Abstract
Background: Although nausea and vomiting are very common in pregnancy, their pathogenesis is poorly understood. We tested the hypothesis that circulating growth and differentiation factor 15 (GDF15) concentrations in early pregnancy, whose gene is implicated in hyperemesis gravidarum, are associated with nausea and vomiting.
Methods: Blood samples for the measurement of GDF15 and human chorionic gonadotrophin (hCG) concentrations were obtained early in the second trimester (median 15.1 (interquartile range 14.4-15.7) weeks) of pregnancy from 791 women from the Cambridge Baby Growth Study, a prospective pregnancy and birth cohort. During each trimester participants completed a questionnaire which included questions about nausea, vomiting and antiemetic use. Associations with pre-pregnancy body mass indexes (BMI) were validated in 231 pregnant NIPTeR Study participants.
Results: Circulating GDF15 concentrations were higher in women reporting vomiting in the second trimester than in women reporting no pregnancy nausea or vomiting: 11,581 (10,977-12,219) (n=175) vs. 10,593 (10,066-11,147) (n=193) pg/mL, p=0.02). In women who took antiemetic drugs during pregnancy (n=11) the GDF15 levels were also raised 13,157 (10,558-16,394) pg/mL (p =0.04). Serum GFD15 concentrations were strongly positively correlated with hCG levels but were inversely correlated with maternal BMIs, a finding replicated in the NIPTeR Study.
Conclusions: Week 15 serum GDF15 concentrations are positively associated with second trimester vomiting and maternal antiemetic use in pregnancy. Given GDF15’s site of action in the chemoreceptor trigger zone of the brainstem and its genetic associations with hyperemesis gravidarum, these data support the concept that GDF15 may be playing a pathogenic role in pregnancy-associated vomiting.
Keywords: antiemetics, nausea, obesity, pregnancy, maternal-fetal relations
Introduction
Nausea and vomiting in pregnancy (NVP) affects 70–90% of all pregnant women. The most severe form of NVP, hyperemesis gravidarum (HG), leads to maternal dehydration and electrolyte imbalance and is the most common cause of hospital admission during early pregnancy 1. Even though the majority of cases of NVP are mild or moderate with little impact upon maternal well-being, HG has substantial consequences for the mother’s quality of life 2, psychological morbidity 3, workplace productivity 4 and decreased caloric intake for the mother 5, 6. Furthermore, HG may have potential adverse effects on the developing fetus, as indicated by higher likelihood of low birth weight, preterm delivery, and small size at birth for gestational age in women with HG 7. While effective pharmacological interventions are available, there are concerns regarding possible fetal teratogenicity of some agents 8.
The pathogenesis of HG is poorly understood. Primiparity, younger maternal age, non-smoking 1 and being underweight 9– 11 may be risk factors. Reproductive hormones, such as human chorionic gonadotropin (hCG), progesterone and estrogen, have been implicated due to their rise in concentrations in the mother’s circulation contemporaneous with the manifestation of NVP 12. However nausea and vomiting are not common side-effects of such agents when administered in other settings, nor are increases in reproductive hormones consistently associated with increased HG severity or duration 13. A family history of HG leads to a 3-fold increase in HG among the female offspring 14, which has led to the hypothesis that it may be genetically driven. Recent studies of HG have tentatively implicated rare variants in TSHR, which encodes the thyrotropin receptor 15, and RYR2 16, which encodes a stress-induced intracellular calcium release channel in some familial cases. Evolutionary theories have been proposed for NVP as a beneficial strategy to protect the fetus from maternal ingestion of noxious substances, particularly during the early stages of pregnancy, coinciding with organogenesis, when the fetus is most vulnerable 12.
Growth and Differentiation Factor 15 (GDF15) signaling through its receptor (a heterodimer of proteins coded for by the GDNF family receptor α–like ( GFRAL) and Rearranged During Transfection ( RET) genes) has recently been identified to activate the mammalian chemoreceptor trigger zone of the medulla to suppress food intake in mice 17– 20 and primates 21. As such it therefore represents a potential mechanism for the aversion to foods and eating behaviors during periods of stress, sickness or high vulnerability to external toxins 12. In the non-pregnant state GDF15 is expressed at low levels in many tissues. In pregnancy, GDF15 is highly expressed in the placenta from early time points. In standard pregnancies circulating levels rise rapidly in maternal blood during the first trimester of pregnancy and remain elevated until delivery 22. A genome wide association study has recently shown that variants in and around the GDF15 locus are strongly associated with the risk of HG in pregnancy 23.
To explore the hypothesis that NVP might relate to circulating GDF15 levels, we measured serum GDF15 in Cambridge Baby Growth Study samples obtained from women who had been prospectively followed throughout their pregnancies. They had answered questionnaires in each of the three trimesters which had incorporated questions regarding nausea, vomiting and antiemetic use. As previous research has variably implicated hCG in the pathogenesis of NVP 24 we examined the relationships between hCG levels, NVP symptoms and GDF15 concentrations in those women in whom these measures were available. As there have been reports that low pre-pregnancy body mass index (BMI) predisposes to NVP 9 we also examined the relationship between pre-pregnancy BMI, GDF15 levels and NVP.
