Abstract
Background
In 1988, 1 of 3 women (W) and heterosexual men living with human immunodeficiency virus (HIV) reported wanting children, but little is known about parenting desires of men who have sex with men (MSM) living with HIV. We examined parenting desires among persons initiating antiretroviral therapy (ART).
Methods
Of 1809 participants in the AIDS Clinical Trials Group (ACTG) Study 5257, 1425 W aged ≤45 years or men completed questionnaires about parenting desires at baseline and 96 weeks after initiating ART. Self-reported desires for children in the future (yes/unsure vs no) and associations between baseline sociodemographics and parenting desires at 96 weeks were examined using multivariable logistic regression, overall and within subgroups.
Results
The 1425 participants were as follows: 36% white, 39% black, 22% Hispanic; median age 36 (interquartile range, 28–44); 70% MSM, 13% men reported sex only with W (MSW), 17% W. At baseline, 42% may want children in the future (42% MSM, 37% MSW, 43% W); at 96 weeks, 41% may want children (41% MSM, 37% MSW, 43% W). At follow-up, approximately 10% of responses changed in each direction. In multivariable analyses, education greater than high school, <30 years, and having no children were significantly associated with future parenting desires among all subgroups. Among MSM, being black was associated with desiring children.
Conclusions
Approximately 40% of MSM, W, and MSW with HIV may want children, both at baseline and 96 weeks after ART initiation. These results highlight the need to regularly assess parenting goals, provide access to comprehensive reproductive services, and address prevention of vertical and heterosexual HIV transmission.
Keywords: ART, HIV, parenting, reproductive health, women
Little is known about parenting desires among individuals currently living with human immunodeficiency virus (HIV) in the United States, making it difficult to address their parenting goals. Furthermore, despite limited information regarding parenting intent, assumptions of lack of interest in having children are common among people living with HIV (PLWH), particularly for men who have sex with men (MSM). Parenting intention may be influenced by many factors, including perceived well being, life expectancy, fear of perinatal HIV transmission, and attitudes about pregnancy among HIV care providers, families, and communities.
Recent data on parenting intent among HIV-positive heterosexual men who have sex with women (MSW) and women in the modern combination antiretroviral therapy (ART) era are limited, and even less is known for MSM living with HIV. In the early years of highly effective ART, Chen et al [1] studied a nationally representative probability sample of women and men living with HIV aged 20–44 in the United States in 1998, but men who only have sex with men were not included. Overall, 28%–29% of this population living with HIV receiving care desired children in the future. For MSM living with HIV, a recent qualitative study reported that men wanted more discussions with providers and services available to address their parenting desires [2]. More recent studies have suggested that among women receiving HIV care in the modern ART era, parenting intent may be more prevalent than these prior estimates suggest [3–6].
Because individuals are living longer, healthier lives with modern ART, their parenting goals may change to become more similar to those of uninfected individuals. In the United States, pregnancy and live-birth rates among women living with HIV have increased, with rates now being similar to those of uninfected women [7]. Furthermore, higher pregnancy rates among women living with HIV are associated with ART use and improved immune status. These results suggest that current parenting intent may be similar to uninfected counterparts and highlight the need for understanding the drivers of parenting desire among women and men with HIV to appropriately address their healthcare needs. Data from a nationally representative sample of childless lesbian, gay, and heterosexual individuals not living with HIV found that heterosexual men and women desired children in the future at higher rates than their gay counterparts [8]. Because provider discussions on parenting goals are often neglected in HIV care [9], there are many missed opportunities to target contraceptive or safe conception counseling, which are effective in reducing both unintended pregnancy and HIV transmission to uninfected partners and children.
To optimize the reproductive health of this population, we sought to understand key factors affecting parenting goals. We examined the parenting desires among men and women living with HIV initiating 1 of 3 modern ART regimens as part of an open-label clinical trial. Focusing on the ART-naive adults enrolling into a prospective antiretroviral study can provide a window in which to explore the impact of ART initiation and early use within a prospective longitudinal study on parenting intentions.
METHODS
Study Population, Design, and Data Collection
We included 1425 (women aged ≤45 years and all men who completed necessary questionnaires) of the 1809 participants enrolled between May 22, 2009 and June 9, 2011 in the AIDS Clinical Trials Group (ACTG) Study 5257, a Phase 3, randomized, open-label trial of 3 modern ART regimens enrolling ART-naive men and women 18 years and older from 57 clinical sites across the United States and Puerto Rico [10]. Study evaluations were completed before entry, at entry, at weeks 4, 8, 16, 24, and 32 and every 16 weeks thereafter. Self-reported, prospectively collected data were analyzed from baseline to 96 weeks after initiating ART, including detailed questions on parenting desires and reproductive choices as well as sociodemographic, clinical, and psychosocial factors collected at baseline, week 48, and week 96.
