Abstract
Temperature is an important environmental factor that can greatly influence the cultivation of Auricularia cornea. In this study, lignin peroxidase, laccase, manganese peroxidase, and cellulose in A. cornea fruiting bodies were tested under five different temperatures (20 °C, 25 °C, 30 °C, 35 °C, and 40 °C) in three different culture periods (10 days, 20 days and 30 days). In addition, the V4 region of bacterial 16S rRNA genes in the substrate of A. cornea cultivated for 30 days at different temperatures were sequenced using next-generation sequencing technology to explore the structure and diversity of bacterial communities in the substrate. Temperature and culture days had a significant effect on the activities of the four enzymes, and changes in activity were not synchronized with changes in temperature and culture days. Overall, we obtained 487,694 sequences from 15 samples and assigned them to 16 bacterial phyla. Bacterial community composition and structure in the substrate changed when the temperature was above 35 °C. The relative abundances of some bacteria were significantly affected by temperature. A total of 35 genera at five temperatures in the substrate were correlated, and 41 functional pathways were predicted in the study. Bacterial genes associated with the membrane transport pathway had the highest average abundance (16.16%), and this increased at 35 °C and 40 °C. Generally, different temperatures had impacts on the physiological activity of A. cornea and the bacterial community in the substrate; therefore, the data presented herein should facilitate cultivation of A. cornea.
Keywords: Auricularia cornea; bacterial community; extracellular enzyme; structure and diversity; temperature
1. Introduction
Auricularia cornea, which belongs to the class Basidiomycota, is a common edible fungus with medicinal value in China [1,2]. The fruiting bodies of A. cornea are abundant, containing many nutrients including protein, crude fiber, carbohydrates, amino acids, and some microelements [3,4]. The polysaccharides in the fruiting bodies have been confirmed to reduce blood fat and have shown antioxidizing, immune enhancing, and cancer reducing activities [5–8]. A. cornea can also nourish and clear the lungs to enable smooth breathing, enrich and circulate blood, and restrain pain and bleeding [9]. Since the early 1980s, A. cornea has been artificially cultivated in China, and its social demands have recently increased greatly owing to its obvious advantages. Like other edible mushrooms, temperature, enzyme and microbiota greatly influence A. cornea during growth, which could lead to changes in polysaccharide and microelements in the sporocarp [10,11]. Among all factors that influence the growth of A. cornea, temperature has the greatest effect and can determine the output and quality of A. cornea. Temperature does not only influence the growth of hyphae, but also the extracellular enzyme activities [12–14]. However, high temperature may result in hyphal gliosis at the surface, causing serious reductions in yield. A. cornea secretes extracellular enzymes to dissolve and absorb outer substances, playing vital roles in its growth. The extracellular enzymes produced during A. cornea growth are related to the substrate, and different temperatures will affect the activity of extracellular enzymes to varying degrees [15]. There are many enzymes associated with A. cornea, primarily laccase, cellulose, lignin peroxidase (Lip), and manganese peroxidase (Mnp). In the macromycetes, laccase is related to formation of pigments and fruiting bodies, degradation of lignin, pathogenicity and detoxification of conidium [16–19]. Among edible mushrooms, especially A. cornea, the nutritional value of fruiting bodies is affected by the substrate. The microbial community structure in the substrate also impacts on the mushroom growth and development [20,21]. The microbial community constitution and activities are closely associated with the ratio of carbon to nitrogen in the substrate, and certain bacterial community structures can indicate the health status of edible mushrooms [22]. Currently, next-generation sequencing technology is being widely applied to explore the distribution and diversity of microbial communities in unique ecological environments [23,24].
Previous studies of A. cornea mainly focused on the active ingredients in the fruiting bodies, cultivation techniques and genetic diversity [1,6,25–30]. The studies have shown that temperature can influence hyphal growth, the formation of sporocarps and the shelf-life of sporocarps [12,31,32]. In addition, temperature has been shown to be important to screen the strains that can produce certain enzymes [13,15,33,34]. However, few studies have been carried out on the changes of extracellular enzymes and bacterial communities in the substrate at different temperatures. Therefore, in this study, we explored the influence of temperature (20 °C, 25 °C, 30 °C, 35 °C, and 40 °C) and culture periods (10 days, 20 days and 30 days) on the activity of A. cornea-associated extracellular enzymes (including Lip, laccase, Mnp, and cellulose), the structure of bacterial community in the substrate and the putative function of the dominant bacteria, with the ultimate goal to facilitate the commercial cultivation of A. cornea.
2. Materials and methods
2.1. A. cornea cultivation
The experimental strains of A. cornea were provided by the Soil and Fertilizer Institute at the Sichuan Academy of Agriculture Science. The substrate was composed of cottonseed hull (10%), crushed corncob (30%), wood chips (33%), rice bran (20%), corn flour (2%), lime (4%), and gypsum (1%).
The substrate was sterilized at 100 °C for 15 h, after which it was placed in polythene cultivation bags (size: 17 cm × 33 cm × 0.005 cm). After cooling to room temperature in a laminar flow cabinet, the substrate was inoculated with A. cornea. Following inoculation, A. cornea was cultivated at 20–25 °C in Tonglin Village, Shifang, China. When the bags were full of hyphae, they were divided equally into five groups and placed into a biochemical incubator for cultivation at five different, but steady temperatures i.e., 20 °C, 25 °C, 30 °C, 35 °C, and 40 °C, respectively.
2.2. Determination of extracellular enzyme activity
Extracellular enzyme activities were measured on the 10th, 20th and 30th days after A. cornea was cultivated at the five different temperatures mentioned above, respectively. Lip, laccase, Mnp, and cellulose of A. cornea were determined using a trace kit (Comin Biotechnology Co., Ltd., Suzhou, China) according to the manufacturer’s instructions.
2.3. Sample collection
A. cornea samples were collected on the 30th day after cultivation at the five different temperatures. Three cultivated bags were selected randomly from each temperature treatment and sent to the laboratory for sampling. Disposable disinfected gloves, sterilized tweezers and knives were prepared and used for sampling.
Substrate materials of each cultivation bag were collected from different parts of the bag and pooled together. More than 500 mg of substrate materials per sample were collected and stored in 2 mL microtubes at –20 °C, prior to DNA extraction.
2.4. DNA extraction, PCR amplification and MiSeq sequencing
The samples collected at 20 °C, 25 °C, 30 °C, 35 °C, and 40 °C were designated as A1, A2, A3, A4, and A5, respectively. Three replications of each temperature treatment were treated independently to evaluate the methodological reproducibility. A MoBio PowerSoil® DNA Isolation Kit (12888, MoBio Laboratories Inc., Carlsbad, CA, USA) was used to isolate DNA from the substrate. The concentration of DNA was quantified using an ultraviolet spectrophotometer (Eppendorf® Bio Photometer, Hamburg, Germany). The size of DNA was determined by 0.8% agarose gel electrophoresis.
