Abstract
Adult skeletal muscles are comprised of multinuclear muscle cells called myofibers. During skeletal muscle development and regeneration, mononuclear progenitor cells (myoblasts) fuse to form multinuclear myotubes, which mature and become myofibers. The molecular events mediating myoblast fusion are not fully understood. Here we report that Shisa2, an endoplasmic reticulum (ER) localized protein, regulates the fusion of muscle satellite cell-derived primary myoblasts. Shisa2 expression is repressed by Notch signaling, elevated in activated compared to quiescent satellite cells, and further upregulated during myogenic differentiation. Knockdown of Shisa2 inhibits the fusion of myoblasts without affecting proliferation. Conversely, Shisa2 overexpression in proliferating myoblasts inhibits their proliferation but promotes premature fusion. Interestingly, Shisa2-overexpressing nascent myotubes actively recruit myoblasts to fuse with. At the molecular level, Rac1/Cdc42-mediated cytoskeletal F-actin remodeling is required for Shisa2 to promote myoblast fusion. These results provide a novel mechanism through which an ER protein regulates myogenesis.
1. Introduction
Skeletal muscle is a dynamic tissue that is capable of regeneration after injury or growth/remodeling in response to physiological stimuli. The regenerative ability of skeletal muscle is primarily due to a population of resident muscle stem cells termed satellite cell (Murphy et al., 2011; Yin et al., 2013). Upon muscle injury, satellite cells are activated, enter the cell cycle and proliferate. The proliferating cells, called myoblasts, subsequently withdraw from the cell cycle and either self-renew to replenish the satellite cell pool, or differentiate into mono-nuclear fusion-competent myocytes, which fuse to form multinuclear myofibers to mediate the contractile function of skeletal muscle (Kuang and Rudnicki, 2008). The paired-box transcription factor Pax7 is essential for the formation and maintenance of quiescence of satellite cells (Kuang et al., 2006; Oustanina et al., 2004). Whereas several basic helix-loop-helix (bHLH) transcription factors including MyoD, Myf5 and myogenin are key regulators in myogenic specification and differentiation (Yin et al., 2013). Myoblast fusion is not only critical for muscle regeneration, but also required for myonuclei accretion under normal physiological conditions, such as muscle growth and maintenance (Bentzinger et al., 2012).
Myoblast fusion is a multistep process that is regulated by a variety of proteins including transcription factors, components of extracellular matrix, and secreted molecules (Hindi et al., 2013). Upon initiation of differentiation, mammalian myoblasts reshape from a fibroblast-like morphology into spindle-like elongated conformation capable of migration towards other differentiated myocytes. Following cell-cell contact, membrane alignment and adhesion occur between partners, accompanied by reorganization of actin filament at the contact site. The cytoskeletal actin dynamics are indispensable for myoblast fusion, and extracellular matrix components including integrin and cadherin have been shown to mediate actin dynamics through activation Rho GTPase (Charrasse et al., 2007; Charrasse et al., 2002a; Huveneers and Danen, 2009). The cadherin family member M-cadherin play a critical role in the fusion of myoblasts (Charrasse et al., 2007; Charrasse et al., 2002b; Kuch et al., 1997b; Zeschnigk et al., 1995). The N-terminal extracellular domain of M-cadherin mediates the initial contact between myoblasts, and the C-terminal intracellular domain interacts with catenin, which is linked to actin cytoskeleton (Wheelock and Johnson, 2003). M-cadherin controls Rac1 activity through Rho-GEF trio during myoblast fusion (Kuch et al., 1997b). Three members of Rho family GTPase (RhoA, Rac1 and Cdc42) appear to have distinct functions in myogenesis. Whereas RhoA positively promotes myogenic differentiation, Rac1 and Cdc42 are more important for the fusion of murine myoblast (Carnac et al., 1998; Vasyutina et al., 2009; Wei et al., 1998). In addition, several other cytoskeletal actin regulatory proteins, for example Nap1, N-Wasp and Dock1 have been reported to regulate myoblast fusion (Gruenbaum-Cohen et al., 2012; Laurin et al., 2008; Nowak et al., 2009). Most recently, a novel muscle-specific membrane protein, myomaker (Tmem26) and its interacting partner (myomixer/myo-merger/minion), was reported as master regulators of myoblast fusion (Millay et al., 2013; Bi et al., 2017; Quinn et al., 2017; Zhang et al., 2017). Myomaker mediated fusion of myoblasts is also dependent on cytoskeleton remodeling, suggesting that a crucial function of actin dynamics during myoblast fusion.
Adaptors of scaffold protein are important mediators of intracellular signaling networks. Shisa family proteins are single-pass transmembrane proteins characterized by a conserved N-terminal cysteine-rich domain and a C-terminal proline-rich region (Pei and Grishin, 2012). Functional analysis reveals that Shisa-like protein acts as adaptor to modulate functions of other membrane proteins, such as cell surface receptors (Pei and Grishin, 2012). Several Shisa homologs were identified in vertebrates and reported to play important roles in development, cancer and apoptosis. The first member of Shisa family, termed xShisa1, was shown to promote head formation in Xenopus by directly inhibiting the maturation of Wnt and FGF receptors (Yamamoto et al., 2005). In addition, Xenopus xShisa2 plays a critical role in the segmental patterning during somitogenesis(Nagano et al., 2006). Human SHISA2 was found to be highly upregulated in several cancer cells lines, and SHISA2 overexpression (OE) led to increased growth and invasion (Zhu et al., 2008) Sequence and phylogenic analysis have identified five Shisa homologs in mouse. Mouse Shisa2 also antagonizes FGF and Wnt signaling (Furushima et al., 2007), and Shisa5 was found to be a proapoptotic gene downstream of p53 (Bourdon et al., 2002). Lastly, Shisa9, a newly identified member of Shisa homolog in mouse, modulates AMPA receptor desensitization in the brain (von Engelhardt et al., 2010).
Notch signaling is involved in the cell-cell communication and cell fate determination of various cell lineages (Kopan and Ilagan, 2009). Notch signaling pathway also plays a key role in satellite cell function and postnatal myogenesis. Defective Notch signaling was shown to be associated with reduced satellite cell activation and proliferation (Conboy et al., 2003; Conboy et al., 2005; Conboy and Rando, 2002). In contrast, other studies reveal that Notch signal is essential to maintain the quiescence of satellite cells (Bjornson et al., 2012; Mourikis et al., 2012). Consistently, constitutive activation of Notch intracellular domain (NICD) inhibits myogenic differentiation and proliferation, and promotes self-renewal of satellite cell by increasing the expression of Pax7 (Wen et al., 2012). However, whether other components downstream of Notch signaling pathway can regulate myoblast fusion is largely unknown. In this study, we report a novel role of Shisa2, whose expression is repressed by Notch, in myogenic differentiation and myoblast fusion.
2. Materials and methods
2.1. Mice and animal care
Mouse maintenance and procedures involving mice were performed in accordance to guidelines of Purdue University Animal Care and Use committee. Pax7CreER mice were kindly provided by Charles Keller (Nishijo et al., 2009). Rosa26dTomato, Rosa26N1ICD and MyoDCre mice were from Jackson laboratory.
