Abstract
Cytochromes P450s (CYPs) constitute a superfamily of enzymes that catalyze the metabolism of drugs and other substances. Endogenous substrates of CYPs include eicosanoids, estradiol, arachidonic acids, cholesterol, vitamin D and neurotransmitters. Exogenous substrates of CYPs include the polycyclic aromatic hydrocarbons and about 80% of currently used drugs. Some isoforms can activate procarcinogens to ultimate carcinogens. Genetic polymorphisms of CYPs may affect the enzyme catalytic activity and have been reported among different populations to be associated with various diseases and adverse drug reactions. With regard of drug metabolism, phenotypes for CYP polymorphism range from ultrarapid to poor metabolizers. In this review, we discuss some of the most clinically important CYPs isoforms (CYP2D6, CYP2A6, CYP2C19, CYP2C9, CYP1B1 and CYP1A2) with respect to gene polymorphisms and drug metabolism. Moreover, we review the role of CYPs in renal, lung, breast and prostate cancers and also discuss their significance for atherosclerosis and type 2 diabetes mellitus.
Keywords: CytochromeP450, polymorphism, T2D, Atherosclerosis, drug metabolism
Introduction
The cytochrome P450 (CYP) is a large superfamily of integral membrane conserved proteins present in animals, plants, and microorganisms (Nebert and Russell, 2002). The CYP isoenzyme superfamily comprises 57 CYP genes and 58 pseudogenes arranged into 18 families and 43 subfamilies in man (Nelson et al., 2004). They are heme-containing proteins that catalyze the oxidative metabolism of many structurally diverse drugs and chemicals (Woo et al., 2002). The reduced cytochrome P450 isoenzymes when bound to CO has a Soret peak at 450 nm (Luthra et al., 2011). This peak is not usual for a hem containing protein molecule. Hence, they are called P450 (Luthra et al., 2011). In mammals, the CYPs system is expressed in all tissues examined (Porter and Coon, 1991). They are expressed predominately in the endoplasmic reticulum membrane as well as in other cellular compartments such as the cell surface and in mitochondria (Neve and Ingelman-Sundberg, 2010). The cytochrome P450 superfamily is located primarily in liver, small intestine and kidney (Thelen and Dressman, 2009; Renaud et al., 2011). CYPs P450 enzymes catalyze different oxidation and some reduction reactions (Guengerich, 2007). Examples of the substrates (Porter and Coon, 1991; Chang and Kam, 1999) of CYPs include exogenous (xenobiotics) and endogenous compounds such as cholesterol, testosterone, progesterone, prostagalandin H2, corticosterone, retinoic acid, vitamin D3 and arachidonic acid (Guengerich, 2017).
Nomenclature and Mechanism of Action of Cytochrome P450
CYPs are grouped into families and subfamilies according to the similarity of amino acid sequence. The enzyme code is started with the CYP followed by a designating number for the family (Petersson, 2009; Sim and Ingelman-Sundberg, 2010; Sim and Ingelman-Sundberg, 2013). Then, a letter for the subfamily and ending with an individual number for the gene (Petersson, 2009). If the CYPs enzymes share more than 40 % identity in amino acid sequence they will be group in the same family, for example (CYP2). If the identity is more than 55%, the CYP enzymes belong to the same subfamily for example CYP2A. Then the CYP enzyme will be given an individual number, for example CYP2A6. An up to date list of CYP is found on a CYP 450 homepage (https://www.pharmvar.org/gene/index_original) (Sim and Ingelman-Sundberg, 2010; Sim and Ingelman-Sundberg, 2013).
All CYP enzymes share a conserved general three dimensional structure that resembles a shape of an inverted triangle (Petersson, 2009). The CYP enzyme consists of a protein moiety and a heme (iron protoporphyrin IX) as prosthetic group of the enzyme (Ortiz de Montellano, 2010). They are involved in the metabolism of the lipophilic endogenous and xenobiotic compounds transform them to hydrophilic or polar compounds such that they can be easily excreted from the body (Chang and Kam, 1999). The biotransformation reactions are divided into two phases. The CYPs catalyze the phase 1 reactions (Sim and Ingelman-Sundberg, 2013), which are oxidation or demethylation (Iyanagi, 2007). The substrate binds the catalytic pocket of the CYP enzyme that contains heme iron. The heme iron is then reduced from the ferric to the ferrous state by an electron transferred from a reduced NADPH (Chang and Kam, 1999). The molecular oxygen then binds temporarily at heme containing active site (Zanger and Schwab, 2013). Thereafter, one oxygen atom is inserted in the substrate molecule and the other atom is reduced to form H2O. The reaction for the monooxygenation can be represented in the formula: RH + NAD (P) H + O2 + H+ -> ROH + NAD (P) + H2O. Where, RH is the substrate containing a hydroxylatable site.
CYP enzymes therefore belong to the enzymes class of monooxygenase that only incorporates one atom of the molecular oxygen into their substrates (Ortiz de Montellano, 2010). The major functions of CYPs are the metabolism of the foreign compounds. For instance, they catalyze the hydroxylation of the drugs barbiturate and the phenobarbital (Zanger and Schwab, 2013) to increase their hydrophilicity such that they can be eliminated via the kidney. Other examples of drugs that are metabolized by the oxidation catalyzed by CYPs are the ibuprofen and the caffeine (Zanger and Schwab, 2013). Therefore, duration of action of many drugs depends on their rate of metabolism by the CYPs enzymes (Fernandez et al., 2011). The polycyclic aromatic hydrocarbons (PAHs)° are wide spreading environmental procarcinogens that inducetumorigenesis when they are activated by CYPs 450 and other enzymes such as epoxide hydrolase, and aldoketoreductase (Shimada et al., 2008; Moorthy et al., 2015). The PAHs activation results in the formation of the reactive and redox-active o-quinones, diolepoxides, or radical cations (Moorthy et al., 2015). All of these compounds react with the DNA and forming DNA adducts. The DNA adducts eventually lead to the mutation and initiation of the carcinogenesis (Moorthy et al., 2015). For instance, it has been reported that CYP1B1 that is found in pulmonary and other tissues (except for liver) has a pivotal role in the metabolic activation of the PAHs (Shimada and Fujii-Kuriyama, 2004; Chun and Kim, 2016). The PAHs that are metabolically activated by the CYP1B1 and CYP1A1 are the Benzo[a]pyrene, dibenzo[a]pyrene, 7,12-dimethylbenz[a]anthracene, 5-methylchrysene (Shimada et al., 2001).
Role of CytochromeP450 in Cancers
A- Renal cancer
The cytochrome P450 (CYP3A) forms was shown to be expressed consistently in kidney cancer cells using the immunohistochemistry, western blot analysis, and reverse transcriptase PCR (Murray et al., 1999). This study has suggested that the expressed CYP3A may be involved in renal cancer development (Murray et al., 1999), and that these forms of CYP3A are the cause of the multidrug resistance observed in this cancer. Moreover, they proposed that the presence CYP3A forms in the renal cells is of benefit for the treatment of renal cancer. For instance, the agent AQ4N, an alkylaminoanthroquinone is bioactivated by CYP3A forms to a highly cytotoxic metabolite in the hypoxic conditions of the tumor cells, but the AQ4N would not be cytotoxic for the normal cells where the conditions are normoxic (Murray et al., 1999). The cytochrome CYP1B1 was also shown to be present in renal cell carcinoma (McFadyen et al., 2004). It is also expressed in wide variety of cancers and not detected in normal cells (Murray et al., 1997; McFadyen et al., 2004). It has been proposed that since CYP1B1 is the metabolizing enzyme for the anticancer drugs (e.g. cyclophosphamide, paclitaxel, doxorubicin, docetaxel, cisplatin, 5-fluorouracil) its inhibition may be a good strategy for cancer therapy (McFadyen et al., 2001). Recently, it has been suggested CYP1B1 is significantly unregulated in renal cell carcinoma, and that it promotes this cancer progression (Mitsui et al., 2015). This study has demonstrated that in renal cell carcinoma cell line attenuating the expression of the CYP1B1 gene results in inhibition of the cancer cell viability, migration, and invasiveness (Mitsui et al., 2015). Moreover, Mitsui et al., (2015) have reported that the expression of the CYP1B1 is positively correlates with the expression of the cell division cycle 20 homolog (CDC20). The CDC20 is an essential factor that drives mitotic phase through activation of anaphase-promoting complex /cyclosome (APC/C) that triggers the transition from metaphase to anaphase (Izawa and Pines, 2011). In addition, Mitsui et al (2015) have suggested that the CYP1B1 promotes renal cell cancer through down regulation of the death-associated protein kinase-1 (DAPK1). DAPK1 is a tumor suppressor gene and its expression is down regulated in many types of carcinoma (Chuang et al., 2008).
B- Breast Cancer (BC)
It has been reported that CYP2E1 contribute to the generation of the reactive oxygen species (ROS) in breast cancer cells (Leung et al., 2013). In addition, CYP2E1 regulates autophagy, stimulate stress of endoplasmic reticulum and suppress metastatic potential of the BC cells (Leung et al., 2013). The expression of the CYP2E1 was shown to increase significantly in BC cells as well as the tissues that are adjacent to the tumor (Vaclavikova et al., 2007). The overexpression of the CYP2E1 enzyme might be beneficial for the cancer patient (Vaclavikova et al., 2007). This benefit comes from CYP2E1 prodrugs activation property and that the metabolism of some substrates of CYP2E1 leads to the production of ROS and oxidative stress (Gonzalez, 2005). This eventually lead to the inhibition of the apoptosis but the necrotic cancer cell death will be accelerated (Gonzalez, 2005; Vaclavikova et al., 2007). In a study aimed to examine the expression profile of CYP450 enzymes in (BC) in Caucasian population (Murray et al., 2010), it has been reported that there are upregulations of the CYP4X1, CYP2S1 and CYP2U1. Moreover, the CYP1B1, CYP3A5 and CYP51 displayed significant correlations with cancer grade (Murray et al., 2010). CYP2S1 is involved in metabolism of important exogenous and endogenous substrates, for example, the retinoic acid that is a metabolite of vitamin A (Madanayake et al., 2013). CYP2S1 may also be involved in activation or deactivation of procarcinogens. The expression of CYP2S1 is comparable to that of the CYP1B1 (Rivera et al., 2007). In addition to tumors, the expression of CYP2S1 is also increased in myocardial infarction, lung hypertension, renal failure and rheumatoid arthritis. This increased expression probably influences the response of these pathological conditions to the treating drugs that are substrate of CYP2S1 (Rivera et al., 2007). Other study has reported that the CYP3A is not expressed in Japanese BC patients and that CYP3A expression in BC population may be of ethnic dependent (Oyama et al., 2005). Studying the expression of CYP3A in BC is of special interest since the tamoxifen is metabolized by CYP3A. The efficacy of tamoxifen treatment is influenced by variation in CYP3A expression (Oyama et al., 2005).
C-Lung Cancer
CYP2A13 belongs to the member of the °CYP2A° subfamily and it has been shown that it is expressed predominantly in the respiratory tract, brain, mammary gland, prostate, testicles, and uterus (Zhu et al., 2006). The highest expression quantified by PCR was in the nasal mucosa, trachea, and lung tissues (Su et al., 2000; Sun and Fan, 2013). CYP2A13 catalyzes the metabolic activation of the major tobacco-specific carcinogen, the 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NMK) through alpha hydroxylation (Su et al., 2000). It has been shown that there is down-regulation of CYP2A13 expression in adenocarcinoma and suggested that CYP2A13 is implicated in the development and progression of lung adenocarcinoma (Sun and Fan, 2013).