Methods
Cohort 1: Cambridge Baby Growth Study
The prospective Cambridge Baby Growth Study recruited 2,229 mothers (and their partners and offspring) attending antenatal ultrasound clinics during early pregnancy at the Rosie Maternity Hospital, Cambridge, United Kingdom, between 2001-9 25. All mothers were over 16 years of age. Pre-pregnancy weight and height were self-reported. In this cohort, 96.9% of the offspring were of white ethnicity, 0.8% were of mixed race, 0.6% were black (African or Caribbean), 0.8% were East-Asian, and 0.9% were Indo-Asian. Research blood samples, from which serum was separated and aliquoted, were collected from 1,177 (52.8%) mothers at recruitment (median 15.0 weeks, interquartile range 1.6 weeks). Around week 14 of pregnancy the participants were offered the chance to have routine blood taken for the measurement of serum alpha-fetoprotein (AFP), hCG and unconjugated estriol (uE3) as the pre-natal screening triple test.
Each mother was given a printed questionnaire at recruitment to fill in and return after the birth of their child 26. The participants were encouraged to fill their questionnaire in as their pregnancy progressed. It included boxes to tick if the participants had experienced NVP during pregnancy 27. If either the nausea or vomiting boxes was ticked there were further boxes to complete concerning the timing (i.e. week(s) of pregnancy) when the nausea or vomiting was experienced. An additional question asked “Have you taken any medicine during this pregnancy?” and a table was provided for positive responses with the following headings: “Name”, “Disease”, “Daily Dose”, “No. of Days” and “Gestational Week(s)”. A total of 1,238 women (54.6%) returned a questionnaire. Of these, only 3 self-reported that they had HG and a further 17 reported treatment with an antiemetic agent: cyclizine (n=7), promethazine (n=5), prochlorperazine (n=4), metoclopramide (n=2), domperidone (n=2), prednisolone (n=2), chlorphenamine (n=1), ondansetron (n=1), chlorpromazine (n=1) and unknown (n=1). The timing of NVP was categorized into trimesters (first: up to gestational week 12; second: 13 to 27 weeks; third: 28+ weeks).
The current analysis was based on 791 women in the Cambridge Baby Growth Study who had an available serum sample collected between gestational age 12 and 18 weeks and returned a completed questionnaire 26, 27. Of these there were only 11 women who reported taking antiemetics during pregnancy. These women were representative of the whole cohort by having similar maternal pre-pregnancy BMIs, parities and offspring birth weights (adjusted for standard confounders) to those women who did not return a pregnancy questionnaire ( Supplementary Table 1).
Cohort 2: NIPTeR Study
The Non-Invasive Prenatal RNA profiling in pregnancy (NIPTeR) study was set up to research the early detection of preeclampsia before symptoms emerge. Included were pregnant women of at least 18 years old at their first antenatal visit. Enrolment took place between September 2015 and November 2017 at the Academic Medical Center, Amsterdam, The Netherlands. Antiemetic use, history of hospital admissions for HG, and pre-pregnancy weight and height were retrieved from the medical charts. In addition to the routine blood samples, a blood sample for the NIPTeR Study was taken. Within 6 hours of blood collection plasma was isolated by a 2-step protocol: first a low speed platelet-rich plasma separation, followed by a general high-speed step for clearance of all cells.
The current analysis was based on data from 231 women whose blood was sampled between 10 and 18 weeks of pregnancy who did not report developing HG/antiemetic use.
Ethics
The Cambridge Baby Growth Study was approved by the Cambridge Research Ethics Committee, Cambridge, United Kingdom (LREC 00/325). All procedures followed were in accordance with Good Clinical Practice guidelines. Written informed consent was obtained from all the study participants. The NIPTeR study was approved by the Academic Medical Center ethics committee (reference 2015_072) and all participating women provided written informed consent.
Assays
GDF15 concentrations were measured in serum (Cambridge Baby Growth Study) and EDTA plasma (NIPTeR) using an in-house Meso Scale Discovery electrochemiluminescence immunoassay (Meso Scale Diagnostics, Rockville, Maryland, U.S.A.) developed using antibodies from R & D Systems Quantikine reagents (BioTechne Ltd., Abingdon, U.K.). The sensitivity of this assay was 3 pg/mL and the working range went up to 32,000 pg/mL. Batch-to-batch variability was 9.8% at 352 pg/mL, 8.1% at 1490 pg/mL and 7.8% at 6667 pg/mL. Pre-natal screening assays were performed using routine AutoDELFIA time-resolved fluoroimmunoassays (PerkinElmer Life Sciences, Wallac Oy, Turku) and the results were expressed as multiples of the median (MOM) 28.
