Abstract
The ability to detect time intervals and temporal patterns is critical to some of the most fundamental computations the brain performs—including the ability to communicate and appraise a dynamically changing environment. Many of these computations take place on the scale of tens-to-hundreds of milliseconds. Electrophysiological evidence shows that some neurons respond selectively to duration, interval, rate, or order. Because the time constants of many time-varying neural and synaptic properties—including short-term synaptic plasticity (STP)—are also in the range of tens-to-hundreds of milliseconds, they are strong candidates to underlie the formation of temporally-selective neurons. Neurophysiological studies indicate that STP is indeed one of the mechanisms that contribute to temporal selectivity, and computational models demonstrate that neurons embedded in local microcircuits can exhibit temporal selectivity if their synapses undergo STP. Together, converging evidence suggests that some forms of temporal selectivity emerge from the dynamic changes in the balance of excitation and inhibition imposed by STP.
Interval Discrimination and Sensory Timing
Animals extract information from a continuous stream of sensory input. Much of this information is contained in the temporal structure of sensory events—or more generally, in the spatiotemporal patterns of activity of sensory afferents. Because of the importance of temporal information, animals have evolved mechanisms to tell time on scales spanning more than ten orders of magnitude [1], but it is on the scale of tens-to-hundreds of milliseconds that our ability to tell time and extract temporal information is at its most sophisticated. Within this range, we are not only able to identify simple temporal intervals but extract higher-order temporal patterns. Speech comprehension, for example, requires extraction of a hierarchy of temporal information: from the voice-onset time of syllables (which contributes to the /ba/ versus /pa/ distinction, for instance), to phrasal boundaries, to prosody [2, 3]. Indeed, speech can be recognized even when spectral information is impoverished but temporal structure is preserved [4, 5]. Humans can recognize speech even when spectral channels are collapsed, meaning that the temporal envelope provides a significant amount of information for speech recognition [4].
Importantly, even on the subsecond scale, timing is not a unitary problem, but encompasses a range of interrelated problems necessary for sensorimotor processing, learning, and cognition [6–8]. Here we focus on the problem of sensory timing—that is, how neural circuits detect and discriminate temporal patterns contained in external stimuli—as opposed to the problem of motor timing, which refers to the ability to actively generate and produce well-timed motor responses. We propose that sensory temporal selectivity is an intrinsic property of local neural circuits, which relies on time-varying synaptic and neuronal properties. We further highlight the role of short-term synaptic plasticity as one of the key mechanisms in the emergence of temporal selectivity.
Temporal selectivity across sensory modalities
Audition is one of the sensory modalities where the relevance of timing information is particularly prominent. Humans and other animals use acoustic signals for communication for many behaviors, including courtship, territoriality, and social affiliation [9, 10]. Acoustic communication relies not only on spectral signatures (e.g., pitch) but on temporal features such as interval, duration, rate, and overall temporal structure. For example, some insects, including cicadas and grasshoppers, use the temporal pattern of acoustical pulses for conspecific recognition [11, 12]. Female crickets exhibit phonotaxis, a behavior characterized by walking or flying toward singing males, and phonotaxis is strongest at pulse durations and intervals that are within the range of the male calling song parameters [13, 14].
Interval timing is relevant to other forms of social communication as well. Weakly electric mormyrid fish use the intervals between successive electric organ discharges to communicate [15]. They produce individual-specific signals called scallops, which consist of distinct temporal patterns of 8–12 electric pulses, and these patterns have been linked to different social behaviors [16]. Similarly, the duration and interval of acoustic pulses are used by some frog species to differentiate between conspecific and heterospecific calls [12]. Indeed, for mating calls, changing the interval between a single pair of pulses – in a call that consists of 10 pulses – significantly decreases the percentage of females showing attraction. In addition to interval duration, the total number of pulses is also important in this mode of communication: females prefer calls that contain ten versus five pulses [17]. Frogs are also able to discriminate between trills that differ in the temporal envelope of acoustic pulses shape [18]. And finally, echolocation in bats provides one of the best studied examples of the behavioral importance of detecting intervals on the scale of milliseconds to tens-of-milliseconds. Specifically, they use the interval between emitted acoustic pulses and the echo of these pulses—the so called pulse-echo delay—to calculate and determine the position of potential prey [19].
In addition to the interval, duration, and rate of acoustic elements, the vocalizations of many birds and mammals relies on more complex temporal features, such as FM sweeps, trills, chirps, and the structure of the overall temporal envelope. For example, the songs of songbirds, much like human speech, are characterized by their complex spectrotemporal structure, as well as the duration of, and interval between, song syllables [20]. Many forms of temporal processing rely on experience, highlighting the role of learning in sensory timing. Rodents, for example, can be trained to make temporal judgments as to whether intervals are short or long relative to each other [21, 22]. And humans are capable of robust temporal perceptual learning, which is generally reported to be interval-specific. For example, repeated interval discrimination of an auditory interval of 100 ms leads to improved discrimination around this interval, but not to shorter or longer intervals [23, 24].
The above examples establish that animals extract information from the temporal features of sensory events. Thus, there must be neural mechanisms in place that allow neurons to detect and represent specific temporal signatures of external stimuli. Indeed, as we will see next, neurons that respond selectively to features such as interval and duration—i.e., temporally-selective neurons—have been identified in many species.
