Abstract
Objective:
To examine the association of total meat (animal flesh) consumption to prostate cancer incidence (PC61) at population level.
Subjects and Methods:
Data from 172 countries were extracted for analysis. Associations between country specific per capita total meat intake and PC61 incidence at country level were examined using Pearson’s r and Spearman rho, partial correlation, stepwise multiple linear regression analyses with ageing, GDP, Is (index of magnitude of prostate cancer gene accumulation at population level), obesity prevalence and urbanization included as the confounding factors. Countries were also grouped for regional association analysis. The data were log-transformed for analysis in SPSS. Microsoft Excel, and ANOVA Post hoc Scheffe tests were applied to calculate and compare mean differences between country groupings.
Results:
Worldwide, total meat intake was strongly and positively associated with PC61 incidence in Pearson’s r (r= 0.595, p<0.001) and Spearman rho (r= 0.637, p<0.001) analyses. This relationship remained significant in partial correlation (r= 0.295, p<0.001) when ageing, GDP, Is, obesity prevalence and urbanization were kept statistically constant. GDP was weakly and insignificantly associated with PC61 when total meat intake was kept statistically constant. Stepwise multiple linear regression identified that total meat was a significant predictor of PC61 with total meat intake and all the five confounders included as the independent variables (R2=0.417). Post hoc Scheffe tests revealed nine significant mean differences of PC61 between the six WHO regions, but all disappeared when the contributing effect of total meat on PC61 incidence rate was removed. GDP was not identified as the statistically significant predictor of PC61 in either of the models including or excluding total meat as the independent variable.
Conclusions:
Total meat intake is an independent predictor of PC61 worldwide, and the determinant of regional variation of PC61. The longitudinal cohort studies are proposed to explore the association further.
Keywords: Total meat (animal flesh), prostate cancer, carcinogen, regional variation
Introduction
Prostate cancer (PC61, abbreviated as per the International Classification of Diseases published by the WHO (IARC 2017)) is the second most common cancer among men in the world (Ferlay et al., 2015). It has become an enormous public health concern in most developed countries and an emerging public health problem in developing countries (Jemal et al., 2011; Stewart 2014). Globally, an estimated 0.9 million men in 2008 were diagnosed with prostate cancer worldwide (Ferlay et al., 2010), but, in 2012, the number increased to 1.1 million (IARC, 2016) and the majority of cases (almost 70%) occur in developed countries (IARC, 2016).
A complete understanding of the aetiology of PC61 remains elusive to the public and professionals (Grönberg, 2003; Hsing and Chokkalingam, 2006). Genetic background is the well-established risk factor through studies of PC61 in family histories (Steinberg et al., 1990; Lichtenstein et al., 2000), and this background may have been accumulated in human population due to the reduced natural selection (You and Henneberg, 2016; Budnik and Henneberg, 2017a; You and Henneberg 2017b; You and Henneberg 2018). Researches into the relationship between ageing and PC61 have revealed that, essentially, ageing process leads to the acquisition of mutations and the formation of a molecular and cellular environment which favours carcinogenesis (Majeed et al., 2000; Campisi, 2003; Shavers et al., 2009). Recent studies have shown that people who are obese may have more exposure to PC61 risk because they have the increased blood levels of insulin and insulin-like growth factor-1 (IGF-1) (Schuurman et al., 2000; Calle et al., 2003). Urbanization has been closely linked to human lifestyle change, such as more meat intake (You and Henneberg, 2016; You and Henneberg, 2017a) and less physical exercise (Allender et al., 2008), due to its process of modernization and industrialization. Therefore, it has been postulated as the risk factor of PC61 (Baade et al., 2011).
PC61 epidemiology has revealed that its incidence varies more than 25-fold worldwide (IARC, 2018). In the past years, researchers from the International Agency for Research on Cancer (IARC) as the specialized cancer agency of the World Health Organization (WHO) published several articles/reports associating the regional variation of PC61 incidence with regional socioeconomic levels (Ferlay et al., 2015; IARC, 2016).
Diet pattern plays a very important role in causing a large percentage of cancers (Doll and Peto 1981; Püssa, 2013). Plant sourced food products, such as vegetables (Key 2010), fruits (Key 2010) and grains (Wang et al., 2015), have been reported as not associated with prostate cancer.
In the last decades, a number of large cohort and case-control studies have controversially and circumstantially linked red meat intake to the development of PC61 (Ma and Chapman, 2009; Vasundara and Laurence, 2010; Gathirua-Mwangi and Zhang, 2014; NCI, 2018). It has been suggested that there was no substantial difference between “red meat” and “white meat” in terms of the nutrient components (Murphy et al., 2014; You and Henneberg, 2016). Therefore, both red meat and white meat might contribute to PC61 together when people had diets which usually include the combination of red meat and white meat. Researches, which simply correlated red meat intake and PC61 risk, may have a defect in the study designs because the contributing effect of white meat intake to PC61 was not removed. Statistically, we may say that white meat intake was not kept constant when the correlation of red meat intake to PC61 was analysed (Alexander et al., 2010; Mandair et al., 2014).
It is proposed to use ecological study for ascertaining a new association between total meat (flesh of animals) intake and PC61 risk at population level. We examined this relationship with the country specific data on total meat intake and PC61 incidence rate published by the United Nations (UN) agencies.
Materials and Methods
Data Collection and Selection
The country specific data were collected for this study:
The most recent IARC data on estimated PC61 incidence rate in 2012 for the adult (aged 15+ years old) part of each population were extracted as the dependent variable (Ferlay et al., 2015).
Total meat intake (expressed in kg/capita/year) in 2011 from the FAOSTAT Food Balance Sheet (FBS) (FAO) was obtained as the independent predictor of PC61. FAO defined total meat as “flesh of animals used for food”, which includes beef and veal, buffalo meat, pig meat, mutton and lamb, goat meat, horse meat, chicken meat, goose meat, duck meat, turkey meat, rabbit meat, game meat and offal (FAO). For the interest of discussing the relationships between PC61 and white meat intake and red meat intake, we extracted poultry meat (flesh) as white meat (expressed in kg/capita/year). We calculated red meat intake by subtracting white meat intake from the total meat intake.
We extracted the following data as the confounding variables as they have been postulated as the risk factors of PC61.
Ageing, expressed with the percentage of males age 65 and above in each country in 2011 was extracted from the World Bank (The World Bank, 2018).
The World Bank data on per capita GDP PPP (gross domestic product converted to international dollars using purchasing power parity rates) in 2011 (The World Bank, 2018). Ferlay et al. indicated that the PC61 incidence rate varies significantly largely because of how widespread the prostate specific antigen (PSA) testing and subsequent biopsy are in practice in those countries and regions (Ferlay et al., 2015). The testing depends on the GDP because of funding for medical services. GDP PPP was incorporated as the confounding factor to reduce/remove the bias on PC61 incidence in addition to other socioeconomic level related factors which may affect the association between meat intake and PC61 incidence.
