Abstract
The name Golovinomyces cynoglossi s. lat. is traditionally applied to a complex of morphologically similar powdery mildews on hosts of the plant family Boraginaceae. The current species-level taxonomy within this complex is ambiguous due to the lack of phylogenetic examinations. The present study applied phylogenetic methods to clarify the taxonomy of G. cynoglossi s. lat. Phylogenetic analysis of rDNA ITS sequences retrieved from Asian, European and North American specimens revealed that G. cynoglossi s. lat. collections from different hosts involved several species in five clearly separated lineages. Clade I consists primarily of Golovinomyces cynoglossi s. str. on Cynoglossum. Clade III consists of Golovinomyces sequences retrieved from the host genera Symphytum and Pulmonaria. The taxa within clade III are now assigned to G. asperifoliorum comb. nov. Clade V encompasses G. cynoglossi s. lat. on the host genera Bothriospermum, Buglossoides, Echium, Myosotis, and Trigonotis. The taxa within clade V are now assigned to G. asperifolii comb. nov. The species concerned in this study were lecto- and epitypified to stabilize their nomenclature.
Keywords: Boraginaceae, co-evolution, Erysiphaceae, Golovinomyces asperifolii, Golovinomyces asperifoliorum, rDNA ITS
1. Introduction
Boraginaceae is a large angiosperm family encompassing around 2740 species in 148 to 156 genera, depending on the recognized generic concept. The highest diversity within this prominent plant family resides in the Mediterranean region, Central Asia, the Near East, and Pacific North America (http://www.spektrum.de/lexikon/biologie-kompakt/boraginaceae/1818; http://data.kew.org/cgi-bin/vpfg1992/genlist.pl?BORAGINACEAE). Numerous boraginaceous species are vulnerable to powdery mildew infections [1,2], from species in the genera Erysiphe, Golovinomyces, Leveillula, and Phyllactinia. Powdery mildew infections from the genus Golovinomyces on Boraginaceae have been traditionally attributed to a single widespread species, G. cynoglossi [2]. Thus, G. cynoglossi is known to exhibit a wide host range of 31 genera belonging to the subfamilies Boraginoideae and Cordioideae [2,3]. Although comprehensive inoculation tests with powdery mildew on Boraginaceae [4] revealed a high degree of biological specialization, the taxonomic unity and status as a single species have not yet been evaluated. Recent molecular phylogenetic analyses of Golovinomyces spp. including G. cynoglossi s. lat. casted doubts on the monophyly of this species. Takamatsu et al. [5] found that a sequence of Golovinomyces on Myosotis sp. (VPRI20429, Australia) did not cluster with other sequences of G. cynoglossi. The morphology of Golovinomyces sp. on Bothriospermum tenellum and Trigonotis peduncularis collected in Korea was recently examined and revealed minimal differences with G. cynoglossi on other hosts. T. peduncularis has been reported as a host of G. cynoglossi in China, Japan, and Korea [1, 6, 7] and B. tenellum has been reported as a host in Japan [1]. Recently, Meeboon and Takamatsu [8] identified the powdery mildew on T. peduncularis as Euoidium sp., due to the lack of the sexual morph. In Korea, asexual and sexual morphs of powdery mildew were first reported on T. peduncularis in 1989 and on B. tenellum in 1999. The initial point of these examinations was to conduct phylogenetic analyses on collections of G. cynoglossi s. lat. and its synonyms on a wide range of hosts and from Europe (the origin of the type localities). The main objectives of these examinations were to (1) clarify the species concept of G. cynoglossi, i.e., to determine whether this species represents a single taxon or a complex of cryptic species, (2) to identify the powdery mildews on Trigonotis and Bothriospermum in Korea, and (3) to elucidate parasite–host relationships and the co-evolutionary aspects of Golovinomyces and its hosts.
2. Materials and methods
2.1. Molecular analyses
Genomic DNA was extracted using Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA) as described by Hirata et al. [9]. The internal transcribed spacer (ITS) regions were amplified with the primers PMITS1/PMITS2 [10]. The PCR products were sequenced with the same primers by Macrogen, a sequencing service company in Seoul, Korea. The sequences were edited by DNASTAR Lasergene software package 7.0 (DNASTAR, Madison, WI). New sequences obtained from the present study were deposited in GenBank under the accession numbers MH189691–189717 (Table 1). These sequences were aligned with the reference sequences of Golovinomyces species used in Takamatsu et al. [5] by MUSCLE implemented in MEGA 7.0 [11]. Phylogenetic trees were constructed by neighbor joining (NJ) and maximum likelihood (ML) methods in MEGA 7.0, and evaluated with 1000 bootstrap (BS) values [12]. All positions containing gaps and missing data were eliminated.
Table 1.
List of herbarium specimens of powdery mildew fungi used for nucleotide sequence analysis in this study.
| Host plant | Location and year of collection | Herbarium specimen no. | GenBank accession no. |
|---|---|---|---|
| Boragineae | |||
| Pulmonaria officinalis | Sachsen, Germany; 2010 | GLM-F100040 | MH189691 |
| Symphytum officinale | Vladivostok, Russia; 2015 | KUS-F28744 | MH189692 |
| S. officinale | Sachsen, Germany; 2004 | GLM-F073207 | MH189693 |
| S. officinale | Sachsen, Germany; 2006 | GLM-F079060 | MH189694 |
| Cynoglosseae | |||
| Asperugo procumbens | Sachsen, Germany; 2001 | GLM-F056808 | MH189695 |
| Bothriospermum tenellum | Seoul, Korea; 1999 | KUS-F15866 | MH189696 |
| B. tenellum | Seoul, Korea; 2010 | KUS-F24884 | MH189697 |
| B. tenellum | Namyangju, Korea; 2016 | KUS-F29208 | MH189698 |
| B. tenellum | Osan, Korea; 2007 | KUS-F22651 | MH189699 |
| Cynoglossum officinale | Sachsen-Anhalt, Germany; 2005 | GLM-F075252 | MH189700 |
| C. officinale | Nordrhein-Westfalen, Germany; 1999 | GLM-F047442 | MH189701 |
| C. officinale | Glenwood, Washington, USA; 2017 | KUS-F30414 | MH189702 |
| C. officinale | Missoula, Montana, USA; 2010 | WSP71856 | MH189703 |
| Myosotis arvensis | Sachsen, Germany; 2007 | GLM-F079306 | MH189704 |
| M. arvensis | Sachsen, Germany; 2007 | GLM-F079292 | MH189705 |
| Myosotis sylvatica | Sachsen, Germany; 2007 | GLM-F079458 | MH189706 |
| M. sylvatica | Sachsen, Germany; 2007 | GLM-F079322 | MH189707 |
| Trigonotis peduncularis | Seoul, Korea; 2016 | KUS-F29281 | MH189708 |
| T. peduncularis | Gapyeong, Korea; 2016 | KUS-F29517 | MH189709 |
| T. peduncularis | Seoul, Korea; 2016 | KUS-F29189 | MH189710 |
| T. peduncularis | Seoul, Korea; 2008 | KUS-F23263 | MH189711 |
| T. peduncularis | Namyangju, Korea; 2016 | KUS-F29206 | MH189712 |
| Lithospermeae | |||
| Buglossoides arvensis | Sachsen-Anhalt, Germany; 2005 | GLM-F073217 | MH189713 |
| B. arvensis | Saxony, Germany; 2007 | GLM-F079145 | MH189714 |
| Cerinthe minor | Sachsen-Anhalt, Germany; 2000 | GLM-F093819 | MH189715 |
| Echium vulgare | Rheinland-Pfalz, Germany; 2005 | GLM-F070123 | MH189716 |
| E. vulgare | Sachsen, Germany; 2006 | GLM-F070465 | MH189717 |
GLM: Senckenberg Gesellschaft für Naturforschung: Senckenberg Museum für Naturkunde Görlitz, Görlitz, Germany; KUS: Korea University, Seoul, Korea; WSP: Washington State University, Pullman, Washington, USA.