Interval and Temporal Pattern Selectivity of Neurons
Neurons that are tuned to temporal features such as interval, duration, pulse rate, and temporal structure of vocalizations have been reported across areas spanning the sensory processing hierarchy [25–29] (Figure 1). Many of the studies of temporally-selective neurons have focused on species that rely on the temporal structure of stimuli for interspecies communication and vocalizations. For example, the temporal features that contribute to reproductive behavior of female crickets is mirrored in the response properties of neurons [14]. And neurons in the midbrain of weakly electric fish have been shown to be selective to the temporal patterns of electrical pulses [30–32]. For example, some neurons are tuned to pulse rate: spiking with low probability for pulse rates of 10 or 100 Hz, but spiking with high probability in response to each pulse at a rate of 20 Hz (Fig. 1A). Importantly, in vivo intracellular recordings showed that these neurons are also sensitive to the precise temporal structure of scallops which consists of a distinctive temporal pattern of 8–12 electric pulses. Subthreshold changes in membrane potential recorded from single neurons discriminated natural scallops from time-reversed, randomized, and jittered sequences [29].
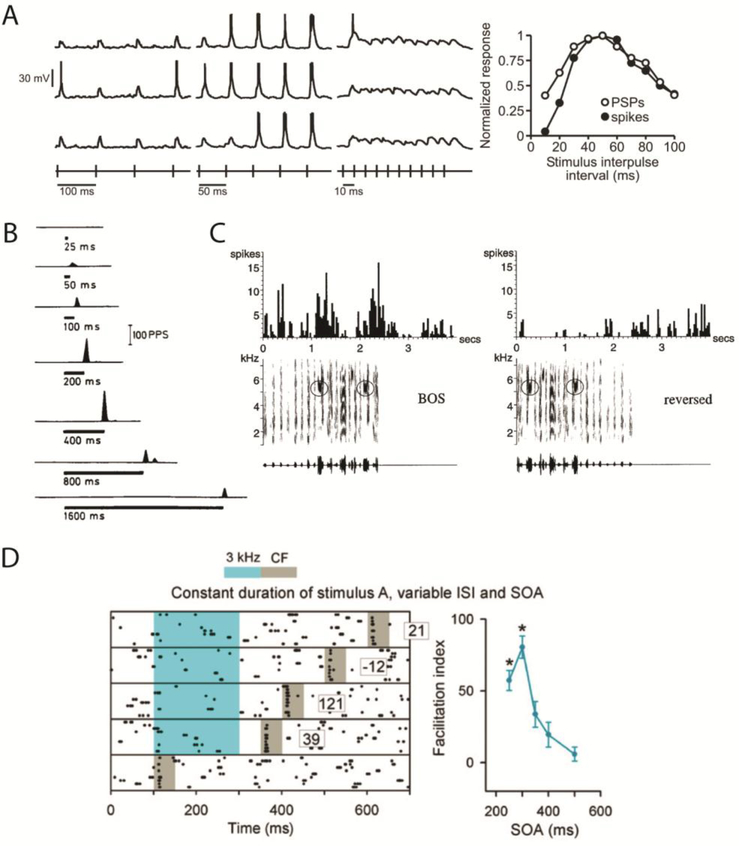
Figure 1. Temporal-selective neurons across species and modalities.
A. Voltage traces from a neuron in the midbrain of an electric fish. Each voltage trace represents the delivery of trains of electrical pulses presented at intervals of 100 (left), 50 (middle) and 10 ms (right). The rows represent three separate repetitions of each train. This neuron was tuned to pulses delivered at intervals of 50 ms (right). The right panel shows the tuning of the amplitude of the postsynaptic potentials (PSPs) and number of spikes. Reproduced from [32]. B. Duration tuned neuron in cat visual cortex. Off responses to a static bar of different durations, this neuron responded maximally to a duration of 400 ms. Of 174 neurons approximately 30% responded differentially to duration, and 3% showed sharp duration tuning curves. Reproduced from [45]. C. Auditory responses of a single unit in lateral portion of the magnocellular nucleus of the anterior neostriatum of an adult zebra finch. Response to the bird’s own song in forward (left) and reversed (right) order. Below each PSTH are shown the sonogram (frequency vs time plot, with energy in each frequency band indicated by the darkness of the signal) and the oscillogram (amplitude waveform) of the song stimulus used. BOS refers to “bird’s own song”. Adapted from [53]. D. Rastergram of a neuron from rat auditory cortex in response to five different stimuli, each composed of a 200 ms 3 kHz tone followed by a 50 ms 7 kHz (CF) tone with different stimulus-onset asynchrony. Numbers represent the facilitation index. Graph (right) shows the average interval tuning curve. Rats were trained to detect an inter-tone onset interval of 300 ms (middle row on left), and this was the spatiotemporal pattern that elicited the maximal response. Reproduced from [57].
Some of the most elegant examples of duration-tuned neurons come from studies in the brainstem of echolocating bats. Specifically, neurons in the inferior colliculus of bats are tuned to pulse duration [33–35]. Importantly, these duration-tuned neurons have been shown to match the range of the durations of echolocation signals [34, 36, 37]. More generally, duration-tuned neurons have been found in the central auditory systems of frogs [38–40], rodents [41, 42], chinchillas [43], and cats [44]. In addition, duration-sensitive neurons have been observed across different modalities. For example, neurons recorded from the cat visual cortex can be tuned to the duration of a stationary bar of light [45] (Fig 1B). The presence of duration-tuned neurons across species and sensory modalities suggests that duration selectivity is a general property of sensory systems.
Other examples of how the temporal structure of sensory stimuli shapes neuronal responses relate to the phenomenon of adaptation. Across sensory modalities, cortical neurons attenuate their responses to identical stimuli when they are repeated on short timescales [46–48]. For example, the vast majority of neurons in the auditory cortex exhibit stimulus-specific adaptation (SSA): neurons selectively reduce their responses to a tone repeated every 300 ms, but respond robustly to an “oddball” tone presented at a different frequency [49].
