Abstract
Objective:
Glucocorticoids are one of the most important drugs in the treatment of acute lymphoblastic leukemia for children. It is very important to response to glucocorticoid in the prognosis of these patients. Therefore, resistance to treatment is a major problem in lymphoid leukemia cases. In, this study, CCRF-CEM cell line was selected as a chemotherapy-resistant model. The aim of this study was to evaluate the effect of high dose prednisolone on induction of apoptosis and changes in BAX and BCL-2 gene expression at different times.
Methods:
CCRF-CEM cell lines were grown in standard conditions. Based on previous studies, a dose of 700 µM as subtoxic dose was selected. Changes in gene expression of BAX and BCL-2 were evaluated by using real time PCR techniques. Also stained with annexin V and the induction of apoptosis was assessed by FACS machine.
Results:
In this study it was found that prednisolone in high doses at different times significantly increased the gene expression of BAX and on the other hand the amount of BCL-2 expression was reduced. Cells that treated for 48 hours had more significant changes in gene expression. Based on flowcytometry data, prednisolone can induce apoptosis in a time dependent manner on this cancerous resistant cell line.
Conclusions:
Apoptosis induced by high-dose prednisolone in the CCRF-CEM cells, which is almost resistant, and possibly mediated by reducing the expression of BCL-2 and BAX up-regulation.
Keywords: Leukemia, Prednisolone, BCL-2 associated X, BCL-2 gene, apoptosis
Introduction
Acute lymphoblastic leukemia (ALL) is an invasive cancer with rapid growth in white blood cells, which are lymphoid precursors involved and uncontrolledly reproduced (Erduran et al., 2006; Yousefi et al., 2017; Heydarabad et al., 2018). About 70 to 80 percent of the cancers belong to blood malignancies and the most common one in children is ALL (Hossain et al., 2016; Aghvami et al., 2018). ALL treatment usually starts with glucocorticoid medications, and Treatment continues on the basis of their response to glucocorticoids, with chemotherapy drugs (Inaba et al., 2013; Bindreither et al., 2014). Glucocorticoids (GCs) play an important role in many biological processes, such as metabolism, growth, differentiation, reproduction, immunity and neurological activity. It also has anti-inflammatory and anti-proliferative effects on immune cells, which is why they are used as immunosuppressive and anti-inflammatory agents and have anti-cancer properties (Tissing et al., 2003; Meng and Yue, 2014; Azimi et al., 2016). Glucocorticoids have significant effects on lymphoid cells and causes cell cycle arrest and apoptosis (Tissing et al., 2003). In case of blood malignancies, patients who treated with glucocorticoids and (induction therapy) respond well, had good prognosis and patient with poor response had bad prognosis(Lambrou et al., 2009). Glucocorticoids, especially prednisolone, play an important role in almost all ALL treatment protocols, and they can prevent the progression of the cell cycle and induces apoptosis in ALL cells (Hulleman et al., 2009). Prednisolone is a synthetic glucocorticoid and is most used in the treatment of ALL, especially in children (Lambrou et al., 2009) and also, used in this study due to lower side effects than other glucocorticoids. The mechanism of glucocorticoid effect is that GCs enter the cell with passive transport and attach to the GRs (glucocorticoid receptors) that located in the cytoplasm, then GCs attachment to the GR causes them to be dimerized and translocated to the nucleus, where they target different genes (transcribing the various genes involved in cell death, activating or deactivating certain genes) (Tissing et al., 2005; Spenerova et al., 2014). The function of glucocorticoid receptors might be vary based on type of