting duct.43,44 Patients should understand that the administration of tolvaptan will result in significant polyuria, a slight reduction in GFR that is less noticeable in advanced CKD and that is reversible after discontinuation of the drug,38,45 and a moderate increase in serum uric acid.1 BP should be monitored and antihypertensive medications adjusted if necessary. An eGFR reduction of 5%–10% can be expected with initiation of tolvaptan and no action is needed other than ensuring adequate hydration. If the drop in eGFR approaches 20%, a reduction of the dose or holding the medication to restart later at a lower dose is appropriate. After initiation of tolvaptan, we recommend monitoring eGFR at 2 and 4 weeks, then monthly for 18 months, and every 3 months thereafter.
Step 7. Initiation, Titration, and Optimization of Tolvaptan Treatment
The goal of treatment with tolvaptan is a sustained suppression of the action of vasopressin on the kidney 24 hours a day, every day.46,47 To achieve this effect while curtailing nocturia, daily split doses of tolvaptan are necessary, with the first dose taken early in the morning and the second taken 8 hours later, in the afternoon (Figure 6). In clinical trials of tolvaptan, 45 mg in the morning and 15 mg in the afternoon were given initially, and then titrated to 60/30 and 90/30 mg, as tolerated.1,2 With the current packaging of the drug dispensed by the approved pharmacies, titration in clinical practice is intended to proceed as in the clinical trials. However, starting titration at lower doses (i.e., 15/15 and 30/15) could reduce early discontinuation, make the titration process more tolerable, and allow for the treatment of patients who are highly sensitive to tolvaptan and otherwise could not be treated. Supplemental Figure 2, A and B illustrate how titration can be performed within the context of monthly pharmacy dispensations, with starting doses of 45/15 and 15/15 mg, respectively.
Figure 6.
The algorithm depicted in the Figure summarizes the recommended steps for the initiation, titration and optimization of tolvaptan treatment and the schedule of laboratory tests to monitor its safety. LFT, liver function tests; Na+, sodium; Q1Mo, every 1 month; Q3Mo, every 3 months.
It can also be argued that tolvaptan needs to be titrated only to the dose required to achieve persistent suppression of the vasopressin effect on the kidney (i.e., urine hypotonicity relative to plasma, a urine osmolality [Uosm] of ≤280 mOsm/kg in a first-void morning sample before the morning dose). In the early dose-finding studies of tolvaptan for ADPKD, efficacy was defined by the capacity to achieve a sustained Uosm of <300 mOsm/kg.48,49 In these studies, approximately 30% of the patients receiving 90/30 mg of tolvaptan were not able to achieve a sustained Uosm of <300 mOsm/kg. For those able to attain this target with lower doses, there is no evidence that further lowering of Uosm is beneficial. On the contrary, it may reduce quality of life and could possibly have detrimental effects due to chronic dehydration. In the TEMPO 3:4 trial, the dose was increased to 90/30 mg if tolerated, but it is possible that the degree of vasopressin V2 receptor suppression achieved by a patient tolerating this dose might also have been attainable by a patient who tolerated only 45/15 mg or even by a patient able to tolerate only 15/15 mg (who would have dropped from the study). Establishing the optimal dose of tolvaptan in ADPKD will require further studies.
