Abstract
Background
Podocyte injury is the hallmark of proteinuric kidney diseases, such as FSGS and minimal change disease, and destabilization of the podocyte’s actin cytoskeleton contributes to podocyte dysfunction in many of these conditions. Although agents, such as glucocorticoids and cyclosporin, stabilize the actin cytoskeleton, systemic toxicity hinders chronic use. We previously showed that loss of the kidney-enriched zinc finger transcription factor Krüppel-like factor 15 (KLF15) increases susceptibility to proteinuric kidney disease and attenuates the salutary effects of retinoic acid and glucocorticoids in the podocyte.
Methods
We induced podocyte-specific KLF15 in two proteinuric murine models, HIV-1 transgenic (Tg26) mice and adriamycin (ADR)-induced nephropathy, and used RNA sequencing of isolated glomeruli and subsequent enrichment analysis to investigate pathways mediated by podocyte-specific KLF15 in Tg26 mice. We also explored in cultured human podocytes the potential mediating role of Wilms Tumor 1 (WT1), a transcription factor critical for podocyte differentiation.
Results
In Tg26 mice, inducing podocyte-specific KLF15 attenuated podocyte injury, glomerulosclerosis, tubulointerstitial fibrosis, and inflammation, while improving renal function and overall survival; it also attenuated podocyte injury in ADR-treated mice. Enrichment analysis of RNA sequencing from the Tg26 mouse model shows that KLF15 induction activates pathways involved in stabilization of actin cytoskeleton, focal adhesion, and podocyte differentiation. Transcription factor enrichment analysis, with further experimental validation, suggests that KLF15 activity is in part mediated by WT1.
Conclusions
Inducing podocyte-specific KLF15 attenuates kidney injury by directly and indirectly upregulating genes critical for podocyte differentiation, suggesting that KLF15 induction might be a potential strategy for treating proteinuric kidney disease.
Keywords: Krüppel-like factor, podocytes, glomerulosclerosis, proteinuria, kidney disease
The Centers for Disease Control and Prevention estimates that 15% of adults in the United States, approximately 30 million Americans, have CKD.1 Failure to maintain renal filtration is the prime indicator during progression of CKD. Podocytes are terminally differentiated epithelial cells in the glomerulus, the major function of which is the maintenance of the renal filtration barrier. Podocyte injury is implicated in many glomerular diseases, including minimal change disease (MCD), FSGS, HIV-associated nephropathy (HIVAN), membranous nephropathy, and diabetic kidney disease.2 In many of these diseased conditions, there is a loss in mature podocyte differentiation markers, and cellular phenotype is marked by destabilization of the actin cytoskeleton, which directly contributes to podocyte dysfunction.
Deciphering the mechanisms by which podocyte injury contributes to the development and progression of FSGS is under active investigation by many laboratories worldwide. Furthermore, previous reports have suggested that regulation of the podocyte transcriptome involved in maintaining mature differentiation markers is critical to preventing podocyte injury.3–5 Also, single-nucleotide polymorphisms in these podocyte-specific transcription factors have been directly implicated in FSGS.6–8 Along with other laboratories, we have previously reported the potential role of Krüppel-Like Factor 15 (KLF15), a kidney-enriched transcription factor, in preventing the development of podocyte injury.9 KLF15 belongs to a 17-member family of DNA binding zinc finger transcription factors that play a critical role in a diverse set of cellular processes, including differentiation, mitochondrial biogenesis, cell cycle, and DNA repair.10,11 We previously showed the essential role of KLF15 in regulating retinoic acid–mediated podocyte differentiation in cultured human podocytes and in proteinuric murine models.9,12 In addition, we recently showed that the podocyte-specific knockdown of Klf15 attenuated the salutary effects of glucocorticoids (GCs) in the three independent proteinuric murine models as well as the level of KLF15 expression in human kidney biopsy specimens correlated with GC responsiveness in primary glomerulopathies.12 Furthermore, we observed that the knockdown of Klf15 in mice increased the susceptibility to podocyte injury in two proteinuric murine models and that the glomerular expression of KLF15 is reduced in human HIVAN and FSGS.12 Conversely, overexpression of KLF15 in cultured human podocytes stabilized the actin cytoskeleton and restored podocyte differentiation markers under cell stress.9,12 On the basis of these findings, we hypothesized that induction of KLF15 specifically in the podocytes will prevent the loss of podocyte differentiation markers, thereby attenuating podocyte injury and eventual FSGS in proteinuric murine models. Here, we show that, using the “tet-on” system, podocyte-specific induction of KLF15 in HIV-1 transgenic mice (Tg26, murine model of FSGS) abrogated podocyte injury, glomerulosclerosis, and tubulointerstitial fibrosis and inflammation, while improving renal function and overall survival. Enrichment analysis of differentially expressed genes extracted from mRNA sequencing in this model showed that podocyte-specific KLF15 induction activates podocyte-specific pathways involved in stabilization of actin cytoskeleton, focal adhesion, and podocyte differentiation. We also validated the renoprotective effects of podocyte-specific KLF15 in an independent proteinuric murine model (adriamycin [ADR] treatment). Finally, we show that the salutary effects of KLF15 are in part mediated by Wilms Tumor 1 (WT1), a podocyte-specific transcription factor, which is also critical in maintaining the podocyte transcriptome under cell stress.
Methods
Cell Culture
Conditionally immortalized human podocytes were gifts from Peter Mundel (Massachusetts General Hospital, Boston, MA). Methods for podocyte cultivation, immortalization, and differentiation were on the basis of the previously described protocol.13 These cells proliferate under permissive conditions (γ-IFN at 33°C) but differentiate under nonpermissive conditions (37°C). Podocytes at 37°C for 14 days are noted to be differentiated.13 To quantify the number of podocytes in cell culture, we followed the manufacturer’s protocol using the Z2 Coulter Particle Counter (Beckman Coulter). Briefly, cells were washed with 1× PBS, trypsinized, and mixed well before addition of saline in cuvette for cell counting.
WT1 knockdown in human podocytes was performed using the Genecopoeia lentiviral shRNA system with the following constructs: HSH01854731LVRH1MP (shRNA1, target sequence cctacagcagtgacaatttat), HSH01854732LVRH1MP (shRNA2, target sequence ccaacttccaagacaagatac), and HSH01854733LVRH1MP (shRNA3, target sequence gggtgaatcttgtctaacatt). In brief, lentiviral particles were produced by transfecting HEK 293T cells with a combination of lentiviral expression plasmid DNA, pCD/NL-BH ΔΔΔ packaging plasmid, and VSV-G–encoding pLTR-G plasmid. For human podocyte infection, viral supernatants were supplemented with 8 μg/ml polybrene and incubated with cells for a 24-hour period. Cells expressing shRNA were selected with puromycin for 2–3 weeks before use in all studies. mCherry expression and Western blot were performed to confirm WT1 knockdown. WT1-shRNA and SC-shRNA podocytes under permissive conditions were transduced with LentiORF-KLF15 to overexpress KLF15, with LentiORF-EV as control. Cells were subsequently transferred to nonpermissive conditions, and podocyte number was quantified after 14 days of differentiation.
LentiORF-WT1 clone was purchased from Genecopoeia, and stable WT1 overexpression was achieved by transduction human podocytes using lentivirus produced from HEK 293T cells. Cells expressing GFP WT1(+KTS) were selected with puromycin for 2–3 weeks before use in all studies. GFP expression and Western blot were performed to confirm WT1 overexpression in lentiORF-WT1 compared with lentiORF-GFP human podocytes. LentiORF-WT1 and LentiORF-EV podocytes under permissive conditions were transduced with KLF15-shRNA to knockdown KLF15, with SC-shRNA as control. Cells were subsequently transferred to nonpermissive conditions, and podocyte number was quantified after 14 days of differentiation.
Coimmunoprecipitation
We cotransfected HEK 293T cells with V5-tagged LentiORF-KLF15-V5 and LentiORF-WT1 vectors compared with LentiORF-EV as control. Cells were harvested 48 hours after transfection, lysed with radioimmunoprecipitation assay buffer with protease inhibitors, immunoprecipitated with rabbit anti-V5 antibody, and subsequently immunoblotted for mouse anti-WT1 antibody. Input (2%) of whole-cell lysates was immunoblotted with KLF15, WT1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to detect protein expression as previously reported.14
Promoter Analyses
Using the TRANSFAC software,15 we scanned the promoters of all mouse genes in the region from (−2000) to the transcription start site with the KLF15 position weight matrix provided by the TRANSFAC system. Enrichment analysis was performed using Enrichr, and the Fisher exact test was used to determine the terms that were over-represented among the genes with KLF15 binding sites.16
Generation of TRE-KLF15 Mice
The TRE-KLF15 transgene contained the (TetO)7/CMV regulatory element driving the full-length human KLF15 coding sequence (CCDS 3036.1) followed by the polyadenylation signal. A map of the plasmid is provided in Supplemental Figure 1A. Transgene was purified from plasmid vector sequences and microinjected into the pronucleus of FVB/N single-celled embryos.
NPHS2-rtTA mice (FVB/N) were acquired from Jackson Laboratory and bred with the TRE-KLF15 mice to generate mice with both transgenes only on the FVB/N background. To induce transgene expression, mice were fed TestDiet Modified LabDiet Rodent Diet 5001 containing 0.15% doxycycline (DOX; El-Mel, Inc., Florissant, MO). Experimental mice remained on DOX food continuously, as did their littermates that were analyzed for comparison.
Genotyping
Genotyping by Extracta DNA prep (Quanta Biosciences) from tails at 2 weeks of age and PCR were performed as described.17,18 Primers for the corresponding mice are provided in Supplemental Table 5. To induce KLF15 expression, mice were fed with DOX in diet beginning at 4 weeks of age (unless otherwise specified).
