Abstract
Background:
Value-based insurance designs are being widely used. We undertook this study to examine whether lowering copayments for blood pressure medications below $0 improves blood pressure control among patients with poorly controlled hypertension.
Methods:
Participants from three Pennsylvania hospitals (n=336) were randomly assigned to (a) get paid $8 per medication per month for filling blood pressure prescriptions, (b) a computerized behavioral intervention (CBI), (c) both payment and CBI, or (d) usual care. The primary outcome was change in blood pressure between baseline and 12 months post-enrollment.
Results:
There were no significant interactions between the incentive and the CBI interventions. Blood pressure decreased among all participants, but to a similar degree between the financial incentive and control groups. Systolic blood pressure (SBP) dropped 13.7 mm for the incentive group vs. 10.0 mm for control group (difference = −3.7, 95% CI = [−9.0, 1.6], p=0.17.) The proportion of patients with blood pressure under control 12 months post-enrollment was 35.6% of the incentive vs. 27.7% of the control group (OR = 1.4, 95% CI = [0.8, 2.5]; p=0.19.). Diabetics in the incentive group had an average drop in SBP of 12.7 mm Hg between baseline and 12 months compared to 4.0 mm Hg in the control group (p = 0.02.) Patients without diabetes experienced average SBP reductions of 15.0 mm Hg, compared to 16.3 for control group non-diabetics (p = 0.71).
Conclusions:
Among patients with poorly controlled blood pressure, financial incentives did not improve blood pressure control or adherence except among diabetics.
Summary
This study extends Value-based insurance design concepts in testing the impact of rewards that provided negative copayments for blood pressure medication on blood pressure control.
Introduction
Insurers are widely adopting Value-based Insurance Designs (VBID) based on the premise that reductions in copayments will significantly increase utilization of beneficial and cost-effective services. These approaches are seen as a way of trying to address widespread problems with adherence to medication for chronic diseases, as there is strong evidence that medication adherence for chronic disease such as hypertension and hypercholesterolemia is low,1–5 limiting the potential for medications with high efficacy in randomized controlled trials to improve the health of the population. However, while observational studies have consistently shown that increases in copayments are associated with both decreases in utilization of medications and worse outcomes,6–14 the impact of decreasing copayments seen in observational studies has been more modest15–21 and the underlying psychology of how people process changes in payments as losses compared to gains suggests that increases and decreases in copayments may not be equivalent.22
Nearly two-thirds of Americans with hypertension (HTN) have poorly controlled hypertension 23 which puts them at risk for substantial morbidity and mortality. Poor adherence is an important factor in poorly controlled hypertension. Taking the logic behind VBID initiatives that lower copayments to $0 with the goal of improving adherence and patient outcomes one step further, we examined whether reducing copayments from $0 to -$8 per medication per month for all anti-hypertensive medications significantly improves blood pressure control among patients with poorly controlled blood pressure at three medical centers in Pennsylvania.
Methods
Study Population
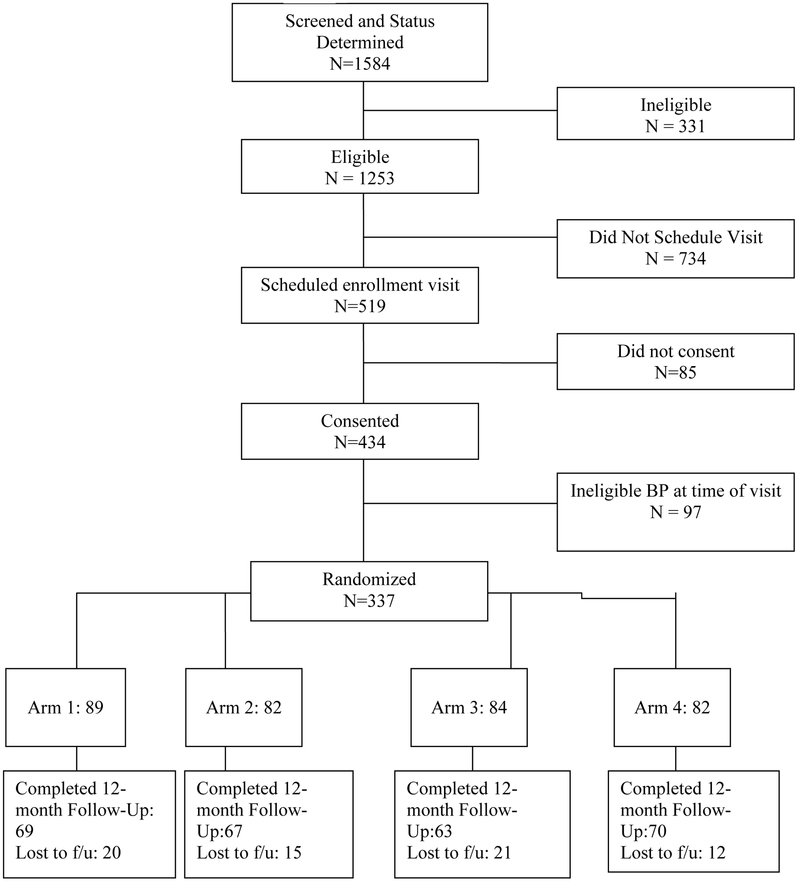