Frequent monitoring of plasma sodium and/or plasma osmolality to ensure that a patient taking tolvaptan is drinking enough water to prevent thirst and maintain adequate hydration is essential for safety and efficacy. Plasma sodium optimally should be maintained between 135 and 143 mEq/L. We recommend monitoring plasma sodium 2 and 4 weeks after initiation of treatment, then monthly for 18 months, and every 3 months thereafter, at the same time that liver function is assessed. Maintaining adequate hydration is important to prevent marked elevations of circulating vasopressin, which can activate V1 receptors and potentially result in unintended effects, such as vasoconstriction. Whether measurements of plasma copeptin can predict or help to monitor the response to tolvaptan deserves study.50 Levels of serum uric acid should be monitored; a uric acid–lowering agent should be considered to reduce the risk of gout if uric acid level exceeds 10 mg/dl or to treat the condition if it develops.51 At present, there is insufficient evidence to recommend treatment of asymptomatic hyperuricemia with the purpose of delaying the progression of CKD.52,53
Step 8. Prevent Aquaresis-Related Complications
Tolvaptan should preferably be started on a day when patients are not at work, to help them adjust to the immediate aquaretic response. They should be instructed to ingest fluids in anticipation of or at the first sign of thirst to avoid thirst or dehydration; to ingest at least 2–3 L of fluid during the day and one to two cups of additional water before bedtime, regardless of perceived thirst; and to replenish fluids after each episode of nocturia. They should also be instructed to monitor their body weight daily and report changes of >3% in a week. The aquaretic effect becomes more tolerable after the first few days or weeks of treatment. Adjustment may also include adapting the schedule, timing, and doses of tolvaptan to the particulars of the patient’s daily activities. Dietary changes that reduce daily osmolar loads, such as moderate reductions in the ingestion of protein and sodium, help to reduce the aquaretic effect.54
Tolvaptan should be held and hydration increased during intercurrent illnesses that lead to dehydration or interfere with adequate hydration, such as food poisoning and gastroenteritis; when conditions such as outdoor activities in warm weather increase insensible water loss; and when water access is restricted, such as during travel and social events. Tolvaptan should also be held 24–48 hours before elective surgeries and not be restarted until the patients are able to maintain adequate hydration. Fluids with high sugar or fat content such as soft drinks, juices, and whole milk should be avoided to prevent excessive caloric intake. The safe and clean quality of the ingested water should be ensured. Tolvaptan can be continued until the decision to start RRT is made. Discontinuation at that time may result in a small increase in eGFR.
Step 9. Evaluation and Management of Liver Enzyme Elevations
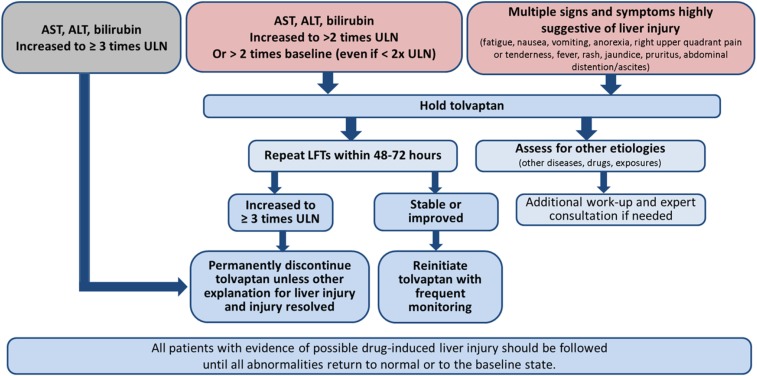
Frequent monitoring with liver function tests is mandated by the FDA as part of the REMS program for prescribing tolvaptan (Figure 6). All physicians prescribing tolvaptan for ADPKD must be trained and certified in its safe use (see the REMS website for details: https://www.jynarquehcp.com/rems-program). Failure to comply with this testing prohibits the specialty pharmacy from dispensing the medication to the patient. Patients and their treating team should be vigilant for any signs or symptoms of hepatic injury, which include fatigue, nausea, vomiting, right upper quadrant pain or tenderness, jaundice, fever, and rash. Tolvaptan should be immediately held at the onset of signs or symptoms consistent with hepatic injury or if ALT or aspartate aminotransferase (AST) levels increase to more than two times the ULN or more than two times the baseline levels even if the latter is less than two times the ULN. Tests for ALT, AST, alkaline phosphatase, and total bilirubin should be repeated as soon as possible (within 48–72 hours) for confirmation and to determine if levels of these biomarkers are increasing or decreasing (Figure 7).
Figure 7.
The algorithm depicted in the figure summarizes the recommendations for evaluation and management of potential drug-induced liver injury. LFT, liver function tests.