Statistical analysis
Women in the Cambridge Baby Growth Study were categorized into one of three groups: vomiting (independent of whether they reported having experienced nausea or not); nausea but no vomiting; and no nausea or vomiting 27. The primary outcome was vomiting during the second trimester, as this coincided with the timing of maternal serum sampling. These were compared to concentrations in women who reported no nausea or vomiting. Maternal pre-pregnancy BMI was calculated dividing body weight prior to pregnancy by height-squared. NIPTeR Study samples were used to validate the relationship between GDF15 and BMI.
Serum GDF15 concentrations were natural logarithm-transformed to achieve a normal distribution and were considered as the dependent variable in linear regression models with adjustment for gestational age at serum sample collection. Where the relationship with adjusted log-transformed GDF15 concentrations did not appear to be linear, data were transformed to approximate linearity prior to analysis (e.g. the reciprocal BMI was used). Statistical analyses were performed using Stata 13.1 (StataCorp LP, College Station, Texas, U.S.A.). P<0.05 was considered to indicate statistical significance.
Results
Maternal nausea and vomiting in pregnancy
37.7% (n=298) of the Cambridge Baby Growth Study women reported vomiting during any trimester of pregnancy. A further 37.9% (n=300) reported nausea but no vomiting, and only 24.4% (n=193) of the Cambridge Baby Growth Study women reported no nausea or vomiting. More women (32.0%, n=253) reported vomiting during the first trimester compared to 22.1% (n=175) in the second trimester, with only 3.8% (n=30) in the third trimester. 86.9% and 56.7% of those reporting vomiting in the second and third trimesters also reported vomiting during the first trimester, respectively. Women who reported vomiting during the second trimester were younger and were carrying relatively more female babies than women who reported no nausea or vomiting during pregnancy ( Table 1), but there were no differences in pre-pregnancy BMI.
Table 1. Clinical characteristics of the women who reported vomiting during the second trimester of pregnancy.
This table shows comparisons of clinical characteristics between those women that reported vomiting during the second trimester of pregnancy and those women that reported no nausea or vomiting throughout pregnancy in the Cambridge Baby Growth Study. Those women who reported second trimester vomiting were very slightly younger and were carrying a higher proportion of female babies. There were no apparent differences in BMI, parity or prevalence of twin pregnancies however.
| Vomiting (2 nd trimester) | No nausea or vomiting | p-value | |
|---|---|---|---|
| n | 175 | 193 | |
| Age at delivery (years) | 32.8 (32.1-33.5) | 33.7 (33.1-34.3) | 0.047 |
| BMI (kg/m 2) | 23.9 (23.2-24.5) | 23.9 (23.3-24.6) | 1.0 |
| Parity (n primiparous (%)) | 84 (48.0%) | 109 (56.5%) | 0.1 |
| Offspring Sex (n females (%)) | 96 (54.9%) | 81 (42.28%) | 0.02 |
| Twin pregnancies | 2 | 0 | 0.2 |
The comparator group are women who reported no nausea or vomiting during pregnancy. Data are geometric means (95% confidence intervals) or numbers of participants.
Maternal GDF15 concentrations
In the Cambridge Baby Growth Study the median GDF15 concentration was 11,004 pg/mL (range 2,378–34,621) in serum samples collected at mean gestational age 15.1 weeks (range 12.0–18.0). Maternal GDF15 concentrations were not associated with gestational age at sampling (linear model with log-GDF15: P=0.4, standardized β=-0.03). In the NIPTeR Study the median GDF15 concentration was 11,014 pg/mL (range 4,106–37,194, n=233) in plasma samples collected at mean gestational age 12.1 weeks (range 10.0–16.1).
Maternal GDF15 concentrations and associations with nausea and vomiting in pregnancy
Maternal GDF15 concentrations around week 15 were higher in women who reported vomiting in the second trimester of pregnancy compared to those who reported no nausea or vomiting during pregnancy (P=0.02; Table 2). This association was unaltered by adjustment for gestation at serum sampling or by maternal BMI. There was no significant elevation of GDF15 concentrations in women reporting nausea alone in the second trimester or in women reporting nausea or vomiting in the first or third trimesters ( Supplementary Table 2).
Table 2. Maternal GDF15 concentrations by self-reported vomiting in the second trimester or antiemetic use during pregnancy.
This table shows comparisons of circulating maternal GDF15 concentrations around week 15 of pregnancy in those women who reported nausea alone or vomiting in the second trimester of pregnancy, those women who reported taking antiemetics during pregnancy and those women who reported no nausea or vomiting in pregnancy in the Cambridge Baby Growth Study. These concentrations were raised in women who reported vomiting whether unadjusted or adjusted for gestational age without or without BMI. Adjusted levels were also higher in women who took antiemetics during pregnancy. No apparent differences were observed in women who reported nausea alone.
| Group | n | Serum GDF15
Concentration (pg/mL) |
Unadjusted | Adjusted for
gestational age |
Additionally adjusted
for maternal BMI |
|---|---|---|---|---|---|
| No nausea or vomiting | 193 | 10,593 (10,066-11,147) | Ref | Ref | Ref |
| Nausea without vomiting
(second trimester) |
325 | 10,772 (10,328-11,235) | P=0.6 | P=0.6 | P=0.5 |
| Vomiting (second
trimester) |
175 | 11,581 (10,977-12,219) | P=0.02 | P=0.02 | P=0.02 |
| Antiemetic use (any
trimester) |
11 | 13,157 (10,558-16,394) | P=0.06 | P=0.04 | P=0.04 |
Data are geometric means (95% confidence intervals).
