Abstract
Dermal fibroblasts play essential roles in wound healing and their dysfunction has been shown to be associated with impaired wound healing in diabetes. In the present study, we aimed at investigating whether Yes-associated protein (YAP), a mediator of mechanotransduction in dermal fibroblasts, is associated with impaired wound healing in diabetic mice. Compared with that in the control, the rate of wound contraction was decreased twofold in db/db type 2 diabetic mice (db/db mice). To mimic diabetic pathological condition, dermal fibroblasts were cultured under high glucose conditions (25.5 mM glucose). Further, dermal fibroblast-mediated contraction of wound was evaluated by in vitro collagen gel contraction assay. Dermal fibroblasts cultured under hyperglycemic condition showed impaired gel contraction and mitochondrial dysfunction, compared to the cells cultured under normoglycemic conditions (5.5 mM glucose). Importantly, compared with the normal dermal fibroblasts, diabetic db/db dermal fibroblasts expressed lower levels of growth factors and cytokines that enhance wound healing, such as insulin-like growth factor-1, stromal cell-derived factor-1, connective tissue growth factor, and transforming growth factor-β (TGF-β). The quantity of YAP mRNA was also lower in diabetic db/db dermal fibroblasts, compared with that in the control fibroblasts. These results indicate that impaired wound healing in diabetics is associated with the dysfunction of dermal fibroblasts, including downregulation of YAP, which plays essential roles in extracellular matrix remodeling and TGF-β-mediated wound healing.
Keywords: Dermal fibroblast, Diabetes, Wound, YAP
Introduction
Wound healing is a complicated biological process that is essential to tissue and organ homeostasis as well as regeneration of injured tissue [1]. Wound contraction following remodeling and stiffening of the extracellular matrix (ECM) is one of the essential factors in wound healing [2]. Abnormal wound contraction is associated with impaired wound healing [2], such as pathologic scarring and chronic non-healing, including diabetic wounds [3, 4].
Dermal fibroblasts are stromal cells that reside in the dermal layer of the skin and play vital roles in wound healing [5, 6]. They produce and secrete molecules that constitute the ECM, including collagens, elastin, and proteoglycan, [7] as well as various cytokines and growth factors such as stromal cell-derived factor-1 (SDF-1) [8]. The functions of dermal fibroblasts in wound healing are regulated by various signaling pathways involving transforming growth factor-β (TGF-β) [9, 10], phosphatidylinositol-3-kinase (PI3K)/Akt [11], Wnt/β-catenin [12], and Yes-associated protein (YAP) [13].
YAP is the terminal effector in the Hippo pathway [14] and regulates cell proliferation, regeneration, epithelial-mesenchymal transition, and stem cell differentiation [15, 16]. YAP also acts as a mediator of mechanotransduction in stromal cells [17]. In the nucleus, YAP contributes to the transcriptional regulation of genes downstream in the Hippo pathway by forming a complex with the transcriptional enhancer associated domain (TEAD) family of transcription factors [14, 16, 18]. In response to the activation of the Hippo pathway, YAP is phosphorylated by upstream kinases, large tumor suppressor kinase 1 and 2 (LATS1 and LATS2), and then phosphorylated YAP is sequestered in the cytosol where it forms a complex with 14-3-3 protein [14]. Moreover, YAP regulates various signaling proteins in each pathway [14]. For example, phosphorylated YAP downregulates Wnt signaling [19] and recruits β-catenin and β-TrCP, leading to the degradation of β-catenin [20]. More importantly, YAP regulates the axis of TGF-β and connective tissue growth factor (CTGF), which is required for wound healing [21]. TGF-β signal controls wound healing via the regulation of CTGF expression [21, 22]. Therefore, the downregulation of YAP results in reduced expression of TGF-β and delayed wound healing [13].
Our previous works demonstrated that YAP activation is associated with acceleration of diabetic dermal wound healing by substance P (in press), an important wound healing stimulator [23]. YAP is also required for cancer-associated fibroblast-mediated regulation of ECM stiffening [17]. Thus, here we sought to determine whether the expression of YAP is reduced in diabetic dermal fibroblasts that are associated with impaired wound healing and defects in wound contraction.