Study participants were drawn from patients at 3 hospitals in Pennsylvania: the Philadelphia Veterans Affairs Medical Center (PVAMC), the VA Pittsburgh Health Care System (VAPitt), and the PinnacleHealth clinic in Harrisburg, with recruitment occurring between March, 2005 and July, 2007. Figure 1 shows the study flow. Potential participation was elicited by sending letters to patients who met eligibility criteria based on electronic or manual screening of records. Eligible patients were aged >= 21 years, with one or more active prescriptions for an anti-hypertensive medication and systolic blood pressure (SBP) of at least 140 (130 in diabetic patients; with eligibility to receive medications without copayments (due to low income or disability). Exclusion criteria included participation in another experimental study, markedly shortened life expectancy (due to diagnosis of metastatic cancer, end-stage renal disease on dialysis, NYHA class IV CHF, or dementia), or atrial fibrillation (because of concerns with accuracy of BP measurement). We enrolled 337of the 1,253 potentially eligible participants in the study (see Figure 1); approximately 20% of screened potentially eligible patients were excluded due to ineligibility.
Figure 1.
Flow diagram of trial participation
Study Protocol
The protocol was approved by the Institutional Review Boards of the PVAMC, VAPitt, PinnacleHealth, and the University of Pennsylvania, and all participants provided written informed consent prior to randomization. The study was registered at http://clinicaltrials.gov as Collaboration to Reduce Disparities in Hypertension, ID # NCT00133068. Participants were randomized to receive either: 1) a financial incentive that effectively lowered copayments to -$8 per medication per month; 2) a computerized behavioral intervention (CBI) provided at enrollment and at the 6-month follow-up visit; 3) both the financial incentives and the CBI; or 4) usual care (with study follow-up visits every 3 months). Financial rewards were calculated such that participants received full reimbursement for all copayments during the 12 months of the study. After an initial visit, participants were requested to return for follow-up blood pressure readings and surveys at 3, 6, 9, and 12 months; financial rewards were paid at each follow-up visit after confirmation that each prescription was filled using either the VA’s computer records, a prescription bottle, or a receipt.
Randomization Procedures
Randomization was carried out using a random number generator and via permuted block randomization with a block size of four. Randomization was stratified by site, income (<100%, 100–200%, 200–300%, and >300% of the federal poverty line), and baseline BP (SBP < 160 or SBP ≥ 160). Randomization was performed after signed written consent forms were received. Allocation assignments were concealed, with staff unable to access randomization assignment for each subject until all eligibility criteria were entered in an electronic tracking system and consent forms were completed. Neither staff nor study participants could be blinded due to the nature of the intervention; investigators and analysts, however, remained blinded to intervention assignments until unblinding occurred, in coordination with the Data Safety Monitoring Board, once follow-ups were nearly complete.
Outcome Assessments
The primary outcome variable was change in blood pressure from enrollment to 12 months post-enrollment. Secondary outcome variables included change in blood pressure 6 months post-enrollment, the percentage of patients with blood pressure in control at 6 and 12 months post-enrollment, self-reported medication adherence, and prescription refill data from the VA electronic medical record system. Blood pressure control was defined as SBP below 140 and DBP below 90 for non-diabetic patients; for diabetic participants, blood pressure control was defined as SBP below 130 and DBP below 85.