Country-specific index of the total opportunity for natural selection in modern populations (Is) was extracted from previous studies (You and Henneberg, 2016a; Budnik and Henneberg, 2017). An Is value signifies here the magnitude of the country to accumulate the PC61 genes(You and Henneberg, 2017; You and Henneberg, 2016a; Budnik and Henneberg, 2017). The calculation methods and significance of Is which was recently published by You and Henneberg (2017) and Saniotis and Henneberg (2013), are based on the Biological State Index as described in the previous publications (Henneberg, 1976; Henneberg and Piontek, 1975). PC61 has strong genetic background which is heritable (Zeegers et al., 2003, Stewart, 2014). Therefore, Is was chosen as the confounding factor to remove the confounding effect of country-specific PC61 genetic background on the association between meat intake and PC61 incidence (You and Henneberg, 2016c; You and Henneberg, 2016d; Budnik and Henneberg, 2017; You and Henneberg, 2017).
The WHO Global Health Observatory (GHO) data on the estimated prevalence rate of obesity (percent of population aged 18+ with BMI ≥ 30 kg/m2) of the male population in 2010 (WHO 2015).
The World Bank data on urbanization (the percent of males living in urban areas in each country in 2011 (The World Bank, 2018).
We simply extracted the country-specific meat intake data from the FAO Food Balance Sheet for 172 countries, that is all countries of the world for which these data were available. And then, we matched the other variables with the meat intake data. All the independent variables were backdated 1-2 years to reflect the exposure with delayed presentation of PC61.
Each country was treated as the individual subject for data analysis in this study. For particular analyses, the number of countries included for variables may have differed somewhat because all information on other variables was not uniformly available for all countries due to unavailability from relevant UN agencies. All the data were extracted and saved in Microsoft Excel® for performing the data analysis.
Statistical analyses
To assess the relationship between PC61 incidence rate and total meat intake, the analysis proceeded in six steps.
1. Scatter plots was produced with the original data in Microsoft Excel® to explore and visualize the strength, shape and direction of association between meat intake and PC61 incidence at the global level.
We also calculated and compared the means of PC61 of the 10 countries with highest and lowest meat intake in the Excel to show how meat consumption changes average incidence rates of PC61
For the data analysis in SPSS (Steps 2 -5), the original data were log-transformed (natural logarithms) to bring their distributions closer to normal, which may increase homoscedasticity of data distributions.
2. Bivariate correlation (Pearson’s and Spearman rho, nonparametric) was used to evaluate the strength and direction of the associations between both dependent variable (PC61 incidence) and all independent variables (Meat intake, Ageing, GDP PPP, Obesity and Urbanization).
3. Partial correlation of Pearson moment-product approach was used to find the relationship between PC61 incidence and meat intake while keeping ageing, GDP PPP, obesity and urbanization statistically constant. Partial correlation was also used to examine separately white and red meat relationship to PC61 incidence.
The independent relationships between PC61 and each of the five variables were explored with partial correlation of Pearson’s moment-product approach while we kept the meat intake statically constant. This allows us to identify how strongly the meat intake affects the association between PC61 and each of the five variables.
A number of previous ecological studies (Siervo et al., 2014; You and Henneberg, 2016b; You and Henneberg 2016; You and Henneberg, 2016c) revealed that meat intake was in significant and strong correlation to GDP. We alternated GDP and meat intake as the predictor and confounding factor for the partial correlation analysis.
4. Stepwise multiple linear regression modelling was performed to identify and rank predictors (independent variables) of PC61. We included and excluded meat intake as the one of the predictors in the two analyses to observe how strongly the meat intake affected the predictor ranking in Stepwise linear analysis.
5. Pearson’s r was calculated to investigate the regional correlation between meat intake and PC61 incidence. Fisher’s r-to-z transformation was performed to test significance of differences between correlation coefficients. We did this analysis because meat intake varies in human diet patterns due to the availability and affordability in different regions, and also because the WHO and its agent the IARC reported that PC61 incidence varies in different regions (Stewart, 2014; Ferlay et al., 2015). The 173 countries were grouped as per WHO region division (WHO, 2018) and the World Bank income classifications (The World Bank, 2015) for correlation analyses.
6. Post hoc Scheffe (Oneway ANOVA) testing was performed to compare the mean difference of meat intake (original data), PC61 incidence (original data), and residual of PC61 incidence standardized on meat intake (original data) between six WHO regions. This may allow us to investigate the importance of meat intake in determining the regional variation of PC61.
The equation (y = 0.7643x + 1.1864) of the best fitting trendline obtained in the scatter plots analysis of correlation between meat intake and PC61 incidence was used to calculate and remove the contributing effect of total meat intake on PC61 incidence rate. Thus, we created a new dependent variable, “PC61 incidence standardized on meat intake” and subsequently “Residual of PC61 incidence standardised on meat intake” after subtracting the “PC61 incidence standardized on meat intake” from the PC61 incidence rate.
The means of PC61 and meat intake of the six WHO regions were compared and the association between meat intake and PC61 incidence in each was obtained in the Excel.
SPSS v. 22 (SPSS Inc., Chicago Il USA) and Microsoft Excel® were used for data analysis. The significance was kept at the 0.05 level, but 0.01 and 0.001 levels are also reported. Stepwise multiple linear regression analysis criteria were set at probability of F to enter ≤ 0.05 and probability of F to remove ≥ 0.10.
Results
Figure 1 showed the unadjusted correlation between meat intake and PC61 incidence. The relationship was noted to be best described by linear equation (y = 0.7643x + 1.1864) with strong correlation (r=0.684, p<0.001).
Figure 1.
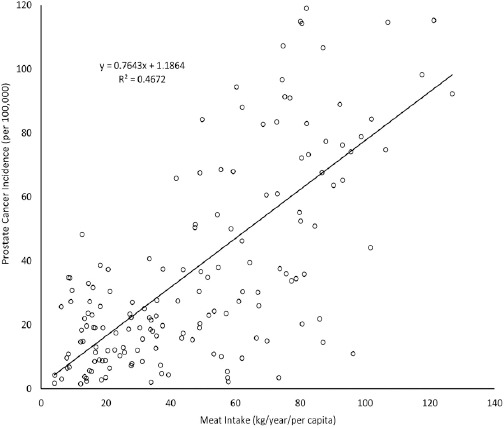
Linear Correlation Plot of Meat Intake and Prostate Cancer Incidence
The average PC61 incidence rate of the 10 countries with highest meat intakes (79.22 per 100,000) was 5.77 times greater than the average of the 10 countries with lowest meat intakes (13.73 per 100,000).
Pearson correlation and nonparametric (Spearman rho) analyses showed that meat intake was in significantly strong correlation to PC61 incidence (r=0.595, p<0.001 and r=0.637, p<0.001 respectively) (Table 1). Pearson r correlation coefficient of meat consumption to PC61 became lower in scatter plots (Figure 1) because the variables were log-transformed. The strong and significant correlations were also observed between PC61 and ageing, GDP PPP, obesity and urbanization respectively. This warranted our selection to include them as the confounding factors in exploring the correlation between meat intake and PC61 incidence.
Table 1.