An important question pertaining to the temporally-tuned neuronal responses mentioned above is whether they reflect innate hardwired circuits, or rather – emerge in an experience-dependent manner as a result of learning and plasticity. It seems likely that in some animals temporal selectivity reflects, at least in part, hardwired circuits. But in other cases, it is clear that temporal neuronal selectivity emerges in an experience-dependent fashion (and as mentioned above, many animals can learn to discriminate intervals and durations). One of the clearest examples of experience-dependent acquisition of complex stimulus selectivity comes from songbirds. Like speech learning, song acquisition occurs early in a songbird’s life, and is critically dependent on auditory experience and feedback [50]. Neurons in multiple areas of adult male finches are strongly selective for both spectral and temporal properties of birdsong; they respond more robustly to the bird’s own song (BOS) than to songs of conspecific individuals, and they respond less well to the BOS if it is played in reverse [20, 51–53] (Fig. 1C).
Such experience-dependent emergence of temporally selective neurons has also been observed in mammals exposed to or trained on stimuli defined by interval, duration, or order of the underlying tones [54–57]. For example, in one study rats were trained on a go/no-go task with a target stimulus composed of a 3 kHz tone followed by a 7 kHz tone with an inter-onset interval of 300 ms [57]. Recordings in A1 revealed a substantial number of neurons that responded optimally at this interval, indicating that learning was accompanied by the formation of auditory neurons that were tuned to the spectrotemporal features of the target stimuli (Fig. 1D).
Tuning to spatial features is among the most widely studied aspects of sensory systems—ranging from selectivity to specific orientations of visual lines to selectivity to the frequency of tones (which we consider “spatial” because of the tonotopic organization of cochlea). The studies discussed earlier suggest that selectivity to temporal features—e.g. duration, interval, rate, and order of sensory events—is perhaps as prevalent among sensory neurons as spatial tuning.
Neural mechanisms of temporal selectivity
The breadth of examples across species and modalities suggests that neural selectivity to temporal features on the order of tens-to-hundreds of milliseconds reflects a general computation within sensory circuits. One hypothesis is that temporal tuning is an intrinsic property of local neural circuits that relies on time-varying synaptic and neuronal properties. Neurons and synapses possess an abundance of functional properties with time constants on the scale of tens-to-hundreds of milliseconds that have been proposed to contribute to sensory timing, including ionotropic and metabotropic receptors [58], ion channels [28, 59, 60], and most notably short-term synaptic plasticity (STP) [31, 61–65]. Below we focus on the contribution of STP to sensory timing, but emphasize that other neural properties have also been implicated perhaps most notably dynamic changes in the excitation/inhibition balance and rebound excitation [31, 66–68]
Short-term synaptic plasticity
STP refers to use-dependent changes in the strength of synaptic connections that take place on time scales of tens to hundreds of milliseconds [69]. At a synapse exhibiting STP, trains of presynaptic spikes that occur within a short timespan can cause progressively smaller or larger postsynaptic potentials (Figure 2). These two opposing forms of STP are referred to as short-term depression (or paired-pulse depression) and short-term facilitation (or paired-pulse facilitation) respectively. These two broad forms of STP, however, can interact to form more complex temporal profiles [70]. Short-term depression results primarily from exhaustion of readily-releasable vesicles in the presynaptic terminal. The mechanisms underlying short-term facilitation, although less precisely understood, involve in part an increase in probability of vesicle release due to residual presynaptic Ca2+ or the activation of specialized presynaptic Ca2+ sensors [69, 71].
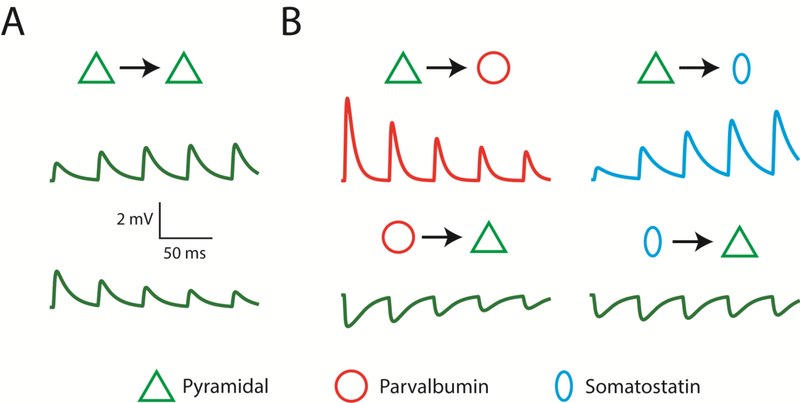
Figure 2. Diversity of short-term synaptic plasticity (STP) at cortical synapses.
Simulations of synaptic transmission with STP based on real whole cell recordings. Traces indicate voltage of the postsynaptic cell as the presynaptic cell fires a train of five action potentials at 20 Hz. Diagrams above each trace indicate the identity of pre- and postsynaptic neurons, including pyramidal (green triangle), Parvalbumin-positive inhibitory (red circle), and Somatostatin-positive inhibitory (cyan oval) cells. A. Facilitating (top) and depressing (bottom) inter-pyramidal synapses. Based on recordings performed for [79]. B. Excitatory-to-inhibitory (top row) and inhibitory-to-excitatory (bottom row) synapses. Based on recordings performed for [77].
STP is remarkably diverse across neurons [72–75], cortical layers [76], brain regions [77, 78], and can be modulated by development [79–82], sensory experience [82], brain state [83], and by neuromodulation [84]. Despite this richness and diversity, some general principles have emerged. For example, although STP is generally attributed to presynaptic mechanisms, the nature of STP of excitatory synapses onto inhibitory interneurons primarily depends on the postsynaptic inhibitory cell type [70, 73]. For example, EPSPs onto fast-spiking inhibitory parvalbumin-positive (PV) interneurons generally undergo depression, whereas EPSPs onto low-threshold-spiking somatostatin-positive (SOM) inhibitory interneurons generally exhibit facilitation (Figure 2B). Furthermore, this differential STP for excitatory-to-PV and excitatory-to-SOM synapses has been hypothesized to contribute to stimulus-specific adaptation [49].
