Abstract
Background.
How diagnosis with a variant of uncertain significance (VUS) in a BRCA gene impacts clinical decision-making is not well known.
Methods.
We queried for all patients attending Mayo Clinic Rochester from 2004 to 2016 who tested positive for BRCA1 or BRCA2 VUS and reviewed patient management choices. Groups were compared by using Wilcoxon rank-sum and Chi-square tests.
Results.
We identified 97 patients (95 females, 2 males) with BRCA VUS. For patients without cancer history (n = 20), 80% had a mother or sister with breast cancer, and median Tyrer-Cuzick (IBIS) lifetime breast cancer risk score was 27% (range 16–62%). Management included bilateral prophylactic mastectomy (BPM) in 39%, where choice for BPM was significantly associated with IBIS score (median 32 vs. 24%, p = 0.02) and first-degree family history of breast cancer (100 vs. 64%, p = 0.03) but not Gail score or total number of family members with cancer. For patients with breast cancer who had known VUS status prior to surgery (n = 9), the rate of contralateral prophylactic mastectomy (CPM) was 22% compared with 25% without known VUS and 83% with known BRCA pathogenic mutation. In 21 of 97 (22%) patients, the BRCA VUS has been reclassified (95% benign, 5% deleterious).
Conclusions.
BRCA VUS carriers with cancer elected surgical choices similar to average-risk breast cancer patients. However, VUS carriers without cancer had high rates of BPM, associated with first-degree family history and IBIS score. Over time, a significant proportion of BRCA VUS were reclassified, illustrating the importance of appropriate counseling regarding VUS.
With the increasing public awareness of breast cancer hereditary syndrome, many women question and consider genetic testing for themselves and their close relatives.1 The National Comprehensive Cancer Network (NCCN) delineates specific guidelines for referral to genetic counseling, including known mutations in the family, early onset cancers, triple-negative cancers, and multiple relatives with cancer.2 With increasing rates of genetic testing come increasing chances of receiving an uninformative result or variant of uncertain significance (VUS).
Guidelines for the management of pathogenic variants in BRCA1 and BRCA2 genes recommend consideration of prophylactic surgeries. However, recommendations for management of VUS suggest a focus on clinical factors, such as personal and family history of cancer when considering breast cancer risk. This is because the disease risk associated with the VUS is unknown. However, this concept of a genetic variant that may or may not have clinical consequence can be confusing and anxiety-provoking to patients.3 In addition, it is not recommended to test family members for VUS, except as part of research protocols aimed at establishing the clinical relevance of the VUS.2
Little is known about the impact of a VUS in BRCA genes on clinical decision-making. Therefore, we sought to explore clinical management options chosen by BRCA1 and BRCA2 VUS patients and to identify factors influencing their decision-making.
METHODS
Study Population
After institutional review board approval, we queried the Mayo Clinic Rochester electronic medical record for all patients with genetic testing who tested positive for a variant classified as VUS in BRCA1 or BRCA2 genes from 2004 to 2016. Retrospective chart review was performed for patient demographic and clinical variables, including patient age, date of VUS diagnosis, date of reclassification, family history, and clinical management options, including type of breast imaging, use of endocrine therapy, timing of surgery, and operation performed.
VUS Classification
Initial date of VUS diagnosis was recorded for each patient. Retrospective chart review was performed to evaluate dates of genetics follow-up appointments or telephone calls and timing when patient was notified of reclassification. In addition, we queried the ClinVar, BRCA Exchange, and BRCA1 and BRCA2 LOVD databases, all of which adhere to ENIGMA consortium rules for variant classification to obtain the most recent information on variant reclassifications.
Statistical Analysis
For statistical testing, we used a two-sided significance level of p = 0.05. Groups were compared using Wilcoxon rank-sum and likelihood-ratio Chi-square tests. Analysis was performed using SAS (Version 9.4).