In the setting of elevated hepatic enzymes, a detailed medical history should be obtained regarding prior or concurrent diseases, concomitant drug use (including nonprescription medications and herbal and dietary supplement preparations), alcohol use, recreational drug use, special diets or change in diet, exposure to environmental chemical agents, or excessive exercise. Other potential explanations should be ruled out, including acute viral hepatitis types A–E, autoimmune or alcoholic hepatitis, nonalcoholic steatohepatitis, hypoxic/ischemic hepatopathy, and biliary tract disease. Gastroenterology or hepatology consultations should be obtained if liver function test abnormalities persist after tolvaptan has been discontinued.
If laboratory abnormalities resolve, tolvaptan may be reinitiated with increased frequency of monitoring (weekly for the first month) as long as ALT and AST have remained below three times ULN. Tolvaptan should not be restarted in patients who experience signs or symptoms consistent with hepatic injury or who have ALT or AST levels that have ever exceeded three times ULN during treatment with tolvaptan, unless there is another explanation for liver injury and the injury had resolved.
All patients with evidence of possible drug-induced liver injury should be followed until all abnormalities return to normal or to the baseline state.
Step 10. Monitor Treatment Efficacy
There is no foolproof way to monitor the efficacy of treatment with tolvaptan in individual patients. We do not recommend yearly TKV volume measurements to monitor the drug’s efficacy. Because of the variability of the measurements and the inability to know how much TKV would have increased without treatment, the rate of TKV increase is not likely to be informative, particularly over a short period of observation. However, it may be instructive to obtain an MRI or CT scan to measure TKV volume every 3–5 years to assess whether the rate of TKV growth compares with that anticipated from the initial imaging class assigned to the patient. Monitoring the rate of eGFR decline during tolvaptan treatment can be used for reassurance that the rate of decline is less than anticipated according to the Mayo imaging class and the derived predictive equation.4 Using quality-of-life and ADPKD-specific questionnaires (the ADPKD Impact Scale)55 at baseline and post-treatment could provide additional measures of treatment efficacy and patient satisfaction.
Access and Cost of Tolvaptan
Tolvaptan has been approved for the treatment of adult patients with ADPKD in the United States, Japan, the European Union, Canada, South Korea, Switzerland, Hong Kong, Australia, Turkey, and Taiwan. It is reimbursed according to varying patient criteria, including in countries with centralized drug coverages such as Japan, the United Kingdom, and France.56–58 In other countries, such as Canada, public payers are not currently covering the tolvaptan cost, although private insurance coverage is available. The Canadian Drug Expert Committee of the Canadian Agency for Drugs and Technology in Health reviewed a pharmacoeconomic evaluation prepared by the manufacturer.59 With the current price of tolvaptan (CAN$34,000 per year), the incremental cost-to-utility ratio per quality-adjusted life year when compared with standard of care was higher than the willingness-to-pay threshold per quality-adjusted life year. The price for tolvaptan used in the evaluation did not account for patent expiration, generic availability, and possible introduction of other vaptans into the market.
No studies have been published looking at the cost effect of the treatment of ADPKD with tolvaptan on the United States health care system. The current wholesale acquisition cost of tolvaptan is $170,000 per year. Likely out-of-pocket costs for patients are not yet known, as United States insurance providers (public and private) are still conducting their initial reviews of the product. Out-of-pocket costs can range from $0 to the full cost of drug, depending on coverage by insurance and through other programs, including a copay program of the manufacturer.
