Version Changes
Revised. Amendments from Version 1
We have revised our manuscript taking into account the reveiwers' suggestions. The main updates include: 1) incorporation of ABO blood group data; 2) expanded discussion of limitations related to self-reported data; 3) discussion of possible mechanisms for the observed association between FUT2 secretor status and kindey disease; 4) re-analysis accounting for two alternative null FUT2 alleles. These changes are detailed in our posted responses to the reviewers' comments.
Abstract
Background: The FUT2 (fucosyltransferase-2) gene determines blood group secretor status. Being homozygous for the inactive “non-secretor” rs601338(A) allele confers resistance to certain infections (e.g. Norovirus, Rotavirus) and susceptibility to others (e.g. Haemophilus influenza, Streptococcus pneumonia). Non-secretors also have an increased risk of type 1 diabetes and inflammatory bowel disease. We examined FUT2 genotype, infections and chronic conditions in a population-based cohort.
Methods: We studied 7,582 pregnant women from the ALSPAC pregnancy cohort. Infections (measles, mumps, chicken pox, whooping cough, meningitis, herpes, gonorrhea and urinary infections) and chronic conditions (kidney disease, hypertension, diabetes, rheumatism, arthritis, psoriasis, hay fever, asthma, eczema and allergies) were self-reported. FUT2 secretor status was determined from the rs601338 genotype. ABO blood type was obtained from clinical records.
Results: Overall, 1920 women (25.3%) were homozygous for the non-secretor allele (AA). Secretor status was associated with mumps, with 68% of non-secretors experiencing this infection, compared to 48% of secretors (RR, 1.40; 95% CI, 1.34–1.46). A weaker association was observed for measles infection (76% vs. 72%; RR, 1.05; 95% CI, 1.02–1.09). Non-secretors also experienced an increased risk of kidney disease (5.4% vs. 3.9%; RR, 1.39; 95% CI, 1.11–1.75). Independent of secretor status, AB blood type was a risk factor for mumps (RR 1.15; 95%CI, 1.03, 1.28 compared to type O). We found no evidence of interaction between secretor status and blood type. For some conditions, including asthma and arthritis, FUT2 heterozygosity (GA) appeared to confer an intermediate phenotype. There was no strong evidence of association between secretor status and other infections or chronic conditions, although statistical power was limited for rare outcomes.
Conclusion: Our results identify an association between FUT2 secretor status and self-reported kidney disease, and confirm a recently reported association with susceptibility to mumps infection. The clinical implications of these associations warrant further investigation.
Keywords: FUT2, infection, chronic disease, secretor status, ALSPAC, mumps, kidney disease
Introduction
The FUT2 (fucosyltransferase 2) gene encodes the alpha (1,2) fucosyltransferase, which determines blood group secretor status. About 20% of Caucasians are homozygous for the nonsense mutation W143X (rs601338G>A), encoding a stop codon that inactivates the FUT2 enzyme 1. Individuals who are homozygous for this “non-secretor” allele (AA) are unable to secrete histo-blood group antigens into bodily fluids, or express them on mucosal surfaces.
Non-secretors have a lower risk of diarrheal illness 2 and ear infections in childhood 3. The non-secretor phenotype also confers resistance to specific pathogens that require FUT2-dependent antigens to infect host cells, including Norovirus 4– 7, Rotavirus 8– 11 and Helicobacter pylori 12, 13. By contrast, the non-secretor phenotype has been associated with increased susceptibility to other pathogens, including Candida 14– 16, Haemophilius influenza 17, Neisseria meningitis 18 and Streptococcus pneumonia 18. Most recently, in a genome-wide association study (GWAS) of common infections, Tian et al. reported an increased susceptibility to mumps in non-secretors 3. In addition, non-secretors appear to be at increased risk for certain autoimmune diseases, including type 1 diabetes 19, psoriasis 20, 21 and inflammatory bowel disease 22, 23.
The above associations have not been simultaneously examined in a single population and several have not been independently replicated. Moreover, the association of FUT2 secretor status with other infectious and chronic diseases has not been widely studied. Finally, previous studies have typically only considered the secretor phenotype as dichotomous, assuming the non-secretor allele to be recessive. In this study, we characterized the association of FUT2 secretor status with a variety of infectious and chronic diseases in the population-based Avon Longitudinal Study of Parents and Children (ALSPAC), and examined the impact of heterozygosity for the non-secretor allele.