Pearson’s r and Nonparametric Correlation Matrix Between All Variables Involved in This Study
| PC61 | Meat | Ageing | GDP PPP | Is | Obesity % | Urbanization | |
|---|---|---|---|---|---|---|---|
| PC61 | 1 | 0.595*** | 0.555*** | 0.529*** | -0.480*** | 0.489*** | 0.470*** |
| Meat | 0.637*** | 1 | 0.648*** | 0.810*** | 0.674*** | 0.761*** | 0.588*** |
| Ageing | 0.587*** | 0.699*** | 1 | 0.706*** | 0.686*** | 0.596*** | 0.498*** |
| GDP | 0.573*** | 0.833*** | 0.750*** | 1 | 0.738*** | 0.717*** | 0.664*** |
| Is | -0.565*** | 0.794*** | 0.864*** | 0.871*** | 1 | 0.708*** | 0.505*** |
| Obesity % | 0.501*** | 0.737*** | 0.630*** | 0.729*** | 0.745*** | 1 | 0.671*** |
| URBAN | 0.516*** | 0.635*** | 0.563*** | 0.737*** | 0.665*** | 0.735*** | 1 |
Pearson r (above diagonal) and nonparametric (below diagonal) correlations were reported. Significance levels: * P <0.05, ** P< 0.01, *** P< 0.001. Numbers of countries range, 157-172. Meat intake (kg/capita/year) sourced from the Food and Agriculture Organization; Ageing (percent of males ages 65 and above) and GDP PPP (gross domestic product converted to international dollars using purchasing power parity rates) and urbanization (the percent of males living in urban areas) were sourced from the World Bank. Male obesity prevalence (percent of males aged 18+ with BMI ≥ 30 kg/m2); Is was extracted from previous publications.
These bivariate correlations were also reflected in the WHO regions showing increased correlation of meat intake with PC61 (Table 2). AFRO region was the exception. In general, the bivariate correlations were also true in country groupings based on economy status as defined by the GDP.
Table 2.
Correlation of Meat Availability to Prostate Cancer Incidence Rate in Different Country Groupings
| Country groupings | Pearson r | p | nonparametric | p |
|---|---|---|---|---|
| Worldwide (n=163) | 0.595 | <0.001 | 0.637 | P<0.001 |
| World Bank income classifications | ||||
| High Income, n=47 | 0.528 | <0.001 | 0.346 | <0.05 |
| Low Income, n=26 | 0.429 | <0.05 | 0.372 | 0.061 |
| Low Middle Income, n=43 | 0.305 | <0.05 | 0.216 | 0.164 |
| Upper Middle, n=47 | 0.402 | <0.01 | 0.419 | P<0.003 |
| WHO regions | ||||
| AFRO, n=38 | 0.180 | 0.28 | 0.049 | 0.771 |
| AMRO, n=29 | 0.570 | <0.001 | 0.555 | <0.01 |
| EMRO, n=18 | 0.524 | <0.05 | 0.556 | <0.05 |
| EURO, n=50 | 0.723 | <0.001 | 0.654 | <0.001 |
| SEARO, n=10 | 0.549 | 0.101 | 0.661 | <0.05 |
| WPRO, n=18 | 0.591 | <0.01 | 0.513 | <0.05 |
Pearson r and nonparametric correlations within country groupings were reported; Meat intake (kg/capita/year) sourced from the Food and Agriculture Organization.
Partial correlation analysis revealed that meat intake was a strong and significant predictor of PC61 independent of ageing, GDP PPP, obesity and urbanization (r=0.295, p<0.001, Table 3). When meat intake was stabilised as a confounding factor in partial correlation analysis, it was revealed that: 1) ageing was identified as a significant independent predictor (r=0.277, p<0.001) of PC61 incidence; 2) urbanization showed weak and significant correlation to PC61 incidence (r=0.185, p<0.05); and 3) GDP, Is and Obesity showed barely a correlation to PC61 incidence (Table 3). This suggested that meat intake had great confounding effects on the correlation between PC61 incidence and GDP PPP, Is, obesity and urbanization respectively.
Table 3.
Partial Correlations between Prostate Cancer Incidence and Independent Variable When Meat Was Included as the Independent and Confounder Respectively
| Partial Correlation to | Partial Correlation to | |||||
|---|---|---|---|---|---|---|
| PC61 | PC61 | |||||
| Variables | r | p | df | r | p | df |
| Meat | 0.295 | <0.001 | 150 | - | - | - |
| Ageing | - | - | - | 0.277 | <0.001 | 160 |
| GDP | - | - | - | 0.100 | 0.209 | 160 |
| Is | - | - | - | -0.041 | 0.608 | 158 |
| Obesity | - | - | - | 0.070 | 0.382 | 158 |
| Urbanization | - | - | - | 0.185 | P<0.05 | 160 |
Partial correlations were reported; Meat intake (kg/capita/year) sourced from the Food and Agriculture Organization; Ageing (percent of males ages 65 and above) and GDP PPP (gross domestic product converted to international dollars using purchasing power parity rates) and urbanization (the percent of males living in urban areas) were sourced from the World Bank. Male obesity prevalence (percent of males aged 18+ with BMI ≥ 30 kg/m2); Is was extracted from previous publications; - Included as the confounding factor.
When meat intake was excluded as the PC61 predictor, ageing and urbanization were selected as the significant predictors of PC61 with R2 = 0.354 in the standard multiple linear regression (Stepwise) analysis. When meat intake was incorporated as an independent variable, it was placed first as the major predictor of PC61 with increasing R2 to 0.417. GDP was not selected as the major predictor of PC61 in Stepwise linear regression. Additionally, it was not in strong or significant correlation to PC61 incidence in partial correlation (Table 2). This may suggest that GDP PPP may not be the strong predictor of PC61, but meat intake is.
Table 5 showed the calculated means of meat intake and PC61 incidence rates in all the six WHO regions. In general, at country grouping level, meat intake was in strong correlation to PC61 incidence based on the best fit trendline (r=0.832, p<0.05). This is consistent with the correlation between meat intake and PC61 incidence at the individual country level (r=0.684, p<0.001) (Table 5).
Table 5.