PCR was conducted using the bacterial 16S rRNA gene V4 region-specific primer 520 F (5′-barcode + GCACCTAAYTGGGYDTAAAGNG-3′) and primer 802 R (5′-TAC NVGGGTATC TA ATCC-3′). Samples were amplified by subjecting mixtures composed of 0.25 μL Q5 high-fidelity DNA polymerase, 5 μL reaction buffer, 5 μL high GC Buffer, 0.5 μL dNTP, 1 μL DNA template, 1 μL forward primer, 1 μL reverse primer, and 11.25 μL sterile water to the following conditions: 98 °C for 2 min (initial denaturation) followed by 25–27 cycles of 98 °C for 15 s (denaturation), 50 °C for 30 s (annealing) and 72 °C for 30 s (extension), after which they were subjected to 72 °C for 5 min (final extension) [35]. High-fidelity DNA polymerase was used to ensure the efficiency and accuracy of PCR amplification. The PCR products were checked by 2% agarose gel electrophoresis and the target fragments were removed and recovered using an Axygen Axy Prep DNA Gel Extraction kit (AxyGen Biosystems, USA). Meanwhile, the products were quantified using a Quant-iT Pico Green dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA) with a Microplate reader (FLx800, BioTek Instruments, Inc., Winooski, Vt.) and then mixed based on the concentration of each sample.
An Illumina TruSeq Nano DNA LT Sample Prep Kit (FC-121-4001 or FC-121-4002, Illumina, San Diego, CA, USA) was used for library construction. Selection of the final segment and purification of the library was conducted using 2% agarose gel electrophoresis. The qualified library was diluted, mixed with each sample and changed to single strand DNA subjected sodium hydroxide, after which a MiSeq Reagent Kit v2 600-cycles-PE (MS-102-3003, Illumina) and the MiSeq platform (Personal Biotechnology Co., Ltd. Shanghai. China) were utilized to accomplish paired-end sequencing of 2 × 300 bp.
2.5. Sequence data processing and statistical analysis
After paired-end sequencing of the DNA, the original data were saved in FASTQ format. The sliding window method was used to screen the quality of sequences in FASTQ format. The FLASH software (version 1.2.7) was used for splicing [36]. The QIIME software (Quantitative Insights Into Microbial Ecology, version 1.8.0) was employed to identify and eliminate the query sequences [37], after which USEARCH (version 5.2.236) was employed to check and remove the chimera sequences to obtain high-quality sequences. The number of high-quality sequences of each sample was then calculated and the length distribution of the high-quality sequences of the whole samples was also calculated using the R software.
High-quality sequences with ≥97% similarity were clustered into operational taxonomic units (OTUs) using UCLUST [38], a sequence alignment tool based on QIIME. The sequence of the greatest abundance per OTU was selected to represent this OTU, whereas those OTUs with a relative abundance lower than 0.001% were removed [39]. The Greengenes database (Release 13.8) was utilized to assign the taxonomy of the OTUs [40]. The OTU table was input into R software and subject to statistical analysis. The shared OTUs were presented in a Venn diagram. Four indices (Chao1, ACE, Shannon and Simpson) were used to reflect the alpha diversity of microbial communities. A heatmap was drawn to cluster and analyze the 50 most abundant genera based on the R heatmap package [41]. Non-metric multidimensional scaling (NMDS) was used to reflect the beta diversity of microbial communities. LEfSe analysis was employed to reveal the bacterial taxa that had differential abundance between different temperature treatments at all taxonomic levels in the substrate of A. cornea [42]. Network analysis was performed using the Mothur software [43]. PICRUSt software was used to predict the metabolic functions of bacterial communities based on the KEGG database [44].
One-way analysis of variance (ANOVA) was used to test the effect of temperature on enzyme activities in SPSS v19.0 (IBM Inc., Armonk, NY, USA). The least significant difference (LSD) was used to identify significant pairwise differences between the means of the treatments. All significance was concluded at p < .05.
3. Results
3.1. The influence of temperature on hyphae and the activities of extracellular enzymes from A. cornea
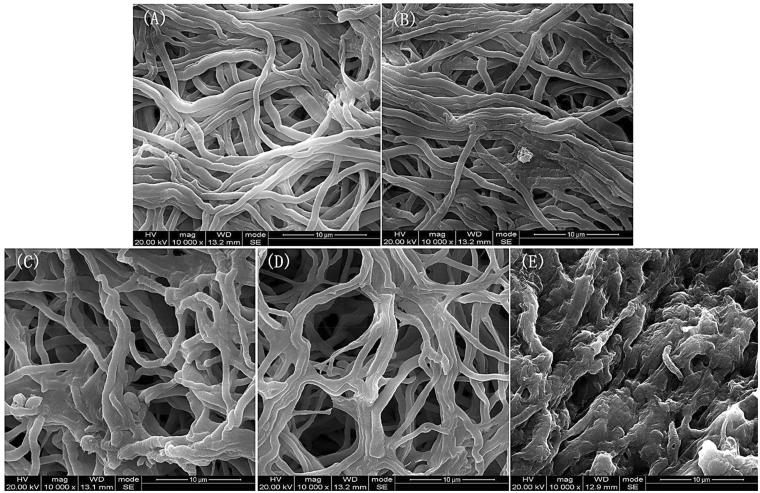
The changes in hyphae under different temperature treatments were evident under an electron microscope in the present study (Figure 1). The five tested temperatures were divided into three groups according to the hyphal presentation: 20 °C and 25 °C, 30 °C and 35 °C, and 40 °C. Hyphal twisting increased with increasing temperature.
Figure 1.
The morphology of A. cornea’s hyphae at five different cultivation temperatures under an electron microscope with magnification of 10000 times. (A) presents the hyphae at 20 °C, (B) at 25 °C, (C) at 30 °C, (D) at 35 °C, (E) at 40 °C.