RESULTS
Patient Characteristics
We identified 97 patients (95 females, 2 males) with BRCA1 or BRCA2 VUS. At the time of VUS identification, 20 had no current or prior breast or ovarian cancer, 67 had current or prior breast cancer, 9 had ovarian cancer, and 1 both breast and ovarian cancer. After initial chart review, we excluded patients with ovarian cancer (n = 10). For further analysis on choice for prophylactic mastectomy, we excluded patients with bilateral breast cancers (n = 5), male patients (n = 2), and a patient with stage IV disease (n = 1), because these groups would not have been candidates for PM. Patient demographic and clinical characteristics are summarized in Table 1.
TABLE 1.
Patient demographics and clinical risk factors among the subset of patients without ovarian cancer (n = 87)
| N = 87 patients | ||
|---|---|---|
| Patients with breast cancer (N = 67) | Patients without breast cancer (N = 20) | |
| Age at VUS diagnosis, median (range) | 49 (27–83) | 45 (30–74) |
| Menopausal status, n (%) | ||
| Premenopausal | 36 (54%) | 8 (40%) |
| Perimenopausal | 3 (4%) | 1 (5%) |
| Postmenopausal | 24 (36%) | 8 (40%) |
| Unknown | 4 (6%) | 3 (15%) |
| Year of VUS diagnosis, n (%) | ||
| 2000–2005 | 7 (10%) | 2 (10%) |
| 2006–2011 | 26 (39%) | 9 (45%) |
| 2012–2016 | 28 (42%) | 9 (45%) |
| Unknown | 6 (9%) | - |
| Gene with VUS, n (%) | ||
| BRCA1 | 21 (31%) | 4 (20%) |
| BRCA2 | 46 (69%) | 16 (80%) |
| Gender, n (%) | ||
| Female | 67 (100%) | 18 (90%) |
| Male | 0 | 2 (10%) |
| Ethnicity, n (%) | ||
| Caucasian | 59 (88%) | 18 (90%) |
| African American | 0 | 0 |
| Hispanic | 2 (3%) | 0 |
| Asian | 2 (3%) | 0 |
| Other/unknown | 3 (4%) | 2 (10%) |
| Marital status, n (%) | ||
| Single | 7 (10%) | 0 |
| Married | 47 (70%) | 18 (90%) |
| Widowed | 6 (9%) | 0 |
| Divorced | 7 (10%) | 2 (10%) |
| Education, n (%) | ||
| High school | 8 (12%) | 4 (20%) |
| College | 34 (51%) | 10 (50%) |
| Graduate | 16 (24%) | 3 (15%) |
| Unknown | 9 (13%) | 3 (15%) |
| Tyrer-Cuzick (IBIS), median (range) | - | 27% (16–62%) |
| Gail (lifetime), median (range) | - | 20% (7–43%) |
| # Relatives with breast cancer, median (range) | 2(0–9) | 3 (1–6) |
| First-degree family history, n (%) (unknown n = 2) | 29/65 (45%) | 16/20 (80%) |
Clinical Management of Patients without Cancer Diagnosis
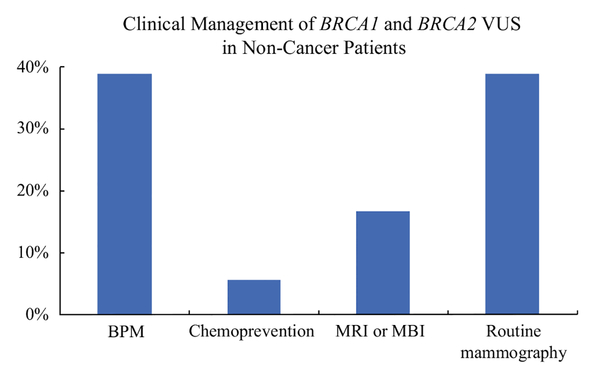
In the 20 patients without current or prior breast or ovarian cancer at time of VUS identification, the median Tyrer-Cuzick (IBIS) lifetime breast cancer risk score was 27% (range 16–62%), and median Gail score (lifetime risk) was 20% (range 7–43%). The median number of relatives with breast cancer was 3 (range 1–6); 16 of 20 (80%) patients had a mother or sister with breast cancer. Thirty-nine percent of patients chose to undergo BPM, and choice for BPM was significantly associated with IBIS score (median 32 vs. 24%, p = 0.02) and first-degree family history (100 vs. 64%, p = 0.03) but not Gail score or total number of family members with breast cancer. Figure 1 demonstrates rates of surgical and non-surgical management for patients without a cancer diagnosis.
