Abstract
Background:
The central giant cell granuloma (CGCG) is generally considered a non-neoplastic lesion. However, some cases show aggressive behavior like neoplasms. Based on clinical observations, a number of researchers have classified this lesion into aggressive and non- aggressive types. This study was aimed to investigate the association between clinical behavior and histopathological features using immunohistochemical vascular CD31 and cellular proliferation Ki67 markers.
Materials and methods:
In this descriptive-analytical, clinicopathological and immunohistochemical study, 50 CGCGs, including 25 aggressive and 25 non-aggressive types were selected according to Chuong’s classification. The samples were then subjected to immunohistochemical staining to analyze positivity for CD31 and Ki67 markers. Numbers of blood vessels and percentage proliferation of underlying fibroendothelial cells were assessed, and the obtained results were analyzed with the t-test and the Mann-Whitney test.
Results:
The results showed a significant difference between aggressive and non-aggressive CGCG lesions in the mean incidences of Ki67 (p=0.044). and CD31 (p=0.003) positivity.
Conclusion:
The present evaluation of expression rates for the vascular CD31 and cellular proliferation Ki67 markers showed there might be a positive relation between the clinical features and histopathology of CGCG. Furthermore, clinical behavior may be predicted based on features such as the number of blood vessels and proliferation of fibroendothelial cells.
Keywords: Aggressive central giant cell granuloma, non-aggressive central giant cell granuloma, vascular CD31, Ki 67
Introduction
Central giant cell granuloma (CGCG) is an bone lesion with unknown etiology (Nevilel et al., 2016). This lesion was first introduced by Jaffe (1953). Recent studies have shown that jaw lesions are monoclonal (Nevilel et al., 2016). Clinically, CGCG occurs during an wide age range (2-80 years), approximately in 60% of cases before the age of 30. This lesion tends to occur in women, and about 70% of cases are seen in mandible (Jaffe, 1953; Peacock et al., 2012; Nevilel et al., 2016; AtarbashiMoghadam and Ghorbanpor, 2017). These lesions are more common in the anterior part of jaws and cross the middle line in the mandible (Jaffe, 1953; Ficarra et al., 1987; Nevilel et al., 2016; AtarbashiMoghadam and Ghorbanpour, 2017). Based on the clinical feature and radiography, CGCG can be classified into aggressive and non-aggressive types (O’Malley et al., 1997; Peacock et al., 2012; El-Attar and Wahba, 2016; Nevilel et al., 2016; AtarbashiMoghadam and Ghorbanpour, 2017).
Most of the lesions are non-aggressive. They are relatively small and have mild symptoms, or are asymptomatic. Their growth is slow, and they do not cause cortical bone perforation or root resorption in the adjacent teeth. They are usually detected during routin radiographic examination or based on the painless swelling of bone (O’Malley et al., 1997; Peacock et al., 2012; El-Attar and Wahba, 2016; Nevilel et al., 2016; AtarbashiMoghadam and Ghorbanpour, 2017). Aggressive lesions are characterized by symptoms such as pain, rapid growth, cortical perforation, root resorption, tooth displacement, paresthesia and certain recurrence potential (Cowson et al., 1997; Nevilel et al., 2016; AtarbashiMoghadam and GHorbanpor, 2017). Spread to the soft tissue and mucosal ulceration may also occur in some cases (Nevilel et al., 2016). Radiographically, CGCG is an ionic lacunar or multi-lacunar radiolucency with defined outlines but non-cortical margins (Ficarra et al., 1987; Torabinia et al., 2011; Nevilel et al., 2016).
Relationship between the histopathological features and clinical behavior of this lesion has remained debatable. It is said that more aggressive lesions may be associated with a greater number of giant cells, more surface occupation by the giant cells and higher mitotic index (O’Malley et al., 1997; Reddy et al., 2012; Nevilel et al., 2016). Few studies have suggested that increased vascular concentration and incidence of markers related to angiogenesis can be attributed to the aggressive clinical behavior of this lesion (Nevilel et al., 2016). For instance, Peacock et al., (2012) reported a higher level of angiogenesis in the aggressive giant cell lesions than non-aggressive ones. Also, Scholzen and Gerdes (2000) reported a higher expression of Ki67 marker in aggressive CGCGs than non-aggressive ones and called it a helpful criteria for diagnosis of these lesions. However, O’Malley et al., (1997) reported that predicting the aggressive and non-aggressive central giant cell lesions based on histology is difficult.