Mean Difference between WHO Regions, and between UN Developed and Developing Regions
| I (Region) | Meat | PC61 incidence rate | Residual of PC61 incidence standardised on meat | |||||
|---|---|---|---|---|---|---|---|---|
| J (Region) | Mean difference (I-J) | I (Region) | J (Region) | Mean difference (I-J) | I (Region) | J (Region) | Mean difference (I-J) | |
| AF n=39 mean= 21.14 |
AM | -43.07*** | AF | AM | -33.75*** | AF | AM | -1.97 |
| EM | -11.98 | n=38 | EM | 10.35 | n=38 | EM | 18.92 | |
| EU | -45.51*** | mean=22.70 | EU | -32.19*** | mean= 5.37 | EU | 2.01 | |
| SEA | 3.4 | SEA | 16.42 | SEA | 13.24 | |||
| WP | -40.83*** | WP | -11.39 | WP | 20.15 | |||
| AM n=36 mean= 33.12 |
AF | 43.07*** | AM | AF | 33.75*** | AM | AF | 1.97 |
| EM | 31.09*** | n=29 | EM | 44.10*** | n=29 | EM | 20.89 | |
| EU | -2.44 | mean=12.35 | EU | 1.56 | mean= 9.44 | EU | 3.98 | |
| SEA | 46.47*** | SEA | 50.17*** | SEA | 15.21 | |||
| WP | 2.23 | WP | 22.36 | WP | 22.13 | |||
| EM n=18 mean= 40.77 |
AF | 11.98 | EM | AF | -10.35 | EM | AF | -18.92 |
| AM | -31.09*** | n=18 | AM | -44.10*** | n=18 | AM | -20.89 | |
| EU | -33.53*** | mean=40.77 | EU | -42.54*** | mean= -14.15 | EU | -16.91 | |
| SEA | 15.38 | SEA | 6.07 | SEA | -5.68 | |||
| WP | -28.86* | WP | -21.74 | WP | 1.23 | |||
| EU n=50 mean=66.95 |
AF | 45.51*** | EU | AF | 32.19*** | EU | AF | -2.01 |
| AM | 2.44 | n=50 | AM | -1.56 | n=50 | AM | -3.98 | |
| EM | 33.53*** | mean=54.89 | EM | 42.54*** | mean=2.77 | EM | 16.91 | |
| SEA | 48.91*** | SEA | 48.61*** | SEA | 11.23 | |||
| WP | 4.68 | WP | 20.8 | WP | 18.15 | |||
| SEA n=10 mean= 17.74 |
AF | -3.4 | SEA | AF | -16.42 | SEA | AF | -13.24 |
| AM | -46.47*** | n=10 | AM | -50.17*** | n=10 | AM | -15.21 | |
| EM | -15.38 | mean= 6.28 | EM | -6.07 | mean= -8.47 | EM | 5.68 | |
| EU | -48.91*** | EU | -48.61*** | EU | -11.23 | |||
| WP | -44.23*** | WP | -27.81 | WP | 6.91 | |||
| WP n= 19 mean =61.97 |
AF | 40.83*** | WP | AF | 11.39 | WP | AF | -20.15 |
| AM | -2.23 | n= 18 | AM | -22.36 | n= 18 | AM | -22.13 | |
| EM | 28.86* | mean=34.09 | EM | 21.74 | mean= -15.38 | EM | -1.23 | |
| EU | -4.68 | EU | -20.8 | EU | -18.15 | |||
| SEA | 44.23*** | SEA | 27.81 | SEA | -6.91 | |||
Mean comparisons between WHO regions (One-way ANOVA, Post hoc Scheffe) were reported; Meat intake (kg/capita/year) sourced from the Food and Agriculture Organization; Ageing (percent of males ages 65 and above) and GDP PPP (gross domestic product converted to international dollars using purchasing power parity rates) and urbanization (the percent of males living in urban areas) were sourced from the World Bank.
A post hoc Scheffe analysis conducted on the multiple mean comparisons revealed that there were numerous significant mean differences in PC61 incidence rates between different WHO regions (Table 5). Mean of PC61 incidence in Africa was significantly lower than that in Americas and Europe. Mean of PC61 incidence in the Eastern Mediterranean was significantly lower than that in Americas and Europe. The mean PC61 incidence in South-Eastern Asia was significantly lower than that in Americas and Europe.
A subsequent ANOVA with post hoc Scheffe procedure performed on the means of “Residual of PC61 standardised on meat intake” in different WHO regions showed no significant differences among and between regions (Table 5). The results from post hoc Scheffe tests conducted on mean comparison between the WHO regions suggested that regional variations of PC61 incidence may only reach statistically significant levels if the contribution of their respective meat intake was included. This result was supported by the findings identified in our previous bivariate and partial correlation (Table 3) and multiple linear regression (Table 4) that meat intake is the major risk factor of PC61 incidence.
Table 4.
Results of Stepwise Multiple Linear Regression Analyses to Sort Significant Predictors of Prostate Cancer Incidence
| Excluding meats | Including meat | ||||
|---|---|---|---|---|---|
| Rank | Variables Entered | Adjusted R Squared | Rank | Variables Entered | Adjusted R Squared |
| 1 | Ageing | 0.31 | 1 | Meat | 0.332 |
| 2 | Urbanization | 0.354 | 2 | Ageing | 0.386 |
| 3 | Ibs | Not a major predictor | 3 | Is | 0.404 |
| 4 | GDP PPP | Not a major predictor | 4 | Urbanization | 0.417 |
| 5 | Obesity % | Not a major predictor | 5 | GDP PPP | Not a major predictor |
| 6 | Obesity | Not a major predictor | |||
Stepwise multiple linear regression modelling is reported. Contribution of variables is listed in order of how much they contribute to prostate cancer incidence; Meat intake (kg/capita/year) sourced from the Food and Agriculture Organization; Ageing (percent of males ages 65 and above) and GDP PPP (gross domestic product converted to international dollars using purchasing power parity rates) and urbanization (the percent of males living in urban areas) were sourced from the World Bank. Male obesity prevalence (percent of males aged 18+ with BMI ≥ 30 kg/m2); Is was extracted from previous publications.
Our data showed that both white meat intake and red meat intake were in strong and significant correlation to PC61 in Pearson r (r=0.515, p<0.001 and r=0.531, p<0.001 respectively) and non-parametric correlations (r=0.560, p<0.001 and r=0.551, p<0.001 respectively) (Table 6). However, only white meat intake, instead of red meat was significantly correlated to PC61 when ageing, GDP, Is, obesity and urbanization were statistically kept constant (r=0.337, p<0.001) (Table 6). Interestingly, when we incorporated red meat as the confounding factor, white meat intake was still significantly correlated to PC61 (r=0.384, p<0.001) (Table 6). This suggested that, if we consume both white and red meat, white meat may be able to contribute to PC61 when we remove the influence of red meat intake on PC61. To the best of our knowledge, statistically, this finding has not been reported by other studies.
Table 6.
Pearson r, Nonparametric and Partial Correlations of Prostate Cancer Incidence to White and Red Meat Respectively
| Pearson r | Spearman rho | Partial | Partial | |||||
|---|---|---|---|---|---|---|---|---|
| r | n | r | n | r | n | r | n | |
| White meat | 0.515*** | 163 | 0.560*** | 163 | 0.337*** | n=150 | 0.3484*** | n=149 |
| Red meat | 0.531*** | 163 | 0.551*** | 163 | 0.092 | n=150 | - | - |
| Ageing | 0.555*** | 163 | 0.587*** | 163 | - | - | - | - |
| GDP | 0.529*** | 157 | 0.573*** | 157 | - | - | - | - |
| Is | 0.274*** | 161 | 0.565*** | 161 | - | - | - | - |
| Obesity % | 0.489*** | 161 | 0.501*** | 161 | - | - | - | - |
| URBAN | 0.470*** | 163 | 0.516*** | 163 | - | - | - | - |
Pearson r, nonparametric and partial correlations were reported. Significance levels: * P <0.05, ** P< 0.01, *** P< 0.001; White meat (poultry) intake (kg/capita/year) sourced from the Food and Agriculture Organization, and red meat intake (kg/capita/year) was calculated through subtracting white meat from total meat intake; Ageing (percent of males ages 65 and above) and GDP PPP (gross domestic product converted to international dollars using purchasing power parity rates) and urbanization (the percent of males living in urban areas) were sourced from the World Bank. Male obesity prevalence (percent of males aged 18+ with BMI ≥ 30 kg/m2); Is was extracted from previous publications.
White meat intake was placed second increasing R2 to 0.363 from 0.315 with ageing selected as the variable having the greatest influence on PC61 (R2 = 0.315) in Stepwise multiple linear regression analysis. When we replaced white meat intake with red meat intake as the independent variable, red meat was not selected as the most influential predictor of PC61.