Methods
Study design and population
This study accessed data from the ALSPAC cohort. ALSPAC recruited 14,541 pregnant women (98% Caucasian) resident in the former county of Avon, UK with expected dates of delivery 1st April 1991 to 31st December 1992 24, 25. The current analysis included a subset of 7,582 Caucasian women who selected and provided written informed consent for genotyping analysis, and reported their personal medical history during pregnancy. ABO blood group was collected from clinical records for the majority of participants (N=6,757). The ALSPAC website contains details of all the data that is available through a fully searchable data dictionary at http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
Genotyping
ALSPAC mothers were genotyped using the Illumina human660W-quad array at Centre National de Génotypage (CNG) (Evry, France) and genotypes were called with Illumina GenomeStudio. PLINK (v1.07) was used to carry out quality control (QC) measures on an initial set of 10,015 participants and 557,124 directly genotyped single nucleotide polymorphisms (SNPs). SNPs were removed if they displayed more than 5% missingness or a Hardy-Weinberg equilibrium P value of less than 1.0×10 −6. Additionally, SNPs with a minor allele frequency of less than 1% were removed. Samples were excluded if they displayed more than 5% missingness, had indeterminate X chromosome heterozygosity or extreme autosomal heterozygosity. Samples showing evidence of population stratification were identified by multidimensional scaling of genome-wide identity by state pairwise distances using the four HapMap populations as a reference, and then excluded 26. Cryptic relatedness was assessed using an identity by descent (IBD) estimate of more than 0.125, which is expected to correspond to roughly 12.5% alleles shared IBD or relatedness at the first cousin level. Related participants that passed all other QC thresholds were retained during subsequent phasing and imputation. In total, 9,048 mothers and 526,688 SNPs passed these QC filters.
Imputation
A total of 477,482 SNP genotypes in common between the sample of mothers described above and a second sample of 9,115 children were combined. SNPs with genotype missingness above 1% due to poor quality (N=11,396 SNPs) were removed and a further 321 participants were removed due to potential ID mismatches. This resulted in a dataset of 17,842 participants, containing 6,305 duos and 465,740 SNPs (112 were removed during liftover and 234 were out of HWE after combination). Haplotypes were estimated using ShapeIT (v2.r644), which utilizes relatedness during phasing. The phased haplotypes were then imputed to the Haplotype Reference Consortium (HRC) panel of approximately 31,000 phased whole genomes using Impute V3. For this study, we excluded the mothers who had removed consent, leaving 8,698 eligible mothers. We further excluded those who did not provide personal medical history, leaving 7,582 for analysis.
Exposure: FUT2 genotype
FUT2 secretor status was defined based on the rs601338 SNP 1, where G is the wild-type “secretor” allele and A is the nonsense W143X “non-secretor” allele. Following previous studies 7, 11, 19, 23, we considered the A allele to be recessive and dichotomized secretor status, combining the GA and GG genotypes as secretors and comparing them to the homozygous AA non-secretors. In addition, we explored the impact of GA heterozygosity at this locus. Two other commonly reported non-secretor alleles were considered. The missense variant at rs1047781, described in Asian populations 27 was not detected in our Caucasian population. The non-synonymous S258G variant at rs602662 28 was highly correlated with rs601338; incorporating this SNP to define secretor status had no impact on 98% of participants’ phenotype classification, and did not materially change our results.
Outcomes: infections and chronic conditions
Infections and chronic conditions were self-reported using a standardized questionnaire during pregnancy. Women were asked if they had ever had various infections (measles, mumps, chicken pox, whooping cough, cold sores, meningitis, genital herpes, gonorrhea and urinary infections) or chronic conditions (diabetes, hypertension, kidney disease, rheumatism, arthritis, psoriasis, hay fever, asthma, eczema, and any allergies, including cat, dust, pollen, insect bites or ‘other’).
Statistical analysis
Demographic characteristics were summarized with descriptive statistics and compared between non-secretors (AA) and secretors (GA or GG combined) using t-tests for continuous variables or chi-squared tests for categorical variables. For each outcome, the relative risk (RR) and 95% confidence interval (95% CI) was calculated for non-secretors versus secretors. A multivariable model was used to determine whether the association of FUT2 secretor status and kidney disease was independent of measles, mumps and urinary tract infections. Multivariable models were also used to mutually adjust for FUT2 secretor status and ABO blood group and to formally test for interaction between these two factors. To explore the potential impact of FUT2 heterozygosity, a three group analysis was also conducted, considering the AA, GA and GG genotypes separately and using homozygous secretors (GG) as the reference group. All statistical analyses were performed in SAS (version 9.4, Carey, NC, US).