CD31 marker is a 130KD type I transmembrane glycoprotein from the large family of immunoglobulins (DeLisser et al., 1997; Peacock et al., 2012; Privratsky et al., 2012), which involves an extracellular site composed of six Ig-like homology domains, one Ig-residue transmembrane domain and one 118 residue cytoplasmic tail (El-Attar and Wahba, 2016). This marker is the most well-known immunohistochemical marker among vascular tumors (Ficarra et al., 1987). This marker, known as platelet endothelial cell adhesionmolecule1 (PEMCAM1), plays different roles in vascular biology, including angiogenesis (Li et al., 2005; Kim et al., 2010).
Ki67 is an antigen with non-histone proteins in cell divisions. Presence in all active stages of mitosis is a significant characteristic of Ki67 so that it is present in all stages of cell cycle, including G1, S, G2 and mitosis, but it is not seen in the resting phase of the cell (Rosai and Ackerman, 2010).
Due to contradictions regarding the relationship between clinical behavior and histological features in aggressive and non-aggressive CGCGs reported by studies up to now, the current study was conducted to investigate the association of histopathological features with clinical behavior of CGCGs via a comparative analysis of the incidence of CD31 and Ki67 markers in aggressive and non-aggressive CGCGs using immunohistochemistry method.
Materials and Methods
In this descriptive-analytical, cross-sectional study, the samples diagnosed with CGCG during 1997-2017 in the archive of pathology department of Isfahan School of Dentistry were assessed, and clinical data, including the patients’ age, history of recurrence and effect on the adjacent structures like teeth and cortical bone were extracted from the patients’ files. From a total of 120 CGCGs, the other types of giant cells like cherubism and brown tumor were excluded from the study, and the remaining samples were included in the analysis. Then, 5 µ microscopic slides stained by hematoxylin and eosin (H and E) staining method were analyzed by two oral pathologists to ensure the diagnosis. Finally, 25 non-aggressive and 25 aggressive CGCGs were confirmed by Chuong’s classification (Chuong et al., 1986) (Table 1) and were coded randomly.
Table 1.
Criteria for Classification of the GCLS of Jaws
| Major Criteria |
|---|
| Recurrence after curettage |
| Lesion size |
| Minor Criteria |
| Rapid growth |
| Root resorption |
| Cortical bone perforation |
| Tooth displacement |
All the selected slides were analyzed in terms of histological parameters such as the presence of fibroendothelial tissue and multinucleated giant cells in the background of lesion and were recorded in a specific table.
For immunohistochemical staining by Biotin-avidin technique, antigen amplification, antigen retrieval, deparaffinization and rehydration and processing were performed, respectively. After irrigating the samples with PBS solution, the slides were put in monoclonal antibody (Monoclonal mouse Anti-human CD31 endothelial cell, JC70A, DAKO, USA) and (Ki67 bio care-SP6, USA) for 10 minutes. Finally, the samples were placed in ethanol with various concentrations for dehydration and then in xylene for clearing the slides, and were mounted by P.V-mounting system. In this study, pyogenic granuloma was used as the positive control for CD31 marker and Burkitt lymphoma was used as positive control for Ki67 marker.
All the slides were survayed by two oral pathologists, who were blind to the samples, using an light microscope (Olympus BX41TF, Tokyo, Japan). The stained cells were randomly counted in 10 microscopic fields at ×400 magnification (hpf). Then, the frequency of blood vessels was determined by counting the blood vessels stained with CD31 marker (as membrane and cytoplasmic staining), and the percentage of proliferation of mono nuclear stromal cells was calculated by counting the cells stained with Ki67 marker (as nuclear staining), Ki67 marker is staining only mono nuclear stroma cells but isn’t staining giant cells because these don’t proliferate. the results of which were recorded in tables. Next, the mean of 10 microscopic fields was determined as the rate of two markers.
