Abstract
Background:
Breast cancer is a complex disease that results from the inheritance of a number of susceptible genes. Intensive search wok was conducted world-wide on molecular bases of breast cancer in order to achieve the best therapeutic modalities; however, breast cancer still remains a challengeable task. It is very important to determine if the biological parameters in metastatic regional lymph nodes are similar to that in the primary breast cancer because therapy is indicated for patients with synchronous metastatic regional lymph nodes of breast cancer. Difference in therapeutic response in cases of breast cancer may be assumed partially to variability in the biological behavior of tumor tissue in primary breast cancer and lymph node metastasis.
Aim:
Our aim is to evaluate any variability in the expression of three types of tissue markers in both the primary breast tumors and corresponding axillary lymph nodes in order to expect the targeted therapeutic effect on both sites.
Material and Methods:
Three markers from different categories; RAGE, EGFR and Ki-67 were immunohistochemicalyl studied for their expression in biopsy specimens from primary breast tumors and their corresponding axillary lymph nodes.
Results:
There was a statistically significant difference in the expression of these markers between benign and malignant breast lesions. Although we found some differences in the expression of the three studied markers between primary breast cancer and corresponding axillary lymph nodes, yet these variations were mostly not statistically significant.
Conclusion:
Our findings support the validity of anti-RAGE and anti-EGFR therapy for treatment of both primary and nodal metastatic breast cancer in immunopositive cases.
Keywords: IHC, RAGE, EGFR, Ki-67, Breast cancer, Lymph node metastasis
Introduction
Breast cancer is the most common form of malignancy that has serious implications on the health of women worldwide, and ranks as the second leading cause of mortality in women. The main cause of breast cancer is still not completely understood (Ye et al., 2004). Breast cancer may develop by different factors such as genetic alteration, life-style, and obesity, higher level of specific hormones, tobacco and smoking. For instance, several studies have suggested that chronic inflammation is derived from smoking and tobacco, which increases the risk of breast cancer (Mink et al., 2002; Hecht, 2002). The pathology of breast cancer is classified into three categories according to its gene expression, receptors and immunohistochemistry characterization. This influences the prognosis of the disease and treatment response. (Gown, 2009; Schnitt, 2010).
Many risk factors increase the chances of developing breast cancer. It is now believed that most solid tumors, including those in the breast, have an inflammatory microenvironment (Quail and Joyce, 2013; Grivennikov et al., 2010).
RAGE is a member of the immunoglobulin superfamily of cell surface receptors, and its interaction with advanced glycation end products and other molecules plays a role in the pathogenesis of cancer progression and metastasis (Hongming et al., 2014). Converging evidence suggested that circulating RAGE level may be a novel clinical biomarker for many types of cancers, such as lung cancer, prostate cancer, colorectal cancer, breast cancer (Zhanga et al., 2013). Experimental studies have demonstrated that to block RAGE signaling in mice can reduce the migration and invasiveness of tumor cells, and possibly cell proliferation and the production of tissue metalloproteinase (Taguchi et al., 2000). RAGE expression is upregulated widely in aggressive triple-negative breast cancer (TNBC) cells, both in primary tumors and in lymph node metastases (Nasser et al., 2015). In an established model of lung metastasis, systemic blockade by injection of a RAGE neutralizing antibody inhibited metastasis development (Rojas et al., 2010). Mechanistic investigations revealed that RAGE bound to the proinflammatory ligand S100A7 and mediated its ability to activate ERK, NF-kB, and cell migration. In an S100A7 transgenic mouse model of breast cancer (mS100a7a15 mice), administration of either RAGE neutralizing antibody or soluble RAGE was sufficient to inhibit tumor progression and metastasis (Lata and Mukherjee, 2014).
The protein encoded by EGFR gene is a transmembrane glycoprotein that is a member of the protein kinase superfamily including also Her 2. This protein is a receptor for members of the epidermal growth factor family. EGFR is a cell surface protein that binds to epidermal growth factor. Binding of the protein to a ligand induces receptor dimerization and tyrosine autophosphorylation and leads to cell proliferation. Mutations in this gene are associated with lung and breast cancer (Sobral-Leite et al., 2017). Molecular targeted therapy has been suggested in the treatment of metastatic breast cancer. Trastuzumab is a high-affinity, humanized, anti-Her-2 antibody for the treatment of advanced breast carcinoma, particularly metastasis. However, it was shown that less than half of patients with a high level of Her-2 expression respond to Trastuzumab treatment (Cobleighet al., 1999 and Slamon el al., 2001). One explanation might be heterogeneity in the expression of Her-2 between primary and metastatic tumor cells (Thor et al., 2001).
In addition, another biomarker, Ki67, is a cell proliferation marker, which is expressed in the nuclei of cells in G1, S and M cell cycle phases. Ki67 has recently emerged as an important marker due to several applications in neoadjuvant therapy in addition to its moderate prognostic value. It is generally accepted that the Ki67 labeling index is a prognostic factor, and there is a tendency for Ki67 labeling index to decrease after chemotherapy (Neubauer et al., 2008, Burcombe et al., 2005 and Arens et al., 2005) and that a larger decrease of Ki67 is correlated with better responsiveness to chemotherapy (Burcombe et al., 2005 and Burcombe et al., 2006). However, only few studies are performed on comparing molecular biomarkers status of primary tumor and axillary lymph node (ALN) metastases in the same patient. (Zhao et al., 2015).
Overall, in the current study, we aim to evaluate RAGE, EGFR and Ki-67 as candidate biomarkers for diagnosis and grading of breast cancer as well as to test the variability of their expression between primary and metastatic breast cancer, that may influence the possible future target therapy.
Materials and Methods
The present study includes 170 cases of breast lesions examined at the pathology department of Theodor Bilharz Research institute from 2013 to 2016. The materials are obtained from surgical training mandate and community out reach commitment.