Objectives, Variable Definitions, and Statistical Analysis
The primary objective was to describe parenting desires among women (W), MSM, and MSW enrolled in ACTG 5257. We defined MSM as men who reported ever having had sex with a man; MSW as men who have never had sex with a man; and W as all women enrolled. The primary outcome was desire to have more children (for example, make a baby or adopt/foster a baby/child) in the future (yes/unsure versus no), as reported at baseline and also at 96 weeks after ART initiation. Because approximately half of pregnancies in the United States (and globally) are unintended [11], we chose to compare those participants who did not want to have children in the future to those who either did want children or may want children in the future (no versus yes/unsure). Potential correlates of parenting desire were evaluated, including self-reported sociodemographic factors (age, education, race/ethnicity, income, insurance, housing, prior children), alcohol and substance use, self-perceived perceptions of infectiousness (visual analog scale from 0 (noninfectious) to 100 [highly infectious]), and HIV viral suppression. Binge drinking was defined as consuming ≥5 drinks of alcohol for men and ≥4 drinks of alcohol for women at least once in the prior month.
For this analysis, the proportion of participants reporting parenting desires was estimated at baseline and at follow-up week 96 after ART initiation; asymptotic 95% confidence intervals (CIs) for the corresponding 96-week outcome percentages were calculated. These respective outcomes were examined overall and stratified by MSM, MSW, and W groups. Proportions of participants reporting parenting desires at baseline, and later at 96 weeks, were compared pairwise among MSM, MSW, and W subgroups using χ2 tests, without adjustment of significance levels for multiple testing.
The χ2 tests were used to explore univariate associations between predictors and covariates of interest (continuous variables were categorized for analysis) and the primary outcomes (described above) at baseline and at 96 weeks. Multivariable logistic regression models were used to evaluate associations between baseline sociodemographic variables and parenting desires at 96 weeks after ART initiation overall, and for MSM, MSW, and W subgroups separately.
All statistical modeling first examined univariate (unadjusted) associations. Any potential covariates of interest with moderate evidence of association (P < .20) were then incorporated into a final multivariable (adjusted) model, along with any potentially important variables identified a priori from the literature. We included all individuals who met our inclusion criteria for all analyses. All analyses were done using the SAS statistical analysis software programs.
RESULTS
A total of 1425 of 1809 (79%) individuals enrolled in ACTG 5257 met the enrollment criteria for this study (eg, women aged ≤45 years and all men completing necessary questionnaires) and were included in this analysis; 992 (70%) were MSM, 189 (13%) MSW, and 244 (17%) W (Table 1). Approximately 30% of participants were under the age of 30, with a median age of 36 years (interquartile range, 28–44). Thirty-nine percent were non-Hispanic black and 36% were non-Hispanic white. Most participants had some post high school education (64%), approximately half had incomes below $20000 per year, and most had government insurance coverage (58%). Although 86% of MSM had no children, only 27% of both MSW and W, respectively, had no children. At pre-ART baseline, most participants were not virologically suppressed (viral load >200 copies/mL), and 30% had baseline CD4 cell counts <200 cells/mm3. At baseline, 34% reported binge drinking in the prior 30 days and 24% reported substance abuse in the prior year, although fewer women had reported binge alcohol or substance use (25% and 20%, respectively).
Table 1.