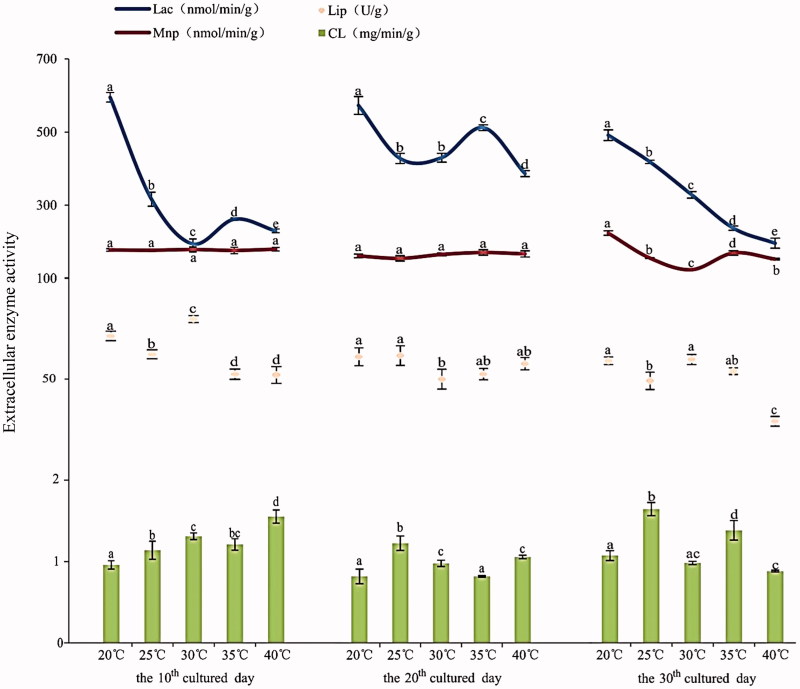
On the same culture day, the activities of each measured extracellular enzyme of A. cornea differed among different temperatures (Figure 2). On the 10th day, the Lip activity reached a maximum of 79.60 U/g at 30 °C (Table S1). Afterwards, the Lip activity decreased greatly with increasing temperature and tended to be the same at 35 °C and 40 °C. On the 20th day, the Lip activity showed no big differences among test temperatures. On the 30th day, the Lip activity was observed lowest at 40 °C (29.27U/g). The laccase activity was highest at 20 °C, regardless of culture days. On the 10th and 20th days, the laccase activity exhibited a fluctuating pattern with increasing temperatures. On the 30th day, the performance of laccase declined with increasing temperature from 20 °C to 40 °C. The Mnp activity did not differ among different temperatures on the 10th and 20th days. However, on 30th day of cultivation, the Mnp activity fluctuated with increasing temperature with the highest activity at 20 °C and the lowest at 30 °C. On 10th day, the cellulose activity increased with increasing temperature and peaked at 40 °C. On the 20th and 30th days, the cellulose activity fluctuated and was observed highest at 25 °C. In general, there were significant differences in enzymes activities between different temperatures on each special culture day.
Figure 2.
The activities of Lip, laccase, Mnp and cellulose at five different temperatures on three different culture days.
3.2. Composition and structure of bacterial community in the substrate
In total, 438,458 high-quality sequences were obtained from the 15 samples, accounting for 89.90% of the total reads. The sequences from all samples were clustered into 915 OTUs, with 97% similarity as the threshold. The number of individual sequences that mapped to each OTU ranged from 36 to 413 (Figure S1). A total of 16 phyla, 29 classes, 47 orders, 101 families, and 177 genera were detected in the 15 samples in the substrate at different temperatures. As shown in Figure S2, the dominant phyla included Firmicutes (average 91.6%), Proteobacteria (5.1%), Bacteroidetes (1.6%), and Actionbacteria (1.2%), all of which showed different relative abundances at different temperatures. In the phylum Firmicutes, Bacilli was the largest class, with an average relative abundance of 90.9%. Bacilli mainly included Paenibacillaceae, Planococcaceae, Bacillaceae, and Streptococcaceae with average relative abundances of 65.0%, 16.8%, 8.1%, and 0.6%, respectively. At the genus level (Figure S3), Paenibacillus (28.2%), Brevibacillus (27.3%) and Lysinibacillus (16.6%) were the dominant genera in the substrate. In addition, Cohnella, Bacillus, Rhodococcus, and Prevotella were relatively rich in abundance, accounting for 8.7%, 7.8%, 1.1%, and 1.1% on average, respectively.
3.3. NMDS analysis
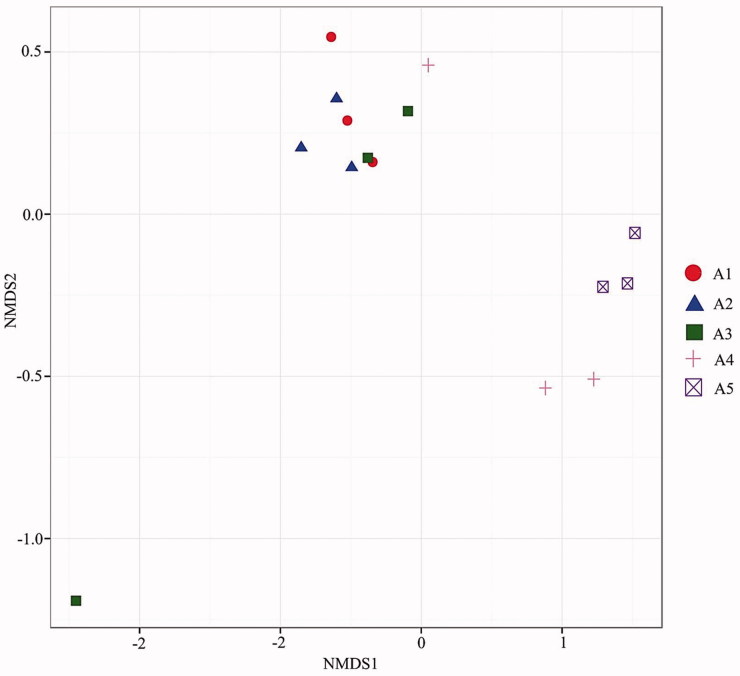
The beta diversity of bacterial communities in the substrate of A. cornea between different temperature treatments was visualized with NMDS ordination (Figure 3). The bacterial community structure was similar under cultivation at 20–30 °C. However, the increase in cultivation temperature above 35 °C resulted in apparent structural changes in bacterial communities in the substrate.
Figure 3.
Nonmetric multidimensional scaling ordination showing the weighted UniFrac dissimilarities of bacterial communities in the substrate of A. cornea subject to five different cultivation temperatures.
3.4. Bacterial alpha diversity indices and community composition at different temperatures
The bacterial alpha diversity indices did not differ between different cultivation temperatures (Table 1).
Table 1.
Community richness and diversity indices of bacteria in the substrate of A. cornea at five different temperatures.
| Samples | Simpson | Chao1 | ACE | Shannon |
|---|---|---|---|---|
| A1 (20 °C) | 0.79 ± 0.05a | 51.00 ± 3.00a | 70.15 ± 14.65a | 3.02 ± 0.22a |
| A2 (25 °C) | 0.84 ± 0.03a | 62.33 ± 11.01a | 91.15 ± 24.82a | 3.44 ± 0.24a |
| A3 (30 °C) | 0.86 ± 0.05a | 83.67 ± 55.77a | 108.07 ± 80.64a | 3.82 ± 0.93a |
| A4 (35 °C) | 0.80 ± 0.03a | 70.33 ± 3.79a | 96.60 ± 5.55a | 3.41 ± 0.16a |
| A5 (40 °C) | 0.86 ± 0.03a | 75.00 ± 10.44a | 92.93 ± 5.74a | 3.86 ± 0.41a |
Each value is mean of three replicates (±SD). Values followed by different lowercase letters indicate significant differences (p < .05) between samples in a line.