Eleven women (1.4%) took antiemetics, ten of whom reported vomiting and one of whom reported nausea without vomiting. Their serum GDF15 concentrations were also raised compared to women who reported no nausea or vomiting during pregnancy (P=0.04, adjusted for gestation; Table 2).
Maternal GDF15 concentrations and associations with prenatal screening markers
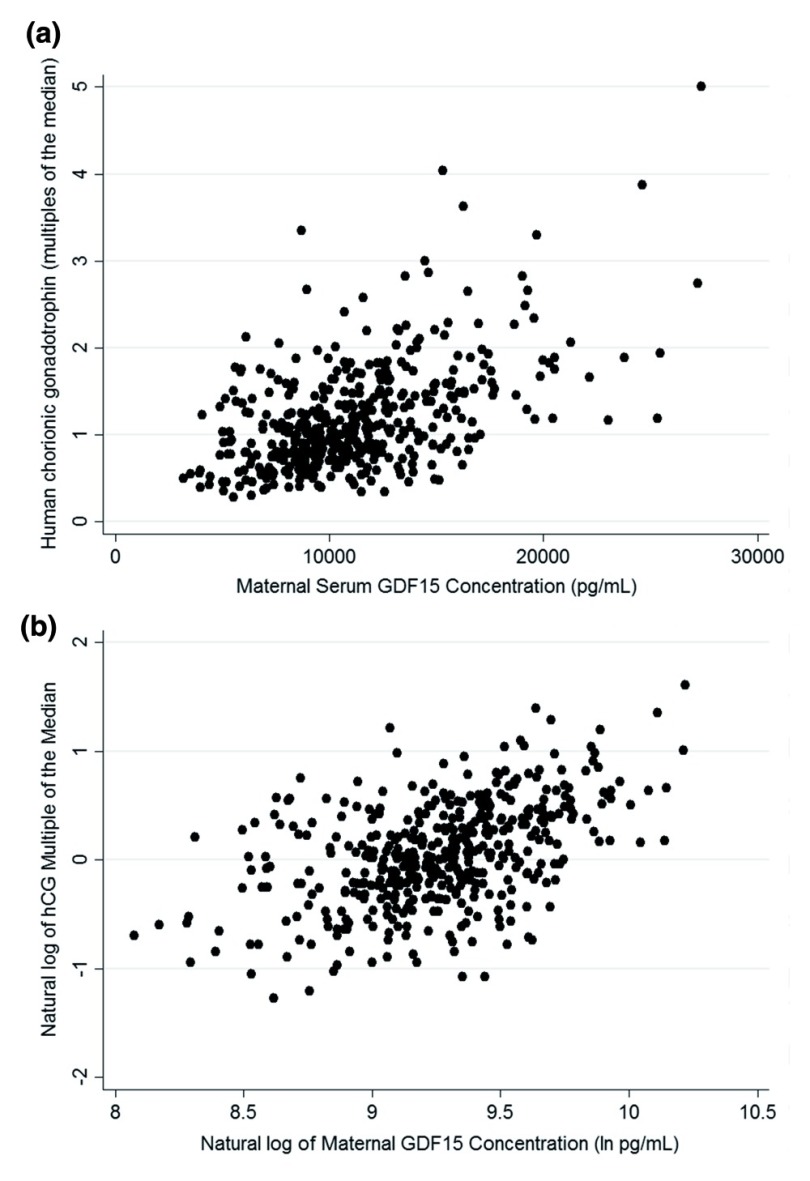
A subset of participants (441) had undergone a 14 week triple test at ~14 weeks gestation measuring AFP, estriol and hCG. Maternal serum GDF15 concentrations were not associated with AFP (standardized β=0.059, P=0.2, n=441). There was a weak positive association with unconjugated estriol (standardized β=0.110, P=0.02, n=440). In contrast there was a strong positive association with hCG ( Figure 1; standardized β=0.436, P=7.2×10 -22, n=441). In contrast to the GDF15 data however, hCG levels were not significantly higher in women reporting vomiting in the second trimester of pregnancy: no nausea or vomiting 1.06 (0.96-1.17) (n=119) v. 1.17 (1.04-1.31) (n=91) (P=0.2).
Figure 1. The relationship between week 15 maternal serum GDF15 concentrations and week 14 hCG MOMs.
( a) A scatter plot of untransformed GDF15 concentration and hCG MOM data from around weeks 14–15 of pregnancy in the Cambridge Baby Growth Study, ( b) a scatter plot of logarithmically-transformed data from the same cohort.