The role of STP in temporal selectivity
Even though STP is observed across virtually all synapses, there is no consensus as to its computational function [85, 86]. STP has been hypothesized to enable dynamic gain control [87, 88] as well as sensory adaptation and sensitization [69, 77, 89, 90]. More generally, it is recognized that STP can implement temporal filters [61–63, 91, 92]—that is, STP transforms temporal patterns of presynaptic spikes into different postsynaptic patterns depending on the STP characteristics of the activated synapses.
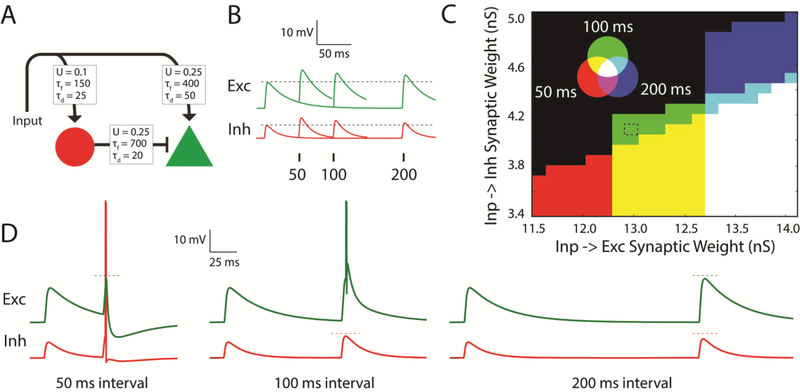
The ability to implement temporal filters at various timescales means that, at least theoretically, STP has the potential to underlie temporal selectivity in neurons [62]. For example, a simulation of a simple circuit composed of integrate-and-fire units demonstrates how STP can be used to generate interval selectivity (Fig. 3). In this simulation, an input unit forms facilitating synapses onto both an excitatory (Ex) and an inhibitory (Inh) unit that provides feedforward inhibition onto the excitatory unit (Fig. 3A). As the input unit generates spike pairs separated by intervals of 50, 100, or 200 ms in separate trials, the resulting EPSPs facilitate to different degrees (Fig. 3B). With appropriate tuning of synaptic weights this simple circuit can function as an interval detector with the excitatory unit playing the role of a readout neuron (Fig. 3C). For example, there is a range of weights of the Input→Ex and Input→Inh connections at which the excitatory units fires exclusively to the 100 ms interval (Fig. 3D). This selectivity emerges because, for the 200 ms interval, short-term facilitation at the Input→Ex synapse has decayed enough such that the Ex unit’s EPSP is subthreshold, yet, for the 50 ms interval, short-term facilitation at the Input→Inh synapse is strong enough to drive the inhibitory unit to spike, thus vetoing what would be a suprathreshold EPSP in the excitatory unit.
Figure 3. Interval selectivity simulated in a simple circuit with STP.
A. Circuit composed of a single inhibitory (red circle) and excitatory (green triangle) neuron. Each synapse is endowed with STP [70]. Parameters used to simulate STP are shown, including the baseline release probability (U) and the time constants of facilitation (τf) and depression (τd). B. Excitatory and inhibitory responses to separate input spike intervals of 50, 100, and 200 ms are overlaid, illustrating short-term facilitation. C. The weights of the Input→Ex synapse (x-axis) and Input →Inh synapse (y-axis) are varied, and the interval selectivity at each point is examined. The color in each region indicates the responsiveness of the Ex unit to one or more of the input intervals. Black areas represent regimes in which the Ex unit fired to the first pulse only, or did not fire at all. D. Voltage traces of the Ex and Inh units during simulations with synaptic weights that resulted in a 100 ms interval detector (dotted rectangle in C). Based on [62].
Over the past decade converging experimental evidence has provided support for hypotheses suggesting that STP contributes to temporal selectivity. For example, STP appears to underlie temporal selectivity in the anuran auditory system [93], in which two broad classes of temporally-selective neurons have been identified. One class consists of short-interval cells that respond best when presented with an optimal number of pulses presented at a fast or intermediate rate [94]. Short-interval cells respond to consecutive inputs with EPSPs followed by large, slow IPSPs. Selectivity appears to result from an enhancement of EPSPs elicited by repeated pulses—that is, a progressive enhancement in EPSP magnitude is eventually able to overcome the strong but stable inhibitory response to each pulse. Importantly, enhancement of excitation is optimal for certain pulse rates [95]. A second class of temporally-selective cells in anuran auditory systems responds well only to slow pulse rates but fails to respond to fast pulse rates. Electrophysiological experiments suggest that the low-pass properties of these neurons resulted from cancellation of temporally-offset excitatory and inhibitory synaptic inputs at fast pulse rates, together with short-term synaptic depression at high stimulation rates [96].
Additional experimental work regarding the mechanistic involvement of STP in pulse rate selectivity comes from whole-cell recordings of neurons in mormyrid electric fish [30–32, 64]. By estimating synaptic conductances during temporally-selective responses, Baker and colleagues determined that both excitatory and inhibitory conductances exhibited short-term depression. However, for high-pass neurons (neurons tuned to faster pulse rates), inhibitory conductances depressed more strongly than excitatory conductances, while for most low-pass neurons excitation depressed more strongly and more quickly [31]. In addition to differences in STP, high and lowpass neurons exhibited differences in the amplitude and duration of excitatory and inhibitory conductances. Analytically reconstructing cellular responses while excluding short-term depression led to drastically reduced diversity in interval tuning [31].
