Abstract
This multicenter phase 1/2 clinical trial evaluated intratumoral SD-101, a TLR9 agonist, and low-dose radiation in patients with untreated indolent lymphoma. 29 enrolled patients received 4 Gy of radiation followed by five weekly intratumoral injections of SD-101 at a single tumor site. No treatment-related grade 4 or serious adverse events occurred. Nearly all patients had tumor reduction at their treated site. More importantly, 24 patients had tumor reduction at their non-treated sites with 5 patients achieving a partial response and one achieving a complete response. Treatment-related increases of CD8+ and CD4+ effector T-cells and decreases of T Follicular Helper and T regulatory cells (Tregs) were observed in the tumor microenvironment. Low pre-treatment levels of CD4+ Tregs, proliferating CD8+ T-cells, and GranzymeB+ CD8+ T-cells were associated with favorable outcomes. Intratumoral SD-101 in combination with low-dose radiation is well tolerated and results in regression of both treated and untreated sites of disease.
Keywords: Indolent Lymphoma, CpG, In Situ Vaccine, Clinical Trial
INTRODUCTION
A wide variety of therapies are now being used to harness the immune system to treat cancer. Some of these treatments are customized for each patient, including chimeric antigen receptor (CAR) T-cells(1), a product of engineered autologous immune cells, or vaccines based on somatic mutations that require the identification of tumor-associated antigens (TAA).(2) While not requiring customization, immune checkpoint antibodies can enhance existing T-cell responses to endogenous TAA, but can also result in autoimmune toxicites.(3) With these clinical challenges in mind, we have developed an alternative approach called in situ vaccination, in which immunostimulatory agents are injected locally into the tumor microenvironment, triggering anti-tumor immune responses that can act against tumors throughout the body. (4, 5)
Multiple preclinical studies have validated this in situ vaccine approach using short synthetic oligodeoxynucleotides containing cytidine- guanosine motifs (CpG) that stimulate innate immunity through the Toll-like receptor 9 (TLR9).(6–11) Our preclinical studies established that the CpG needs to be injected directly into the tumor microenvironment. Furthermore, the addition of a T cell stimulatory antibody against OX40 markedly enhanced the therapeutic effect of in situ vaccination and resulted in cure of established lymphoma, colon cancer and even a spontaneous model of breast cancer.(11) We previously conducted a clinical trial for patients with relapsed indolent lymphoma, testing the combination of local low-dose radiation and intratumoral CpG (PF-3512676).(12) We observed regressions of both treated and distant, non-injected sites of disease in this small 15 patient trial. Notably, this study was conducted with a CpG sequence that had shown little therapeutic activity when given systemically.(13, 14) However, PF-3512676 is no longer in clinical development after it caused increased toxicities without improving outcomes when combined with chemotherapy in non-small cell lung cancer.(15, 16)
Based on these findings, we designed a dose escalating phase 1/2 clinical trial of in situ vaccination in patients with low-grade B-cell lymphoma using a novel CpG compound, SD-101, a class C CpG which induces high-levels of interferon-alpha (IFN-α) as well as dendritic cell maturation. The primary endpoints were safety and the induction of interferon-regulated gene expression in peripheral blood cells. Secondary endpoints included clinical efficacy and modulation of the immune microenvironment at both treated and untreated sites of tumor.