2.2. Cell culture and chemical treatment
C2C12 myoblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Primary myoblast were isolated from adult mice following a procedure described previously with few modifications (Rando and Blau, 1994). Briefly, muscles from hindlimbs were minced and digested with type I collagenase and dispase B mixture (Roche applied science). The slurry was then filtered through 100 μm cell strainer. Cells were then centrifuged and cultured in F-10 Ham’s medium supplemented with 20% FBS, 4 ng/ml basic fibroblasts growth factors and 1% penicillin-streptomycin (growth medium; GM). Primary myoblasts were seeded on collagen-coated cell culture plate at 37 °C, 5% CO2. For differentiation assay, growth medium was replaced with DMEM containing 2% horse serum and 1% penicillin-streptomycin (differentiation medium; DM) when cells were 80%–90% confluence. The Cdc42 inhibitor ML141(Hong et al., 2013) and Rac 1 inhibitor NSC23766 (Gao et al., 2004) were dissolved in DMSO to a stock solution (100 mM). Working concentrations of 10 μM ML141 and 100 μM NSC23766 were used to block Cdc42 and Rac1 activity, respectively.
2.3. Immunofluorescence
Cells were fixed with 4% paraformaldehyde for 10 min and incubated with blocking buffer containing 5% horse serum, 2% bovine serum albumin, 0.2% Triton X-100 and 0.1% sodium azide for 1 h. Cells were incubated with primary antibody for 1 h, and then incubated with secondary antibody and DAPI for 30 min. Fluorescent images were taken by a Leica DMI 6000B fluorescent microscope (Mannheim, Germany). For actin cytoskeleton staining, fixed cells were incubated with Alexa-fluor-568 conjugated phalloidin (Invitrogen) diluted in PBS, and then washed with PBS for three times. ER staining is performed with ER-tracker Red (Invitrogen) according to manufacture instruction.
2.4. Differentiation and fusion assays
After differentiation, cells were fixed and performed with immunofluorescence procedure. The cells were incubated with an antibody (MF20) reacting with pan-sarcomeric myosin heavy chain. To analysis the differentiation, the percentage of nuclei in MF20 positive cells was analyzed. Fusion index was determined by dividing the nuclei numbers in fused myotubes (MF20 positive cells with 2 or more nuclei) by the total number of nuclei. For the fusion assay, the nuclei numbers of myotube were counted from 50 to 100 myotubes/replicate. Each experiment is performed in at least triplicates.
2.5. Western blot and antibodies
For total protein extraction, cells were collected and lysed with ice-cold cell RIPA buffer (50 mM Tris–HCl, pH 7.5, 2 mM EDTA, 100 mM NaCl, 1% SDS, 1% Triton-×100, 0.5% deoxycholate and protease inhibitors). Extracts were centrifuged at 12000 g for 20 min at 4 °C and supernatant were collected. Protein concentrations were determined by the BCA protein assay reagent (Pierce Biotechnology, Rockford, IL, USA). Proteins were resolved by SDS-PAGE and transferred to PVDF membrane (Millipore Corp., Billerica, MA, USA) for blotting. Antibodies used were: anti-GAPDH (1:4000, Santa Cruz Biotechnolog, CA, USA), anti-myogenin (Clone F5D, 1:1000, Santa Cruz Biotechnolog, CA, USA), anti-Shisa2 (1:1000, Sigma-Aldrich, St. Louis, MO).
2.6. RNA extraction and quantitative real-time RT-PCR
Total RNA were extracted with Trizol reagent, and the DNA contamination were removed with DNase I. Two micrograms of RNA were reverse transcribed with MLV transcriptase according to the manufacture’s instruction. Quantitative PCR was performed using the Roche LightCycler 480 system with SYBR green master mix reagents (Roche). The relative quantification Delta-delta Ct method (Yoshida et al., 2003) was used for analysis. 18 s was used as a housekeeping gene to normalize all data.
2.7. Plasmid construction and virus transfection
The full length codon sequence of mouse Shsia2 was amplified from primary myoblast by RT-PCR and inserted into the pEGFP-C1 vector (Addgene). C2C12 cells were transfected using the Neon™ Transfection System (Invitrogen). The recombinant adenovirus overexpressing Shisa2 or GFP (control) was generated by use of the AdEasy technology as described (Luo et al., 2007). For infection, the Adeno-GFP or Adeno-Shisa2 virus were directly added to the medium and incubated for 24 h, then washed away with PBS. The efficiency of adenovirus transfection was 80%–90% as determined by GFP expression. For the preparation of lentivirus vectors, Shisa2 shRNA lentiviral vectors (Thermo Scientific) was cotransfected with envelope plasmid pCMV-VSVG and packaging plasmid pCMV-dR8.2 DVPR into HEK293 cells using Lipofectamine 2000 reagent (Invitrogen). Culture supernatant containing virus was collected at 48 h after transfection. The C2C12 myoblasts stably expressing Shisa2-specific shRNA were generated by puromycin selection at a concentration of 1.5 μg/ml.
2.8. Co-culture experiments
Co-culture of red (TdTomato) and green (GFP) labeled myoblasts were used to distinguish the relative role of Shisa2 in fusion competent mononuclear myoblasts versus nascent myotubes. To examine the role of Shisa2 in nascent myotubes, myoblasts were differentiated for 48 h and infected with adeno-Shisa2 or adeno-GFP virus. Equal numbers of non-differentiated tdTomato-labeled myoblasts were then mixed with the adenovirus infected nascent myotubes, and cultured for additional 48 h. To examine the role of Shisa2 in fusion competent myoblasts, adeno-Shisa2 or adeno-GFP infected myoblasts (24 h incubation) were added to Td-Tomato labeled nascent myotubes that had been cultured in differentiation medium for 48 h, and the coculture were further differentiated for another 48 h.
3. Results
3.1. Shisa2 expression is correlated with activation and differentiation of myoblasts
We recently reported that cell-autonumous activation of Notch signaling through OE of Notch1 intracellular domain (N1ICD) promotes satellite cell self-renewal and inhibits myogenic differentiation (Wen et al., 2012). To understand how Notch signaling regulates differentiation, we screened genes repressed by N1ICD OE in primary myo-blasts. This screen identified Shisa2, whose expression was down-regulated by N1ICD OE. To track the expression pattern of Shisa2 in vivo, we constructed an EGFP-Shisa2 fusion vector and control GFP vector. Transfection of these vectors into C2C12 cells revealed that the EGFP-Shisa2 fusion protein co-localizes with the ER-Tracker vital dye that specifically labels the endoplasmic reticulum (Fig. 1A).
Fig. 1.