Disclosures
R.D.P. is a member of the steering committee for the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) and Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD (REPRISE) clinical trials, has received research/clinical trial support from Otsuka, Kadmon, and Sanofi-Genzyme, and has consulted for Vertex and Palladio. A.B.C. is a member of the steering committee for the TEMPO and REPRISE clinical trials and has received research/clinical trial support from Otsuka. N.K.D. has received research/clinical trial support from Otsuka and Kadmon and has consulted for Otsuka. P.C.H. has received research support from Otsuka and consulted for Vertex. M.M. has received research/clinical trial support from and has consulted for Otsuka and Sanofi-Genzyme. A.R. has received research/clinical trial support from Sanofi-Genzyme, Kadmon, Amgen, Astra Zeneca, Bayer, Otsuka, Quesctor, Sandoz, and Reata. T.W. has received research/clinical trial support from Otsuka and Kadmon. A.S.L.Y. has consulted for Regulus Therapeutics and Sanofi-Genzyme. V.E.T. is a member of the steering committees for the TEMPO and REPRISE clinical trials, has received research support from Otsuka, and consulted for Vertex, Sanofi-Genzyme, and Palladio. F.T.C. and R.A.M. have no conflicts of interest.
Supplementary Material
Acknowledgments
This study has been supported in part by the Mayo Clinic Robert M. and Billie Kelley Pirnie Translational Polycystic Kidney Disease Center and the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK090728).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018060590/-/DCSupplemental.
References
- 1.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. : TEMPO 3:4 Trial Investigators: Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. : REPRISE Trial Investigators: Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Dandurand A, et al. : TEMPO 4:4 Trial Investigators: Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 Trial. Nephrol Dial Transplant 32: 1262, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Edwards ME, Chebib FT, Irazabal MV, Ofstie TG, Bungum LA, Metzger AJ, et al. : Long-term administration of tolvaptan in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 13: 1153–1161, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, et al. : Conference Participants: Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 88: 17–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong AC, Devuyst O, Knebelmann B, Walz G; ERA-EDTA Working Group for Inherited Kidney Diseases: Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet 385: 1993–2002, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Cornec-Le Gall E, Torres VE, Harris PC: Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol 29: 13–23, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, et al. : Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei Y, Hwang YH, Conklin J, Sundsbak JL, Heyer CM, Chan W, et al. : Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol 26: 746–753, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornec-Le Gall E, Olson RJ, Besse W, Heyer CM, Gainullin VG, Smith JM, et al. : Genkyst Study Group; HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease: Monoallelic mutations to DNAJB11 cause atypical autosomal-dominant polycystic kidney disease. Am J Hum Genet 102: 832–844, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besse W, Dong K, Choi J, Punia S, Fedeles SV, Choi M, et al. : Isolated polycystic liver disease genes define effectors of polycystin-1 function. J Clin Invest 127: 1772–1785, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornec-Le Gall E, Chebib FT, Madsen CD, Senum SR, Heyer CM, Lanpher BC, et al. : HALT Progression of Polycystic Kidney Disease Group Investigators: The value of genetic testing in polycystic kidney diseases illustrated by a family with PKD2 and COL4A1 mutations. Am J Kidney Dis 72: 302–308, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulati A, Bae KT, Somlo S, Watnick T: Genomic analysis to avoid misdiagnosis of adults with bilateral renal cysts. Ann Intern Med 169: 130–131, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, et al. : Genkyst Study Group, HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease: Mutations in GANAB, encoding the glucosidase IIα subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet 98: 1193–1207, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug administration, April 23 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/204441Orig1s000Approv.pdf. Access September 6, 2018 [Google Scholar]