It has been suggested that there is association between estrogen metabolism and cigarette smoking and there is growing evidences proposing that estrogen levels is elevated in lung cancer (Chakraborty et al., 2010; Slowikowski et al; 2017). CYP1B1 is induced by tobacco (Port et al., 2004), and can convert the tobacco procarcinogens benzo[a]pyrene (B[a]P) to carcinogenic intermediates (Port et al., 2004). Estrogen also is metabolized with CYP1B1 (Samavat and Kurzer, 2015). In lung tissues during the metabolism of the estradiol and the tobacco specific carcinogens by CYP1B1, DNA adducts and ROS can form which may cause DNA damage which can be mutagenic (Slowikowski et al., 2017). It has been shown that there are lower expressions of CYP1B1 in non-small cell lung cancer at both mRNA and protein levels in the middle stage of non-small cell lung cancer (NSCLC) irrespective of the age, gender or the histopathological type of lung cancer (Slowikowski et al., 2017). However, there is overexpression of CYP1B1 in advanced cases of NSCLC (Su et al., 2009). The gene polymorphism of CYP1B1 Leu432Val was associated with increased lung cancer risk in nonsmokers (Wenzlaff et al., 2005).
D-Prostate Cancer (PC)
It has been shown with real time PCR and western blot analysis that the CYP3A5 is the most expressed CYP isoform in basolateral cells of normal prostate tissue, but not expressed tumor cells (Leskela et al., 2007). Leskelä et al., (2007) reported that the amount of CYP3A5 mRNA expressed in normal prostate cells was about 20% of the amount present in hepatic tissue. This study suggested that CYP3A5 functions in metabolism of intra-prostatic androgens and promotes luminal cell growth, and that gene polymorphisms in CYP3A5 are associated with PC risk and aggressiveness (Leskela et al., 2007). CYP1B1 has been shown to be overexpressed in PC cells and this overexpression is caused by hypomethylation of promoter/enhancer of the CYP1B1 gene (Tokizane et al., 2005). It is proposed that CYP1B1 is implicated in prostate carcinogenesis by its ability to activate metabolic carcinogens, as there is association between exposure to PAHs and increased risk to PC (Tokizane et al., 2005). CYP1A1 is involved in metabolism of estrogen and can activate procarcinogens (He and Feng, 2015). It has been reported that CYP1A1 polymorphism is associated with PC development (Ding et al., 2013). In a study conducted in Indian cohort reported that CYP1A1 gene polymorphism that leads to Ile462Val change was associated with a reduced PC risk, while polymorphism T3801C at 3’UTR of CYP1A1 gene was associated with increased PC risk (Vijayalakshmi et al., 2005). Furthermore, it has been suggested that CYP27B1 controls the normal growth of prostate cells through its role in vitamin D metabolism (Chen et al., 2012), and that CYP27B1 is regulated by epidermal growth factor (Chen et al., 2012). CYP27B1 has anti-tumor activity and when desregulated by epidermal growth factor the PC develops (Chen, 2008; Chen et al., 2012). Another CYP isoform of interest in PC is CYP17A1. CYP17A1 is involved in androgen biosynthesis and has been implicated in PC proliferation (Gomez et al., 2015). The use of CYP17A1 inhibitors (such as abiraterone acetate) is a recent strategy for treatment of castration-resistant PC (Gomez et al., 2015).
Roles of CytochromeP450 in Diabetes
CYP2E1 is induced by and oxidize ethanol, and it activates procarcinogens such as N-nitrosodimethylamine, benzene and N-alkylformamides (Wang et al., 2003). It has been shown that CYP2E1 is overexpressed in alcohol-induced liver damage as well as non-alcoholic steatosis (Niemela et al., 2000). Diabetes is commonly associated with fat mobilization as it will be the first energy source which will lead to the development of non-alcoholic fatty liver disease (Hazlehurst et al., 2016). It has been shown that there is elevated activity of CYP2E1 in liver of obese Type 2 diabetic patients (Wang et al., 2003). It is hypothesized that in the case of fat mobilization as in DM, the hyperketonemia and other small organic molecules are both substrates and inducers of CYP2E1 that will lead to non-alcoholic fatty liver disease (Wang et al., 2003, Oh et al., 2012). This overexpressed CYP2E1 exhibits a high capacity to produce free radicals that are probably the cause of liver damage and lipid peroxidation in obese T2D patients (Wang et al. 2003). On the other hand, another study has suggested that DM significantly reduced the catalytic activity as well as the protein level of CYP 3A4 (Dostalek et al., 2011). The CYP 3A4 is the most expressed CYP in the liver and intestine and may represent about 60% of total all CYP in liver (Dostalek et al., 2011). It belongs to the CYP3A subfamily (other members include CYP 3A5, 3A7 and 3A43) that metabolizing more that 50% of currently used medication and some endogenous substrates for instance, cortisol, estradiol, progesterone, and testosterone (Dostalek et al., 2011). Members of CYP3A subfamily exhibit a divergent expression pattern, but they have overlapping substrate specificity (Dostalek et al., 2011). The reduced activity and decreased expression of CYP 3A4 as in DM would influence the diabetic patients in respect to the biotransformation of drugs that are substrates of CYP3A. Moreover, downregulated CYP3A4 would probably decrease the oxidation of testosterone. This would reduce the amount of the biologically active form of dihydrotestosterone that is required for the normal growth of the prostate gland (Dostalek et al., 2011). The reduced CYP3A4 activity and protein level in diabetic patients may be because of obesity, and elevated pro-inflammatory cytokines (IL-2 and interferon-γ) (Sunman et al., 2004; Dostalek et al., 2011), noncytokine components and oxidative stress (Dostalek et al., 2011). In an experiment conducted in T2D nonobese Goto–Kakizaki rats (Oh et al., 2012), it has been shown that hepatic expression of CYP3A2 was increased, whereas, CYP1A2 and CYP3A1 expression was reduced. In another experiment conducted in streptozotocin-induced diabetic Sprague-Dawley male rats (Sindhu et al., 2006), it has been reported that the of CYP1B1, CYP2B1, CYP1A2 and CYP2E1 is upregulated in diabetic rats, in contrast to CYP2C11 that was decreased to more than 90% in the diabetic rats. Both of the above studies indicated that in diabetes, the CYP expression is in an isoform-specific manner and that this altered expression can partially be treated with insulin (Sindhu et al., 2006, Oh et al., 2012). It has also been reported that metformin decreases the expression of CYP1B1 by down regulation of the aryl hydrocarbon receptor (AhR) expression in breast cancer cells (Do et al., 2014). This study suggested that metformin could be used against breast cancer cells development. Furthermore, It has been reported that CYP2C8*3 (rs10509681), CYP2C9*2 (rs1799853), CYP3A4 (Ile118Val), CYP2C19*2 and CYP1B1*2 (rs1056827) polymorphisms were associated with increased susceptibility to T2D in Indian, Japanese, Mexican and Saudi populations, respectively (Yamada et al., 2007; Hoyo-Vadillo et al., 2010; Mahdi et al., 2016; Elfaki et al., 2018).
Roles of CytochromeP450 in Atherosclerosis
Arachidonic acid is metabolized by the CYP450 (CYP 2B, 2C8, 2C9, 2C10, 2J2) or arachidonic acid epoxygenase to epoxyeicosatrienoic acids (EETs). The EETs act as an endothelium-derived hyperpolarizing factor (EDHF) that functions as vasodilator in all vasculatures including the coronary arteries (Chawengsub et al. 2009). It has been shown in cultured human endothelial cells and native porcine coronary artery endothelial cells that the EDHF metabolite of arachidonic acid by CYP2C is the most vital cause of endothelium relaxation (Fisslthaler et al., 2000). Other relaxing factors include prostacyclin (PGI2) and nitric oxide (NO) (Chawengsub et al., 2009). These CYP450 enzymes therefore prevent or regress the atherosclerosis (Luoma, 2007). On the other hand, reactive oxygen species (ROS) are also generated by CYP2C catalytic reactions (particularly by CYP2C9) in coronary artery endothelial cells (Fleming et al., 2001). When the CYP2C is over produced, it will generate more ROS (Ercan et al., 2008). The deleterious effects of the ROS include the inhibition of the relaxation mediated by the NO and the elevated activity of the redox-sensitive transcription factor (NF-κB) (Fleming et al., 2001). The NF-κB pathway plays important roles in the development of metabolic disease such as atherosclerosis and T2D (Baker et al., 2011). NF-κB pathway contributes to the inflammatory response by the production of cytokines, recruitment of WBCs, differentiation of immune cells, and survival of the cells for example the macrophage foam cells, whose necrosis initiated the atherosclerotic plaques (Baker et al., 2011). Furthermore, ROS enhances the expression of vascular cell adhesion molecule1 (VCAM-1) (Fleming et al., 2001). The role of VCAM-1 in the inflammatory response and atherosclerosis is well established (Skeoch et al., 2014, Denys et al. 2016). Moreover, the CYP1B1 has been shown to promote the generation of ROS and contribute to the development of hyperlipidemia and atherosclerosis in apolipoprotein E–deficient mice fed atherogenic diet (Song et al., 2016). This study demonstrated that the CYP1B1 inhibitors such as 2,3’,4,5’-tetramethoxystilbene (TMS) can be used for treatment of atherosclerosis (Song et al., 2016).
CYP7A1 plays a protective role against atherosclerosis (Li et al., 2011). CYP7A1 (also known as cholesterol 7α-hydroxylase) catalyzes the committed step in cholesterol and bile acids synthesis in hepatocytes (Li et al., 2011), and is critical for their homeostasis (Li et al., 2011). CYP7A1 maintains this homeostasis by enhancing formation of bile acids from cholesterol, and increases free cholesterol secreted in bile without increasing cholesterol reabsorption by intestinal cells (Li et al., 2011). In man, a frame shift mutation in CYP7A1 gene (L413fsX414) that lead to enzyme loss of function would result in hypercholesterolemia and premature coronary and peripheral vascular disease (Pullinger et al., 2002). Recently, it has been shown in mice that the overexpression of CYP7A1 attenuates atherosclerosis by increasing secretion of bile, decreasing serum LDL-C, and decreasing acculturation of visceral fat deposits (Krishnamurthy et al., 2016).
CYP4A11 also exerts anti- atherosclerosis effect and metabolize arachidonic acid into the vasoactive 20-hydroxyeicosatetraenoic acid (20-HETE) (Fu et al., 2013; White et al., 2013). SNP in the promoter of CYP4A11 (rs9332978 T>C) has been reported to be associated with coronary artery disease in females of Russian cohort (Sirotina et al., 2018). This study proposed that the estradiol inhibits expression of CYP4A11 in the carriers of CC genotype which would result in reduced production of the vasoactive 20-HETE (Sirotina et al., 2018). Other polymorphism in CYP4A11gene is (T8590C) polymorphism which reduces its catalytic activity (White et al., 2013). This polymorphism was associated with a reduced HDL-C level and elevated C-reactive protein concentration in females (White et al., 2013). It is suggested that CYP4A11with reduced activity would lead to decreased synthesis of ω-hydroxylated epoxyeicosatrienoic acids which is an endogenous agonist of peroxisome proliferator-activated receptor-α (PPARα) (White et al., 2013). PPAR α is modulated by estrogen, and its agonist reduces the blood lipids, increases HDL-C and exert anti-inflammatory effect (Cuzzocrea et al., 2006; Nissen et al., 2007; White et al., 2013).