RESULTS
Patient Characteristics
29 patients with untreated low-grade lymphoma were enrolled between October 2014 and October 2016 with baseline characteristics summarized in Table 1 and were treated as summarized in Figure 1. Diagnoses included FL (n=21), MZL (n=4), SLL/CLL (n=3), and CBCL (n=1). At the time of enrollment, patients mean age was 59.8 years (range 22 to 84) with the majority of patients having Stage III/IV disease (90%).
Table 1:
Baseline Characteristics
| Baseline Characteristics | |||||
|---|---|---|---|---|---|
| Dost Cohort | 1 mg | 2 mg | 4 mg | s mg | All |
| (n=10) | (n=3) | (n=3) | (n=13) | (n=29) | |
| Age (yr) | |||||
| Mean (range) | 56.9 (22, 69) | 56(50, 67) | 61 (50, 80) | 62.7(34, 84) | 59.8 (22, 84) |
| Sex | |||||
| Female | 4(40.0%) | 2 (66.7%) | 1(33.3%) | 6(46.2%) | 13 (44.8%) |
| Male | 6(60.0%) | 1(33.3%) | 2(66.7%) | 7(53.9%) | 16(55.2%) |
| ECOG PS at Screening | |||||
| 0 | 6(60.0%) | 3(100%) | 3(100%) | 11 (84.6%) | 23(79.3%) |
| 1 | 4(40.0%) | 0 | 0 | 2(15.4%) | 6 (20.7%) |
| Disease Type | |||||
| Cutaneous B cell | 0 | 0 | 0 | 1 (7.7%) | 1(3.5%) |
| Follicular | 8(80.0%) | 3(100%) | 2(66.7%) | 8(61.5%) | 21(72.4%) |
| Marginal | 1(10.0%) | 0 | 0 | 3(23.1%) | 4(13.8%) |
| SLL/CLL | 1(10.0%) | 0 | 1(33.3%) | 1(7.7%) | 3(10.3%) |
| Stage | |||||
| I | 0 | 0 | 0 | 0 | 0 |
| II | 1(10.0%) | 0 | 0 | 2(15.4%) | 3 (10.3%) |
| III | 3 (30.0%) | 0 | 1(33.3%) | 2(15.4%) | 6(20.6%) |
| IV | 6 (60.0%) | 3 (100%) | 2(66.7%) | 9 (69.2%) | 20(69.0%) |
| Grade (Follicular Only) | |||||
| n | 8 | 3 | 2 | 8 | 21 |
| 1 | 0 | 3(100%) | 1(50.0%) | 6(46.2%) | 9(42.9%) |
| 2 | 6(75.0%) | 0 | 1(50.0%) | 2(15 4%) | 9(42.9%) |
| 3A | 2 (25.0%) | 0 | 0 | 0 | 2(9.5%) |
| FLIPI Score (Follicular Only) | |||||
| n | 8 | 3 | 2 | 8 | 21 |
| 0 | 0 | 0 | 0 | 1(12.5%) | 1(4.8%) |
| 1 | 2(25.0%) | 0 | 0 | 1(12.5%) | 3(14.3%) |
| 2 | 4(50.0%) | 3(100%) | 1(50.0%) | 3(37.5%) | 11(52.4%) |
| 3 | 2(25.0%) | 0 | 1(50.0%) | 3(37.5%) | 6 (28.6%) |
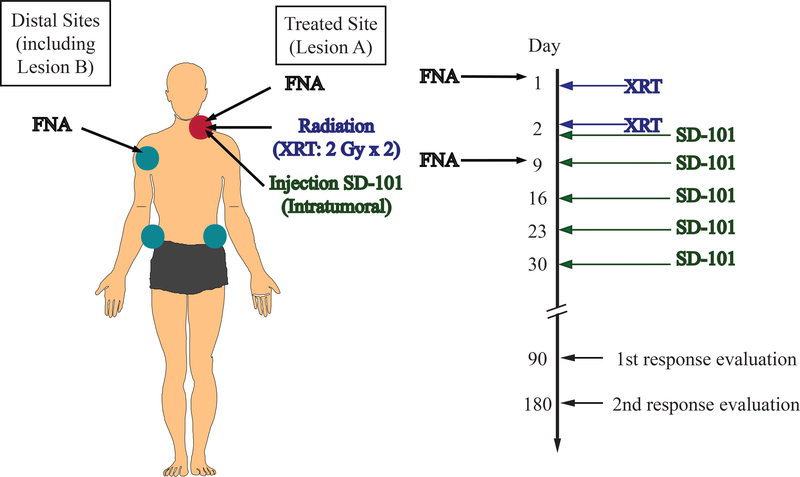
Figure 1. Schema of Clinical Trial.
Patients underwent treatment at Lesion A with radiation (XRT) with 2 Gy per day on Days 1 and 2. Starting after XRT on Day 2, patients received five weekly intratumoral injections at the treated site (Lesion A) with the TLR9 agonist, SD-101. Treatment response was evaluated 90-day and 180-days post-treatment and every 6 months thereafter. FNA biopsies were collected pretreatment (Day 1) and post-initial treatment (Day 9) at Lesion A and, if available, at a single distal site, Lesion B.
Safety
No treatment related DLTs were observed. Drug-related AEs of grade 1–2 were reported by all patients with eight patients having grade 3 drug-related AEs (Table 2 and Supplemental Table 1). No drug-related grade 4 or serious adverse events were experienced by any patients. The most common treatment-related side effect was a flu-like systemic reaction consisting of malaise, chills, headache, fatigue, and fever lasting typically between 24–48 hours after the injections, a rate similar to that observed in prior studies of TLR9 agonists.(12) More grade 3 drug-related AEs were seen at the 8 mg dose (46.2%) compared with the 1 mg dose (20%); more nausea and vomiting was seen at the 8 mg dose. Four patients required a delay in treatment due to treatment-related AEs; three patients were delayed due to neutropenia and one patient was delayed due to pain at the treatment site. Only one patient discontinued treatment because of a treatment-related AE due to fever and confusion that rapidly improved.