Maternal GDF15 concentrations and associations with pre-pregnancy BMI
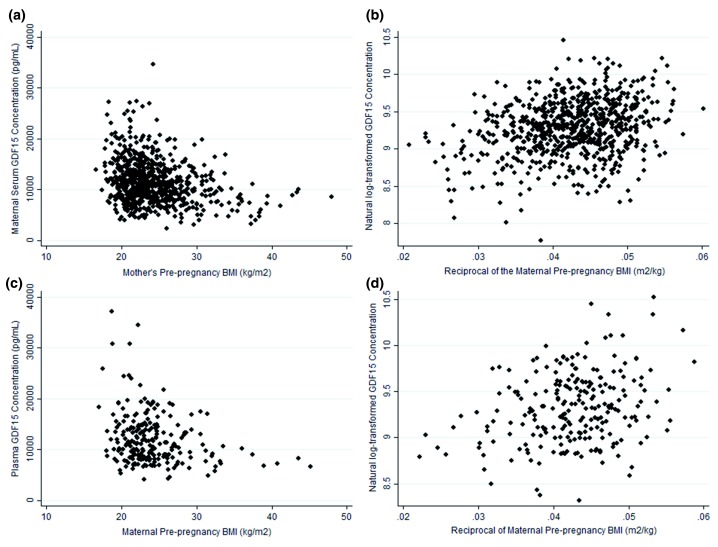
In the Cambridge Baby Growth Study the distribution of the relationship of week 15 GDF15 concentrations and maternal pre-pregnancy BMI was asymptotic ( Figure 2a), with higher GDF15 levels being seen exclusively in leaner mothers. The data were analyzed using log transformation of GDF15 levels ( Figure 2b) and a highly significant relationship with the reciprocal of pre-pregnancy BMI was apparent (standardized β=0.266, p=4.1×10 -13, n=721).This relationship was replicated in the NIPTeR Study ( Figure 2c & d; standardized β=0.280, p=1.5×10 -5, n=231).
Figure 2. The relationship between maternal serum GDF15 concentrations around week 15 of pregnancy and pre-pregnancy BMIs.
( a) A scatter plot of untransformed GDF15 concentrations from around week 15 of pregnancy and pre-pregnancy BMI data from the Cambridge Baby Growth Study, ( b) a scatter plot of transformed data from the same cohort, ( c) a scatter plot of untransformed GDF15 and BMI data from the NIPTeR Study and ( d) a scatter plot of transformed data from the same study.
Discussion
In this large prospective pregnancy cohort study, maternal circulating GDF15 concentrations around week 15 of pregnancy were higher in women who reported vomiting in the second trimester and were even higher in women who reported taking antiemetics during pregnancy, compared to those of women who reported no nausea or vomiting during pregnancy. The results from the women who took antiemetics during pregnancy probably reflect the severity of their symptoms rather than their treatment. We also found that week 15 GDF15 concentrations were related to maternal pre-pregnancy BMIs, with the highest circulating GDF15 concentrations found in mothers with the lowest BMIs.
To our knowledge, this is the first report relating GDF15 concentrations to vomiting during pregnancy. Circulating GDF15 concentrations rise rapidly in maternal blood during early pregnancy and several studies have reportedly substantially lower concentrations at around 6–13 weeks gestation in those pregnancies that subsequently miscarried 29, 30. Possible explanations for this highly reproducible phenomenon have included the suggestion that maternal circulating GDF15 is a biomarker of successful placentation. Alternatively it has been suggested that GDF15 may promote fetal viability through an immunomodulatory action 31. However the recent discovery of the highly specific expression of the receptor for GDF15 in the hindbrain makes this less likely 32. Despite that uncertainty, our findings provide a possible mechanistic explanation for the widely observed associations between NVP and lower rates of miscarriage 33.
There are at least three possible interpretations of the inverse association between serum GDF15 concentrations and maternal pre-pregnancy BMI which we found in two independent studies. It is possible that those women who develop high levels of GDF15 in pregnancy have an intrinsic tendency to be GDF15 overproducers. Even in the non-pregnant state, given the known effects of this hormone on appetite 34 this could be directly related to their low weight. Such a hypothesis would need to take into account the fact that a substantial amount of the GDF15 found in maternal blood is likely to be secreted by the trophoblast, which is fetally-encoded and only shares ~50% genetic identity with the mother. An alternative interpretation is that women with a low pre-pregnancy BMI are particularly vulnerable to the stressful effects of pregnancy. Consistent with this GDF15 appears to be overproduced by a variety of tissues in response to different stress states, including undernutrition 35. Finally our findings could be consistent with the idea that early placentation is less successful in women with higher BMIs. This notion is supported by the fact that there is a graded increase in pregnancy loss with increasing maternal BMI 36, as well as an increase in maternal placental syndromes, including preeclampsia and gestational hypertension as maternal BMI increases 37. However this also has to be contrasted with the increased birth weights of babies born to women with high BMIs.