Discussion
The results from our study suggested, at population level, total meat (flesh) intake was strongly and significantly associated with incidence rate of PC61 globally and regionally. Worldwide, total meat intake may be a major predictor of PC61 regardless of the influence from other risk factors, such as ageing, GDP, Is, obesity and urbanization. Our results also suggested that meat consumption, instead of GDP, may be a determinant of the regional variation of PC61.
Red and processed meat increasing risk of PC61 has been a central dogma reported in the majority of the studies into relationship between meat intake and PC61. The dogma, which is supported by the IARC (International Agency for Research on Cancer, 2015), stipulates multiple etiologies through which red and processed meat intake contributes to PC61 risk (Sinha et al., 2009):
1) Carcinogens such as heterocyclic amines (PhIP), 2-amino-3,8-dimethylimidazo-[4,5-b]quinoxaline (MeIQx), and 2-amino-3, 4,8-trimethylimidazo-[4,5-f]quinoxaline (DiMeIQx), and polycyclic aromatic hydrocarbons may be formed when meat is cooked at high-temperature (Knize et al., 1995; Knize et al., 1996; Knize et al., 1998; Sinha et al., 1998; Sinha et al., 2000; Kazerouni et al., 2001).
2) N-nitroso compounds (NOCs) may be produced endogenously from meat itself or preservatives added to processed meats (Cross et al., 2000; Hughes et al., 2001; Cross and Sinha, 2004).
3) Heme iron has catalytic effects on (i) the endogenous formation of carcinogenic N-nitroso compounds and (ii) the formation of cytotoxic and genotoxic aldehydes by lipoperoxidation (Cross et al., 2000; Cross et al., 2002; Cross et al., 2003; Cross et al., 2006; Lewin et al., 2006., Grant, 2008).
4) Meat may cause metabolic syndrome (MetS) (Babio et al. 2012), which plays a role in the development of PC61 (De Nunzio et al., 2011).
Recent studies reported that meat protein from both red meat and white meat may be digested slowly and later than other maco-nutrients, such as carbohydrates and fat (You and Henneberg, 2016; You and Henneberg, 2017a). This may highlight the role of meat in contribution to PC61.
However, the results from these studies may not be rigorous as they only focused on the relationship between red meat intake, instead of total meat intake, and PC61. It may not be wise to exclude white meat from the studies because: 1) The contents of red meat and white meat are quite similar although the quantities of the specific compounds are different. 2) Both red and white meat can produce the same mutagens or carcinogens when they are cooked at high temperature (Sinha et al., 1998; Sugimura et al., 2004; Gu et al., 2010). 3) Fat (Mandair et al. 2014) and heme iron (Lewin et al. 2006) (Cross et al., 2002; Cross et al., 2003; Grant, 2008) in red meat have been postulated as the carcinogen. However, red meat has been leaner than ever over the past few decades due to leaner animals being bred and improved butchery and feeding techniques that make fat content fall significantly (Lawrie and Ledward, 2006; Pearce et al., 2010). Blood, which contains lots of heme iron, has been extensively consumed in Asian cuisines for thousands of years, but the PC61 incidence in Asia (9.4 per 100,000) is much lower than in other continents, such as Africa (23.2 per 100,000), Americas (75.0 per 100,000), Europe (61.3 per 100,000) and Oceania (101.9 per 100,000) (Ferlay et al., 2013). Additionally, The National Pork Board of the United States used to classify pork, a major “red meat”, as “the other white meat” (Levere, 2005). Therefore, the contribution of white meat to PC61 may not be ignored in those studies. However, those studies into the relationship between red meat and PC61 did not remove the influence of white meat on PC61. In other words, statistically, there may be a defect in these studies as they did not establish the relationship independent of white meat consumption.
Some studies do not support that red meat should be the only meat category to be associated with PC61. Globally, the overall consumption of white meat (poultry in per capita per year) between 1990 and 2009 has increased by 76.6% (Henchion et al., 2014). Accompanying this process, the PC61 incidence keeps increasing (Ferlay et al., 2010; Jemal et al., 2011; Stewart, 2014; IARC, 2016) worldwide. At the specific country level, for instance, in Australia, between 1982 and 2009, poultry meat has increased by 105%, but red meat has decreased by 22%. However, during this period, the PC61 incidence rate increased from 79.4 (per 100,000) in 1982 to 193.9 (per 100,000) in 2009 (FAO, 2018).
Although, statistically, we found that white meat intake may be a major predictor of PC61, it may not be proper to conclude that white meat intake is a major predictor of PC61, while red meat intake is not, considering the similarities between white meat and red meat (see above for details) and the controversial and circumstantial findings in previous studies.
A cohort study based on the dietary habits of 917 subjects with PC61 concluded that there were no association between chicken intake and the risk of aggressive prostate cancer (Amin et al., 2008). This result may not conflict with our finding that white meat was a major and independent predictor of PC61 because of a couple differences in study designs: 1) Only chicken which is main component, but not all, of white (poultry) meat. Our study included all the meat from poultry. 2) The research subjects in this study were PC61 patients, but our study chose all the males. 3) Cooked chicken was used as the independent variable in the previous study, but poultry flesh was included as the independent variable in our study.
The association between processed meat intake and PC61 has been tentative (WCRF/AICR 2007, Alexander et al. 2010). Processed meat is usually composed of both red meat and white meat (Pearson and Gillett, 2012). Therefore, this may support that white meat also contributes to PC61. There may be several issues with those studies. Firstly, the cariogenic effects of processing aids, such as sodium nitrite (E250) on PC61 were (or could) not be removed from the association between processed meat intake and PC61. Secondly, the total processed meat, such as hotdogs and sausages, instead of pure meat were included for study. Therefore, the quality of the data may be questionable. Similarly, statistically, the influence of unprocessed meat intake on PC61 was not removed from the association between processed meat and PC61. This may be the defect in these studies as well.
A recent study conducted by Murphy et al. concluded that, due to similarities between pork, beef and chicken diets, people on these three diets for three months did not have different changes of the Body Mass Index (BMI) or any other marker of adiposity (Murphy et al., 2014). Similarly, another study did not deem that it was necessary to differentiate meat into different categories for investigating the relationship between meat intake and obesity (You and Henneberg, 2016).
Categorizing meats and associating some meat types, such as red meat and processed meat, with detrimental health effects in the different circumstances is not supported by the health eating guideline published by the authorities from different governments, such as Australia (NHMRC, 2012; EUFIC, 2018), Canada (Government of Canada-Health-Food and Nutrition-Healthy Eating, 2015), Europe (EUFIC, 2018) and United States (USDA, 2015). One of the reasons may be that the conclusions from these studies are still controversial and not convincing enough.