Measurement of blood pressure was done following a standardized protocol using an automated BP cuff (Omron HEM-90R) ensuring that the correct cuff size was used.24 Participants were instructed to relax for five minutes before their BP was taken; then the patient’s arm (the dominant arm unless the patient expressed a preference to use the other arm) was supported on a chair or desk, and the BPs were measured while the patient was sitting. Three measurements were taken, two minutes apart, and averaged. The BP measurements were not revealed to the study participants. Although the study nurses could not be blinded to the randomization, the use of an automated BP cuff and a standardized protocol protected against systematic differences among groups in the way BP was measured.
Medication adherence was measured using self-report based on the Hill Bone Scale,25 with supplemental assessment using electronic prescription fill records where available. For these records, we calculated medication possession ratios (number of days a patient had a filled prescription divided by 360 days) and gap ratios of 30, 60, and 90 days (percentage of patients who had gaps in filled prescriptions of at least 30, 60, or 90 days).26
Covariates
Baseline blood pressure levels were assessed with other factors including height, weight, and creatinine level. We also collected information on income, baseline health status, health history, medication use, age, gender, and self-reported race or ethnicity. We used information on income and family size to calculate income as a percent of the federal poverty line.
Statistical analysis
To evaluate the similarity of the treatment groups with respect to baseline covariates, we compared groups using Student’s t test for continuous variables and χ2 test for categorical variables, with Fisher’s Exact tests used for analyses with five or fewer subjects per cell. Because of the factorial design, we first assessed whether receipt of CBI affected the impact of incentive payments. We then collapsed the arms to compare all subjects receiving incentive payments to all subjects receiving no payments; the primary unadjusted analyses tested the mean differences in the degree of change in SBP and DBP between the incentive and control groups from baseline to 12 months post-enrollment using Student’s t-test. We similarly calculated differences in change in blood pressure from baseline to 6 months post-enrollment. Missing values for 6-month and 12-month SBP and DBP readings were handled using the Markov Chain Monte Carlo (MCMC) multiple imputation method, utilizing 10 imputations.27 Separate imputation regression models were implemented for SBP and DBP. The primary analyses were conducted on each of the ten imputed data sets and the results combined using the standard approach to yield a single result. Unadjusted odds ratios for achieving in-control blood pressure were estimated via logistic regression using the imputed data in the same manner.
Regression coefficients and their 95% confidence intervals were estimated from an unadjusted linear regression model that incorporated only a factor indicating receipt of incentives vs. control; these were compared with regression coefficients estimated from a model adjusted for the stratification variables (site, high SBP, and income), in all cases using the imputed data. In addition to pre-specified subgroup analyses on race and income, we examined changes in SBP and DBP in subgroups defined by study site, initial SBP (>=160 mmHg vs. below 160 mmHg), presence of diabetes, and education level (high school or lower, some college or college degree, beyond college). Homogeneity of the association between treatment groups and blood pressure change across subgroups was tested by assessing the significance of appropriate interaction terms included in the linear regression models described above.
The trial was powered to ensure that clinically meaningful differences in SBP of 10 mm Hg and of DBP of 5 mm Hg28–30 could be detected in any of the contrasts discussed above, assuming an interaction between the CBI and incentive interventions. We used an α of 0.05 and standard deviations of change in SBP and DBP of 20 and 10, respectively (based on the upper limit of standard deviation directly measured in clinical trials).31,32 Based on these estimates, we estimated we would need 63 subjects per arm to detect the clinically meaningful difference in BPs discussed above. To accommodate an estimated 20% loss to follow-up, recruitment goals for each arm were increased to 79 subjects, for a total of 316 subjects.
Results
Study characteristics were generally balanced across the arms of the study (Table 1); exceptions are noted below. Average age was 61, with approximately 81% males, 5% Hispanic, and 61% black; there were significantly more blacks in the control group (p=0.01). About 45% had incomes below 100% FPL, 26% at 100–200% FPL, 12% at 200–300% FPL, and the remainder above 300% FPL. Baseline SBP and DBP readings averaged 154 and 84`mm Hg, respectively.
Table 1.