Cytochrome P450 Gene Polymorphism
Genetic polymorphisms in CYPs are a major cause of the inter individuals variation in drug metabolism. They lead to the occurrence of variation in response to the drugs ranging from adverse effects to lack of efficacy (Ingelman-Sundberg et al., 1999). From the 50 identified CYPs isoenzymes that catalyze the drug metabolism, there are more than 20 genes of CYPs are functionally polymorphic, for instance the CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP1B1, and CYP1A2. Therefore, about 40% of drug metabolism is catalyzed by the polymorphic CYPs (Bernard et al., 2006). Furthermore, polymorphisms of CYPs have been reported to confer diseases susceptibility (Table 2) (Pikuleva and Waterman, 2013; Meng, Ma et al. 2015, Mittal et al., 2015) as well as disease protection or reduced risk (Silvestri et al., 2003; Yamada et al., 2007; Ur Rasheed et al., 2017). In the following paragraphs we discuss some clinically important polymorphic CYPs.
Table 1.
Some CYPs with Their Endogenous, Drug Substrates, Inhibitors and Their Capability to Activate Procarcingens
| CYP Enzyme | Endogenous substrates | Drug substrates | Inhibitors | Importance in Procarcinogen activation |
|---|---|---|---|---|
| 1- CYP2D6 | Hydroxytryptamines, neurosteroids (Wang et al. 2009) | Tamoxifen, sparteine, dextromethorphan, debrisoquine (Ferraldeschi et al., 2010, Farooq et al., 2016) | Bupropion (Kotlyar et al., 2005) | No (Johansson and Ingelman-Sundberg, 2011) |
| 2- CYP2A6 | Retinoid acids, steroids (Di et al., 2009). | Coumarin, Methoxyflurane, Letrozole (Raunio et al., 2001) | N1-(4-fluorophenyl) cyclopropane-1-carboxamide, 4-chloroben zylamine, 4-bromobenzylamine, 2-chlorobenzylamine (Rahnasto et al., 2008). | No (Johansson and Ingelman-Sundberg, 2011). |
| 3- CYP2C9 | steroids, melatonin, retinoids, arachidonic acid (Zhou et al., 2009). | Angiotensin II blocker, Tamoxifen, Diclofenac, Ibuprofen, Glibenclamide, Glipizide, Warfarin, Tolbutamide, phenytoin (Van Booven et al., 2010) | Benzbromarone, benzofuran derivatives (Locuson et al., 2003). | No (Johansson and Ingelman-Sundberg, 2011). |
| 4- CYP2C19 | Estradiol, progesterone, testosterone, estrone (Persson et al., 2014) | clopidogrel, diazepam, omeprazole, citalopram (Sanford et al. 2013, Zhou et al., 2013) | Cannabidiol (Jiang et al., 2013) | No (Johansson and Ingelman-Sundberg, 2011). |
| 5- CYP1B1 | Estradiol (Halberg et al., 2008) | docetaxel, paclitaxel, mitoxantrone and flutamide (McFadyen, et al., 2004). | Quinazoline derivatives (Mohd Siddique et al., 2017). | Yes (Halberg et al. 2008) |
| 6- CYP1A2 | Steroids, retinols, melatonin, arachidonic acids, uroporphyrinogen (Zhou et al. 2009) | Paracetamol, theophylline, propranolol, lidocaine (Vasanthanathan et al. 2009, Zhou et al., 2009) | Artemisinin and thiabendazole (Bapiro Sayi et al., 2005). | Yes (Ayari et al. 2013) |
Table 2.
Examples of Some Diseases Associated with Cytochrome P450 Enzymes
| CYP | Polymorphism | Disease | Population | Reference |
|---|---|---|---|---|
| CYP2D6 | RS3892097 | Increased risk to Parkinson disease | Pakistan | Anwarullah et al., 2017 |
| CYP19A1 | rs3751592 | Alzheimer disease | Chinese | Zheng et al., 2016 |
| CYP2C9 | rs4918758 | Reduced risk Coronary heart disease | Russian | Polonikov et al., 2017 |
| CYP2C9 | rs9332242 and rs61886769 | epistatic interactions to Coronary heart disease susceptibility | Russian | Polonikov et al., 2017 |
| CYP1B1 | Wild | Atherosclerosis | mice | Song et al., 2016 |
| CYP4A11 | rs1126742 rs3890011 |
essential hypertension | Chinese | Zhang et al., 2017 |
| CYP4F2 | rs2108622 and rs3093105 | Coronary heart disease | Chinese | Yu et al, 2014 |
| CYP8A1 | C1117A | Left main coronary artery disease | Greece | Bousoula et al., 2012 |
| CYP2J2 | -50G/T | Ischemic stroke | Chinese | Wang et al., et al. 2017 |
| CYP1B1 | rs1056827 | T2D | Saudi | Elfaki et al., 2018 |
| CYP24A1* | rs6068812 rs114368325 |
idiopathic infantile hypercalcemia | German, Russia, Turkey | Schlingmann et al., 2011 |
| CYP7A1 | rs3808607 | Tuberculosis | Moroccan | Qrafli et al 2014 |
| CYP1A2 | T3801C at 3’UTR | Prostate cancer | Indian | Vijayalakshmi et al 2005 |
| CYP1A2 | rs762551 | Cancers | Caucasians | Wang et al., 2012 |
| CYP17A1 | Wild | Prostate cancer | General | Gomez, et al. 2015 |
| CYP17 | rs743572 | Gallbladder cancer /breast cancer | Indian (Tobacco users)/Chinese | Sun et al. 2018 |
CYP24A1 catalyzed the catabolism of the 1, 25-dihydroxyvitamin D. Failure of 1, 25-dihydroxyvitamin degradation leads to elevated calcium in the blood and vomiting, loss of fluids, and nephrocalcinosis (Schlingmann et al., 2011).
a. Cytochrome P450 2D6 (CYP2D6)
The CYP 2D6 represents a small percentage of all hepatic cytochrome P 450s (Ingelman-Sundberg, 2005). It is encoded by the CYP2D6 gene that is localized on chromosome 22q13.1, and catalyzes the biotransformation of about 20-25% of the clinically used drugs (Ingelman-Sundberg, 2005). The endogenous substrates of CYP2D6 include neurotransmitters and neurosteroids, for examples, the serotonin, or hydroxytryptamines, 5-methoxyindolethylamine O-demethylase, and pinoline, progesterone and Hydroxyprogesterone (Wang et al., 2014). The crystal structure of human CYP 2D6 at a resolution of 3.0 Å has been described (Rowland et al., 2006). The CYP2D6 fold similarly to the characteristic P450 fold as for all CYP family member, with lengths and orientations of the alpha helices and sheets very similar to CYP 2C9 (Rowland et al., 2006). The most important difference is that the F helix, the F-G loop, the B’ helix, β sheet 4, and part of β sheet 1 are found on the distal face of CYP2D6 (Rowland et al., 2006). The CYP 2D6 enzyme has a well-defined active site cavity above the heme group (Figure 1A). The active site was described as having the shape of a right foot (Rowland et al., 2006). The polymorphism of the enzyme results in poor, intermediate, efficient or ultra-rapid metabolizers (UMs) of CYP2D6 drugs substrates (Ingelman-Sundberg, 2005). The drug substrate include antidepressants, neuroleptics, some antiarrhythmics, lipophilic β-adrenoceptor blockers and opioids (Bertilsson et al., 2002). Some individuals carry nonfunctional gene copies of the CYP2D6 in which there is one or more nucleotide is changed. This mutated gene results in faulty mRNA, which is translated to incomplete proteins (Yu et al., 2002). The incomplete protein is unable to bind the heme. This result in enzyme has no catalytic activity. Some CYP2D6 alleles contain insertion or deletion mutations resulting in one or more change in amino acid residues (Yu et al., 2002). For example, the variant allele CYP2D6*17 result from the mutations Thr107Iso, Arg296Cys, and Ser486Thr. These mutations lead to a CYP2D6 with reduced catalytic activity (Ingelman-Sundberg., 2005). Also the CYP2D6*4 that result from a defective splicing and produce an inactive verstion CYP2D6 (Ingelman-Sundberg 2005). These changes may result in the structural change or misfolded protein that will have a reduced or no catalytic activity (Yu et al., 2002). This variation produces different phenotypes of the 2D6.1 (wild types), for example the 2D6.2, 2D6.10, and 2D6.17 Allelic Isoforms (Yu et al., 2002). The catalytic activities of these CYP2D6 phenotypes in comparison to the wild type 2D6.1, decrease in the order 2D6.2 > 2D6.17 > 2D6.10 (Yu et al., 2002). The (CYP2D6) ultra-rapid metabolizer has been reported to be protected against Parkinson disease, whereas, the poor metabolizer is being susceptible (Ur Rasheed et al., 2017).
Figure 1.
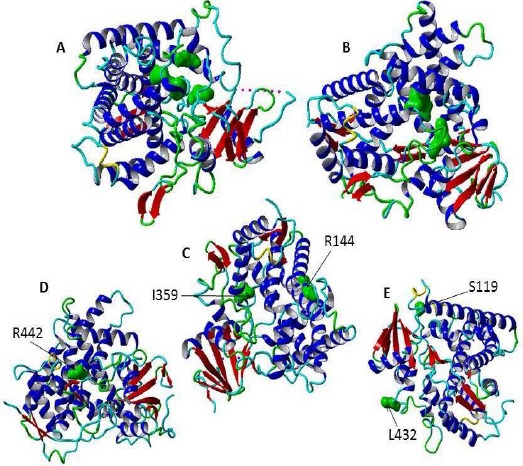
The Three Dimensional Structures (3D) of Cytochromes P450 Enzymes in Ribbon Representations, A- The secondary structure (PDB ID, 2F9Q) of CYP2D6 has a well-defined binding pocket above the heme group containing amino acid (illustrated in green and surface structure) residues that are implicated in recognition and binding of substrate (Asp-301, Glu-216, Phe-483, and Phe-120) [95]. B- The secondary structure of CYP2A6 (PDB 3T3Q). The amino acid forming the active site is indicated in surface structure and green color (Phe 300, Ala 301, Ser208, Ser369 and Leu370). C- The secondary structure of P450 2C9 (PDB ID, 1R9O). The sites of two important mutations are shown in surface structure and green color (R144C and 359I>L). D- The secondary structure of CYP2C19 (PDB code ID, 4GQS). The site of SNP that results in alteration of the amino acid residue R442C is illustrated in surface structure and green color. E. the secondary structure of CYP1B1 (PDB ID, 3PM0). There are two important single nucleotide variations in CYP1B1 gene in A119S and L432V that resulted in disease phenotypes. The heme molecule is not shown. This figure has been prepared using YASARA View (version 17.7.30).