Table 2:
Drug-related Adverse Events Drug-related adverse events that occurred in at least 10% of patients
| Drug-related Adverse Events | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SD-101 Dose | 1mg(n=10) | 2 mg (n=3) | 4 mg (n=3) | 8 mg(n=13) | Total (n=29) | ||||||
| Grade | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | ALL |
| Malaise | 9(90) | 0 | 2(67) | 0 | 3(100) | 0 | 8(62) | 5(38) | 22 (76) | 5(17) | 27 (93) |
| Chills | 8(80) | 0 | 2(67) | 0 | 3(100) | 0 | 9(69) | 4(31) | 22(76} | 4 (14) | 26(90) |
| Fatigue | 7(70) | 0 | 3(109) | 0 | 3(100) | 0 | 19(77) | 2(15) | 23(79) | 2(7) | 25(86) |
| Headache | 7(70) | 1(19) | 3(109) | 0 | 3(100) | 0 | 7(54) | 4(31) | 20(69) | 5(17) | 25(86) |
| Myalgia | 6(60) | 0 | 3(100) | 0 | 3(100) | 0 | 10(77) | 3(23) | 22 (76) | 3(10) | 25(86) |
| Fever | 1(40) | 0 | 1(33) | 0 | 2(67) | 0 | 12(92) | 0 | 19(66) | 0 | 19(66) |
| Hau sea | 2(20) | 0 | 1(33) | 0 | 0 | 0 | 8(62) | 0 | 11(38) | 0 | 11(38) |
| Diarrhea | 3(30) | 0 | 0 | 0 | 1(33) | 0 | 4(31) | 0 | 8(28) | 0 | 8(28) |
| Injection Site Erythema | 1(40) | 0 | 0 | 0 | 1(33) | 0 | 3(23) | 0 | 8(28) | 0 | 8(28) |
| Vomiting | 0 | 0 | 1(33) | 0 | 0 | 0 | 5(38) | 0 | 6(21) | 0 | 6(21) |
| Neutropenia | 1(10) | 1(19) | 0 | 0 | 0 | 0 | 2(15) | 1(8) | 3(10) | 2(7) | 5(17) |
| Decrease Appetite | 2(20) | 0 | 0 | 0 | 0 | 0 | 2(15) | 0 | 4(14) | 0 | 4(14) |
| Injection Site Swelling | 3(30) | 0 | 1(33) | 0 | 0 | 0 | 0 | 0 | 4(14) | 0 | 4(14) |
| Night Sweats | 2(20) | 0 | 0 | 0 | 1(33) | 0 | 0 | 0 | 3(10) | 0 | 3(10) |
| Sore throat | 1(10) | 0 | 0 | 0 | 0 | 0 | 2(15) | 0 | 3(10) | 0 | 3(10) |
| Thrombocytopenia | 0 | 0 | 1(33) | 0 | 0 | 0 | 2(15) | 0 | 3(10) | 0 | 3(10) |
Clinical Responses
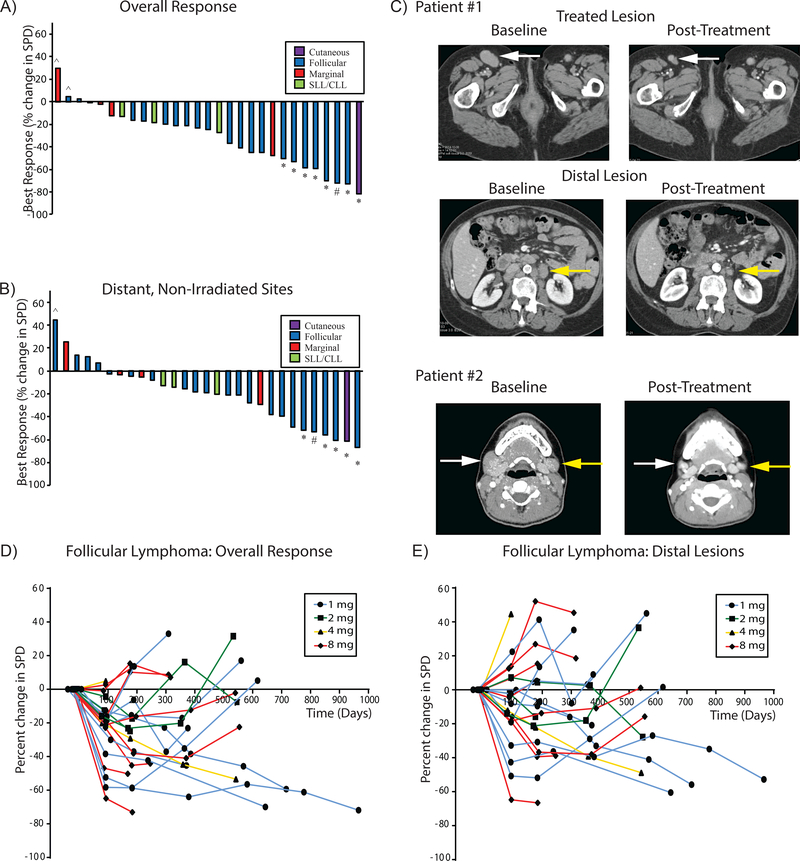
All 29 enrolled patients were evaluable for clinical response with a median follow-up of 12 months. Tumor response at the treated site was expected due to the low dose radiation and occurred in virtually all the patients (Supplemental Figure 1). 26 out of 29 treated patients demonstrated a reduction in overall tumor burden with seven patients achieving a partial response and one patient achieving a complete response. The best overall clinical response is shown in the waterfall plots by disease subtype and dose (Figure 2A and in Supplemental Figure 1). Systemic responses at the distant, non-irradiated lesions were seen in 24 of 29 patients (Figure 2B and Supplemental Figure 1). The response of distant, non-irradiated lesions correlated with response at the treated site (Supplemental Figure 2). Figure 2C shows examples of the pre and post treatment responses at treated and untreated sites in two FL patients. Tumor responses were typically durable and could deepen over time as has been described in previous studies of tumor immunotherapy (Figure 2D/E and Supplemental Figure 3).(12) Neither initial tumor burden, stage, nor follicular lymphoma international prognostic indices (for those patients with FL), nor the development of the flu-like symptoms during therapy correlated with clinical response (Supplemental Table 2). During the expansion phase of the trial, patients were permitted to receive a second cycle at the same doses they had received initially. Four patients, who had a minimal response initially, received a second cycle of treatment, again with minimal clinical response. In these four patients the treatment-related AEs were similar for both cycles.
Figure 2. SD-101 and low-dose radiation induces responses in patients with indolent lymphoma.