Characteristics of Study Participants
| Variable | Negative Copay n = 164 |
Control n = 173 |
p – value |
|---|---|---|---|
| Demographics | |||
| Average age (years) (std dev) |
62.2 (11.5) |
59.8 (11.4) |
0.05 |
| Male (%) | 78.7 | 82.7 | 0.35 |
| White (%) | 37.4 | 30.6 | 0.01 |
| Black (%) | 54.6 | 67.1 | |
| Other race (%) | 8.0 | 2.3 | |
| Hispanic Ethnicity (%) | 6.8 | 3.5 | 0.22 |
| Site | |||
| Philadelphia VA (%) | 46.3 | 49.1 | 0.79 |
| Pinnacle (%) | 21.3 | 22.0 | |
| Pittsburgh VA (%) | 32.3 | 28.9 | |
| Education | |||
| High School or lower (%) | 56.3 | 54.1 | 0.92 |
| Some College or College (%) | 36.9 | 39.0 | |
| Beyond college (%) | 6.9 | 7.0 | |
| Poverty | |||
| <100% Poverty line (%) | 43.9 | 45.7 | 0.88 |
| 100-200% Poverty line (%) | 25.0 | 27.2 | |
| 200-300% Poverty line (%) | 13.4 | 11.6 | |
| >300% Poverty line (%) | 17.7 | 15.6 | |
| Baseline Blood pressure | |||
| Systolic blood pressure (mm Hg) (std dev) |
155.3 (14.5) |
153.3 (15.5) |
0.22 |
| Diastolic blood pressure (mm Hg) (std dev) |
83.8 (13.5) |
83.9 (13.9) |
0.93 |
| Medication taking | |||
| # hypertensive medications (std dev) |
2.6 (1.3) |
2.5 (1.4) |
0.62 |
| # medications overall (std dev) |
6.2 (2.9) |
5.8 (3.0) |
0.26 |
| Comorbidities | |||
| Diabetes | 56.7 | 51.5 | 0.33 |
| Congestive Heart Failure | 14.1 | 13.9 | 0.95 |
| Heart Attack or AMI | 15.2 | 13.3 | 0.61 |
| Kidney Failure | 6.1 | 3.5 | 0.31 |
| Stroke | 9.2 | 9.3 | 0.99 |
| TIA or Mini Stroke | 11.7 | 15.6 | 0.29 |
| High Cholesterol | 67.1 | 57.8 | 0.08 |
Follow-up rates were slightly higher among incentive arm participants at 12 months but the difference in follow-up rates was not significantly different (84% in incentive arms, 76% in non-incentive arms; p=0.10). Baseline systolic blood pressure, number of anti-hypertensive medications, or number of medications overall did not differ between those who were lost to follow-up and those participants in whom we had data at 12 months.
Mean changes in systolic blood pressure were −9.8 mm Hg [95% CI −15.0, −4.5] in the control group, −12.6 [95% CI −18.0, −7.1] in the copayment reduction group, −10.2 [95% CI −15.4, −5.1] in the CBI group, and −14.8 [95% CI −19.9, −9.7] in the combined copayment/CBI group. There were no significant interactions between incentive payments and receipt of the computerized behavioral intervention with respect to 12-month outcomes (p-value = 0.76); therefore, the primary and all subsequent analyses are collapsed across CBI status to focus on the impact of reduction of copayments to -$8 on blood pressure and adherence (hereafter comparison of ‘incentive’ vs. ‘control’).
Primary and Secondary Outcomes
We found no significant difference in blood pressure reduction between the incentive and control groups (Table 2). The incentive group lowered their SBP by 13.7 mm Hg on average, vs. 10.0 mm Hg for the control group (p=0.17). The drop in DBP was 6.8 mm Hg for the incentive group, compared to 4.1 mm Hg for the control group (p=0.07). At the end of 12 months, 35.6% of the incentive group had their blood pressure in control vs. 27.7% for the control group (OR = 1.4, 95% CI = [0.8, 2.5], p= 0.19). Results of sensitivity analyses in which we assumed that the blood pressure in all patients lost to follow-up was equal to their last measured value or their baseline blood pressure (as opposed to the imputed blood pressure value) were qualitatively similar.
Table 2.