b. The Cytochrome P450 2A6 (CYP2A6)
The CYP2A6 enzyme participates in the biotransformation of several xenobiotics that has been categorized as pharmaceuticals and toxic agents (Raunio et al., 2001). Examples of drugs substrates of CYP2A6 include Coumarin, Methoxyfurane, Halothane, Valproic acid, whereas, the toxic substrates of the CYP2A6 include the Nicotine, Quinoline and methyl tertbutyl ether (Raunio et al., 2001). Inhibitors of the CYP2A6 include methoxsalen (8-methoxypsoralen), menthofuran, pilocarpine and the monoamine oxidase inhibitor tranylcypromine (Yano et al., 2005). The inhibition of the CYP2A6 has two important aspects. Firstly, its inhibitor the methoxsalen (8-methoxypsoralen) reduces nicotine metabolism, and hence methoxsalen have been suggested as a novel approach for smoking cessation (Bagdas et al., 2014). Secondly, inhibition of CYP 2A6 inhibited the formation tobacco-specific carcinogens N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone to mutagenic compounds in human liver microsomes (Patten et al., 1997, Yano et al., 2005). The crystal structure of the CYP2A6 complexed with coumarin and methoxsalen was determined (Yano et al., 2005). The CYP2A6 crystal structure revealed that the CYP2A6 catalytic domain fold in 16 alpha helices and 4 beta sheets like all mammalian microsomal CYPs. However, its binding pocket is hydrophobic, compact and represent about one quarter of the binding pockets of some important drug-metabolizing CYPs like CYPs 2C8 (PDB code 1PQ2), 2C9 (PDB code 1R9O) and 3A4 (PDB code 1TQN)(Yano et al., 2005). Site-directed mutagenesis and homology modeling studies have indicated that the amino acids residues (e.g. Phe300, Ala 301, Ser 208 red, Ser369, and Leu370) may play have a key role in recognition of substrates, Figure 1 (Di et al., 2009).
CYP2A6 gene polymorphism has been associated with smoking behavior, drug metabolism and lung cancer risk (Di et al., 2009). This polymorphism resulted in a variety of phenotypes of the CYP2A6. These phenotypes are wild type CYP2A6*1A with normal enzyme activity (Hukkanen et al., 2005; Akrodou, 2015). The phenotypes CYP2A6*4A, CYP2A6*4B, and CYP2A6*4D have no enzyme activity due to the whole gene deletion (Hukkanen et al., 2005), or due to single nucleotide substitution (CYP2A6*2). There is also the allele CYP2A6*5 that encoded a very unstable enzyme with no catalytic activity because the conserved Glycin-479 has been substituted with the valine (Oscarson et al., 1999). The CYP2A6 is able to activate several procarcinogens such as nitrosamines and the aflatoxins. Logically, the phenotypes that are without enzyme activity (CYP2A6*4A, CYP2A6*4B, and CYP2A6*4D) are protected from the cancers caused by the procarcinogens that are substrates of CYP2A6 (Raunio et al., 2001).
c. The Cytochrome P450 2C9 (CYP2C9)
CYP2C9 plays a major role in phase I metabolism of xenobiotic and some endogenous compounds (Van Booven et al., 2010). CYP2C9 is the second main CYP expressed in hepatocytes, whereas the CYP3A4 is most expressed CYP in liver cells (Van Booven et al., 2010). The CYP2C9 is the drug-metabolizing enzyme for about 15-20% of all drugs undergoing phase I metabolism. For instance, the nonsteroidal anti-inflammatories, oral anticoagulants, and oral hypoglycemic (Rettie and Jones, 2005). It has been shown that CYP2C9 is induced by treatment with rifampicin (Van Booven et al., 2010). The metabolic rates of P450 2C9 substrates for examples the phenytoin, tolbutamide, S-warfarin, losartan are increased to about two times in patients treated with rifampicin (Kanebratt et al., 2008). Inhibitors of the CYP2C9 include amiodarone, fluconazole, and sulphaphenazole (Miners and Birkett, 1998; Van Booven et al., 2010). The crystal structure of the human CYP2C9 unliganded and bound to the anti-coagulant drug warfarin has been elucidated (Williams et al., 2003). The crystal structure (Protein Data Bank ID: 1OG2 and 1OG5) revealed a novel binding pocket in CYP2C9 and unexpected interactions between the CYP2C9 and the drug Warfarin (Williams et al., 2003). The presence of this novel binding pocket suggests CYP2C9 may binds multiple ligands and undergo allosteric mechanism during its biological function (Williams et al., 2003). The crystal structure of CYP2C9 in complex with NSAID flurbiprofen has also been elucidated (Protein Data Bank ID: 1R9O) (Wester et al., 2004). It has revealed that the residue Arginine 108 is responsible for the binding of flurbiprofen and other substrates like naproxen, ibuprofen, diclofenac to CYP2C9 (Wester et al., 2004). Two residues of on helix I (Aspartate 293 and Asparagine 289) are interacting and stabilizing Arginine 108 (Wester et al., 2004). Polymorphism of CYP2C9 has been associated with adverse drug events (Van Booven et al., 2010). Individuals with low CYP2C9 catalytic activity develops adverse drug reactions particularly with substrates with a narrow therapeutic index, for instance, S-warfarin, phenytoin, glipizide, and tolbutamide (Pirmohamed and Park, 2003). Examples of two important mutations in CYP2C9 are the CYP2C9 (Arg144Cys, rs1799853 and Iso359Leu, rs1057910 Figure 1C) that result in CYP2C9 poor metabolic activities (Van Booven et al., 2010). These two mutations are significantly highly frequent in Caucasian populations than in African and Asian populations (Sistonen et al., 2009, Van Booven et al., 2010). When treated with anti-coagulant warfarin, the patients with these two mutations have prolonged clotting time as well as an elevated possibility of severe bleeding complications (Aithal et al., 1999). In treatment with the hypoglycemic agent, glipizide and tolbutamide, these patients (with Arg144Cys, rs1799853 and Iso359Leu, rs1057910 mutations) may develop very low blood glucose levels (Kidd et al., 1999). Moreover, these individuals develop symptoms of phenytoin overdose when treated with it (Ninomiya et al. 2000), since they are poor metabolizers of these drugs. Furthermore, recently, it has been reported that patients taking long-term oral anti-coagulants treatment with Vitamin K antagonists are at a high risk of thrombotic and/or major hemorrhage adverse events if they are carriers of one of three polymorphisms (Misasi et al., 2016), and the (Iso359Leu rs1057910) is one of them (Misasi et al., 2016). The sulphonylureas are antidiabetic medications. They stimulate insulin secretion from the Pancreatic Beta cells (Rendell, 2004). Sulphonylureas bind the ATP-sensitive potassium channel. This binding closes the potassium channel and open the calcium channel which results in insulin secretion from pancreatic Beta cells (Rendell, 2004). Sulphonylureas are metabolized by CYP2C9 in hepatocytes. Therefore, CYP2C9 gene variations might be associated with risk of diabetes and/or altered drug reaction to the Sulphonylureas (Pollastro et al., 2015). It has been shown that CYP2C9 (Iso359Leu rs1057910) polymorphism is associated with a more reduction of fasting blood glucose level and a higher rate of treatment response to gliclazide (Zeng et al., 2016).
d. Cytochrome P450 2C19 (CYP2C19)
The Cytochrome P450 2C19 is encoded by chromosome 10, and is expressed in the human liver (Uehara et al., 2015). It is the most polymorphic member of CYP2 C subfamily (Lee, 2012). It catalyzes the metabolism of drugs for example the S-mephenytoin, omeprazole, in addition to some of antidepressants and serotonin reuptake inhibitors (Reynald et al., 2012). Genetic polymorphism in CYP2C19 results in inter-individual differences in the enzyme expression and in metabolic activity. The Allelic variation (gene polymorphism) classified the population according to the catalytic activity of CYP2C19 to poor, extensive and ultra-rapid phenotypes for drug clearance (Reynald et al., 2012). For instance, the individuals who are carriers of the loss-of-function alleles of CYP2C19 have shown to have a decreased antiplatelet effect of clopidogrel as well as lower blood concentrations of the active form of clopidogrel (Brandt et al., 2007; Simon et al., 2009). This is since the clopidogrel (pro-drug) should be sufficiently metabolized by the CYP2C19 such that it is converted to an effective inhibitor of platelet aggregation (Dean, 2012). It is therefore, the patients that are homozygous for the CYP2C19 loss-of-function alleles develop adverse clinical reactions when treated with clopidogrel after acute myocardial infarction (Simon et al., 2009). On the other hand, the individuals that are ultrarapid metabolizers with the CYP2C19*17 allele can exhibit a good therapeutic response that is associated with an increased effect of clopidogrel and probably an increased bleeding risk (Sibbing et al., 2010, Tiroch et al., 2010). Moreover, it has been reported the SNP (168946C>T) in exon 9 of CYP2C19 gene would lead to change in arginine 442 to cysteine (Morita et al., 2004). This change from a positively-charged amino acid arginine 442 to the sulphur-containing amino acid cysteine is located proximal to the CYP2C19 heme binding region (Figure 1 D) and reduces it catalytic activity (Morita et al. 2004). The crystal structure of CYP2C19 complexed with the inhibitor inhibitor (2-methyl-1-benzofuran-3-yl)-(4- hydroxy-3,5-dimethylphenyl) methanone was to 2.87 Å resolution by x-ray crystallography(Reynald, Sansen et al. 2012). The 3D structure of CYP2C19 is highly similar to that of CYP2C9 and CYP2C8. However, its binding pockets are completely different in term of topography, polarity and hydrophobicity since the amino acid residues are divergent in this region which may explain why these enzymes exhibit different substrate/inhibitor specificities (Reynald et al., 2012).
e. Cytochrome P450 1B1 (CYP1B1)
The cytochrome P450 1B1 (CYP1B1) catalyzes the metabolism of number of xenobiotic such as theophylline, ethoxyresorufin, and caffeine (Rochat et al., 2001; Zanger and Schwab, 2013). It also activates some procarcinogens, for instance, heterocyclic amines, polycyclic hydrocarbons, aromatic amines, and nitropolycyclic hydrocarbons (Chun and Kim, 2016). It is encoded by the CYP1B1 gene that is regulated by the AHR (Nebert et al., 2004), and other transcription factors such as AHR nuclear translocator (ARNT) complex (AHR/ARNT), the Sp1 transcription factor, a cyclic AMP (cAMP)–response element–binding protein (CREB), and the estrogen receptor (Sissung et al., 2006). The crystal structure of the CYP1B1 was determined (Wang et al., 2011), it has a narrow slot-like active site that is similar to that of CYP1A1, however, the residues around the edge of the active sites are different for specific binding of substrates and inhibitors (Wang et al., 2011). Using the reverse transcriptase-coupled PCR, CYP1B1 mRNA was detected in hepatocytes, lymphocytes, and uterus (Hakkola et al., 1997). CYP1B1 gene is expressed in different amounts among tissues, the highest mRNA expression levels were found in extrahepatic tissues such as renal, uterine, heart, brain, lung, skeletal muscle (Pavek and Dvorak, 2008). CYP1B1 is highly expressed in estrogen related organ such as uterus, ovaries and mammary glands (Tsuchiya et al, 2004). The CYP1B1 catalyzes the metabolism of endogenous substrates such as 17 β-estradiol into reactive metabolites, such as 4-hydroxyestradiol (Smerdova et al., 2014). The 4-hydroxyestradiol, a catechol metabolite reduces the activity of estrogen. However, it generates free radicals from the reductive-oxidative cycling with the corresponding quinone and semiquinone forms, which cause cellular damage. Therefore, 4-hydroxyestradiol is toxicologically active and may have a role in induction of cancer (Bolton and Thatcher, 2008). The 4-hydroxyestradiol level is elevated in human uterine and breast cancers in comparison to normal tissue (Bolton and Thatcher, 2008). Mutations in CYP1B1 were reported to be causes of disease phenotype such as the primary congenital glaucoma (Badeeb et al., 2014). Moreover, CYP1B1 gene polymorphisms L432V and A119S (rs1056827) were reported to be risk of developing endometrial (Zhu et al., 2011), laryngeal cancers and T2D (Yu et al., 2015; Elfaki et al., 2018).