Materials and methods
Mice
Male db/db mice and C57BL/6 male mice were purchased from Laboratory Animal Resource Center of Korea Research Institute of Bioscience & Biotechnology (Daejeon, South Korea) and Central Lab Animal Inc. (Seoul, South Korea), respectively. Mice were maintained under controlled temperature (21–22 °C) and light (12 hours light/12 hours dark cycles) with free access to drinking water and food. Eight to ten-week old mice were used for primary culture of dermal fibroblasts. All animal experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use of Kyung Hee University (KHMC-IACUC 15-020).
Wound healing model
Eight-week old mice were anesthetized with ketamine (8 mg/kg, Yuhan corporation, Seoul, Korea) and rompun (Xylazine, 5.6 mg/kg, Bayer Korea, Seoul, Korea). The dorsal skin was shaved and wounded using a 4 mm biopsy punch. Hydrogel (Poloxamer F127, Sigma, St Louis, MO, USA) was applied to the wounds. Then, the wounds were dressed with Mepitel (Mölnlycke Health Care, Gothenburg, Sweden) and Tegaderm (3 M, Maplewood, MN, USA). Digital images of the wounds were obtained at 7 days post-wound. Wound size was analyzed using Adobe Photoshop CS6. The wounds were harvested for further analyses at 7 days post-wounding.
Dermal fibroblasts
Fibroblasts were isolated from the dermis of normal or db/db mice through treatment with type I collagenase (Washington Biochemical Corp., Freefold, NJ, USA). Dermal fibroblasts were cultured in fibroblast growth media (FGM2, Lonza, Switzerland) containing 2% fetal bovine serum, 5 μg/mL of insulin, 1 ng/mL of basic fibroblast growth factor, and 50 μg/mL of gentamicin. Cells between passages 6 to 10 were used for experiments. Human dermal fibroblasts were obtained from MTCC (Seoul, Korea) and maintained in FGM-2 (Lonza). For mimicking diabetic conditions, the human dermal fibroblasts (passage 6–9) were maintained in high-glucose (25.5 mM) Dulbecco’s modified eagle’s medium (DMEM, GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% FBS (JR scientific, Inc., Woodland, CA, USA) and 1% penicillin and streptomycin (P/S; Invitrogen). As the control group, the human dermal fibroblasts were maintained in low-glucose (5.5 mM) DMEM. All cells were maintained at 37 °C in a humidified incubator containing 5% CO2.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from fibroblasts, using TRIzol™ reagent (Life Technologies) according to the manufacturer’s instructions. Five μg of total RNA were used for single strand cDNA synthesis using Superscript First-Strand cDNA Synthesis System (Life Technologies) according to the manufacturer’s instructions. Real-time PCR was performed using Power SYBR Green PCR Master Mix (Life Technologies). Mouse ribosomal protein 36B4 gene was used as an endogenous control. The following primers were used to detect the expression of insulin-like growth factor 1 (IGF-1), stromal cell-derived factor-1 (SDF-1), connective tissue growth factor (CTGF), transforming growth factor-β (TGF-β), connective Yes-associated protein (YAP), and 36B4: IGF-1 (sense): 5′-TCATGTCGTCTTCACACCTCTTCT-3′, IGF-1 (antisense): 5′-CCACACACGAACTGAAGAGCAT-3′, SDF-1 (sense): 5′-GTCCTCTTGCTGTCCAGCTC-3′, SDF-1 (antisense): 5′-AGATGCTTGACGTTGGCTCT-3′, CTGF (sense): 5′-AAGACACATTTGGCCCAGAC-3′, CTGF (antisense): 5′-GACAGGCTTGGCGATTTTAG-3′, TGF-β (sense): 5′-TGTTAAAACTGGCATCTGA-3′, TGF-β (antisense): 5′-GTCTCTTAGGAAGTAGGT-3′, YAP (sense): 5′-TACATAAACCATAAGAACAAGACCACA-3′, YAP (antisense): 5′-GCTTCACTGGAGCACTCTGA-3′, 36B4 (sense): 5′-GAACATCTCCCCCTTCTCCTT-3′, and 36B4 (antisense): 5′-GCTTCACTGGAGCACTCTGA-3′.