We have to point out a strong advantage of this study. This study does not list any circumstance for the existing relationship between total meat intake and PC61. The majority of the previous studies categorized meats for investigating the association between specific meat groups, such as red meat, and PC61 in the specific circumstances (Koutros et al., 2008). However, generally, people do not eat individual meats but rather meats in combination in broad circumstances (Tantamango-Bartley et al., 2015; You and Henneberg, 2016). We used the total meat intake, defined as the “flesh of animals used for food”, as the independent variable in this study (Lawrie and Ledward, 2006; The FAO, 2018). The cooking methods, processing methods or nutritional function were not used to differentiate meat types. However, previous studies always listed one or more circumstances (categorizing meat) when the relationship between meat intake and PC61 was investigated. The circumstances may include, but limited to, level of doneness (Sinha et al., 1998; Sugimura et al., 2004; Koutros et al., 2008; Gu et al., 2010), myoglobin content (red and white meat) (Joshi et al., 2012; IARC, 2015; Wolk, 2017), modification methods (processed meat) (John et al., 2011; Wolk, 2017), ethnicity (John et al., 2011; Richman et al., 2011; Sarwar et al., 2013) and stage of the PC61 (Koutros et al., 2008; John et al., 2011; Joshi et al., 2012). The definitions of these circumstances varied greatly and were not crystal clear. These ambiguous circumstances may have produced the controversial relationships between specific meat intake and PC61 (Richman et al., 2011). Without any circumstance, the relationship between total meat intake and PC61 identified in our study may offer the new insight into the study of the adverse health effects of meat intake (Tantamango-Bartley et al., 2015).
There have been a couple of investigations into the relationship between total meat intake and PC61 risk. John et al. concluded that total meat intake was not associated with the risk of advanced prostate cancer (John et al., 2011). Compared with our findings, the relationship in this study is very circumstantial because the results were based on the specific ethnicity (Non-Hispanic White and African-American men) and specified cooking methods and degree of doneness and stage of PC61 (advanced). In addition to the multiple circumstances included in this study, the data on total meat intake may be biased because newly diagnosed PC61 patients were included as the main research subjects in this study. These patients may more easily recall the negative life events (total meat intake) which have been considered as PC61 risks (Blaney, 1986; Cohen et al., 1988; Brett et al., 1990; Courtney et al., 1993; Bai et al., 2016). Our results were in agreement with the findings reported by Koutros et al. that total meat was in weak association with the increased risk of incidence PC61 and increased risk of advanced PC61 although in this study “well or very well done total meat” was indicated as the independent variable (Koutros et al., 2008).
Several limitations in this study need to be declared. Firstly, the total meat intake data analysed were calculated for per capita in each country. Therefore, the relationship between meat intake and PC61 may only be demonstrated at a country level, which does not necessarily correspond to the same relationship holding true at the individual level. Furthermore, the general market availability of total meat, not the actual human consumption, were tracked for this study. We could not be able to access the direct measures of actual meat consumed by humans as we did not have the data to measure food wastage and provide actual meat intake at country level. Secondly, we included ageing, GDP, magnitude of PC61 accumulation, obesity and urbanization as the potential confounding variables in partial correlation analysis, but other confounding factors, e.g. saturated fat, alpha-linolenic acid, dairy food, elevated intraprostatic androgen and elevated IGF-1, may still have influenced the associations reported in this study. For instance, meat intake varies worldwide due to availability, cultural beliefs or religious preferences. However, we could not locate and include other variables as the confounding factors in this study. Thirdly, the PC61 incidence rate was extracted from the GLOBOCAN database. It is probable that datasets from developing countries are less complete than those from developed countries due to issues of underdiagnoses. We attempted to remove the different levels of PC61 diagnoses through controlling for GDP and urbanization, but this removal might not be sufficient. Fourthly, total meat (“flesh of animals”) was used as the independent predictor of PC61 in this study. However, it is constantly reported that specific types, cooking methods, doneness levels and processing methods of meat may be the factors which make meat contribute to PC61.
In conclusion, per capita total meat (flesh of animals) consumption may be an independent predictor of PC61 incidence at a global level. However, this needs to be confirmed by other studies since our study may be affected by ecological fallacy. Major shifts in dietary habits featured with more meat intake should be investigated globally to determine its adverse health effects. It is novel to include total meat as the predictor of the worldwide non-communicable disease epidemic. The studies on the diet patterns of PC61 patients may be useful.
Declarations
Ethics approval and Availability of data.
All the data used in this study were freely downloaded from the United Nations (UN) agencies’ websites. No ethical approval or written informed consent for participation was applicable.
Authors’ contributions
WY and MH conceived the idea for this study. WY extracted the data, and MH and WY analysed and interpreted the data. WY reviewed the literature and drafted the manuscript. WY and MH edited and approved the manuscript for submission to the journal.
Competing interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This research was supported by the Mäxi Foundation, Zurich, Switzerland.
References
- 1.Alexander DD, Mink PJ, Cushing CA, Sceurman B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr J. 2010;9:50. doi: 10.1186/1475-2891-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allender S, Foster C, Hutchinson L, Arambepola C. “Quantification of urbanization in relation to chronic diseases in developing countries:a systematic review. J Urban Health. 2008;85:938–51. doi: 10.1007/s11524-008-9325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin M, Jeyaganth S, Fahmy N, et al. Dietary habits and prostate cancer detection:a case–control study. Can Urol Assoc J. 2008;2:510. [PMC free article] [PubMed] [Google Scholar]
- 4.Baade PD, Youlden DR, Coory MD, Gardiner RA, Chambers SK. Urban- rural differences in prostate cancer outcomes in Australia:what has changed? Med J Aust. 2011;194:293. doi: 10.5694/j.1326-5377.2011.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 5.Babio N, Sorlí M, Bulló M, et al. Association between red meat consumption and metabolic syndrome in a Mediterranean population at high cardiovascular risk:cross-sectional and 1-year follow-up assessment. Nutr Metab Cardiovasc Dis. 2012;22:200–7. doi: 10.1016/j.numecd.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Bai A, Li H, Huang Y, et al. A survey of overall life satisfaction and its association with breast diseases in Chinese women. Cancer Med. 2016;5:111–9. doi: 10.1002/cam4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaney PH. Affect and memory:A review. Psychol Bull. 1986;99:229–46. [PubMed] [Google Scholar]
- 8.Brett JF, Brief AP, Burke MJ, George JM, Webster J. Negative affectivity and the reporting of stressful life events. Health Psychol. 1990;9:57–68. doi: 10.1037//0278-6133.9.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Budnik A, Henneberg M. Worldwide increase of obesity is related to the reduced opportunity for natural selection. PLoS One. 2017;12:e0170098. doi: 10.1371/journal.pone.0170098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez CK, Walker-Thurmond MJ Thun. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;2003:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 11.Campisi J. Cancer and ageing:rival demons? Nat Rev Cancer. 2003;3:339–49. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LH, Towbes LC, Flocco R. Effects of induced mood on self- reported life events and perceived and received social support. J Pers Soc Psychol. 1988;55:669–74. doi: 10.1037//0022-3514.55.4.669. [DOI] [PubMed] [Google Scholar]
- 13.Courtney JG, Longnecker MP, Theorell T, Deverdier MG. Stressful life events and the risk of colorectal cancer:Results from a case-control study. Epidemiology. 1993;138:628. doi: 10.1097/00001648-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Cross A, Pollock J, Bingham S. Increased endogenous N-nitrosation in the human colon:a response to red and white meat? Br J Cancer. 2000;83:81. [Google Scholar]
- 15.Cross A, Pollock J, Bingham S. Red meat and colorectal cancer risk:the effect of dietary iron and haem on endogenous N-nitrosation. IARC Sci Publ. 2002;156:205. [PubMed] [Google Scholar]
- 16.Cross AJ, Gunter MJ, Wood RJ, et al. Iron and colorectal cancer risk in the α-tocopherol, β-carotene cancer prevention study. Int J Cancer. 2006;118:3147–52. doi: 10.1002/ijc.21780. [DOI] [PubMed] [Google Scholar]
- 17.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–60. [PubMed] [Google Scholar]
- 18.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 19.De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons KJ. The correlation between metabolic syndrome and prostatic diseases. Eur Urol. 2011;61:560–70. doi: 10.1016/j.eururo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Doll R, Peto R. The causes of cancer:quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191. [PubMed] [Google Scholar]
- 21.EUFIC. Food-based dietary guidelines in Europe:The European Food Information Council.“. 2018. Retrieved 14 January 2018, from http://www.eufic.org/en/healthy-living/article/food-based-dietary-guidelines-in-europe .