f. Cytochrome P450 1A2 (CYP1A2)
CYP1A2 is primarily expressed in hepatocytes and is about 13% of all CYP 450s in liver (Ren et al., 2016). About 5% of currently used drugs are metabolized by CYP1A2 (Zhou et al., 2009). Drug substrates of CYP1A2 include acetaminophen, paracetamol, theophylline, propranolol, lidocaine, tacrine and triamterene (Vasanthanathan et al., 2009; Zhou et al., 2009). Endogenous substrates of CYP1A2 are steroids, retinols, melatonin, uroporphyrinogen, and arachidonic acids (Zhou et al., 2009). CYP1A2 is also able to activate procarcinogens (Ayari et al. 2013). Examples of CYP1A2 inducers include tobacco, flutamide, and caffeine (Wang et al., 2005). The crystal structure reveal the CYP1A2 folds as the general CYP structure into 4 Beta sheets designated from 1-4, and 12 alpha helices designated from A-L, in addition to several other alpha helices designated by prime or double prime (Sansen et al., 2007). The substrate binding pocket of CYP1A2 is narrow, compact and closed to fit binding and oxidation of relatively large and planar substrates such as PAHs (Sansen et al., 2007). It has been reported that there is a SNP C>A (rs762551) in intron 1 of CYP1A2 with AA homozygote carriers metabolize the caffeine at highest rate (Womack et al., 2012). This polymorphism influences the inducibility of CYP1A2 particularly in smokers (Koonrungsesomboon et al. 2017). The highest CYP1A2 induction rate was reported in AA genotype and no difference between AC and CC genotypes (Sachse et al., 1999). It was postulated that the differences in CYP1A2 expression levels are due altered regulatory proteins binding or due to linkage disequilibrium with other mutations influencing inducibility of CYP1A2 (Cornelis et al., 2004). It was reported that carriers of CYP1A2 with high activity were at more risk to lung cancer (Seow et al., 2001; Bu, et al. 2014), confirming the role of CYP1A2 in activating heterocyclic arylamines. Furthermore, Cornelis et al reported that individuals had low inducibility CYP1A2 were more susceptible to myocardial infarction (MI) than those had high inducibility CYP1A2 (Cornelis et al., 2004). It has been proposed that this increased susceptibility was because MI patients who had low inducibility CYP1A2 had increased cytokines levels (Cornelis et al., 2004). The role of cytokines in the pathogenesis of coronary heart disease is well-established (Tousoulis et al., 2016). CYP1A2 also metabolizes 17β-estradiol to 2-hydroxyestradiol which is then converted to 2-methoxyestradiol by catechol-O-methyltransferase (Ren et al., 2016). The 2-methoxyestradiol inhibits hepatocellular carcinoma (HCC) cells proliferation by inducing apoptosis (Ren et al., 2016). This study suggested that CYP1A2 downregualtion would result in progression of HCC.
Concluding Remarks
Cytochrome 450 (CYP450) is a large superfamily comprised of 18 families. There are 57 genes encoding these hemoprotein enzymes. CYP540s catalyze various vital reactions. They present in mitochondria and endoplasmic reticulum of different tissues, but mainly in hepatocytes and intestinal cells. CYP450 are involved in the metabolism of certain endogenous substrate such as cholesterol, estrogen, vitamin D and arachidonic acid. They are also catalyzing phase 1 in metabolism of xenobiotics such as drugs and carcinogens. Some CYP450 isoforms can activate procarcinogens to the ultimate carcinogens, for example CYP1A1, CYP1A2 and CYP1B1. Polymorphisms have been reported in CYP450s genes and cause no or altered enzyme activity. These CYP450 gene polymorphisms have been associated with unexpected drug responses as well as susceptibility to diseases such as cancers, type 2 diabetes, and atherosclerosis.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This project is funded by the Deanship of Scientific research, university of Tabuk grant for project number (No.0178-1437-S) for IE and FMA, and grant number (No.0121-1439-S) for IE.
References
- 1.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–9. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 2.Akrodou YM. CYP2A6 polymorphisms may strengthen individualized treatment for nicotine dependence. Scientifica (Cairo) 2015;2015:491514. doi: 10.1155/2015/491514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwarullah Aslam M, Badshah M, Abbasi R, et al. Further evidence for the association of CYP2D6*4 gene polymorphism with Parkinson's disease: a case control study. Genes Environ. 2017;30:18. doi: 10.1186/s41021-017-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayari I, Fedeli U, Saguem S, et al. Role of CYP1A2 polymorphisms in breast cancer risk in women. Mol Med Rep. 2013;7:280–6. doi: 10.3892/mmr.2012.1164. [DOI] [PubMed] [Google Scholar]
- 5.Badeeb OM, Micheal S, Koenekoop RK, den Hollander AI, Hedrawi MT. CYP1B1 mutations in patients with primary congenital glaucoma from Saudi Arabia. BMC Med Genet. 2014;15:109. doi: 10.1186/s12881-014-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagdas D, Muldoon PP, Zhu AZ, Tyndale RF, Damaj MI. Effects of methoxsalen, a CYP2A5/6 inhibitor, on nicotine dependence behaviors in mice. Neuropharmacology. 2014;85:67–72. doi: 10.1016/j.neuropharm.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bapiro TE, Sayi J, Hasler JA, et al. Artemisinin and thiabendazole are potent inhibitors of cytochrome P450 1A2 (CYP1A2) activity in humans. Eur J Clin Pharmacol. 2005;61:755–61. doi: 10.1007/s00228-005-0037-3. [DOI] [PubMed] [Google Scholar]
- 9.Bernard S, Neville KA, Nguyen AT, Flockhart DA. Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implications. Oncologist. 2006;11:126–35. doi: 10.1634/theoncologist.11-2-126. [DOI] [PubMed] [Google Scholar]
- 10.Bertilsson L, Dahl ML, Dalen P, Al-Shurbaji A. Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol. 2002;53:111–22. doi: 10.1046/j.0306-5251.2001.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol. 2008;21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bousoula E, Kolovou V, Vasiliadis I, et al. CYP8A1 gene polymorphisms and left main coronary artery disease. Angiology. 2012;63:461–5. doi: 10.1177/0003319711425230. [DOI] [PubMed] [Google Scholar]
- 13.Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–36. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 14.Bu ZB, Ye M, Cheng Y, Wu WZ. Four polymorphisms in the cytochrome P450 1A2 (CYP1A2) gene and lung cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:5673–9. doi: 10.7314/apjcp.2014.15.14.5673. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty S, Ganti AK, Marr A, Batra SK. Lung cancer in women: role of estrogens. Expert Rev Respir Med. 2010;4:509–18. doi: 10.1586/ers.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang GW, Kam PC. The physiological and pharmacological roles of cytochrome P450 isoenzymes. Anaesthesia. 1999;54:42–50. doi: 10.1046/j.1365-2044.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- 17.Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;297:H495–507. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen TC. 25-Hydroxyvitamin D-1 alpha-hydroxylase (CYP27B1) is a new class of tumor suppressor in the prostate. Anticancer Res. 2008;28:2015–7. [PubMed] [Google Scholar]
- 19.Chen TC, Sakaki T, Yamamoto K, Kittaka A. The roles of cytochrome P450 enzymes in prostate cancer development and treatment. Anticancer Res. 2012;32:291–8. [PubMed] [Google Scholar]
- 20.Chuang YT, Fang LW, Lin-Feng MH, Chen RH, Lai MZ. The tumor suppressor death-associated protein kinase targets to TCR-stimulated NF-kappa B activation. J Immunol. 2008;180:3238–49. doi: 10.4049/jimmunol.180.5.3238. [DOI] [PubMed] [Google Scholar]
- 21.Chun YJ, Kim D. Cancer activation and polymorphisms of human cytochrome P450 1B1. Toxicol Res. 2016;32:89–93. doi: 10.5487/TR.2016.32.2.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of CYP1A2 increases the risk of myocardial infarction. J Med Genet. 2004;41:758–62. doi: 10.1136/jmg.2004.022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzzocrea S, Mazzon E, Di Paola R, et al. The role of the peroxisome proliferator-activated receptor-alpha (PPAR-alpha) in the regulation of acute inflammation. J Leukoc Biol. 2006;79:999–1010. doi: 10.1189/jlb.0605341. [DOI] [PubMed] [Google Scholar]
- 24.Dean L. Clopidogrel therapy and CYP2C19 genotype. In: Pratt V, McLeod H, Dean L, et al., editors. Medical genetics summaries [Internet] Bethesda MD: National Center for Biotechnology Information(US); 2012. [Google Scholar]
- 25.Denys A, Clavel G, Lemeiter D. Aortic VCAM-1: an early marker of vascular inflammation in collagen-induced arthritis. J Cell Mol Med. 2016;20:855–63. doi: 10.1111/jcmm.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di YM, Chow VD, Yang LP, Zhou SF. Structure, function, regulation and polymorphism of human cytochrome P450 2A6. Curr Drug Metab. 2009;10:754–80. doi: 10.2174/138920009789895507. [DOI] [PubMed] [Google Scholar]
- 27.Ding G, Xu W, Liu H, et al. CYP1A1 MspI polymorphism is associated with prostate cancer susceptibility: evidence from a meta-analysis. Mol Biol Rep. 2013;40:3483–91. doi: 10.1007/s11033-012-2423-0. [DOI] [PubMed] [Google Scholar]
- 28.Do MT, Kim HG, Tran TT, et al. Metformin suppresses CYP1A1 and CYP1B1 expression in breast cancer cells by down-regulating aryl hydrocarbon receptor expression. Toxicol Appl Pharmacol. 2014;280:138–48. doi: 10.1016/j.taap.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Dostalek M, Court MH, Yan B, Akhlaghi F. Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus. Br J Pharmacol. 2011;163:937–47. doi: 10.1111/j.1476-5381.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elfaki I, Almutairi MF, Mir R, Khan R, Abu-Duhier FM. Cytochrome P450 CYP1B1*2 gene and its Association with T2D in Tabuk Population, Northwestern Region of Saudi Arabia. Asian J Pharm Clin Res. 2018;11:55–9. [Google Scholar]
- 31.Ercan B, Ayaz L, Cicek D, Tamer L. Role of CYP2C9 and CYP2C19 polymorphisms in patients with atherosclerosis. Cell Biochem Funct. 2008;26:309–13. doi: 10.1002/cbf.1437. [DOI] [PubMed] [Google Scholar]