3-(4,5)-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
After culturing the human dermal fibroblasts under normoglycemic or hyperglycemic condition for two passages (passage 6–8), the cells were seeded into 48-well plates at a density of 1.25 × 104 cells/mL or 5 × 104 cells/mL and cultured for 6 hours under normoglycemic or hyperglycemic condition. Then, the cells were incubated at 37 °C for 4 hours in 2 mL of 1 × MTT solution, which was made with 10 × MTT solution and DMEM media without FBS. Next, the cells were incubated in 2 mL of (1:1) dimethyl sulfoxide (DMSO) and ethanol for 30 min. The optical density (OD) of the solution was measured at 540 nm.
Collagen gel contraction assay
Human dermal fibroblasts were cultured under normoglycemic or hyperglycemic condition for two passages (passage 6–8). Each group of the cultured cells were trypsinized and resuspended in low-(5.5 mM) or high-glucose (25.5 mM) DMEM at a cell density of 2.5 × 104 cells/mL or 5 × 104 cells/mL. Thereafter, equal volumes of the cells and 2 mg/mL type I collagen solution (Life Technologies) were mixed and added into 48-well plates, which were coated with 1% bovine serum albumin (BSA, Sigma), for 1 hour at 37 °C. Then, the mixture was allowed to polymerize for 2 hours at 37 °C and the solidified mixture was incubated in high-(25.5 mM) or low-glucose (5.5 mM) DMEM for 3 days. Collagen gel contraction was analyzed by weighing the gels and imaging them.
Statistical analysis
Quantitative data were presented as mean ± SD. Unpaired student t-test was applied to evaluate differences between two groups. Values of p < 0.05 were considered statistically significant. All statistical analyses were performed using GraphPad version 5.01 (GraphPad Software, USA; http://www.graphpad.com).
Results
Wound healing process is delayed in diabetic mice
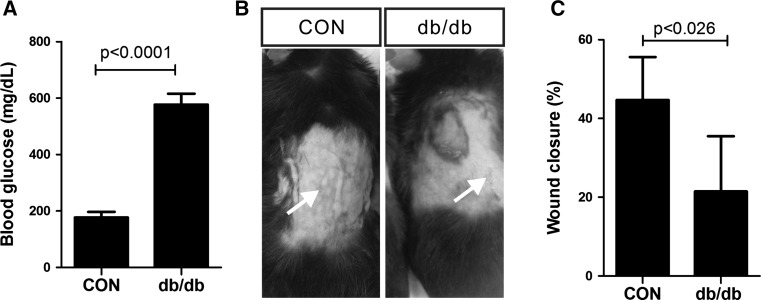
Previous studies demonstrated that wound healing is impaired in individuals with diabetes [24, 25]. Therefore, we examined wound contraction in a type 2 diabetic (db/db) mouse model [26] compared with that in a control group. Type 2 diabetic (db/db) mice showed higher levels of blood glucose (576.9 mg/dL ± 6.22, n = 10) than the control (117.1 mg/dL ± 12.19, n = 10) (Fig. 1A). Seven days after wound creation, full-thickness closure of the excision wound was significantly delayed (p < 0.026) in db/db mice (21.48% ± 6.99, n = 4), compared with that in the control mice (44.69% ± 4.88, n = 5) (Fig. 1B and C). Collectively, these results indicate that wound healing was impaired in type 2 diabetic (db/db) mice.
Fig. 1.
Impaired wound contraction in db/db type 2 diabetic mice. A Blood glucose level in the control (CON, n = 10) and db/db type 2 diabetic (db/db, n = 10) mice. B Representative images of wounds from the control (CON) and db/db type 2 diabetic (db/db) mice, 7 days after wounding. Arrows indicate the wounds. C Quantitative results of wound contraction in the control (CON, n = 5) and db/db type 2 diabetic (db/db, n = 4) mice. Data are expressed as mean ± SD
Mitochondrial dysfunction is induced by high glucose levels in human dermal fibroblasts
Mitochondrial dysfunction is associated with insulin resistance in peripheral tissues and hyperglycemia caused by malfunction of pancreatic β-cells [27, 28]. Oxidative damage caused by mitochondrial dysfunction is significantly associated with cellular dysfunction in various cells, including dermal fibroblasts that induce impaired wound healing [29–31]. Human skin derived dermal fibroblasts had been cultured in vitro under hyperglycemic condition or normoglycemic for two passages. They were then analyzed by MTT assay following short in vitro culture for 5 hours to examine mitochondrial function without any effect from proliferation. Human skin-derived dermal fibroblasts that had been cultured in vitro under hyperglycemic condition showed mitochondrial dysfunction, compared to the cells cultured under normoglycemic conditions (Fig. 2). This result was confirmed with cells cultured at two different densities (Fig. 2; A = 5 × 104 cells/mL). We concluded that human fibroblasts cultured under hyperglycemia condition mimic dermal fibroblasts derived from diabetes, and used the dermal fibroblasts to carry out collagen gel contraction assay.