Waterfall plot showing the best overall change in the sum of the product of the diameters in all target lesions (A) and distal sites (B) by lymphoma subtype. Patients achieving a partial response (*), complete response (#), or progression (^) by the Revised 2007 International Working Group criteria are shown. A patient with follicular lymphoma treated in the right inguinal lesion (white arrow, upper panels) has both local and systemic responses (para-aortic lesion, yellow arrow, middle panels) as seen in the initial pretreatment imaging and 6 months post-treatment. A second patient with follicular lymphoma treated in the right cervical lesion (white arrow, lower panels) has both local and systemic responses (left cervical lesion (yellow arrow, lower panels) as seen in the initial pretreatment imaging and 21 months post-treatment.(C). Spider plot showing change over time in the sum of the product of the diameters in all lesions (D), or just distal sites (excluding Lesion A) (E) by dose in patients with Follicular Lymphoma.
Pharmacokinetics.
The majority of post-dose samples were below the limit of quantitation (BLOQ) indicating that there is little systemic SD-101 following intratumoral injections at these doses. For those few samples that had values above the lower limit of quantitation (LLOQ), half were at the 1hr post-dose time point with the rest scattered among different time points (Supplemental Table 3).
IFN-responsive gene signature
SD-101 is a “C” class CpG, optimized to induce high-levels IFN-α after engaging TLR9 on plasmacytoid dendritic cells. Blood samples were collected before and 24 hours after the second intratumoral injection of SD-101 to measure induction of mRNA transcripts of well-characterized IFN responsive genes. As Supplemental Figure 1C shows, IFN responsive genes were upregulated at 24 hours at all dose levels..
Treatment Related Immune Changes in Patients with Follicular Lymphoma
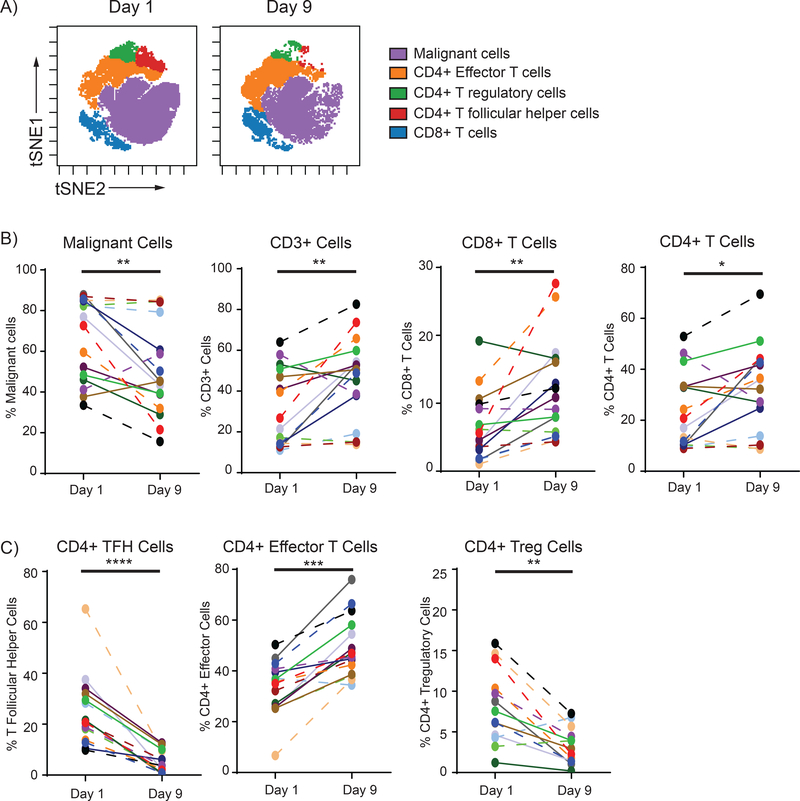
FNA biopsies were collected from patients at pretreatment (Day 1) and post-treatment (Day 9) from the treated site (Lesion A) and, if available, at a second un-injected, distal site (Lesion B). Since the microenvironment of each lymphoma subtype is different, we focused our analysis on the most prevalent histology, Follicular Lymphoma (FL). 16 of 21 patients with FL had sufficient Day 1 and 9 samples for paired analysis from Lesion A and two additional patients had just a Day 9 sample. The pretreatment intratumoral immune cell composition was remarkably similar between different tumor sites of the same patient but varied considerably between patients. (Supplemental Figure 4). We observed a significant reduction in malignant cells and an increase in CD3+, CD8+, and CD4+ T-cells post-treatment (Day 9) in the treated site (Figure 3A/B and Supplemental Figure 5). Further investigation of CD4+ T-cell subsets in the treated site revealed a marked decrease in TFH and Treg cells, and a significant increase in effector CD4+ T-cells (Figure 3C and Supplemental Figure 5B).
Figure 3. Treatment Induced Immune Cell Changes.
Initial (Day 1) and post-treatment (Day 9) lymphoma–infiltrating immune subsets were gated and visualized in tSNE (t-Distributed Stochastic Neighbor Embedding) space using Cytobank software. (A) Malignant cells, CD3+ cells, CD8+ T-cells, and CD4+ T-cells were evaluated pre- (Day 1) and post-treatment (Day 9). As a percentage of all cells, the percent of intratumoral malignant cells decreased (p=0.0052) and CD3+, CD8+, and CD4+ T-cells increased post-treatment (p=0.0079, p=0.0036, and p=0.0248, respectively, using a two-tailed paired t-test) (B). CD4+ T-cell subsets percentages of T-cells were analyzed pre- and post-treatment. TFH cells (CD4+ FoxP3- CXCR5+ PD-1high ICOS+) and T regs (CD4+ CD25+ CD127-) decreased (p<0.0001 and p=0.0012, respectively) and effector cells (remaining CD4+ FoxP3- cells) increased (p=0.0010) using a two-tailed paired t-test. (C) Each patient is tracked by a specific color and patients achieving at least an overall partial response are connected by solid lines and those who did not by dashed lines.