Results
Overall, 1920 women were homozygous for the FUT2 non-secretor allele (AA, 25.3%), 1906 were homozygous for the secretor allele (GG, 25.1%) and 3756 were heterozygous (GA, 49.5%). Almost half (46%) were first-time mothers, 21% were unmarried, 21% smoked, and 14% had a university degree. The mean (± standard deviation) age was 26.9 (± 5.9) years and the mean body mass index was 22.9 (± 3.7) kg/m 2. These demographic characteristics were not associated with FUT2 secretor status ( Table 1). The lifetime incidence of infections ranged from <1% for meningitis to 87% for chicken pox, while the incidence of chronic conditions ranged from 1% for diabetes to 43% for allergies.
Table 1. Demographics of mothers in the ASLPAC cohort according to FUT2 secretor status.
|
FUT2 Secretor Status
(rs601338 genotype) |
|||||
|---|---|---|---|---|---|
| Non-Secretors
(AA) |
Secretors
(GG or GA) |
P value | |||
| N=1920 | N=5662 | ||||
| Age, years | 26.9 | ± 5.9 | 26.9 | ± 5.8 | 0.94 |
| BMI, kg/m 2 | 22.8 | ± 3.6 | 23.0 | ± 3.7 | 0.05 |
| Married | |||||
| No | 423 | (22.4) | 1173 | (21.2) | 0.28 |
| Yes | 1468 | (77.6) | 4364 | (78.8) | |
| Parity | |||||
| 0 | 861 | (46.3) | 2539 | (46.2) | 0.67 |
| 1 | 641 | (34.4) | 1957 | (35.6) | |
| 2 | 264 | (14.2) | 738 | (13.4) | |
| 3 or more | 95 | (5.1) | 259 | (4.7) | |
| Smoking | |||||
| No | 1480 | (78.8) | 4324 | (77.9) | 0.37 |
| Yes | 397 | (21.2) | 1229 | (22.1) | |
| Education | |||||
| <O level | 457 | (24.6) | 1418 | (25.8) | 0.44 |
| O level | 642 | (34.5) | 1946 | (35.4) | |
| A level | 461 | (24.8) | 1306 | (23.8) | |
| University degree | 276 | (14.8) | 762 | (13.9) | |
Values are mean ± standard deviation or n (%). AA, homozygous for non-secretor alleles; GG, homozygous for secretor alleles; GA, heterozygous; ALSPAC, Avon Longitudinal Study of Parents and Children; BMI, body mass index. Comparisons by t-test for continuous variables or chi-squared test for categorical variables.
Dichotomous FUT2 secretor status and infections
The homozygous AA non-secretor genotype was associated with mumps infection, with 68% of non-secretors experiencing this infection, compared to 48% of secretors (RR, 1.40; 95% CI, 1.34–1.46; p<0.0001) ( Table 2). Weaker associations were observed for measles infection (76% vs. 72%; RR, 1.05; 95% CI, 1.02–1.09; p=0.0008) and urinary infections (57% vs. 55%; RR, 1.05; 95% CI, 1.00–1.10; p=0.05). There was no strong evidence of association between FUT2 secretor status and whooping cough, chicken pox or cold sores ( Table 2).
Table 2. Lifetime incidence and relative risk of infectious and chronic conditions among mothers in the ALSPAC cohort according to dichotomized FUT2 secretor status.