Types of examined materials include 125 tissue biopsies, 40 cases of fine needle aspiration cytology (FNAC) and 5 cases as slide consultations. The surgical procedures done were lumpectomy-excision biopsies, breast-conserving surgery wide local excision with axillary clearance and standard modified radical mastectomy
Sections from these biopsies were subjected to the following procedures:
-
1) Routine histopathological examination using paraffin sections stained by hematoxylin and eosin stain, with special reference to:
- - Diagnosis of benign and malignant lesions
- - Diagnosis of grade, stage and type of breast carcinoma
- - Diagnosis of metastatic deposits in regional lymph nodes
2) Immunohistochemical study of tissue sections using monoclonal antibodies for detection of RAGE, EFGR and Ki-67.
Immunohistochemical Method for RAGE and EGFR and Ki-67
Tissue sections were processed for IHC analysis of RAGE, EGFR and Ki-67 antigens as follows. IHC examinations were carried out on 4 μm thick sections. For anti-RAGE IHC, unmasking was performed with 10 mM sodium citrate buffer, pH 6.0, at 90°C for 30 min. Sections were incubated in 0.03% hydrogen peroxide for 10 min at room temperature, to remove endogenous peroxidase activity, and then in blocking serum (0.04% bovine serum albumin, A2153, Sigma-Aldrich, Shanghai, China, and 0.5% normal goat serum X0907, Dako Corporation, Carpinteria, CA, USA, in PBS) for 30 min at room temperature. Anti-RAGE antibody (A11): sc- 80652 RAGE Antibody (A11) is a mouse monoclonal IgG2a provided at 200 μg/ml, raised against a truncated extracellular domain of RAGE of human origin (Santa Cruz Biotechnology, USA). The antibody was used at a dilution of 1:100. Antibody for EGFR (F4) with Cat. number (sc-53274) mouse monoclonal IgG was used in a dilution of 1 to 200. Anti body for Ki-67 (Roche, CONFIRM anti-Ki-67 (30-9) Rabbit Monoclonal Primary Antibody), Cat. Number: 790-4286) was used in a dilution 1:200.
The sections were incubated with the antibodies overnight at 4°C. Sections were then washed three times for 5 min in PBS. Non-specific staining was blocked 5% normal serum for 30 min at room temperature. Finally, staining was developed with diaminobenzidine substrate and sections were counterstained with hematoxylin. PBS replaced the antibody in negative controls.
Quantification of RAGE and EGFR and Ki-67 expression
The expression of RAGE was semi quantitatively estimated as the total membrane-cytoplasmic immunostaining scores, which were calculated as the product of a proportion score and an intensity score. The proportion and intensity of staining was evaluated independently. The proportion score reflected the fraction of positive staining cells (score 0: <5%, score 1: 5%-10%, score 2: 10%-50%, score 3: 50%-75%, score 4: >75%) and the intensity score represented the staining intensity (score 0: no staining, score 1: weak positive, score 2: moderate positive, score 3: strong positive). Finally, a total expression score was given ranging from 0 to 12 Based on the analysis in advance, RAGE was regarded as negative expression in gastric cancer tissues if the score <2, and positive expression if the score ≥2 (Dai et al., 2014).
The EGFR positive staining was indicated by brown cytoplasmic, membranous, or both cytoplasmic and membranous staining of tumor cells. The score used for EGFR interpretation according to Morinaga et al., (2006); Buckley et al., (2008) and Harder et al., (2009) is the number of positive cells evaluated under x400 magnification (Extent of expression) and was assessed as:
0 = no positive cells
1+ = 1-10% positive cells,
2+ = 11-50% positive cells,
3+ = > 51% of cells with positive staining.
The Ki-67 index was obtained by the percentage of tumor cells that were labeled at nuclei by Ki-67 (Zhao et al., 2015).
Ethical Considerations
Confidentiality of data in medical records was maintained in this study. Data were available to researcher without identification of patients. Approval was exempted for the used data with no interference to patients and their confidentiality. Archived samples.
Statistical analysis
Pearson’s Chi square test was used to compare the differences in percentages of positive results between groups. ANOVA and student t-tests were used to compare groups’ means. Spearmann’s test was used to correlate different parameters of the studied groups. SPSS 20.0 for Windows was used for all statistical analyses. Significant differences between groups were achieved if (p<0.05).
Figure 1.
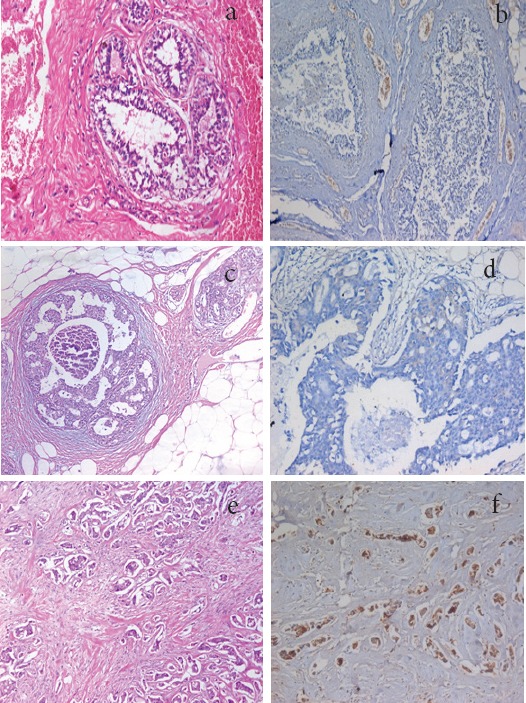
a, Section in Breast Tissue Showing Benign Fibrocystic Changes (Hematoxylin and eosin stain, X100); b, section in case of fibrocystic disease showed negative RAGE expression in ductal epithelium (IHC for RAGE, X100); c, Section in breast tissue showing Duct Carcinoma In Situ (DCIS), with cribriform pattern (Hematoxylin and eosin stain, X100), d, section in duct carcinoma showing weak expression for RAGE (IHC for RAGE, X100); e, Section in breast tissue showing Invasive Duct Carcinoma (IDC) of moderate differentiation (G2) (Hematoxylin and eosin stain, X200); f, a case of invasive duct carcinoma showing positive expression for RAGE in tumor cells (IHC for RAGE, X200).