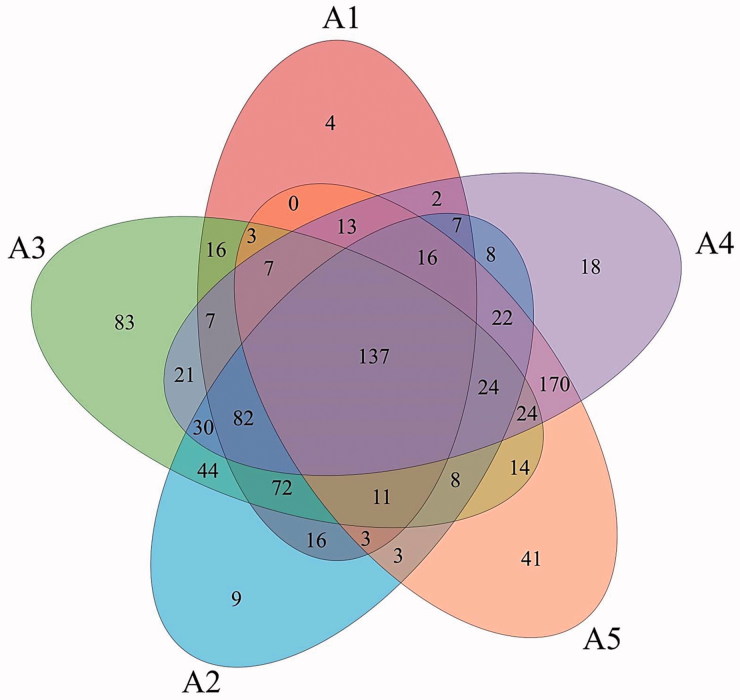
A Venn diagram was used to display the degree of overlap of bacterial OTUs among the five temperature treatments (Figure 4). Samples at different temperatures had different numbers of OTUs, with 396, 492, 583, 588, and 496 OTUs being observed at 20 °C, 25 °C, 30 °C, 35 °C, and 40 °C, respectively. Moreover, there were 4, 9, 83, 18, and 41 unique OTUs at each temperature, respectively. Additionally, a total of 137 common OTUs existed at all the temperatures, accounting for 14.97% of the total.
Figure 4.
Venn diagram displaying the overlaps of bacterial OTUs between different temperature treatments.
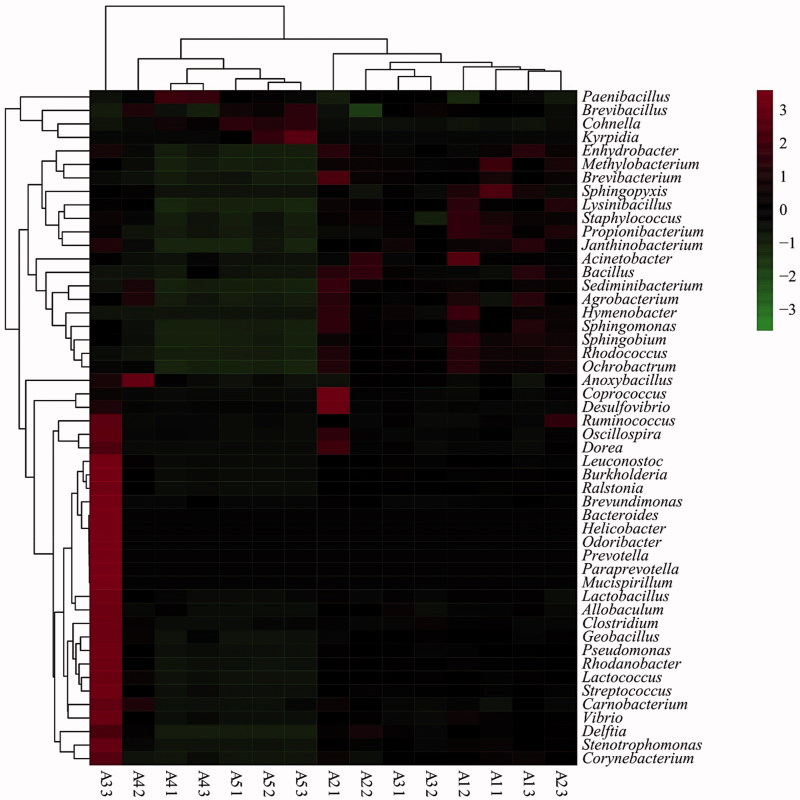
The abundance of several phyla differed at the five tested temperatures (Figure S4). The average abundance of Actinobacteria decreased as temperature increased. The richest phylum, Firmicutes, decreased at 20 °C–30 °C, then increased at 30–40 °C. The 50 most abundant genera can be seen in the heatmap (Figure 5). The bacterial communities of the samples at 20 °C and 25 °C were similar, as were those of samples at 35 °C and 40 °C; however, these two groups differed significantly. In the samples from 20 °C and 25 °C, the dominant genera were Sphingopyxis, Acinetobacter, Coprococcus, Desulfovibrio, and Brevibacterium, belonging to Proteobacteria, Actinobacteria and Firmicutes. However, the genera Kyrpidia, Cohnella, Brevibacillus, and Paenibacillus (all Firmicutes) were dominant at 35 °C and 40 °C, while Kyrpidia was the only genus observed at 40 °C.
Figure 5.
Heat map showing the relative abundance of genera in the substrate of A. cornea at five different temperatures. The color of scale reflects the density of OTUs abundance. The 50 most abundant OTUs were clustered in R software.
3.5. Biomarker discovery
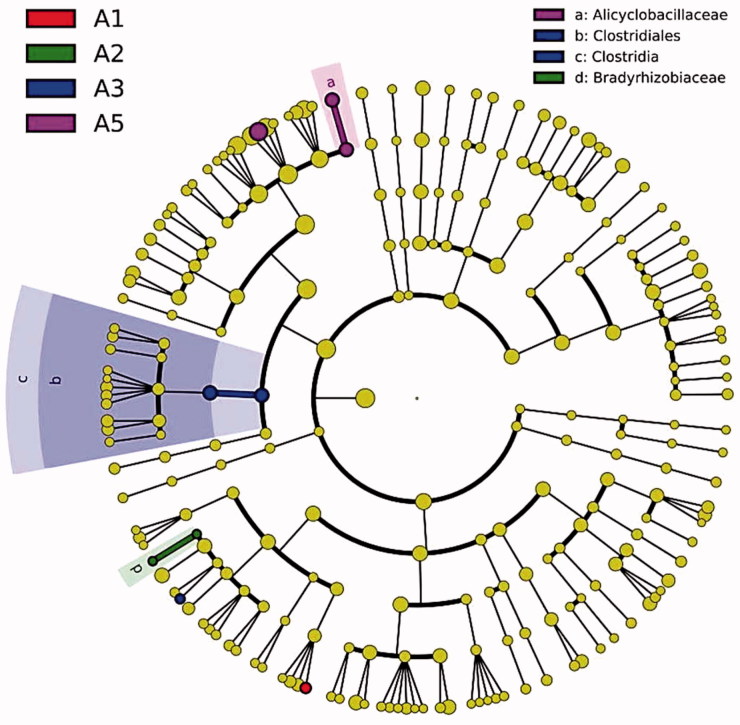
LEfSe analysis was used to reveal the substrate bacterial taxa that showed differential abundance between different temperatures on the 30th day of A. cornea cultivation at all taxonomic levels (Figure 6). One unidentified genus showed the greatest abundance at 20 °C. The family Bradyrhizobiaceae was significantly enriched in the substrate at 25 °C relative to other temperature conditions. A significantly higher abundance of the class Clostridia (e.g., order Clostridiales) was observed in the substrate at 30 °C than at the other four temperatures. The family Alicyclobacillaceae and an unidentified genus exhibited significantly higher abundance at 40 °C. However, no distinct species were observed in the substrate of A. cornea cultivated at 35 °C.