- 16.Soroka S, Alam A, Bevilacqua M, Girard LP, Komenda P, Loertscher R, et al. : Assessing risk of disease progression and pharmacological management of autosomal dominant polycystic kidney disease: A Canadian expert consensus. Can J Kidney Health Dis 4: 2054358117695784, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, et al. : Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: A position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 31: 337–348, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, et al. : CRISP Investigators: Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, et al. : Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu ASL, Shen C, Landsittel DP, Harris PC, Torres VE, Mrug M, et al. : Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in Autosomal Dominant Polycystic Kidney Disease. Kidney Int 93: 691–699, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrone RD, Neville J, Chapman AB, Gitomer BY, Miskulin DC, Torres VE, et al. : Therapeutic area data standards for autosomal dominant polycystic kidney disease: A report from the Polycystic Kidney Disease Outcomes Consortium (PKDOC). Am J Kidney Dis 66: 583–590, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Perrone RD, Mouksassi MS, Romero K, Czerwiec FS, Chapman AB, Gitomer BY, et al. : Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep 2: 442–450, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, et al. : CRISP Investigators: Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irazabal MV, Abebe KZ, Bae KT, Perrone RD, Chapman AB, Schrier RW, et al. : HALT Investigators: Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: The HALT-PKD clinical trial. Nephrol Dial Transplant 32: 1857–1865, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girardat-Rotar L, Braun J, Puhan MA, Abraham AG, Serra AL: Temporal and geographical external validation study and extension of the Mayo Clinic prediction model to predict eGFR in the younger population of Swiss ADPKD patients. BMC Nephrol 18: 241, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irazabal MV, Blais JD, Perrone RD, Gansevoort RT, Chapman AB, Devuyst O, et al. : Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: The TEMPO 3:4 clinical trial. Kidney Int Rep 1: 213–220, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Neill WC, Robbin ML, Bae KT, Grantham JJ, Chapman AB, Guay-Woodford LM, et al. : Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: The Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP). Am J Kidney Dis 46: 1058–1064, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Bhutani H, Smith V, Rahbari-Oskoui F, Mittal A, Grantham JJ, Torres VE, et al. : CRISP Investigators: A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int 88: 146–151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kline TL, Korfiatis P, Edwards ME, Blais JD, Czerwiec FS, Harris PC, et al. : Performance of an artificial multi-observer deep neural network for fully automated segmentation of polycystic kidneys. J Digit Imaging 30: 442–448, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kline TL, Edwards ME, Korfiatis P, Akkus Z, Torres VE, Erickson BJ: Semiautomated segmentation of polycystic kidneys in T2-weighted MR images. AJR Am J Roentgenol 207: 605–613, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards ME, Blais JD, Czerwiec FS, Erickson BJ, Torres VE, Kline TL: Standardizing total kidney volume measurements for clinical trials of ADPKD [published online ahead of print August 29, 2018]. Am J Nephrol doi: 10.1093/ckj/sfy078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornec-Le Gall E, Audrézet MP, Rousseau A, Hourmant M, Renaudineau E, Charasse C, et al. : The PROPKD score: A new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 942–951, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornec-Le Gall E, Audrézet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, et al. : Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossetti S, Burton S, Strmecki L, Pond GR, San Millán JL, Zerres K, et al. : The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 13: 1230–1237, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Magistroni R, He N, Wang K, Andrew R, Johnson A, Gabow P, et al. : Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 1164–1174, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Chebib FT, Torres VE: Recent advances in the management of autosomal dominant polycystic kidney disease [published online ahead of print July 26, 2018]. Clin J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Torres VE, Czerwiec F, et al. : Potential impact of tolvaptan on blood pressure in the TEMPO 3:4 population. Poster presented at the annual European Renal Association/European Dialysis and Transplant Association, May 24–27, 2018, Copenhagen, Denmark, 2018 [Google Scholar]