Figure 2.
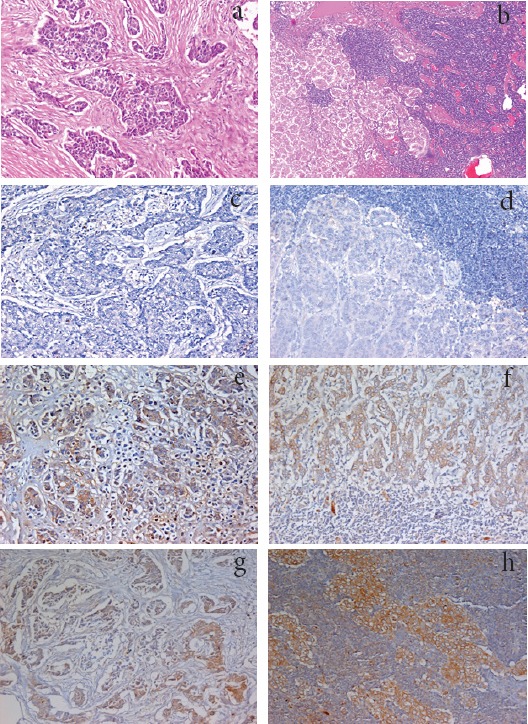
a, Section in Breast Tissue Showing Invasive Duct Carcinoma (IDC) (G2) (Hematoxylin and eosin stain, X200); b, Section in axillary lymph node showing metastatic deposits of Invasive Duct Carcinoma (IDC) of moderate differentiation (G2) (Hematoxylin and eosin stain, X200); c, Section in breast tissue showing Invasive Duct Carcinoma (IDC) of low grade (G2), showing negative EGFR expression (IHC for EGFR-DAB, X200); d, Section in axillary lymph node showing metastatic deposits of Invasive Duct Carcinoma (IDC) with negative expression for EGFR (IHC for EGFR-DAB, X200); e, Section in case of invasive duct carcinoma showing positive expression for RAGE in tumor cells (IHC for RAG, X200); f, Section in axillary lymph node showing metastatic deposits of duct carcinoma with positive expression for RAGE (IHC for RAGE, X200); g, Section in case of invasive duct carcinoma showing positive expression for EGFR in tumor cells (IHC for EGFR, X200); h, Section in a case of metastatic deposits of invasive duct carcinoma in axillary lymph node showing positive expression for EGFR (IHC for EGFR, X200).
Figure 3.
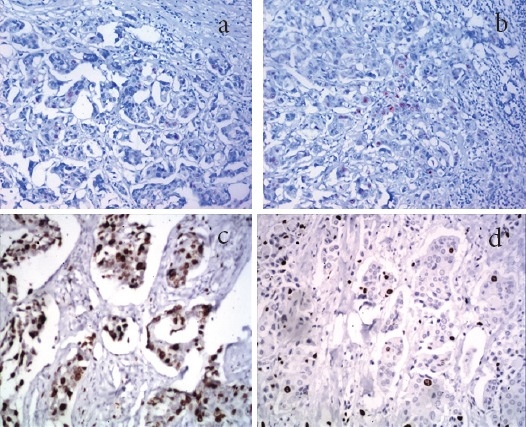
a, Section in Case of Invasive Duct Carcinoma of G2 Showing Few Scattered Positive Nuclear Staining for Ki-67 in Some Tumor Cells (IHC for Ki-67, X200); b, section in the metastatic deposit in axillary lymph node of the same case in (a); showing nearly the same percentage of Ki-67 positive nuclei (IHC for KI-67, X200). c, Section in case of invasive duct carcinoma showing high expression of Ki-67 (IHC for Ki-67, X400). d, Section in axillary LN showing moderate expression of Ki-67 in metastatic deposits of duct carcinoma (IHC for Ki-67, X400).
Figure 4.

Difference in EGFR Intensity in Different Stages of Breast Cancer
Figure 5.

Difference in EGFR Percentage in Different Stages of Breast Cancer. Graph, show Difference in percentage of cellular expression of EGFR between different grades of Breast cancer and variants of lymph node.
Results
The present study includes 170 cases of breast lesions examined at the pathology department of Theodor Bilharz Research institute from 2013 to 2016. Types of examined materials include 125 tissue biopsies, 40 cases of fine needle aspiration cytology (FNAC) and 5 cases as slide consultations. All slide consultations showed malignant results. However, most of biopsies and FNACs showed benign breast lesions, with no statistically significant differences (Table 1).
Table 1.
Types and Diagnoses of Studied Cases
| Type of examined specimen | Pathological Result | |||
|---|---|---|---|---|
| benign | malignant | Total | ||
| Biopsy | Count | 65a | 60a | 125 |
| % within type | 52% | 48% | 100.0% | |
| FNAC | Count | 26a | 14a | 40 |
| % within type | 65% | 35% | 100.0% | |
| Slides | Count | 0a | 5a | 5 |
| % within type | 0.0% | 100.0% | 100.0% | |
| Total | Count | 91 | 79 | 170 |
| % within type | 53.53% | 46.47% | 100.0% | |
Our study was conducted on breast and lymph node biopsies from 170 female patients ranging in age from 18 to 55 years. The mean age for benign cases was 31.45±9.22 years and for malignant cases was 48.65±13.81 (p <0.01). Tissue samples include 65 excision biopsies from benign cases and 60 modified radical mastectomy biopsies from malignant cases.