2.2. Morphology
In order to take micrographs, fresh samples were mounted in sterile water, and dried specimens in lactic acid [13]. Measurements of asexual and sexual structures were performed by means of an Olympus BX51 microscope (Olympus, Tokyo, Japan) under bright-field. Micrographs were acquired using a Zeiss AX10 microscope equipped with an AxioCam MRc5 camera (Carl Zeiss, Göttingen, Germany) under differential-interference contrast microscopy. Each structure was measured at least 30 times at magnifications of 400× and 1000×.
3. Results
3.1. Phylogenetic analyses
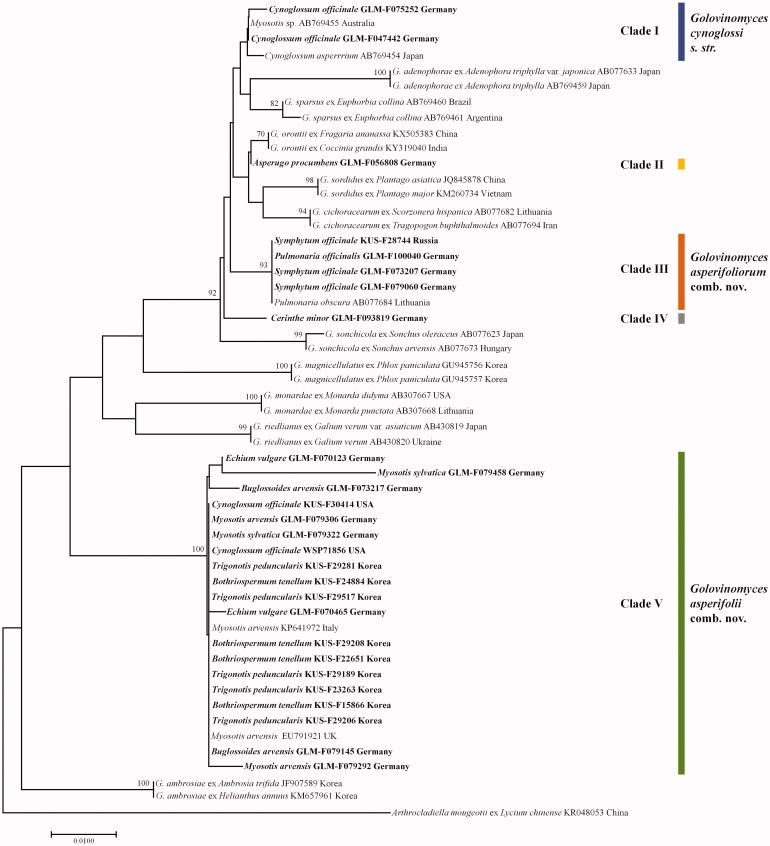
In total, 27 ITS sequences were analyzed in this study (Table 1). Each sequence was aligned with 25 Golovinomyces sequences. A sequence of Arthrocladiella mougeotii was retrieved from GenBank and used as outgroup according to Takamatsu et al. [5]. Two trees constructed by NJ and ML methods were consistent with each other, except for minor branch orders and branch lengths. The NJ tree with BS values higher than 50% is shown in Figure 6. In the phylogenetic trees, the G. cynoglossi complex is separated into five distinct clades. Clade I consists of four sequences, including three powdery mildew collections on Cynoglossum from Germany and Japan, and an Australian specimen on Myosotis. However, it should be noted that the BS value of this clade is low. Clade III is composed of sequences of five specimens on Pulmonaria and Symphytum and is supported with a high BS value (BS =94). Clade II and IV contain only a single sequence originated from Asperugo and Cerinthe, respectively. Clade V encompasses 21 samples from Bothriospermum, Buglossoides, Cynoglossum, Echium, Myosotis, and Trigonotis, and is highly supported with a BS value of 100.
Figure 6.
Phylogenetic relationship between Golovinomyces cynoglossi, G. asperifoliorum, and G. asperifolii isolates and some reference isolates retrieved from GenBank, inferred by maximum likelihood method using the internal transcribed spacer regions. Bootstrap values based on 1000 replications are indicated above the branches. The scale bar represents 0.01 nucleotide substitutions per site. The isolates presented in this study are indicated in bold.
3.2. Taxonomy
The phylogenetic and morphological analyses reveal that powdery mildew collections on Cynoglossum within clade I represent G. cynoglossi s. str., whereas G. cynoglossi s. lat. on Pulmonaria and Symphytum (clade III) constitutes a species of its own for which the name G. asperifoliorum comb. nov., based on Erysiphe asperifoliorum, is introduced. Powdery mildews on the genera Bothriospermum, Buglossoides, Cynoglossum [only in the USA, i.e., outside the natural range], Echium, Myosotis, and Trigonotis within clade V represent an additional species of Golovinomyces that occurs on a wider range of boraginaceous plants. Myosotis species are common hosts of this taxon in Europe for which the name G. asperifolii comb. nov., based on Oidium asperifolii, is introduced. Identification and application of G. cynoglossi, G. asperifoliorum, and G. asperifolii by means of phylogenetic methods are supported by epitypifications and ex-epitype reference sequences.
It is too early to settle the identification and taxonomic status of the single specimens of Asperugo and Cerinthe in clades II and IV. Broader sampling is needed to clarify the taxonomy of these clades.
The three species constituting the former G. cynoglossi complex are characterized as follows:
Golovinomyces cynoglossi (Wallr.) Heluta, Ukrayins’k. Bot. Zhurn. 45(5): 62, 1988, emend. (s. str.) (Figure 1)
≡Alphitomorpha cynoglossi Wallr., Ann. Wetterauischen Ges. Gesammte Naturk., N. F., 4: 240, 1819.
≡Erysiphe cynoglossi (Wallr.) U. Braun, Mycotaxon 15: 136, 1982.
=Erysiphe horridula var. cynoglossi Sorok., Rev. Mycol. 11: 147, 1889.
=Erysiphe horridula f. sp. cynoglossi S. Blumer, Jahrb. Philos. Fakult. II, Univ. Bern 2: 30, 1922.
=Erysiphe horridula f. sp. cynoglossi S. Blumer, Centralbl. Bakteriol., 2. Abth., 55: 291, 1922.
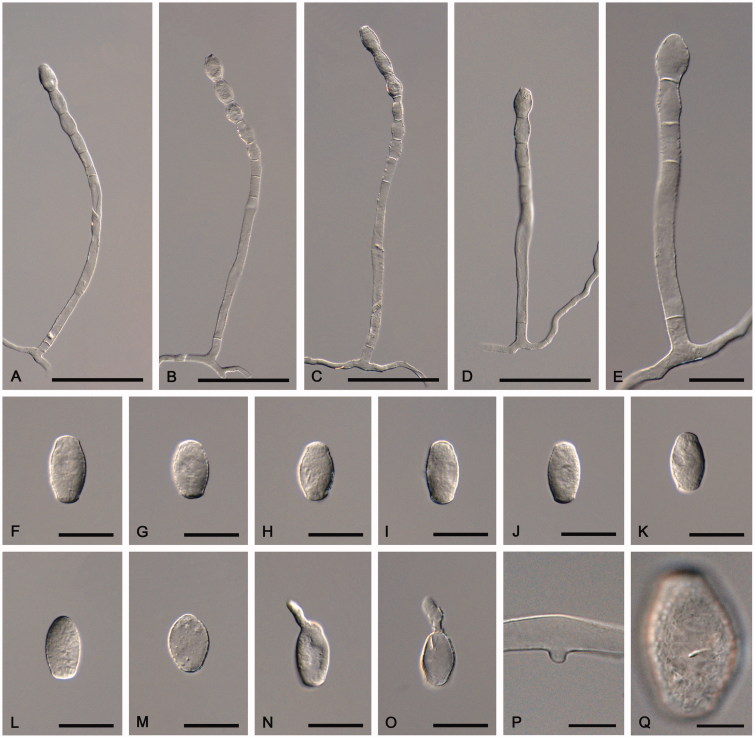
Figure 1.