ADR Treatment of Mice
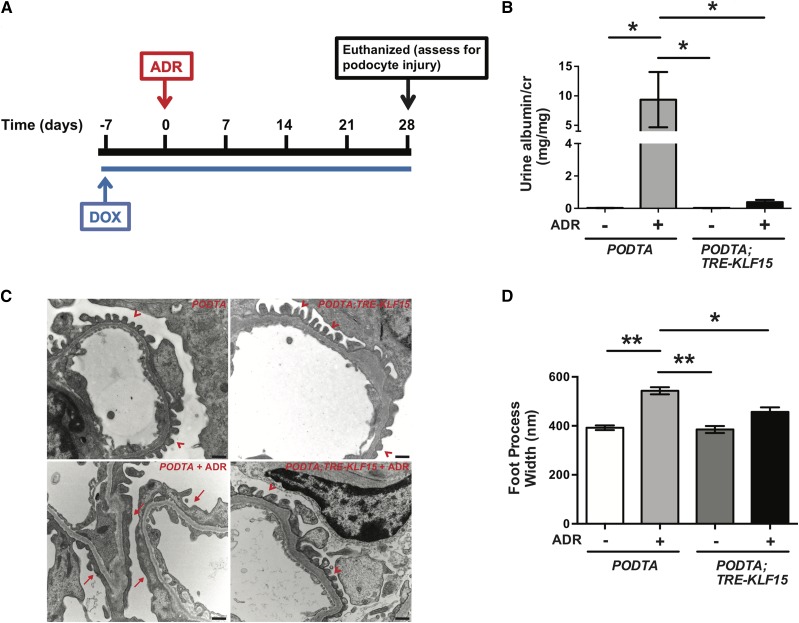
Baseline urine was collected in the PODTA and PODTA;TRE-KLF15 mice (12 weeks of age, FVB/N background). All mice were administered ADR (18 mg/kg) intravenously by tail vein injection19 starting at day 7 post-DOX treatment. Urine was collected weekly, and all mice were euthanized at 4 weeks after ADR treatment. Significant podocyte injury has been described typically at 4 weeks after ADR treatment19; as such, these mice were euthanized, and kidneys were harvested for analysis at 4 weeks after ADR treatment. A schematic of the ADR treatment protocol is provided (Figure 7A).
Figure 7.
Podocyte-specific induction of Krüppel-like factor 15 (KLF15) attenuates podocyte injury in an adriamycin (ADR)-induced proteinuric murine model. Podocin-rtTA (PODTA) and PODTA;TRE-KLF15 mice were initially fed doxycycline (DOX) at 12 weeks of age for 1 week before treatment with ADR (18 mg/kg). Urine was collected weekly, mice were euthanized, and renal cortex was fixed for histology 4 weeks post-ADR treatment. (A) A schematic diagram of the experimental protocol is shown. (B) Albuminuria (urine albumin-to-creatinine ratio) was measured (n=6). *P<0.05 (Kruskal–Wallis test with Dunn post-test). (C) Electron microscopy was performed to assess ultrastructural changes in podocyte morphology. The representative images from four mice in each group are shown (×18,500). Red arrowheads show normal upright foot processes. Red arrows show foot process effacement. (D) Foot process width was quantified by counting the number of slits per length of glomerular basement membrane with ImageJ (n=3). *P<0.05 (Kruskal–Wallis test with Dunn post-test); **P<0.01 (Kruskal–Wallis test with Dunn post-test).
Measurement of Urine Albumin and Creatinine
Urine albumin was quantified by ELISA using a kit from Bethyl Laboratory Inc. Urine creatinine levels were measured in the same samples using the Creatinine (Urinary) Colorimetric Assay Kit (500701; Cayman) according to the manufacturer’s instruction. The urine albumin excretion rate was expressed as the ratio of albumin to creatinine.
Measurement of Serum Urea Nitrogen and Creatinine Levels
Serum urea nitrogen levels were measured by a colorimetric detection method (Arbor Assay, Ann Arbor, MI) according to the manufacturer’s protocol. Serum creatinine levels were measured using the isotope dilution liquid chromatography-tandem mass spectrometer at the University of Alabama at the Birmingham O’Brien Core Center.
Isolation of Glomeruli from Mice for RNA Extraction
Mouse glomeruli were isolated as described.20 Briefly, mice were perfused with HBSS containing 2.5 mg/ml iron oxide and 0.1% BSA. At the end of perfusion, kidneys were removed, decapsulated, minced into 1-mm3 pieces, and digested in HBSS containing 1 mg/ml collagenase A and 100 U/ml deoxyribonuclease I. Digested tissue was then passed through a 100-μm cell strainer and collected by centrifugation. The pellet was resuspended in 2 ml of HBSS, and glomeruli were collected using a magnet. The purity of glomerular was verified under microscopy. Total RNA was isolated from kidney glomeruli of mice using the RNAeasy kit (Qiagen).
Isolation of Primary Glomerular Epithelial Cells
After glomerular isolation (as described above), primary mouse podocytes were isolated as previously described.21,22 In brief, isolated glomeruli were initially cultured on collagen I–coated culture dishes in RPMI 1640 containing 10% FBS (Cansera International) supplemented with 1% Insulin-Transferin-Selenium-A liquid media supplement (Life Technologies) and 100 U/ml penicillin. Cultures were incubated in a 37°C humidified incubator. Subculture of primary podocytes was performed after 5 days of culture of isolated glomeruli. Cellular outgrowths were detached with Trypsin-ethylenediaminetetraacetic acid solution (Corning) and passed through a 40-μm sieve to remove the remaining glomerular cores. The filtered cells were cultured on collagen I–coated dishes and processed for RNA or protein preparation. The purity of isolation was confirmed by testing for podocyte-specific markers by real-time PCR as previously shown.9
Real-Time PCR
Total RNA was extracted by using TRIzol (Life Technologies). First strand cDNA was prepared from total RNA (1.5 μg) using the SuperScript IV VILO Master Mix (Life Technologies), and diluted cDNA (1 μl) was amplified in triplicate using PowerUp SYBR qPCR Master Mix on an ABI QuantStudio 3 (Applied Biosystems). All primers used in the studies were designed using NCBI Primer-BLAST, and they were validated for efficiency before application (Supplemental Table 6). Light cycler analysis software was used to determine crossing points using the second derivative method. Data were normalized to housekeeping genes (GAPDH or β-actin) and presented as fold increase compared with RNA isolated from control group using the 2−ΔΔCT method.
Chromatin Immunoprecipitation Assay
Before performing the chromatin immunoprecipitation (ChIP) assay, immortalized human podocytes with LentiORF-KLF15-V5 tag or empty vector (EV) control were differentiated at 37°C for 14 days and then treated with ADR or vehicle for 24 hours. The ChIP assay was performed using a kit from Cell Signaling Technology as per the manufacturer’s protocol. Briefly, 2×107 cultured human podocytes were crosslinked with 1% formaldehyde for 10 minutes followed by the addition of 1/10 volume of 1.25 M glycine to quench unreacted formaldehyde. Cells were lysed using a series of lysis buffers as per the manufacturer’s protocol. Chromatin extracted from the lysed cells was digested with Micrococcal nuclease and sonicated using a Sonic Dismembrator 550 sonicator (Fisher Scientific) with microtip to generate chromatin fragments of between 150 and 1000 bp. Immunoprecipitation of KLF15-crosslinked chromatin was carried out using rabbit anti-V5 (ab15828; Abcam) antibody. To control for nonspecific IgG binding, normal rabbit IgG (Cell Signaling Technology) was used. After incubation of chromatin with antibody at 4°C overnight, protein G–coupled magnetic beads were added and further incubated for 2 hours; then, the beads were washed several times, and immunoprecipitated chromatin complexes were eluted from the beads. DNA-protein crosslinks were reversed by incubation at 65°C for 16 hours, and then, RNAase A and proteinase K were added sequentially to remove RNA and proteins. Purified DNA was used for the analysis of the WT1 proximal promoter region by real-time PCR on an ABI QuantStudio 3 RT-PCR system using PowerUp SYBR Green Master Mix. PCR primers for the KLF15 binding sites in the human WT1 promoter region are listed in Supplemental Table 7. The relative amplification of the promoter sequence of each site was calculated using the 2−ΔΔCT method, and normalization was performed against the 1:50 diluted input of DNA.
Luciferase Reporter Assay
For human WT1 promoter luciferase assay, a 1.6-kb fragment of the human WT1 promoter upstream of the ATG start codon was cloned into pEZX-PG04. We cotransfected HEK 293T cells into six-well plates with pEZX-PG04-WT1 or pEZX-PG04-control and pReceiver-Lv216 (LentiORF-EV)–, pReceiver-Lv216-KLF15 (LentiORF-KLF15)–, psi-LVRH1GP-scramble–, or psi-LVRH1GP-KLF15shRNA–expressing constructs. Forty-eight hours after transfection, the Secrete-Pair Gaussia Luciferase Dual Kit (Genecopoeia) was used to determine the secreted Gaussia luciferase and secreted Alkaline Phosphatase activities. For data analysis, the values were normalized by using Gaussia luciferase and secreted Alkaline Phosphatase, respectively.
Western Blot
Primary mouse podocytes were lysed with a buffer containing 1% Triton, a protease inhibitor cocktail, and tyrosine and serine-threonine phosphorylation inhibitors. Lysates were subjected to immunoblot analysis using rabbit anti-KLF15 (ABC471; Millipore), rabbit anti-WT1 (13580S; Cell Signaling Technology), rabbit anti–β-actin (A1978; Sigma-Aldrich), and mouse anti-GAPDH (MAB374; Millipore). Densitometry was performed to quantify the change in expression by ImageJ.