Benign lesions include fibrocystic disease of breast (34 cases) and fibroadenoma (31 cases), while malignant tumors include invasive duct carcinoma (50 cases), invasive lobular carcinoma (2 cases), medullary carcinoma (1 case), mucoid carcinoma (1 case) and intraduct carcinoma (6 cases). Invasive duct carcinoma cases include 3 cases of tubular carcinoma, and 47 cases of non-otherwise specified (NOS) carcinoma.
Most of the studied cases were of low grade but high stage of malignancy (GIandII, T2 and 3). There were high significant differences in means of all RAGE expression parameters between benign and malignant breast lesions. Parameters of RAGE expression were also higher in lymph node metastatic deposits in relation to primary breast tumors (Table 2). Low grade invasive breast cancer show higher percentage of RAGE expression compared to DCIS and high grade invasive breast cancer with significant difference between groups (p<0.01).RAGE intensity and RAGE score showed increasing values from DCIS up to high grade invasive breast carcinoma. However, the difference between groups was not statistically significant (p>0.05).
Table 2.
Difference in RAGE Expression Parameters between Benign and Malignant Breast Lesions, Compared to Lymph Node (LN) Metastasis
| diagnosis | RAGE percent | RAGE intensity | RAGE score | LN RAGE percent | LN RAGE Intensity | LN RAGE score | |
|---|---|---|---|---|---|---|---|
| benign | Mean | 3.33 | 1.00 | 1.00 | - | - | - |
| Number of cases | 65 | 65 | 65 | - | - | - | |
| Std. Deviation | 1.36 | 0.00 | 0.00 | - | - | - | |
| malignant | Mean | 63.61 | 2.02 | 6.57 | 86.66 | 2.41 | 9.45 |
| Number of cases | 60 | 60 | 60 | 48 | 48 | 48 | |
| Std. Deviation | 29.13 | 0.74 | 3.80 | 8.58 | 0.49 | 2.04 | |
| Total | Mean | 57.37 | 1.89 | 6.00 | 86.66 | 2.41 | 9.45 |
| Number of cases | 66 | 66 | 66 | 48 | 48 | 48 | |
| Std. Deviation | 33.20 | 0.76 | 3.98 | 8.58 | 0.49 | 2.04 | |
| ANOVA | P<0.001 | P<0.01 | P<0.01 | - | - | - | |
Intensity and score of RAGE expression were higher in lymph node metastatic deposits of high grade invasive breast cancer compared to the same parameters in case of low grade invasive breast cancer with statistical significance (p<0.05 and p<0.01 respectively). On the other hand the percentage of RAGE expression in lymph node of metastatic deposits was non-significantly higher in low grade invasive breast cancer compared to cases of high grade invasive breast cancer (p>0.05).
Also, there is increase in the value of all RAGE expression parameters from DCIS up to high stage of invasive breast cancer, however, the difference was significant in case of RAGE percent (p<0.001) and non-significant in RAGE intensity and RAGE score parameters (p>0.1 and p>0.05 respectively). All parameters were higher in lymph node metastatic deposits than in invasive breast cancer (Table 3).
Table 3.
Difference in RAGE Expression Parameters in Relation to Different Grades and Stages of Malignancy and Lymph Node Metastatic Deposits
| GRADE | RAGE percent | RAGE intensity | RAGE score | LN RAGE percent | LN RAGE intensity | LN RAGE score | |
|---|---|---|---|---|---|---|---|
| DCIS | Mean | 4.00 | 1.00 | 1.00 | - | - | - |
| Number of cases | 6 | 6 | 6 | - | - | - | |
| Std. Deviation | 1.41 | 0.00 | 0.00 | - | - | - | |
| Low grade | Mean | 66.95 | 2.24 | 6.69 | 86.81 | 2.36 | 9.22 |
| Number of cases | 46 | 46 | 46 | 44 | 44 | 44 | |
| Std. Deviation | 27.02 | 0.73 | 3.65 | 8.83 | 0.48 | 1.970 | |
| High grade | Mean | 55.00 | 2.50 | 8.00 | 85.00 | 3.00 | 12.00 |
| Number of cases | 8 | 8 | 8 | 8 | 8 | 8 | |
| Std. Deviation | 28.86 | 0.57 | 4.61 | 5.77 | 0.00 | 0.00 | |
| ANOVA | P<0.01 | p>0.05 | p>0.05 | p>0.05 | P<0.05 | P<0.01 | |
| STAGE | |||||||
| DCIS | Mean | 4.00 | 1.00 | 1.00 | |||
| Number of cases | 6 | 6 | 6 | -- | -- | -- | |
| Std. Deviation | 1.41 | 0.00 | 0.00 | ||||
| Low stage | Mean | 36.66 | 2.17 | 5.33 | 95.00 | 3.00 | 12.00 |
| Number of cases | 10 | 10 | 10 | 8 | 8 | 8 | |
| Std. Deviation | 49.05 | 0.89 | 5.16 | 5.77 | 0.00 | 0.00 | |
| High stage | Mean | 70.00 | 2.04 | 7.00 | 85.90 | 2.36 | 9.22 |
| Number of cases | 44 | 44 | 44 | 44 | 44 | 44 | |
| Std. Deviation | 20.45 | 0.71 | 3.49 | 8.44 | 0.48 | 1.970 | |
| ANOVA | P<0.001 | p>0.1 | p>0.05 | P<0.05 | P<0.05 | P<0.01 | |
The tumor grade correlates significantly with both the RAGE intensity and RAGE score of both the primary tumor and the metastatic lymph node deposits. (P<0.05). The tumor stage on the other hand correlates positively with RAGE percent and score of primary tumor tissue (p<0.05) and correlates negatively with all parameters of RAGE expression in metastatic lymph node deposits (p<0.05). RAGE percent of primary tumor tissue shows high significant correlation with RAGE percent of metastatic lymph node deposits (p< 0.01).