- 32.Farooq M, Kelly EJ, Unadkat JD. CYP2D6 is inducible by endogenous and exogenous corticosteroids. Drug Metab Dispos. 2016;44:750–7. doi: 10.1124/dmd.115.069229. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Fernandez E, Perez R, Hernandez A, et al. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics. 2011;3:53–72. doi: 10.3390/pharmaceutics3010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferraldeschi R, Howell SJ, Thompson AM, Newman WG. Avoidance of CYP2D6 inhibitors in patients receiving tamoxifen. J Clin Oncol. 2010;28:584–5. doi: 10.1200/JCO.2010.30.1887. author reply e586. [DOI] [PubMed] [Google Scholar]
- 35.Fisslthaler B, Fleming I, Busse R. EDHF: a cytochrome P450 metabolite in coronary arteries. Semin Perinatol. 2000;24:15–9. doi: 10.1016/s0146-0005(00)80048-8. [DOI] [PubMed] [Google Scholar]
- 36.Fleming I, Michaelis UR, Bredenkotter D, et al. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 37.Fu Z, Ma Y, Xie X, et al. A novel polymorphism of the CYP4A11 gene is associated with coronary artery disease. Clin Appl Thromb Hemost. 2013;19:60–5. doi: 10.1177/1076029611436197. [DOI] [PubMed] [Google Scholar]
- 38.Gomez L, Kovac JR, Lamb DJ. CYP17A1 inhibitors in castration-resistant prostate cancer. Steroids. 2015;95:80–7. doi: 10.1016/j.steroids.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–10. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Guengerich FP. Mechanisms of cytochrome P450 substrate oxidation: MiniReview. J Biochem Mol Toxicol. 2007;21:163–8. doi: 10.1002/jbt.20174. [DOI] [PubMed] [Google Scholar]
- 41.Guengerich FP. Intersection of the roles of cytochrome P450 enzymes with xenobiotic and endogenous substrates, relevance to toxicity and drug interactions. Chem Res Toxicol. 2017;30:2–12. doi: 10.1021/acs.chemrestox.6b00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakkola J, Pasanen M, Pelkonen O, et al. Expression of CYP1B1 in human adult and fetal tissues and differential inducibility of CYP1B1 and CYP1A1 by Ah receptor ligands in human placenta and cultured cells. Carcinogenesis. 1997;18:391–7. doi: 10.1093/carcin/18.2.391. [DOI] [PubMed] [Google Scholar]
- 43.Halberg RB, Larsen MC, Elmergreen TL, et al. Cyp1b1 exerts opposing effects on intestinal tumorigenesis via exogenous and endogenous substrates. Cancer Res. 2008;68:7394–7402. doi: 10.1158/0008-5472.CAN-07-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He X, Feng S. Role of metabolic enzymes P450 (CYP) on activating procarcinogen and their polymorphisms on the risk of cancers. Curr Drug Metab. 2015;16:850–63. doi: 10.2174/138920021610151210164501. [DOI] [PubMed] [Google Scholar]
- 46.Hoyo-Vadillo C, Garcia-Mena J, Valladares A, et al. Association of CYP2C19 genotype with type 2 diabetes. Health. 2010;2:1184–90. [Google Scholar]
- 47.Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 48.Imig JD. Epoxyeicosatrienoic acids and 20-Hydroxyeicosatetraenoic acid on endothelial and vascular function. Adv Pharmacol. 2016;77:105–41. doi: 10.1016/bs.apha.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5:6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- 50.Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci. 1999;20:342–9. doi: 10.1016/s0165-6147(99)01363-2. [DOI] [PubMed] [Google Scholar]
- 51.Iyanagi T. Molecular mechanism of phase I and phase II drug-metabolizing enzymes: implications for detoxification. Int Rev Cytol. 2007;260:35–112. doi: 10.1016/S0074-7696(06)60002-8. [DOI] [PubMed] [Google Scholar]
- 52.Izawa D, Pines J. How APC/C-Cdc20 changes its substrate specificity in mitosis. Nat Cell Biol. 2011;13:223–33. doi: 10.1038/ncb2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28:332–8. doi: 10.2133/dmpk.dmpk-12-rg-129. [DOI] [PubMed] [Google Scholar]
- 54.Johansson I, Ingelman-Sundberg M. Genetic polymorphism and toxicology--with emphasis on cytochrome p450. Toxicol Sci. 2011;120:1–13. doi: 10.1093/toxsci/kfq374. [DOI] [PubMed] [Google Scholar]
- 55.Jonsdottir SO, Ringsted T, Nikolov NG, et al. Identification of cytochrome P450 2D6 and 2C9 substrates and inhibitors by QSAR analysis. Bioorg Med Chem. 2012;20:2042–53. doi: 10.1016/j.bmc.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 56.Kanebratt KP, Diczfalusy U, Backstrom T, et al. Cytochrome P450 induction by rifampicin in healthy subjects: determination using the Karolinska cocktail and the endogenous CYP3A4 marker 4beta-hydroxycholesterol. Clin Pharmacol Ther. 2008;84:589–94. doi: 10.1038/clpt.2008.132. [DOI] [PubMed] [Google Scholar]
- 57.Kidd RS, Straughn AB, Meyer MC, et al. Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics. 1999;9:71–80. doi: 10.1097/00008571-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Koonrungsesomboon N, Khatsri R, Wongchompoo P, Teekachunhatean S. The impact of genetic polymorphisms on CYP1A2 activity in humans: a systematic review and meta-analysis. Pharmacogenomics J. 2017;10:11–3. doi: 10.1038/s41397-017-0011-3. [DOI] [PubMed] [Google Scholar]
- 59.Kotlyar M, Brauer LH, Tracy TS, et al. Inhibition of CYP2D6 activity by bupropion. J Clin Psychopharmacol. 2005;25:226–9. doi: 10.1097/01.jcp.0000162805.46453.e3. [DOI] [PubMed] [Google Scholar]
- 60.Krishnamurthy K, Glaser S, Alpini GD. Heat shock factor-1 knockout enhances cholesterol 7alpha-hydroxylase (CYP7A1) and multidrug transporter (MDR1) gene expressions to attenuate atherosclerosis. Cardiovasc Res. 2016;111:74–83. doi: 10.1093/cvr/cvw094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SJ. Clinical application of CYP2C19 pharmacogenetics toward more personalized medicine. Front Genet. 2012;3:318. doi: 10.3389/fgene.2012.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leskela S, Honrado E, Montero-Conde C, et al. Cytochrome P450 3A5 is highly expressed in normal prostate cells but absent in prostate cancer. Endocr Relat Cancer. 2007;14:645–54. doi: 10.1677/ERC-07-0078. [DOI] [PubMed] [Google Scholar]
- 63.Leung T, Rajendran R, Singh S, et al. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast Cancer Res. 2013;15:R107. doi: 10.1186/bcr3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li T, Matozel M, Boehme S. Overexpression of cholesterol 7alpha-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology. 2011;53:996–1006. doi: 10.1002/hep.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Locuson CW, Wahlstrom JL, Rock DA, Rock DA, Jones JP. A new class of CYP2C9 inhibitors: probing 2C9 specificity with high-affinity benzbromarone derivatives. Drug Metab Dispos. 2003;31:967–71. doi: 10.1124/dmd.31.7.967. [DOI] [PubMed] [Google Scholar]
- 66.Luoma PV. Cytochrome P450-physiological key factor against cholesterol accumulation and the atherosclerotic vascular process. Ann Med. 2007;39:359–70. doi: 10.1080/07853890701379767. [DOI] [PubMed] [Google Scholar]
- 67.Luthra A, Denisov IG, Sligar SG. Spectroscopic features of cytochrome P450 reaction intermediates. Arch Biochem Biophys. 2011;507:26–35. doi: 10.1016/j.abb.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma MK, Woo MH, McLeod HL. Genetic basis of drug metabolism. Am J Health Syst Pharm. 2002;59:2061–9. doi: 10.1093/ajhp/59.21.2061. [DOI] [PubMed] [Google Scholar]
- 69.Madanayake TW, Lindquist IE, Devitt NP, Mudge J, Rowland AM. A transcriptomic approach to elucidate the physiological significance of human cytochrome P450 2S1 in bronchial epithelial cells. BMC Genomics. 2013;14:833. doi: 10.1186/1471-2164-14-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahdi F, Raza ST, Rizvi S, Abbas S, Karoli R. Distribution of genetic polymorphisms in drug metabolizing gene cytochrome P450 (CYP2C8*3 and CYP2C9*2) in a North Indian type 2 diabetes population. Explor Res Hypothesis Med. 2016;1:42–6. [Google Scholar]
- 71.McFadyen MC, McLeod HL, Jackson FC, et al. Cytochrome P450 CYP1B1 protein expression: a novel mechanism of anticancer drug resistance. Biochem Pharmacol. 2001;62:207–12. doi: 10.1016/s0006-2952(01)00643-8. [DOI] [PubMed] [Google Scholar]
- 72.McFadyen MC, Melvin WT, Murray GI. Cytochrome P450 CYP1B1 activity in renal cell carcinoma. Br J Cancer. 2004;91:966–71. doi: 10.1038/sj.bjc.6602053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McFadyen MC, Melvin WT, Murray GI. Cytochrome P450 enzymes: novel options for cancer therapeutics. Mol Cancer Ther. 2004;3:363–71. [PubMed] [Google Scholar]
- 74.Meng FD, Ma P, Sui CG, Tian X, Jiang YH. Association between cytochrome P450 1A1 (CYP1A1) gene polymorphisms and the risk of renal cell carcinoma: a meta-analysis. Sci Rep. 2015;5:8108. doi: 10.1038/srep08108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–38. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Misasi S, Martini G, Paoletti O, et al. VKORC1 and CYP2C9 polymorphisms related to adverse events in case-control cohort of anticoagulated patients. Medicine (Baltimore) 2016;95:e5451. doi: 10.1097/MD.0000000000005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitsui Y, Chang I, Fukuhara S, et al. CYP1B1 promotes tumorigenesis via altered expression of CDC20 and DAPK1 genes in renal cell carcinoma. BMC Cancer. 2015;15:942. doi: 10.1186/s12885-015-1951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mittal B, Tulsyan S, Kumar S, Mittal RD, Agarwal G. Cytochrome P450 in cancer susceptibility and treatment. Adv Clin Chem. 2015;71:77–139. doi: 10.1016/bs.acc.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Mohd Siddique MU, McCann GJ, Sonawane VR, et al. Quinazoline derivatives as selective CYP1B1 inhibitors. Eur J Med Chem. 2017;130:320–7. doi: 10.1016/j.ejmech.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 80.Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci. 2015;145:5–15. doi: 10.1093/toxsci/kfv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morita J, Kobayashi K, Wanibuchi A, et al. A novel single nucleotide polymorphism (SNP) of the CYP2C19 gene in a Japanese subject with lowered capacity of mephobarbital 4'-hydroxylation. Drug Metab Pharmacokinet. 2004;19:236–8. doi: 10.2133/dmpk.19.236. [DOI] [PubMed] [Google Scholar]
- 82.Murray GI, McFadyen MC, Mitchell RT, et al. Cytochrome P450 CYP3A in human renal cell cancer. Br J Cancer. 1999;79:1836–42. doi: 10.1038/sj.bjc.6690292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray GI, Patimalla S, Stewart KN, Miller ID, Heys SD. Profiling the expression of cytochrome P450 in breast cancer. Histopathology. 2010;57:202–11. doi: 10.1111/j.1365-2559.2010.03606.x. [DOI] [PubMed] [Google Scholar]
- 84.Murray GI, Taylor MC, McFadyen MC, et al. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57:3026–31. [PubMed] [Google Scholar]