Change in blood pressure from baseline to 12 months
| Outcome measures | Negative Copay n = 162 |
Control n = 173 |
Difference Between Groups (95% CI) |
p -value |
|---|---|---|---|---|
| Change in blood pressure | ||||
| Systolic blood pressure (mm Hg) | −13.7 | −10.0 | −3.7 (−9.0, 1.6) |
0.17 |
| Diastolic blood pressure (mm Hg) | −6.8 | −4.1 | −2.7 (−5.6, 0.2) |
0.07 |
|
Negative Copay n = 239 |
Control n = 240 |
Odds Ratio (95% CI) |
p -value | |
| % who reached goal | 35.6 | 27.7 | 1.4 (0.8, 2.5) |
0.19 |
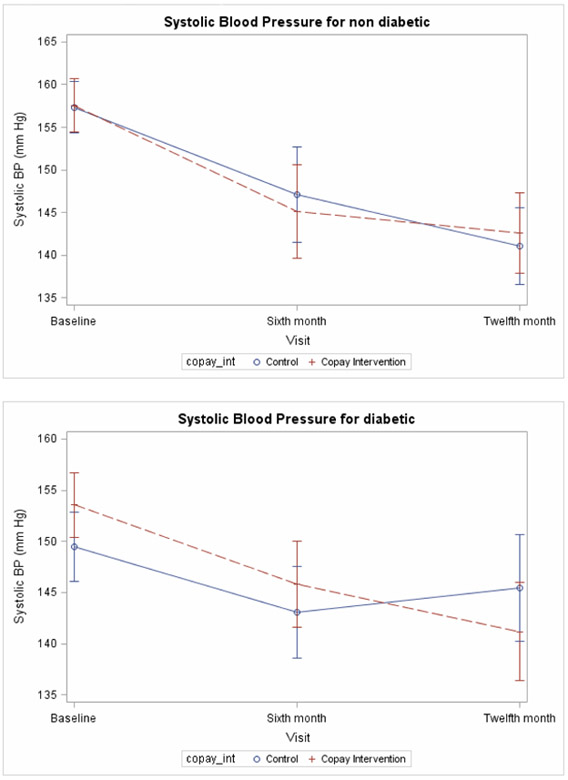
The pattern of changes in blood pressure from baseline to 6 months was similar, with no significant differences observed between the incentive and control group conditions (Figure 2). Adjusted estimates of changes in SBP indicated no significant differences between incentive and control groups in the degree of change in blood pressure (Table 3).
Figure 2.
Change in Systolic Blood Pressure over Time for Diabetics and Non-Diabetics
Table 3.
12 Month Systolic BP Change - Regression Coefficient Estimates
| Copay Exempt n = 335 |
||
|---|---|---|
| Group | Parameter | BP Change (95% CI) |
| Unadjusted Model | ||
| Intercept | No Intervention | −10.0 (−13.5, −6.5) |
| Study Arm | Copay Intervention | −3.7 (−9.0, 1.6) |
| Adjusted for Stratification Variables Only | ||
| Intercept | No Intervention Site = Philadelphia VA SBP at baseline <160 Income >300% Poverty Line |
−7.1 (−14.3, 0.0) |
| Study Arm | Copay Intervention | −2.5 (−7.6, 2.7) |
| Site | Pinnacle | 1.3 (−6.0, 8.5) |
| Pittsburgh | −0.7 (−6.7, 5.3) |
|
| SBP at baseline |
SBP >=160 | −14.7 (−20.2, −9.3) |
| Income | <100% Poverty line (%) | 3.2 (−4.1, 10.4) |
| 100-200% Poverty line (%) | −0.3 (−7.9, 7.4) |
|
| 200-300% Poverty line (%) | −0.3 (−9.6, 9.1) |
|
Changes in adherence as measured by medication possession ratios indicated no relative change in the proportion of participants who had MPR>0.8 between baseline and 12 months (OR = 1.7, [95% CI 0.8, 3.4], p-value 0.14). Changes as measured by gaps in medication possession of 30 days (OR = 0.7, [95% CI 0.3, 1.4], p-value 0.30), 60 days (OR = 1.5, [95% CI 0.6, 3.6], p-value 0.39), or 90 days (OR = 1.7, [95% CI 0.6, 5.0], p-value 0.32) indicated no differences between the control and incentive groups.
Subgroup analyses
The degree of change in SBP between the incentive and control group was compared among several subgroups of the populations in the study, including those with and without diabetes, those with a baseline SBP above or below 160, and subgroups determined by race, income, and education. The subgroup of patients with diabetes did show a significant difference between the incentive and control groups (p value for interaction = 0.04). Specifically, diabetics in the incentive group had an average drop in SBP of 12.7 mm Hg between baseline and 12 month follow-up, while diabetics in the control group had an average SBP reduction of only 4.0 mm Hg (p-value for the difference = 0.02). Patients without diabetes experienced an average SBP reduction of 15.0 mm Hg, compared to an average reduction of 16.3 for non-diabetics in the control group (p-value for the difference = 0.71.) None of the other subgroups experienced any significant differences.