Light Microscopy
Mice were perfused with HBSS. The kidneys were fixed in 10% phosphate-buffered formalin overnight and switched to 70% ethanol before processing for histology. Kidney tissue was embedded in paraffin by American Histolabs, and 3-μm-thick sections were stained with periodic acid–Schiff and Masson Trichrome (Sigma-Aldrich).
Transmission Electron Microscopy
Mice were perfused with PBS and then immediately fixed in 2.5% glutaraldehyde for electron microscopy as previously described.17 After embedding of kidney tissues in epoxy resin, ultrathin sections were stained with uranyl acetate and lead citrate, mounted on a copper grid, and photographed under a Hitachi H7650 microscope. Briefly, negatives were digitized, and images with a final magnitude of approximately 18,500 were obtained. Podocyte effacement was quantified as previously described.23
Immunofluorescence and Immunohistochemistry
Specimens were initially baked for 60 minutes in a 55°C to 60°C oven and then processed as previously described.18,24 Briefly, formalin-fixed and paraffin-embedded sections were deparaffinized, and endogenous peroxidase was inactivated with H2O2. All kidney sections from these mice were prepared in identical fashion. Similarly, all paraffin-embedded human kidney biopsy specimens were prepared in identical fashion. Immunofluorescence was performed using polycolonal rabbit anti-KLF15 (GenScript Inc.), mouse anti-WT1 (SC-7385; Santa Cruz), rabbit anti-Nephrin (ALX-810–016-R100; Enzo Life Sciences), goat anti-Synaptopodin (sc21537; Santa Cruz), mouse anti–Gr-1 (RB6–8C5; Abd Serotec), mouse anti–Claudin-1 (sc81796; Santa Cruz), rat anti-CD44 (103001; BioLegend), rabbit anti-Ki67 (Biocare), mouse anti-αSMA (A5228; Sigma-Aldrich), and rabbit antiphospho–β-catenin (Ser552; 9566S; Cell Signaling Technology). After washing, sections were incubated with the appropriate fluorophore-linked secondary antibody (Alexa Fluor 488 donkey anti-chicken IgG; Jackson Immune Research and Alexa Fluor 679 donkey anti-mouse, Alexa Fluor 568 donkey anti-goat IgG, and Alexa Fluor 568 donkey anti-rabbit from Life Technologies). After counterstaining with Hoechst (Thermo Fisher), slides were mounted in Prolong Gold mounting media (Thermo Fisher) and photographed under a Nikon Eclipse 90i microscope with a digital camera.
EdU Injection and EdU Click-iT Reaction
To label proliferated cells, a single intraperitoneal injection of EdU (1 mg per mouse) in sterile PBS was administered to each mouse 3 hours before perfusion.
EdU incorporation into DNA was detected using the Click-iT EdU Alexa Fluor 647 Imaging Kit (Invitrogen). The Click-iT kit was removed from −20°C storage and allowed to thaw in a light-protected box. The amount of reaction cocktail needed was determined and made according to the chart. Tissues were then incubated in the EdU cocktail for 20 minutes at room temperature, protected from light, and subsequently rinsed three times in TBST for 5 minutes per rinse. On completion of the EdU Click-iT reaction, tissues were mounted in Prolong Gold mounting media onto glass slides.
Quantification of Immunostaining
Quantification of KLF15 staining in the podocytes was determined by quantifying the intensity of KLF15 staining (OD) in +WT1+Hoechst staining using ImageJ 1.26t software (National Institutes of Health; rsb.info.nih.gov/ij). Gr-1 staining was quantified by counting the number of Gr-1+ cells per high-power field (30 high-power field micrographs at a final magnification of approximately ×20 were used). Quantification of Nephrin, Synaptopodin, and αSma staining was performed by measuring area staining using ImageJ.12 Quantification of Claudin-1, Ki67, and EdU staining was determined by calculating the percentage of glomeruli with +Claudin-1, +Ki67, and +EdU staining.
RNA Sequencing and Enrichment Analyses
RNA sequencing data were processed as previously described.25 Briefly, sequencing reads were first aligned to the mouse genome (mm10) using Spliced Transcripts Alignment to a Reference (STAR 2.4.1c).26 Aligned reads were then quantified to the transcriptome (UCSC mm10 annotation) at the gene level using featureCounts (v1.4.6).27 Read counts were normalized to count per million, and differentially expressed genes were identified using the Characteristic Direction method.28 Enrichment analyses of the differentially expressed genes were performed with Enrichr.16,29 The single-cell RNA sequencing raw data are available in the Gene Expression Omnibus database (accession no. GSE117987).
Statistical Analyses
A t test was used to compare continuous data between two groups, and two-way ANOVA with Tukey post-test was used to compare continuous data between more than two groups. Because we could not assume normality on some of the other datasets with smaller sample sizes, nonparametric statistical tests were performed using the Mann–Whitney test to compare continuous data between two groups and Kruskal–Wallis test with Dunn post-test to compare continuous data between more than two groups. The exact test used for each experiment is denoted in the figure legends, and data were expressed as the mean±SEM. All experiments were repeated a minimum of three times, and representative experiments are shown. Statistical significance was considered when P<0.05. All statistical analyses were performed using GraphPad Prism 7.0.
Determination of the significance of overlap between gene sets was on the basis of a hypergeometric test performed using the phyper function on R (phyper(q,m,n,k,lower.tail = FALSE): q = overlap between gene sets 1 and 2, m = number of genes in set 1, n = total number of genes in genome or being compared, k = number of genes in set 2).
Study Approval
Stony Brook University Animal Institute Committee approved all animal studies, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals was followed strictly.
Results
Podocyte-Specific KLF15 Induction in Mice
Our recent findings suggest that the loss of Klf15 increases the susceptibility to podocyte injury in proteinuric murine models.12 Furthermore, we recently observed that KLF15 is required to mediate the renoprotective effects of retinoic acid and GCs.9,12 As such, we hypothesized that the induction of human KLF15 specifically in the podocyte might prevent podocyte injury in proteinuric murine models. Because KLF15 is expressed in several cell types in the kidney,9,14 we initially generated mice with podocyte-specific expression of human KLF15 (KLF15) using the “tet-on” system on the FVB/N background, where the binding of chimeric tetracycline transactivator protein (rtTA) to tet-operator and gene activation only occurs in the presence of DOX (Supplemental Figure 1A). We bred the TRE-KLF15 mice with the Podocin-rtTA (PODTA) mice to generate mice with podocyte-specific expression of KLF15 in the setting of DOX administration on the FVB/N background strain. Real-time PCR and Western blot confirmed the increased expression of KLF15 in glomeruli and glomerular epithelial cells (GECs) compared with the tubular compartment or other tissues fractions (i.e., liver lysates) (Supplemental Figure 1, B and C). Furthermore, podocyte-specific induction of KLF15 was validated by immunostaining and colocalizing KLF15 with WT1 (podocyte-specific marker) (Supplemental Figure 1D). At baseline, DOX-treated PODTA;TRE-KLF15 mice were viable and showed no significant changes in proteinuria or podocyte injury compared with the DOX-treated PODTA or TRE-KLF15 mice.
Podocyte-Specific KLF15 Induction Attenuates Kidney Injury in Tg26 Mice
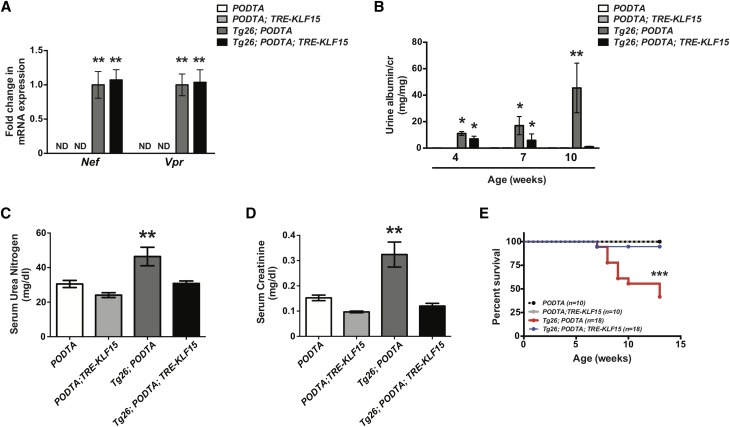
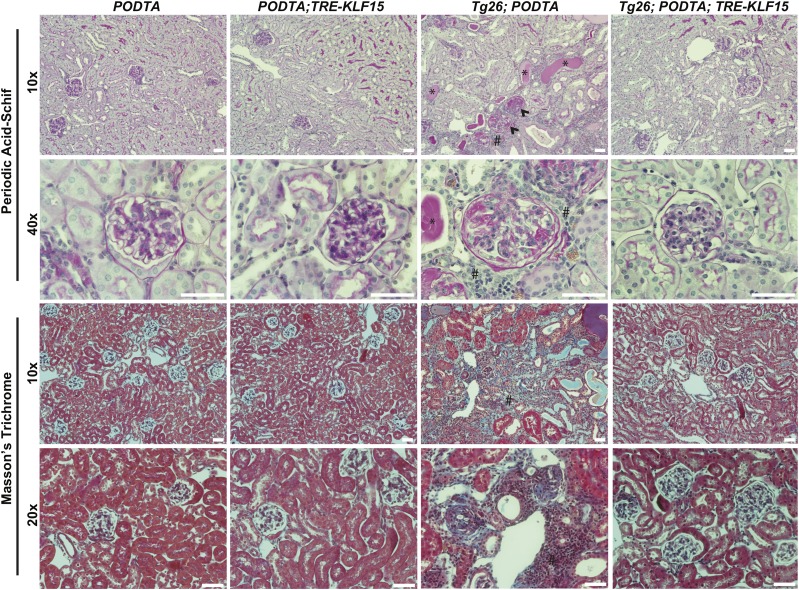
To assess the renoprotective role of podocyte-specific KLF15, we initially used HIV-1 transgenic (Tg26) mice. Tg26 mice are known to develop significant podocyte injury, extensive proteinuria, and collapsing FSGS starting at 4–6 weeks of age.30 Furthermore, glomerular KLF15 expression is reduced in Tg26 mice compared with wild-type mice.12 We bred the PODTA;TRE-KLF15 mice with the Tg26 mice to generate the PODTA, PODTA;TRE-KLF15, Tg26;PODTA, and Tg26;PODTA;TRE-KLF15 (all mice on the FVB/N background). We observed no significant changes in viral gene expression (Nef and Vpr) between DOX-treated Tg26;PODTA and Tg26;PODTA;TRE-KLF15 mice, suggesting that the observed phenotypic changes are not due to altered viral gene expression (Figure 1A). Before DOX treatment, both Tg26;PODTA and Tg26;PODTA;TRE-KLF15 mice exhibited a significant increase in albuminuria at 4 weeks of age compared with PODTA or PODTA;TRE-KLF15 mice (Figure 1B). However, administration of DOX significantly reduced albuminuria in Tg26;PODTA;TRE-KLF15 mice compared with Tg26;PODTA mice by 10 weeks of age (Figure 1B). In addition, induction of KLF15 improved renal function as determined by a reduction in serum urea nitrogen and creatinine, while also improving overall survival in the Tg26 mice (Figure 1, C–E). Interestingly, nonsurviving Tg26;PODTA mice exhibited an increase in albuminuria compared with surviving Tg26;PODTA mice at 10 weeks of age, suggesting that underlying kidney disease might be driving reduced survival in these mice (Supplemental Figure 2). Periodic acid–Schiff and Masson Trichrome staining showed a significant increase in glomerulosclerosis, tubulocystic dilation with proteinaceous casts, and interstitial inflammation and fibrosis in the Tg26;PODTA, which were significantly improved in the Tg26;PODTA;TRE-KLF15 mice at 12 weeks of age (Figure 2). These histologic changes were also quantified by an independent renal pathologist in a blinded fashion (Supplemental Table 1).