| Condition * |
FUT2 Secretor Status
(rs601338 genotype) |
Relative Risk
Non-Secretors vs. Secretors |
||||
|---|---|---|---|---|---|---|
| Non-Secretors
(AA) |
Secretors
(GA or GG) |
P value | ||||
| N=1920 | N=5662 | |||||
| cases | (%) | cases | (%) | RR (95% CI) | ||
| Infections | ||||||
| measles | 1458 | (75.9) | 4076 | (72.0) | 1.05 (1.02, 1.09) | 0.0008 |
| mumps | 1299 | (67.7) | 2734 | (48.3) | 1.40 (1.34, 1.46) | <0.0001 |
| chicken pox | 1656 | (86.3) | 4925 | (87.0) | 0.99 (0.97, 1.01) | 0.41 |
| whooping cough | 222 | (11.6) | 638 | (11.3) | 1.03 (0.89, 1.18) | 0.73 |
| cold sores | 843 | (43.9) | 2458 | (43.4) | 1.01 (0.95, 1.07) | 0.71 |
| meningitis | 15 | (0.8) | 61 | (1.1) | 0.73 (0.41, 1.27) | 0.26 |
| genital herpes | 45 | (2.3) | 108 | (1.9) | 1.23 (0.87, 1.73) | 0.24 |
| gonorrhea | 29 | (1.5) | 70 | (1.2) | 1.22 (0.80, 1.88) | 0.36 |
| urinary infection | 1095 | (57.0) | 3085 | (54.5) | 1.05 (1.00, 1.10) | 0.05 |
| Chronic conditions | ||||||
| kidney disease | 103 | (5.4) | 218 | (3.9) | 1.39 (1.11, 1.75) | 0.004 |
| hypertension | 272 | (14.2) | 804 | (14.2) | 1.00 (0.88, 1.13) | 0.94 |
| diabetes | 23 | (1.2) | 55 | (1.0) | 1.23 (0.76, 2.00) | 0.40 |
| rheumatism | 93 | (4.8) | 230 | (4.1) | 1.19 (0.94, 1.51) | 0.14 |
| arthritis | 77 | (4.0) | 188 | (3.3) | 1.21 (0.93, 1.57) | 0.15 |
| psoriasis | 59 | (3.1) | 213 | (3.8) | 0.82 (0.62, 1.08) | 0.16 |
| hay fever | 573 | (29.8) | 1742 | (30.8) | 0.97 (0.90, 1.05) | 0.45 |
| asthma | 215 | (11.2) | 652 | (11.5) | 0.97 (0.84, 1.12) | 0.71 |
| eczema | 469 | (24.4) | 1271 | (22.4) | 1.09 (0.99, 1.19) | 0.07 |
| any allergies | 837 | (43.6) | 2412 | (42.6) | 1.02 (0.97, 1.09) | 0.42 |
AA, homozygous for non-secretor alleles; GG, homozygous for secretor alleles; GA, heterozygous; ALSPAC, Avon Longitudinal Study of Parents and Children; RR, relative risk; CI, confidence interval. *Self-reported during pregnancy: "Have you ever had…?"
Dichotomous FUT2 secretor status and chronic conditions
Homozygous AA non-secretors experienced a 39% increased risk of self-reported kidney disease compared to secretors (5.4% vs. 3.9%; RR, 1.39; 95% CI, 1.11–1.75; p=0.004) ( Table 2). This association was essentially unchanged in a multivariable model controlling for mumps, measles and urinary infections (adjusted RR, 1.39; 95% CI, 1.10–1.75; p=0.005). Directionally consistent results were also observed for diabetes (RR, 1.23; 95% CI, 0.76–2.00; p=0.40), rheumatism (RR 1.19, 95%CI: 0.94–1.51, p=0.14) and arthritis (RR, 1.21; 95% CI, 0.93–1.57; p=0.15), although power was lacking for these relatively rare outcomes. There was no strong evidence of association between FUT2 secretor status and hypertension, hay fever, asthma or allergies ( Table 2).
ABO blood group
Since secretor status determines the ability to secrete blood group antigens, we also explored the impact of ABO blood group on associations observed for mumps infection and kidney disease ( Table 3). For both conditions, the effect estimate for FUT2 secretor status was essentially unchanged following adjustment for ABO blood group and there was no significant interaction between FUT2 and ABO blood group (p for interaction: 0.60 for mumps, 0.57 for kidney disease). Independent of FUT2 secretor status, women with type AB blood had an increased risk of mumps infection (59.4%) compared to women with type A, B, or O blood (52.2%, 52.0%, 52.9%, respectively; adjusted RR 1.15, 95% CI: 1.03, 1.28 for AB vs O, p=0.01). ABO blood group was not associated with kidney disease.