tissue and cell (Lambrou et al., 2009). We used the CCRF-CEM cell line as a system of study because other studies have shown that these cells are resistant to GCs in routine doses (Kofler, 2000; Lambrou et al., 2009). As well as, previous studies have shown that low-dose of prednisolone has proliferative effect on lymphoid cell lines and the higher doses have apoptotic effects (Lambrou et al., 2009). Therefore, we used a high dose of prednisolone in our study to evaluate the performance of CCRF-CEM cells. We chose the acute lymphoblastic cell line with mutation in GRs to evaluate the effect of massive dose of prednisolone, because the routine doses do not have a significant effect on this cell line. Induction of apoptosis is one of the basic goals of the therapeutic protocols (Hassan et al., 2013). The main proteins of the apoptosis pathways include, the BCL-2 family, which is associated abnormal expression in lymphoid leukemia. The role of BCL-2 family proteins in glucocorticoid resistance is noteworthy. bcl2 and bax are the most important proteins in balancing apoptosis. These proteins have anti-apoptotic and pro-apoptotic properties (Alshatwi, 2011). In lymphoid leukemia this balance was disrupt (Azimi et al., 2018). BAX expression sometimes increases the proliferation of cancerous cells, For instance, in breast and ovarian cancers, BAX expression increases reproductive capacity (Marx et al., 1997; Narayan et al., 2007). BCL-2 in various conditions, such as radiation therapy, Chemotherapy, GCs, cytotoxic lymphocains, and thermal shocks inhibit apoptosis (Narayan et al., 2007). It has been shown that BCL-2 retained T-ALL cell lines (CCRE-CEM) against apoptosis induced by GCs (Hartmann et al., 1999). As well as, previous studies showed that BCL-2 overexpression associated with poor treatment results in hematologic malignancies such as lymphoma, CLL and AML (Narayan et al., 2007; Ghafouri-Fard et al., 2012; Esmaili et al., 2016). Bcl2 prevents apoptosis via a direct effect on mitochondria and prevent the increased permeability of membranes and interactions with other proteins (Yang and Korsmeyer, 1996). Studies on certain tumor cell lines in vitro, indicating that excessive expression of BCL-2 prevents cell death (Findley et al., 1997). Coustan-Smith et al. (1995) found high levels of bcl-2 in both cancerous T and B blasts (Coustan-Smith et al., 1996). In a study also, the relationship between bcl-2 / bax ratio and clinical outcome was evaluated and shown that, High levels of bcl-2 / bax ratios are associated with failure in complete amelioration in acute leukemia cases (Stoetzer et al., 1996). In our study we aimed to evaluate the effect of massive dose of prednisolone in BAX and BCL2 gene expression levels and apoptosis induction in GR mutated Acute lymphoblastic leukemia cell line (CCRF-CEM).
Materials and Methods
Cell culture and cell line
The CCRF-CEM cell line was purchased from Pasteur Institute (Tehran, Iran) and cultured in RPMI 1640 (GiBCO, USA) containing 10% FBS (GIBCO, USA) and Antibiotic (containing 1000 U / ML penicillin and 10 mg / ml of Streptomycin), then incubated at 37 °C in a humid chamber containing 5% Co2.
Treatment
Prednisolone (Purity 98%, Sigma Aldrich, Germany) was dissolved in 1% ethanol and added to a culture medium of CCRF-CEM at a concentration of 700 μM. Finally, the cells were harvested from the medium at 12, 24 and 48 hours after extraction of total RNA.
RNA Extraction
Total RNA was extracted using TRIZOL (Invitrogen, USA) reagent, according to the protocols. For RT-PCR analysis of both gene expression and quality, the NanoDROP UV-VIS 2000C spectrophotometer (THERMO, USA) were determined. RNA quality parameters recommended for RT-PCR analysis include: UV SPECTROSCOPY A260/280 ratio of 1.8 -2.0 and A260/A230 ratio greater than 1.8, 18 s/28 s rRNA ratio of 1.8-2.1.