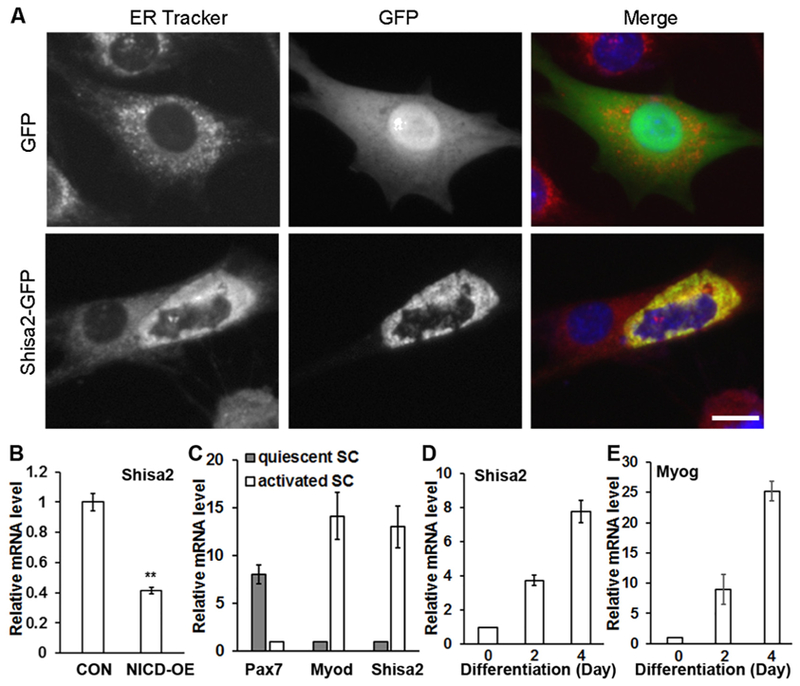
Shisa2 subcellular localization and expression pattern in myoblasts.
Next we cultured primary myoblasts from Rosa26N1ICD mice (Murtaugh et al., 2003) and induced N1ICD OE by adenovirus mediated Cre (Adeno-Cre) transduction. After infection with adeno-Cre, Notch targets genes Hey1 and Heyl were dramatically increased compared to cells infected with Adeno-GFP control virus (Supplementary Fig. S1). Quantitative RT-PCR (qPCR) confirmed that expression of Shisa2 was suppressed by 60% in N1ICD OE cells compared with control cells (Fig. 1B). As Notch signaling is more active in quiescent compared to activated satellite cells (Mourikis et al., 2012), we further addressed whether Shisa2 expression also distinguishes quiescent and activated satellite cells. To do this, we established Pax7creER/ROSA-tdTomato mice, in which Tamoxifen (TMX) injection leads to tdTomato expression in Pax7 expressing satellite cells. Adult mice were injected with TMX for 5 consecutive days to activate CreER, and injected with cardiotoxin (CTX) 7 days later to activate satellite cells. Satellite cells from CTX-injected and saline-injected muscles were then purified by Fluorescent Activation Cell Sorting (FACS) based on the red fluorescence of tdTomato at day 5 post injection. As a confirmation of the effectiveness of CTX to activate satellite cells, Myod expression was 13 times higher in satellite cells isolated from CTX treated muscles, and Pax7 expression was 7 times higher in saline treated quiescent satellite cells (Fig. 1C). Notch target genes Hey1 and Heyl were also expressed at higher levels in quiescent than activated satellite cells (Supplemental Fig. S1). Importantly, Shisa2 mRNA level was 12 times higher in activated than in quiescent satellite cells (Fig. 1C). Thus, Shisa2 expression is inversely correlated to Notch signaling activation.
We further investigated the expression pattern of Shisa2 during myogenic differentiation. Shisa2 mRNA levels rapidly increases during myoblast differentiation (Fig. 1D), in a pattern similar to the expression of myogenin (Fig. 1E), an established marker of early myogenic differentiation. These results suggest that Shisa2 may play a role in satellite cell activation and myogenic differentiation.
3.2. Shisa2 OE inhibits proliferation and promotes premature fusion of myoblasts
To directly investigate the role of Shisa2 in myogenesis, We examined the growth and differentiation of C2C12 cells transiently transfected with the EGFP-Shisa2 plasmid, which led to 300-fold increases in Shisa2 mRNA level (Fig. 2C). Interestingly, Shisa2 OE (GFP+) cells are predominantly negative for the proliferation marker Ki67 staining, while the untransfected GFP− cells in the same treatment were mostly Ki67+ (Fig. 2A). Similarly, control cells transfected with GFP vector were also predominantly Ki67+ (Fig. 2A). Overall, Shisa2 OE reduced the abundance of Ki67+ cells by 80% (Fig. 2D). These results provide compelling evidence that Shisa2 OE inhibits proliferation in C2C12 cells.
Fig. 2.
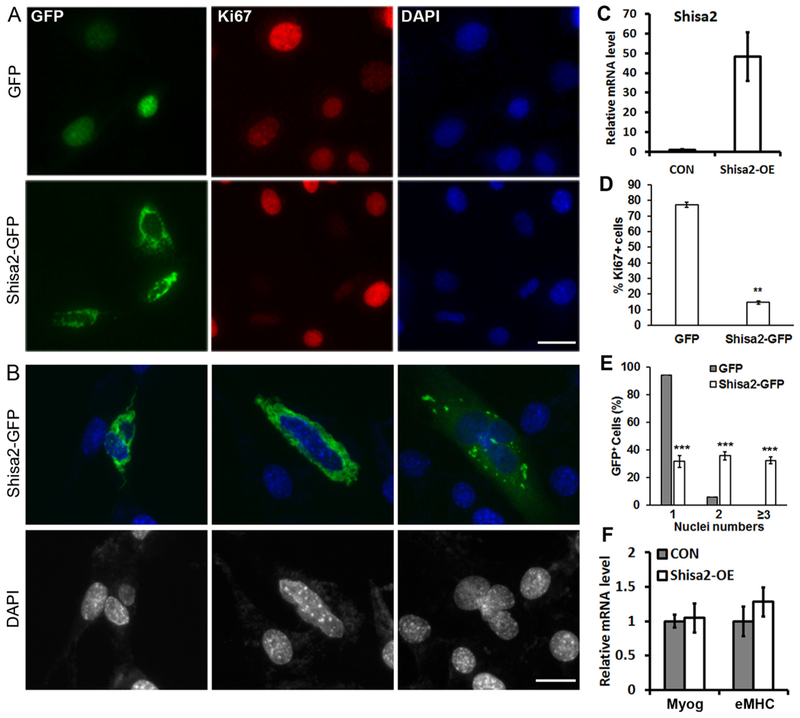
Overexpression Shisa2 inhibits proliferation and promotes premature fusion of myoblasts.
Morphological analysis of Shisa2 OE (GFP+) cells also revealed some interesting features. A significant portion of Shisa2-GFP cells contained multiple nuclei, most ranging from 2 to 4 nuclei/cell (Fig. 2B), even though they are still cultured in proliferation medium. Quantitative analysis showed that 94% of the control cells (transfected with GFP vector) were mononuclear; the remainder 6% cells had two nuclei – presumably because they are at the M-phase of cell cycle (Fig. 2E). In contrast, only one-third of the Shisa2-GFP OE cells were mononuclear, another one-third contained 2 nuclei and the remainder cells had 3 or more nuclei (Fig. 2E). As myoblasts fusion typically occurs following differentiation, we thus examined the differentiation genes expression. However, the expression of myogenin and embryonic myosin heavy chain (eMHC) was comparable between GFP and Shisa2-GFP OE cells. These data suggests that Shisa2 OE promotes pre-mature fusion of myoblasts without affecting differentiation.