Baseline Characteristics for Men Who Have Sex With Men, Men Who Have Sex With Women, and Women ≤45 Years Enrolled in ACTG Study 5257
| All | MSM | MSW | Women | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | N | (%) | N | (%) | N | (%) | N | (%) |
| Entire Sample | 1425 | (100) | 992 | (69.6) | 189 | (13.3) | 244 | (17.1) |
| Age Categories (N = 1425) | ||||||||
| 18–29 | 428 | (30.0) | 334 | (33.7) | 27 | (14.3) | 67 | (27.5) |
| 30–39 | 440 | (30.9) | 299 | (30.1) | 51 | (27.0) | 90 | (36.9) |
| 40–45 | 557 | (39.1) | 359 | (36.2) | 111 | (58.7) | 87 | (35.7) |
| Education (N = 1418) | ||||||||
| <High School | 269 | (19.0) | 123 | (12.4) | 71 | (37.6) | 75 | (31.1) |
| High School Grad/GED | 246 | (17.3) | 146 | (14.8) | 45 | (23.8) | 55 | (22.8) |
| Post High School | 903 | (63.7) | 719 | (72.8) | 73 | (38.6) | 111 | (46.1) |
| Race/Ethnicity (N = 1425) | ||||||||
| White Non-Hispanic | 517 | (36.3) | 456 | (46.0) | 25 | (13.2) | 36 | (14.8) |
| Black Non-Hispanic | 558 | (39.2) | 307 | (30.9) | 108 | (57.1) | 143 | (58.6) |
| Hispanic (regardless of race) | 308 | (21.6) | 195 | (19.7) | 51 | (27.0) | 62 | (25.4) |
| Other | 42 | (2.9) | 34 | (3.4) | 5 | (2.6) | 3 | (1.2) |
| Income Category (N = 1241) | ||||||||
| <20K | 621 | (50.0) | 373 | (42.5) | 115 | (69.7) | 133 | (66.8) |
| $20–$49999K | 381 | (30.7) | 300 | (34.2) | 34 | (20.6) | 47 | (23.6) |
| ≥50K | 239 | (19.3) | 204 | (23.3) | 16 | (9.7) | 19 | (9.5) |
| Insurance Category (N = 1377) | ||||||||
| Government | 793 | (57.6) | 506 | (52.3) | 129 | (72.1) | 158 | (68.7) |
| Private | 484 | (35.1) | 392 | (40.5) | 36 | (20.1) | 56 | (24.3) |
| Self-pay | 100 | (7.3) | 70 | (7.2) | 14 | (7.8) | 16 | (7.0) |
| Number of Children (N = 1341) | ||||||||
| No Children | 913 | (68.1) | 803 | (85.9) | 47 | (27.3) | 63 | (26.9) |
| 1–2 Children | 259 | (19.3) | 95 | (10.2) | 71 | (41.3) | 93 | (39.7) |
| 3+ Children | 169 | (12.6) | 37 | (4.0) | 54 | (31.4) | 78 | (33.3) |
| Housing Status (N = 1399) | ||||||||
| Owned/rented home | 1243 | (88.8) | 889 | (90.8) | 143 | (78.1) | 211 | (89.0) |
| Lives with friends or family | 103 | (7.4) | 60 | (6.1) | 29 | (15.8) | 14 | (5.9) |
| No stable housing | 53 | (3.8) | 30 | (3.1) | 11 | (6.0) | 12 | (5.1) |
| Week 96 Self-Perceived Infectiousness (N = 1326) | ||||||||
| Low (0%–33%) | 588 | (44.3) | 447 | (48.1) | 60 | (34.5) | 81 | (36.5) |
| Medium (34%–66%) | 257 | (19.4) | 176 | (18.9) | 34 | (19.5) | 47 | (21.2) |
| High (67%–100%) | 481 | (36.3) | 307 | (33.0) | 80 | (46.0) | 94 | (42.3) |
| Baseline CD4 Cell Count (N = 1425) | ||||||||
| ≤200 cells/mm3 | 426 | (29.9) | 273 | (27.5) | 86 | (45.5) | 67 | (27.5) |
| >200 cells/mm3 | 999 | (70.1) | 719 | (72.5) | 103 | (54.5) | 177 | (72.5) |
| Week 48 HIV VL (N = 1425) | ||||||||
| VL ≤200 copies/mL | 68 | (4.8) | 41 | (4.1) | 5 | (2.6) | 22 | (9.0) |
| VL >200 copies/mL | 1357 | (95.2) | 951 | (95.9) | 184 | (97.4) | 222 | (91.0) |
| Baseline Binge Drinking (N = 1041) | ||||||||
| Nonbinge drinker | 691 | (66.4) | 494 | (64.5) | 80 | (67.2) | 117 | (75.0) |
| Binge drinker | 350 | (33.6) | 272 | (35.5) | 39 | (32.8) | 39 | (25.0) |
| Baseline Substance Abuse (N = 1334) | ||||||||
| Never used | 750 | (56.2) | 494 | (52.9) | 114 | (66.7) | 142 | (61.7) |
| Past use | 267 | (20.0) | 197 | (21.1) | 27 | (15.8) | 43 | (18.7) |
| Used within past year | 317 | (23.8) | 242 | (25.9) | 30 | (17.5) | 45 | (19.6) |
Abbreviations: ACTG, AIDS Clinical Trials Group; GED, General Education Diploma; Grad, graduate; HIV, human immunodeficiency virus; MSM, men who have sex with men; MSW, heterosexual men who have sex with women; VL, viral load.