Table 3. Mutually-adjusted associations of FUT2 secretor status and ABO blood group with mumps infection and kidney disease in the ALSPAC cohort.
| Mumps | Kidney Disease | |||||||
|---|---|---|---|---|---|---|---|---|
| n/N | % | RR (95% CI) | p | n/N | % | RR (95% CI) | p | |
| FUT2 Genotype | ||||||||
| Non-Secretor (AA) | 1299/1920 | 67.7 | 1.39 (1.33, 1.46) | <.0001 | 103/1920 | 5.4 | 1.32 (1.04, 1.69) | 0.02 |
| Secretor (AG or GG) | 2734/5662 | 48.3 | 1.00 (ref) | 218/5662 | 3.9 | 1.00 (ref) | ||
| Blood Group | ||||||||
| A | 1522/2917 | 52.2 | 1.00 (0.95, 1.05) | 0.93 | 135/2917 | 4.6 | 1.09 (0.56, 2.11) | 0.14 |
| B | 316/608 | 52.0 | 0.99 (0.91, 1.07) | 0.78 | 26/608 | 4.3 | 1.10 (0.73, 1.67) | 0.64 |
| O | 1595/3015 | 52.9 | 1.00 (ref) | 117/3015 | 3.9 | 1.00 (ref) | ||
| AB | 129/217 | 59.4 | 1.15 (1.03, 1.28) | 0.01 | 9/217 | 4.1 | 1.09 (0.56, 2.11) | 0.80 |
RR, relative risk; CI, confidence interval. Models are mutually adjusted for FUT2 genotype and blood group.
FUT2 heterozygosity
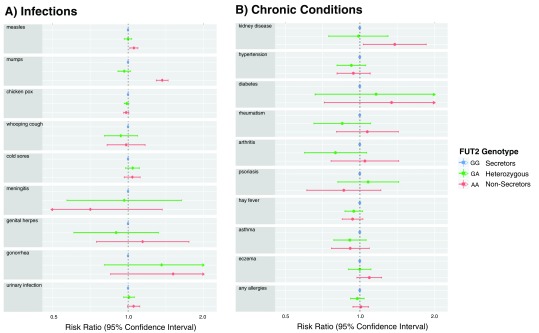
Compared to homozygous GG secretors, GA heterozygotes experienced a similar risk of mumps infection (RR, 0.97; 95% CI, 0.91–1.02; p=0.24) and kidney disease (RR, 0.99; 95% CI, 0.75–1.30; p=0.93), suggesting that increased susceptibility to these conditions (as described above) is likely to be a recessive trait experienced only in homozygous AA non-secretors. Similar evidence was found for measles, urinary infections and eczema, where disease risk was comparable for individuals with GG and GA genotypes. However, this pattern was not consistent across all conditions. For example, the risk of asthma was similarly reduced in GA heterozygotes (11.1%) and AA non-secretors (11.2%) compared to GG secretors (12.2%), and the risk of arthritis was lower in GA heterozygotes (3.0%) compared to either homozygous genotype (4.0% in AA, 3.8% in GG), although statistical evidence of association was weak for these relatively rare outcomes ( Figure 1).
Figure 1.
Relative risk of ( A) infectious and ( B) chronic conditions among 7,582 mothers in the ALSPAC cohort according to FUT2 genotype at rs601338.
Discussion
Our findings from the population-based ALSPAC cohort confirm and extend previous research associating the FUT2 genotype with susceptibility to infections and chronic diseases. Specifically, we confirmed a recently reported association with mumps infection 3 and identified an association with self-reported kidney disease. We also evaluated a number of other common conditions (e.g. whooping cough, chicken pox and asthma) but found no strong evidence of association with FUT2 secretor status, indicating that FUT2 influences pathogen- or disease-specific processes, rather than overall innate or adaptive immunity. Finally, our results suggest that FUT2 heterozygosity may confer an intermediate phenotype for certain conditions, although further research is required to replicate these findings.
Our results confirm the association reported in a recent GWAS for common infections among 23andMe research participants by Tian et al. 3, where the FUT2 rs516316(C) allele was associated with mumps infection (odds ratio, 1.25; 95% CI, 1.24–1.27). This risk allele is in complete linkage disequilibrium with the non-secretor rs601338(A) allele evaluated in our study, where a strong association was also observed (RR, 1.40; 95% CI, 1.34–1.46). Tian et al. hypothesized that non-secretors are more susceptible to mumps infection because binding of the mumps virus to host cell sialic acid receptors is enhanced in the absence of FUT2-dependent antigens on the cell surface. Indeed, using x-ray crystallography and functional assays, Kubota et al. 29 recently showed that mumps virus preferentially uses a trisaccharide containing α2,3-linked sialic acid in unbranched sugar chains as a receptor. Our results provide further evidence that susceptibility to mumps infection is modulated by FUT2 secretor status.