3.3. Shisa2 promotes fusion of newly differentiated primary myoblasts
We further investigated the role of Shisa2 in primary myoblasts by adenovirus mediated OE approach. The Shisa2 expression was dramatically increased 2 days after infected with the Shisa2 adenovirus (Supplemental Fig. S2A and E). However, we found that Shisa2 OE led to strong ER stress, manifested by high levels of stress related Hspa5 mRNA and increased abundance of spliced XBP1 (Supplemental Fig. S2B-S2C). After induced to differentiate by serum withdrawal, the Shisa2 OE myoblasts exhibited signs apoptosis, showing by elevated levels of cleaved PARP (Supplemental Fig. S2E). The expression of myogenin and Myosin heavy chain, however, is not affected by Shisa2 OE (Supplemental Fig. S2D-S2E).
To investigate the role of Shisa2 in myoblast fusion, we infected newly differentiated myocytes with Shisa2 OE adenovirus at 48 h after induced to differentiate. After infection, myocytes were kept in the differentiation medium for another 48 h. Cells were stained with MF20 that reacts with sarcomeric myosin heavy chain. Shisa2 OE resulted in the appearance of large myotubes containing many myonuclei (Fig. 3A). Quantification of nuclei numbers per myotube indicated percentage of myotube containing > 10 nuclei were significantly increased in adeno-Shisa2 cells compared with adeno-GFP comtrol (Fig. 3B). As the mRNA and protein levels of differentiation markers myogenin and myosin heavy chain were comparable in Shisa2 OE and control cells (Supplemental Fig. S2D-S2E), this observation suggests that Shisa2 OE promotes myoblast fusion upon induced to differentiate.
Fig. 3.
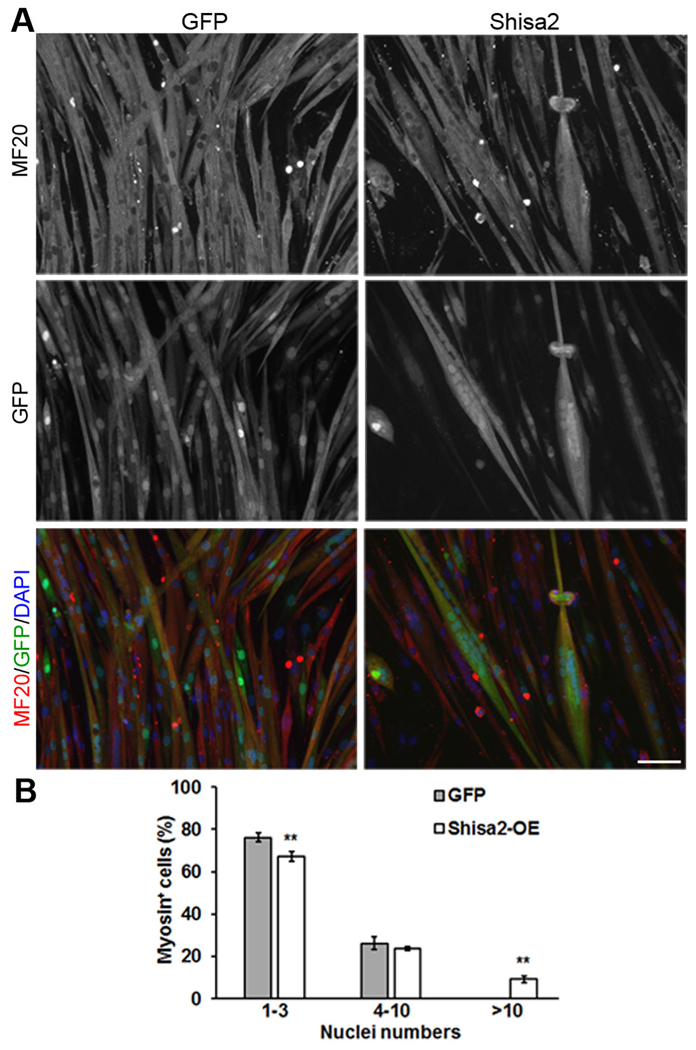
Shisa2 OE during myoblast differentiation promotes fusion.
Efficient recruitment of mononuclear myoblasts to fuse with nascent myotubes is a critical process that contributes to muscle development and regeneration. To investigate the relative role of Shisa2 in nascent myotubes versus fusion-competent myoblasts during fusion, we performed co-culture experiments involving nascent myotubes and mononuclear myoblasts. Nascent myotubes infected with GFP control and Shisa2-GFP adenovirus were cocultured with tdTomato-labeled myoblasts isolated from MyoDcre/ ROSA-tdTomato mice. Fusion events between the GFP-labeled nascent myotubes and tdTomato-labeled myoblasts were monitored by the appearance of myotubes containing both GFP and tdTomato signal (Fig. 4A, arrows). A significant increase in the percentage (33%) of mixed color myotubes was observed in the Shsia2 OE cocultures compared to GFP (12%) control cocultures (Fig. 4B). In contrast, when nascent tdTomato-labeled myotubes were cocultured with mononuclear myoblasts infected with adeno-GFP or adeno-Shisa2-GFP, the abundance of mixed color myotubes was comparable (Fig. 4C and D). These results suggest that Shisa2 expression in nascent myotubes, but not in mononuclear myoblasts, enhances the fusion process during myogenesis.
Fig. 4.
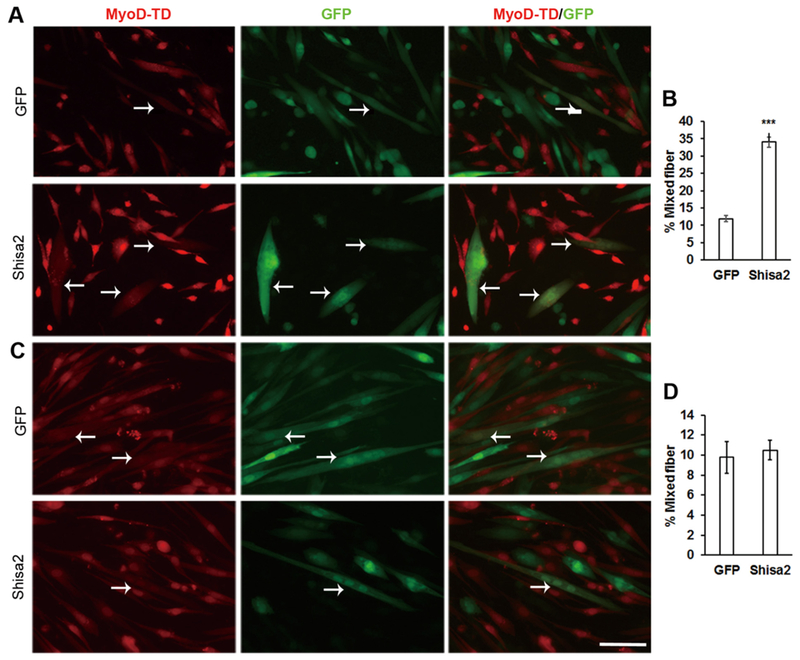
Enhanced fusion capacity of Shisa2-OE myotube.