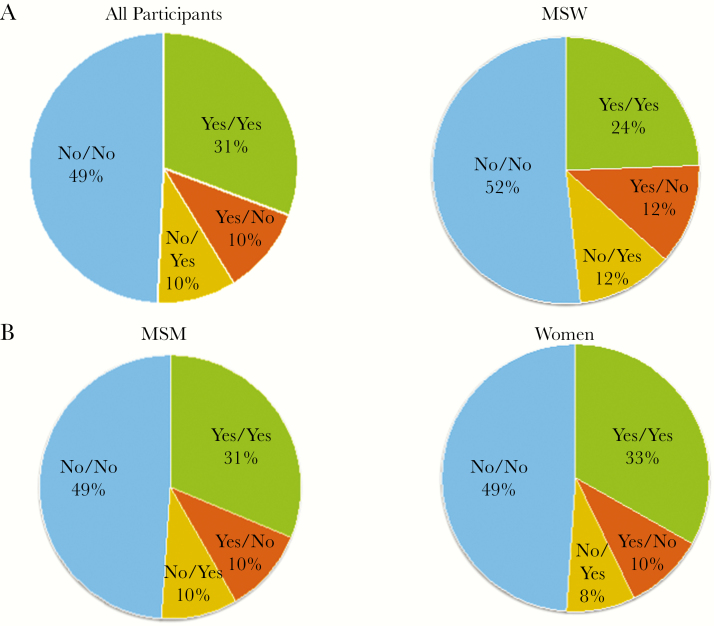
Overall, 41% of participants (95% CI, 38%–43%) wanted or may want children in the future (yes/unsure) at baseline and at 96 weeks, respectively. At baseline, 42%, 37%, and 43% of MSM, MSW, and W, respectively, desired children in the future. At 96 weeks, 41%, 37%, and 43% of MSM, MSW, and W, respectively, desired children in the future. There were no statistically significant differences in the desire for children in the future among MSM, MSW, and W at baseline and at 96 weeks (Figure 1). After 96 weeks of follow-up, approximately 10% of each group who initially desired children changed their preferences, and an equal percentage who initially did not desire children became unsure or desired children in the future (Figure 1).
Figure 1.
Parenting desires (%) at baseline/96 weeks after antiretroviral therapy initiation among (a) all participants, (b) men who have sex with men (MSM), (c) men reported sex only with women (MSW), and (d) Women*. *There were no statistically significant differences in parenting desires (yes/unsure versus no) among all participants, or in the MSM, MSW, and W subgroups at baseline, week 96 or the change between baseline and week 96. (NOTE: “Yes” in the above charts refers to “Yes/Unsure” responses to the parenting desire questions.)
In univariate analysis, factors significantly associated with parenting desires in the future (Appendix Table 1) included being younger, not having prior children, and being black non-Hispanic race. In multivariable analysis among all participants, there was an increased odds of considering having children in the future among participants whose age was <30 years, were black, had more than a high school degree, and had no children (Table 2). In the MSM group, participants whose age was <30 years and were black were more likely to desire children in the future, whereas for MSW group, participants whose age was <30 years were more likely to desire children in the future compared to those whose age was ≥40 years. Women aged <30 years and who had fewer than 3 children were more likely to desire children in the future. It is notable that CD4 count, viral load at week 48, or self-perceived infectiousness were not associated with desire for children in the future, nor were income, insurance status, substance abuse, or binge drinking. Despite the fact that 95% of participants overall had HIV viral suppression at 48 weeks, the proportion of participants desiring children did not change appreciably after starting ART (from baseline to 96 weeks) among all participants and within each subgroup.
Table 2.
Multivariable Logistic Regression Models for Baseline Factors Associated With Parenting Desires at 96 Weeks Among All Participants, MSM, MSW, and Women Participating in ACTG Study 5257*
| All | MSM | MSW | Women | |
|---|---|---|---|---|
| (N = 1425) | (N = 992) | (N = 189) | (N = 244) | |
| Desires Children** | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| Entire Sample | 580 (41%) (38%–43%) | 406 (41%) (38%–44%) | 70 (37%) (30%–44%) | 104 (43%) (36%–49%) |
| Age Categories | ||||
| 18–29 | REF | REF | REF | REF |
| 30–39 | 0.32 (0.22–0.48)a | 0.35 (0.22–0.54)a | 0.38 (0.09–1.59) | 0.17 (0.06–0.49)a |
| 40–45 | 0.11 (0.07–0.17)a | 0.09 (0.05–0.15)a | 0.12 (0.03–0.50)b | 0.09 (0.03–0.27)a |
| Education | ||||
| <High School | REF | REF | REF | REF |
| High School Grad/GED | 1.25 (0.64–2.44) | 1.67 (0.69–4.06) | 1.07 (0.28–4.04) | 0.70 (0.22–2.19) |
| Post-High School | 1.93 (1.08–3.44)c | 2.12 (0.99–4.55) | 2.93 (0.85–10.09) | 0.91 (0.34–2.46) |
| Race/Ethnicity | ||||
| White Non-Hispanic | REF | REF | REF | REF |