Network Models of Temporal Pattern Selectivity Based on STP
The theoretical and experimental evidence discussed above indicate that STP plays a role in temporal filtering and the formation of temporally selective neurons. Indeed, as shown in Figure 3, it is relatively straightforward to create interval selective neurons in disynaptic circuits that exhibit short-term facilitation. However, in this example, interval selectivity relies on the careful tuning of synaptic weights and STP. Far more general models of cortical computation referred to as state-dependent network models or liquid state machines [61, 62, 97, 98] propose that STP provides a rich mechanism to endow cortical networks with the ability to decode the spatiotemporal structure of stimuli. Specifically, STP functions as a memory of what happened within the past few hundred milliseconds. Consider the case of two identical tones arriving in the auditory cortex 100 ms apart during an interval discrimination task. Even if we assume the second tone activates the same pattern of thalamocortical inputs into the cortex as the first tone, it will arrive in a different cortical state, where some synapses will be depressed and others facilitated. Thus, the same tone should have a different net effect on the circuit, depending on the recent input history. While some neurons will be activated by both events, others are likely to be activated by one or the other, and these neurons can provide information about the length of the interval or the order of events.
In these models, STP (and other time-varying properties) provides a memory buffer that ensures that each event is encoded in the context of the previous events. Thus if two tones A and B are presented 100 ms apart, the response to B does not simply encode the stimulus B, but ‘B preceded by A’. This view predicts that it should be possible to decode previous stimuli based on the population response to the current stimulus. This prediction has been confirmed, by showing that in the visual cortex, when a pair of images is sequentially presented it is possible to determine the first image based on the response to the second [99]. Another prediction is that interval discrimination should be impaired by preceding stimuli, and indeed psychophysical experiments show that simply presenting two intervals to be judged close together in time impairs interval discrimination [100, 101]. While these results are consistent with the role of STP in establishing the state-dependence of the local network (the memory buffer), it remains to be determined whether STP is indeed one of the mechanisms underlying these results. Some support to this possibility comes from computer simulations, which have established that randomly connected recurrent neural networks endowed with STP are intrinsically capable of discriminating simple intervals [61, 62, 97, 100, 102]. Furthermore, the presence of STP in such networks enhances their ability to discriminate complex temporal patterns such as speech [62, 103, 104].
Concluding Remarks and Future Perspectives
Sensory neurons can be selective to temporal features such as interval, duration, and overall spatiotemporal structure. However, in contrast to the neural mechanisms underlying spatial selectivity, relatively little is known about how neurons in the sensory hierarchy respond selectively to the temporal features of stimuli. The experimental and theoretical data reviewed here supports the notion that sensory timing relies on the intrinsic dynamics of time-varying synaptic and neural properties. Among these properties, we propose that STP plays a fundamental role in implementing temporal filters and the generation of temporally-selective neurons. While some experimental evidence provides direct support for this hypothesis, a causal relationship between STP and sensory timing remains to be established. This, however, is a challenging endeavor because STP is a universal property of synapses and difficult to manipulate without altering baseline synaptic transmission. Nevertheless, STP can be altered through pharmacological means. Interestingly, recent studies show that Synaptotagmin 7 knockout animals do not exhibit short-term facilitation [71], opening up the possibility of employing genetic manipulations in examining the relationship between STP and temporal selectivity. This will lead, no doubt, to novel and exciting lines of research aimed at elucidating the neural mechanisms underlying sensory timing. Future studies will rely in part at establishing a causal relationship between time-varying neural properties such as STP and simple sensory timing tasks
Outstanding Questions.
Do “hardwired” temporally-selective neurons rely on the same neural mechanisms as those that emerge in an experience-dependent manner?
How does diversity of short-term plasticity relate to diversity in coding of temporal features?
How is short-term plasticity regulated by development and sensory experience?
Is short-term plasticity causally related to neuronal temporal selectivity?
Highlights:
Animals have evolved mechanisms to track time and extract temporal information on the scale of tens-to-hundreds of milliseconds. It is within this range that animals and humans are not only able to identify simple temporal intervals but extract higher-order temporal patterns.
Across species and modalities, researchers have identified neurons that selectively respond to temporal features including interval, duration, rate, and complex temporal structure.
We propose that temporal selectivity is an intrinsic property of local neural circuits that relies on time-varying synaptic and neuronal properties, most notably short-term synaptic plasticity.
Computational models establish that temporally selective neurons can emerge from neural microcircuits that incorporate short-term synaptic plasticity.