Tian et al. 3 also reported an association between the ABO gene and mumps infection, and suggested that ABO antigens may disrupt binding of the mumps virus to host cell receptors. Our results using clinical ABO blood group data support and extend this finding by confirming an association and specifically identifying blood type AB as a risk factor for mumps infection. Since FUT2 secretor status determines whether ABO antigens are secreted into body fluids and onto cell surfaces, we hypothesized that secretor status and blood type may interact to influence susceptibility to mumps infection; however, we found no evidence of this interaction. Thus, our study suggests that FUT2 genotype and ABO blood group are independently associated with mumps infection, with increased risk among non-secretors and blood type AB.
Our study also provides new evidence that non-secretors may be predisposed to kidney disease (RR, 1.39; 95% CI, 1.11–1.75), although we lacked clinical information to confirm and classify this self-reported diagnosis. To our knowledge, the FUT2 genotype has not previously been associated with kidney disease in the general population, although some studies have used traditional blood group assays to evaluate secretor status in patients with pyelonephritis (kidney inflammation, typically due to bacterial infection). One study of women with acute uncomplicated pyelonephritis found that non-secretor status was significantly more common in these patients than in the general population 30, and another found that renal scarring in girls with recurrent pyelonephritis was more common in non-secretors than secretors 31. It has been hypothesized that these associations reflect an increased susceptibility to uropathogenic Escherichia coli infection among non-secretors, resulting from the enhanced expression of preferred binding receptors in the vaginal epithelium and kidneys of non-secretor women 32, 33. Notably, in our study, the association between FUT2 genotype and self-reported kidney disease appeared to be independent of self-reported urinary infections. However, there are multiple clinically-distinct causes of “kidney disease” and “urinary infections”, and we lacked clinical information to define the etiology of these conditions in our study. Thus, additional research is needed to replicate our observations with confirmed and clinically-defined kidney disease and urinary infections, and to examine the possible relationship between these conditions and FUT2 genotype.
Consistent with previous studies 19– 21, we observed a trend towards an increased risk of arthritis, rheumatism and diabetes among non-secretors, although we lacked statistical power for the analysis of these relatively uncommon autoimmune disorders.
Finally, we examined disease risk among GA heterozygotes, who are typically considered secretors because the non-secretor rs601338(A) allele is assumed to be recessive. Our results for mumps and kidney disease support this assumption, as increased susceptibility was only seen in homozygous AA non-secretors. However, we observed different patterns of association for some other conditions, including a potentially increased risk of gonorrhea and reduced risk of arthritis among GA heterozygotes, although our effect estimates were imprecise for these relatively rare conditions. Further research is warranted to replicate these observations in larger populations, and explore whether heterozygosity may impart an intermediate risk or unique protection from certain conditions.
Limitations of this work include the reliance on self-reported medical histories and low power for rare outcomes (such as meningitis, diabetes and other autoimmune diseases). Power was also limited for interaction analyses. Also, we could not identify the specific pathogens responsible for urinary infections, and we lacked clinical data to confirm, classify and define the etiology of multifactorial disorders (such as allergies and kidney disease). Finally, our analysis of the ALSPAC pregnancy cohort was limited to women, so the results may not be generalizable to men, and potential sex differences could not be investigated.
In conclusion, our results identify a novel association between FUT2 non-secretor status and increased risk of kidney disease, and confirm a recently-reported association with increased susceptibility to mumps infection. The clinical implications of these associations warrant further investigation.
Data availability
The ALSPAC data management plan ( http://www.bristol.ac.uk/alspac/researchers/data-access/documents/alspac-data-management-plan.pdf) describes in detail the policy regarding data sharing, which is through a system of managed open access. The steps below highlight how to apply for access to the data included in this paper and all other ALSPAC data. The datasets used in this analysis are linked to ALSPAC project number B3047; please quote this project number during your application.
-
1.
Please read the ALSPAC access policy (PDF, 627kB) which describes the process of accessing the data and samples in detail, and outlines the costs associated with doing so.
-
2.
You may also find it useful to browse the fully searchable ALSPAC research proposals database, which lists all research projects that have been approved since April 2011.
-
3.
Please submit your research proposal for consideration by the ALSPAC Executive Committee. You will receive a response within 10 working days to advise you whether your proposal has been approved.
If you have any questions about accessing data, please email alspac-data@bristol.ac.uk.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. We also thank Faisal Atakora (University of Manitoba) for assistance with data visualization, Laura Corbin (University of Bristol) for assistance with genotyping methodology, and Lars Bode (University of California San Diego) for assistance with interpretation of results.