| Black Non-Hispanic | 2.20 (1.45–3.34)b | 2.33 (1.46–3.74)a | 1.04 (0.16–6.96) | 0.93 (0.31–2.78) |
| Hispanic (regardless of race) | 1.07 (0.64–1.79) | 1.12 (0.64–1.97) | 0.82 (0.09–7.22) | 0.83 (0.20–3.47) |
| Other | 1.28 (0.49–3.33) | 1.05 (0.37–2.97) | 0.92 (0.01–70.63) | 0.93 (0.04–20.91) |
| Income Category | ||||
| <20K | REF | REF | REF | REF |
| $20-$49999K | 0.82 (0.54–1.25) | 0.81 (0.50–1.31) | -- | 1.08 (0.44–2.66) |
| ≥50K | 0.75 (0.44–1.26) | 0.72 (0.40–1.30) | -- | 3.07 (0.81–11.57) |
| Insurance Category | ||||
| Government | REF | REF | REF | REF |
| Private | 0.97 (0.65–1.45) | 1.04 (0.65–1.64) | 0.47 (0.12–1.80) | -- |
| Self-pay | 1.22 (0.66–2.24) | 1.12 (0.55–2.28) | 2.18 (0.35–13.72) | -- |
| Number of Children | ||||
| No Children | REF | REF | REF | REF |
| 1–2 Children | 0.50 (0.31–0.82)b | 0.49 (0.23–1.03) | 0.56 (0.17–1.91) | 0.41 (0.16–1.09) |
| 3+ Children | 0.47 (0.23–0.94)c | 0.42 (0.09–2.03) | 0.52 (0.12–2.23) | 0.21 (0.07–0.64)b |
| Housing Status | ||||
| Owned/rented home | REF | REF | REF | REF |
| Lives with friends or family | 1.61 (0.78–3.33) | 1.46 (0.61–3.52) | -- | 0.09 (0.01–1.16) |
| No stable housing | 0.98 (0.4–2.39) | 0.87 (0.29–2.55) | -- | 0.45 (0.08–2.53) |
| Week 96 Self-Perceived Infectiousness | ||||
| Low (0%–33%) | REF | REF | REF | REF |
| Medium (34%–66%) | 0.93 (0.64–1.37) | 0.96 (0.61–1.51) | -- | -- |
| High (67%–100%) | 0.71 (0.46–1.11) | 0.73 (0.44–1.22) | -- | -- |
| Baseline CD4 Cell Count | ||||
| >200 cells/mm3 | REF | REF | REF | REF |
| ≤200 cells/mm3 | 0.78 (0.53–1.14) | 0.88 (0.56–1.39) | -- | 0.58 (0.24–1.40) |
| Week 48 HIV VL | ||||
| VL ≤200 copies/mL | REF | REF | REF | REF |
| VL >200 copies/mL | 1.65 (0.69–3.99) | 1.57 (0.55–4.45) | -- | -- |
| Baseline Binge Drinking | ||||
| Nonbinge Drinker | REF | REF | REF | REF |
| Binge Drinker | 1.19 (0.84–1.70) | 1.08 (0.72–1.61) | 3.01 (0.99–9.18) | -- |
| Baseline Substance Use | ||||
| Never used | REF | REF | REF | REF |
| Past use | 0.90 (0.58–1.40) | 0.99 (0.60–1.63) | 1.49 (0.38–5.89) | 0.51 (0.16–1.60) |
| Used within past year | 0.76 (0.51–1.14) | 0.82 (0.52–1.31) | 0.25 (0.05–1.24) | 1.45 (0.57–3.70) |
Abbreviations: ACTG, AIDS Clinical Trials Group; CI, confidence interval; GED, General Education Diploma; HIV, human immunodeficiency virus; MSM, men who have sex with men; MSW, heterosexual men who have sex with women; OR, odds ratio; REF, reference; VL, viral load.
a P < .001, bP < .01, cP < .05.
*, Only covariates with a univariate association with parenting desires (yes/unsure versus no) (P < .2) were included in the multivariable models.
**, Parenting desires variable is characterized as “Yes/Unsure” versus “No.”
DISCUSSION
This study examined the parenting desires among a diverse group of ART-naive persons living with HIV who entered a large ART clinical trial conducted across the United States [10]. We found a surprisingly similar desire to have children in the future (yes/unsure) both before and 2 years after successful treatment with ART. These parenting desires did not differ among MSM, MSW, or W. Although previous studies have reported on parenting desires among women and MSW living with HIV both before and after suppressive ART, this study is the first to report on the desire of MSM living with HIV to have children in the future. Approximately 40% of MSM, MSW, and W living with HIV were considering having children at each visit before and 96 weeks after starting ART, with a similar proportion who changed from not wanting to considering having children and the reverse. Across all 3 groups, younger people and those with fewer children were more likely to consider having children in the future.