Figure 1.
Podocyte-specific induction of Krüppel-like factor 15 (KLF15) attenuates kidney injury and improves overall survival in Tg26 mice. Podocin-rtTA (PODTA), PODTA;TRE-KLF15, Tg26;PODTA, and Tg26;PODTA;TRE-KLF15 mice were treated with doxycycline at 4 weeks of age and euthanized at 12 weeks of age. (A) Nef and Vpr mRNA expressions were measured in glomerular fractions (n=8). ND, not determined. **P<0.01 versus PODTA and PODTA;TRE-KLF15 mice (Kruskal–Wallis test with Dunn post-test). (B) Albuminuria was measured at 4, 7, and 10 weeks of age in all four groups (n=8). *P<0.05 versus PODTA and PODTA;TRE-KLF15 mice (Kruskal–Wallis test with Dunn post-test); **P<0.01 versus all other groups (Kruskal–Wallis test with Dunn post-test). (C) Serum urea nitrogen and (D) serum creatinine were measured at 12 weeks of age in all four groups (n=8). **P<0.01 (Kruskal–Wallis test with Dunn post-test). (E) Survival curves for all four groups are shown until age 12 weeks old (log rank [Mantel–Cox] test). ***P<0.001.
Figure 2.
Podocyte-specific induction of Krüppel-like factor 15 (KLF15) attenuates glomerulosclerosis and tubulointerstitial inflammation and fibrosis in Tg26 mice. Podocin-rtTA (PODTA), PODTA;TRE-KLF15, Tg26;PODTA, and Tg26;PODTA;TRE-KLF15 mice were treated with doxycycline at 4 weeks of age and euthanized at 12 weeks of age. All mice were euthanized and renal cortex fixed for histology. Periodic acid–Schiff and Masson Trichrome staining was performed to evaluate for tubulointerstitial changes. The representative images from four mice in each group are shown. Arrowheads show sclerotic glomeruli. *Tubulocystic dilation and proteinaceous casts; #interstitial inflammation and fibrosis.
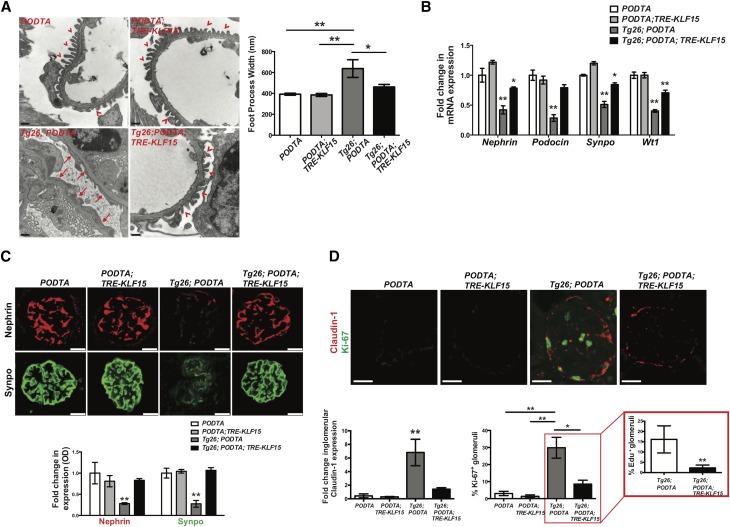
Because initial podocyte injury is a key driver of eventual FSGS in Tg26 mice31 and the podocyte-specific expression of KLF15 attenuated glomerulosclerosis in the Tg26 mice, we inspected each group of mice for the extent of podocyte injury. Initially, we observed a significant improvement in foot process effacement in the Tg26;PODTA;TRE-KLF15 mice compared with Tg26;PODTA mice (Figure 3A). Furthermore, mRNA expression of mature differentiated podocyte markers (Nephrin, Podocin, Synaptopodin, and Wt1) was increased in the Tg26;PODTA;TRE-KLF15 compared with Tg26;PODTA mice (Figure 3B). In addition, Tg26;PODTA;TRE-KLF15 mice exhibited an increase in Nephrin and Synaptopodin protein expression compared with Tg26;PODTA mice (Figure 3C). In combination with a loss in podocyte differentiation markers and collapsing FSGS lesions, previous studies have shown an increase in GEC proliferation in the Tg26 mice.32–34 Similarly, we observed an increase in GEC proliferation as determined by an increase in glomerular Claudin-1 expression and percentage of glomeruli with Ki67+ cells in the Tg26;PODTA mice compared with all other groups (Figure 3D). Conversely, glomerular Claudin-1 expression and percentage of glomeruli with Ki67+ cells were significantly reduced in the Tg26;PODTA;TRE-KLF15 mice compared with Tg26;PODTA mice, which was further validated with a reduction in the percentage of glomeruli with EdU+ cells (Figure 3D). Because Claudin-1 can be expressed in injured podocytes as well as activated parietal epithelial cells (PECs),35,36 we stained for CD44, a marker of activated PECs, to show that podocyte-specific induction of KLF15 reduces the percentage of glomeruli with activated PECs in Tg26 mice (Supplemental Figure 3).
Figure 3.
Podocyte-specific induction of Krüppel-like factor 15 (KLF15) restores podocyte differentiation markers in Tg26 mice. Podocin-rtTA (PODTA), PODTA;TRE-KLF15, Tg26;PODTA, and Tg26;PODTA;TRE-KLF15 mice were treated with doxycycline (DOX) at 4 weeks of age and euthanized at 12 weeks of age. (A) Electron microscopy was performed to determine the extent of foot process effacement. The left panel shows representative images (×18,500) from each of the four groups. The right panel shows quantification of foot process width (n=6). *P<0.05 (Kruskal–Wallis test with Dunn post-test); **P<0.01 (Kruskal–Wallis test with Dunn post-test). (B) Glomeruli were isolated and RNA was extracted for real-time PCR. Nephrin, Podocin, Synaptopodin (Synpo), and Wilms Tumor 1 (Wt1) mRNA expression levels are shown relative to DOX-treated PODTA mice (n=6). *P<0.05 (Kruskal–Wallis test with Dunn post-test); **P<0.01 versus all other groups (Kruskal–Wallis test with Dunn post-test). (C) Immunostaining for Nephrin and Synpo was performed. The upper panel shows representative images from six mice in each group (×20). The glomerular region was selected, and OD was measured and quantified as a relative fold change to DOX-treated PODTA mice for Nephrin and Synpo (lower panel; n=6). **P<0.01 versus all other groups (Kruskal–Wallis test with Dunn post-test). (D) Immunostaining for Claudin-1 and Ki67 was performed. The upper panel shows representative images from six mice in each group (×20). The lower left panel shows fold change in glomerular Claudin-1 expression (n=6). The lower right panel shows the percentage of Ki67+ glomeruli (n=6). *P<0.05 (Kruskal–Wallis test with Dunn post-test); **P<0.01 (Kruskal–Wallis test with Dunn post-test). D, Inset shows the percentage of EdU+ glomeruli in Tg26;PODTA, and Tg26;PODTA;TRE-KLF15 mice (n=6). **P<0.01 (Mann–Whitney test).