CDNA Synthesis and Real-Time PCR (RT-PCR)
After 12, 24 and 48 hours after treatment with prednisolone, cDNA was determined using the SYBERGREEN qRT-PCR kit (Invitrogen, USA) according to the protocols from total RNA. PCR reaction was performed using reverse primers and forward primer for each gene (Takapouzist, Tehran, Iran). The β-actin as a housekeeping gene was used to normalize the cDNA variation. The sequence of forward and reverse primers for PCR was designed based on previous studies. Primer sequence for training:BAX forward primer: 5`-GCCCTTTTGCTTCAGGGTTT-C`; BAX reverse primer: 5`- TCCAATGTCCAGCCTTTG-3`; BCL-2 forward primer: 5`-CGGAGGCTGGGATGCCTTTG-3`; BCL2 revers primer:5`TTTGGGGCAGG CATGTTGAC-3`; β-actin revers primer: 5`-GAGACCTTCAACACCCCAGCC-3`; β-actin revers primer: 5`-AGACGCAGGATGGCATGGG-3`. All reactions were triplicated.
Apoptosis analysis by annexin V staining
CCRF-CEM cells were cultured (2-5 × 105 per well) and treated with prednisolone for 24 and 48 hours. The incubation time of 12 hrs, because of less efficacy on both gene expression was excluded for flow cytometry analysis. The cells were washed with PBS and then centrifuged. The remaining components absorbed onto the float and the remaining components were suspended, then 5 µL was added from Annexin V for each 100 µL suspension and then incubated for 15 minutes in a dark place at room temperature. Finally, the apoptosis induction was analyzed by FACS machine after each treatment (BD, FACS CALIber, USA).
Statistical analysis
The statistical calculations were performed with GraphPad Prism software (GraphPad Prism softwareInc, CA, USA). Student’s t-test was used for a comparison between control and test group. P<0.05 was considered statistically significant.
Results
The effect of prednisolone on the expression of BAX and BCL-2
Based on our results and other similar studies, a high dose of 700 μM of prednisolone was used to induce apoptosis in the CCRF-CEM cell line that was mutated in its glucocorticoid receptor (Lambrou et al., 2009). This cell line is a model of relapse T- acute lymphoblastic leukemia. Other studies suggested that prednisolone in massive dose might be induce apoptosis through different pathways from glucocorticoid receptors. To evaluate the effects of high dose prednisolone on the expression of BAX and BCL-2 genes in control and treated group, RT-PCR technique were used for analysis of the these two genes. High dose of prednisolone significantly up-regulated BAX expression and down-regulated BCL-2 in a time dependent manner. The most effect was observed in 48 hours.
Analysis of apoptotic cells with flow cytometry
At 24 and 48 hours after treatment, the induction of apoptosis in the treated group was evaluated in comparison with the control group. The control group is treated with 1% ethanol as a vehicle control. As shown in Figure 2, high dose of prednisone induces apoptosis in a time dependent manner in glucocorticoid-resistant cell line. Apoptosis in the control groups were about 2.1% and 2.6% after 24 and 48 hours of treatment, respectively. The amount of apoptosis induction after 24 and 48 hours were about 4.3% and 19.8%, respectively. Induction of apoptosis significantly increased in a time dependent manner.
Figure 1.
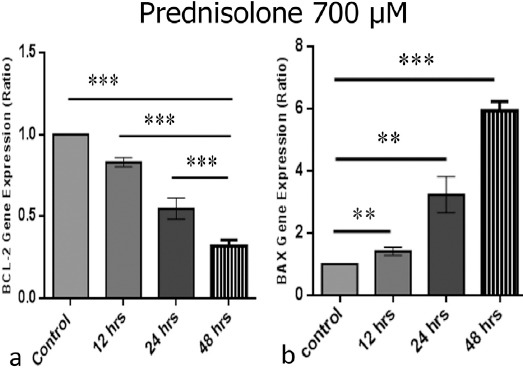
The Expression of BAX and BCL-2 Genes after Treatment with 700 μM Prednisolone after 12, 24 and 48 hours. a) BCL-2 gene expression after treatment at different times b) BAX gene expression level after treatment at different times (*P<0.05, **P<0.01, ***P<0.001).
Figure 2.