Discussion
In the first randomized controlled trial to effectively lower copayments below $0 for anti-hypertensive medications among patients with poorly controlled hypertension, we found no overall improvement in blood pressure control. We did see a relative improvement in blood pressure among diabetics but not among other subgroups.
These findings are important to ongoing discussions about value-based insurance design and specifically efforts to improve patient outcomes through reduction in copayments for high-value prescription medications.33,34 Recent efforts to reduce the degree of patient cost sharing based on the value of prescriptions have garnered extensive interest among payers and employers.35,36 While increases in prescription copayments have been associated with decreases in medication adherence and worse outcomes in numerous studies,6,7,26 however, only two clinical trials have examined the question of to what degree decreasing copayments improves outcomes. The most definitive randomized trial on the impact of cost sharing on health care utilization, the RAND Health Insurance Experiment (HIE) conducted 25 years ago, found sizable effects of patient cost-sharing on use and expenditures, but more modest effects on health status. This was also performed more than 20 years ago when less effective medications were available, excluded the elderly, was over 80% Caucasian, and included a population with relatively few comorbidities,37 the populations for whom prescription drug coverage is most likely to be cost-effective. Of note, among low-income persons with high blood pressure free care resulted in significant improvements in blood pressure. However, the HIE randomly assigned roughly 2000 families to one of 14 experimental health plans that varied in their cost-sharing arrangements for all medical goods and services. The recently published MI-FREEE study, demonstrated that making medications free post-AMI increased medication possession ratios (MPR) by about 5 to 6 percentage points (control group average MPR 38.9%), which was associated with reductions in the rate of total major vascular events or revascularization without any increase in total health care spending.35
Several observational studies have now indicated that approaches that involve lowering copayments to zero for generics and by about 30% for brand name medications are associated with increases in MPR of about 1 to 4 percentage points on a base of about 60–80%. While such effects are statistically significant in large populations, these studies did not measure clinical outcomes, and it seems unlikely that changes of this magnitude in MPR would have important clinical effects within a population. The findings of this study suggest that small reductions in copayment below $0 do not significantly improve blood pressure or MPRs.
There are a number of reasons why reductions in copayments may have less of an impact on health than increases in copayments. First, increases in copayments affect utilization primarily affect utilization among adherent patients, whereas decreases in copayments are targeted at affecting utilization among non-adherent patients, in whom a change in copayment of a given magnitude is likely to have less impact. Second, increases in copayments are likely processed as a loss by patients, and behavioral economists have demonstrated that losses are felt much more strongly than equivalent gains.22 Third, copayment reductions may be a bit like the ‘dog that didn’t bark’; for a non-adherent patient who doesn’t come to the pharmacy or fill prescriptions, communications about a reduction in copayments may be largely ignored.
Because studies have indicated that unbundling rewards from other payments make the rewards more effective38 and due to the fact that the PinnacleHealth system patients filled prescriptions at a large number of pharmacies in the Harrisburg area in which we could not control point of service pricing, we provided post-hoc rebates rather than reducing the price of the medications at point of service. The lack of an overall effect on blood pressure or adherence could be due to this design feature of the trial. This made our approach different than many other VBID initiatives by providing rebates as opposed to up-front payments, but had the disadvantage of introducing time delays in feedback after the incented behavior occurred. Other limitations include copayment reduction magnitude, as it may have been too small to induce changes in behavior, though previous work suggested that in low income populations, copayment increases as low as $0.50-$1per prescription (approximately $2–3 adjusting for inflation) can reduce drug utilization.39 The study was conducted primarily among veterans at two VA hospitals and thus had primarily male participants, though there are no obvious reasons to believe that the interventions would be less effective in men than women.
In conclusion, incentives that lowered copayments below $0 for blood pressure medications had little impact on blood pressure control except among diabetics. Because this was an isolated finding in one subgroup, we conclude this intervention did not systematically improve patient outcomes. Further initiatives should examine the comparative effectiveness of different ways of delivering such incentives, the relationship between magnitude of incentives and effectiveness, and the impact on different populations.