All data were fed into SPSS software (IBM-SPSS-Statistics22) and analyzed by Mann-Whitney and t-test. P<0.05 was considered significant.
Results
The results of this descriptive-analytical study on 50 CGCGs, including 25 aggressive and 25 non-aggressive CGCGs showed the mean expression of CD31 marker was 8.4% in non-aggressive CGCGs and 12.54% in aggressive CGCGs (Figure 1). Also, the mean expression of Ki67 marker was 16% in non-aggressive CGCGs and 22.15% in aggressive CGCGs (Figure 2). The findings of t-test indicated a significant difference between the mean expression of CD31 and KI67 markers in non-aggressive and aggressive CGCGs (Pv Ki67=0.044 and Pv CD31=0.003) (Table 2).
Figure 1.
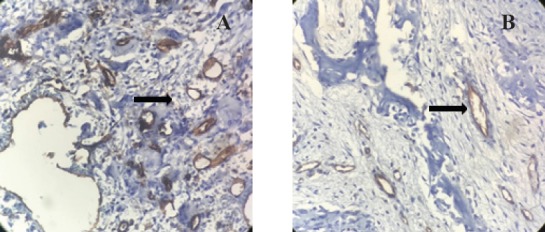
Expression of CD31 Marker in Stained Endothelial Cells of Capillaries in Non- Aggressive CGCG (A) and Aggressive CGCG (B) (×400). Notice the higher expression of CD31 marker in aggressive CGCG.
Figure 2.
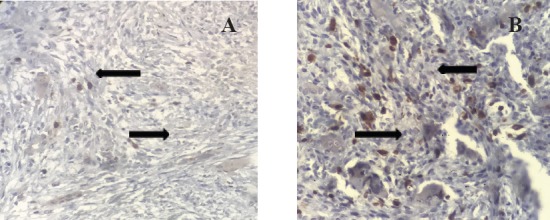
Expression of Ki67 Marker in Mono Nuclear Stromal Cells of Capillaries in Non- Aggressive CGCG (A) and Aggressive CGCG (B) (×400). Notice the higher expression of Ki67 marker in aggressive CGCG.
Table 2.
Expression of CD31 and Ki67 Markers in Aggressive and Non-Aaggressive CGCGsa
| Tissue | Ki67 | CD31 | |
|---|---|---|---|
| Aggressive | Mean | 22.15 | 12.54 |
| Minimum | 6 | 3.1 | |
| Maximum | 46 | 22 | |
| N | 25 | 25 | |
| Non-aggressive | Mean | 16.04 | 8.41 |
| Minimum | 0 | 0.9 | |
| Maximum | 60 | 19 | |
| N | 25 | 25 | |
| Total | Mean | 19.09 | 10.48 |
| Minimum | 0 | 0.9 | |
| Maximum | 60 | 22 | |
| N | 50 | 50 |
Discussion
Central giant cell granuloma (CGCG) is considered a non-neoplastic lesion (Nevilel et al., 2016). Based on the clinical feature and radiography, CGCG can be divided into aggressive and non-aggressive types (Nevilel et al., 2016). There has always been disagreement on whether the clinical behavior of CGCG (aggressive or non-aggressive) can be determined according to the histopathological features of the lesion.
This study investigated the association of histopathological features with clinical behavior of CGCG through analysis of the incidence of CD31 vascular marker and Ki67 proliferation marker in aggressive and non-aggressive CGCGs based on Chuong’s classification. The findings showed the incidence of CD31 and Ki67 markers was higher in aggressive than non-aggressive CGCG. Further, the incidence rate of CD31 marker was 12.54% in aggressive CGCG and 8.4% in non-aggressive CGCG, indicating a significant difference between them (P=0.003). On the other hand, the increased vascular proliferation in aggressive CGCGs may be responsible for the aggressive behavior of this lesion. In line with this theory that vascular proliferation in aggressive CGCGs may be responsible for the aggressive behavior of this lesion, it can be argued that increased angiogenesis can differentiate the blood vessels into osteoclasts, which in turn induces the rapid growth, osteolysis and enlargement of this lesion.