Funding Statement
This research was undertaken, in part, thanks to funding from the Canada Research Chairs program. The UK Medical Research Council and the Wellcome Trust (102215/2/13/2) and the University of Bristol provide core support for ALSPAC. A comprehensive list of grants funding is available on the ALSPAC website. GWAS data was generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corporation of America) using support from 23andMe. MBA holds the Canada Research Chair in Developmental Origins of Chronic Disease. N.J.T. is a Wellcome Trust Investigator (202802/Z/16/Z), works within the University of Bristol NIHR Biomedical Research Centre (BRC) (IS-BRC-1215) and as part of the Cancer Research UK Integrative Cancer Epidemiology Programme (C18281/A19169). K.H.W. is funded equally by two programs of the Medical Research Council Integrative Epidemiology Unit (MC_UU_12013/3 and MC_UU_12013/4). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This publication is the work of the authors and M.B.A. will serve as guarantor for the contents of this paper.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 2 approved]
References
- 1. Kelly RJ, Rouquier S, Giorgi D, et al. : Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene ( FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270(9):4640–4649. 10.1074/jbc.270.9.4640 [DOI] [PubMed] [Google Scholar]
- 2. Bustamante M, Standl M, Bassat Q, et al. : A genome-wide association meta-analysis of diarrhoeal disease in young children identifies FUT2 locus and provides plausible biological pathways. Hum Mol Genet. 2016;25(18):4127–4142. 10.1093/hmg/ddw264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tian C, Hromatka BS, Kiefer AK, et al. : Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections. Nat Commun. 2017;8(1): 599. 10.1038/s41467-017-00257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlsson B, Kindberg E, Buesa J, et al. : The G428A Nonsense Mutation in FUT2 Provides Strong but Not Absolute Protection against Symptomatic GII.4 Norovirus Infection. PLoS One. 2009;4(5):e5593. 10.1371/journal.pone.0005593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kindberg E, Akerlind B, Johnsen C, et al. : Host Genetic Resistance to Symptomatic Norovirus (GGII.4) Infections in Denmark. J Clin Microbiol. 2007;45(8):2720–2722. 10.1128/JCM.00162-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruvoën-Clouet N, Belliot G, Le Pendu J: Noroviruses and histo-blood groups: the impact of common host genetic polymorphisms on virus transmission and evolution. Rev Med Virol. 2013;23(6):355–366. 10.1002/rmv.1757 [DOI] [PubMed] [Google Scholar]
- 7. Thorven M, Grahn A, Hedlund KO, et al. : A homozygous nonsense mutation (428G-->A) in the human secretor ( FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J Virol. 2005;79(24):15351–15355. 10.1128/JVI.79.24.15351-15355.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenberg HB, Estes MK: Rotaviruses: From Pathogenesis to Vaccination. Gastroenterology. 2009;136(6):1939–1951. 10.1053/j.gastro.2009.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu L, Crawford SE, Czako R, et al. : Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 2012;485(7397):256–259. 10.1038/nature10996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang P, Xia M, Tan M, et al. : Spike Protein VP8* of Human Rotavirus Recognizes Histo-Blood Group Antigens in a Type-Specific Manner. J Virol. 2012;86(9):4833–4843. 10.1128/JVI.05507-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imbert-Marcille BM, Barbé L, Dupé M, et al. : A FUT2 Gene Common Polymorphism Determines Resistance to Rotavirus A of the P[8] Genotype. J Infect Dis. 2014;209(8):1227–1230. 10.1093/infdis/jit655 [DOI] [PubMed] [Google Scholar]
- 12. Azevedo M, Eriksson S, Mendes NM, et al. : 612 Infection By Helicobacter pylori Expressing the BaBa Adhesin Is Influenced By the Secretor Phenotype. Gastroenterology. 2008;134(4, Supplement 1):A–86. 10.1016/S0016-5085(08)60402-3 [DOI] [PubMed] [Google Scholar]
- 13. Ikehara Y, Nishihara S, Yasutomi H, et al. : Polymorphisms of two fucosyltransferase genes ( Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti- Helicobacter pylori IgG antibody. Cancer Epidemiol Biomarkers Prev. 2001;10(9):971–977. [PubMed] [Google Scholar]