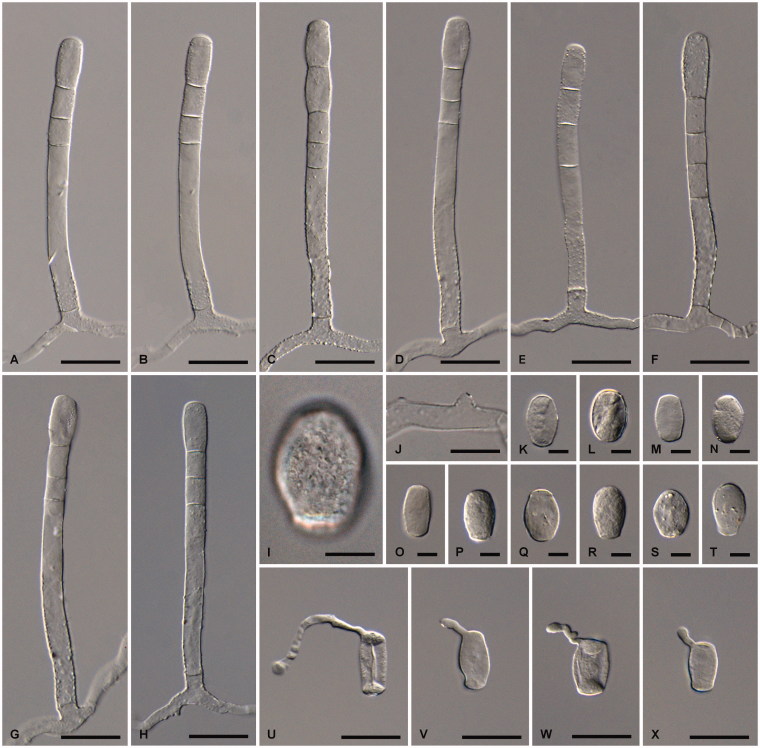
Golovinomyces cynoglossi s. str. examined from GLM-F047442 ex Cynoglossum officinale. (A–E) Conidiophores. (F–K) Conidia. (L–M) Primary conidia. (N–O) Conidia in germination. (P) Appressorium. (Q) Surface of a conidium. Scale bar =100 μm for A–D, 30 μm for E–O, and 10 μm for P–Q.
Illustrations: Braun and Cook (2002: 311, fig. 333, upper chasmothecium with short appendages) [2], Chen et al. (1987: 80, Figure 27) [14].
Mycelium amphigenous, on stems and sepals, dense, forming irregular white patches or effuse; hyphae hyaline, thin-walled, smooth, 4–7 µm wide; hyphal appressoria nipple-shaped, 4–8 µm diam; conidiophores erect, arising from the upper surface of hyphal mother cells and always towards one end of the cell, i.e., not centrally positioned, 130–250 µm long, foot-cells straight, cylindrical, subcylindrical or slightly increasing in width from base to top, 55–200 × 9–15 µm, basal septum of the foot-cell raised above the junction with the hyphal mother cell, 5–25 µm, rarely directly at the branching point, followed by 1–3 shorter cells, forming catenescent conidia; conidia ellipsoid-ovoid to doliiform, 25–40 × 15–20 µm, length/width ratio 1.4–2.0; primary conidia apically rounded and basally sub-truncate; germ tubes perihilar, short, apex with somewhat swollen appressorium (Euoidium type). Chasmothecia amphigenous, scattered to gregarious, often immersed in mycelial patches, 85–150 µm diam; peridium cells rounded, irregular to daedaleoid in shape, (5–)10–25(–30) µm diam; appendages usually numerous, equatorially arising and in the lower half, mycelioid, usually unbranched, 0.5–1.5(–2) times as long as the chasmothecial diam (gregarious chasmothecia immersed in dense mycelial patches often with very short appendages), 3.5–8 µm wide, at first hyaline, subhyaline to yellowish, later pale to medium brown throughout or paler towards the tip, septate, wall thin, 1–1.5(–2) µm, smooth or almost so; asci 8–16, subglobose-ovoid, saccate to clavate, stalked, 45–75 × 25–40 µm, apex rounded to frequently subtruncate, wall thin, 1–1.5(–2) µm, terminal oculus not very conspicuous, 8–18 µm wide, 2-spored, rarely with 3 spores; ascospores broad ellipsoid-ovoid, 15–24 × 10–15 µm, colorless.
Lectotype (designated in Braun and Cook 2012): Germany, on Cynoglossum officinale, herb. Wallroth, without any further data (STR). Epitype (designated here, MycoBank, MBT381246): Germany, Nordrhein-Westfalen, Kreis Soest, Erwitte, near Eikeloh, on Cynoglossum officinale, October 16 1999, U. Raabe (GLM-F047442).
Additional specimens examined: On Cynoglossum officinale – Armenia, Megrinskij Rajon, 2300–2600 m alt., July 15 1958, M. Manukyan & R. Bagalyan (HAL 870 F). Germany, Sachsen-Anhalt, Halle (Saale), September 1976, U. Braun (HAL 882 F), Halle (Saale), Dölauer Heide, Heidesee, November 4 1977, U. Braun (HAL 883 F), Merseburg, Buna stockpile, August 9 1978, S. Klotz (HAL 878 F), Saalekreis, Niemberg, October 18 2005, H. Jage 3223/05 (GLM-F075252). Russia, Bashkortostan, 10 km west of Starye Bogdaly, July 17 1977, U. Braun (HAL 881 F). On Cynoglossum sp. – China, Xingjian Uyghur Autonomous Region, July 3 1959, H.-Y. Liu & R. Liu 674 (HAL 871 F).
Golovinomyces asperifoliorum (Grev.) U. Braun & H.D. Shin, comb. nov. (Figures 2 and 3)
Basionym: Erysiphe asperifoliorum Grev., Fl. Edin.: 461, 1824.
MycoBank, MB 824901
=Erysiphe horridula f. sp. pulmonariae S. Blumer, Jahrb. Philos. Fakult. II, Univ. Bern 2: 30, 1922.
=Erysiphe horridula f. sp. symphyti S. Blumer (l.c.).
=Erysiphe horridula f. sp. pulmonariae S. Blumer, Centralbl. Bakteriol., 2. Abth., 55: 491, 1922.
=E. horridula f. sp. symphyti S. Blumer (l.c.: 490).
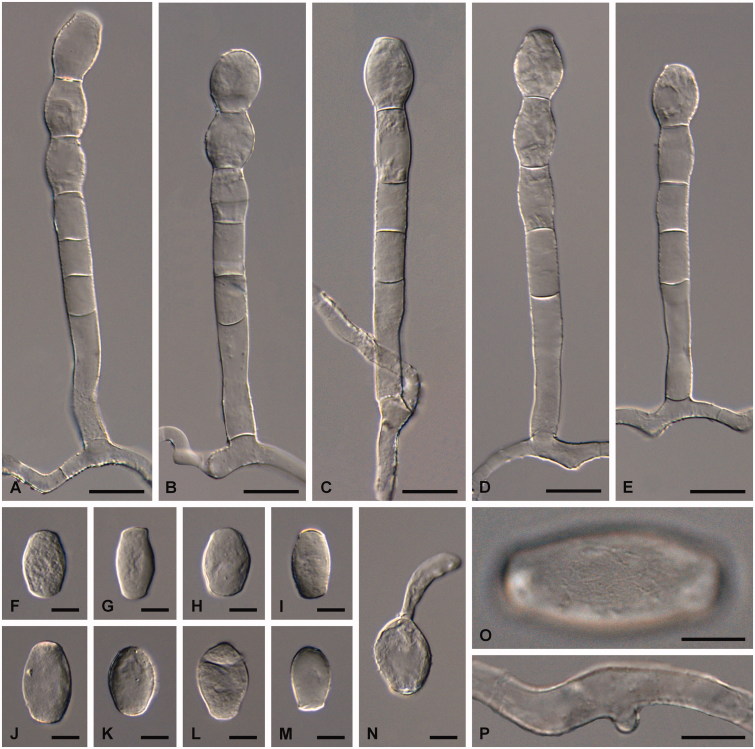
Figure 2.