Data for EGFR
There were high significant differences in means of all EGFR expression parameters between benign lesions that were all negative for EGFR, and malignant breast lesions. Parameters of EGFR expression were lower in lymph node metastatic deposits in relation to primary breast tumors but without significant difference (p>0.05) (Table 4).
Table 4.
Difference in EGFR Expression between Benign and Malignant Breast Lesions, Compared to Lymph Node (LN) Metastasis
| diagnosis | Breast Mass EGFR percent | Breast Mass EGFR intensity | Breast Mass EGFR positivity | LN EGFR percent | LN EGFR Intensity | LN EGFR positivity | |
|---|---|---|---|---|---|---|---|
| Benign (65) | Mean | 23.75 | 0 | 0/65 (0%) | - | - | |
| Number of cases | 3 | 0 | - | - | |||
| Std. Deviation | 12.45 | 0.00 | - | - | - | ||
| Malignant (60) | Mean | 84.54 | 2.33 | 7/60 (11.67%) | 63.38 | 1.91 | 5/48 (10.42%) |
| Number of cases | 7 | 7 | 5 | 5 | |||
| Std. Deviation | 36.03 | 0.46 | 30.47 | 0.42 | |||
| t- test | P<0.05 | - | - | - | - | ||
Low grade invasive breast cancer show higher percentage of EGFR expression compared to DCIS and high grade invasive breast cancer with significant difference between groups (p<0.01).
EGFR intensity and EGFR score showed increasing values from DCIS up to high grade invasive breast carcinoma. However, the difference between groups was not statistically significant (p>0.05).
Intensity and score of EGFR expression were higher in lymph node metastatic deposits of high grade invasive breast cancer compared to the same parameters in case of low grade invasive breast cancer with statistical significance (p<0.05 and p<0.01 respectively). On the other hand the percentage of EGFR expression in lymph node of metastatic deposits was non-significantly higher in low grade invasive breast cancer compared to cases of high grade invasive breast cancer (p>0.05) (Table 5).
Table 5.
Difference in EGFR Expression in Relation to Different Grades and Stages of Malignancy and Lymph Node Metastatic Deposits
| Grade | Breast Ca EGFR Positivity P/T (%) | LN EGFR Positivity P/T (%) |
|---|---|---|
| DCIS (6) | 1/6 (16.67%) | - |
| Low grade (46) | 5/46 (10.87%) | 4/40 (10%) |
| High grade (8) | 1/8 (12.5%) | 1/8 (12.5%) |
| Total | 7/60 (11.67%) | 5/48 (10.42%) |
| Stage | ||
| DCIS (6) | 1/6 (16.67%) | - |
| Low stage (10) | 1/10 (10%) | 0/4 (0%) |
| High stage (44) | 5/44 (11.36%) | 5/44 (11.36%) |
| Total | 7/60 (11.67%) | 5/48 (10.42%) |
There is an increase in the value of all EGFR expression parameters from DCIS up to high stage of invasive breast cancer, however, the difference was significant in case of EGFR percent (p<0.001) and non-significant in EGFR intensity and EGFR score parameters (p>0.1 and p>0.05 respectively). All parameters were higher in lymph node metastatic deposits than in invasive breast cancer, but without significant difference (p>0.05).
As regard the correlations; Spearmann’s test showed that the tumor grade correlates significantly with both the EGFR intensity and EGFR score of both the primary tumor and the metastatic lymph node deposits (P<0.05). The tumor stage on the other hand correlates positively with EGFR percent and score of primary tumor tissue (p<0.05) and correlates negatively with all parameters of EGFR expression in metastatic lymph node deposits (p<0.05). EGFR percentage of primary tumor tissue shows highly significant correlation with EGFR percentage of metastatic lymph node deposits (p< 0.01).
Ki-67 expression data
There was a highly significant difference in the mean value of Ki-67 expression between low and high grades of primary breast carcinoma (p<0.001). Also, There was a high significant difference in Ki-67 expression between axillary lymph node metastatic deposits of low and high grades of breast carcinoma. (p<0.001). However, there was no significant differences in Ki-67 expression between the primary breast carcinoma and their metastatic deposits in axillary lymph nodes in its low or high grades. (p>0.1).
On the other hand, There was no significant differences in Ki-67 expression values between either low and high stages of breast carcinoma or between their metastatic deposits in axillary lymph nodes. (p>0.1). Also, there was no significant difference in the Ki-67 expression values between the primary breast carcinoma and their metastatic deposits in axillary lymph nodes in its low or high stages.(p>0.1) (Table 6).
Table 6.
Differential Expression of Ki-67 in Primary Breast Tumors and Metastatic Lymph Node Deposits in Relation to Grade and Stage
| GRADE | Number | Mean | Std. Deviation | |
|---|---|---|---|---|
| Tumor Ki67 | low | 46 | 14.57 | 10.29 |
| high | 8 | 40.60** | 20.31 | |
| LN Ki67 | low | 46 | 16.52 | 14.69 |
| high | 8 | 43.73## | 18.84 | |
| P value | ||||
| STAGE | <0.001 | |||
| Tumor Ki67 | Low | 10 | 22.17 | 18.43 |
| High | 44 | 21.03 | 15.86 | |
| LN Ki67 | Low | 10 | 24.02 | 15.01 |
| High | 44 | 22.21 | 17.15 | |
| P value | N.S |
represent high significant difference in means of Ki-67 expression between low and high grades of primary breast carcinoma (p<0.001).
represent high significant difference in means of Ki-67 expression between low and high grades of axillary lymph node metastatic breast carcinoma (p<0.001)+.