There are several important implications of our findings for improving the care of persons living with HIV. Healthcare providers should assess reproduction desires and contraception needs of all of their patients routinely while providing HIV care. They should be prepared to provide information on safer conception practices with the highest likelihood of pregnancy success, to give thoughtful and tailored preconception counseling, and to support persons living with HIV to enable them to have children safely while minimizing the risk of transmitting HIV to uninfected partners and future children [12]. In contrast, those who do not want to have children currently should be offered safe and appropriate contraception, including highly effective methods such as long-acting contraceptive methods. If men and women living with HIV are interested in considering having children in the future, healthcare providers should provide education on the merits of obtaining and maintaining viral suppression and promoting healthy behaviors overall that would optimize future fertility (for example, smoking cessation and treatment of other concurrent health conditions). In addition, healthcare providers should be aware of available community and medical resources for MSM living with HIV who desire children, including adoption and surrogacy.
Factors known to be predictive of parenting desires from previous cross-sectional studies of populations with HIV, such as age and current children, were also found to be important in this current study of individuals starting modern ART, and similar among MSM, MSW, and W. In our study, black MSM were twice as likely to consider having children compared with MSM who were white or Hispanic. We did not observe similar associations between race/ethnicity and parenting desires among MSW and W, potentially because participants in these groups were more likely to be black and least likely to be white, and these groups had fewer participants, thus decreasing the power to find such differences. Many of the factors thought to be important in making certain health decisions such as income, education, insurance, binge drinking, and substance use were not associated with desires for children in the future in our analysis. Although data were available on the number of sexual partners, we did not know whether sexual partners were new and whether sexual partners desired children, factors that have been showed to be predictive among women living with HIV [13].
Our data show that MSM and heterosexual men and women living with HIV commonly desire children at rates similar to their HIV-negative counterparts and with higher frequency than in the early HIV era. Riskind and Patterson [8] used data from the 2002 US National Center for Health Statistics National Survey of Family Growth, which did not include HIV status. They found that 54% of all gay male participants without children and 75% of all heterosexual male participants without children expressed parenting desires, with younger, non-white, and heterosexual male participants being the most likely to express parenting desires [8]. In our study, in which 86% of MSM had never had children and over half were non-white, we found that 41% desired children in the future, only approximately 25% lower than the national sample reported by Riskind and Patterson [8]. For heterosexual men and women living with HIV, compared with the earlier nationally representative sample from 1998, the rate of desiring children was approximately 50% higher for women in our population (41% versus 28%) and approximately 25% higher for heterosexual men (36% versus 29%) [1]. These higher parenting desires among our sample could reflect the greater optimism with managing HIV disease in the setting of modern ART.
Although this study had many strengths, there were some limitations. The numbers of heterosexual men and women were smaller than for MSM, and most of them were non-white, limiting our ability to explore race/ethnicity differences in parenting desires in these subgroups. We did not enroll HIV-negative individuals and thus cannot comment on comparative desires based on HIV status. Because this is a secondary analysis, some factors that may contribute to parenting intentions may not have been assessed in the parent study. For example, the parent study did not assess whether a participant had a new partner during the study follow-up period, a factor that may influence parenting desires. The MSM status was determined at entry and maintained through this study, although some men who did not report ever having had sex with other men at entry could have changed their status during the study, and we would not have captured this. It is important that future studies evaluate the impact of participant education, knowledge of perinatal transmission risk, depression or psychiatric conditions, relationship status, partners’ serostatus and their desires for children, and contraceptive use on parenting desires. Furthermore, we were unable to evaluate the impact of surgical sterilization on parenting desires, recognizing that many women may have had sterilization regret [13, 14]. Because many questions covered sensitive topics, social desirability bias and stigma may have affected the results. However, questionnaires were completed in a private room and on a periodic basis and were not reviewed for completeness by study personnel before being forwarded to the data center, so social desirability bias should be minimal. Furthermore, generalizability of these findings may be limited by the fact that individuals were enrolled in a clinical trial. However, the demographics of the cohort in this study closely emulated persons in the United States living with HIV who are starting ART.
CONCLUSIONS
Our data showed that overall, MSM, MSW, and W have similar parenting desires. Hence, all of these populations would benefit from preconception counseling, counseling about methods of contraception, and understanding how to prevent transmitting HIV to their uninfected partners or to their future children. Effective strategies for safe conception for men and women living with HIV are important for the prevention of vertical and horizontal transmission. Regular ongoing assessment of parenting goals for both men and women in HIV care settings is critical because many individuals, regardless of sexual orientation, may desire having children, and some individuals who did not want children may change their minds over time. Furthermore, to reduce barriers, HIV care services should include integrated comprehensive reproductive health, fertility, and contraceptive services available to men and women.