Figure 6.
Cladogram showing the differentially abundant bacterial taxa in the substrate of A. cornea at five different temperatures based on LEfSe analysis (p < .05, LDA score >2). There were no A4-enriched OTUs, so no legend was assigned for A4.
3.6. Association network analysis of the dominant genera
The 35 most abundant genera showed certain correlations with each other (Figure S5). The related behaviors of Paraprevotella and Mucispirillum differed from those of other genera. Specifically, variations in their abundances were not correlated with other changes in abundance, and the two genera only appeared in the samples at 30 °C with a low abundance (about 0.1%). The other 33 genera were in a complex network and closely connected, forming mutual inhibition or mutual promotion relationships. The abundance of Cohnella was negatively correlated with Acinetobacter, Propionibacterium, Sphingobium, Staphylococcus, and Janthinobacterium. Some genera on the edge of the network, such as Acinetobacter, Sphingopyxis and Brevundimonas [Ruminococcus], had less contact with other members, while others were involved in the complex relationship.
3.7. Predicted bacterial community function
In this study, seven functional genotypes with 41 pathways were predicted by PICRUSt based on KEGG database (Table 2), including environmental information processing, genetic information processing, metabolism, cellular processes, human diseases, organismal systems and unclassified. Overall, 45.03% of these pathways were related to metabolism, followed by environmental information processing (19.15%) and genetic information processing (15.58%). There were relatively few pathways of human diseases, biological systems and cell physiology. In addition, 14.68% of these pathways were unclassified. The membrane transport pathway was the most abundant pathway, accounting for 16.16% on average. Although its abundance changed slightly among 20 °C, 25 °C and 30 °C, it was much higher at 35 °C and 40 °C. The average abundances of amino acid metabolism and carbohydrate metabolism were also higher (9.56% and 9.44%), with both decreasing slightly with increasing temperature. Surprisingly, the proportion of replication, repair and translation pathways decreased from 25 °C, while their abundance increased at 40 °C. Some pathways related to metabolism, such as terpenoid and polyketide metabolism, lipid metabolism and xenobiotics biodegradation and metabolism, decreased in abundance with increasing temperature.
Table 2.
Predicted functional pathways of bacterial communities in the substrate of A. cornea.
| Function type | KEGG pathways | Bacteria abundance (%) |
||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | ||
| Environmental information processing | Membrane transport | 15.41% | 14.84% | 15.06% | 17.58% | 17.93% |
| Signaling molecules and interaction | 0.16% | 0.15% | 0.17% | 0.14% | 0.14% | |
| Signal transduction | 2.78% | 2.66% | 2.68% | 3.01% | 3.04% | |
| Genetic information processing | Folding, sorting and degradation | 1.82% | 1.87% | 1.93% | 1.81% | 1.79% |
| Replication and repair | 6.39% | 6.55% | 6.71% | 6.55% | 6.66% | |
| Transcription | 3.16% | 3.13% | 3.11% | 3.42% | 3.52% | |
| Translation | 3.76% | 3.88% | 4.01% | 3.88% | 3.93% | |
| Metabolism | Xenobiotics biodegradation and metabolism | 3.48% | 3.42% | 3.14% | 2.58% | 2.34% |
| Metabolism of cofactors and vitamins | 3.80% | 3.87% | 3.82% | 3.59% | 3.60% | |
| Metabolism of other amino acids | 1.80% | 1.80% | 1.76% | 1.64% | 1.56% | |
| Metabolism of terpenoids and polyketides | 1.95% | 1.96% | 1.84% | 1.58% | 1.52% | |
| Nucleotide metabolism | 2.92% | 2.98% | 3.07% | 3.02% | 3.07% | |
| Lipid metabolism | 3.97% | 3.94% | 3.70% | 3.43% | 3.38% | |
| Energy metabolism | 4.74% | 4.82% | 4.88% | 4.51% | 4.54% | |
| Enzyme families | 2.20% | 2.12% | 2.19% | 2.30% | 2.37% | |
| Amino acid metabolism | 10.02% | 10.04% | 9.80% | 9.03% | 8.91% | |
| Biosynthesis of other secondary metabolites | 0.80% | 0.81% | 0.84% | 0.90% | 0.88% | |
| Carbohydrate metabolism | 9.42% | 9.57% | 9.57% | 9.34% | 9.29% | |
| Glycan biosynthesis and metabolism | 1.27% | 1.34% | 1.56% | 1.35% | 1.21% | |
| Cellular processes | Transport and catabolism | 0.32% | 0.33% | 0.32% | 0.30% | 0.28% |
| Cell communication | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | |
| Cell growth and death | 0.45% | 0.46% | 0.46% | 0.43% | 0.42% | |
| Cell motility | 3.33% | 3.36% | 3.33% | 3.53% | 3.64% | |
| Human diseases | CancersCardiovascular diseases | 0.11%0.01% | 0.11%0.01% | 0.11%0.01% | 0.13%0.01% | 0.12%0.00% |
| Immune system diseases | 0.03% | 0.03% | 0.04% | 0.03% | 0.03% | |
| Infectious diseases | 0.36% | 0.38% | 0.37% | 0.31% | 0.27% | |
| Metabolic diseases | 0.06% | 0.06% | 0.06% | 0.05% | 0.05% | |
| Neurodegenerative diseases | 0.30% | 0.30% | 0.29% | 0.27% | 0.24% | |
| Organismal systems | Circulatory system | 0.01% | 0.01% | 0.02% | 0.01% | 0.00% |
| Digestive system | 0.06% | 0.06% | 0.05% | 0.06% | 0.06% | |
| Endocrine system | 0.30% | 0.32% | 0.29% | 0.22% | 0.20% | |
| Environmental adaptation | 0.15% | 0.14% | 0.15% | 0.16% | 0.17% | |
| Excretory system | 0.04% | 0.04% | 0.03% | 0.03% | 0.03% | |
| Immune system | 0.05% | 0.05% | 0.06% | 0.09% | 0.09% | |
| Nervous system | 0.08% | 0.08% | 0.08% | 0.08% | 0.08% | |
| Sensory system | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | |
| Unclassified | Cellular processes and signaling | 4.65% | 4.65% | 4.56% | 4.84% | 4.94% |
| Metabolism | 2.68% | 2.64% | 2.69% | 2.53% | 2.50% | |
| Poorly characterized | 5.17% | 5.17% | 5.16% | 5.17% | 5.10% | |
| Genetic information processing | 2.01% | 2.05% | 2.12% | 2.08% | 2.10% | |
4. Discussion
The temperature is an important limiting factor during the cultivation of A. cornea. Therefore, it is necessary to understand the changes in its characteristics during the vegetative reproductive process at different temperatures. The changes of the mycelium growth could be observed obviously in this process. In our results, hyphal twisting increased with increasing temperature and the morphology of hyphae was significantly different among group 1 (20 °C, 25 °C), group 2 (30 °C, 35 °C) and group 3 (40 °C). The different morphology of hyphae at different temperatures means that the temperature has a great influence on the growth of hyphae. Temperatures and other environmental factors play a vital role in the growth of hyphae and directly influence the entire cultivation process [45]. Many scholars have shown that different temperatures may lead to a distinctive growth rate, vigor and hyphal formation [31,46]. Under high temperature stress, the hyphae emerged with twisted, shortened branches, which diminished and concentrated the cell nucleus [47,48]. In a study conducted by Devi, A. polytricha showed the highest biomass and maximum radial growth of 7.2 mm/day when cultivated at 30 °C (optimum temperature) in solid and liquid media [49].