In a similar study, Peacock (2012) also evaluated the expression of VEGF, CD31 and CD34 markers in CGCGs. They found the angiogenesis level was higher in the aggressive samples than non-aggressive samples. Therefore, the incidence rate of these markers can be a basis for predicting the clinical behavior of CGCG (Peacock et al., 2012). However, in another study these researchers carried out in the same year on 41 CGCG samples, use of histopathological criteria including, the number of giant cells or giant cell nuclei to differentiate the aggressive and non-aggressive CGCGs was not considered sufficient (Peacock et al., 2012).
Moreover, in the study of El-Attar and Wahba (2016) on Ki67, P53, CD31 and CD68 markers in aggressive and non-aggressive lesions, the expression of CD31 and CD68 markers did not show a significant difference between the two groups, but the number of Ki67 and P53 markers was higher in aggressive lesions. Accordingly, it was concluded that the expression of Ki67 and P53 markers was helpful in determining the clinical behavior of CGCGs. On the other hand, AttarbashiMoghadam, (2016) reported no significant difference in expression of CD34 vascular marker between 16 aggressive and non-aggressive CGCG samples. Hence, it was concluded that CD34 vascular marker was of no use in determining the clinical behavior of CGCGs (AtarbashiMoghadam and Ghorbanpour, 2017).
The positive point of the present study is that along with use of CD31 marker, as the most well-known immunohistochemical vascular marker, the researchers decided to use Ki67 marker, which is a sensitive cellular proliferation marker. An important characteristic of this marker is that it is present in all active stages of mitosis, so it can be well associated with cell proliferation (Gejman et al., 2008; Rosai and Ackerman, 2010).
Based on the obtained results, the mean incidence of Ki67 was 22.15% in aggressive CGCG samples and 16% in non-aggressive samples, which indicated a significant difference between the two groups (p=0.044). On the other hand, the incidence of Ki67 marker in CGCG group was significantly higher than that of non-aggressive CGCG. Accordingly, cell proliferation by itself can be used as a reliable histopathological parameter to predict the clinical behavior of CGCG lesions. In this line, the study of El-Attar and Wahba (2016) on the expression of Ki67, P53, CD31 and CD68 markers in 10 CGCG non-aggressive samples and 8 aggressive CGCG samples showed estimating the expression rate of Ki67 and P53 markers could be helpful in determining the clinical behavior of CGCG lesions.
However, Al Sheddi et al., (2004) conducted a study on 18 CGCG cases and investigated the expression of CD68, CD34 and Ki67 markers. They reported no difference between aggressive and non-aggressive CGCG lesions with regard to histopathological and immunohistochemical features; therefore, these indices could not be used for predicting the clinical behavior of CGCG (19). O’Melly (1997) also reported the same point in their study in 1997 on 16 aggressive CGCG cases and 12 non-aggressive CGCG ones along with the expression of Ki67, P53, Cd68 and CD34. According to their results, the expression of Ki67 and CD34 showed no significant difference between the two groups, as P53 marker was not expressed in none of these two lesions. Hence, they concluded it is not possible to predict the clinical behavior of CGCG by histological analyses and proliferation parameters.
Although the results of these two studies are not in agreement with the findings of the present study, a point worth mentioning is that in the current study the number of studied samples was by far more than those of the aforementioned studies. Accordingly, it can be argued that the results of the present research are more reliable. Thus, considering the obtained results in the current study, it can be argued that the incidence of Ki67 marker can also be used as a reliable histopathological parameter to anticipate the clinical behavior of CGCG.
In conclusion, the findings of this study showed that the clinical behavior of CGCG lesions can be partly predicted by using immunohistochemical markers and analyzing their expression in CGCG lesions. On the other hand, the histological criteria can probably be a proper index for early diagnosis of aggressive CGCG and consequently a guideline for the clinician or surgeon in making use of a proper treatment plan, including anti-angiogenesis treatments in order to prevent the outcomes occurring afterward, such as spread of lesion, destruction of the surrounding tissues and recurrence.