Golovinomyces asperifoliorum examined from GLM-F079060 ex Symphytum officinale. (A–E) Conidiophores. (F–K) Conidia. (L–M) Primary conidia. (N) Conidium in germination. (O) Surface of a conidium. (P) Appressorium. Scale bar =20 μm for A–E and 10 μm for F–P.
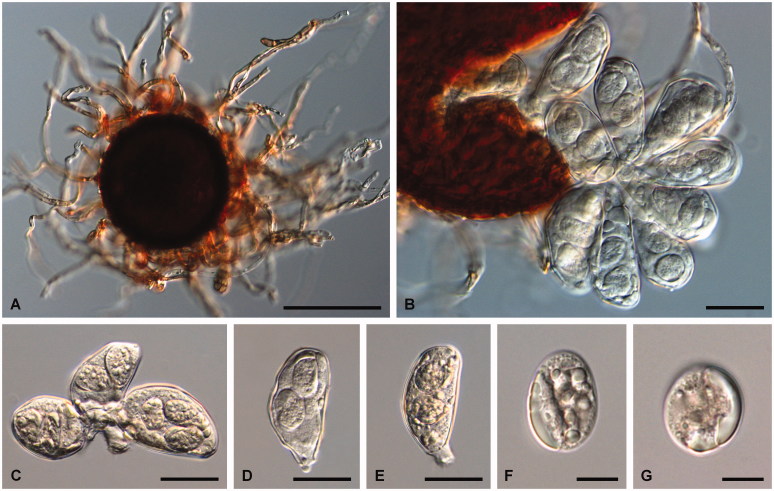
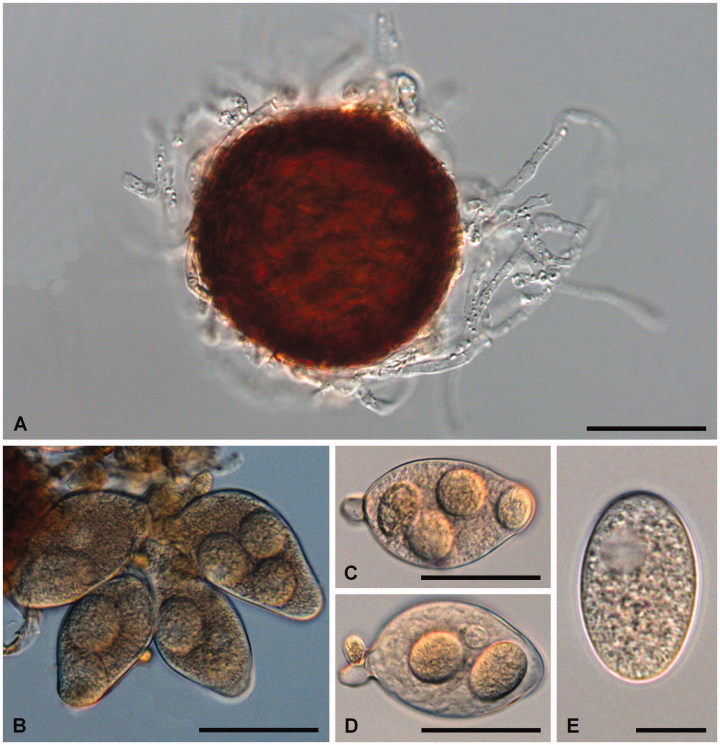
Figure 3.
Golovinomyces asperifoliorum examined from GLM-F079060 ex Symphytum officinale. (A) Chasmothecium with mycelioid appendages. (B) Cluster of asci containing 2 ascospores each. (C–E) Asci containing 2 or 3 ascospores. (F–G) Ascospores. Scale bar =100 μm for A, 30 μm for B–E, and 10 μm for F–G.
Illustration: Braun and Cook (2002: 311, Figure 333, lower chasmothecium and asexual morph) [2].
Mycelium amphigenous, on stems and sepals, dense, forming regular or irregular white patches, effuse; hyphae hyaline, thin-walled, smooth, 3.5–7 µm wide; hyphal appressoria nipple-shaped, 4–7 µm diam; conidiophores erect, arising from the upper surface of hyphal mother cells, always towards one end of the cell, i.e., not centrally positioned, 80–165 µm long; foot-cells straight, cylindrical or subcylindrical, 40–120 × 8–14 µm, basal septum of foot-cell at the junctions with the mother cells or only slightly raised, 2.5–5 µm, foot-cell followed by 1–3 shorter cells, forming catenescent conidia; conidia ovoid-oblong to doliiform, 27–40 × 15–20 µm, length/width ratio 1.5–2.3, primary conidia apically rounded and sub-truncate at the base; germ tubes perihilar, short, apex with somewhat swollen appressorium (Euoidium type). Chasmothecia gregarious or somewhat scattered, often densely aggregated and immersed in the mycelial felt, forming brown patches composed of ascomata, appendages and hyphae that may become pigmented with age, sub-globose, 70–145 µm diam; peridium cells polygonal to daedaleoid, 8–25 µm diam; appendages equatorial and in the lower half of the chasmothecia, numerous, mycelioid, simple, interlaced with each other and with the mycelium, 0.5–2(–3) times as long as the chasmothecial diam, 4–10 µm wide, sometimes up to 12 µm at the very base, septate, walls thin, smooth, pale to medium dark brown throughout or brown below and paler towards the tips; asci 5–15, ellipsoid-obovoid, clavate-saccate, apex rounded to truncate, 50–80 × 20–35 µm, stalked, wall 1–2 µm wide, terminal oculus not very conspicuous, 10–18 µm diam, 2-spored, rarely with 3 spores; ascospores ellipsoid-ovoid to almost globose, 13–28 × (10–)12–18 µm, colorless.
Lectotype (designated here, MycoBank, MBT381247): Scotland, Roslin, on Symphytum sp., undated, R.K. Greville (E 456091). Epitype (designated here, MycoBank, MBT381248): Germany, Sachen-Anhalt, Roßlau, Ragösen, Rathsbruch, S. officinale, August 28 2004, A. Hoch (GLM-F073207).