Because we observed an improvement in interstitial fibrosis and inflammation in the Tg26 mice with podocyte-specific KLF15 induction, we interrogated the change in expression of specific fibrotic and inflammatory markers. We observed a significant reduction in the expression of fibrotic markers (Fibronectin, Col1α1, Vimentin, and αSma) in Tg26;PODTA;TRE-KLF15 compared with Tg26;PODTA mice (Supplemental Figure 4A). In addition, immunostaining for αSMA validated its reduced expression with induction of KLF15 (Supplemental Figure 4B). We subsequently evaluated the expression of key inflammatory markers (Il-1, Tnf-α, Ifn-γ, Il-6, Tnfr1, and Tnfr2) in the renal cortex, which were examined previously in Tg26 mice.37 Tg26;PODTA mice showed an increase in Il-1, Tnf-α, Il-6, and Tnfr2, which was reduced in the Tg26;PODTA;TRE-KLF15 mice (Supplemental Figure 4C). Furthermore, Tg26;PODTA mice exhibited an increase in Gr-1+ cells in the renal cortex, which was reduced with KLF15 induction (Supplemental Figure 4D). Finally, previous studies have shown that dysregulation of Wnt/β-catenin signaling contributes to kidney injury observed in the Tg26 mice.38 Furthermore, we recently observed that KLF15 binds and attenuates the activation of Wnt/β-catenin signaling.14 Interestingly, we observed a significant reduction in downstream Wnt/β-catenin targets (c-Myc, Tcf7l2, and Lef1) as well as nuclear colocalization of active phospho–β-catenin (Ser552) in the Tg26;PODTA;TRE-KLF15 mice compared with the Tg26;PODTA mice (Supplemental Figure 5). Combined, these findings suggest that podocyte-specific induction of KLF15 restored podocyte differentiation markers and improved glomerular and tubulointerstitial injury in Tg26 mice.
Induction of KLF15 in Tg26 Mice Activates Pathways Specific to Actin Cytoskeleton, Focal Adhesion, and Differentiation
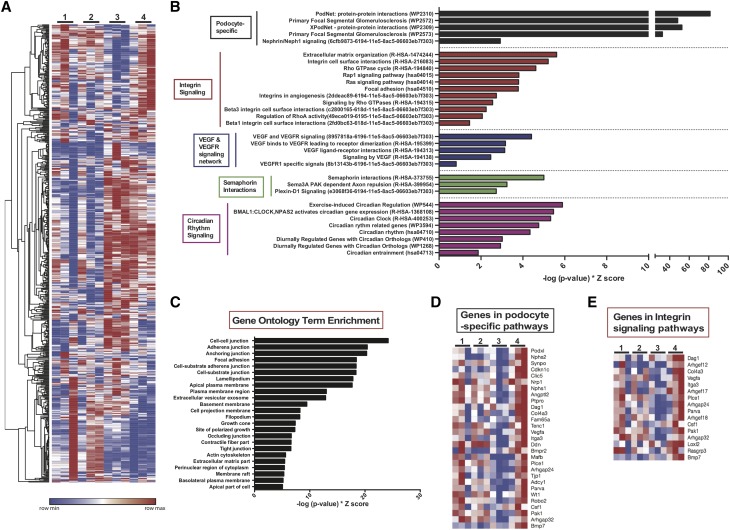
Because we observed that podocyte-specific induction of KLF15 ameliorated kidney injury and improved overall survival in the Tg26 mice, we sought to determine the changes that occur at the transcriptome level mediated by KLF15 in the setting of podocyte injury. To investigate the renoprotective pathways mediated by KLF15, we initially performed mRNA sequencing in glomerular extracts from all four groups of DOX-treated mice at 12 weeks of age (PODTA, PODTA;TRE-KLF15, Tg26;PODTA, and Tg26;PODTA;TRE-KLF15 mice). Initially, we visualized the expression profiles of 600 differentially expressed genes between Tg26;PODTA and Tg26;PODTA;TRE-KLF15 groups (Figure 4A). The ranked differentially expressed upregulated and downregulated genes between the Tg26;PODTA and Tg26;PODTA;TRE-KLF15 groups are provided in Supplemental Tables 2 and 3. Next, we performed gene list enrichment analysis by applying the tool Enrichr16 to the list of genes differentially upregulated in Tg26;PODTA;TRE-KLF15 compared with Tg26;PODTA glomerular fractions. Enrichment analysis against the gene set libraries WikiPathways,39 NCI-Nature Pathway Interaction Database,40 and KEGG pathways41 revealed a significant increase in pathways involved in podocyte differentiation, Integrin signaling, VEGF-VEGFR signaling, Semaphorin interactions, and circadian rhythm signaling (Figure 4B). Furthermore, Gene Ontology Cellular Component enrichment analysis of these differentially expressed genes identified enrichment in cell-cell junction, adherens junction, anchoring junction, and focal adhesion (Figure 4C). Interestingly, the majority of the differentially upregulated genes in Tg26;PODTA;TRE-KLF15 mice are markers of mature differentiated podocytes as well as genes critical for preventing effacement and detachment of podocytes (Figure 4, D and E). We also validated several of these differentially expressed genes (Nephrin, Podocin, Synaptopodin, and Wt1) that were significantly increased in Tg26;PODTA;TRE-KLF15 mice compared with Tg26;PODTA mice by real-time PCR (Figure 3B). In addition, we further validated several of these key upregulated (Cdkn1c, Clic5, Inf2, Plcε1, Vegfa, and Podxl) and downregulated (Neat1, Thrombospondin 1, and Egr1) genes involved in restoring the mature podocyte phenotype in Tg26;PODTA;TRE-KLF15 mice compared with Tg26;PODTA mice by real-time PCR (Supplemental Figure 6). Specifically, Thrombospondin 1, a critical cell matrix glycoprotein implicated in proteinuric kidney disease,42–44 was markedly increased in Tg26;PODTA mice by RNA sequencing and real-time PCR, but expression levels were reduced with podocyte-specific KLF15 induction (Supplemental Figure 6, Supplemental Table 3). These data indicate that the podocyte-specific induction of KLF15 rescues podocytes from injury in the Tg26 mice by upregulating genes critical to maintaining podocyte differentiation and preventing cell detachment and effacement.
Figure 4.
Differentially expressed genes with enrichment analysis demonstrates upregulation of genes critical to maintaining podocyte differentiation and preventing cell detachment and effacement. Podocin-rtTA (PODTA) (lane 1), PODTA;TRE-KLF15 (lane 2), Tg26;PODTA (lane 3), and Tg26;PODTA;TRE-KLF15 (lane 4) mice were treated with doxycycline at 4 weeks of age and euthanized at 12 weeks of age. Glomeruli were isolated, and RNA was extracted for mRNA sequencing (n=3 per group). (A) Heat map analyses of all 600 transcripts differentially expressed between the Tg26;PODTA (lane 3) and Tg26;PODTA;TRE-KLF15 (lane 4) mice are shown in all four groups. (B) A combination of WikiPathway, KEGG Pathways, and NCI-Nature PID enrichment analyses of differentially expressed transcripts upregulated in the Tg26;PODTA;TRE-KLF15 (lane 4) compared with Tg26;PODTA (lane 3). (C) Gene ontology analysis of differentially expressed transcripts upregulated in the Tg26;PODTA;TRE-KLF15 (lane 4) compared with Tg26;PODTA (lane 3). (D) Heat map analysis of the average expression of differentially expressed transcripts related to podocyte-specific pathways between all four groups. (E) Heat map of the average expression of differentially expressed transcripts related to integrin signaling pathways between all four groups.
Salutary Effects of KLF15 Are Partially Mediated by WT1
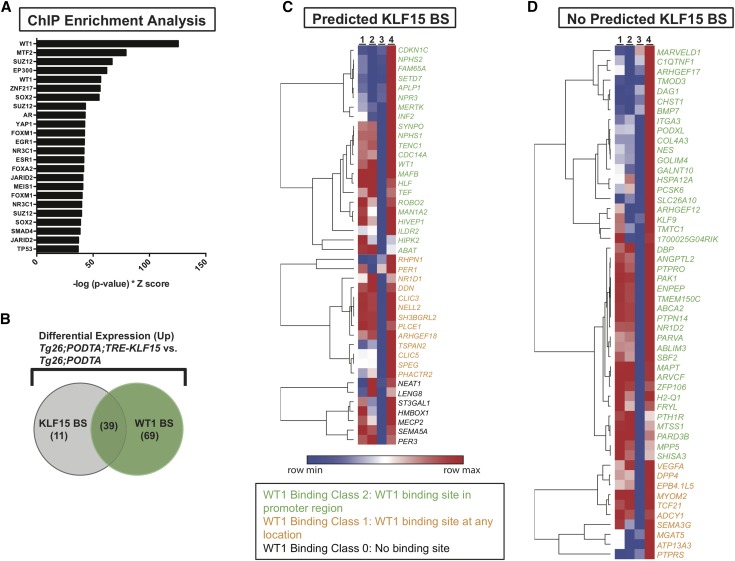
Although we previously reported that KLF15 might transcriptionally regulate some of the mature podocyte differentiation markers by ChIP studies,12 the mechanism by which these other differentially expressed genes are regulated by KLF15 remains unclear. To identify additional mechanisms by which KLF15 restores genes essential to podocyte differentiation and structural integrity, we performed ChIP enrichment analysis45 to identify other transcription factors that might coregulate or mediate the renoprotective effects of KLF15. The ChIP enrichment analysis output, given the 149 differentially upregulated genes in the Tg26;PODTA;TRE-KLF15 mice compared with Tg26;PODTA mice, revealed WT1 as the most statistically significant transcription factor (Fisher exact test, P value of <0.001) to cobind to the promoter of the same set of genes (Figure 5A). To determine the genes that are transcriptionally coregulated by KLF15 and WT1, KLF15 only, or WT1 only, we also performed TRANSFAC position weight matrix analysis46 to identify which of these differentially upregulated genes (minimum of 1.2-fold change) possesses transcriptional binding sites for KLF15 (Supplemental Table 4). We subsequently compared this list of KLF15 target genes to the WT1 ChIP sequencing database deposited in the Sequence Read Archive of NCBI (25; series GSE64063). Of the 149 upregulated transcripts, 108 had WT1 binding sites (P value of <0.001), and 50 had KLF15 binding sites (P value of 0.003), with 39 genes having both WT1 and KLF15 binding sites (P value of 0.003) (Figure 5, B–D). Furthermore, we previously showed that Wt1 expression is increased with KLF15 induction in the Tg26 mice (Figure 3B), which was also confirmed with mRNA sequencing (Figure 4D). These findings suggest that WT1 might coregulate as well as mediate the KLF15 transcriptional network required to prevent glomerulosclerosis in the Tg26 mice.