In the current study we found a remarkably strong association between GDF15 concentrations around week 15 of pregnancy and week 14 hCG levels. Both are major endocrine products of the placenta and it is likely that they at least in part reflect the functional mass of placenta. Fewer women has hCG measured in our study which may explain why we did not demonstrate a relationship of hCG levels and symptoms. Although it the most widely implicated hormone thought to stimulate NVP, hCG’s link to NVP is inconsistent 13 and largely relates to associations between the timing of changes in its concentrations and NVP symptoms rather than to a known pathogenesis 24. Given that there is a potential mechanism linking GDF15 concentrations in pregnancy with vomiting 38, the results from the present study raise the possibility that GDF15 is actually the causal factor (or at least one of them) and that reported associations between hCG and NVP 24 actually reflect GDF15 concentrations and bioactivity. These findings require confirmation in other studies.
Although we have uniquely shown associations between circulating GDF15 concentrations and vomiting in pregnancy, pre-pregnancy BMI and circulating hCG levels, this study has a number of limitations. Ideally, we would have measured maternal GDF15 concentrations in samples collected at gestational age 9 weeks, which is the peak for NVP symptoms 39. However, many women have not yet presented to maternal health services at that stage, and indeed for many women NVP represents the first indication of pregnancy. A further limitation is that maternal BMI was only available pre-pregnancy although weight is unlikely to have changed much during the initial 15 weeks of pregnancy. Finally and unsurprisingly, reflecting recruitment of the cohort at routine antenatal clinics, cases of HG were under-represented. Future case-control study designs are therefore needed to test whether our findings can be extrapolated to HG.
On the basis of a substantial body of recently emerging data we have previously proposed that the role of GDF15 in the adult organism is to provide a signal to the brain that the organism is engaging in damaging behavior 38. Its hindbrain-localized receptor activates a signal which is likely to be aversive and promote the future avoidance of this particular behavior. We propose that the placenta has evolved to use the GDF15 system to promote a state in which the mother is sensitized to other adverse stimuli, particularly those that might come from food, in order to protect the fetus from exposure to maternal ingestion of potential teratogens during the vulnerable stages of organ development. In the context of the recently revealed biology of GDF15 these data suggest that antagonism of GDF15 may have some potential for therapeutic benefit in NVP.
Data availability
Open Science Framework: Data for associations of vomiting and antiemetic use in pregnancy with levels of circulating GDF15 early in the second trimester: A nested case-control study. https://doi.org/10.17605/OSF.IO/5JT3K 40.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Data are present from both the Cambridge Baby Growth and the NIPTeR Studies, merged from the individual central record databases for each study. Anonymized data for the Cambridge Baby Growth study are available to other investigators through collaborative agreements, and the co-investigators welcome formal or informal proposals and will consider these at their bimonthly meetings. Please contact Dr Carlo Acerini [ cla22@cam.ac.uk]. Concerning NIPTeR study data, please contact Dr. Gijs Afink [ g.b.afink@amc.uva.nl].
Acknowledgments
We thank all the families who took part in the Cambridge Baby Growth Study, and we acknowledge the crucial role played by the research nurses especially Suzanne Smith, Ann-Marie Wardell and Karen Forbes, staff at the Addenbrooke’s Wellcome Trust Clinical Research Facility, and midwives at the Rosie Maternity Hospital. We also thank Dianne Wingate for expert assistance in sample preparation and Ashley Clarke for developing and validating the GDF15 immunoassay. In addition, we would like to acknowledge the contribution of all clinicians and lab technicians that took care of the inclusions and sample preparation for the NIPTeR study. In particular Vera Manders for initiating the NIPTeR study and Naomi Min for managing the clinical database.
An earlier version of this article is available on BioRxiv as a preprint https://doi.org/10.1101/221267.
Funding Statement
This work was supported by the Wellcome Trust [100574; Strategic Award]; and an unrestricted award from the Novo Nordisk Foundation (International Prize for Excellence in diabetes research) (both SOR). The Cambridge Baby Growth Study has been funded by the Medical Research Council (7500001180) (CLA), European Union Framework 5 (QLK4-1999-01422) (IAH), the Mothercare Foundation (RG54608) (IAH), Newlife Foundation for Disabled Children (07/20) (IAH), and the World Cancer Research Fund International (2004/03) (DBD). It is also supported by the National Institute for Health Research Cambridge Biomedical Research Centre. KKO and JRB are supported by the Medical Research Council (Unit Programme MC_UU_12015/2). The NIPTeR study was in part supported by an AMC-VUMC Alliance grant.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
Supplementary material
Supplementary Tables: Clinical characteristics of women in the Cambridge Baby Growth Study who filled in and returned their questionnaires in comparison to those that did not, and week 15 GDF15 concentrations in women who reported nausea or vomiting in the first or third trimesters of pregnancy are appended.