- 22.FAO. FAOSTAT-Food Balance Sheet. 2015. Retrieved [11.26.2015], from http://faostat3.fao.org/
- 23.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008:Globocan 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 24.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide:sources, methods and major patterns in Globocan 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 25.Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012 v1.0, Cancer incidence and mortality worldwide:IARC CancerBase No. 11 [Internet].”. 2013. Retrieved 28.05.2016, from http://globocan.iarc.fr .
- 26.Food and Agriculture Organization. FAOSTAT:Food supply - livestock and fish primary equivalent. 2018. from http://www.fao.org/faostat/en/#data/CL .
- 27.Gathirua-Mwangi WG, Zhang J. Dietary factors and risk of advanced prostate cancer. Eur J Cancer Prev. 2014;23:96. doi: 10.1097/CEJ.0b013e3283647394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Government of Canada-Health-Food and Nutrition-Healthy Eating. (2015, 2015-06-12). Build a healthy meal:use the Eat Well Plate. “Retrieved 14 January 2018, from http://www.healthycanadians.gc.ca/eating-nutrition/healthy-eating-saine-alimentation/tips-conseils/interactive-tools-outils-interactifs/eat-well-bien-manger-eng.php .
- 29.Grant WB. An ecological study of cancer mortality rates including indices for dietary iron and zinc. Anticancer Res. 2008;28:1955–63. [PubMed] [Google Scholar]
- 30.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 31.Gu D, McNaughton L, LeMaster D, et al. A comprehensive approach to the profiling of the cooked meat carcinogens 2-amino-3, 8-dimethylimidazo [4, 5-f] quinoxaline, 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine, and their metabolites in human urine. Chem Res Toxicol. 2010;23:788–801. doi: 10.1021/tx900436m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henchion M, McCarthy M, Resconi VC, Troy D. Meat consumption:Trends and quality matters. Meat Sci. 2014;98:561–8. doi: 10.1016/j.meatsci.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Henneberg M. Reproductive possibilities and estimations of the biological dynamics of earlier human populations. J Hum Evol. 1976;5:41–8. [Google Scholar]
- 34.Henneberg M, Piontek J. Biological state index of human groups. Przeglad Anthropologiczny. 1975;XLI:191–201. [Google Scholar]
- 35.Hsing A, Chokkalingam A. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 36.Hughes R, Cross A, Pollock J, Bingham S. Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis. 2001;22:199–202. doi: 10.1093/carcin/22.1.199. [DOI] [PubMed] [Google Scholar]
- 37.IARC. Lyon, France: IARC Monographs evaluate consumption of red meat and processed meat; 2015. [Google Scholar]
- 38.IARC. Globocan cancer fact sheets:Prostate cancer. 2016. Retrieved 01.05.2016, from http://globocan.iarc.fr .
- 39.IARC. Cancer. 2017. Retrieved 22 December 2017, from http://globocan.iarc.fr/Pages/cancer.aspx .
- 40.IARC. Globocan cancer fact sheets:prostate cancer. 2018. from http://globocan.iarc.fr/old/FactSheets/cancers/prostate-new.asp .
- 41.International Agency for Research on Cancer. Lyon, France: IARC Monographs evaluate consumption of red meat and processed meat; 2015. [PubMed] [Google Scholar]
- 42.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 43.John EM, Stern MC, Sinha R, Koo J. Meat consumption, cooking practices, meat mutagens, and risk of prostate cancer. Nutr Cancer. 2011;63:525–37. doi: 10.1080/01635581.2011.539311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi AD, Corral R, Catsburg C, et al. Red meat and poultry, cooking practices, genetic susceptibility and risk of prostate cancer:results from a multiethnic case–control study. Carcinogenesis. 2012;33:2108–18. doi: 10.1093/carcin/bgs242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N. Analysis of 200 food items for benzo [a] pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol. 2001;39:423–36. doi: 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 46.Key TJ. Fruit and vegetables and cancer risk. Br J Cancer. 2010;104:6. doi: 10.1038/sj.bjc.6606032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knize M, Sinha R, Brown E, et al. Heterocyclic amine content in restaurant-cooked hamburgers, steaks, ribs, and chicken. J Agric Food Chem. 1998;46:4648–51. [Google Scholar]
- 48.Knize M, Sinha R, Rothman N, et al. Heterocyclic amine content in fast-food meat products. Food Chem Toxicol. 1995;33:545–51. doi: 10.1016/0278-6915(95)00025-w. [DOI] [PubMed] [Google Scholar]
- 49.Knize M, Sinha R, Salmon C, et al. Formation of heterocyclic amine mutagens/carcinogens during home and commercial cooking of muscle foods. J Muscle Foods. 1996;7:271–9. [Google Scholar]
- 50.Koutros SAJ, Cross DP, Sandler JA, et al. Meat and meat mutagens and risk of prostate cancer in the Agricultural Health Study. Cancer Epidemiol Prev Biomarkers. 2008;17:80–7. doi: 10.1158/1055-9965.EPI-07-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawrie RA, Ledward D. Lawrie's meat science. 7th ed. Cambridge: Woodhead Publishing Limited; 2006. pp. 41–74. [Google Scholar]
- 52.Lewin MH, Bailey N, Bandaletova T, et al. Red meat enhances the colonic formation of the DNA adduct O6-carboxymethyl guanine:implications for colorectal cancer risk. Cancer Res. 2006;66:1859–65. doi: 10.1158/0008-5472.CAN-05-2237. [DOI] [PubMed] [Google Scholar]
- 53.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 54.Ma RL, Chapman K. A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet. 2009;22:187–99. doi: 10.1111/j.1365-277X.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 55.Majeed A, Babb P, Jones J, Quinn M. Trends in prostate cancer incidence, mortality and survival in England and Wales 1971–1998. BJU Int. 2000;85:1058–62. doi: 10.1046/j.1464-410x.2000.00661.x. [DOI] [PubMed] [Google Scholar]
- 56.Mandair D, Rossi RE, Pericleous M, Whyand T, Caplin ME. Prostate cancer and the influence of dietary factors and supplements:a systematic review. Nutr Metab (Lond) 2014;11:30. doi: 10.1186/1743-7075-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy KJ, Parker B, Dyer KA, et al. A comparison of regular consumption of fresh lean pork, beef and chicken on body composition:a randomized cross-over trial. Nutrients. 2014;6:682–96. doi: 10.3390/nu6020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.NCI. Chemicals in meat cooked at high temperatures and cancer risk - National Cancer Institute.”. 2018. from https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/cooked-meats-fact-sheet .