Temperature also significantly influenced the growth of A. cornea through effects on the extracellular enzyme activity. Specifically, temperature and culture time led to changes in the activity of Lip, laccase, Mnp and cellulose, which were closely related to the growth of A. cornea. However, the trends in extracellular enzyme activity differed significantly among enzymes. Lip, laccase and Mnp all belongs to ligninolytic enzymes [50]. The optimum temperature of laccase was 20 °C in our study, when its activity was highest on the 10th day of cultivation. At all other temperatures, the laccase activity was highest on the 20th day. Other study reported that the optimal fermentation condition of laccase by A. cornea was 27 °C, 6 days [34]. There is some discrepancy which may be caused by the different strains and different substrate. The maximum activity of Mnp was observed at 20 °C on the 30th day and the maximum activity of Lip was observed at 30 °C on 10th day. Ganoderma lucidum had the good lignin degradation and the optimal temperatures of Lip, laccase and Mnp were all 30 °C [51]. The cellulose activity was relatively low and was higher at 40 °C on 10th day and at 25 °C on 30th day. As for cellulose enzymes, temperature has a significant effect on their activity in the stage of reproductive growth, moreover, with the increase of temperature, the enzyme activity increased, and the higher temperature is more conducive to the secretion of cellulose [15]. Overall, in our study, the influence of temperatures and cultivation days on the four enzymes varied; Lip, laccase and Mnp were more active at 20 °C on 10th day, but cellulose activity was not high under these conditions. Extracellular enzymes are enzymes produced inside cells and play roles outside the cell. These enzymes can decompose lignocelluloses and detoxify and degrade contaminants [52]. In the process of growth and development of edible fungi, hyphae can secrete various enzymes in outside substrate, previous studies showed that A. cornea can control the mechanism of extracellular enzymes in order to dissolve and make use of substances in the substrate [53]. Then the hyphae absorb the small molecule materials to complete the growth and development of A. cornea [54]. So there is an inevitable relationship between the extracellular enzyme activity and the growth of A. cornea [54]. In other words, measuring the activity of the enzyme can understand the variation regularity of the growth of edible fungi to some extent. The activity of these four extracellular enzymes is regulated by A. cornea themselves, and the enzymes may interact with each other. Some scholars have shown that the lignin degrading enzymes including Lip, laccase and Mnp exerted synergistic activity, but could lead to mutual inhibition with environmental changes [55]. Moreover, the proportion of these enzymes will directly affect the results of lignin and cellulose degradation, although the specific mechanism is not clear [56].We can infer that the temperature may influence regulatory mechanism of extracellular enzymes and then affect the absorption of nutrients by hyphae, so the growth of hyphae was different at different temperature.
Microbial communities in the substrate play an important role in the growth of edible fungi, because they can provide nutrition for the organism and resist virus infection, creating a stable growth environment for edible fungi [22,57]. The detection of microbial communities can reflect physiological changes in A. cornea to some extent. In our conclusion, bacterial community composition and structure in the substrate changed when the temperature was above 35 °C. Although the bacterial alpha diversity indices did not change with the increasing temperature, the relative abundances of certain bacteria were significantly affected by temperature. In terms of phyla, the abundance of Firmicutes maintained at a high level but its abundance decreased at 20–30 °C and then increased to a maximum at 40 °C. However, the variation of Acidobacteria, Bacteroidetes and Proteobacteria abundances was just contrary to that of Firmicutes. In addition, the abundance of Actinobacteria decreased with the rise in temperature. Firmicutes and Bacteroidetes were found as the two most abundant populations in anaerobic digestion processes and there were negative correlation between the relative abundance of Firmicutes and Bacteroidetes [58,59]. During the fermentation process of Agaricus bisporus, Firmicutes, and Actinobacteria was observed to increase as fermentation progressed, which indicated that these two phyla played prominent roles in the conversion of wheat straw into utilisable sugars [60]. At 40 °C, Bacilli, belonging to Firmicutes, accounted for the vast majority (99%) of bacteria in the substrate of A. cornea. Bacilli have strong environmental adaptability because of the formation of highly resistant spores [61]. In terms of genera, Sphingopyxis, Acinetobacter, Coprococcus, Desulfovibrio, and Brevibacterium dominated at 20 °C and 25 °C, while Firmicutes Kyrpidia, Cohnella, Brevibacillus, and Paenibacillus dominated at 35 °C and 40 °C with Kyrpidia only being observed at 40 °C. These results showed that the composition and structure of bacterial communities in the substrate, change with different temperatures. In previous studies, the amount of bacteria in the soil matrix used to cultivate mushrooms was found to be closely related to the metabolic activity of mushroom mycelia [62]. Specifically, the mycelia of mushrooms have been found to selectively induce and promote the growth and reproduction of certain bacteria in the overlying soil. Temperature can significantly influence the growth and metabolism of mycelium, and the abundance of each bacterium varies with temperature. In biological systems, different microbial communities interact, and stable community structures in the environment are more conducive to the growth of crops and edible fungi [63,64]. There are some specific interactions influenced by temperature between microbial communities. In the substrate of A. cornea, the abundances of 35 genera were correlated with each other. With the exception of Cohnella, the abundances of these genera were positively correlated; however, they exerted their effects at different temperatures. Proteobacteria and Acidobacteria both existed in the anthropogenic soil, and that they were both negatively correlated with Actinobacteria [65].