Acknowledgments
We acknowledge and appreciate the sites and study personnel conducting the study and the men and women who participated in this study.
Financial support. This work was funded in part by the Clinical Sciences Core of the Third Coast Center for AIDS Research (National Institutes of Health [NIH] Grant P30 AI117943 and NIH/National Institute of Allergy and Infectious Diseases [NIAID] Grant AI69471 [to S. E. C.]; NIH/NIAID Grant K23 AI 114407 [to A. N. S.]; and Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 1K23HD078153-01A1 [to L. B. H.]).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix Table 1. Univariable Logistic Regression Models for Baseline Factors Associated with Parenting Desires (Yes/Unsure Versus No) at 96 Weeks Among All Participants, MSM, MSW, and WOMEN Participating in ACTG 5257
| ALL | MSM | MSW | WOMEN | |
|---|---|---|---|---|
| (N = 1425) | (N = 992) | (N = 189) | (N = 244) | |
| Unadjusted OR | Unadjusted OR | Unadjusted OR | Unadjusted OR | |
| Desire Children* | (95% CI) | (95% CI) | (95% CI) | (95% CI) |
| Entire Sample | 580 (41%)(38%–43%) | 406 (41%)(38%–44%) | 70 (37%)(30%–44%) | 104 (43%)(36%–49%) |
| Age Categories | ||||
| 18–29 | REF | REF | REF | REF |
| 30–39 | 0.23 (0.18–0.31)c | 0.22 (0.15–0.30)c | 0.41 (0.15–1.09) | 0.23 (0.12–0.45)c |
| 40+ | 0.08 (0.06–0.10)c | 0.06 (0.04–0.09)c | 0.13 (0.05–0.33)c | 0.09 (0.04–0.19)c |
| Education | ||||
| <High School | REF | REF | REF | REF |
| High School Grad/GED | 1.39 (0.97–1.98) | 1.63 (1.00–2.66) | 0.91 (0.40–2.05) | 1.08 (0.52–2.23) |
| Post-High School | 1.35 (1.01–1.79)a | 1.11 (0.75–1.65) | 2.05 (1.04–4.05)a | 1.99 (1.09–3.64)a |
| Race/Ethnicity | ||||
| White Non-Hispanic | REF | REF | REF | REF |
| Black Non-Hispanic | 2.04 (1.60–2.62)c | 3.11 (2.30–4.21)c | 0.61 (0.25–1.48) | 0.83 (0.40–1.73) |
| Hispanic (Regardless of Race) | 1.23 (0.91–1.65) | 1.41 (0.99–2.00) | 0.89 (0.34–2.34) | 0.44 (0.19–1.03) |
| Other | 1.54 (0.82–2.93) | 1.40 (0.68–2.87) | 1.91 (0.27–13.49) | 2.00 (0.17–24.07) |
| Income Category | ||||
| <20K | REF | REF | REF | REF |
| $20-$49999K | 0.97 (0.75–1.26) | 0.82 (0.60–1.12) | 1.22 (0.56–2.66) | 1.39 (0.71–2.72) |
| ≥50K | 0.68 (0.50–0.93)a | 0.52 (0.40–0.74)c | 0.58 (0.18–1.91) | 3.71 (1.33–10.40)a |
| Insurance Category | ||||
| Government | REF | REF | REF | REF |
| Private | 0.81 (0.64–1.02) | 0.68 (0.52–0.90)b | 1.27 (0.59–2.73) | 1.24 (0.67–2.29) |
| Self-pay | 1.24 (0.82–1.88) | 1.01 (0.61–1.67) | 2.67 (0.87–8.17) | 1.43 (0.51–4.01) |
| Number of Children | ||||
| No Children | REF | REF | REF | REF |
| 1–2 Children | 0.60 (0.45–0.81)b | 0.49 (0.30–0.78)b | 0.54 (0.26–1.14) | 0.26 (0.13–0.52)c |
| 3+ Children | 0.26 (0.18–0.40)c | 0.09 (0.02–0.31)c | 0.2 (0.08–0.49)c | 0.14 (0.07–0.29)c |
| Housing Status | ||||
| Owned/ rented home | REF | REF | REF | REF |
| Lives with friends or family | 1.51 (1.01–2.25)a | 2.13 (1.25–3.61)b | 1.54 (0.69–3.43) | 0.33 (0.09–1.23) |
| No stable housing | 0.76 (0.43–1.36) | 1.16 (0.56–2.42) | 0.37 (0.08–1.76) | 0.41 (0.11–1.55) |
| Week 96 Self-perceived Infectiousness | ||||
| Low (0–33%) | REF | REF | REF | REF |
| Medium (34–66%) | 0.77 (0.60–0.99)a | 0.75 (0.56–1.01) | 0.89 (0.44–1.81) | 0.80 (0.44–1.46) |
| High (67–100%) | 0.97 (0.72–1.30) | 0.98 (0.69–1.40) | 1.47 (0.62–3.47) | 0.70 (0.34–1.46) |
| Baseline CD4 cell count | ||||
| >200 cells/mm3 | REF | REF | REF | REF |
| ≤200 cells/mm3 | 0.74 (0.59–0.94)a | 0.81 (0.61–1.08) | 0.70 (0.39–1.28) | 0.57 (0.31–1.02) |
| Week 48 HIV viral load (VL) | ||||
| VL ≤200 copies/mL | REF | REF | REF | REF |