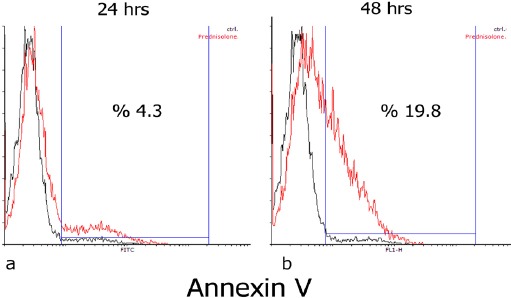
Staining with Annexin V to Evaluate Apoptosis after Treatment with Prednisolone 700 μM after 24 and 48 hours. a) Evaluation of apoptosis by staining with Annexin V after 24 hours b) Evaluation of apoptosis by staining with Annexin V after 48 hours
Disscution
Glucocorticoids (GCs) are medications that have been used for more than half a century. One of the most important GCs in the treatment of ALL is prednisolone, a type of synthetic glucocorticoid and usually affects itself through binding to glucocorticoid receptors (GRs) (Pruett et al., 2003). Prednisolone is commonly used as a selective drug in the primary care of pediatric ALL. Resistance to glucocorticoids is one of the problems encountered in chemotherapy-resistant cases, especially in patients with T-ALL recurrence. This chemo-resistant phenomenon caused poor prognosis in ALL patients (Cecchinato et al., 2007; Goossens and Van Vlierberghe, 2016). In this study, CCRF-CEM cells were used to evaluate the effects of prednisolone on the chemo-resistant cell line model. Previous studies have shown that prednisolone in high doses can affect different pathways from GRs (Lambrou et al., 2009). Therefore, we also used a subtoxic high dose of Prednisolone in our study. Induction of apoptosis is a key mechanism for anti-cancer therapy and chemo-sensitizing is considered as an application to reduce the dose of other chemo-therapy agents in ALL treatment protocol (Cecchinato et al., 2007; Zhang et al., 2014; Yousefi et al., 2016). Resistance to glucocorticoid-induced apoptosis is associated with poor prognosis and ultimately results in poor clinical outcome. Some studies have shown that prednisone has a proliferative effect on lymphoid cells at low doses and has an apoptotic effect on this cell line at high doses (Sterzer et al., 2004; Lambrou et al., 2009), which is why we used high doses. In this study, we used a high dose of prednisolone to induce apoptosis in CCRF-CEM cells that resistant to GCs and observed that high dose of prednisolone induces apoptosis in this cell line in a time-dependent manner. As we showed, time has a critical role in efficacy of prednisolone. The anti-apoptotic protein BCL-2 plays an important role in the development of resistance to apoptosis, leading to the progression of cancer cells and their survival, and usually increases in leukemia (Bhadri et al., 2012; Yao et al., 2015). We investigated the effect of high prednisolone on the expression of BCL-2 and BAX genes, and we found that a high dose of prednisolone led to a decrease in BCL-2 and increase of BAX gene expression, which may be these alteration in gene expression is one of the mechanisms that high dose of prednisolone, predisposed these chemoresistant cancerous cells to apoptosis induction. In our study, treatment time of 12 hours was eliminated due to less efficacy than other times, in case of apoptosis evaluation by anxin V staining. Therefore, it can be said that the BCL-2 down-regulation and BAX up-regulation in CCRF-CEM cells, increases the cell sensitivity to apoptosis. The results of this study can be confirmed by the work of Hartman et al., (1999) who reported that BCL-2 inhibited the induction of apoptosis by GCs in the CCRF-CEM cell line. It has also been shown in his studies that expression of exogenous BCL-2 in negative BCL-2 cells, suppresses apoptosis (Findley et al., 1997). In some other studies, it has been observed that expression of high levels of BCL-2 transgenes in immature T-cells in transgenic mice significantly contributes to glucocorticoid resistance in vivo and in vitro (Miyashita and Reed, 1993), which is related to our study. Also, some studies have shown that in people with ALL, resistance to GCs in vivo and in vitro can be related to BCL-2 (Haarman et al., 1999; Salomons et al., 1999). Even expressing BCL-2 gene in pre-B-cell leukemia cells leads to high resistance to these GCs (Miyashita and Reed, 1993). Pro Apoptotic bax protein is also a member of the BCL-2 family of important proteins of the apoptotic pathway. Studies have shown that increased expression of BAX leads to an increase in the sensitivity of the ratio of apoptosis (Yao et al., 2015), which is similar to the results of our work. Finally, the results of our study suggest that in case of relapse pediatric ALL, high dose of prednisolone could sensitized these cancerous cells to apoptosis through changes in BCL-2 and BAX gene expression, While these GR mutated cells are resistant to routine doses.