A growing number of studies have used the next-generation sequencing technology methods to predict their genetic functions to show the importance of each genus and understand their genetic variations [66]. In this study, a total of seven functional genotypes with 41 pathways were predicted. Among the metabolic pathways, the membrane transport pathway showed the highest average relative abundance. Moreover, the abundance of this pathway increased at 35 °C and 40 °C, indicating that the membrane transport metabolism will be enhanced at high temperature. A previous study revealed that there was an important mechanism responsible for temperature sensing in organisms that controls the vesicular transportation of temperature sensory receptors to adjust the transduction of temperature information, which may be an important means of adapting to the variations in environmental temperature [67]. Overall, the proportion associated with metabolism was highest, and the abundances of other functional pathways varied with temperature, despite being a few differences among some functional pathways. Taken together, these results indicate that the metabolic activities of microbial communities in the substrate of A. cornea at different temperatures need to be further explored.
Supplementary Material
Funding Statement
This work was supported by the Chinese Agriculture Research System (CARS-20), as well as the innovation mushroom team in Sichuan province.
Acknowledgments
We would like to thank LetPub (www.LetPub.com) for providing linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1. Sheu F, Chien PJ, Chien AL, et al. Isolation and characterization of an immunomodulatory protein (APP) from the jew's ear mushroom Auricularia polytricha . Food Chem. 2004;87:593–600. [Google Scholar]
- 2. Zhang D, Zheng Y. An introduction of the current studies on basidiomycete fungus Auricularia polytricha . Southwest China J Agric Sci. 2004;17:668–673. [Google Scholar]
- 3. Ghorai S, Banik SP, Verma D, et al. Fungal biotechnology in food and feed processing. Food Res Int. 2009;42:577–587. [Google Scholar]
- 4. Du P, Cui BK, Dai YC. Genetic diversity of wild Auricularia polytricha in Yunnan province of south-western China revealed by sequence-related amplified polymorphism (SRAP) analysis. J Med Plant Res. 2011;5:1374–1381. [Google Scholar]
- 5. Yoon SJ, Yu MA, Pyun YR, et al. The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thromb Res. 2003;112:151–158. [DOI] [PubMed] [Google Scholar]
- 6. Yu M, Xu X, Qing Y, et al. Isolation of an anti-tumor polysaccharide from Auricularia polytricha (jew's ear) and its effects on macrophage activation. Eur Food Res Technol. 2009;228:477–485. [Google Scholar]
- 7. Yu J, Sun R, Zhao Z, et al. Auricularia polytricha polysaccharides induce cell cycle arrest and apoptosis in human lung cancer A549 cells. Int J Biol Macromol. 2014;68:67–71. [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Wang W. Research progress on polysaccharide from Auricularia polytricha Sacc . J Zhangzhou Norm Univ. 2010;23:103–107. [Google Scholar]
- 9. Shen C, Luo X, Jang N, et al. Research progress on pharmacological activities of Auricularia polytricha polyaccharide. J Anhui Agric Sci. 2011;39:13407–13408. [Google Scholar]
- 10. Stajic M, Milenkovic I, Brceski I, et al. Mycelial growth of edible and medicinal oyster mushroom [Pleurotus ostreatus (Jacq.: Fr.) Kumm.] on selenium-enriched media. Int J Med Mushr. 2002;4:4–8. [Google Scholar]
- 11. Tanaka M, Knowles W, Brown R, et al. Biomagnetic recovery and bioaccumulation of selenium granules in magnetotactic bacteria. Appl Environ Microbiol. 2016;82:3886–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tommerup IC. Temperature relations of spore germination and hyphal growth of vesicular-arbuscular mycorrhizal fungi in soil. Transact Brit Mycolog Soc. 1983;81:381–387. [Google Scholar]
- 13. Ni X, Guo Q. Optimal pH and optimal temperature for enzyme action of several extracellular enzymes from Agaricus blazei . Zhongguo Shiyongjun. 2001;20:35–37. [Google Scholar]
- 14. Curran M, Lu Y, Taylor J, et al. The temperature response of fungal enzyme kinetics. AGU Fall Meeting Abstracts. 2013; abstract id. B33C–0501. [Google Scholar]
- 15. Wei W, Yu M, Xu X, et al. Effects of temperatures on the substance content and extracellular enzyme activity of culture substrate during Ganoderma lucidum reproductive stage. Zhongguo Shiyongjun. 2014;33:49–55. [Google Scholar]
- 16. Ander P, Eriksson KE. The importance of phenol oxidase activity in lignin degradation by the white-rot fungus Sporotrichum pulverulentum . Arch Microbiol. 1976;109:1–8. [Google Scholar]
- 17. Leatham GF. Extracellular enzymes produced by the cultivated mushroom Lentinus edodes during degradation of a lignocellulosic medium. Appl Environ Microbiol. 1985;50:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DE Vries OMH, Kooistra WHCF, Wessels JGH. Formation of an extracellular laccase by a Schizophyllum commune dikaryon. Microbiology. 1986;132:2817–2826. [Google Scholar]
- 19. Carbajo JM, Junca H, Terrón MC, et al. Tannic acid induces transcription of laccase gene cglcc1 in the white-rot fungus Coriolopsis gallica . Can J Microbiol. 2002;48:1041–1047. [DOI] [PubMed] [Google Scholar]
- 20. Cho YS, Kim JS, Crowley DE, et al. Growth promotion of the edible fungus Pleurotus ostreatus by fluorescent pseudomonads. FEMS Microbiol Lett. 2003;218:271–276. [DOI] [PubMed] [Google Scholar]
- 21. Liang C, Wu C, Lu P, et al. Biological efficiency and nutritional value of the culinary-medicinal mushroom Auricularia cultivated on a sawdust basal substrate supplement with different proportions of grass plants. Saudi J Biol Sci. 2016. In press. DOI: 10.1016/j.sjbs.2016.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Hou L, Liu M, et al. Primary effects of extracellular enzyme activity and microbial community on carbon and nitrogen mineralization in estuarine and tidal wetlands. Appl Microbiol Biotechnol. 2015;99:2895–2909. [DOI] [PubMed] [Google Scholar]
- 23. Patel RK, Jain M. NGS QC toolkit: a platform for quality control of next-generation sequencing data. Encyclop Metagenom. 2015;7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meier R. From Malaise traps to phylogenetic diversity: developing rapid biodiversity assessment techniques based on NGS. In: 2016 International Congress of Entomology. Annapolis: Entomological Society of America; 2016. [Google Scholar]
- 25. Yang Z. Study of optimum meteorological conditions for Auricularia polytricha cultivation. Shiyongjun. 1993;15:5–6. [Google Scholar]
- 26. Yu M, Ma B, Luo X, et al. Molecular diversity of Auricularia polytricha revealed by inter-simple sequence repeat and sequence-related amplified polymorphism markers. Curr Microbiol. 2008;56:240–245. [DOI] [PubMed] [Google Scholar]