Figure 5.
Wilms Tumor 1 (WT1) mediates the Krüppel-like factor 15 (KLF15) transcriptome. (A) Chromatin immunoprecipitation (ChIP) enrichment analysis of the differentially upregulated genes in glomerular extracts from the Tg26;Podocin-rtTA (PODTA);TRE-KLF15 mice compared with the Tg26;PODTA mice. We subsequently crossmatched with the previously experimentally validated WT1 ChIP-seq data70 with predicted KLF15 binding sites (TRANSFAC promoter analysis). (B) The Venn diagram shows the overlap in KLF15 and WT1 binding sites (BSs) in the differentially upregulated genes. In addition, the heat map shows the differentially upregulated genes (with a minimum of a 1.2-fold change) in the Tg26;PODTA;TRE-KLF15 mice compared with the Tg26;PODTA mice (C) with KLF15 binding sites and (D) without KLF15 binding sites. Genes shown in green are WT1 binding class 2 (WT1 binding sites in the promoter region). Genes shown in yellow are WT1 binding class 1 (WT1 binding sites at any location). Genes shown in black are WT1 binding class 0 (no WT1 binding sites).
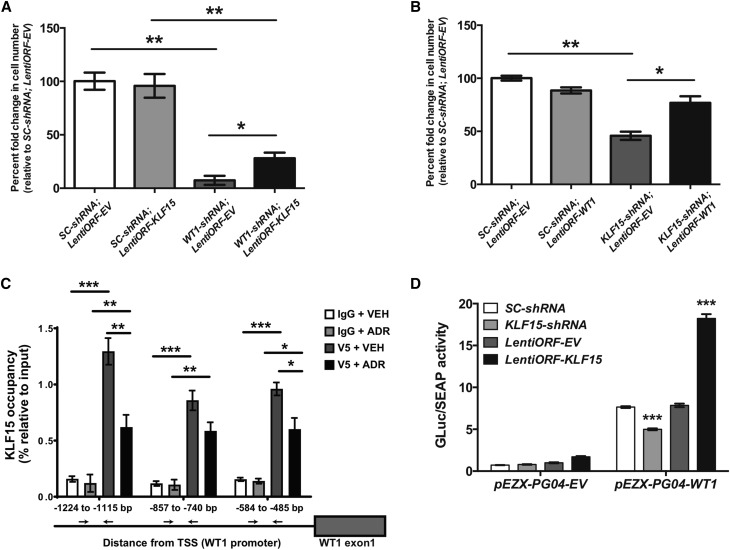
To determine whether the renoprotective effects of KLF15 are in part mediated by WT1, we initially generated human podocytes with stable knockdown for WT1 using three shRNA constructs: WT1-shRNA1, WT1-shRNA2, and WT1-shRNA3. Although all three constructs achieved significant knockdown in WT1 expression by real-time PCR (Supplemental Figure 7A), the WT1-shRNA1 cell line exhibited a more robust knockdown in WT1 protein expression compared with EV-shRNA (control) cells by Western blot (Supplemental Figure 7B). Because WT1 expression is required for podocyte differentiation,47,48 we initially interrogated the role of WT1 under permissive (33°C) and nonpermissive (37°C) conditions. Although all cell lines exhibited no significant changes in survival during permissive conditions, under nonpermissive conditions, the WT1-shRNA1 podocytes exhibited a significant reduction in survival compared with EV-shRNA podocytes (Figure 6A), suggesting that WT1 is required for cell differentiation. We previously observed a similar phenotype with KLF15 knockdown under nonpermissive conditions.9 Because we hypothesized that the renoprotective effects of KLF15 are in part mediated by WT1, we postulated that overexpression of KLF15 will not completely rescue WT1-shRNA1 podocytes from cell death under nonpermissive conditions. To assess this, we overexpressed KLF15 (lentiORF-KLF15) in WT1-shRNA1 and EV-shRNA podocyte lines and assessed for cell survival under nonpermissive conditions. Validation of KLF15 overexpression by Western blot has been previously reported.9 Although KLF15 overexpression partially improved survival of WT1-shRNA1 cells, knockdown of WT1 reduced podocyte survival compared with control cells, regardless of KLF15 overexpression under nonpermissive conditions (Figure 6A). These findings show that KLF15 overexpression only partially rescues the detrimental effects of WT1 knockdown in podocytes under nonpermissive conditions, suggesting that WT1 might be required for the salutary effects of KLF15 in the podocyte.
Figure 6.
Krüppel-like factor 15 (KLF15) regulates Wilms Tumor 1 (WT1) expression. Human podocytes with WT1 knockdown (WT1-shRNA) and KLF15 overexpression (LentiORF-KLF15) along with the corresponding controls (SC-shRNA, LentiORF-EV) were generated. Human podocytes were transferred from 33°C (permissive) to 37°C (nonpermissive) to induce cell differentiation. (A) Cell survival was determined by counting the number of cells at 14 days at 37°C (n=6). *P<0.05 (Kruskal–Wallis test with Dunn post-test); **P<0.01 (Kruskal–Wallis test with Dunn post-test). (B) Conversely, human podocytes with KLF15 knockdown (KLF15-shRNA) and WT1 overexpression (LentiORF-WT1) along with the corresponding controls were generated. Cell survival was determined by counting the number of cells at 14 days at 37°C (n=6). *P<0.05 (Kruskal–Wallis test with Dunn post-test); **P<0.01 (Kruskal–Wallis test with Dunn post-test). (C) Subsequently, chromatin immunoprecipitation assay was performed to show the presence of KLF15 binding in the WT1 promoter in LentiORF-EV and LentiORF-KLF15-V5 cells treated with adriamycin (ADR) compared with vehicle (VEH) treatment. Immunoprecipitation with IgG and V5 was performed. IgG serves as control (n=6). TSS, transcription start site. *P<0.05 (Kruskal–Wallis test with Dunn post-test); **P<0.01 (Kruskal–Wallis test with Dunn post-test); ***P<0.001 (Kruskal–Wallis test with Dunn post-test). (D) Human podocytes were transfected with reporter construct directed at the WT1 promoter region (pEZX-PG04-WT1) or empty vector (pEZX-PG04-EV). Fold induction in WT1 promoter activity is shown with KLF15 knockdown (KLF15-shRNA) and KLF15 overexpression (LentiORF-KLF15) compared with their respective controls (n=6). GLuc, Gaussia luciferase; SEAP, secreted Alkaline Phosphatase. ***P<0.001 (two-way ANOVA test with Tukey post-test).
As we previously observed, knockdown of KLF15 contributes to the loss of podocyte differentiation markers and increases cell death under nonpermissive conditions.9 Because some of the genes regulated by KLF15 might in part be mediated by WT1, we postulated that overexpression of WT1 will partially rescue KLF15-shRNA podocytes from cell death under nonpermissive conditions. The most common isoforms of WT1 are WT1(+KTS) and WT1(−KTS). To determine which isoform is involved in the maintenance of the KLF15 transcriptome in the podocytes, we measured the mRNA expression of both WT1 isoforms in the previous Tg26 model. We observed a significant increase in the WT1(+KTS) isoform in the DOX-treated Tg26;PODTA;TRE-KLF15 mice compared with the DOX-treated Tg26;PODTA mice (Supplemental Figure 7C). Therefore, we overexpressed WT1(+KTS) (lentiORF-WT1) in KLF15-shRNA and Scramble-shRNA podocyte lines and assessed for cell survival under nonpermissive conditions. Validation of WT1(+KTS) overexpression was confirmed by Western blot (Supplemental Figure 7D). Under nonpermissive conditions, KLF15-shRNA;lentiORF-WT1 podocytes exhibited improved survival compared with KLF15-shRNA;lentiORF-EV podocytes (Figure 6B). Similar to the effects of KLF15 overexpression in WT1 knockdown podocytes, WT1 overexpression partially attenuated the detrimental effects of KLF15 knockdown under nonpermissive conditions, suggesting that KLF15 and WT1 are both required for the maintenance of podocyte differentiation markers.
To determine the mechanism by which WT1 might mediate the KLF15 transcriptome, we overexpressed KLF15 and performed ChIP followed by real-time PCR to show that KLF15 occupies the promoter region of WT1 (Figure 6C). We interrogated regions in the WT1 promoter predicted to be occupied by KLF15 using the previously reported KLF15 consensus binding sequence12 (Figure 6C). We also determined that KLF15 binding to the WT1 promoter is reduced with ADR treatment compared with vehicle treatment, suggesting that cell stress might attenuate the transcriptional regulatory role of KLF15 on WT1 in podocytes. To further investigate the mechanism by which KLF15 induces WT1 expression, we transfected HEK 293T cells with reporter construct directed at the WT1 promoter region (pEZX-PG04-hWT1), 1.6 kb upstream of the WT1 transcription start site, and investigated the change in WT1 promoter activity with modulation of KLF15 expression. WT1 promoter activity was significantly increased in pEZX-PG04-hWT1 cells with KLF15 overexpression compared with control cells, which were attenuated in cells with KLF15 knockdown (Figure 6D). We also determined that there are no significant protein-protein interactions between KLF15 and WT1 under basal conditions by overexpressing KLF15 and WT1 and immunoprecipitating KLF15 with anti-V5 antibody and immunoblotting for WT1 (Supplemental Figure 8). Combined, these findings suggest that the renoprotective effects of KLF15 under podocyte stress are in part mediated through the activation of the WT1 transcriptional network.