Acknowledgements
D.V.B would like to acknowledge the funding support of the NIH (MH60163, NS100050).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buonomano DV (2007) The biology of time across different scales. Nat Chem Biol. 3, 594–7. [DOI] [PubMed] [Google Scholar]
- 2.Tallal P, ed. In the perception of speech time is of the essence Temporal Coding in the Brain, ed. Buzsaki G, et al. , Springer-Verlag: Berlin: 291–299. [Google Scholar]
- 3.Aasland WA and Baum SR (2003) Temporal parameters as cues to phrasal boundaries: A comparison of processing by left- and right-hemisphere brain-damaged individuals. Brain and Language. 87, 385–399. [DOI] [PubMed] [Google Scholar]
- 4.Shannon RV, et al. (1995) Speech recognition with primarily temporal cues. Science. 270, 303–4. [DOI] [PubMed] [Google Scholar]
- 5.Drullman R (1995) Temporal envelope and fine structure cues for speech intelligibility. J Acoust Soc Am. 97, 585–92. [DOI] [PubMed] [Google Scholar]
- 6.Meck WH and Ivry RB (2016) Editorial overview: Time in perception and action. Current Opinion in Behavioral Sciences. 8, vi–x. [Google Scholar]
- 7.Buzsaki G and Llinas R (2017) Space and time in the brain. Science. 358, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauk MD and Buonomano DV (2004) The Neural Basis of Temporal Processing. Ann. Rev. Neurosci 27, 307–340. [DOI] [PubMed] [Google Scholar]
- 9.Hebets EA and Papaj DR (2005) Complex signal function: Developing a framework of testable hypotheses. Behavioral Ecology and Sociobiology. 57, 197–214. [Google Scholar]
- 10.Bradbury JW and Vehrencamp SL (1998) Animal communication. Sinauer Assoc., Inc., Sunerland, Mass. [Google Scholar]
- 11.Pollack G (2000) Who, what, where? Recognition and localization of acoustic signals by insects. Curr Opin Neurobiol. 10, 763–7. [DOI] [PubMed] [Google Scholar]
- 12.Gerhardt HC and Huber F (2002) Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. 2002. [Google Scholar]
- 13.Thorson J, Weber T, and Huber F (1982) Auditory Behavior of the Cricket - II. Simplicity of Calling-Song Recognition in Gryllus, and Anomalous Phonotaxis at Abnormal Carrier Frequencies. Journal of Comparative Physiology A. 146, 361–378. [Google Scholar]
- 14.Kostarakos K and Hedwig B (2012) Calling Song Recognition in Female Crickets: Temporal Tuning of Identified Brain Neurons Matches Behavior. The Journal of Neuroscience. 32, 9601–9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson BA (2002) Electric signaling behavior and the mechanisms of electric organ discharge production in mormyrid fish. J Physiol Paris. 96, 405–19. [DOI] [PubMed] [Google Scholar]
- 16.Carlson BA and Hopkins CD (2004) Central control of electric signaling behavior in the mormyrid Brienomyrus brachyistius: segregation of behavior-specific inputs and the role of modifiable recurrent inhibition. J Exp Biol. 207, 1073–84. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz JJ, et al. (2010) Effect of anomalous pulse timing on call discrimination by females of the gray treefrog (Hyla versicolor): behavioral correlates of neurobiology. J Exp Biol. 213, 2066–72. [DOI] [PubMed] [Google Scholar]
- 18.Gerhardt HC and Doherty JA (1988) Acoustic Communication in the Gray Treefrog, Hyla-Versicolor - Evolutionary and Neurobiological Implications. Journal of Comparative Physiology a-Sensory Neural and Behavioral Physiology. 162, 261–278. [Google Scholar]
- 19.Covey E and Casseday JH (1999) Timing in the auditory system of the bat. Annu. Rev. Physiol 61, 457–76. [DOI] [PubMed] [Google Scholar]
- 20.Doupe AJ and Kuhl PK (1999) Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 22, 567–631. [DOI] [PubMed] [Google Scholar]
- 21.Gouvea TS, et al. (2015) Striatal dynamics explain duration judgments. Elife. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pai SS, et al. (2011) Minimal impairment in a rat model of duration discrimination following excitotoxic lesions of primary auditory and prefrontal cortices. Frontiers in Systems Neuroscience. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright BA, et al. (1997) Learning and generalization of auditory temporalinterval discrimination in humans. J. Neurosci 17, 3956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bueti D and Buonomano DV (2014) Temporal Perceptual Learning. Timing and Time Perception. 2, 261–289. [Google Scholar]
- 25.Sakai M, et al. (2009) Neural mechanisms of interstimulus interval-dependent responses in the primary auditory cortex of awake cats. BMC Neurosci. 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel A and Buonomano DV (2014) Timing as an intrinsic property of neural networks: evidence from in vivo and in vitro experiments. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 369, 20120460–20120460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose G and Capranica RR (1983) Temporal selectivity in the central auditory system of the leopard frog. Science. 219, 1087–9. [DOI] [PubMed] [Google Scholar]
- 28.Fortune ES and Rose GJ (1997) Passive and active membrane properties contribute to the temporal filtering properties of midbrain neurons in vivo. J Neurosci. 17, 3815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pluta SR and Kawasaki M (2010) Temporal selectivity in midbrain electrosensory neurons identified by modal variation in active sensing. J Neurophysiol. 104, 498–507. [DOI] [PubMed] [Google Scholar]
- 30.Baker CA, et al. (2016) Behavioral and Single-Neuron Sensitivity to Millisecond Variations in Temporally Patterned Communication Signals. The Journal of Neuroscience. 36, 8985–9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker CA and Carlson BA (2014) Short-Term Depression, Temporal Summation, and Onset Inhibition Shape Interval Tuning in Midbrain Neurons. The Journal of Neuroscience. 34, 14272–14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlson BA (2009) Temporal-Pattern Recognition by Single Neurons in a Sensory Pathway Devoted to Social Communication Behavior. J. Neurosci 29, 9417–9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casseday JH, Ehrlich D, and Covey E (1994) Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science. 264, 847–50. [DOI] [PubMed] [Google Scholar]
- 34.Ehrlich D, Casseday JH, and Covey E (1997) Neural tuning to sound duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol. 77, 2360–72. [DOI] [PubMed] [Google Scholar]
- 35.Fuzessery ZM and Hall JC (1999) Sound duration selectivity in the pallid bat inferior colliculus. Hear Res. 137, 137–54. [DOI] [PubMed] [Google Scholar]