3.4. Shisa2 knockdown (KD) inhibits the fusion of primary myoblasts
To further investigate the role of Shisa2 in myoblasts fusion, we used lentivirus mediated short hairpin RNA (shRNA) to knockdown Shisa2 expression in myoblasts. We knocked down Shisa2 expression in newly differentiated myocytes 48 h after induced to differentiate. After infection, myocytes were kept in the differentiation medium for another 48 h. (Fig. 5A). Shisa2 KD significantly inhibited fusion of myotubes (Fig. 5A). Quantification of nuclei numbers per myotube indicated percentage of myotube containing > 4 nuclei were significantly reduced in Shisa2 KD cells compared with control (Fig. 5B). qPCR analysis confirmed that Shisa2 mRNA level was reduced by over 40% in the shRNA KD group. The expression of myogenin was slightly reduced in Shisa2 KD cells (Fig. 5C and D). However, the expression of terminal differentiation marker gene, myosin heavy chain, was not affected by Shisa2 KD (Fig. 5C and D). To examine if Shisa2 affects myoblast proliferation, we selected stable clones of Shisa2 KD C2C12 cells, which had 80% reduction in Shisa2 mRNA level (Supplemental Fig. S3A). However, the Shisa2 KD cells had similar growth and proliferation kinetics as control cells (Supplemental Fig. S3B-S3D). These results indicate that Shisa2 KD inhibits the fusion of myoblasts.
Fig. 5.
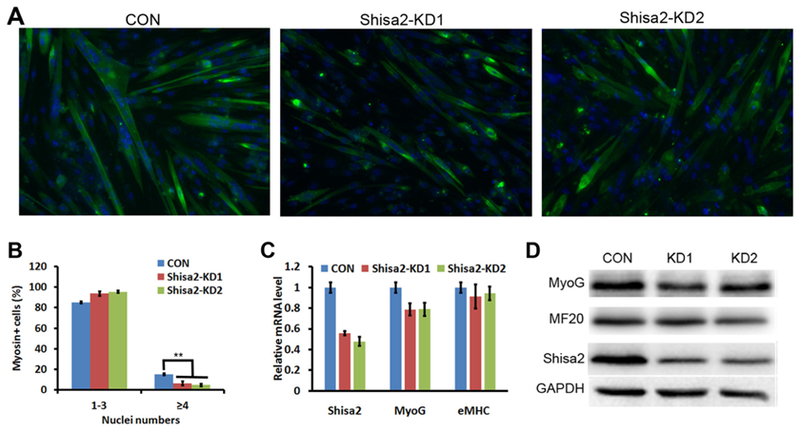
Knockdown (KD) of Shisa2 inhibits fusion of primary myoblasts.
3.5. Rac1 and Cdc42 are indispensable for Shisa2’s pro-fusion function
Myoblast fusion requires actin cytoskeletal rearrangement. The Rho family small GTPase Rac1 and Cdc42 are two key intercellular components that control the actin remolding and myoblast fusion (Chen and Olson, 2001; Chen et al., 2003; Gruenbaum-Cohen et al., 2012; Laurin et al., 2008; Nowak et al., 2009; Vasyutina et al., 2009). To test whether Rac1/Cdc42 mediates the function of Shisa2 in myoblast fusion, we used Rac1 and Cdc42 specific inhibitors. The chemical compound NSC23766 blocks the binding of Rac1 to Rac-specific guanine nucleotide exchange factors Trio and Trim1 (Gao et al., 2004), and ML141 inhibits GTP binding to Cdc42, thus blocking the activity of Cdc42(Hong et al., 2013). Either NSC23766 or ML141 blocked the appearance of medium to large myotubes containing 5 or more nuclei (Fig. 6A). Quantitatively, the abundance of small myotubes containing 1–4 nuclei were significantly increased in NSC23755 and ML141 treated cells, regardless of Shisa2 expression (Fig. 6B). However, the abundance of medium myotubes containing 5–9 nuclei were significantly lower in NSC23755 and ML141 treated cells, and the sole presence of large myotubes with 10 or more nuclei in Shisa2 OE cells was completely blocked by NSC23755 or ML141 treatment (Fig. 6B). These data indicate that Rac1/Cdc42 mediated cytoskeletal remodeling is necessary for Shisa2 to promote myoblast fusion.
Fig. 6.
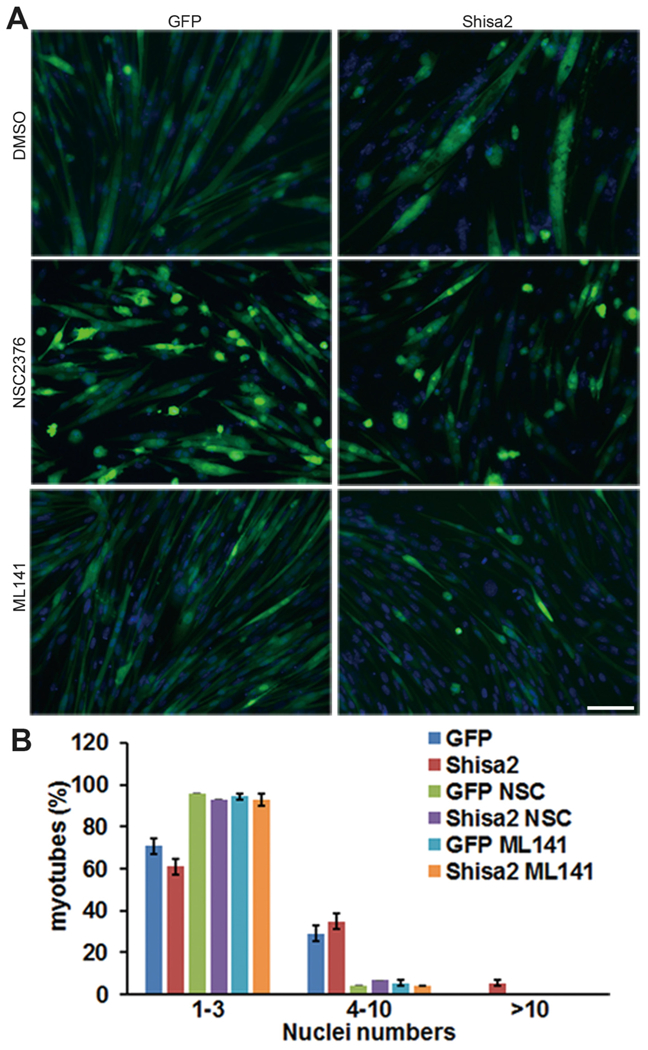
Multinucleated myotube formation of Shisa2-OE myoblast were blocked by Rac1/cdc42 inhibitor.