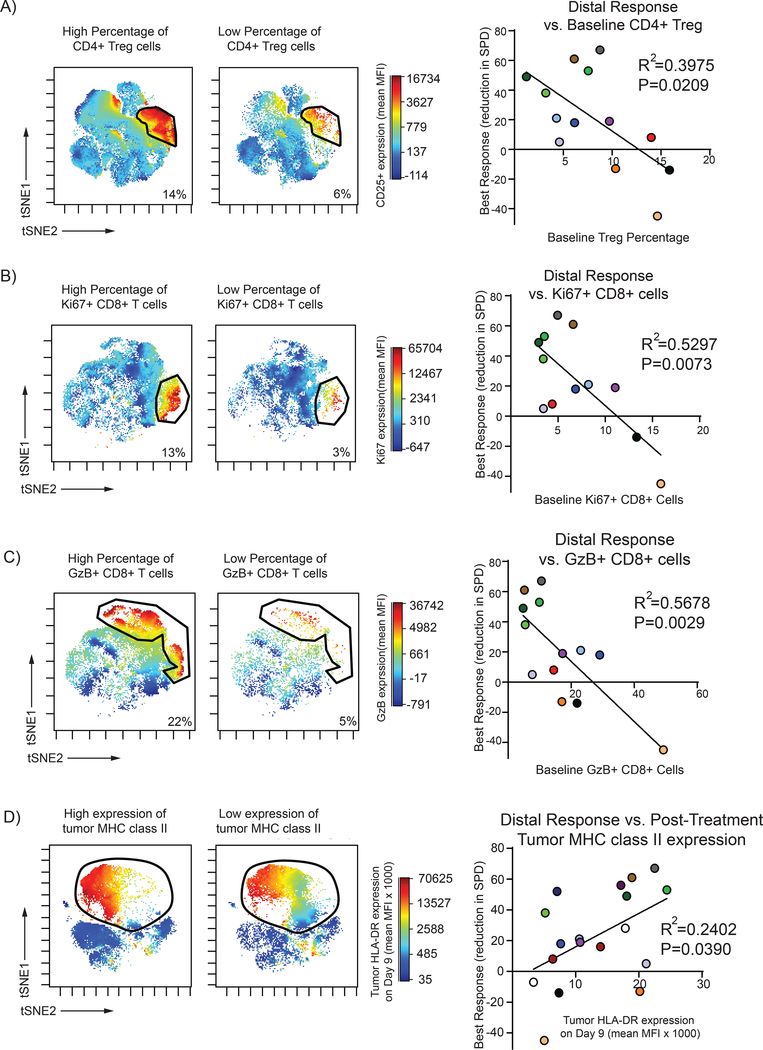
Next, we investigated if any baseline (Day 1) characteristics of CD4+ or CD8+ T-cells subsets correlated to distal and overall tumor responses. Interestingly, we found that low baseline percentage of CD4+ Treg cells correlated with better clinical responses (Figure 4A). Similarly, within the CD8+ subsets we found a low initial percentage of proliferating (Ki67+) and granzyme B+ (GzB+) CD8+ T-cells correlated with better clinical outcomes (Figure 4B and C). Finally, we noted a significant relationship between the expression of MHC II on the tumor cells at the treated site and the clinical outcomes. High tumor-expressing MHC class II post-treatment was associated with improved clinical outcomes. (Figure 4D)
Figure 4. Low initial levels of CD4+ Tregs, proliferating CD8+, and Granzyme B+ CD8+ T-cells and high post-treatment tumor MHC class II expression predict better response to treatment.
Baseline percentage of CD4+ Treg of all T-cells were gated (CD3+ CD4+ CD25+ CD127-) and visualized in tSNE space using Cytobank software. A low baseline percentage of CD4+ Treg correlated to a better distal clinical response (p=0.0209) by linear regression analysis. (A) Baseline percentage of proliferating (Ki67+) CD8+ cells as a total of CD8+ T-cells were gated and visualized in tSNE space. A lower percentage of proliferating CD8+ T-cell correlated to better distal response (p=0.0073) by linear regression analysis. (B) Baseline percentage of Granzyme B+ (GzB) CD8+ cells as a total of CD8+ T-cells were gated and visualized in tSNE space. A lower percentage of GzB+ CD8+ T-cells correlated to better distal response (p=0.0029) by linear regression analysis. (C) Post-treatment (Day 9) tumor cells were gated and visualized in tSNE space to evaluate MHC class II (HLA-DR) expression. Tumor cell MHC class II expression as measured by mean fluorescence intensity (MFI) positively correlated to distal response (p=0.0390) by linear regression analysis. (D) Each patient is tracked by a specific color.
DISCUSSION
The ultimate goal of cancer immunotherapy is to harness the immune system to trigger durable antineoplastic immune responses without inducing significant auto-immune toxicities. We now know that patients with B-cell lymphomas possess tumor-specific CD4+ T-cells that are capable of recognizing MHC class II presented peptides derived from the idiotype, renewing an interest in creating anti-lymphoma vaccines.(17) Therefore it is possible that such pre-existing immune potential can be enhanced by delivering the appropriate immunostimulatory signals directly into the tumor microenvironment, where the T-cell repertoire may be enriched for tumor-reactive T-cells. In situ vaccination may have the advantage of inducing such immune responses to the tumor while avoiding the induction of auto-immunity.