- 36.Faure PA, et al. (2003) Temporal masking reveals properties of sound-evoked inhibition in duration-tuned neurons of the inferior colliculus. J Neurosci. 23, 3052–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinheiro AD, Wu M, and Jen PH (1991) Encoding repetition rate and duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Comp Physiol A. 169, 69–85. [DOI] [PubMed] [Google Scholar]
- 38.Narins PM and Capranica RR (1980) Neural adaptations for processing the two-note call of the Puerto Rican treefrog, Eleutherodactylus coqui. Brain Behav Evol. 17, 48–66. [DOI] [PubMed] [Google Scholar]
- 39.Gooler DM and Feng AS (1992) Temporal coding in the frog auditory midbrain: the influence of duration and rise-fall time on the processing of complex amplitude-modulated stimuli. J Neurophysiol. 67, 1–22. [DOI] [PubMed] [Google Scholar]
- 40.Potter HD (1965) Patterns of Acoustically Evoked Discharges of Neurons in Mesencephalon of Bullfrog. Journal of Neurophysiology. 28, 1155-&. [DOI] [PubMed] [Google Scholar]
- 41.Brand A, Urban R, and Grothe B (2000) Duration Tuning in the Mouse Auditory Midbrain. Journal of Neurophysiology. 84, 1790–1799. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Gonzalez D, et al. (2006) Duration selective neurons in the inferior colliculus of the rat: topographic distribution and relation of duration sensitivity to other response properties. J Neurophysiol. 95, 823–36. [DOI] [PubMed] [Google Scholar]
- 43.Chen GD (1998) Effects of stimulus duration on responses of neurons in the chinchilla inferior colliculus. Hear Res. 122, 142–50. [DOI] [PubMed] [Google Scholar]
- 44.He J, et al. (1997) Temporal integration and duration tuning in the dorsal zone of cat auditory cortex. J Neurosci. 17, 2615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duysens J, Schaafsma SJ, and Orban GA (1996) Cortical Off Response Tuning for Stimulus Duration. Vision Res. 36, 3243–3251. [DOI] [PubMed] [Google Scholar]
- 46.Das A and Gilbert CD (1999) Topography of contextual modulations mediated by short-range interactions in primary visual cortex. Nature. 399, 655–61. [DOI] [PubMed] [Google Scholar]
- 47.Asari H and Zador AM (2009) Long-lasting context dependence constrains neural encoding models in rodent auditory cortex. J Neurophysiol. 102, 2638–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen-Kashi Malina K, et al. (2013) Imbalance between Excitation and Inhibition in the Somatosensory Cortex Produces Postadaptation Facilitation. The Journal of Neuroscience. 33, 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Natan RG, et al. (2015) Complementary control of sensory adaptation by two types of cortical interneurons. eLife. 4, e09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konishi M (1965) Effects of deafening on song development in American robins and black-headed grosbeaks. Z Tierpsychol. 22, 584–99. [PubMed] [Google Scholar]
- 51.Margoliash D and Fortune ES (1992) Temporal and harmonic combinationsensitive neurons in the Zebra Finch’s HVc. J. Neursoci. 12, 4309–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boari S and Amador A (2018) Neural coding of sound envelope structure in songbirds. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 204, 285–294. [DOI] [PubMed] [Google Scholar]
- 53.Doupe AJ (1997) Song- and order-selective neurons in the songbird anterior forebrain and their emergence during vocal development. J. Neurosci. 17, 1147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kilgard MP and Merzenich MM (2002) Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci U S A. 99, 3205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kilgard MP and Merzenich MM (1998) Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1, 727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yin P, et al. (2008) Early stages of melody processing: stimulus-sequence and task-dependent neuronal activity in monkey auditory cortical fields A1 and R. J Neurophysiol. 100, 3009–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou X, et al. (2010) Successive-signal biasing for a learned sound sequence. Proceedings of the National Academy of Sciences. 107, 14839–14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fiala JC, Grossberg S, and Bullock D (1996) Metabotropic glutamate receptor activation in cerebellar Purkinje Cells as substrate for adaptive timing of the classically conditioned eye-blink response. J Neurosci. 16, 3760–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hooper SL, Buchman E, and Hobbs KH (2002) A computational role for slow conductances: single-neuron models that measure duration. Nat. Neurosci 5, 551–556. [DOI] [PubMed] [Google Scholar]
- 60.Kohashi T and Carlson BA (2014) A fast BK-type K(Ca) current acts as a postsynaptic modulator of temporal selectivity for communication signals. Frontiers in Cellular Neuroscience. 8, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buonomano DV and Merzenich MM (1995) Temporal information transformed into a spatial code by a neural network with realistic properties. Science. 267, 1028–30. [DOI] [PubMed] [Google Scholar]
- 62.Buonomano DV (2000) Decoding temporal information: a model based on short-term synaptic plasticity. J Neurosci. 20, 1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fortune ES and Rose GJ (2001) Short-term synaptic plasticity as a temporal filter. Trends Neurosci. 24, 381–5. [DOI] [PubMed] [Google Scholar]
- 64.George AA, et al. (2011) A Diversity of Synaptic Filters Are Created by Temporal Summation of Excitation and Inhibition. Journal of Neuroscience. 31, 14721–14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rose G, Leary C, and Edwards C (2011) Interval-counting neurons in the anuran auditory midbrain: factors underlying diversity of interval tuning. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 197, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alluri RK, et al. (2016) Phasic, suprathreshold excitation and sustained inhibition underlie neuronal selectivity for short-duration sounds. Proceedings of the National Academy of Sciences. 113, E1927–E1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aubie B, Becker S, and Faure PA (2009) Computational Models of Millisecond Level Duration Tuning in Neural Circuits. J. Neurosci 29, 9255–9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Large EW and Crawford JD (2002) Auditory Temporal Computation: Interval Selectivity Based on Post-Inhibitory Rebound. Journal of Computational Neuroscience. 13, 125–142. [DOI] [PubMed] [Google Scholar]