3.6. Shisa2 affects F-actin distribution in myotube
During myoblast fusion, various protein complexes are assembled at the adhesion sites of fusion competent myoblast, leading to actin filament reorganization that is necessary for fusion (Charrasse et al., 2002a; Knudsen et al., 1990; Kuch et al., 1997a; Swailes et al., 2004). The cadherin proteins mediate cell-cell contact and regulate Rho GTPase activity, resulting in F-actin accumulation in the adhesion sites (Charrasse et al., 2007; Charrasse et al., 2002b). To investigate whether shisa2 regulates myoblast fusion through F-actin cytoskeletal remodeling, we stained the F-actin by Alexa fluor 594 conjugated phalloidin. Our results revealed the distribution of F-actin was dramatically altered in Shisa2 OE myotubes. Particularly, polarized aggregation of F-actin was found at the edge of the Shisa2 OE myotubes (Fig. 7A arrow). Quantitative analysis revealed that the percentage of myotubes with polarized F-actin distribution was significantly higher in Shisa2 OE myotubes compared with GFP control (Fig. 7E). In addition, the polarized distribution of F-actin in Shisa2-OE myotubes was abolished by Rac1 or Cdc42 inhibitor treatment (Fig. 7B and C). As polarized asymmetrical distribution of F-actin often exists in migrating cells, we hypothesized that F-acting polarization induced by Shisa2 OE might increase myoblast mobility, which is mediated by lamellipodia (Meriane et al., 2002; Ridley et al., 1992). We thus investigated the formation of lamellipodia in Shisa2 KD C2C12 cells (Fig. 7D). Lamellipodia were detected in 81% of the control cells (Fig. 7F, arrows). In contrast, only 38% of the Shisa2 KD cells had lamelliphodia (Fig. 7F). Instead, we observed filopodia-like extrusions in the Shisa2 KD cells (Fig. 7D, arrowheads). Our results demonstrate that Shisa2 regulates F-actin remodeling, and subsequently promotes the myoblast fusion.
Fig. 7.
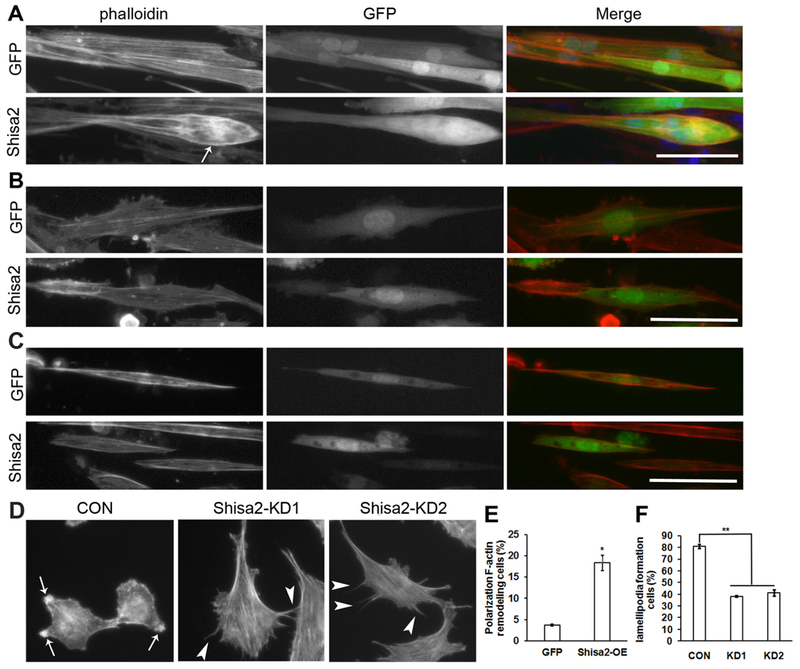
Shisa2 affects actin cytoskeletal organization in myoblast.
4. Discussion
Myoblast fusion is a critical step in the formation and regeneration of myofibers. In this report, we found mouse Shisa2, an ER localized protein, plays an important role in myoblast fusion. Shisa2 OE in newly differentiated myocytes dramatically increased nuclei number of myo-tubes. As the expression of myogenin and myosin heavy chain was not altered by Shisa2 OE, we interpret that Shisa2 specifically controls myoblast fusion without affecting differentiation.
Several cell surface proteins were identified to be involved in the myoblast fusion process, including β1 integrin, M-cadherin, brain-specific angiogenesis inhibitor 1 and myomaker (Charrasse et al., 2007; Hochreiter-Hufford et al., 2013; Kuch et al., 1997a; Millay et al., 2013; Quach et al., 2009). Importantly, the fusion process mediated by these membrane proteins all requires cytoskeletal F-actin remodeling. Consistent to this, Shisa2 function is abolished by Rho GTPase Rac1 and Cdc42 inhibitor, and we also found Shisa2 affects F-actin distribution, indicating Shisa2 depends on cytoskeleton to exert its function. ER is critical for membrane protein synthesis and modification, especially glycosylation (Breitling and Aebi, 2013). Interestingly, the glycosylation is crucial for E-cadherin mediated cell adhesion (Lommel et al., 2013; Vester-Christensen et al., 2013). Given xShisa directly regulating maturation of Wnt and FgF receptors, Shisa2 maybe mediates the maturation or modification of the membrane protein for myoblast fusion.
Our co-culture experiments revealed that Shisa2 mainly enhanced the fusion ability of myotubes, but not fnascent myotubes secrets IL-4 to recruit mononuclear myoblasts (Horsley et al., 2003). However, Shisa2 OE myotube conditioned medium fails to enhance fusion of myoblasts (Data not shown), suggesting that Shisa2 mediated fusion is not through secreted factors. In normal conditions myoblast fusion only happens after myogenic differentiation. Interestingly, our observation on the premature fusion of Shisa2 OE C2C12 myoblast in proliferation stage reveals novel phenomena of myoblast fusion. Furthermore, we also found Shisa2 overexpression in Stromal vascular fraction (SVF) cells from white adipose tissue led to multinucleated cells (Supplemental Fig. S4), suggesting that Shisa2 is a new factor mediating membranes fusion in multiple cell types. Considering the different source of cells in SVF culture, the multinucleated cell might result from macrophage fusion, which can generate osteoclast and giant cells (Aguilar et al., 2013).
Activation of Notch signaling markedly inhibits myogenic differentiation by repressing MyoD and myogenin expression (Buas et al., 2010; Kopan et al., 1994; Kuroda et al., 1999; Wen et al., 2012). We found that Shisa2 expression was inhibited by N1ICD. Our previous study has demonstrated constitutive activation of Notch signaling promotes satellite cell self-renewal/quiescence by directly regulating Pax7 transcription. Consistently, we also found Shisa2 expression was much lower in the quiescent satellite cells compared with activated cells. We propose maintaining low level of Shisa2 may be important for quiescent of satellite cells by preventing their premature fusion.
Cell-cell fusion is important for normal development of multicellular organism (Aguilar et al., 2013). The somatic cells fusion is the strategy to sculpt organs, such as muscle, bone and placenta. Sexual reproduction also requires fusion between two gametes. Discovery of new fusion protein is critical to understand the genetically programmed fusion process. The putative mechanisms of action of Shisa family protein are proposed to interact with the transmembrane protein by the C-terminal motif and affect the function of its target (Pei and Grishin, 2012). It is critical to search for direct targets of Shisa2 in ER to elucidate mechanism through which Shisa2 regulate myoblast fusion. Moreover, the function of Shisa2 in vivo need to be further elucidated.
4.1. Statistical analysis
Data are present as mean ± standard error (S.E.). P-values were calculated using two tailed Student’s t-test. P-values < .05 were considered to be statically significant.