- 59.NHMRC. “The Five Food Groups |Eat For Health.”. 2015. Retrieved 14 January 2018, from https://www.eatforhealth.gov.au/food-essentials/five-food-groups .
- 60.Pearce KL, Norman HC, Hopkins DL. The role of saltbush-based pasture systems for the production of high quality sheep and goat meat. Small Rumin Res. 2010;91:29–38. [Google Scholar]
- 61.Pearson AM, Gillett TA. Processed meats. Springer; 2012. pp. 23–52. [Google Scholar]
- 62.Püssa T. Toxicological issues associated with production and processing of meat. Meat Sci. 2013;95:844–853. doi: 10.1016/j.meatsci.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 63.Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Chan JM. Egg, red meat, and poultry intake and risk of lethal prostate cancer in the prostate-specific antigen-era:incidence and survival. Cancer Prev Res. 2011;4:2110–21. doi: 10.1158/1940-6207.CAPR-11-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saniotis A, Henneberg M. Evolutionary medicine and future of humanity:Will evolution have the final word? Humanities. 2013;2:278–91. [Google Scholar]
- 65.Sarwar MH, Sarwar MF, Sarwar M, Qadri NA, Moghal S. The importance of cereals (Poaceae:Gramineae) nutrition in human health:A review. J Cereals Oilseeds. 2013;4:32–5. [Google Scholar]
- 66.Schuurman AG, Goldbohm RA, Dorant E, van Den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–9. doi: 10.1093/oxfordjournals.aje.a010241. [DOI] [PubMed] [Google Scholar]
- 67.Shavers VL, Underwood W, Moser RP. Race/ethnicity and the perception of the risk of developing prostate cancer. Am J Prev Med. 2009;37:64–7. doi: 10.1016/j.amepre.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siervo M, Montagnese C, Mathers JC, et al. Sugar consumption and global prevalence of obesity and hypertension:an ecological analysis. Public Health Nutr. 2014;17:587–96. doi: 10.1017/S1368980013000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinha R, Gustafson DR, Kulldorff M, et al. 2-Amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine, a carcinogen in high-temperature-cooked meat, and breast cancer risk. J Natl Cancer Inst. 2000;92:1352–4. doi: 10.1093/jnci/92.16.1352. [DOI] [PubMed] [Google Scholar]
- 70.Sinha R, Knize M, Salmon C, et al. Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem Toxicol. 1998;36:289–97. doi: 10.1016/s0278-6915(97)00159-2. [DOI] [PubMed] [Google Scholar]
- 71.Sinha R, Park Y, Graubard BI, et al. Meat and meat- related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am J Epidemiol. 2009;170:1165. doi: 10.1093/aje/kwp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinha R, Rothman N, Salmon C, et al. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem Toxicol. 1998;36:279–87. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- 73.Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh BP. Family history and the risk of prostate cancer. Prostate. 1990;17:337–47. doi: 10.1002/pros.2990170409. [DOI] [PubMed] [Google Scholar]
- 74.Stephan CN, Henneberg M. Medicine may be reducing the human capacity to survive. Med Hypotheses. 2001;57:633–7. doi: 10.1054/mehy.2001.1431. [DOI] [PubMed] [Google Scholar]
- 75.Stewart BW. World cancer report 2014. Lyon, Lyon, FRA: International Agency for Research on Cancer; 2014. [PubMed] [Google Scholar]
- 76.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines:Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–9. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tantamango-Bartley Y, Knutsen SF, Knutsen R, et al. Are strict vegetarians protected against prostate cancer? Am J Clin Nutr. 2015;103:153–60. doi: 10.3945/ajcn.114.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.The FAO. [Retrieved 22 January 2018];Products from slaughtered animals. 2018 [Google Scholar]
- 79.The New York Times (March 4, 2005). The Pork Industry's 'Other White Meat'Campaign Is Taken in New Directions (Media, Authored by Levere, Jane) Retrieved 14 January 2018. http://www.nytimes.com/2005/03/04/business/media/04adco.html .
- 80.The World Bank. Country and lending groups |Data.“. 2015. Retrieved 11.26.2015, from http://data.worldbank.org/about/country-and-lending-groups .
- 81.The World Bank. Indicators |Data.”. 2018. from https://data.worldbank.org/indicator .
- 82.USDA. All about the Protein Foods Group.”. 2015, 2015-02-23. Retrieved 14 January 2018, from https://www.choosemyplate.gov/protein-foods .
- 83.Vasundara V, Laurence HK. Diet and prostate cancer:mechanisms of action and implications for chemoprevention. Nat Rev Urol. 2010;7:442. doi: 10.1038/nrurol.2010.102. [DOI] [PubMed] [Google Scholar]
- 84.Wang R-J, Tang j-E, Chen Y, Gao J-G. Dietary fiber, whole grains, carbohydrate, glycemic index, and glycemic load in relation to risk of prostate cancer. (Original Reseaech)(Report) 2015;8:2415. doi: 10.2147/OTT.S88528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.WCRF/AICR (2007, 12.05.2015). Food, nutrition, physical activity, and the prevention of cancer:A global perspective. from http://www.dietandcancerreport.org/cancer_resource_center/downloads/Second_Expert_Report_full.pdf .
- 86.WHO. Global health observatory, the data repository.”WHO. 2015. Retrieved [11.26.2015, from http://www.who.int/gho/database/en/
- 87.WHO. WHO regional offices. 2018. Retrieved [11.26.2015, from http://www.who.int .
- 88.Wolk A. Potential health hazards of eating red meat. J Int Med. 2017;281:106–22. doi: 10.1111/joim.12543. [DOI] [PubMed] [Google Scholar]
- 89.You W-P, Henneberg M. Type 1 diabetes prevalence increasing globally and regionally:the role of natural selection and life expectancy at birth. BMJ Open Diabetes Res Care. 2016a;4:e000161. doi: 10.1136/bmjdrc-2015-000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.You W, Henneberg M. Cereal crops are not created equal:Wheat consumption associated with obesity prevalence globally and regionally. AIMS Public Health. 2016b;3:313–28. doi: 10.3934/publichealth.2016.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.You W, Henneberg M. Meat consumption providing a surplus energy in modern diet contributes to obesity prevalence:an ecological analysis. BMC Nutr. 2016c;2:1–11. [Google Scholar]
- 92.You W, Henneberg M. Meat in modern diet, just as bad as sugar, correlates with worldwide obesity:an ecological analysis. J Nutr Food Sci. 2016d;6:517. [Google Scholar]
- 93.You W, Henneberg M. Cancer incidence increasing globally:The role of relaxed natural selection. Evol Appl. 2017a;00:1–13. doi: 10.1111/eva.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.You W, Henneberg M. Meat in modern diet, Just as bad as sugar, correlates with worldwide obesity:An ecological analysis. J Nutr Food Sci. 2017b;6:517. [Google Scholar]
- 95.You W, Henneberg M. Relaxed natural selection contributes to global obesity increase more in males than in females due to more environmental modifications in female body mass. PLoS One. 2018;13:e0199594. doi: 10.1371/journal.pone.0199594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeegers M, Jellema A, Ostrer H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma. Cancer. 2003;97:1894–1903. doi: 10.1002/cncr.11262. [DOI] [PubMed] [Google Scholar]