Take-away points:
Improving medication adherence through small rewards that lower copayments below zero may be effective in subgroups of the population but may not improve outcomes overall. Consideration should be given to further testing of this approach in carefully selected populations which are high risk and where increased adherence could have significant economic and health benefits.
Acknowledgements
Other coauthors include Jingsan Zhu, Yuanyuan Tao, Wei Yang, John H. Holmes, Dominick L. Frosch, Katrina Armstrong, Shiriki Kumanyika, Kjell Enge, Raymond R. Townsend, Nirmal Joshi. They could not be listed on the cover sheet because of the limitations to how many authors could be listed.
The work in this paper was primarily supported by a grant from the Commonwealth of Pennsylvania, titled Collaboration to Reduce Disparities in Hypertension, grant number ME-02-382. Supplemental support was received from Pfizer, Inc. The sponsors/funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Volpp and Dr. Kimmel had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Volpp has received research funding from the Aetna Foundation, Aramark, Discovery (South Africa), Horizon Blue Cross and Blue Shield, Humana, Mckinsey, Weight Watchers (all unrelated to the topic of this paper), research funding and consulting income from CVS Caremark, and consulting income from VALHealth. Dr. Kimmel has done consulting for several pharmaceutical companies, including Pfizer, all unrelated to the topic of this paper. Other than what is listed above, there are no known financial conflicts of interest among any of the authors including but not limited to employment/affiliation, all grants or funding, honoraria, paid consultancies, expert testimony, and patents filed, received or pending.
REFERENCES
- 1.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 2006;166:1842–7. [DOI] [PubMed] [Google Scholar]
- 2.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- 3.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA : the journal of the American Medical Association 2002;288:462–7. [DOI] [PubMed] [Google Scholar]
- 4.Haynes RB. Improving Patient Adherence: State of the art, with a special focus on medication taking for cardiovascular disorders In: Burke LE, Ockene IS, eds. Compliance in Healthcare and Research. Armonk, NY: Futura Publishing Company, Inc.; 2001:3–21. [Google Scholar]
- 5.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–41. [DOI] [PubMed] [Google Scholar]
- 6.Goldman DP, Joyce GF, Escarce JJ, et al. Pharmacy benefits and the use of drugs by the chronically ill. JAMA : the journal of the American Medical Association 2004;291:2344–50. [DOI] [PubMed] [Google Scholar]
- 7.Hsu J, Price M, Huang J, et al. Unintended consequences of caps on Medicare drug benefits. N Engl J Med 2006;354:2349–59. [DOI] [PubMed] [Google Scholar]
- 8.Soumerai SB, McLaughlin TJ, Ross-Degnan D, Casteris CS, Bollini P. Effects of a limit on Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med 1994;331:650–5. [DOI] [PubMed] [Google Scholar]
- 9.Soumerai SB, Ross-Degnan D, Avorn J, McLaughlin T, Choodnovskiy I. Effects of Medicaid drug-payment limits on admission to hospitals and nursing homes. N Engl J Med 1991;325:1072–7. [DOI] [PubMed] [Google Scholar]
- 10.Tamblyn R, Laprise R, Hanley JA, et al. Adverse Events Associated with Prescription Drug Cost-sharing Among Poor and Elderly Persons. JAMA : the journal of the American Medical Association 2001;285:421–9. [DOI] [PubMed] [Google Scholar]
- 11.Roblin DW, Platt R, Goodman MJ, et al. Effect of increased cost-sharing on oral hypoglycemic use in five managed care organizations: how much is too much? Med Care 2005;43:951–9. [DOI] [PubMed] [Google Scholar]
- 12.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. Health Economics 2007;298:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson TB, McLaughlin CG, Smith DG. A copayment increase for prescription drugs: the long-term and short-term effects on use and expenditures. Inquiry 2005;42:293–310. [DOI] [PubMed] [Google Scholar]
- 14.Trivedi AN, Moloo H, Mor V. Increased ambulatory care copayments and hospitalizations among the elderly. N Engl J Med 2010;362:320–8. [DOI] [PubMed] [Google Scholar]