Conflict of interest
The authors have no conflict of interest.
Acknowledments
We are grateful to all the members of our research group for their support and advice regarding this study. This work was supported by the Maragheh University of Medical Sciences. We thank the Drug Applied Research Center for laboratory facilities and excellent research environment.
References
- 1.Aghvami M, Ebrahimi F, Zarei MH, et al. Matrine induction of ROS mediated apoptosis in human aLL B-lymphocytes via mitochondrial targeting. Asian Pac J Cancer Prev. 2018;19:555–60. doi: 10.22034/APJCP.2018.19.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alshatwi A. Anti-proliferative effects of organic extracts from root bark of Juglans Regia L.(RBJR) on MDA-MB-231 human breast cancer cells:role of Bcl-2/Bax, caspases and Tp53. Asian Pac J Cancer Prev. 2011;12:525–30. [PubMed] [Google Scholar]
- 3.Azimi A, Hagh MF, Yousefi B, et al. The effect of prednisolone on miR 15a and miR16-1 expression levels and apoptosis in acute lymphoblastic leukemia cell line:CCRF-CEM. Drug Res (Stuttg) 2016;66:432–5. doi: 10.1055/s-0042-108640. [DOI] [PubMed] [Google Scholar]
- 4.Azimi A, Majidinia M, Shafiei-Irannejad V, et al. Suppression of p53R2 gene expression with specific siRNA sensitizes HepG2 cells to doxorubicin. Gene. 2018;642:249–55. doi: 10.1016/j.gene.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Bhadri VA, Trahair TN, Lock RB. Glucocorticoid resistance in paediatric acute lymphoblastic leukaemia. J Paediatr Child Health. 2012;48:634–40. doi: 10.1111/j.1440-1754.2011.02212.x. [DOI] [PubMed] [Google Scholar]
- 6.Bindreither D, Ecker S, Gschirr B, et al. The synthetic glucocorticoids prednisolone and dexamethasone regulate the same genes in acute lymphoblastic leukemia cells. BMC Genomics. 2014;15:662. doi: 10.1186/1471-2164-15-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecchinato V, Chiaramonte R, Nizzardo M, et al. Resveratrol-induced apoptosis in human T-cell acute lymphoblastic leukaemia MOLT-4 cells. Biochem Pharmacol. 2007;74:1568–74. doi: 10.1016/j.bcp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Coustan-Smith E, Kitanaka A, Pui C-H, et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996;87:1140–6. [PubMed] [Google Scholar]
- 9.Erduran E, Tekelioglu Y, Karakas T, et al. Comparision of the apoptotic effects on lymphoblasts and on increase of myeloid lineage cells of a short-time, high-dose methylprednisolone and the conventional-dose prednisolone treatments in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2006;23:587–98. doi: 10.1080/08880010600857053. [DOI] [PubMed] [Google Scholar]
- 10.Esmaili H, Vahedi A, Mohajeri S, et al. Evaluation of the pathogenesis of tumor development from endometriosis by estrogen receptor, P53 and Bcl-2 immunohistochemical staining. Asian Pac J Cancer Prev. 2016;17:5247. doi: 10.22034/APJCP.2016.17.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Findley HW, Gu L, Yeager AM, et al. Expression and regulation of Bcl-2, Bcl-xl, and Bax correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood. 1997;89:2986–93. [PubMed] [Google Scholar]