- 69.Zucker RS and Regehr WG (2002) Short-Term Synaptic Plasticity. Annual Review of Physiology. 64, 355–405. [DOI] [PubMed] [Google Scholar]
- 70.Markram H, Wang Y, and Tsodyks M (1998) Differential signaling via the same axon of neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA 95, 5323–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackman SL, et al. (2016) The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature. 529, 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beierlein M (2003) Two Dynamically Distinct Inhibitory Networks in Layer 4 of the Neocortex. Journal of Neurophysiology. 90, 2987–3000. [DOI] [PubMed] [Google Scholar]
- 73.Reyes A, et al. (1998) Target-cell-specific facilitation and depression in neocortical circuits. Nature neuroscience. 1, 279–285. [DOI] [PubMed] [Google Scholar]
- 74.Gupta A, Wang Y, and Markram H (2000) Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 287, 273–8. [DOI] [PubMed] [Google Scholar]
- 75.Ma Y, Hu H, and Agmon A (2012) Short-Term Plasticity of Unitary Inhibitoryto-Inhibitory Synapses Depends on the Presynaptic Interneuron Subtype. Journal of Neuroscience. 32, 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Viaene AN, Petrof I, and Sherman SM (2011) Synaptic Properties of Thalamic Input to the Subgranular Layers of Primary Somatosensory and Auditory Cortices in the Mouse. Journal of Neuroscience. 31, 12738–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reyes AD (2011) Synaptic short-term plasticity in auditory cortical circuits. Hearing Research. 279, 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atzori M, et al. (2001) Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nature Neuroscience. 4, 1230–1237. [DOI] [PubMed] [Google Scholar]
- 79.Reyes a. and Sakmann B (1999) Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 19, 3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar SS and Huguenard JR (2001) Properties of excitatory synaptic connections mediated by the corpus callosum in the developing rat neocortex. Journal of Neurophysiology. 86, 2973–2985. [DOI] [PubMed] [Google Scholar]
- 81.Oswald AMM and Reyes AD (2008) Maturation of Intrinsic and Synaptic Properties of Layer 2/3 Pyramidal Neurons in Mouse Auditory Cortex. Journal of Neurophysiology. 99, 2998–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finnerty GT, Roberts LS, and Connors BW (1999) Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 400, 367–71. [DOI] [PubMed] [Google Scholar]
- 83.Diaz-Quesada M, et al. (2014) Diverse Thalamocortical Short-Term Plasticity Elicited by Ongoing Stimulation. Journal of Neuroscience. 34, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsieh CY, Cruikshank SJ, and Metherate R (2000) Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Research. 880, 51–64. [DOI] [PubMed] [Google Scholar]
- 85.Abbott LF and Regehr WG (2004) Synaptic computation. Nature. 431, 796–803. [DOI] [PubMed] [Google Scholar]
- 86.Anwar H, et al. (2017) Functional roles of short-term synaptic plasticity with an emphasis on inhibition. Current Opinion in Neurobiology. 43, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abbott LF, et al. (1997) Synaptic depression and cortical gain control. Science (New York, N.Y.). 275, 220–224. [DOI] [PubMed] [Google Scholar]
- 88.Cook D, et al. (2003) Synaptic depression in the localization of sound. Nature. 421, 66–70. [DOI] [PubMed] [Google Scholar]
- 89.Ulanovsky N (2004) Multiple Time Scales of Adaptation in Auditory Cortex Neurons. Journal of Neuroscience. 24, 10440–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kastner DB and Baccus SA (2011) Coordinated dynamic encoding in the retina using opposing forms of plasticity. Nat Neurosci. 14, 1317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rotman Z, Deng P-Y, and Klyachko VA (2011) Short-Term Plasticity Optimizes Synaptic Information Transmission. The Journal of Neuroscience. 31, 14800–14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenbaum R, Rubin J, and Doiron B (2012) Short term synaptic depression imposes a frequency dependent filter on synaptic information transfer. PLoS Computational Biology. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rose GJ (2014) Time computations in anuran auditory systems. Front Physiol. 5, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Edwards CJ, Alder TB, and Rose GJ (2002) Auditory midbrain neurons that count. Nat Neurosci. 5, 934–936. [DOI] [PubMed] [Google Scholar]
- 95.Edwards CJ, Leary CJ, and Rose GJ (2007) Counting on inhibition and ratedependent excitation in the auditory system. J Neurosci. 27, 13384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edwards CJ, Leary CJ, and Rose GJ (2008) Mechanisms of long-interval selectivity in midbrain auditory neurons: roles of excitation, inhibition, and plasticity. J Neurophysiol. 100, 3407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maass W, Natschläger T, and Markram H (2002) Real-time computing without stable states: a new framework for neural computation based on perturbations. Neural Comput. 14, 2531–2560. [DOI] [PubMed] [Google Scholar]
- 98.Buonomano DV and Maass W (2009) State-dependent Computations: Spatiotemporal Processing in Cortical Networks. Nat Rev Neurosci. 10, 113–125. [DOI] [PubMed] [Google Scholar]
- 99.Nikolić D, et al. (2009) Distributed Fading Memory for Stimulus Properties in the Primary Visual Cortex. PLoS Biol. 7, e1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karmarkar UR and Buonomano DV (2007) Timing in the absence of clocks: encoding time in neural network states. Neuron. 53, 427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spencer RM, Karmarkar U, and Ivry RB (2009) Evaluating dedicated and intrinsic models of temporal encoding by varying context. Philos Trans R Soc Lond B Biol Sci. 364, 1853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perez O and Merchant H (2018) The synaptic properties of cells define the hallmarks of interval timing in a recurrent neural network. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carvalho TP and Buonomano DV (2011) A novel learning rule for long-term plasticity of short-term synaptic plasticity enhances temporal processing. Front Integr Neurosci. 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee TP and Buonomano DV (2012) Unsupervised formation of vocalization-sensitive neurons: a cortical model based on short-term and homeostatic plasticity. Neural Comput. 24, 2579–603. [DOI] [PubMed] [Google Scholar]