- 38.Boertien WE, Meijer E, de Jong PE, Bakker SJ, Czerwiec FS, Struck J, et al. : Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int 84: 1278–1286, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, et al. : Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: Analysis of clinical trials database. Drug Saf 38: 1103–1113, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodhead JL, Brock WJ, Roth SE, Shoaf SE, Brouwer KL, Church R, et al. : Application of a mechanistic model to evaluate putative mechanisms of tolvaptan drug-induced liver injury and identify patient susceptibility factors. Toxicol Sci 155: 61–74, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres VE, Gansevoort RT, Perrone RD, Devuyst O, Chapman AB, Higashihara E, et al. : Statins, ADPKD severity and progression in the TEMPO 3:4 ADPKD clinical trial. Presented at American Society of Nephrology Kidney Week, San Diego, CA, November 3–8, 2015, 2015 [Google Scholar]
- 42.Kramers BJ, van Gastel MDA, Meijer E, Gansevoort RT: Case report: A thiazide diuretic to treat polyuria induced by tolvaptan. BMC Nephrol 19: 157, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG: Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol 16: 1920–1928, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Bachmann S, Mutig K: Regulation of renal Na-(K)-Cl cotransporters by vasopressin. Pflugers Arch 469: 889–897, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Torres VE, Higashihara E, Devuyst O, Chapman AB, Gansevoort RT, Grantham JJ, et al. : TEMPO 3:4 Trial Investigators: Effect of tolvaptan in autosomal dominant polycystic kidney disease by CKD stage: Results from the TEMPO 3:4 trial. Clin J Am Soc Nephrol 11: 803–811, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aihara M, Fujiki H, Mizuguchi H, Hattori K, Ohmoto K, Ishikawa M, et al. : Tolvaptan delays the onset of end-stage renal disease in a polycystic kidney disease model by suppressing increases in kidney volume and renal injury. J Pharmacol Exp Ther 349: 258–267, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE: Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, et al. : TEMPOFormula and 156-05-002 Study Investigators: Tolvaptan in autosomal dominant polycystic kidney disease: Three years’ experience. Clin J Am Soc Nephrol 6: 2499–2507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoaf SE, Chapman AB, Torres VE, Ouyang J, Czerwiec FS: Pharmacokinetics and pharmacodynamics of tolvaptan in autosomal dominant polycystic kidney disease: Phase 2 trials for dose selection in the pivotal phase 3 trial. J Clin Pharmacol 57: 906–917, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gansevoort RT, van Gastel MDA, Chapman AB, Blais J, Czerwiec FS, Perrone RD, et al.: Copeptin, a surrogate for vasopressin, predicts disease progression and tolvaptan treatment efficacy in ADPKD. Results of the TEMPO 3:4 trial. Oral abstract presented at American Society of Nephrology Kidney Week, Chicago, IL, November 15–20, 2016 [Google Scholar]
- 51.Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK: Serum uric acid and the risk of incident and recurrent gout: A systematic review. J Rheumatol 44: 388–396, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, et al. : Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: Report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis 71: 851–865, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sampson AL, Singer RF, Walters GD: Uric acid lowering therapies for preventing or delaying the progression of chronic kidney disease. Cochrane Database Syst Rev 10: CD009460, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amro OW, Paulus JK, Noubary F, Perrone RD: Low-osmolar diet and adjusted water intake for vasopressin reduction in autosomal dominant polycystic kidney disease: A pilot randomized controlled trial. Am J Kidney Dis 68: 882–891, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberdhan D, Cole JC, Krasa HB, Cheng R, Czerwiec FS, Hays RD, et al. : Development of the Autosomal Dominant Polycystic Kidney Disease Impact Scale: A new health-related quality-of-life instrument. Am J Kidney Dis 71: 225–235, 2018 [DOI] [PubMed] [Google Scholar]
- 56. Haute Autorité de Santé: HAdS: Commission de la Transparence. Tolvaptan. Available at: https://www.has-sante.fr/portail/upload/docs/evamed/CT-14555_JINARC_PIC_INS_Avis2_CT14555.pdf. Accessed December 2, 2015.
- 57. National Institute for Health and Care Excellence: NIfHaCE: Tolvaptan for Treating Autosomal Dominant Polycystic Kidney Disease. Technology Appraisal Guidance. Available at: https://www.nice.org.uk/guidance/ta358. Accessed October 28, 2015.
- 58. Scottish Medicines Consortium: Tolvaptan 15mg, 30mg, 45mg, 60mg and 90mg Tablets (Jinarc®). Available at: https://www.scottishmedicines.org.uk/files/advice/tolvaptan_Jinarc_FINAL_December_2015_for_website.pdf. Accessed January 11, 2016.
- 59.Canadian Agency for Drugs and Technologies in Health: CADTH Canadian Drug Expert Committee Final Recommendation for Jinarc (Tolvaptan). Available at: https://www.cadth.ca/sites/default/files/cdr/complete/SR0435_complete_Jinarc-Feb_26_16_e.pdf. Accessed February 26, 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.