- 27. Cheng L, Cheng Z, Wang C, et al. Experimental research on screening suitable strains of Auricularia polytricha for cultivation by sawdust of mulberry branch. Southern Hortic. 2013;24:15–18. [Google Scholar]
- 28. Wang B, Jia D, Gao J, et al. Study on genetic differences and yields within Auricularia cornea mutants. Southwest China J Agric Sci. 2015;28:2832–2834. [Google Scholar]
- 29. Zhang B, Miao R, Zhou J, et al. Effects of cultivating substrates with different nitrogen sources on agronomic traits, quality and production efficiency of Auricularia cornea . J Southern Agric. 2017;48:2210–2217. [Google Scholar]
- 30. Du P, Cui B, Dai Y. High genetic diversity in wild culinary-medicinal wood ear mushroom, Auricularia polytricha (Mont.) Sacc., in tropical China revealed by ISSR analysis. Int J Med Mushr. 2011;13:289–297. [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Zhang J, Ma Q, et al. Research on black fungus secondary mycelium forms on different conditions of temperature and light. Heilongjiang Sci. 2014;5:13–15. [Google Scholar]
- 32. Zervakis G, Philippoussis A, Ioannidou S, et al. Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia Microbiol (Praha). 2001;46:231–234. [DOI] [PubMed] [Google Scholar]
- 33. Ma H. Research on partial enzymological property of crude cellulase and xylanase from three kinds of edible fungus residues. J Anhui Agric Sci. 2010;38:15479–15480. [Google Scholar]
- 34. Zhu Q, Gao F, Yu X, et al. Study on femention condition of laccase from Auricularia polytricha(Mont.)Sacc . Resour Dev Market. 2012;28:679–680. [Google Scholar]
- 35. Langenheder S, Szekely AJ. Species sorting and neutral processes are both important during the initial assembly of bacterial communities. Isme J. 2011;5:1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mago T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. [DOI] [PubMed] [Google Scholar]
- 39. Bokulich NA, Mills DA. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl Environ Microbiol. 2013;79:2519–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Galili T, O’Callaghan A, Sidi J, et al. Heatmaply: an R package for creating interactive cluster heatmaps for online publishing. Bioinformatics. 2018;34:1600–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60– R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Langille MGI, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoa HT, Wang C. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology. 2015;43:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guo Y, Ye X, Gan B, et al. Study on three kinds of edible fungi hyphae colony and hyphae form in different temperature and light training conditions. Southwest China J Agric Sci. 2011;24:2301–2306. [Google Scholar]
- 47. Song C, Chen Q, Wu X, et al. Heat stress induces apoptotic-like cell death in two Pleurotus species. Curr Microbiol. 2014;69:611–616. [DOI] [PubMed] [Google Scholar]
- 48. Abrashev R, Stoitsova S, Krumova E, et al. Temperature-stress tolerance of the fungal strain Aspergillus niger 26: physiological and ultrastructural changes. World J Microbiol Biotechnol. 2014;30:1661–1668. [DOI] [PubMed] [Google Scholar]
- 49. Devi MN. Comparative study for production of extracellular ligninolytic enzymes from commercially cultivated Auricularia polytricha and wild type mushrooms. Shoolini University of Biotechnology and Management Sciences ; 2015. Available from: http://hdl.handle.net/10603/43048.
- 50. Abrahao MC, Gugliotta AM, Silva R, et al. Ligninolytic activity from newly isolated basidiomycete strains and effect of these enzymes on the azo dye orange II decolourisation. Ann Microbiol. 2008;58:427–432. [Google Scholar]
- 51. Wang M, Yong L, Shizhong L, et al. Screening and enzyme-producing conditions of edible fungus strains with higher lignin degradation ability. Guizhou Agric Sci. 2013;41:83–86. [Google Scholar]
- 52. Dong X, Yuan H, Gao T. Progress in studies of ligninolytic enzymes and genes. Biotechnol Bull. 2014;11:62–72. [Google Scholar]
- 53. Lv C, Sun T, Zhang J, et al. Study on the measurement methods of several kinds of common edible fungi extracellular enzyme activity. Forest By-Prod Spec China. 2013;5:93–96. [Google Scholar]
- 54. Wang Q, Hong LU, Wang L. Culture conditions optimization for Auricularia polytrichain vitex chips. Northern Hortic. 2017;12:146–150. [Google Scholar]
- 55. Wang H, Li Z. Three important enzymes for lignin degradation. J Biol. 2003;20:9–11. [Google Scholar]
- 56. Reddy CA. An overview of the recent advances on the physiology and molecular biology of lignin peroxidases of Phanerochaete chrysosporium . J Biotechnol. 1993;30:91–107. [DOI] [PubMed] [Google Scholar]
- 57. Saha A, Pipariya A, Bhaduri D. Enzymatic activities and microbial biomass in peanut field soil as affected by the foliar application of tebuconazole. Environ Earth Sci. 2016;75:558–571. [Google Scholar]
- 58. Chen S, Cheng H, Wyckoff KN, et al. Linkages of Firmicutes and Bacteroidetes populations to methanogenic process performance. J Ind Microbiol Biotechnol. 2016;43:771–781. [DOI] [PubMed] [Google Scholar]
- 59. Kampmann K, Ratering S, Kramer I, et al. Unexpected stability of Bacteroidetes and Firmicutes communities in laboratory biogas reactors fed with different defined substrates. Appl Environ Microbiol. 2012;78:2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mcgee CF, Byrne H, Irvine A, et al. Diversity and dynamics of the DNA and cDNA-derived bacterial compost communities throughout the Agaricus bisporus mushroom cropping process. Ann Microbiol. 2017;67:751–761. [Google Scholar]
- 61. Song Z-Q, Wang F-P, Zhi X-Y, et al. Diversities of Firmicutes in four hot springs in Yunnan and Tibet. Environ Microbiol. 2013;15:1160–1486. [DOI] [PubMed] [Google Scholar]
- 62. Ming C, Ying Y, Lin F, et al. Microbial community structure of casing soil during mushroom growth. Pedosphere. 2009;19:446–452. [Google Scholar]
- 63. Gigliotti G, Pezzolla D, Zadra C, et al. Dynamics of organic matter and microbial populations in amended soil: a multidisciplinary approach. Soil Biol Biochem. 2015;82:9–20. [Google Scholar]
- 64. Vajna B, Nagy A, Sajben E, et al. Microbial community structure changes during oyster mushroom substrate preparation. Appl Microbiol Biotechnol. 2010;86:367–375. [DOI] [PubMed] [Google Scholar]
- 65. Durrer A, Gumiere T, Taketani RG, et al. The drivers underlying biogeographical patterns of bacterial communities in soils under sugarcane cultivation. Appl Soil Ecol. 2017;110:12–20. [Google Scholar]
- 66. Harrison RJ. Understanding genetic variation and function- the applications of next generation sequencing. Semin Cell Dev Biol. 2012;23:230–236. [DOI] [PubMed] [Google Scholar]
- 67. Li C, Ma W, Yin S, et al. Sorting nexin 11 regulates lysosomal degradation of plasma membrane TRPV3. Traffic. 2016;17:500–514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.