- 15.Choudhry NK, Fischer MA, Avorn J, et al. At Pitney Bowes, value-based insurance design cut copayments and increased drug adherence. Health affairs (Project Hope) 2010;29:1995–2001. [DOI] [PubMed] [Google Scholar]
- 16.Gibson TB, Wang S, Kelly E, et al. A value-based insurance design program at a large company boosted medication adherence for employees with chronic illnesses. Health affairs (Project Hope) 2011;30:109–17. [DOI] [PubMed] [Google Scholar]
- 17.Gibson TB, Mahoney J, Ranghell K, Cherney BJ, McElwee N. Value-based insurance plus disease management increased medication use and produced savings. Health affairs (Project Hope) 2011;30:100–8. [DOI] [PubMed] [Google Scholar]
- 18.Maciejewski ML, Farley JF, Parker J, Wansink D. Copayment reductions generate greater medication adherence in targeted patients. Health affairs (Project Hope) 2010;29:2002–8. [DOI] [PubMed] [Google Scholar]
- 19.Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Affairs 2008;27:103–12. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney JJ. Reducing patient drug acquisition costs can lower diabetes health claims. The American journal of managed care 2005;11:S170–6. [PubMed] [Google Scholar]
- 21.Nair K, Miller K, Saseen J, Wolfe P, Allen R, Park J. Prescription Copay Reduction Program for Diabetic Employees: Impact on Medication Compliance and Healthcare Costs and Utilization. American Health and Drug Benefits 2009;2:14–24. [PMC free article] [PubMed] [Google Scholar]
- 22.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica 1979;47:263–91. [Google Scholar]
- 23.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension 2007;49:69–75. [DOI] [PubMed] [Google Scholar]
- 24.Perloff D, Grim C, Flack J, et al. Human Blood Pressure Determination by Sphygmomanometry. Circulation 1993;88:2460–70. [DOI] [PubMed] [Google Scholar]
- 25.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog Cardiovasc Nurs 2000;15:90–6. [DOI] [PubMed] [Google Scholar]
- 26.Doshi JA, Zhu J, Lee B, Kimmel S, Volpp KG. Impact of a Prescription Copayment Increase on Lipid Lowering Medication Adherence in Veterans. Circulation 2009;119:365–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley; 2002. [Google Scholar]
- 28.Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000;355:865- [DOI] [PubMed] [Google Scholar]
- 29.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335:827–38. [DOI] [PubMed] [Google Scholar]
- 30.MacMahon S, Peto R, Cutler JA, et al. Blood Pressure, Stroke, and Coronary Heart Disease Part 1: Prolonged Differences in Blood Pressure: Prospective Observational Studies Corrected for the Regression Dilution Bias. Lancet 1990;335:765–74. [DOI] [PubMed] [Google Scholar]
- 31.Hooper L, Bartlett C, Davey Smith G, Ebrahim S. Systematic review of long term effects of advice to reduce dietary salt in adults BMJ (Clinical research ed) 2002;325:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma AM, Golay A. Effect of orlistat-induced weight loss on blood pressure and heart rate in obese patients with hypertension. Journal of hypertension 2002;20:1873–8. [DOI] [PubMed] [Google Scholar]
- 33.Fendrick AM, Chernew ME. Value-based insurance design: a “clinically sensitive” approach to preserve quality of care and contain costs. The American journal of managed care 2006;12:18–20. [PubMed] [Google Scholar]
- 34.Fendrick AM, Smith DG, Chernew ME, Shah SN. A benefit-based copay for prescription drugs: patient contribution based on total benefits, not drug acquisition cost. The American journal of managed care 2001;7:861–7. [PubMed] [Google Scholar]
- 35.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med 2011;365:2088–97. [DOI] [PubMed] [Google Scholar]
- 36.Husten L MI FREEE: How Much do Free Medications Really Cost? Forbes November 14, 2011.
- 37.Lohr KN, Brook RH, Kamberg CJ, et al. Use of medical care in the Rand Health Insurance Experiment. Diagnosis- and service-specific analyses in a randomized controlled trial. Med Care 1986;24:S1–87. [PubMed] [Google Scholar]
- 38.Thaler RH. Mental Accounting and Consumer Choice. Marketing Science 1985;4:199–214. [Google Scholar]
- 39.Soumerai SB, Ross-Degnan D, Fortess EE, Abelson J A critical analysis of studies of state drug reimbursement policies: research in need of discipline. Milbank Q 1993;71:217–52. [PubMed] [Google Scholar]