FIG. 1.
Comparison of rates of surgical choices versus nonsurgical choices in patients without a cancer diagnosis who have a BRCA1 or BRCA2 variant of uncertain significance
Clinical Management of Patients with Cancer Diagnosis
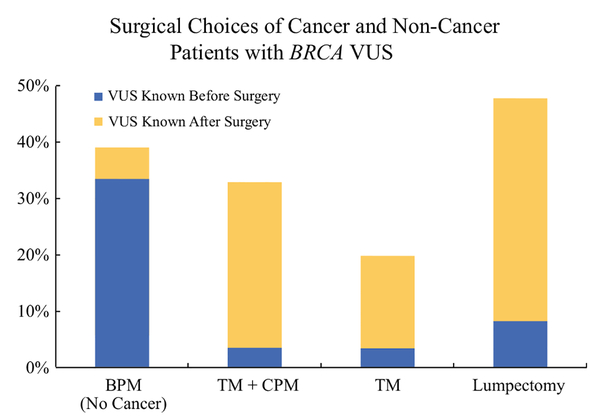
Of 67 patients with a VUS who had breast cancer, the VUS diagnosis was known before initial surgery in 10 patients. After excluding one patient with bilateral cancer, five of nine (56%) elected lumpectomy, two of nine (22%) had unilateral therapeutic mastectomy (TM), and two of nine (22%) underwent therapeutic along with contralateral prophylactic mastectomy (TM + CPM). In the 52 patients with breast cancer surgery preceding VUS identification (after excluding 4 with bilateral cancer and 1 with stage IV disease), 24 (46%) had lumpectomy, 10 (19%) unilateral therapeutic mastectomy, 17 (33%) therapeutic mastectomy with CPM, and 1 (2%) lumpectomy converted to therapeutic mastectomy and CPM. Thus, rate of CPM at initial surgery did not differ significantly between those where the VUS was identified before surgery compared with those where VUS was identified after surgery (22 vs. 35%, respectively, p = 0.45) when excluding those who would not have been offered CPM (bilateral cancers, ovarian cancers, or stage IV disease). An additional three patients underwent delayed CPM after VUS identification: two underwent initial lumpectomy but required delayed mastectomy for margin control and had CPM at that time, and one who initially underwent unilateral therapeutic mastectomy opted for delayed CPM after VUS identification. Rates of prophylactic mastectomy were slightly higher for noncancer patients than among those diagnosed with cancer but exceeded 32% for both groups (Fig. 2).
FIG. 2.
Comparison of surgical choices of patients with and without cancer who were diagnosed with a BRCA1 or BRCA2 variant of uncertain significance
Breast cancer patients with a VUS had a median of two family members with breast cancer (range 0–9), and 45% had a mother or sister with breast cancer. Choice for CPM after VUS was not significantly associated with number of relatives with breast cancer (p = 0.62) or presence of first-degree family history (40 vs. 49%, p = 0.71).