(A) Co-location of Shisa2 and ER. C2C12 myoblast were transfected with Shisa2-GFP plasmids. ER staining was shown with ER Tracker (Red). Nuclei were labeled with DAPI (Blue). (B) Relative expression of Shisa2 in control and N1ICD overexpression (OE) primary myoblasts.(C) Relative expression of Shisa2, Pax7 and Myod in quiescent and activated satellite cells. (D-E) Relative expression of Shisa2 and myogenin during myoblast differentiation. Relative expression in B-D was determined by realtime PCR and 18 s was used as an internal control for quantification. Error bars represent ± SE. *P < .05, ** P < .01. n = 3. Scale bar, 20 μm.
(A) Representative photographs of GFP and Shisa2-GFP myoblast staining with Ki67+ (Red). Nuclei were labeled with DAPI (Blue). (B) Representative photograph of pre-mature fusion of Shisa2-GFP C2C12 myoblast. Nuclei were labeled with DAPI. (C) Relative expression of Shisa2 transfected with GFP or Shisa2-GFP plasmid in C2C12 cells. (D) Percentage of Ki67+ cells in GFP and Shisa2-GFP cells. (E) Percentage of Shisa2-GFP cells containing indicated nuclei numbers. (F) Relative expression of Myog and eMHC transfected with GFP or Shisa2-GFP plasmid in C2C12 cells. Data are from 3 independent experiments. Error bars represent ± SE. *P < .05. ** P < .01. *** P < .001. Scale bar, 50 μM.
(A) Representative photographs of GFP and Shisa2-OE primary myoblast after differentiation. Primary myoblast were induced to differentiate in DM for 48 h, and infected with Ad-GFP or Ad-Shisa2 virus. After infection myoblast were allowed to differentiate for 48 h. Myocyte differentiation was visualized by immunostaining myosin heavy chain (MHC) with MF20 antibody (Red) and nuclei was labeled with DAPI (Blue). (B) The number of nuclei present in GFP and Shisa2-OE cells. All of data are from 3 independent experiments. Error bars represent ± SE. *P < .05. ** P < .01. Scale bar, 50 μM.
(A) GFP or Shisa2-OE nascent myotube were cocultured with Myod-td mononucleated myoblast in DM for 48 h. (C) Myod-td nascent myo-tube were cocultured with either GFP or Shias2-OE mononucleated myoblast in DM for 48 h. (B) and (D) Quantification of percentage of multinucleated myotube displaying combined red and green signal in GFP+ cells. Arrows show mixed myotubes. Error bars represent ± SE, ***P < .001. Scale bar, 50 μM.
(A) Representative photographs of control and Shisa2 KD primary myoblasts after differentiation. Primary myoblast were induced to differentiate in DM for 48 h, and infected with control or Shisa2 shRNAs lentivirus. After infection myoblast were allowed to differentiate for 48 h. Myocyte differentiation was visualized by immunostaining myosin heavy chain (MHC) with MF20 antibody (Green) and nuclei was labeled with DAPI (Blue). Scale bar, 50 μM. (B) The number of nuclei present in control and Shisa2-KD cells. All of data are from 3 independent experiments. Error bars represent ± SE. ** P < .01. (C) Relative expression of myogenin and myosin heavy chain in control and Shisa2 KD myocytes. 18 s was used as the internal control. (D) Western blot showing relative levels of myogenin and myosin heavy chain proteins in control and Shisa2-KD myocytes.
(A) Representative photographs of GFP and Shisa2-OE primary myoblast after differentiation treated with or without Rac1/cdc42 inhibitor. Primary myoblast were induced to differentiate in DM for 48 h, and infected with Ad-GFP or Ad-Shisa2 virus. After infection myoblast were allowed to differentiate for 48 h with or without NSC23766/ML141. Nuclei were labeled with DAPI. (B) The number of nuclei present in GFP and Shisa2-OE cells. All of data are from 3 independent experiments. Error bars represent ± SE. Scale bar, 50 μM.
(A) Representative photographs of GFP and Shisa2-OE primary myoblast after differentiation. 2-day differentiated myoblast were infected with Ad-GFP or Ad-Shisa2 virus. After infection myoblast were allowed to differentiate for 48 h. F-actin were labeled with phalloidin (red), nuclei were labeled with DAPI (blue). (B) and (C) Representative photographs of differentiated GFP and Shisa2-OE primary myoblast treated with or without Rac1 or cdc42 inhibitor, respectively. Primary myoblast were induced to differentiate in DM for 48 h, and infected with Ad-GFP or Ad-Shisa2 virus. After infection myoblast were allowed to differentiate for 48 h with or without NSC23766/ML141. F-actin were labeled with phalloidin (red), nuclei were labeled with DAPI (blue). (D) Representative photographs of lamellipodia (arrows) and filopodia (arrowheads) formation in control and Shisa2-KD C2C12. F-actin distribution was stained by phalloidin. (E) Quantification analysis of percentage of differentiated GFP and Shisa2-OE primary myoblast with polarized F-actin distribution. (F) Quantification of percentage of control and Shisa2-KD C2C12 myoblast with lamellipodia formation. Data are from 3 independent experiments. Scale bar, 50 μM.
Supplementary Material
Acknowledgements
This work is partially supported by National Institute of Health (NIH) through grant (R01AR071649) to SK, and a grant from National Natural Science Foundation of China (81601215) to ZL.
Footnotes
Author disclosure statements
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scr.2018.07.004.