This multicenter clinical trial tested an in situ vaccination strategy using a novel “C” class CpG, SD-101, in contrast to a previous clinical trial that used a “B” class CpG, PF- 3512676, that is no longer available for clinical testing.(12) Whether these class differences in CpGs translate to meaningful differences in therapeutic efficacy or toxicity is unknown. In addition, the current trial tested treatment-naïve patients in contrast to the previous trial that tested only patients with relapsed disease Despite these differences, the rate of overall, and distant tumor responses was remarkably similar between these two trials. In the current trial 26 of 29 patients had a reduction in total tumor volume with seven patients achieving an overall partial response and one patient achieving a complete response. Abscopal responses to radiation are known to occur rarely in patients with lymphoma.(18, 19) Therefore the benefit of intratumoral CpG plus radiation over radiation alone in the current study while suggestive, is not proven. Additionally, patients with indolent lymphoma can occasionally have spontaneous remissions, but again, the response rate seen in this trial surpasses what has been previously observed. (20)
A variety of preclinical studies have demonstrated that potent systemic antitumor immune responses can be generated without the use of cytotoxic chemotherapy/radiotherapy by combining local CpG with other immunostimulating agents. Treatment with local CpG combined with ibrutinib or with monoclonal antibodies (mAbs) targeting GITR, CTL4, and/or OX40 generate potent systemic antineoplastic T-cell responses in multiple transplantable and spontaneous mouse tumor models.(8–11) Similarly, intratumoral SD-101 combined with anti-PD-1 mAbs induces strong, systemic anti-tumor responses, even in tumor models unresponsive to anti-PD-1 alone.(21) Ongoing studies continue to determine the optimal therapeutic partner to combine with intratumoral CpG for the treatment of lymphoma and other malignancies. Trials evaluating intratumoral SD-101 and local radiation with intratumoral ipilimumab (NCT02254772) or with oral ibrutinib (NCT02927964) have been initiated; and trials combining SD-101 with an anti-OX40 mAb in both indolent lymphomas (NCT03410901) and solid malignancies are planned given encouraging preclinical data.(11) Additionally, SD-101 in combination with pembrolizumab without the use of radiation, (NCT02521870) has shown early promise in patients with melanoma.(22) Beyond TLR9 agonists, other immunostimulatory agents such as TLR3 agonists,(23, 24) TLR4 agonists,(25) TLR7/8 agonists,(26–28) and STING agonists,(29) have shown early therapeutic promise and going/future studies will be needed to determine optimal in situ vaccination combinations.
The combination of SD-101 and local radiotherapy rapidly induced changes including a reduction in tumor percentage associated with an increase of CD8+ T-cells and CD4+ effector cells. This treatment combination also substantially reduces the percentage of TFH cells; cells thought to promote follicular lymphoma immune escape by inducing an immunosuppressive tumor microenvironment.(30) Additionally, we observed a treatment induced reduction of CD4+ Treg in the treated site. Interestingly, those patients that exhibited low initial levels of CD4+ Treg cells had better responses to treatment. While substantial data suggests that Treg contribute to tumor escape from host immune surveillance, the relationship between Tregs and patient outcomes is idiosyncratic. Low levels of Tregs can be associated with either an improved or unfavorable prognosis depending on the treatment modality and tumor type.(31) Intriguingly, high induced levels of CD4+ Tregs in an ex vivo assay was associated with poor response to TLR9 agonists in indolent lymphoma.(12) In addition, in our current trial low levels of proliferating CD8+ and GzB+ CD8+ T-cells correlated with better response to treatment. Similar findings have been seen for other tumor types.(32, 33) These findings are the result of an initial exploratory analysis and will need to be validated.
Preclinical studies evaluating TLR9 agonists have demonstrated that systemic tumor clearance was partially CD4+ T-cell-dependent.(11) Here, we find high expression of tumor MHC class II was associated with a better overall clinical response, further supporting a CD4+ T-cell-dependent antitumor mechanism. Additionally, the observed variability in MHC class II expression on FL cells may have a genetic basis. Mutations in CREBBP, which is commonly mutated in FL, is associated with a down-regulation of MHC class II.(34, 35) Therefore it will be of interest to examine the relationship between CREBBP mutation status and outcomes of in situ vaccination in future trials.
Overall, these results and associated preclinical studies provide the rationale for expanded studies of in situ vaccination with TLR9 agonists in conjunction with other immune modulating agents in patients with lymphoma.
METHODS
Study Design
This multicenter, open-label, dose-escalation phase 1/2 study (ClinicalTrials.gov Identifier: NCT02266147) was designed to evaluate safety, pharmacodynamics and preliminary efficacy of intratumoral SD101 together with low dose radiotherapy. Part 1 consisted of 4 cohorts of escalating doses of SD-101 (1 mg, 2 mg, 4 mg, and 8 mg) in a standard 3+3 design, and Part 2 studied expanded cohorts to further evaluate the 1 mg and 8 mg doses. Subjects in Part 2 had the option to undergo a second cycle of treatment at the same dose level they received in cycle 1. All subjects underwent safety, PK, and PD assessments, tumor response determinations, and sampling of the treated (Lesion A) and untreated (Lesion B) tumor sites just prior to treatment and at Day 9 by fine needle aspiration (FNA) for correlative biomarker analysis.