Additional specimens examined: On Pulmonaria angustifolia – Germany, Thuringia, Steinach, Holzberg, July 21 1979, St. Rauschert (HAL 850 F). On Pulmonaria mollis – Russia, Bashkortostan, Pavlovaka, water reservoir, July 14 1977, U. Braun (HAL 849 F). On Pulmonaria obscura – Germany, Sachen-Anhalt, Bad Düben, cemetery, 25 Aug. 1977, U. Braun (HAL 846 F); Russia, Bashkortostan, Yumatovo, July 3 1977, U. Braun (HAL 861 F), Pavlovaka, water reservoir, 14 Jul. 1977, U. Braun (HAL 879 F). On Pulmonaria officinalis – Germany, Saxony, Königshain, Schloßpark, May 22 2010, S. Hoeflich (GLM-F100040). On Symphytum officinale – Germany, Sachsen-Anhalt, Halle (Saale), Dölauer Heide, August 17 1975, U. Braun (HAL 860 F), Ziegelrodaer Forst, Jägerhof, August 28 1976, U. Braun (HAL 880 F), Greifenhagen, Aug. 1980, U. Braun (HAL 851 F), Grimmen, September 14 1982, U. Braun (HAL 853 F), Saxony, Westerzgebirge, Annaberg-Buchholz, June 1986, W. Dietrich (HAL 868 F), Görlitz-Biesnitz, Kunnewiter Grund, Reiherweiher, October 31 2006, S. Hoeflich (GLM-F079060); Russia, Bashkortostan, Ufa, Belaja, Kalinin Park, July 4 1977, U. Braun (HAL 864 F), Tujmazinskij Rayon, Lake Kandrykul, July 12 1977, U. Braun (HAL 845 F), Vladivostok, Skver Imeni K. Sukhanova, July 18 2015, B.S. Kim (KUS-F28744); Scotland, Stirlingshire, road ascending to Wallace Monument, July 1 2008, R. Watling (E 278591).
Notes: The name Erysiphe asperifoliorum is available for clade III, which comprises European Golovinomyces collections on Pulmonaria and Symphytum spp. Greville [15] described Erysiphe asperifoliorum from Scotland (“Symphytum tuberosum, Lycopsis arvensis, & c., about Edinburgh, Autumn”). Junell [16] discovered in Edinburgh (herb. E) a single original specimen examined by R.K. Greville and cited it as “type” of this species, which did not constitute a formal lectotypification and cannot be corrected as “lectotype” according to Art. 9.9 of the Code. Therefore, a formal lectotypification in the sense of Junell is designated herein. In order to fix and establish the application of the name G. asperifoliorum, epitypification with German material on S. officinale is proposed, including an ex-epitype sequence serving as a reference sequence. Attempts to retrieve DNA from the Scottish material (E 278591, see collections examined) failed for unknown reasons and prevented to designate this material as an epitype. However, since this species is common in Europe on Symphytum spp., an epitype from Germany is reasonable and undoubtedly acceptable.
Golovinomyces asperifolii (Erikss.) U. Braun & H.D. Shin, comb. nov. (Figures 4 and 5)
Basionym: Oidium asperifolii Erikss., Fungi Paras. Scand. Exs., Fasc. 8, no. 386, 1891.
=Oidium myosotidis Rabenh., Fungi Eur. Exs., Ed. Nov., Ser. Sec., Cent. 26, no. 2558, 1881, nom. inval. (Art. 36.1).
≡Oidium myosotidis Rabenh. ex Jacz., Karm. Opred. Grib., Vip. 2, Muchn.-rosj. Griby (Leningrad): 460, 1927, nom. illeg. (Art. 52.1).
=Erysiphe horridula f. sp. echii-myosotidis S. Blumer, Jahrb. Philos. Fakult. II, Univ. Bern 2: 30, 1922.
=Erysiphe horridula f. sp. echii-myosotidis S. Blumer, Centralbl. Bakteriol., 2. Abth., 55: 491, 1922.
Figure 4.
Golovinomyces asperifolii examined from GLM-F79292 ex Myosotis arvensis. (A–H) Conidiophores. (I) Surface of a conidium. (J) Appressorium. (K–Q) Conidia. (R–T) Primary conidia. (U–X) Conidia in germination. Scale bar =30 μm for A–H, 10 μm for I–T, and 30 μm for U–X.
Figure 5.
Golovinomyces asperifolii examined from KUS-F29281 ex Trigonotis peduncularis. (A) Chasmothecium. (B–D) Asci containing 2–4 ascospores. (E) Ascospore. Scale bar =50 μm for A, 30 μm for B–D, and 10 μm for E.
Illustration: Liu (2010: 156, Figure 74 A, based on a collection on Trigonotis peduncularis) [6].
Mycelium amphigenous, on stems and sepals, dense, persistent, forming regular or irregular white patches, effuse; hyphae hyaline, thin-walled, smooth, 3.5–7 µm wide; hyphal appressoria nipple-shaped, 3.5–7 µm diam; conidiophores erect, arising from upper surface of hyphal mother cells and always towards one end of the cell, i.e., not centrally positioned, 100–250 µm long; foot-cells straight, cylindrical, 40–130 × 8–14 µm, basal septum of the foot-cell somewhat raised, 5–25 µm above the junction with the hyphal mother cells, foot-cells followed by 1–3 shorter cells, forming catenescent conidia; conidia ovoid to doliiform, 22–38 × 12–20 µm, length/width ratio 1.4–2.4, primary conidia apically rounded and sub-truncate at the base; germ tubes perihilar, short, apex with somewhat swollen appressorium (Euoidium type). Chasmothecia gregarious to somewhat scattered, usually gregarious and immersed in dense mycelial patches, sub-globose to globose, 80–140 µm diam; peridium cells irregularly polygonal to daedaleoid, 10–25 µm diam; appendages more or less equatorial and in the lower half of the chasmothecia, always numerous, mycelioid, simple, interlaced with each other and with the mycelium, 0.5–2.5 times as long as the chasmothecial diam, 4–8 µm wide, septate, walls thin, smooth or almost so, hyaline or brown below and paler or colorless towards the tips; asci 5–15, with oil drops, ellipsoid-obovoid, clavate-saccate, 38–65 × 22–35 µm, short-stalked, 2–4-spored; ascospores ellipsoid-ovoid to almost globose, 10–20 × 10–18 µm, colorless.
Lectotype (designated here, MycoBank, MBT381249): Sweden, Stockholm, Experimental-fältet, on Myosotis sylvatica [M. “alpestris” hort.], July 11 1882, J. Eriksson [Erikss., Fungi Paras. Scand Exs. 386] (S-F270062). Isolectotypes: Erikss., Fungi Paras. Scand Exs. 386, e.g., BPI 409301, HAL. Epitype (designated here, MycoBank, MBT381250): Germany, Saxony, Görlitz-Bresnitz, on Myosotis sylvatica, May 10 2007, H. Boyle (GLM-F079322).