- 12.Ghafouri-Fard S, Abdollahi DZ, Omrani M, et al. shRNA mediated RHOXF1 silencing influences expression of BCL2 but not CASP8 in MCF-7 and MDA-MB-231 cell lines. Asian Pac J Cancer Prev. 2012;13:5865–9. doi: 10.7314/apjcp.2012.13.11.5865. [DOI] [PubMed] [Google Scholar]
- 13.Goossens S, Van Vlierberghe P. Overcoming steroid resistance in T cell acute lymphoblastic leukemia. PLoS Med. 2016;13:e1002208. doi: 10.1371/journal.pmed.1002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haarman E, Kaspers G, Pieters R, et al. In ‘Drug Resistance in Leukemia and Lymphoma III’. New York: Plenum Press; 1999. BCL-2 expression in childhood leukemia versus spontaneous apoptosis, drug induced apoptosis, and in vitro drug resistance; pp. 325–33. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann BL, Geley S, Löffler M, et al. Bcl-2 interferes with the execution phase, but not upstream events, in glucocorticoid-induced leukemia apoptosis. Oncogene. 1999;18:713. doi: 10.1038/sj.onc.1202339. [DOI] [PubMed] [Google Scholar]
- 16.Hassan ZK, Elamin MH, Omer SA, et al. Oleuropein induces apoptosis via the p53 pathway in breast cancer cells. Asian Pac J Cancer Prev. 2013;14:6739–42. doi: 10.7314/apjcp.2013.14.11.6739. [DOI] [PubMed] [Google Scholar]
- 17.Heydarabad MZ, Nikasa M, Vatanmakanian M, et al. Regulatory effect of resveratrol and prednisolone on MDR1 gene expression in acute lymphoblastic leukemia cell line (CCRF-CEM):An epigenetic perspective. J Cell Biochem. 2018;119:4890–96. doi: 10.1002/jcb.26709. [DOI] [PubMed] [Google Scholar]
- 18.Hossain T, Mannan M, Nahar S, et al. Effectiveness of dexamethasone compared with Prednisolone in induction therapy of childhood acute lymphoblastic leukemia. J Paediatr Surg Bangladesh. 2016;6:3–9. [Google Scholar]
- 19.Hulleman E, Kazemier KM, Holleman A, et al. Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood. 2009;113:2014–21. doi: 10.1182/blood-2008-05-157842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–55. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kofler R. The molecular basis of glucocorticoid-induced apoptosis of lymphoblastic leukemia cells. Histochem Cell Biol. 2000;114:1–7. doi: 10.1007/s004180000165. [DOI] [PubMed] [Google Scholar]
- 22.Lambrou GI, Vlahopoulos S, Papathanasiou C, et al. Prednisolone exerts late mitogenic and biphasic effects on resistant acute lymphoblastic leukemia cells:Relation to early gene expression. Leuk Res. 2009;33:1684–95. doi: 10.1016/j.leukres.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Marx D, Binder C, Meden H, et al. Differential expression of apoptosis associated genes bax and bcl-2 in ovarian cancer. Anticancer Res. 1997;17:2233–40. [PubMed] [Google Scholar]
- 24.Meng X-G, Yue S-W. Dexamethasone disrupts cytoskeleton organization and migration of T47D human breast cancer cells by modulating the AKT/mTOR/RhoA pathway. Asian Pac J Cancer Prev. 2014;15:10245–50. doi: 10.7314/apjcp.2014.15.23.10245. [DOI] [PubMed] [Google Scholar]
- 25.Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–7. [PubMed] [Google Scholar]
- 26.Narayan S, Chandra J, Sharma M, et al. Expression of apoptosis regulators Bcl-2 and Bax in childhood acute lymphoblastic leukemia. Hematology. 2007;12:39–43. doi: 10.1080/10245330600938125. [DOI] [PubMed] [Google Scholar]