Discussion
Breast cancer is a complex disease that results from the inheritance of a number of susceptible genes. Although exhaustive investigations from single-locus to genome-wide association studies have been conducted, to unravel the ultimate genetic underpinnings of breast cancer still remains a challengeable task (Hana et al., 2015).
Metastasis is a major cause of mortality in Breast Cancer (BC) patients. Among the different types of BC, triple negative BC (TNBC) (ER-, PR-, and HER2-) has been associated the most with poor prognosis and survival due to early metastasis to other organs and a lack of clinically established targeted therapies. Hence, elucidating novel mechanisms that regulate metastasis would lead to the development of targeted therapies and new treatments for TNBC and metastatic breast cancers (Cheng et al., 2013).
Our study was conducted on breast and lymph node biopsies from 170 female patients ranging in age from 18 to 55 years. The mean age for benign cases was 31.45±9.22 years and for malignant cases was 48.65±13.81 (p <0.01). It is well documented that the risk of getting breast cancer increases with age. That is, 3 or 4 out of every 100 women who are 60 years old today will get breast cancer by the age of 70 (Howlader et al., 2015).
Tissue samples include 65 excision biopsies from benign cases and 60 modified radical mastectomy biopsies from malignant cases.
Benign lesions include fibrocystic disease of breast (34 cases) and fibroadenoma (31 cases), while malignant tumors include invasive duct carcinoma (50 cases), invasive lobular carcinoma (2 cases), medullary carcinoma (1 case), mucoid carcinoma (1 case) and intraduct carcinoma (6 cases). Invasive duct carcinoma cases include 3 cases of tubular carcinoma, and 47 cases of non-otherwise specified (NOS) carcinoma.
It is now well accepted that solid tumors, including those in the breast, have an inflammatory microenvironment. Receptor for advanced glycation end products (RAGE) is a member of the immunoglobulin superfamily of cell surface molecules which has been associated with chronic inflammation, which in turn enhances the progression of various cancers (Nasser et al., 2015).
The selection of RAGE gene as a research candidate is biologically plausible. RAGE is a member of the immunoglobulin superfamily of cell surface receptors, and its interaction with advanced glycation end products and other molecules plays a role in the pathogenesis of cancer progression and metastasis (Tesarova et al., 2007). There were high significant differences in means of all RAGE expression parameters between benign and malignant breast lesions, with higher levels achieved in malignant cases. This was in agreement with Hongming et al., (2014).
Low grade invasive breast cancer show higher percentage of RAGE expression compared to DCIS and high grade invasive breast cancer with significant difference between groups (p<0.01).
RAGE intensity and RAGE score showed increasing values from DCIS up to high grade invasive breast carcinoma. However, the difference between groups was not statistically significant (p>0.05). In this aspect, our results were in concordance with the results of Hongming et al., (2014).
We found also that, the intensity and score of RAGE expression were higher in lymph node metastatic deposits of high grade invasive breast cancer compared to the same parameters in case of low grade invasive breast cancer with statistical significance (p<0.05 and p<0.01 respectively). On the other hand the percentage of RAGE expression in lymph node of metastatic deposits was non-significantly higher in low grade invasive breast cancer compared to cases of high grade invasive breast cancer (p>0.05). These results were in agreement with Nasser et al., (2015) showing that RAGE is preferentially expressed in invasive and lymph node metastasis tissues and with Radia et al., (2013).
Our results were confirmed by the fact that the tumor grade correlates significantly with both the RAGE intensity and RAGE score of both the primary tumor and the metastatic lymph node deposits. (P<0.05). The tumor stage on the other hand correlates positively with RAGE percent and score of primary tumor tissue (p<0.05) and correlates negatively with all parameters of RAGE expression in metastatic lymph node deposits (p<0.05). RAGE percent of primary tumor tissue shows high significant correlation with RAGE percent of metastatic lymph node deposits (p< 0.01). In evaluating the functional contributions of RAGE in breast cancer, found that RAGE-deficient mice displayed a reduced propensity for breast tumor growth. In an established model of lung metastasis, systemic blockade by injection of a RAGE neutralizing antibody inhibited metastasis development (Nasser et al., 2015). Decades of research in molecular oncology have brought about promising new therapies that are designed to target specific molecules that promote tumor growth and survival. The epidermal growth factor receptor (EGFR) is one of the first identified important targets of these novel antitumor agents.(Masuda et al., 2012). Dysregulation of EGFR pathways by overexpression or constitutive activation can promote tumor processes including angiogenesis and metastasis and is associated with poor prognosis in many human malignancies (Salomon et a., 1995; Lurje and Lenz, 2009; Martinazzi et al.,1993).
In our study there were high significant differences in means of EGFR expression percentage and intensity between benign (that were all negative for EGFR), and malignant breast lesions. Our study showed also that EGFR expression was moderate in lower grade breast cancer, while marked expression was seen in higher grades of IBC. This was in agreement with Magkou et al., (2008) who stated that with regard to EGFR, its expression was positively associated with nuclear grade (P = 0.001).
In addition, parameters of EGFR expression were non significantly lower in lymph node metastatic deposits in relation to primary breast tumors (p>0.05). Percentage of EGFR cellular positivity and intensity of staining were higher in primary breast cancers of all stages compared to metastatic deposits in lymph nodes, however, the differences between both groups were non-significant using t-test (p>0.05). This was in agreement with (Cho et al., 2008) who stated that EGFR expressions showed a concordance between the primary lesion and the metastatic regional lymph nodes in 90% of cases.