Podocyte-Specific KLF15 Induction Attenuates Podocyte Injury in ADR-Treated Mice
To validate that the renoprotective effects of KLF15 extend to other proteinuric murine models, we used the ADR-induced podocyte injury model. Although mice on the FVB/N background are resistant to classic FSGS with ADR treatment,49 previous studies have shown that treatment with ADR at 18 mg/kg induces albuminuria with extensive podocyte effacement on the FVB/N background.19,50 We initially determined that glomerular KLF15 expression is reduced in ADR-treated wild-type mice compared with vehicle-treated wild-type mice (Supplemental Figure 9A). To assess whether podocyte-specific induction of KLF15 attenuates ADR-induced nephropathy, 12-week-old PODTA and PODTA;TRE-KLF15 mice were administered DOX for 7 days before treatment with ADR. A schematic of the treatment protocol is provided (Figure 7A), with a detailed description of the protocol in Methods. At 4 weeks post-ADR administration, ADR-treated PODTA;TRE-KLF15 mice exhibited a significant reduction in albuminuria and podocyte effacement compared with in ADR-treated PODTA mice (Figure 7, B–D). Finally, previously reported expression arrays from isolated glomeruli in human kidney biopsies with MCD and diabetic kidney disease also show a reduction in glomerular KLF15 expression compared with healthy donor nephrectomies (Supplemental Figure 9B).51 Combined, these findings suggest that the renoprotective effects of podocyte-specific induction of KLF15 in Tg26 mice might extend to other proteinuric murine models.
Discussion
A large body of evidence has shown that initial podocyte injury and subsequent loss of mature differentiation markers directly contribute to the development of FSGS. Identification of targets to ameliorate podocyte injury in proteinuric diseases has focused recently on stabilizing the actin cytoskeleton and enhancing the expression of critical structural proteins required for maintenance of the mature podocyte. We previously showed that the global loss of Klf15 in mice increased the susceptibility to podocyte injury in proteinuric murine models.12 In addition, we recently reported that podocyte-specific Klf15 was required to mediate the direct salutary effects of GCs in proteinuric murine models.9 Here, we show that podocyte-specific induction of KLF15 attenuated podocyte injury, glomerulosclerosis, and tubulointerstitial inflammation and fibrosis while improving survival in Tg26 mice. We also observed that podocyte-specific induction of KLF15 activated pathways involved in focal adhesion, stabilization of actin cytoskeleton, and restoration of podocyte differentiation markers.
The expression of several key transcription factors is critical for podocyte development as well as maintenance of the podocyte transcriptome in adult mice.5 Furthermore, recent studies by Hayashi et al.50 showed that podocyte-specific induction of KLF4 ameliorated podocyte injury after ADR treatment. However, to date, this is the first study to show that induction of a zinc finger transcription factor specifically in the podocyte attenuated kidney injury in a murine model of collapsing FSGS. Tg26 mice lose their mature podocyte differentiation markers and develop significant albuminuria with early FSGS lesions by 4 weeks of age, but significant glomerulosclerosis and tubulointerstitial fibrosis do not occur until later age.52 We observed that podocyte-specific induction of KLF15 at 4 weeks prevented worsening of FSGS and development of tubulointerstitial fibrosis, while improving overall survival. We suspect that this improvement in renal disease directly reduced mortality in these Tg26 mice, because induction of KLF15 was specific to the podocyte. Future studies will focus on whether induction of KLF15 in older Tg26 mice will reverse glomerulosclerosis and tubulointerstitial fibrosis. In addition, this will also need to be tested in other FSGS murine models, such as ADR-induced nephropathy on the BALB/c background, to determine whether podocyte-specific induction of KLF15 can reverse sclerotic lesions consistent with FSGS after ADR administration. Finally, DOX-inducible podocyte-specific induction of KLF15 was generated using the Podocin promoter, because both the Tg26 and PODTA mice were on the FVB/N background. Additional studies will also need to be performed to validate our findings with other podocyte-specific promoters on the FVB/N background.
We previously showed that Klf15 expression is reduced in Tg26 mice and that the loss of Klf15 increases the susceptibility to podocyte injury.12 Here, we observed that induction of KLF15 in podocytes abrogated this injury in two proteinuric models, suggesting that KLF15 might have a therapeutic role in several other glomerular diseases. Similar to HIVAN and FSGS,12 analysis of expression arrays from Ju et al.51 showed a decrease in glomerular KLF15 expression in MCD and diabetic kidney disease. On the basis of these data, examining the mechanisms by which podocyte-specific KLF15 ameliorates these other glomerular diseases will be a focus of future investigations. Along with other laboratories, we have shown that GCs and retinoic acid are potent inducers of KLF15 expression.9,12,53,54 In addition, we recently observed that the salutary effects of GCs are attenuated with podocyte-specific knockdown of Klf15 in multiple proteinuric murine models.12 Although we did not observe any deleterious effects of persistent podocyte-specific induction of KLF15 from 4 to 12 weeks of age in this study, the effects of prolonged podocyte-specific as well as global induction of KLF15 in mice remain to be determined. Finally, because the loss of Klf15 has been implicated in cardiovascular disease,55–57 airway smooth muscle hyper-responsiveness,58 and adipocyte differentiation,59 future studies should focus on using these TRE-KLF15 mice to study the salutary effects of KLF15 induction in a tissue-specific manner in these other diseases.
Of particular interest in our studies is the observation that the salutary effects of KLF15 might in part be mediated by WT1. Several studies have highlighted the essential role of WT1 in kidney development, and single-nucleotide polymorphisms in WT1 have been implicated in FSGS and HIVAN.6,7,47,60–63 In addition, we observed that the majority of the transcripts critical to maintaining podocyte differentiation and preventing cell detachment and effacement are concurrently mediated by KLF15 and WT1. We hypothesize that both KLF15 and WT1 are required to maintain mature differentiation markers in the setting of cell stress. Although our coimmunoprecipitation studies did not show a significant protein-protein interaction between KLF15 and WT1 under basal conditions, this interaction might be dynamic and depend on the activation of the transcriptional machinery under cell stress. Furthermore, similar to KLF15, the critical role of other transcription factors, including WT1, in regulating WT1 expression cannot be neglected.64–67 These include SP1, EGR1, and ZHX2 as well as reciprocal regulation by PAX2 and isoforms of WT1, which are essential in regulating WT1 expression in development and disease.64–67 Additional studies are required to determine the mechanism by which these other transcription factors interact with KLF15 and WT1 in maintaining podocyte differentiation markers under cell stress. Other KLFs, such as KLF4 and KLF6, have also been previously reported to play a role in podocytopathies.18,50 Induction of KLF15 in our studies might regulate the expression and/or function of these other KLFs. Our RNA sequencing was performed in isolated glomeruli, and as such, it is difficult to interrogate podocyte-specific changes in expression of other KLFs. Nonetheless, additional studies in isolated podocytes are necessary to determine whether the induction of KLF15 regulates these other KLFs. In addition, examining the protein-protein interactions between these KLFs at baseline and in cell stress might provide a framework to understand the dynamic nature of the transcriptional network in podocytopathies.
Endogenous KLF15 expression is not restricted to podocytes, because previous immunostaining has shown expression in tubules, endothelial cells, and renal stromal cells.14 We also recently showed that the loss of renal stromal–specific Klf15 exacerbated kidney injury in murine models of unilateral uretic obstruction and chronic Angiotensin II administration.14 Although renal stromal– or tubule-specific induction of KLF15 might also improve kidney injury in the Tg26 mice, we focused on the role of KLF15 induction in podocytes, because previous studies have shown that podocyte injury is the main contributing factor to FSGS and eventual tubulointerstitial injury in the Tg26 mice. Interestingly, along with restoring podocyte differentiation markers, we observed a reduction in activated PECs with podocyte-specific induction of KLF15 in Tg26 mice. Mechanisms by which podocyte-specific induction of KLF15 reduces PEC activation remain to be explored, but potential pathways include activated Notch and STAT3 signaling as previously reported.22,68,69 Nonetheless, to test whether the salutary effects of KLF15 extend beyond the podocyte, future studies will need to focus on the role of tubule- and/or stromal-specific induction of KLF15 in murine models with primarily tubulointerstitial disease.
To the best of our knowledge, this is the first study to show that podocyte-specific induction of a zinc finger transcription factor attenuates kidney injury in a murine model of collapsing FSGS. In addition, we show that KLF15 attenuates kidney injury by restoring the transcriptome required to maintain mature podocyte differentiation markers, which is in part mediated by WT1. Collectively, these studies show the critical need to study KLF15 as a therapeutic target in podocytopathies.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases grants DK078897 (to J.C.H.), DK102519 (to S.K.M.), and DK112984 (to S.K.M.); Veterans Affairs Merit grant 1I01BX003698 (to S.K.M.); and Dialysis Clinic Inc. Paul Teschan Research Grant (to S.K.M.) and partially supported by NIH grants U54HL127624 (to A.M.) and U24CA224260 (to A.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018030324/-/DCSupplemental.