- 27.Pruett SB, Fan R, Zheng Q. Characterization of glucocorticoid receptor translocation, cytoplasmic IκB, nuclear NFκB, and activation of NFκB in T lymphocytes exposed to stress-inducible concentrations of corticosterone in vivo. Int Immunopharmacol. 2003;3:1–16. doi: 10.1016/s1567-5769(02)00081-4. [DOI] [PubMed] [Google Scholar]
- 28.Salomons G, Smets L, Verwijs-Janssen M, et al. Bcl-2 family members in childhood acute lymphoblastic leukemia:relationships with features at presentation, in vitro and in vivo drug response and long-term clinical outcome. Leukemia. 1999;13:1574. doi: 10.1038/sj.leu.2401529. [DOI] [PubMed] [Google Scholar]
- 29.Spenerova M, Dzubak P, Srovnal J, et al. Combination of prednisolone and low dosed dexamethasone exhibits greater in vitro antileukemic activity than equiactive dose of prednisolone and overcomes prednisolone drug resistance in acute childhood lymphoblastic leukemia. Biomed Pap. 2014;158:422–7. doi: 10.5507/bp.2012.059. [DOI] [PubMed] [Google Scholar]
- 30.Sterzer P, Wiegers GJ, Reul JM. Long-term in vivo administration of glucocorticoid hormones attenuates their capacity to accelerate in vitro proliferation of rat splenic T cells. Endocrinology. 2004;145:3630–8. doi: 10.1210/en.2003-1578. [DOI] [PubMed] [Google Scholar]
- 31.Stoetzer O, Nüssler V, Darsow M, et al. Association of bcl-2, bax, bcl-xL and interleukin-1 beta-converting enzyme expression with initial response to chemotherapy in acute myeloid leukemia. Leukemia. 1996;10:18–22. [PubMed] [Google Scholar]
- 32.Tissing W, Meijerink J, Den Boer M, et al. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 2003;17:17. doi: 10.1038/sj.leu.2402733. [DOI] [PubMed] [Google Scholar]
- 33.Tissing WJ, Meijerink JP, den Boer ML, et al. Genetic variations in the glucocorticoid receptor gene are not related to glucocorticoid resistance in childhood acute lymphoblastic leukemia. Clin Cancer Res. 2005;11:6050–6. doi: 10.1158/1078-0432.CCR-04-2097. [DOI] [PubMed] [Google Scholar]
- 34.Yang E, Korsmeyer SJ. Molecular thanatopsis:a discourse on the BCL2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 35.Yao K, Xing H, Wu B, et al. Effect of TIEG1 on apoptosis and expression of Bcl-2/Bax and Pten in leukemic cell lines. Genet Mol Res. 2015;14:1968–74. doi: 10.4238/2015.March.20.6. [DOI] [PubMed] [Google Scholar]
- 36.Yousefi B, Azimi A, Majidinia M, et al. Balaglitazone reverses P-glycoprotein-mediated multidrug resistance via upregulation of PTEN in a PPARγ-dependent manner in leukemia cells. Tumor Biol. 2017;39:1010428317716501. doi: 10.1177/1010428317716501. [DOI] [PubMed] [Google Scholar]
- 37.Yousefi B, Shafiei-Irannejad V, Azimi A, et al. PPAR-gamma in overcoming kinase resistance in chronic myeloid leukemia. Cell Mol Biol (Noisy-le-grand) 2016;62:52–5. [PubMed] [Google Scholar]
- 38.Zhang S-F, Wang X-L, Yang X-Q, et al. Autophagy-associated targeting pathways of natural products during cancer treatment. Asian Pac J Cancer Prev. 2014;15:10557–63. doi: 10.7314/apjcp.2014.15.24.10557. [DOI] [PubMed] [Google Scholar]