Comparison of Rates of Prophylactic Mastectomy
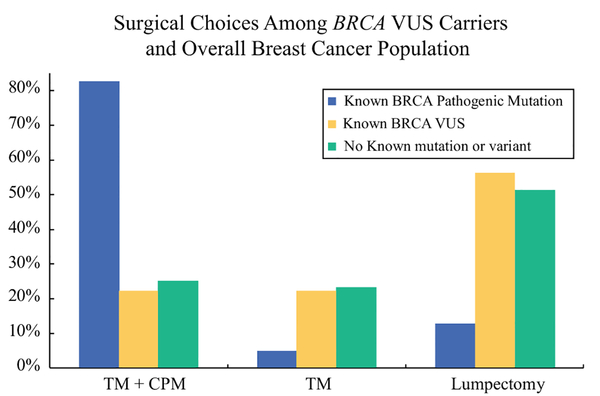
Focusing on the patients who had known diagnosis of BRCA VUS (n = 9) at the time of cancer surgery, we compared rates of prophylactic mastectomy in this group with the PM rates in known BRCA pathogenic mutation carriers, as well as the average risk breast cancer surgical population at Mayo Clinic Rochester. When evaluating BRCA known pathogenic mutation carriers with newly diagnosed unilateral stage 0–III breast cancer (n = 63), BRCA pathogenic mutation carriers had expectedly high rates of TM + CPM of (83%) compared with lumpectomy (13%) or TM alone (5%).4 In comparison, in the average risk breast cancer population at Mayo Clinic Rochester (excluding patients with BRCA VUS or BRCA deleterious mutation), 25% chose TM + CPM compared with 51% lumpectomy and 24% TM.5 Comparison of these two groups to our BRCA VUS carriers who had known VUS status before surgery demonstrates similar rates of prophylactic mastectomy between those with a VUS and those with no known pathogenic mutation (22 vs. 25%, respectively; Fig. 3).
FIG. 3.
Surgical choices of patients with breast cancer diagnosed with a BRCA1 or BRCA2 variant of uncertain significance compared with breast cancer patients at Mayo Clinic Rochester with known BRCA1 or BRCA2 deleterious mutations and breast cancer patients with no known mutation or variant4,5
Reclassification of Variant of Uncertain Significance
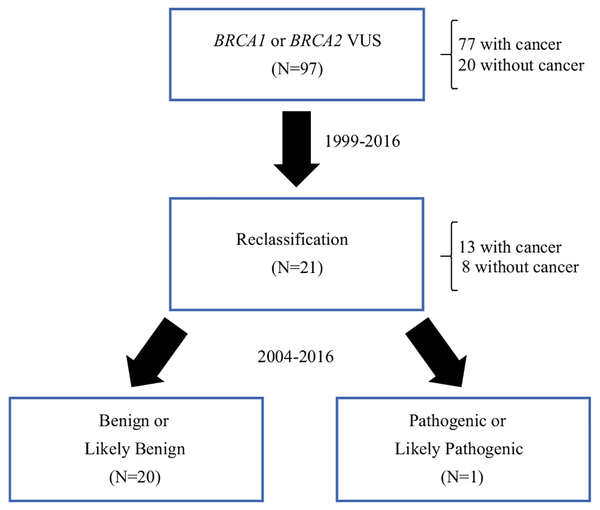
Of the 97 variants initially identified as a VUS, 20 (21%) have been subsequently reclassified to benign or likely benign and 1 (1%) to pathogenic or likely pathogenic as demonstrated in Fig. 4.
FIG. 4.
Illustration of reclassification of BRCA VUS
DISCUSSION
In our investigation of clinical decision-making in patients diagnosed with variant of uncertain significance in BRCA1 or BRCA2 genes, we found that VUS may influence surgical decision-making for women. For those diagnosed with breast cancer, it is reassuring that there was no difference in rate of CPM whether VUS was diagnosed before compared to after initial breast cancer operation, suggesting in this group VUS may not have been the only factor influencing surgical decision-making. In those without cancer, BPM rates were associated with first-degree family history and IBIS scores, so it is difficult to establish the degree of influence of VUS on decision-making compared with family history.