References
- 1.Al Sheddi M, Mosadomi H, Al Dayel F. Central giant cell granuloma of the jaws and giant cell tumor of long bones.A clinicopathologic, cytometric, and immunohistochemical comparative study. J Oral Maxillofac Surg Med Pathol. 2004;4:195–6. [Google Scholar]
- 2.Atarbashi Moghadam S, Ghorbanpour M. Central giant cell granuloma of the Jaws:Correlation between vascularity and biologic behavior. J Mashad Dent Sch. 2017;1:35–9. [Google Scholar]
- 3.Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws:a clinicopathologic study. J Oral Maxillofac Surg. 1986;44:708. doi: 10.1016/0278-2391(86)90040-6. [DOI] [PubMed] [Google Scholar]
- 4.Cowson RA, Binnie WH, Speight PM, Barrett AW. Wright JM. Lucas pathology of tumors of the oral tissues. 5thed. Philadelphia: Churchill living stone; 1999. pp. 107–10. [Google Scholar]
- 5.DeLisser HM, Christofidou-Solomidou M, Strieter RM, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. J Pathol. 1997;3:671–7. [PMC free article] [PubMed] [Google Scholar]
- 6.El-Attar RHM, Wahba OM. Expression of Ki67, CD31, CD68 and P53 in peripheral and central giant cell granuloma of the Jaws. Arch Cancer Res. 2016;4:2. [Google Scholar]
- 7.Ficarra G, Kaban LB, Hansen LS. Central giant cell lesions of the mandible and maxilla:a clinicopathologic and cytometric study. Oral Surg Oral Med Oral Pathol. 1987;1:44–9. doi: 10.1016/0030-4220(87)90115-0. [DOI] [PubMed] [Google Scholar]
- 8.Gejman R, Swearingen B, Hedley-Whyte ET. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. 2008;5:758–66. doi: 10.1016/j.humpath.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe HL. Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-osseous) dysplasia of the jawbones. J Oral Pathol Med. 1953;1:159–75. doi: 10.1016/0030-4220(53)90151-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Cho HJ, Kim SW, et al. CD31+cells represent highly angiogenic and vasculogenic cells in bone marrow:novel role of nonendothelial CD31+cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–14. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li ZJ, Wang ZZ, Zheng YZ, et al. Kinetic expression of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) during embryonic stem cell differentiation. J Cell Biochem. 2005;3:559–7. doi: 10.1002/jcb.20436. [DOI] [PubMed] [Google Scholar]
- 12.Nevilel BW, Damm DD, Allen CM, Chi AC. Oral and maxillofacial pathology. 4^thed. saunders Co; 2016. pp. 584–86. [Google Scholar]
- 13.O'Malley M, Pogrel MA, Stewart JC, Silva RG, Regezi JA. Central giant cell granulomas of the jaws:phenotype and proliferation-associated markers. J Oral Pathol Med. 1997;4:159–63. doi: 10.1111/j.1600-0714.1997.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 14.Peacock ZS, Resnick CM, Susarla SM, et al. Do histologic criteria predict biologic behavior of giant cell lesions? J Oral Maxillofac Surg. 2012;11:2573–80. doi: 10.1016/j.joms.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Peacock ZS, Jordan RC, Schmidt BL. Giant cell lesions of the jaws:does the level of vascularity and angiogenesis correlate with behavior? J Oral Maxillofac Surg. 2012;8:1860–6. doi: 10.1016/j.joms.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Privratsky JR, Newman DK, Newman PJ. PECAM-1:conflicts of interest in inflammation. Life Sci. 2012;3:69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy V, Saxena S, Aggarwal P, Sharma P, Reddy M. Incidence of central giant cell granuloma of the jaws with clinical and histological confirmation:an archival study in Northern India. J Oral Maxillofac Surg. 2012;7:668–72. doi: 10.1016/j.bjoms.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Rosai G, Ackerman LV. Surgical pathology. 4thed. Philadelphia: Mosby; 2010. pp. 66–7. [Google Scholar]
- 19.Scholzen T, Gerdes J. The Ki-67 protein:from the known and the unknown. J Cell Physiol. 2000;3:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Torabinia N, Razavi SM, Shokrolahi Z. A comparative immunohistochemical evaluation of CD68 and TRAP protein expression in central and peripheral giant cell granulomas of the jaws. J Oral Pathol Med. 2011;4:334–7. doi: 10.1111/j.1600-0714.2010.00944.x. [DOI] [PubMed] [Google Scholar]