- 85.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–50. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 86.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–62. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 87.Nelson DR, Zeldin DC, Hoffman SM, et al. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 88.Neve EP, Ingelman-Sundberg M. Cytochrome P450 proteins: retention and distribution from the endoplasmic reticulum. Curr Opin Drug Discov Devel. 2010;13:78–85. [PubMed] [Google Scholar]
- 89.Niemelä O, Parkkila S, Juvonen RO, et al. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 90.Ninomiya H, Mamiya K, Matsuo S, et al. Genetic polymorphism of the CYP2C subfamily and excessive serum phenytoin concentration with central nervous system intoxication. Ther Drug Monit. 2000;22:230–2. doi: 10.1097/00007691-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 91.Nissen SE, Nicholls SJ, Wolski K, et al. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA. 2007;297:1362–73. doi: 10.1001/jama.297.12.1362. [DOI] [PubMed] [Google Scholar]
- 92.Oh SJ, Choi JM, Yun KU, et al. Hepatic expression of cytochrome P450 in type 2 diabetic Goto-Kakizaki rats. Chem Biol Interact. 2012;195:173–9. doi: 10.1016/j.cbi.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 93.Ortiz de Montellano PR. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem Rev. 2010;110:932–48. doi: 10.1021/cr9002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oscarson M, McLellan RA, Gullstén H. Identification and characterisation of novel polymorphisms in the CYP2A locus: implications for nicotine metabolism. FEBS Lett. 1999;460:321–7. doi: 10.1016/s0014-5793(99)01364-2. [DOI] [PubMed] [Google Scholar]
- 95.Oyama T, Morita M, Isse T, et al. Immunohistochemical evaluation of cytochrome P450 (CYP) and p53 in breast cancer. Front Biosci. 2005;10:1156–61. doi: 10.2741/1608. [DOI] [PubMed] [Google Scholar]
- 96.Patten CJ, Smith TJ, Friesen MJ, et al. Evidence for cytochrome P450 2A6 and 3A4 as major catalysts for N'-nitrosonornicotine alpha-hydroxylation by human liver microsomes. Carcinogenesis. 1997;18:1623–30. doi: 10.1093/carcin/18.8.1623. [DOI] [PubMed] [Google Scholar]
- 97.Pavek P, Dvorak Z. Xensobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr Drug Metab. 2008;9:129–43. doi: 10.2174/138920008783571774. [DOI] [PubMed] [Google Scholar]
- 98.Persson A, Sim SC, Virding S, et al. Decreased hippocampal volume and increased anxiety in a transgenic mouse model expressing the human CYP2C19 gene. Mol Psychiatry. 2014;19:733–41. doi: 10.1038/mp.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petersson H. Steroid metabolizing cytochrome P450 (CYP) enzymes in the maintenance of cholesterol and sex hormonelevels PhD, Uppsala University. 2009 [Google Scholar]
- 100.Pikuleva IA, Waterman MR. Cytochromes p450: roles in diseases. J Biol Chem. 2013;288:17091–8. doi: 10.1074/jbc.R112.431916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pirmohamed M, Park BK. Cytochrome P450 enzyme polymorphisms and adverse drug reactions. Toxicology. 2003;192:23–32. doi: 10.1016/s0300-483x(03)00247-6. [DOI] [PubMed] [Google Scholar]
- 102.Pollastro C, Ziviello C, Costa V, Ciccodicola A. Pharmacogenomics of drug response in type 2 diabetes: Toward the definition of tailored therapies? PPAR Res. 2015;2015:415149. doi: 10.1155/2015/415149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Polonikov A, Kharchenko A, Bykanova M, et al. Polymorphisms of CYP2C8, CYP2C9 and CYP2C19 and risk of coronary heart disease in Russian population. Gene. 2017;627:451–9. doi: 10.1016/j.gene.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 104.Port JL, Yamaguchi K, Du B, et al. Tobacco smoke induces CYP1B1 in the aerodigestive tract. Carcinogenesis. 2004;25:2275–81. doi: 10.1093/carcin/bgh243. [DOI] [PubMed] [Google Scholar]
- 105.Porter TD, Coon MJ. Cytochrome P-450 multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991;266:13469–72. [PubMed] [Google Scholar]
- 106.Pullinger CR, Eng C, Salen G, et al. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–17. doi: 10.1172/JCI15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qrafli M, Amar Y, Bourkadi J, et al. The CYP7A1 gene rs380↟variant is associated with susceptibility of tuberculosis in Moroccan population. Pan Afr Med J. 2014;18:1. doi: 10.11604/pamj.2014.18.1.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rahnasto M, Wittekindt C, Juvonen RO, et al. Identification of inhibitors of the nicotine metabolising CYP2A6 enzyme--an in silico approach. Pharmacogenomics J. 2008;8:328–38. doi: 10.1038/sj.tpj.6500481. [DOI] [PubMed] [Google Scholar]
- 109.Rai R, Sharma KL, Misra S, Kumar A, Mittal B. CYP17 polymorphism (rs743572) is associated with increased risk of gallbladder cancer in tobacco users. Tumour Biol. 2014;35:6531–7. doi: 10.1007/s13277-014-1876-2. [DOI] [PubMed] [Google Scholar]
- 110.Raunio H, Rautio A, Gullstén H, Pelkonen O. Polymorphisms of CYP2A6 and its practical consequences. Br J Clin Pharmacol. 2001;52:357–63. doi: 10.1046/j.0306-5251.2001.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ren J, Chen GG, Liu Y, et al. Cytochrome P450 1A2 metabolizes 17beta-estradiol to suppress hepatocellular carcinoma. PLoS One. 2016;11:e0153863. doi: 10.1371/journal.pone.0153863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Renaud HJ, Cui JY, Khan M, Klaassen CD. Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice. Toxicol Sci. 2011;124:261–77. doi: 10.1093/toxsci/kfr240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004;64:1339–58. doi: 10.2165/00003495-200464120-00006. [DOI] [PubMed] [Google Scholar]
- 114.Rettie AE, Jones JP. Clinical and toxicological relevance of CYP2C9: drug-drug interactions and pharmacogenetics. Annu Rev Pharmacol Toxicol. 2005;45:477–94. doi: 10.1146/annurev.pharmtox.45.120403.095821. [DOI] [PubMed] [Google Scholar]
- 115.Reynald RL, Sansen S, Stout CD, Johnson EF. Structural characterization of human cytochrome P450 2C19: active site differences between P450s 2C8, 2C9, and 2C19. J Biol Chem. 2012;287:44581–91. doi: 10.1074/jbc.M112.424895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rivera SP, Wang F, Saarikoski ST, et al. A novel promoter element containing multiple overlapping xenobiotic and hypoxia response elements mediates induction of cytochrome P4502S1 by both dioxin and hypoxia. J Biol Chem. 2007;282:10881–93. doi: 10.1074/jbc.M609617200. [DOI] [PubMed] [Google Scholar]
- 117.Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL. Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J Pharmacol Exp Ther. 2001;296:537–41. [PubMed] [Google Scholar]
- 118.Rowland P, Blaney FE, Smyth MG, et al. Crystal structure of human cytochrome P450 2D6. J Biol Chem. 2006;281:7614–22. doi: 10.1074/jbc.M511232200. [DOI] [PubMed] [Google Scholar]
- 119.Sachse C, Brockmöller J, Bauer S, Roots I. Functional significance of a C-->A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–9. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamed S, Mindy SK. Estrogen metabolism and breast cancer. Cancer Lett. 2015;356:231–43. doi: 10.1016/j.canlet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanford JC, Guo Y, Sadee W, Wang D. Regulatory polymorphisms in CYP2C19 affecting hepatic expression. Drug Metabol Drug Interact. 2013;28:23–30. doi: 10.1515/dmdi-2012-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sansen S, Yano JK, Reynald RL, et al. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J Biol Chem. 2007;282:14348–55. doi: 10.1074/jbc.M611692200. [DOI] [PubMed] [Google Scholar]
- 123.Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365:410–21. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- 124.Seow A, Zhao B, Lee EJ, et al. Cytochrome P4501A2 (CYP1A2) activity and lung cancer risk: a preliminary study among Chinese women in Singapore. Carcinogenesis. 2001;22:673–7. doi: 10.1093/carcin/22.4.673. [DOI] [PubMed] [Google Scholar]
- 125.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shimada T, Murajama N, Tanaka K, et al. Interaction of polycyclic aromatic hydrocarbons with human cytochrome P450 1B1 in inhibiting catalytic activity. Chem Res Toxicol. 2008;21:2313–23. doi: 10.1021/tx8002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shimada T, Murayama N, Yamazaki H, et al. Metabolic activation of polycyclic aromatic hydrocarbons and aryl and heterocyclic amines by human cytochromes P450 2A13 and 2A6. Chem Res Toxicol. 2013;26:529–37. doi: 10.1021/tx3004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shimada T, Oda Y, Gillam EM, Guengerich FP, Inoue K. Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos. 2001;29:1176–82. [PubMed] [Google Scholar]
- 129.Sibbing D, Koch W, Gebhard D, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–8. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 130.Silvestri L, Sonzogni L, De Silvestri A, et al. CYP enzyme polymorphisms and susceptibility to HCV-related chronic liver disease and liver cancer. Int J Cancer. 2003;104:310–7. doi: 10.1002/ijc.10937. [DOI] [PubMed] [Google Scholar]
- 131.Sim SC, Ingelman-Sundberg M. The human cytochrome P450 (CYP) allele nomenclature website: a peer-reviewed database of CYP variants and their associated effects. Hum Genomics. 2010;4:278–81. doi: 10.1186/1479-7364-4-4-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sim SC, Ingelman-Sundberg M. Update on allele nomenclature for human cytochromes P450 and the human cytochrome P450 allele (CYP-allele) nomenclature database. Methods Mol Biol. 2013;987:251–9. doi: 10.1007/978-1-62703-321-3_21. [DOI] [PubMed] [Google Scholar]
- 133.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 134.Sindhu RK, Koo JR, Sindhu KK, et al. Differential regulation of hepatic cytochrome P450 monooxygenases in streptozotocin-induced diabetic rats. Free Radic Res. 2006;40:921–8. doi: 10.1080/10715760600801272. [DOI] [PubMed] [Google Scholar]
- 135.Sirotina S, Ponomarenko I, Kharchenko A, et al. A novel polymorphism in the promoter of the CYP4A11 gene is associated with susceptibility to coronary artery disease. Dis Markers. 2018;2018:5812802. doi: 10.1155/2018/5812802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sissung TM, Price DK, Sparreboom A, Figg WD. Pharmacogenetics and regulation of human cytochrome P450 1B1: implications in hormone-mediated tumor metabolism and a novel target for therapeutic intervention. Mol Cancer Res. 2006;4:135–50. doi: 10.1158/1541-7786.MCR-05-0101. [DOI] [PubMed] [Google Scholar]