- 14. Ben-Aryeh H, Blumfield E, Szargel R, et al. : Oral Candida carriage and blood group antigen secretor status. Mycoses. 1995;38(9–10):355–358. 10.1111/j.1439-0507.1995.tb00064.x [DOI] [PubMed] [Google Scholar]
- 15. Chaim W, Foxman B, Sobel JD: Association of recurrent vaginal candidiasis and secretory ABO and Lewis phenotype. J Infect Dis. 1997;176(3):828–830. 10.1086/517314 [DOI] [PubMed] [Google Scholar]
- 16. Thom SM, Blackwell CC, MacCallum CJ, et al. : Non-secretion of blood group antigens and susceptibility to infection by Candida species. FEMS Microbiol Immunol. 1989;1(6–7):401–405. 10.1016/0378-1097(89)90265-6 [DOI] [PubMed] [Google Scholar]
- 17. Blackwell CC, Jonsdottir K, Hanson MF, et al. : Non-secretion of ABO blood group antigens predisposing to infection by Haemophilus influenzae. Lancet. 1986;2(8508):687. 10.1016/S0140-6736(86)90193-5 [DOI] [PubMed] [Google Scholar]
- 18. Blackwell CC, Jónsdóttir K, Hanson M, et al. : Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet. 1986;2(8501):284–285. 10.1016/S0140-6736(86)92103-3 [DOI] [PubMed] [Google Scholar]
- 19. Smyth DJ, Cooper JD, Howson JM, et al. : FUT2 nonsecretor status links type 1 diabetes susceptibility and resistance to infection. Diabetes. 2011;60(11):3081–3084. 10.2337/db11-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellinghaus D, Ellinghaus E, Nair RP, et al. : Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Genet. 2012;90(4):636–647. 10.1016/j.ajhg.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang H, Jin X, Li Y, et al. : A large-scale screen for coding variants predisposing to psoriasis. Nat Genet. 2013;46(1):45. 10.1038/ng.2827 [DOI] [PubMed] [Google Scholar]
- 22. Jostins L, Ripke S, Weersma RK, et al. : Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGovern DP, Jones MR, Taylor KD, et al. : Fucosyltransferase 2 ( FUT2) non-secretor status is associated with Crohn's disease. Hum Mol Genet. 2010;19(17):3468–3476. 10.1093/hmg/ddq248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyd A, Golding J, Macleod J, et al. : Cohort Profile: The ‘Children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–127. 10.1093/ije/dys064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fraser A, Macdonald-Wallis C, Tilling K, et al. : Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. 10.1093/ije/dys066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pembrey M, ALSPAC Study Team: The Avon Longitudinal Study of Parents and Children (ALSPAC): a resource for genetic epidemiology. Eur J Endocrinol. 2004;151 Suppl 3:U125–9. [DOI] [PubMed] [Google Scholar]
- 27. Ferrer-Admetlla A, Sikora M, Laayouni H, et al. : A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26(9):1993–2003. 10.1093/molbev/msp108 [DOI] [PubMed] [Google Scholar]
- 28. Barrett JC, Hansoul S, Nicolae DL, et al. : Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40(8):955–62. 10.1038/ng.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kubota M, Takeuchi K, Watanabe S, et al. : Trisaccharide containing α2,3-linked sialic acid is a receptor for mumps virus. Proc Natl Acad Sci U S A. 2016;113(41):11579–11584. 10.1073/pnas.1608383113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishitoya S, Yamamoto S, Mitsumori K, et al. : Non-secretor status is associated with female acute uncomplicated pyelonephritis. BJU Int. 2002;89(9):851–4. 10.1046/j.1464-410X.2002.02782.x [DOI] [PubMed] [Google Scholar]
- 31. Lomberg H, Hellström M, Jodal U, et al. : Secretor state and renal scarring in girls with recurrent pyelonephritis. FEMS Microbiol Immunol. 1989;1(6–7):371–375. 10.1016/0378-1097(89)90260-7 [DOI] [PubMed] [Google Scholar]
- 32. Stapleton A, Nudelman E, Clausen H, et al. : Binding of uropathogenic Escherichia coli R45 to glycolipids extracted from vaginal epithelial cells is dependent on histo-blood group secretor status. J Clin Invest. 1992;90(3):965–72. 10.1172/JCI115973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stapleton AE, Stroud MR, Hakomori SI, et al. : The globoseries glycosphingolipid sialosyl galactosyl globoside is found in urinary tract tissues and is a preferred binding receptor In vitro for uropathogenic Escherichia coli expressing pap-encoded adhesins. Infect Immun. 1998;66(8):3856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]