Additional specimens examined: On Bothriospermum tenellum – Korea, Seoul, Forestry Research Institute, April 27 1999, H.D. Shin (KUS-F15700), Seoul, Korea University, May 1 1999, H.D. Shin (KUS-F15715), Seoul, Korea University, May 25 1999, H.D. Shin (KUS-F15866), Jinju, Southern Forest Resources Research Center, May 28 2003, H.D. Shin (KUS-F19530), Cheongju, Chungbuk National University, May 1 2004, H.D. Shin (KUS-F20150), Osan, Mulhyanggi Botanical Garden, May 30 2007, H.D. Shin (KUS-F22651), Suwon, Seodun-dong, 8 May 2009, H.D. Shin (KUS-F24054), Seoul, Korea University, May 8 2010, H.D. Shin (KUS-F24884), Namyangju, Chukryeongsan Recreational Forest, June 8 2016, H.D. Shin (KUS-F29208). On Buglossoides arvensis – Armenia, Martuninsij Rayon, 2700–2800 m alt., August 12 1980, S. Simonyan (HAL 876 F); Germany, Sachsen-Anhalt, Merseburg, south of Klötzschen, June 16 2005, H. John (GLM-F073217), Saxony, Kringelsdorf, May 6 2007, leg. H. Boyle (GLM-F079145), Görlitz-Tauchitz, July 6 2006, leg. H. Boyle (GLM-F079081); Ukraine, Altagir, May 31 1984, U. Braun (HAL 852 F). On Cynoglossum officinale – USA, Montana, Missoula, August 29 2010, unknown collector (WSP71856), Washington, Glenwood, October 24 2017, M. Bradshaw (KUS-F30414). On Echium vulgare – Germany, Rheinland-Pfalz, Koblenz, Knappenrode, July 21 2005, S. Hoeflich (GLM-F070123), Sachsen, Sproitz, May 31 2006, H. Boyle & S. Hoeflich (GLM-F070465). On Myosotis arvensis – Germany, Sachsen-Anhalt, Greifenhagen, September 1981, U. Braun (HAL 848 F), Saxony, Westerzgebirge, Annaberg-Buchholz, August 26 1981, W. Dietrich (HAL 869 F), Olbersdorf, May 17 2007, S. Höflich (GLM-F079292), Olbersdorf, May 10 2007, H. Boyle (GLM-F079306). On Myosotis sylvatica – Germany, Saxony, Görlitz, May 6 2007, R. Franke (GLM-F079458). On Myosotis sp., Bulgaria, Rila Mts., Sokolvec, July 27 1978, U. Braun (HAL 847 F). On Trigonotis peduncularis – Korea, Gangneung, Ponam-dong, May 17 1989, H.D. Shin (KUS-F10143), Gangneung, Ponam-dong, May 30 1991, H.D. Shin (KUS-F10748), Gangneung, Ponam-dong, May 17 1992, H.D. Shin (KUS-F11590), Gangneung, Gangneung National University, April 23 1994, H.D. Shin (KUS-F12759), Gangneung, Eoheul-ri, May 22 1994, H.D. Shin (KUS-F12783), Seoul, Korea University, May 17 1997, H.D. Shin (KUS-F13776), Seoul, Korea University, October 11 1997, H.D. Shin (KUS-F14397), Seoul, Korea University, March 27 1999, H.D. Shin (KUS-F15668), Seoul, Korea University, April 3 1999, H.D. Shin (KUS-F15671), Seoul, Korea University, April 5 1999, H.D. Shin (KUS-F15673), Seoul, Korea University, April 24 1999, H.D. Shin (KUS-F15688), Chuncheon, Udu-dong, May 13 1999, H.D. Shin (KUS-F15783), Namyangju, Deokso Farm, May 28 1999, H.D. Shin (KUS-F15889), Seoul, Forestry Research Institute, May 31 1999, H.D. Shin (KUS-F15926), Samcheok, Miro-myeon, May 11 2000, H.D. Shin (KUS-F17290), Seoul, Forestry Research Institute, May 19 2000, H.D. Shin (KUS-F17338), Hongcheon, Bukbang-myeon, November 4 2005, H.D. Shin (KUS-F21629), Seoul, Forestry Research Institute, November 1 2006, H.D. Shin (KUS-F22434), Gimhae, Inje University, November 25 2006, H.D. Shin (KUS-F22503), Seoul, Korea University, April 7 2008, H.D. Shin (KUS-F23263), Suwon, Seodun-dong, May 8 2009, H.D. Shin (KUS-F24053), Seoul, Korea University, June 6 2016, T.T. Zhao (KUS-F29189), Namyangju, Chukryeongsan Recreational Forest, June 8 2016, H.D. Shin & S.E. Cho (KUS-F29206), Seoul, Seoul National University, June 30 2016, H.D. Shin & S.E. Cho (KUS-F29281), Gapyeong, Homyeongsan, September 29 2016, H.D. Shin & S.E. Cho (KUS-F29517).
Notes: Clade V encompasses powdery mildew collections occurring on a wider range of boraginaceous genera, including Myosotis spp. This powdery mildew species is common on Myosotis spp. in Europe. All sequences based on European powdery mildew samples on Myosotis spp. cluster within clade V for which teleomorph-typified names are not available. However, names based on asexual morphs on Myosotis have to be taken into consideration. The oldest name, Oidium myosotidis, proposed in Rabenhorst, Fungi Eur. Exs., Ed. Nov., Ser. Sec., Cent. 26, no. 2558, 1881, is invalid due to it was not being accepted by Rabenhorst, i.e., it was designated as “ad int.” (Art. 36.1). Rabenhorst (l.c.) failed to describe O. myosotidis, only stating: “Die Konidien unterscheiden sich von den O. ruborum etc. in keiner Weise” [the conidia differ from those of O. ruborum etc. not at all]. Jaczewski [17] took up the name O. myosotidis and added a brief description of the conidia. Jaczewski [17] failed to validate this name due to his citation of the valid name O. asperifolii as synonym. By doing so, he made O. myosotidis Rabenh. ex Jacz. a superfluous name (nom. illeg.). O. asperifolii, introduced in Eriksson, Fungi Paras. Scand. Exs., Fasc. 8, no. 386, 1891 (with brief description of the conidia), for a powdery mildew collected in an experiment field near Stockholm, Sweden, on Myosotis “alpestris” [hort.] (= M. sylvatica, see Junell 1967: 23), refers to clade V and has to be utilized for the taxon involved. A new specimen on M. sylvatica collected in Sweden, suitable for phylogenetic analyses, was not available for epitypification purposes. Therefore, a German powdery mildew sample on M. sylvatica is designated as epitype to establish the application of G. asperifolii. There is a certain degree of genetic variation within clade V. Most sequences in this clade are identical or almost so, however, some of them retrieved from the German samples on Buglossoides arvensis (GLM-F073217), E. vulgare (GLM-F070123, 070405), and Myosotis spp. (GLM-F079458, 079292) are genetically deviating and need a further investigation. Currently we prefer to maintain all collections and corresponding sequences of clade V in G. asperifolii, at least tentatively.