There are more than 1500 different known VUS results for BRCA1 and BRCA2 genes. The rate of VUS depends on the testing prevalence in the population as well as ancestry of patients. Over time, as more information has been gained, the rate of VUS in BRCA1 and BRCA2 has declined from 13% a decade ago to 2% today.6 However, incidence of identification of VUS in other breast-cancer related genes continues to increase (as high as 20–40%) with the increasing use of multigene panel testing.7
Many of these VUS will be reclassified over time, and we have demonstrated at the time of our review, 20% of the VUS in our patients were reclassified to benign and only 1% to pathogenic. Similarly, in another study where patients with VUS in BRCA1 or BRCA2 were followed from 1998 to 2009 with up to 9 years of follow-up, 45% were later reclassified with 81% as benign, only 6% as deleterious, and the remaining 6.3% suspected deleterious and 6.3% favor polymorphism. Of those who were followed for 8 or more years, only 22% still were classified as VUS.3 Our lower rates are likely secondary to both the shorter interval of follow-up in our study as well as the more recent time period where the starting prevalence of VUS was lower in 2004 than 1999. Nonetheless, both of these studies highlight the ongoing advances in understanding of genetic variants and the importance of appropriate counseling for patients about VUS result before making any surgical decisions.
Little is known about the impact of a VUS in BRCA gene on clinical decision-making. Limited prior studies have shown VUS patients have similar mastectomy rates to BRCA negative patients but experience less cancer distress reduction.8 Other work has shown that at follow-up one-third of VUS patients did not recall the significance of this result.9 In contrast, our work showed higher rates of contralateral prophylactic mastectomy in BRCA1 and BRCA2 VUS patients compared with BRCA-negative and untested patients (33 vs. 25%) but lower rates compared with those with BRCA pathogenic mutations (33 vs. 83%). However, when we investigated this further, we found by limiting our analysis of rates of CPM to those who had known BRCA VUS results before surgery, despite small sample size, the rate of CPM was only 22%, suggesting the majority of our BRCA VUS population that chose TM + CPM had already made this decision based on other factors (such as family history) before their genetic testing.
Concerningly, we also demonstrated very high rates of bilateral prophylactic mastectomy, exceeding 38% in patients with BRCA VUS and no personal cancer history who underwent genetic testing based on the presence of a significant family history of breast cancer.
Implications of a BRCA VUS diagnosis can include difficulty in counseling patients. BRCA VUS patients have been shown to have higher levels of anxiety and distress compared to those with definite benign or with pathogenic variants.3 Our study raises the question whether diagnosis of VUS, particularly in the noncancer patients as most of these had VUS diagnosis before surgery, might be contributing to the high rates of bilateral prophylactic mastectomy, which may potentially result from higher anxiety.
Even among physicians, results can be confusing, leading to discrepancies in patient understanding and counseling. Among breast cancer specialists in the United Kingdom (including surgeons, medical, and radiation oncologists) when interpreting VUS results, 71% of physicians felt uncomfortable interpreting the results, and 39% said they did not know how to counsel their patients, again highlighting the need for appropriate referral to genetic counseling.10
Among those VUS carriers without current or prior cancer, rates of prophylactic surgery were as high as 39%. This was associated with first-degree family history and high IBIS score. The majority of these patients made their surgical decision after report of the VUS result. While it is difficult to differentiate whether prophylactic surgery was chosen secondary to the VUS result, the family history, or a combination of both, it is concerning and warrants further investigation.
Interestingly, we also identified a small percentage of patients with cancer who underwent initial cancer operation prior to genetic testing followed by delayed CPM after their VUS diagnosis. In this group there was no association with first-degree family history or number of relatives with breast cancer. This raises the question of whether these patients might have chosen CPM solely due to the VUS and what their understanding of VUS may be.
There are some limitations to this study including the retrospective nature and the inability to establish the degree to which psychological factors such as anxiety or overestimation/fear of cancer risk influenced surgical decision-making. Additionally, there may be selection bias introduced in that the majority of patients with recorded genetic testing results at our institution have been evaluated in a specialized medical genetics clinic and have undergone comprehensive counseling by certified genetic counselors. In addition, those pursuing prophylactic mastectomy are further educated by both breast—specialized internists and breast surgeons. Therefore, we could potentially be underestimating the impact of lack of understanding, particularly if patients are seen in other settings with less thorough counseling where trained genetic counselors are not available. Nonetheless, despite extensive counseling, there are still a large number of patients choosing prophylactic procedures.