| VL >200 copies/mL | 2.46 (1.49–4.06)b | 2.61 (1.36–4.98)b | 7.15 (0.78–65.32) | 1.70 (0.70–4.09) |
| Baseline Binge Drinking | ||||
| Non-Binge Drinker | REF | REF | REF | REF |
| Binge Drinker | 1.31 (1.01–1.70)a | 1.29 (0.96–1.74) | 1.92 (0.86–4.25) | 1.11 (0.54–2.30) |
| Baseline Substance Use | ||||
| Never used | REF | REF | REF | REF |
| Past use | 0.70 (0.52–0.93)a | 0.74 (0.53–1.05) | 0.58 (0.23–1.43) | 0.57 (0.28–1.17) |
| Used within past year | 0.74 (0.57–0.97)a | 0.84 (0.61–1.15) | 0.28 (0.10–0.77)a | 0.65 (0.33–1.31) |
a P < .001, bP < .01, cP < .05
*Parenting desires variable is characterized as “Yes/Unsure” versus “no.”
Presented in part: Conference on Retroviruses and Opportunistic Infections (CROI) 2017, February 13–16, 2017, Seattle, WA.
References
- 1. Chen JL, Philips KA, Kanouse DE, et al. Fertility desires and intentions of HIV-positive men and women. Fam Plann Perspect 2001; 33:144–52, 165. [PubMed] [Google Scholar]
- 2. Weber S, Zakaras JM, Hilliard S, et al. “Is it all right for me to have a baby or not?”: Men living with HIV discuss fertility desires and interactions with providers. J Assoc Nurses AIDS Care 2017; 28:118–29. [DOI] [PubMed] [Google Scholar]
- 3. Haddad LB, Machen LK, Cordes S, et al. Future desire for children among women living with HIV in Atlanta, Georgia. AIDS Care 2016; 28:455–9. [DOI] [PubMed] [Google Scholar]
- 4. Finocchario-Kessler S, Sweat MD, Dariotis JK, et al. Understanding high fertility desires and intentions among a sample of urban women living with HIV in the United States. AIDS Behav 2010; 14:1106–14. [DOI] [PubMed] [Google Scholar]
- 5. Bedimo-Rung AL, Clark AR, Dumestre J, et al. Reproductive decision-making among HIV-infected women. J Natl Med Assoc 2005; 97:1403–10. [PMC free article] [PubMed] [Google Scholar]
- 6. Nattabi B, Li J, Thompson SC, et al. A systematic review of factors influencing fertility desires and intentions among people living with HIV/AIDS: implications for policy and service delivery. AIDS Behav 2009; 13:949–68. [DOI] [PubMed] [Google Scholar]
- 7. Haddad LB, Wall KM, Mehta CC, et al. Trends of and factors associated with live-birth and abortion rates among HIV-positive and HIV-negative women. Am J Obstet Gynecol 2017; 216:71.e1–71.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riskind RG, Patterson CJ. Parenting intentions and desires among childless lesbian, gay, and heterosexual individuals. J Fam Psychol 2010; 24:78–81. [DOI] [PubMed] [Google Scholar]
- 9. Steiner RJ, Finocchario-Kessler S, Dariotis JK. Engaging HIV care providers in conversations with their reproductive-age patients about fertility desires and intentions: a historical review of the HIV epidemic in the United States. Am J Public Health 2013; 103:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lennox JL, Landovitz RJ, Ribaudo HJ, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 2016; 374:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawwass JF, Smith DK, Kissin DM, et al. Strategies for preventing HIV infection among HIV-uninfected women attempting conception with HIV-infected men - United States. MMWR Morb Mortal Wkly Rep 2017; 66:554–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanwood NL, Cohn SE, Heiser JR, Pugliese M. Contraception and fertility plans in a cohort of HIV-positive women in care. Contraception 2007; 75:294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Badell ML, Lathrop E, Haddad LB, et al. Reproductive healthcare needs and desires in a cohort of HIV-positive women. Infect Dis Obstet Gynecol 2012; 2012:107878. [DOI] [PMC free article] [PubMed] [Google Scholar]