Key to three recognized species of Golovinomyces cynoglossi complex based on morphological characteristics:
1 Conidiophores 80–165 µm long, basal septum at the junction with the mother cell or only slightly elevated (2.5–5 µm); on Pulmonaria and Symphytum spp. as principal hostsG. asperifoliorum
1* Conidiophores longer, up to 250 µm, basal septum usually 5–25 µm above the junction with the mother cell2
2 Conidia 25–40 × 15–20 μm, length/width ratio 1.4–2.0; chasmothecial appendages pale to medium brown throughout or paler towards the tip; asci usually 2-spored; on Cynoglossum spp. as principal hosts, widespread in the natural range of the species
G. cynoglossi
2* Conidia 22–38 × 12–20 µm, length/width ratio 1.4–2.4; chasmothecial appendages hyaline or brown below and paler or colorless towards the tip, asci 2–4-spored; on various hosts, including Bothriospermum, Buglossoides, Echium (only outside of the range in North America), Myosotis, and Trigonotis spp.G. asperifolii
4. Discussion
Golovinomyces cynoglossi, previously known as Erysiphe asperifoliorum and E. horridula, is the most common and widespread powdery mildew on hosts of the Boraginaceae [1,2,7,18]. Other powdery mildews on hosts of this family pertain to the genera Erysiphe, Leveillula, and Phyllactinia. Species of the genus Podosphaera, including Sphaerotheca, which are common on hosts of many other plant families, are not known to infect hosts within Boraginaceae. Sphaerotheca lappulae [19], introduced as a powdery mildew on Lappula heteracantha in China, is an excluded and doubtful species (incorrect host identification and description of the sexual morph based on chasmothecia deposited as contamination) [2]. Almost all host species of G. cynoglossi s. lat. belong to genera of the subfamily Boraginoideae, which is currently split into four tribes, i.e., Boragineae, Cynoglosseae, Echiochilieae, and Lithospermeae [3]. Of the 31 boraginaceous genera recorded as hosts of this species [1,2], 17 are in tribe Cynoglosseae, six are in tribe Lithospermeae, and nine are in tribe Boragineae. Blumer [4,20] performed comprehensive inoculation tests and morphological examinations on powdery mildew referred to as Erysiphe horridula. Blumer’s [4,20] research dictated the current wide concept of G. cynoglossi. He revealed a high degree of biological specialization and proposed to split this species into seven taxonomic entities for which he used the category “forma specialis”, i.e., he introduced informal taxa not regulated by the Code (ICN): Erysiphe horridula f. sp. anchusae, f. sp. asperuginis, f. sp. cerinthes, f. sp. cynoglossi, f. sp. echii-myosotidis, f. sp. pulmonariae, and f. sp. symphyti [20]. On the basis of his own results and inoculation experiments carried out by Neger [21] and Hammarlund [22], Blumer [18] provided the following classification and overview: (1) Erysiphe asperifoliorum f. sp. anchusae, on Anchusa officinalis as principal host, ascus 2–5-spored, conidia formed singly [unrelated to G. cynoglossi and corresponding to Erysiphe lycopsidis]. Ascus 2–3-spored, conidia formed in chains (catenescent), corresponding to G. cynoglossi s. lat.: (2) E. asperifoliorum f. sp. echii-myosotidis, on Echium and Myosotis spp. as principal hosts, and Borago officinalis, Omphalodes linifolia, Cerinthe major, and C. glabra as secondary hosts, appendages thin, short, almost hyaline, conidia 33–38 µm long. Appendages thicker, more or less brown: (3) E. asperifoliorum f. sp. lithospermi, mainly on Lithospermum arvense, conidia 33–38 µm long. (4) E. asperifoliorum f. sp. asperuginis, on Asperugo procumbens and Cerinthe major, conidia 38–41 µm long. (5) E. asperifoliorum f. sp. cynoglossi, on Cynoglossum officinale, conidia 38–41 µm long. (6) E. asperifoliorum f. sp. symphyti, mainly on Symphytum spp., secondary hosts Lappula echinata, Cerinthe major, C. glabra, Lycopsis arvensis and Anchusa azurea, conidia 40–45 µm long. (7) E. asperifoliorum f. sp. pulmonariae, mainly on Pulmonaria spp., secondary hosts Symphytum officinale, and Cerinthe major, conidia 40–45 µm long. The biological heterogeneity of G. cynoglossi s. lat., the slight morphological differences between collections from different host genera and the first molecular sequence analyses raised serious doubts as to the monophyly of this species. Phylogenetic analyses recently performed on the basis of specimens from Asia, Europe and North America clearly showed that G. cynoglossi s. lat. is genetically divided into several clades which are in concordance with Blumer’s [18] concept of formae speciales, at least to a certain degree.
Clade I consists of three sequences retrieved from powdery mildew on Cynoglossum (Cynoglosseae) in Germany and Japan, and a single sequence of Golovinomyces on Myosotis from Australia. Although ITS sequences are commonly and widely used for powdery mildews, they are often not sufficiently reliable for final taxonomic conclusions on the species level [23,24]. However, in this scenario, sequence data are indispensable for the clarification of the G. cynoglossi complex. All sequences obtained from Golovinomyces on Cynoglossum in Europe and Asia cluster together, and in spite of a low BS value and a limited number of included sequences, the results from clade I corroborated with the epitypification of a German collection and an ex-epitype sequence (due to the original description of this species from Germany on the Eurasian host species, Cynoglossum officinale), allows the position and circumscription of G. cynoglossi s. str. to be fixed. The type material for the G. cynoglossi complex is old and is thus inappropriate for phylogenetic analyses. Because of this, epitypification with appropriate material for analyses is the currently preferred method. A single sequence in clade I originates from Golovinomyces on Myosotis in Australia which raises the assumption that G. cynoglossi may cause accidental infections on other hosts, including those outside of this powdery mildew species’ natural range. Otherwise, Blumer [4,18,20] found a strict specialization of the C. officinale powdery mildew, without any secondary hosts. On the other hand, it is worth noting that a sequence obtained from Golovinomyces on Cynoglossum in Montana, USA, clustered in clade V. The Golovinomyces within clade V represent G. asperifolii, a distinct, not closely allied species with a wider host range amongst boraginaceous genera. This suggests that the genus Cynoglossum may be infected by at least two Golovinomyces species, with G. cynoglossi as the principal powdery mildew species within the natural range of Cynoglossum spp. However, many questions remain open. Seventeen genera of tribe Cynoglosseae were listed as hosts of G. cynoglossi s. lat. [1,2,7], including Amsinkia, Cryptantha, Lindefolia, and Plagiobotrys, which are considered as phylogenetically closely allied to Cynoglossum [3]. The identity of Golovinomyces on hosts of these genera is still unclear and needs to be morphologically and phylogenetically re-examined.
Clade III contains sequences of powdery mildews from two genera, Symphytum and Pulmonaria, which belong to tribe Boragineae. This clade is well supported, is clearly distinct from G. cynoglossi s. str., and corresponds to Blumer’s E. horridula f. sp. pulmonariae and f. sp. symphyti which were classified to have identical morphology and overlap in their host range (S. officinale was listed as a secondary host for f. sp. pulmonariae) [18]. Golovinomyces species within clade III constitutes a species of its own, Golovinomyces asperifoliorum, which is also morphologically distinguished from the powdery mildews of clade I (G. cynoglossi s. str.) and clade V (G. asperifolii). The conidiophores are relatively short, 90–165 µm, and the basal septum of the conidiophores is located at the junction with the mother hypha or only slightly elevated, 2.5–5 µm (vs. conidiophores longer, up to 250 µm, basal septum 5–25 µm elevated in G. cynoglossi and G. asperifolii). The chasmothecial appendages in G. asperifoliorum are usually pigmented throughout and the asci are 2-spored, rarely 3-spored, in contrast to appendages pigmented below and paler or hyaline towards the tip and asci 2–4-spored in G. asperifolii.
E. asperifoliorum was introduced as powdery mildew on Symphytum tuberosum, Lycopsis arvensis and other boraginaceous hosts [15], which necessitates a lectotypification to define the application of this name. A collection on Symphytum sp. is the only original material examined by Greville that is maintained in the herbarium in Edinburgh (E). This specimen is designated as lectotype and enables the application of this name for clade III.
Anchusa, Borago, Brunnera, Nonea, Pectocarya, Rindera, and Solenanthus are additional host genera belonging in tribe Boragineae. Species of these genera are known to be hosts of G. cynoglossi s. lat. [1,2,7]. Morphological re-examinations and phylogenetic analyses are necessary to clarify the affinity of the powdery mildews on the hosts concerned.
Clade II and IV comprise Golovinomyces on Asperugo and Cerinthe, respectively. The two clades contain a single genus and, in each case, only one sequence. The positions of the two clades suggest the possible involvement of additional species on Boraginaceae, but the examined collections were not sufficient for final taxonomic conclusions. A larger sampling is necessary to discern the involvement of additional species. Cerinthe belongs to tribe Lithospermeae, but sequences retrieved from Golovinomyces on hosts of two other genera belonging to tribe Lithospermeae, i.e., Echium and Buglossoides, are located in clade V. Alkanna and Onosma spp. are common, widespread hosts of Golovinomyces reported from many European countries [1,7,25]. However, the identity of Golovinomyces on hosts of these genera is unclear and needs to be examined on the basis of molecular and morphological methods. Although Asperugo belongs to tribe Cynoglosseae and contains a close phylogenetic relationship to Trigonotis and Bothriospermum [3], a sequence obtained from Golovinomyces on Asperugo procumbens collected in Germany clusters separately from clade I (G. cynoglossi s. str.), as well as from clade V (G. asperifolii), which includes powdery mildew on Trigonotis and Bothriospermum. Other genera, such as Mertensia and Omphalodes, which have close phylogenetic relationships to Asperugo, Trigonotis and Bothriospermum, are also host plants of G. cynoglossi s. lat. [1,7,25], but have not yet been included in phylogenetic analyses.