Overall, BRCA VUS carriers with cancer are electing similar choices to average risk breast cancer patients. However, VUS carriers without cancer have rates of BPM above 38% and appear to be influenced by both first-degree family history and Tyrer-Cuzick risk assessment. Comprehensive genetic counseling for these patients is essential. With the common misunderstanding and anxiety over VUS as well as the increasing use of multi-gene panels which may add to this problem, we must ensure appropriate understanding and advocate for access to genetic counseling to allow patients to make appropriately informed decisions regarding management of VUS and breast cancer risk.
ACKNOWLEDGEMENTS
This research was supported in part by an NCI breast cancer Specialized Program of Research Excellence (SPORE) award (P50 CA116201) to the Mayo Clinic, NIH Grant CA116167, and the Mayo Clinic Breast Cancer Registry.
Footnotes
DISCLOSURES
No commercial interest, financial, or material support to disclose.
REFERENCES
- 1.Nash R, Goodman M, Lin CC, Freedman RA, Dominici LS, Ward K, Jemal A. State variation in the receipt of a contralateral prophylactic mastectomy among women who received a diagnosis of invasive unilateral early-stage breast cancer in the United States, 2004–2012. JAMA Surg 2017. doi: 10.1001/jamasurg. 2017.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed 30 March 2017.
- 3.Murray ML, Cerrato F, Bennett RL, Jarvik GP. Follow-up of carriers of BRCA1 and BRCA2 variants of unknown significance: variant reclassification and surgical decisions. Genet Med. 2011;13(12):998–1005. [DOI] [PubMed] [Google Scholar]
- 4.Chiba A, Hoskin TL, Hallberg EJ, Cogswell JA, Heins CN, Couch FJ, Boughey JC. Impact that timing of genetic mutation diagnosis has on surgical decision making and outcome for BRCA1/BRCA2 mutation carriers with breast cancer. Ann Surg Oncol. 2016;23(10):3232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoskin TL, Hieken TJ, Degnim AC, Jakub JW, Jacobson SR, Boughey JC. Use of immediate breast reconstruction and choice for contralateral prophylactic mastectomy. Surgery. 2016;159(4):1199–209. [DOI] [PubMed] [Google Scholar]
- 6.Eggington JM, Bowles KR, Moyes K, et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin Genet. 2014; 86(3):229–37. [DOI] [PubMed] [Google Scholar]
- 7.Carter NJ, Hiraki S, Yackowski L, et al. Yield of pathogenic/likely pathogenic variants in breast cancer patients undergoing an inherited cancer panel based upon ethnic background. 2016; https://www.genedx.com/wp-content/uploads/2016/04/ACMG-2016-Ethnicity-and-IC-Panel-Testing-Results.pdf. Accessed 30 March 2017.
- 8.Culver JO, Brinkerhoff CD, Clague J, Yang K, Singh KE, Sand SR, Weitzel JN. Variants of uncertain significance in BRCA testing: evaluation of surgical decisions, risk perception, and cancer distress. Clin Genet. 2013;84(5):464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter S, Haroun I, Graham TC, Eisen A, Kiss A, Warner E. Variants of unknown significance in BRCA testing: impact on risk perception, worry, prevention and counseling. Ann Oncol 2013;24 Suppl 8:viii69–viii74. doi: 10.1093/annonc/mdt312. [DOI] [PubMed] [Google Scholar]
- 10.Eccles BK, Copson E, Maishman T, Abraham JE, Eccles DM. Understanding of BRCA VUS genetic results by breast cancer specialists. BMC Cancer. 2015;15:936. [DOI] [PMC free article] [PubMed] [Google Scholar]