All investigators obtained written informed consent from patients prior to participate in the study, and this study was conducted according to the Declaration of Helsinki principles. This study was approved by institutional review boards prior to enrollment. This study was conducted in accordance with good clinical practice (GCP) as defined in International Conference on Harmonisation (ICH) guidelines and US Code of Federal Regulations Title 21, Parts 11, 50, 54, 56, 312, and Title 45 Parts 46, 160 and 164.
Patient Selection
Patients had untreated low-grade B-cell lymphomas, including grade 1–3A follicular lymphoma (FL), marginal zone lymphoma (MZL), small lymphocytic lymphoma/chronic lymphocytic leukemia (CLL/SLL), and cutaneous B cell lymphoma (CBCL) with multiple lymph node involvement that could be managed by a “watch and wait” approach. Patients were required to have at least two sites of disease amenable for injection and/or FNA sampling. One of these sites was used for treatment and the second site outside of the treatment field was used for FNA sampling and for response assessment. Patients were ≥18 years with adequate hematopoietic, renal, and hepatic function; and excluded for the presence of central nervous system lymphoma involvement, hepatitis B or C, HIV, or any active infection; clinically significant cardiovascular disease; or who were previously diagnosed with another cancer requiring treatment within the past 3 years or autoimmune disease, pregnancy, or an Eastern Cooperative Oncology Group performance status of ≥2.
Treatment Schema
A single palpable site of disease, Lesion A, was irradiated with 4 Gy, a standard treatment for single sites of low grade lymphoma, known to kill some tumor cells while sparing the antigen-presenting cells in the tumor microenvironment. (24) The patients received this treatment over two consecutive days (Days 1 and 2) and then were injected 5 times at one week intervals with SD101 at the assigned dose (Figure 1). Blood was collected for measurement of interferon responsive gene induction, a PD endpoint. FNAs were performed at both the treated lesion (A) and at a second, untreated lesion, (B), before and one week after the initiation of therapy and the resulting cell suspensions were shipped overnight at 4 °C in RPMI medium containing 5% Fetal Calf Serum to Stanford where flow cytometry analysis was performed.
Study Assessments
Safety
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Treatment delays occurred for Grade ≥2 neutropenia (ANC < 1500/mm3). Dose limiting toxicities (DLTs) were defined as any non-hematological toxicity Grade ≥3 except for alopecia or nausea uncontrolled by medical management; Grade 4 thrombocytopenia or grade 3 thrombocytopenia with bleeding or any requirement for platelet transfusion; febrile neutropenia; Grade 4 neutropenia lasting >5 days; Grade 4 anemia, unexplained by underlying disease; and/or any Grade ≥ 2 toxicity related to SD-101 that does not resolve to Grade ≤ 1 with standard treatment by the time of the next treatment.
Efficacy
Disease assessment included CT scans (or PET/CT) at screening, at 3 and 6 months post-treatment, and then every 6 months for the remainder of the trial. There was an additional CT scan at 9 months for subjects who received Cycle 2. Overall tumor responses were assessed according to the Revised 2007 International Working Group criteria.(36) In addition, we scored tumor responses separately at the treated site and at untreated sites of disease. Patients were not permitted to receive lymphoma-directed therapy, including steroids, during the follow-up period and were off study if they received any such treatments.
Pharmacokinetics
Plasma samples were collected for PK analysis before and 24 hours after the Day 9 injection and analyzed for SD101 level by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
IFN Responsive Gene Signature
Before and 24 hours after treatment on Day 9, whole blood was collected in PAXgene tubes (Qiagen) and frozen until RNA was isolated. The expression of IFN responsive genes (MCP-1, GBP-1, ISG-54, and MxB), was performed via quantitative PCR and normalized to the expression level of ubiquitin. To assess the engagement of TLR9, a composite score was generated by calculating the geometric mean of the fold activity of each of the 4 genes on Day 10 relative to the Day 9 baseline.
FNA analysis by Flow Cytometry
Serial FNA samples were collected on Day 1 and Day 9 from both a treated (Lesion A) and a single distal lesion (Lesion B), if available. A single cell suspension was stained with 3 panels of fluorochrome-conjugated antibodies, fixed and permeabilized using Fix/Perm solution (BD Biosciences), and then stained for intracellular proteins. Panel 1 included antibodies against CD3, CD4, CD8, CD25, CD27, CD127, CD134, CD278(ICOS), CD279(PD-1), CXCR5, HLA-DR, and intracellular FoxP3 (BD Biosciences). Panel 2 included antibodies against CD3, CD8, CD25, CD45RO+, CD62L, CD127, CD279, and intracellular Eomes, Ki-67, and Granzyme B (GzB). Panel 3 included antibodies against CD3, CD19, CD20, and lambda light chain. Flow cytometry was performed with an LSR II cytometer (BD Immunocytometry Systems) and the data was analyzed using Cytobank software. Relationships between individual markers, on T,B and myeloid-cell subsets were interrogated in relation to therapy and to clinical outcomes. Statistical significance in the differences in cell populations between Day 1 and 9 was determined using Prism 6.0 (GraphPad). P-values of <0.05 were considered significant.
Supplementary Material
SIGNIFICANCE.