References
- Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B, Wang H, Wong M, 2013. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 29, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA, 2012. Building muscle: molecular regulation of Myogenesis. Cold Spring Harb. Perspect. Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi P, Ramirez-Martinez A, Li H, Cannavino J, McAnally JR, Shelton JM, Sanchez-Ortiz E, Bassel-Duby R, Olson EN, 2017. Control of muscle formation by the fusogenic micropeptide myomixer. Science 356, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CRR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA, 2012. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 30, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon JC, Renzing J, Robertson PL, Fernandes KN, Lane DP, 2002. Scotin, a novel p53-inducible proapoptotic protein located in the ER and the nuclear membrane. J. Cell Biol 158, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling J, Aebi M, 2013. N-linked protein glycosylation in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol 5, a013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buas MF, Kabak S, Kadesch T, 2010. The notch effector Hey1 associates with myogenic target genes to repress Myogenesis. J. Biol. Chem 285, 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnac G, Primig M, Kitzmann M, Chafey P, Tuil D, Lamb N, Fernandez A, 1998. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell 9, 1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouviere C, 2007. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol. Biol. Cell 18, 1734–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C, 2002a. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J. Cell Biol 158, 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C, 2002b. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J. Cell Biol 158, 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EH, Olson EN, 2001. Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev. Cell 1, 705–715. [DOI] [PubMed] [Google Scholar]
- Chen EH, Pryce BA, Tzeng JA, Gonzalez GA, Olson EN, 2003. Control of myo-blast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell 114, 751–762. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA, 2003. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA, 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA, 2002. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397–409. [DOI] [PubMed] [Google Scholar]
- Furushima K, Yamamoto A, Nagano T, Shibata M, Miyachi H, Abe T, Ohshima N, Kiyonari H, Aizawa S, 2007. Mouse homologues of Shisa antagonistic to Wnt and Fgf signalings. Dev. Biol 306, 480–492. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y, 2004. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. U. S. A 101, 7618–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum-Cohen Y, Harel I, Umansky KB, Tzahor E, Snapper SB, Shilo BZ, Schejter ED, 2012. The actin regulator N-WASp is required for muscle-cell fusion in mice. Proc. Natl. Acad. Sci. U. S. A 109, 11211–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindi SM, Tajrishi MM, Kumar A, 2013. Signaling mechanisms in mammalian myoblast fusion. Sci. Signal 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA, Klibanov AL, Yan Z, Mandell JW, Ravichandran KS, 2013. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature 497, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L, Kenney SR, Phillips GK, Simpson D, Schroeder CE, Noth J, Romero E, Swanson S, Waller A, Strouse JJ, et al. , 2013. Characterization of a Cdc42 protein inhibitor and its use as a molecular probe. J. Biol. Chem 288, 8531–8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Jansen KM, Mills ST, Pavlath GK, 2003. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113, 483–494. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Danen EHJ, 2009. Adhesion signaling - crosstalk between integrins, Src and Rho. J. Cell Sci 122, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Myers L, McElwee SA, 1990. A role for the Ca2(+)-dependent adhesion molecule, N-cadherin, in myoblast interaction during myogenesis. Exp. Cell Res 188, 175–184. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX, 2009. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Nye JS, Weintraub H, 1994. The intracellular domain of mouse notch - a constitutively activated repressor of Myogenesis directed at the basic helix-loop-helix region of Myod. Development 120, 2385–2396. [DOI] [PubMed] [Google Scholar]
- Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA, 2006. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol 172, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA, 2008. The emerging biology of satellite cells and their therapeutic potential. Trends Mol. Med 14, 82–91. [DOI] [PubMed] [Google Scholar]
- Kuch C, Winnekendonk D, Butz S, Unvericht U, Kemler R, Starzinskipowitz A, 1997a. M-cadherin-mediated cell adhesion and complex formation with the catenins in myogenic mouse cells. Exp. Cell Res 232, 331–338. [DOI] [PubMed] [Google Scholar]
- Kuch C, Winnekendonk D, Butz S, Unvericht U, Kemler R, Starzinski-Powitz A, 1997b. M-cadherin-mediated cell adhesion and complex formation with the catenins in myogenic mouse cells. Exp. Cell Res 232, 331–338. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Tani S, Tamura K, Minoguchi S, Kurooka H, Honjo T, 1999. Delta-induced notch signaling mediated by RBP-J inhibits MyoD expression and myogenesis. J. Biol. Chem 274, 7238–7244. [DOI] [PubMed] [Google Scholar]
- Laurin M, Fradet N, Blangy A, Hall A, Vuori K, Cote JF, 2008. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc. Natl. Acad. Sci. U.S. A 105, 15446–15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommel M, Winterhalter PR, Willer T, Dahlhoff M, Schneider MR, Bartels MF, Renner-Muller I, Ruppert T, Wolf E, Strahl S, 2013. Protein O-mannosylation is crucial for E-cadherin-mediated cell adhesion. Proc. Natl. Acad. Sci. U. S. A 110, 21024–21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al. , 2007. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc 2, 1236–1247. [DOI] [PubMed] [Google Scholar]
- Meriane M, Charrasse S, Comunale F, Mery A, Fort P, Roux P, Gauthier-Rouviere C, 2002. Participation of small GTPases Rac1 and Cdc42Hs in myoblast transformation. Oncogene 21, 2901–2907. [DOI] [PubMed] [Google Scholar]
- Millay DP, O’Rourke JR, Sutherland LB, Bezprozvannaya S, Shelton JM, Bassel-Duby R, Olson EN, 2013. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 499, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S, 2012. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30, 243–252. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G, 2011. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA, 2003. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. U. S. A 100, 14920–14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Takehara S, Takahashi M, Aizawa S, Yamamoto A, 2006. Shisa2 promotes the maturation of somitic precursors and transition to the segmental fate in Xenopus embryos. Development 133, 4643–4654. [DOI] [PubMed] [Google Scholar]
- Nishijo K, Hosoyama T, Bjornson CR, Schaffer BS, Prajapati SI, Bahadur AN, Hansen MS, Blandford MC, McCleish AT, Rubin BP, et al. , 2009. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J 23, 2681–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak SJ, Nahirney PC, Hadjantonakis AK, Baylies MK, 2009. Nap1-mediated actin remodeling is essential for mammalian myoblast fusion. J. Cell Sci 122, 3282–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T, 2004. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 23, 3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JM, Grishin NV, 2012. Unexpected diversity in Shisa-like proteins suggests the importance of their roles as transmembrane adaptors. Cell. Signal 24, 758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA, 2009. Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion. Mol. Biol. Cell 20, 3422–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn ME, Goh Q, Kurosaka M, Gamage DG, Petrany MJ, Prasad V, Millay DP, 2017. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun 8. 10.1038/ncomms15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Blau HM, 1994. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol 125, 1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A, 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410. [DOI] [PubMed] [Google Scholar]
- Swailes NT, Knight PJ, Peckham M, 2004. Actin filament organization in aligned prefusion myoblasts. J. Anat 205, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasyutina E, Martarelli B, Brakebusch C, Wende H, Birchmeier C, 2009. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc. Natl. Acad. Sci. U. S. A 106, 8935–8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, Vakhrushev SY, Clausen H, 2013. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc. Natl. Acad. Sci. U. S. A 110, 21018–21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Mack V, Sprengel R, Kavenstock N, Li KW, Stern-Bach Y, Smit AB, Seeburg PH, Monyer H, 2010. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science 327, 1518–1522. [DOI] [PubMed] [Google Scholar]
- Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, Balasubramanyam A, Schwartz RJ, 1998. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J. Biol. Chem 273, 30287–30294. [DOI] [PubMed] [Google Scholar]
- Wen Y, Bi P, Liu W, Asakura A, Keller C, Kuang S, 2012. Constitutive notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol 32, 2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR, 2003. Cadherins as modulators of cellular phenotype.Annu. Rev. Cell Dev. Biol 19, 207–235. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S, 2005. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell 120, 223–235. [DOI] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki MA, 2013. Satellite cells and the muscle stem cell niche.Physiol. Rev 93, 23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Suzuki N, Nakano Y, Kawada M, Oho T, Koga T, 2003. Development of a 5′ nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus. J. Clin. Microbiol 41, 4438–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeschnigk M, Kozian D, Kuch C, Schmoll M, Starzinskipowitz A, 1995. Involvement of M-cadherin in terminal differentiation of skeletal-muscle cells. J. Cell Sci 108, 2973–2981. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Vashisht AA, O’Rourke J, Corbel SY, Moran R, Romero A, Miraglia L, Zhang J, Durrant E, Schmedt C, et al. , 2017. The microprotein Minion controls cell fusion and muscle formation. Nat. Commun 8. 10.1038/ncomms15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tsuchida A, Yamamoto A, Furukawa K, Tajima O, Tokuda N, Aizawa S, Urano T, Kadomatsu K, 2008. Expression and roles of a xenopus head-forming gene homologue in human cancer cell lines. Nagoya J. Med. Sci 70, 73–82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