In our study, there was a highly significant difference in the mean value of Ki-67 expression between low and high grades of primary breast carcinoma (p<0.001). Also, There was a high significant difference in Ki-67 expression between axillary lymph node metastatic deposits of low and high grades of breast carcinoma. (p<0.001). However, there was no significant differences in Ki-67 expression between the primary breast carcinoma and their metastatic deposits in axillary lymph nodes in its low or high grades. (p>0.1).
Once again, the differentiation of tumors showed obvious correlation with Ki-67. G1 tumors had Ki-67-labeling indices of 10 %, G2 tumors of 16 %, and G3 tumors of 37 %. Regarding the tumor size, there were no substantial differences in Ki-67 distribution. Regarding the tumor size, there were no substantial differences in Ki-67 distribution (Inwald et al., 2013).
On the other hand, There was no significant differences in Ki-67 expression values between either low and high stages of breast carcinoma with their metastatic deposits in axillary lymph nodes (p>0.1). Also, there was no significant difference in the Ki-67 expression values between the primary breast carcinoma and their metastatic deposits in axillary lymph nodes in its low or high stages (p>0.1).
Previous study reported the median Ki67 expression in primary and metastatic tumors was 20% and 15%, respectively (Tawfik et al., 2015). We compared primary tumors with their corresponding metastatic lesions, and found that there were no significant differences in the expression of Ki67 between them, which is same with studies carried out by Tawfik et al., (2015), but different from studies have found greater expression of Ki67 in metastatic tumors compared with primary tumors (Park et al., 2007; Buxant et al., 2002; Tokes et al., 2015). That Ki67 expression is useful in metastatic tumors but not in primary tumors has been reported (Park et al., 2007). Interestingly, it was demonstrated that a high Ki67 in ALN but not in breast is significantly associated with shorter patient survival, sug¬gesting that patients with higher Ki67 level in LN metastases might require more aggressive therapy and closer clinical monitoring of their disease. Therefore, further studies are recommended to further investigate the heterogeneity of both stem and tumor cells in primary and metastatic tumors to design therapies that are tailored to target the specific clones.
In conclusion, our overall results give additional evidence that not only primary breast cancer but also metastatic breast cancer within axillary lymph nodes would be also a subject for anti-RAGE and anti EGFR targeted therapy.
Acknowledgments
Authors of this paper are greatly thankful to Mr. Magdy Wahbi, Mrs. Nadia Abdullah and Mr. Hany Ramadan; histopathology technicians at the pathology department, Theodor Bilharz Research Institute, for their help in routine and immunohistochemical techniques.
References
- 1.Arens N, Bleyl U, Hildenbrand R. HER2/neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virchows Arch. 2005;446:489–96. doi: 10.1007/s00428-005-1244-0. [DOI] [PubMed] [Google Scholar]
- 2.Buckley AF, Burgart LJ, Sahai V, Kakar S. Epidermal growth factor receptor expression and gene copy number in conventional hepatocellular carcinoma. Am J Clin Pathol. 2008;129:245–51. doi: 10.1309/WF10QAAED3PP93BH. [DOI] [PubMed] [Google Scholar]
- 3.Burcombe R, Wilson GD, Dowsett M, et al. Evaluation of Ki-67 proliferation and apoptotic index before, during and after neoadjuvant chemotherapy for primary breast cancer. Breast Cancer Res. 2006;8:R31. doi: 10.1186/bcr1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burcombe RJ, Makris A, Richman PI, et al. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer. 2005;92:147–55. doi: 10.1038/sj.bjc.6602256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxant F, Anaf V, Simon P, Fayt I, Noel JC. Ki-67 immunostaining activity is higher in positive axillary lymph nodes than in the primary breast tumor. Breast Cancer Res Trea. 2002;75:1–3. doi: 10.1023/a:1016504129183. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Herndon ME, Lawler J. Systemics RAGE is a biomarker of emphysemaand with AGER genetic variants in COPD patients. Am J Respir Crit Care Med Metastasis. 2013;19:1423–37. [Google Scholar]
- 7.Cho Eun Y, Jae Joon H, Yoon-La C, Kyoung-Mee K, Young LO. Comparison of Her-2, EGFR and Cyclin D1 in primary breast cancer and paired metastatic lymph nodes:An immunohistochemical and chromogenic in situ hybridization study. J Korean Med Sci. 2008;23:1053–61. doi: 10.3346/jkms.2008.23.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti- HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 9.Dai L, Liu M, Zhu Q, et al. Using immunomic approach to enhance tumor-associated autoantibody detection in diagnosis of hepatocellular carcinoma. Cancer Res. 2014;74:885. doi: 10.1016/j.clim.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inwald EC, Klinkhammer-Schalke M, Hofsta¨dter F, et al. Ki-67 is a prognostic parameter in breast cancer patients:results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–52. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gown AM. Molecular vs. immunohistochemical classification of breast cancer. Breast Cancer. 2009. pp. 28–30. https://pdfs.semanticscholar.org/c2e8/6f2b50779ec877d705f1d9aa5b1d5d.e471f3.pdf .