In situ vaccination with the TLR9 agonist, SD-101, along with low dose radiation was safe and induced systemic responses in patients with indolent lymphoma. Low levels of CD4+ Tregs, proliferating CD8+ T-cells, and GranzymeB+ CD8+ T-cells in the tumor microenvironment predicted favorable response to treatment.
ACKNOWLEDGEMENTS:
The authors thank all of the participating investigators, patients, and their families.
Financial support: This work was supported by grants from the NCI R35CA19735301 and Dynavax Corporation. This work was supported by a grant to MJF from the American Cancer Society’s Grand View League.
Footnotes
Conflict of Interest Disclosure: R.J., A.F.C. and R.L.C. are employees of Dynavax Corporation. The remaining authors declare no conflict of interest
REFERENCES:
- 1.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017. 12;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010. July;363(5):411–22. [DOI] [PubMed] [Google Scholar]
- 3.Wolchok JD, Rollin L, Larkin J. Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017. 12;377(25):2503–4. [DOI] [PubMed] [Google Scholar]
- 4.Hammerich L, Binder A, Brody JD. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol Oncol. 2015. December;9(10):1966–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammerich L, Bhardwaj N, Kohrt HE, Brody JD. In situ vaccination for the treatment of cancer. Immunotherapy. 2016;8(3):315–30. [DOI] [PubMed] [Google Scholar]
- 6.Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdörfer B, et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002. October;169(7):3892–9. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Song W, Czerwinski DK, Varghese B, Uematsu S, Akira S, et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007. August;179(4):2493–500. [DOI] [PubMed] [Google Scholar]
- 8.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009. April;113(15):3546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013. June;123(6):2447–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagiv-Barfi I, Kohrt HE, Burckhardt L, Czerwinski DK, Levy R. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood. 2015. March;125(13):2079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018. January;10(426). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010. October;28(28):4324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betting DJ, Yamada RE, Kafi K, Said J, van Rooijen N, Timmerman JM. Intratumoral but not systemic delivery of CpG oligodeoxynucleotide augments the efficacy of anti-CD20 monoclonal antibody therapy against B cell lymphoma. J Immunother. 2009 2009. Jul-Aug;32(6):622–31. [DOI] [PubMed] [Google Scholar]
- 14.Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012. February;53(2):211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsh V, Paz-Ares L, Boyer M, Rosell R, Middleton G, Eberhardt WE, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011. July;29(19):2667–74. [DOI] [PubMed] [Google Scholar]
- 16.Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska-Morawiec M, et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol. 2012. January;23(1):72–7. [DOI] [PubMed] [Google Scholar]
- 17.Khodadoust MS, Olsson N, Wagar LE, Haabeth OA, Chen B, Swaminathan K, et al. Antigen presentation profiling reveals recognition of lymphoma immunoglobulin neoantigens. Nature. 2017. 03;543(7647):723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015. June;41(6):503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rees GJ. Abscopal regression in lymphoma: a mechanism in common with total body irradiation? Clin Radiol. 1981. July;32(4):475–80. [DOI] [PubMed] [Google Scholar]
- 20.Ardeshna KM, Qian W, Smith P, Braganca N, Lowry L, Patrick P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol. 2014. April;15(4):424–35. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A. 2016. November;113(46):E7240–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung ACF, Kummar S, Agarwala SS, Nemunaitis JJ, Gonzalez R, Drabick JJ. Phase 1b/2, open label, multicenter, study of intratumoral SD-101 in combination with pembrolizumab in anti-PD1 naïve & experienced metastatic melanoma patients. Journal of Clinical Oncology 2017. [Google Scholar]
- 23.Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res. 2014. August;2(8):720–4. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Ruiz ME, Perez-Gracia JL, Rodríguez I, Alfaro C, Oñate C, Pérez G, et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann Oncol. 2018. May;29(5):1312–9. [DOI] [PubMed] [Google Scholar]
- 25.Flowers C, Panizo C, Isufi I, Herrera AF, Okada C, H. CE, et al. Intratumoral G100 Induces Systemic Immunity and Abscopal Tumor Regression in Patients with Follicular Lymphoma: Results of a Phase 1/ 2 Study Examining G100 Alone and in Combination with Pembrolizumab. Blood 2017. p. 2771.28331056 [Google Scholar]
- 26.Smyth EC, Flavin M, Pulitzer MP, Gardner GJ, Costantino PD, Chi DS, et al. Treatment of locally recurrent mucosal melanoma with topical imiquimod. J Clin Oncol. 2011. Nov;29(33):e809–11. [DOI] [PubMed] [Google Scholar]
- 27.Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, et al. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009. May;182(9):5217–24. [DOI] [PubMed] [Google Scholar]
- 28.Singh M, Khong H, Dai Z, Huang XF, Wargo JA, Cooper ZA, et al. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol. 2014. November;193(9):4722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015. May;11(7):1018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawal S, Chu F, Zhang M, Park HJ, Nattamai D, Kannan S, et al. Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. J Immunol. 2013. June;190(12):6681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther. 2015;4:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003. November;94(11):1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCoy MJ, Nowak AK, van der Most RG, Dick IM, Lake RA. Peripheral CD8(+) T cell proliferation is prognostic for patients with advanced thoracic malignancies. Cancer Immunol Immunother. 2013. March;62(3):529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green MR, Kihira S, Liu CL, Nair RV, Salari R, Gentles AJ, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci U S A. 2015. March;112(10):E1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011. March;471(7337):189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007. February;25(5):579–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.