References
- 1. Jarvis S, Nelson-Piercy C: Management of nausea and vomiting in pregnancy. BMJ. 2011;342:d3606. 10.1136/bmj.d3606 [DOI] [PubMed] [Google Scholar]
- 2. Wood H, McKellar LV, Lightbody M: Nausea and vomiting in pregnancy: blooming or bloomin' awful? A review of the literature. Women Birth. 2013;26(2):100–104. 10.1016/j.wombi.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell-Jones N, Gallos I, Farren J, et al. : Psychological morbidity associated with hyperemesis gravidarum: a systematic review and meta-analysis. BJOG. 2017;124(1):20–30. 10.1111/1471-0528.14180 [DOI] [PubMed] [Google Scholar]
- 4. Trovik J, Vikanes Å: Hyperemesis Gravidarum is associated with substantial economic burden in addition to severe physical and psychological suffering. Isr J Health Policy Res. 2016;5:43. 10.1186/s13584-016-0099-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birkeland E, Stokke G, Tangvik RJ, et al. : Norwegian PUQE (Pregnancy-Unique Quantification of Emesis and nausea) identifies patients with hyperemesis gravidarum and poor nutritional intake: a prospective cohort validation study. PLoS One. 2015;10(4):e0119962. 10.1371/journal.pone.0119962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Stuijvenberg ME, Schabort I, Labadarios D, et al. : The nutritional status and treatment of patients with hyperemesis gravidarum. Am J Obstet Gynecol. 1995;172(5):1585–1591. 10.1016/0002-9378(95)90501-4 [DOI] [PubMed] [Google Scholar]
- 7. Veenendaal MV, van Abeelen AF, Painter RC, et al. : Consequences of hyperemesis gravidarum for offspring: a systematic review and meta-analysis. BJOG. 2011;118(11):1302–1313. 10.1111/j.1471-0528.2011.03023.x [DOI] [PubMed] [Google Scholar]
- 8. Koren G: Safety considerations surrounding use of treatment options for nausea and vomiting in pregnancy. Expert Opin Drug Saf. 2017;16(11):1227–1234. 10.1080/14740338.2017.1361403 [DOI] [PubMed] [Google Scholar]
- 9. Ben-Aroya Z, Lurie S, Segal D, et al. : Association of nausea and vomiting in pregnancy with lower body mass index. Eur J Obstet Gynecol Reprod Biol. 2005;118(2):196–198. 10.1016/j.ejogrb.2004.04.026 [DOI] [PubMed] [Google Scholar]
- 10. Roseboom TJ, Ravelli AC, van der Post JA, et al. : Maternal characteristics largely explain poor pregnancy outcome after hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):56–59. 10.1016/j.ejogrb.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 11. Fiaschi L, Nelson-Piercy C, Tata LJ: Hospital admission for hyperemesis gravidarum: a nationwide study of occurrence, reoccurrence and risk factors among 8.2 million pregnancies. Hum Reprod. 2016;31(8):1675–1684. 10.1093/humrep/dew128 [DOI] [PubMed] [Google Scholar]
- 12. Patil CL, Abrams ET, Steinmetz AR, et al. : Appetite sensations and nausea and vomiting in pregnancy: an overview of the explanations. Ecol Food Nutr. 2012;51(5):394–417. 10.1080/03670244.2012.696010 [DOI] [PubMed] [Google Scholar]
- 13. Niemeijer MN, Grooten IJ, Vos N, et al. : Diagnostic markers for hyperemesis gravidarum: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211(2):150.e1–15. 10.1016/j.ajog.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 14. Vikanes A, Skjaerven R, Grjibovski AM, et al. : Recurrence of hyperemesis gravidarum across generations: population based cohort study. BMJ. 2010;340:c2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coulon AL, Savagner F, Briet C, et al. : Prolonged and Severe Gestational Thyrotoxicosis Due to Enhanced hCG Sensitivity of a Mutant Thyrotropin Receptor. J Clin Endocrinol Metab. 2016;101(1):10–11. 10.1210/jc.2015-3670 [DOI] [PubMed] [Google Scholar]
- 16. Fejzo MS, Myhre R, Colodro-Conde L, et al. : Genetic analysis of hyperemesis gravidarum reveals association with intracellular calcium release channel (RYR2). Mol Cell Endocrinol. 2017;439:308–316. 10.1016/j.mce.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mullican SE, Lin-Schmidt X, Chin CN, et al. : GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017;23(10):1150–1157. 10.1038/nm.4392 [DOI] [PubMed] [Google Scholar]
- 18. Emmerson PJ, Wang F, Du Y, et al. : The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017;23(10):1215–1219. 10.1038/nm.4393 [DOI] [PubMed] [Google Scholar]
- 19. Yang L, Chang CC, Sun Z, et al. : GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017;23(10):1158–1166. 10.1038/nm.4394 [DOI] [PubMed] [Google Scholar]
- 20. Hsu JY, Crawley S, Chen M, et al. : Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550(7675):255–259. 10.1038/nature24042 [DOI] [PubMed] [Google Scholar]