- 137.Sistonen J, Fuselli S, Palo JU, et al. Pharmacogenetic variation at CYP2C9, CYP2C19, and CYP2D6 at global and microgeographic scales. Pharmacogenet Genomics. 2009;19:170–9. doi: 10.1097/FPC.0b013e32831ebb30. [DOI] [PubMed] [Google Scholar]
- 138.Skeoch S, Haque S, Pemberton P, Bruce IN. Cell adhesion molecules as potential biomarkers of nephritis, damage and accelerated atherosclerosis in patients with SLE. Lupus. 2014;23:819–24. doi: 10.1177/0961203314528061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Słowikowski BK, Gałęcki B, Dyszkiewicz W, Jagodziński PP. Decreased expression of cytochrome p450 1B1 in non-small cell lung cancer. Biomed Pharmacother. 2017;95:339–45. doi: 10.1016/j.biopha.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 140.Smerdová L, Šmerdová J, Kabátková M, et al. Upregulation of CYP1B1 expression by inflammatory cytokines is mediated by the p38 MAP kinase signal transduction pathway. Carcinogenesis. 2014;35:2534–43. doi: 10.1093/carcin/bgu190. [DOI] [PubMed] [Google Scholar]
- 141.Song CY, Ghafoor K, Ghafoor HU, et al. Cytochrome P450 1B1 contributes to the development of atherosclerosis and hypertension in apolipoprotein E-deficient Mice. Hypertension. 2016;67:206–13. doi: 10.1161/HYPERTENSIONAHA.115.06427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Su JM, Lin P, Wang CK, Chang H. Overexpression of cytochrome P450 1B1 in advanced non-small cell lung cancer: a potential therapeutic target. Anticancer Res. 2009;29:509–15. [PubMed] [Google Scholar]
- 143.Su T, Bao Z, Zhang QY, et al. Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–9. [PubMed] [Google Scholar]
- 144.Sun J, Zhang H, Gao M, et al. Association between CYP17 T-34C rs743572 and breast cancer risk. Oncotarget. 2018;9:4200–13. doi: 10.18632/oncotarget.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun L, Fan X. Expression of cytochrome P450 2A13 in human non-small cell lung cancer and its clinical significance. J Biomed Res. 2013;27:202–7. doi: 10.7555/JBR.27.20120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sunman JA, Hawke RL, LeCluyse EL, Kashuba AD. Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab Dispos. 2004;32:359–63. doi: 10.1124/dmd.32.3.359. [DOI] [PubMed] [Google Scholar]
- 147.Thelen K, Dressman JB. Cytochrome P450-mediated metabolism in the human gut wall. J Pharm Pharmacol. 2009;61:541–58. doi: 10.1211/jpp/61.05.0002. [DOI] [PubMed] [Google Scholar]
- 148.Tiroch KA, Sibbing D, Koch W, et al. Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J. 2010;160:506–12. doi: 10.1016/j.ahj.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 149.Tokizane T, Shiina H, Igawa M, et al. Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin Cancer Res. 2005;11:5793–5801. doi: 10.1158/1078-0432.CCR-04-2545. [DOI] [PubMed] [Google Scholar]
- 150.Tousoulis D, Oikonomou E, Economou EK, Crea F, Kaski JC. Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur Heart J. 2016;37:1723–32. doi: 10.1093/eurheartj/ehv759. [DOI] [PubMed] [Google Scholar]
- 151.Tsuchiya Y, Nakajima M, Kyo S, et al. Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Res. 2004;64:3119–25. doi: 10.1158/0008-5472.can-04-0166. [DOI] [PubMed] [Google Scholar]
- 152.Uehara S, Uno Y, Inoue T, et al. Novel marmoset cytochrome P450 2C19 in livers efficiently metabolizes human P450 2C9 and 2C19 substrates, S-warfarin, tolbutamide, flurbiprofen, and omeprazole. Drug Metab Dispos. 2015;43:1408–16. doi: 10.1124/dmd.115.066100. [DOI] [PubMed] [Google Scholar]
- 153.Ur Rasheed MS, Mishra AK, Singh MP. Cytochrome P450 2D6 and parkinson's disease: polymorphism, metabolic role, risk and protection. Neurochem Res. 2017;42:3353–61. doi: 10.1007/s11064-017-2384-8. [DOI] [PubMed] [Google Scholar]
- 154.Vaclavikova R, Hubackova M, Stribrna-Sarmanova J, et al. RNA expression of cytochrome P450 in breast cancer patients. Anticancer Res. 2007;27:4443–50. [PubMed] [Google Scholar]
- 155.Van Booven D, Marsh S, McLeod H, et al. Cytochrome P450 2C9-CYP2C9. Pharmacogenet Genomics. 2010;20:277–81. doi: 10.1097/FPC.0b013e3283349e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vasanthanathan P, Taboureau O, Oostenbrink C, et al. Classification of cytochrome P450 1A2 inhibitors and noninhibitors by machine learning techniques. Drug Metab Dispos. 2009;37:658–64. doi: 10.1124/dmd.108.023507. [DOI] [PubMed] [Google Scholar]
- 157.Vijayalakshmi K, Vettriselvi V, Krishnan M. Cytochrome p4501A1 gene variants as susceptibility marker for prostate cancer. Cancer Biomark. 2005;1:251–8. doi: 10.3233/cbm-2005-14-508. [DOI] [PubMed] [Google Scholar]
- 158.Wang A, Savas U, Stout CD, Johnson EF. Structural characterization of the complex between alpha-naphthoflavone and human cytochrome P450 1B1. J Biol Chem. 2011;286:5736–43. doi: 10.1074/jbc.M110.204420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang B, Yang LP, Zhang XZ, et al. New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme. Drug Metab Rev. 2009;41:573–643. doi: 10.1080/03602530903118729. [DOI] [PubMed] [Google Scholar]
- 160.Wang H, Zhang Z, Han S, et al. CYP1A2 rs762551 polymorphism contributes to cancer susceptibility: a meta-analysis from 19 case-control studies. BMC Cancer. 2012;12:528. doi: 10.1186/1471-2407-12-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang HX, Liu X, Xu CJ, et al. Induction of liver cytochrome P450 1A2 expression by flutamide in rats. Acta Pharmacol Sin. 2005;26:1382–6. doi: 10.1111/j.1745-7254.2005.00211.x. [DOI] [PubMed] [Google Scholar]
- 162.Wang SY, Xing PF, Zhang CY, Deng BQ. Association of CYP2J2 gene polymorphisms with ischemic stroke and stroke subtypes in Chinese population. Medicine (Baltimore) 2017;96:e6266. doi: 10.1097/MD.0000000000006266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang X, Li J, Dong G, Yue J. The endogenous substrates of brain CYP2D. Eur J Pharmacol. 2014;724:211–18. doi: 10.1016/j.ejphar.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 164.Wang Z, Hall SD, Maya JF, et al. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol. 2003;55:77–85. doi: 10.1046/j.1365-2125.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wenzlaff AS, Cote ML, Bock CH, et al. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: a population-based study. Carcinogenesis. 2005;26:2207–12. doi: 10.1093/carcin/bgi191. [DOI] [PubMed] [Google Scholar]
- 166.Wester MR, Yano JK, Schoch GA. The structure of human cytochrome P450 2C9 complexed with flurbiprofen at 2.0-A resolution. J Biol Chem. 2004;279:35630–7. doi: 10.1074/jbc.M405427200. [DOI] [PubMed] [Google Scholar]
- 167.White CC, Feng Q, Cupples LA, et al. CYP4A11 variant is associated with high-density lipoprotein cholesterol in women. Pharmacogenomics J. 2013;13:44–51. doi: 10.1038/tpj.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Williams PA, Cosme J, Ward A, et al. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature. 2003;424:464–8. doi: 10.1038/nature01862. [DOI] [PubMed] [Google Scholar]
- 169.Womack CJ, Saunders MJ, Bechtel MK, et al. The influence of a CYP1A2 polymorphism on the ergogenic effects of caffeine. J Int Soc Sports Nutr. 2012;9:7. doi: 10.1186/1550-2783-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yamada Y, Matsuo H, Watanabe S, et al. Association of a polymorphism of CYP3A4 with type 2 diabetes mellitus. Int J Mol Med. 2007;20:703–7. [PubMed] [Google Scholar]
- 171.Yano JK, Hsu MH, Griffin KJ, Stout CD, Johnson EF. Structures of human microsomal cytochrome P450 2A6 complexed with coumarin and methoxsalen. Nat Struct Mol Biol. 2005;12:822–3. doi: 10.1038/nsmb971. [DOI] [PubMed] [Google Scholar]
- 172.Yu A, Kneller BM, Rettie AE, Haining RL. Expression, purification, biochemical characterization, and comparative function of human cytochrome P450 2D6.1, 2D6.2, 2D6.10, and 2D6.17 allelic isoforms. J Pharmacol Exp Ther. 2002;303:1291–1300. doi: 10.1124/jpet.102.039891. [DOI] [PubMed] [Google Scholar]
- 173.Yu C, Yan Q, Fu C, et al. CYP4F2 genetic polymorphisms are associated with coronary heart disease in a Chinese population. Lipids Health Dis. 2014;13:83. doi: 10.1186/1476-511X-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu PJ, Chen WG, Feng QL, et al. Association between CYP1B1 gene polymorphisms and risk factors and susceptibility to laryngeal cancer. Med Sci Monit. 2015;21:239–45. doi: 10.12659/MSM.893084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 176.Zeng W, Guo Y2, Chen P, et al. CYP2C93 variant is associated with antidiabetes efficacy of gliclazide in Chinese type 2 diabetes patients. J Diabetes Investig. 2016;7:764–8. doi: 10.1111/jdi.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang H, Jin L, Mu T, et al. Associations of CYP4A11 gene-gene and gene-smoking interactions with essential hypertension in the male eastern Chinese Han population. Clin Exp Hypertens. 2017;39:448–53. doi: 10.1080/10641963.2016.1267201. [DOI] [PubMed] [Google Scholar]
- 178.Zheng J, Yan H, Shi L, et al. The CYP19A1 rs3751592 variant confers susceptibility to Alzheimer disease in the Chinese Han population. Medicine (Baltimore) 2016;95:e4742. doi: 10.1097/MD.0000000000004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou B, Song Z, Qian M, et al. Functional polymorphisms in the CYP2C19 gene contribute to digestive system cancer risk: evidence from 11,042 subjects. PLoS One. 2013;8:e66865. doi: 10.1371/journal.pone.0066865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou SF, Yang LP, Zhou ZW, Liu YH, Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11:481–94. doi: 10.1208/s12248-009-9127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou SF, Zhou ZW, Yang LP, Cai JP. Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16:3480–3675. doi: 10.2174/092986709789057635. [DOI] [PubMed] [Google Scholar]
- 182.Zhu LR, Thomas PE, Lu G, et al. CYP2A13 in human respiratory tissues and lung cancers: an immunohistochemical study with a new peptide-specific antibody. Drug Metab Dispos. 2006;34:1672–6. doi: 10.1124/dmd.106.011049. [DOI] [PubMed] [Google Scholar]
- 183.Zhu ZY, Mu YQ, Fu XM, Li SM, Zhao FX. Association of CYP1B1 gene polymorphisms and the positive expression of estrogen alpha and estrogen beta with endometrial cancer risk. Eur J Gynaecol Oncol. 2011;32:188–91. [PubMed] [Google Scholar]