Clade V is the largest clade with the broadest host range, consisting of host species belonging to genera of the tribes Cynoglosseae (Bothriospermum, Myosotis, and Trigonotis) and Lithospermeae (Echium and Buglossoides), suggesting a plurivorous, widespread species. All sequences of Golovinomyces on Myosotis ssp. from various European countries belong to clade V. Hence, the name Oidium asperifolii, described from Sweden on Myosotis sylvatica, can be used for this clade and its application is determined by a corresponding epitypification. A sequence retrieved from Golovinomyces on Myosotis in Australia clustering in clade I seems to be the results of an unusual infection, under exotic conditions, outside of the natural range of G. cynoglossi. Clade V is well supported and clearly distinct from clade I and III, i.e., the treatment of this clade as species of its own, G. asperifolii, is fully justified. In addition, there are some morphological peculiarities such as longer conidiophores with distinctly raised basal septum (in comparison with G. asperifoliorum) and paler chasmothecial appendages as well as 2–4-spored asci (compared to G. cynoglossi).
In conclusion, it can be stated that the three species, G. cynoglossi s. str., G. asperifoliorum, and G. asperifolii, discovered and substantiated within the course of the present examinations, are phylogenetically and morphologically distinct. Differences in the characters of the conidiophores have been found for the first time. Previous analyses of asexual characters just focused on the conidial size [4,18,26]. The germ tubes within G. cynoglossi s. lat. are rather uniform (short, subclavate, apex somewhat swollen, occasionally twined), which was already stated in Neger [21] for powdery mildew on Cerinthe, Echium, Buglossoides (Lithospermum), Pulmonaria, and Symphytum. Blumer [4,18,26] and Junell [16] discussed morphological differences in the sexual morphs of boraginaceous powdery mildews, which could be confirmed in the course of our own examinations. These differences are, however, gradual and difficult to discern, and thus are only applicable in combination with characters of the asexual morphs and phylogenetic data.
G. cynoglossi s. lat. has a wide host range covering host species of numerous genera. Only powdery mildew on hosts of a limited number of these genera could be included in our analyses, i.e., the present work is just a first step in the revision of this species complex and should be seen as a basis for further analyses and taxonomic examinations. The splitting of the G. cynoglossi (s. lat.) complex into three species, G. cynoglossi s. str., G. asperifoliorum and G. asperifolii, is reasonable and supported by sequence analyses, biological aspects and morphological differences. The initial question regarding the identity of powdery mildew on Trigonotis and Bothriospermum in Korea could also be answered, i.e., it does not belong to G. cynoglossi s. str., but to G. asperifolii, a species with a wide host range and distribution. In general, it can be stated that the particular clades and corresponding Golovinomyces species on boraginaceous host genera are not aligning confidently with the phylogenetic-taxonomic affinity of the host genera to tribes of the Boraginaceae. However, they reflect to a certain extent, previous biological analyses and classifications into formae speciales [4,21,22].
Funding Statement
This work was financially supported by the BK21 Plus program in 2016-2020 funded by the National Research Foundation of Korea (NRF) and also in part by a special grant from Korea University to HDS.
Acknowledgments
Particular thanks are due to the curators of the herbaria in Edinburgh, Scotland (E), Görlitz, Germany (GLM), and Washington, USA (WSP) for allowing us to examine material in their keeping and to use it for molecular analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Amano K. Host range and geographical distribution of the powdery mildew fungi. Tokyo: Japan Science Society Press; 1986. [Google Scholar]
- 2.Braun U, Cook RTA. Taxonomic manual of the Erysiphales (Powdery Mildews). CBS Biodiversity Series 11. Utrecht: CBS; 2012. [Google Scholar]
- 3.Nazaire M, Hufford L. A broad phylogenetic analysis of Boraginaceae: implications for the relationships of Mertensia. Syst Bot. 2012;37:758–783. [Google Scholar]
- 4.Blumer S. Specialization of Erysiphe horridula Lév. on Boraginaceae. Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten, 2. Abtheilung. 1922;55:480–506. [Google Scholar]
- 5.Takamatsu S, Matsuda S, Grigaliunaite B. Comprehensive phylogenetic analysis of the genus Golovinomyces (Ascomycota: Erysiphales) reveals close evolutionary relationships with its host plants. Mycologia. 2013;105:1135–1152. [DOI] [PubMed] [Google Scholar]
- 6.Liu TZ. The Erysiphaceae of Inner Mongolia. Chifeng: Inner Mongolia Science and Technology Press; 2010. [Google Scholar]
- 7.Farr DF, Rossman AY Fungal Databases. Syst Mycol Microbiol Lab., 2018. Online publication, ARS, USDA. Retrieved March 26. https://nt.ars-grin.gov/fungaldatabases/index.cfm
- 8.Meeboon J, Takamatsu S. Notes on powdery mildews (Erysiphales) in Japan: III. Golovinomyces and Podosphaera. Mycoscience. 2015;56:243–251. [Google Scholar]
- 9.Hirata T, Takamatsu S. Nucleotide diversity of rDNA internal transcribed spacers extracted from conidia and cleistothecia of several powdery mildew fungi. Mycoscience. 1996; 37:283–288. [Google Scholar]
- 10.Cunnington JH, Takamatsu S, Lawrie AC, et al. Molecular identification of anamorphic powdery mildew fungi. Austral Plant Pathol. 2003;32:421–428. [Google Scholar]
- 11.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA. 2004;101:11030–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin HD. Erysiphaceae of Korea. Suwon: National Institute of Agricultural Science and Technology; 2000. [Google Scholar]
- 14.Chen GQ, Han SJ, Lai YQ, et al. Flora Fungorum Sinicorum. Vol. 1, Erysiphales. Beijing: Science Press; 1987. [Google Scholar]
- 15.Greville RK. Flora Edinensis. Edinburgh: William Blackwood; 1824. [Google Scholar]
- 16.Junell L. Erysiphaceae of Sweden. Symbolae Botanicae Upsaliensis. 1967;14:1–117. [Google Scholar]
- 17.Jaczewski AA. Pocket identification book. Part. 2. Powdery mildews. Leningrad: Mycological Laboratory A.A. Jaczewski. State Institute of Experimental Agronomy; 1927. [Google Scholar]
- 18.Blumer S. Powdery mildews (Erysiphaceae). Jena: G. Fischer Verlag; 1967 [Google Scholar]
- 19.Liu TZ, Xu ZJ. A new species of Sphaerotheca. Mycosystema. 2004;23:464–465. [Google Scholar]
- 20.Blumer S. Contributions to the specialization of Erysiphe horridula Lév. on Boraginaceae. Jahrbuch der Philosophischen Fakultät II. Universität Bern. 1922;2:28–34. [Google Scholar]
- 21.Neger FW. Contributions to the biology of the Erysiphaceae. 2. Communication. 1902;90:221–272. [Google Scholar]
- 22.Hammarlund C. To the genetics, biology and physiology of some Erysiphaceae. Hereditas. 2010;6:1–126. [Google Scholar]
- 23.Kiss L. Limits of nuclear ribosomal DNA internal transcribed spacer (ITS) sequences as species barcodes for fungi. Proc Natl Acad Sci USA. 2012;109:E1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scholler M, Schmidt A, Siahaan SAS, et al. A taxonomic and phylogenetic study of the Golovinomyces biocellatus complex (Erysiphales, Ascomycota) using asexual state morphology and rDNA sequences data. Mycol Progress. 2016;15:56. [Google Scholar]
- 25.Braun U. The Powdery Mildews (Erysiphales) of Europe. Jena: G. Fischer Verlag; 1995. [Google Scholar]
- 26.Blumer S. The Erysiphaceae of Central Europe with special reference to Switzerland. Beiträge Zur Kryptogamenflora Der Schweiz. 1933;7:1–483. [Google Scholar]