- 21. Xiong Y, Walker K, Min X, et al. : Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Sci Transl Med. 2017;9(412): pii: eaan8732. 10.1126/scitranslmed.aan8732 [DOI] [PubMed] [Google Scholar]
- 22. Moore AG, Brown DA, Fairlie WD, et al. : The transforming growth factor-ss superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J Clin Endocrinol Metab. 2000;85(12):4781–4788. 10.1210/jcem.85.12.7007 [DOI] [PubMed] [Google Scholar]
- 23. Fejzo MS, Sazonova OV, Sathirapongsasuti JF, et al. : Placenta and appetite genes GDF15 and IGFBP7 are associated with hyperemesis gravidarum. Nat Commun. 2018;9(1):1178. 10.1038/s41467-018-03258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bustos M, Venkataramanan R, Caritis S: Nausea and vomiting of pregnancy - What's new? Auton Neurosci. 2017;202:62–72. 10.1016/j.autneu.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petry CJ, Ong KK, Hughes IA, et al. : Early Pregnancy-Associated Plasma Protein A Concentrations Are Associated With Third Trimester Insulin Sensitivity. J Clin Endocrinol Metab. 2017;102(6):2000–2008. 10.1210/jc.2017-00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petry CJ, Ong KK, Hughes IA, et al. : Associations between bacterial infections and blood pressure in pregnancy. Pregnancy Hypertens. 2017;10:202–206. 10.1016/j.preghy.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petry CJ, Ong KK, Beardsall K, et al. : Vomiting in pregnancy is associated with a higher risk of low birth weight: a cohort study. BMC Pregnancy Childbirth. 2018;18(1):133. 10.1186/s12884-018-1786-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Babbur V, Lees CC, Goodburn SF, et al. : Prospective audit of a one-centre combined nuchal translucency and triple test programme for the detection of trisomy 21. Prenat Diagn. 2005;25(6):465–469. 10.1002/pd.1163 [DOI] [PubMed] [Google Scholar]
- 29. Tong S, Marjono B, Brown DA, et al. : Serum concentrations of macrophage inhibitory cytokine 1 (MIC 1) as a predictor of miscarriage. Lancet. 2004;363(9403):129–130. 10.1016/S0140-6736(03)15265-8 [DOI] [PubMed] [Google Scholar]
- 30. Tong S, Ngian GL, Onwude JL, et al. : Diagnostic accuracy of maternal serum macrophage inhibitory cytokine-1 and pregnancy-associated plasma protein-A at 6-10 weeks of gestation to predict miscarriage. Obstet Gynecol. 2012;119(5):1000–1008. 10.1097/AOG.0b013e3182518fd3 [DOI] [PubMed] [Google Scholar]
- 31. Corre J, Hébraud B, Bourin P: Concise review: growth differentiation factor 15 in pathology: a clinical role? Stem Cells Transl Med. 2013;2(12):946–952. 10.5966/sctm.2013-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Segerer SE, Rieger L, Kapp M, et al. : MIC-1 (a multifunctional modulator of dendritic cell phenotype and function) is produced by decidual stromal cells and trophoblasts. Hum Reprod. 2012;27(1):200–209. 10.1093/humrep/der358 [DOI] [PubMed] [Google Scholar]
- 33. Koren G, Madjunkova S, Maltepe C: The protective effects of nausea and vomiting of pregnancy against adverse fetal outcome--a systematic review. Reprod Toxicol. 2014;47:77–80. 10.1016/j.reprotox.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 34. Tsai VW, Lin S, Brown DA, et al. : Anorexia-cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15. Int J Obes (Lond). 2016;40(2):193–197. 10.1038/ijo.2015.242 [DOI] [PubMed] [Google Scholar]
- 35. Zhang M, Sun W, Qian J, et al. : Fasting exacerbates hepatic growth differentiation factor 15 to promote fatty acid β-oxidation and ketogenesis via activating XBP1 signaling in liver. Redox Biol. 2018;16:87–96. 10.1016/j.redox.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaskins AJ, Rich-Edwards JW, Colaci DS, et al. : Prepregnancy and early adulthood body mass index and adult weight change in relation to fetal loss. Obstet Gynecol. 2014;124(4):662–669. 10.1097/AOG.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rahman MM, Abe SK, Kanda M, et al. : Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: a systematic review and meta-analysis. Obes Rev. 2015;16(9):758–770. 10.1111/obr.12293 [DOI] [PubMed] [Google Scholar]
- 38. O'Rahilly S: GDF15-From Biomarker to Allostatic Hormone. Cell Metab. 2017;26(6):807–808. 10.1016/j.cmet.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 39. Gadsby R, Barnie-Adshead AM, Jagger C: A prospective study of nausea and vomiting during pregnancy. Br J Gen Pract. 1993;43(6):245–248. [PMC free article] [PubMed] [Google Scholar]
- 40. Petry C: Data for GDF15 paper. 2018. http://www.doi.org/10.17605/OSF.IO/5JT3K [Google Scholar]