References
- 1.Centers for Disease Control and Prevention: Chronic Kidney Disease Surveillance System Website, 2017. Available at: http://www.cdc.gov/ckd. Accessed March 9, 2017
- 2.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Miner JH, Morello R, Andrews KL, Li C, Antignac C, Shaw AS, et al.: Transcriptional induction of slit diaphragm genes by Lmx1b is required in podocyte differentiation. J Clin Invest 109: 1065–1072, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quaggin SE: Transcriptional regulation of podocyte specification and differentiation. Microsc Res Tech 57: 208–211, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS: Transcriptional regulation of podocyte disease. Transl Res 149: 237–242, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orloff MS, Iyengar SK, Winkler CA, Goddard KA, Dart RA, Ahuja TS, et al.: Variants in the Wilms’ tumor gene are associated with focal segmental glomerulosclerosis in the African American population. Physiol Genomics 21: 212–221, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Woroniecki RP, Kopp JB: Genetics of focal segmental glomerulosclerosis. Pediatr Nephrol 22: 638–644, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu H, Artomov M, Brähler S, Stander MC, Shamsan G, Sampson MG, et al.: A role for genetic susceptibility in sporadic focal segmental glomerulosclerosis. J Clin Invest 126: 1067–1078, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallipattu SK, Guo Y, Revelo MP, Roa-Peña L, Miller T, Ling J, et al.: Krüppel-like factor 15 mediates glucocorticoid-induced restoration of podocyte differentiation markers. J Am Soc Nephrol 28: 166–184, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bialkowska AB, Yang VW, Mallipattu SK: Krüppel-like factors in mammalian stem cells and development. Development 144: 737–754, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallipattu SK, Estrada CC, He JC: The critical role of Krüppel-like factors in kidney disease. Am J Physiol Renal Physiol 312: F259–F265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallipattu SK, Liu R, Zheng F, Narla G, Ma’ayan A, Dikman S, et al.: Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem 287: 19122–19135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al.: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Gu X, Mallipattu SK, Guo Y, Revelo MP, Pace J, Miller T, et al.: The loss of Krüppel-like factor 15 in Foxd1+ stromal cells exacerbates kidney fibrosis. Kidney Int 92: 1178–1193, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matys V, Fricke E, Geffers R, Gössling E, Haubrock M, Hehl R, et al.: TRANSFAC: Transcriptional regulation, from patterns to profiles. Nucleic Acids Res 31: 374–378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al.: Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14: 128, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallipattu SK, Liu R, Zhong Y, Chen EY, D’Agati V, Kaufman L, et al.: Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int 83: 626–634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallipattu SK, Horne SJ, D’Agati V, Narla G, Liu R, Frohman MA, et al.: Krüppel-like factor 6 regulates mitochondrial function in the kidney. J Clin Invest 125: 1347–1361, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee VW, Harris DC: Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, et al.: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T: An improved method for primary culture of rat podocytes. Kidney Int 69: 2101–2106, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Dai Y, Gu L, Yuan W, Yu Q, Ni Z, Ross MJ, et al.: Podocyte-specific deletion of signal transducer and activator of transcription 3 attenuates nephrotoxic serum-induced glomerulonephritis. Kidney Int 84: 950–961, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallipattu SK, Gallagher EJ, LeRoith D, Liu R, Mehrotra A, Horne SJ, et al.: Diabetic nephropathy in a nonobese mouse model of type 2 diabetes mellitus. Am J Physiol Renal Physiol 306: F1008–F1017, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong Y, Wu Y, Liu R, Deng Y, Mallipattu SK, Klotman PE, et al.: Roflumilast enhances the renal protective effects of retinoids in an HIV-1 transgenic mouse model of rapidly progressive renal failure. Kidney Int 81: 856–864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Ma’ayan A: An open RNA-Seq data analysis pipeline tutorial with an example of reprocessing data from a recent Zika virus study. F1000Res 5: 1574, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al.: STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y, Smyth GK, Shi W: featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Clark NR, Hu KS, Feldmann AS, Kou Y, Chen EY, Duan Q, et al.: The characteristic direction: A geometrical approach to identify differentially expressed genes. BMC Bioinformatics 15: 79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al.: Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44[W1]: W90–W97, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barisoni L, Kriz W, Mundel P, D’Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 31.He JC, Lu TC, Fleet M, Sunamoto M, Husain M, Fang W, et al.: Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol 18: 93–102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barisoni L, Bruggeman LA, Mundel P, D’Agati VD, Klotman PE: HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int 58: 173–181, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D’Agati V, Alpers CE: Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: Role in podocyte proliferation and maturation. Kidney Int 58: 674–683, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Feng X, Lu TC, Chuang PY, Fang W, Ratnam K, Xiong H, et al.: Reduction of Stat3 activity attenuates HIV-induced kidney injury. J Am Soc Nephrol 20: 2138–2146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, et al.: Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med 19: 1496–1504, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong Y, Sunq A, Roth RA, Hou J: Inducible expression of claudin-1 in glomerular podocytes generates aberrant tight junctions and proteinuria through slit diaphragm destabilization. J Am Soc Nephrol 28: 106–117, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruggeman LA, Drawz PE, Kahoud N, Lin K, Barisoni L, Nelson PJ: TNFR2 interposes the proliferative and NF-κB-mediated inflammatory response by podocytes to TNF-α. Lab Invest 91: 413–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shkreli M, Sarin KY, Pech MF, Papeta N, Chang W, Brockman SA, et al.: Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med 18: 111–119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pico AR, Kelder T, van Iersel MP, Hanspers K, Conklin BR, Evelo C: WikiPathways: Pathway editing for the people. PLoS Biol 6: e184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, et al.: PID: The pathway interaction database. Nucleic Acids Res 37: D674–D679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanehisa M, Goto S: KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, et al.: Impaired angiogenesis in the remnant kidney model. I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol 12: 1434–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, et al.: Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 115: 3451–3459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeisberg M, Tampe B, LeBleu V, Tampe D, Zeisberg EM, Kalluri R: Thrombospondin-1 deficiency causes a shift from fibroproliferative to inflammatory kidney disease and delays onset of renal failure. Am J Pathol 184: 2687–2698, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lachmann A, Xu H, Krishnan J, Berger SI, Mazloom AR, Ma’ayan A: ChEA: Transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics 26: 2438–2444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, et al.: TRANSFAC and its module TRANSCompel: Transcriptional gene regulation in eukaryotes. Nucleic Acids Res 34: D108–D110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison AA, Viney RL, Saleem MA, Ladomery MR: New insights into the function of the Wilms tumor suppressor gene WT1 in podocytes. Am J Physiol Renal Physiol 295: F12–F17, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Toska E, Roberts SG: Mechanisms of transcriptional regulation by WT1 (Wilms’ tumour 1). Biochem J 461: 15–32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, et al.: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Hayashi K, Sasamura H, Nakamura M, Azegami T, Oguchi H, Sakamaki Y, et al.: KLF4-dependent epigenetic remodeling modulates podocyte phenotypes and attenuates proteinuria. J Clin Invest 124: 2523–2537, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, et al.: ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium : Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 7: 316ra193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratnam KK, Feng X, Chuang PY, Verma V, Lu TC, Wang J, et al.: Role of the retinoic acid receptor-α in HIV-associated nephropathy. Kidney Int 79: 624–634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asada M, Rauch A, Shimizu H, Maruyama H, Miyaki S, Shibamori M, et al.: DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Krüppel-like factor 15 gene expression. Lab Invest 91: 203–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasse SK, Mailloux CM, Barczak AJ, Wang Q, Altonsy MO, Jain MK, et al.: The glucocorticoid receptor and KLF15 regulate gene expression dynamics and integrate signals through feed-forward circuitry. Mol Cell Biol 33: 2104–2115, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang B, Haldar SM, Lu Y, Ibrahim OA, Fisch S, Gray S, et al.: The Kruppel-like factor KLF15 inhibits connective tissue growth factor (CTGF) expression in cardiac fibroblasts. J Mol Cell Cardiol 45: 193–197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, et al.: Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 483: 96–99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y, Zhang L, Liao X, Sangwung P, Prosdocimo DA, Zhou G, et al.: Kruppel-like factor 15 is critical for vascular inflammation. J Clin Invest 123: 4232–4241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masuno K, Haldar SM, Jeyaraj D, Mailloux CM, Huang X, Panettieri RA Jr, et al.: Expression profiling identifies Klf15 as a glucocorticoid target that regulates airway hyperresponsiveness. Am J Respir Cell Mol Biol 45: 642–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, et al.: Role of Krüppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem 280: 12867–12875, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, et al.: WT-1 is required for early kidney development. Cell 74: 679–691, 1993 [DOI] [PubMed] [Google Scholar]
- 61.Roberts SG: Transcriptional regulation by WT1 in development. Curr Opin Genet Dev 15: 542–547, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Nabet B, Tsai A, Tobias JW, Carstens RP: Identification of a putative network of actin-associated cytoskeletal proteins in glomerular podocytes defined by co-purified mRNAs. PLoS One 4: e6491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lefebvre J, Clarkson M, Massa F, Bradford ST, Charlet A, Buske F, et al.: Alternatively spliced isoforms of WT1 control podocyte-specific gene expression. Kidney Int 88: 321–331, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Dehbi M, Ghahremani M, Lechner M, Dressler G, Pelletier J: The paired-box transcription factor, PAX2, positively modulates expression of the Wilms’ tumor suppressor gene (WT1). Oncogene 13: 447–453, 1996 [PubMed] [Google Scholar]
- 65.Cohen HT, Bossone SA, Zhu G, McDonald GA, Sukhatme VP: Sp1 is a critical regulator of the Wilms’ tumor-1 gene. J Biol Chem 272: 2901–2913, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Klamt B, Koziell A, Poulat F, Wieacker P, Scambler P, Berta P, et al.: Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/-KTS splice isoforms. Hum Mol Genet 7: 709–714, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Liu G, Clement LC, Kanwar YS, Avila-Casado C, Chugh SS: ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J Biol Chem 281: 39681–39692, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Sharma M, Magenheimer LK, Home T, Tamano KN, Singhal PC, Hyink DP, et al.: Inhibition of Notch pathway attenuates the progression of human immunodeficiency virus-associated nephropathy. Am J Physiol Renal Physiol 304: F1127–F1136, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu L, Dai Y, Xu J, Mallipattu S, Kaufman L, Klotman PE, et al.: Deletion of podocyte STAT3 mitigates the entire spectrum of HIV-1-associated nephropathy. AIDS 27: 1091–1098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kann M, Ettou S, Jung YL, Lenz MO, Taglienti ME, Park PJ, et al.: Genome-wide analysis of Wilms’ Tumor 1-controlled gene expression in podocytes reveals key regulatory mechanisms. J Am Soc Nephrol 26: 2097–2104, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.