- 12.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hana S, Sabine M, Wang-Zaki R, et al. Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochim Biophys Acta. 2015;1852:429–41. doi: 10.1016/j.bbadis.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Harder J, Waiz Otto F, Geissler M, et al. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol. 2009;15:4511–7. doi: 10.3748/wjg.15.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht SS. Tabacco smoke carcinogens and breast cancer. Environ Mol Mutagen. 2002;39:119–26. doi: 10.1002/em.10071. [DOI] [PubMed] [Google Scholar]
- 16.Hongming P, Lan H, Bin W, Wenquan N. The relationship between RAGE gene four common polymorphisms and breast cancer risk in northeastern Han Chinese. Sci Rep. 2014;4:4355. doi: 10.1038/srep04355. 10.1038/srep04355 DOI:10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer. Stat Rev. 2015;1975–2012 https://seer.cancer.gov/archive/csr/1975_2014/ [Google Scholar]
- 18.Lata K, Mukherjee TK. Knockdown of receptor for advanced glycation end productsattenuat e17alpha-ethinyl-estradioldependentproliferationand survival of MCF-7 breast cancer cells. Biochim Biophys Acta. 2014;1840:1083–91. doi: 10.1016/j.bbagen.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–10. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 20.Magkou C, Lydia N, Christina Z, et al. Expression of the epidermal growth factor receptor (EGFR) and the phosphorylated EGFR in invasive breast carcinomas. Breast Cancer Res. 2008;10:R49. doi: 10.1186/bcr2103. (doi:10.1186/bcr21030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinazzi M, Crivelli F, Zampatti C, Martinazzi S. Relationships between epidermal growth factor receptor (EGF-R) and other predictors of prognosis in breast carcinomas. An immunohistochemical study. Pathologica. 1993;85:637–44. [PubMed] [Google Scholar]
- 22.Masuda H, Dongwei Z, Chandra B, et al. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat. 2012;136 doi: 10.1007/s10549-012-2289-9. 10.1007/s10549-012-2289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mink PJ, Shahar E, Rosamond WD, Alberg AJ. Serum insulin and glucose level and breast cancer incidence. Am J Epidemiol. 2002;4:349353. doi: 10.1093/aje/kwf050. [DOI] [PubMed] [Google Scholar]
- 24.Morinaga S, Nakamura Y, Sugano N, et al. Expression of epidermal growth factor receptor (EGFR) associates with proliferation, apoptosis and histologically aggressive features in hepatocellular carcinoma. Ann Meeting Proceedings. 2006;24:14039. [Google Scholar]
- 25.Nasser MW, Wani NA, Ahirwar DK, et al. RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer Res. 2015;75:974–85. doi: 10.1158/0008-5472.CAN-14-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neubauer H, Gall C, Vogel U, et al. Changes in tumour biological markers during primary systemic chemotherapy (PST) Anti Cancer Res. 2008;28:1797–1804. [PubMed] [Google Scholar]
- 27.Park D, Karesen R, Noren T, Sauer T. Ki-67 expression in primary breast carcinomas and their axillary lymph node metastases:clinical implications. Virchows Arch. 2007;451:11–8. doi: 10.1007/s00428-007-0435-2. [DOI] [PubMed] [Google Scholar]
- 28.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radia AM, Yaser AM, Ma X, et al. Specific siRNA targeting receptor for advanced Glycation end products (RAGE) decreases proliferation in human breast cancer cell lines. Int J Mol Sci. 2013;14:7959–78. doi: 10.3390/ijms14047959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment:the role of multiligand/RAGE axis. Carcinogenesis. 2010;31:334–41. doi: 10.1093/carcin/bgp322. [DOI] [PubMed] [Google Scholar]
- 31.Salomon DS, Brandt R, Ciardiello F, Normanno N. Crit Epidermal growth factor-related peptides and their receptors in human malignancies. Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 32.Schnitt SS. Classification and prognosis of invasive breast cancer:from morphology to molecular taxonomy. Mod Pathol. 2010;23:60–4. doi: 10.1038/modpathol.2010.33. [DOI] [PubMed] [Google Scholar]
- 33.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 34.Sobral-Leite M, Lips EH, Vieira-Monteiro HA, et al. Evaluation of the EGFR polymorphism R497K in two cohorts of neoadjuvantly treated breast cancer patients. PLoS One. 2017;12:e0189750. doi: 10.1371/journal.pone.0189750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taguchi A, Blood DC, Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 36.Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Ki-67 expression in axillary lymph node metastases in breast cancer is prognostically significant. Hum Pathol. 2012;44:39–46. doi: 10.1016/j.humpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Tesarová P, Kalousová M, Jáchymová M, et al. Receptor for advanced glycation end products (RAGE)-soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Invest. 2007;25:720–5. doi: 10.1080/07357900701560521. [DOI] [PubMed] [Google Scholar]
- 38.Thor A. Are patterns of HER-2/neu amplification and expression among primary tumors and regional metastases indicative of those in distant metastases and predictive of Herceptin response? J NatlCancer Inst. 2001;93:1120–1. doi: 10.1093/jnci/93.15.1120. [DOI] [PubMed] [Google Scholar]
- 39.Tokes AM, Szasz AM, Geszti F, et al. Expression of proliferation markers Ki67, cyclin A, geminin and aurora-kinase A in primary breast carcino-mas and corresponding distant metastases. J Clin Pathol. 2015;68:274–82. doi: 10.1136/jclinpath-2014-202607. [DOI] [PubMed] [Google Scholar]
- 40. WWW.ijcep.com . ISSN:1936-2625/IJCEP0007867.
- 41.Ye Y, Qiu TH, Kavanaugh C, Green JE. Molecular mechanism of breast cancer progression:Lessons from mouse mammary cancer models and gene expression profiling. J Breast Cancer. 2004;19:69–82. doi: 10.3233/bd-2004-19109. [DOI] [PubMed] [Google Scholar]
- 42.Zhanga S, Xuwei H, Sihua Z, et al. Polymorphisms of receptor for advanced glycation end products and risk of epithelial ovarian cancer in Chinese patients. Cell Physiol Biochem. 2013;31:525–31. doi: 10.1159/000350073. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S, Lanwei X, Wenjun L, et al. Comparison of the expression of prognostic biomarkers between primary tumor and axillary lymph node metastases in breast cancer. Int J Clin Exp Pathol. 2015;8:5744–8. [PMC free article] [PubMed] [Google Scholar]