Fig. 2.
Mitochondrial dysfunction of dermal fibroblasts treated with high glucose. MTT assay was performed using human dermal fibroblasts cultured under normoglycemic (Normoglycemia) or hyperglycemic (Hyperglycemia) conditions to examine their mitochondrial function. The cells were cultured at two different densities. The results of the MTT assay are shown in OD at 540 nm. Data are expressed as mean ± SD. 0.25 A, 1.25 × 104 cells/mL; A, 5 × 104 cells/mL
Extracellular matrix contraction is impaired in dermal fibroblasts cultured under hyperglycemia
Collagen gel contraction assay was performed to investigate whether impaired wound contraction is associated with dysfunction of dermal fibroblasts. Collagen gel contraction was quantified as loss of gel weight (Fig. 3A) and change in gel size (Fig. 3B). Collagen gel embedded with human dermal fibroblasts contracted in a dose-dependent manner (Fig. 3). Importantly, collagen gel containing the dermal fibroblasts cultured under hyperglycemic induced a slow gel contraction, compared to the cell under normoglycemic (Fig. 3A and B; 0.25 A: 19.75 ± 0.63 mg, n = 4 gels mixed with diabetic dermal fibroblasts s versus 15.00 ± 0.82 mg, n = 4 gels mixed with the control; 0.5 A: 15.00 ± 1.225 mg, n = 4 gels mixed with diabetic dermal fibroblasts s versus 10.00 ± 1.225 mg, n = 4 gels mixed with the control). Therefore, it is likely that hyperglycemia caused a defect in dermal fibroblasts mediated gel contraction and the decreased gel contraction might cause impaired wound contraction in diabetes.
Fig. 3.
Impaired gel contraction of dermal fibroblasts treated with high glucose. A Collagen gel contraction assay was performed using human dermal fibroblasts cultured under normoglycemic (Normoglycemia) or hyperglycemic (Hyperglycemia) conditions to examine their contraction activity. The results of collagen gel contraction assay are shown in wet weight of gels. Data are expressed as mean ± SD. 0 A, 0 × 104 cells/mL; 0.25 A, 1.25 × 104 cells/mL; 0.5 A, 2.5 × 104 cells/mL. (*p < 0.03, **p < 0.0037, NS; not significant). B Representative images of the gels from A
Expression of wound healing-associated genes is impaired in diabetic dermal fibroblasts
Various cell types mediate the complicated process of wound healing. Dermal fibroblasts play an essential role in orchestrating wound repair, including wound contraction [4, 5], via extracellular matrix remodeling, growth factors, and cytokines [6]. We investigated whether impaired wound contraction in diabetic mice would be associated with differences in expression of growth factors and cytokines between dermal fibroblasts established from the control and from type 2 diabetic (db/db) mice. Compared to the control, type 2 diabetic (db/db) mice-derived dermal fibroblasts showed lower expression levels of insulin-like growth factor-1 (IGF-1: 0.34 ± 0.03, diabetic dermal fibroblasts versus 1.00 ± 0.14, the control), stromal cell-derived factor-1 (SDF-1: 0.45 ± 0.05, diabetic dermal fibroblasts versus 1.00 ± 0.22, the control), and connective tissue growth factor (CTGF: 0.47 ± 0.03, diabetic dermal fibroblasts versus 1.07 ± 0.16, the control), which are essential in wound healing and expressed by dermal fibroblasts [6–11] (Fig. 4A–C). TGF-β is one of the essential wound-associated growth factors [11] and is differentially regulated in normal and impaired wound healing [11, 12]. Expression of TGF-β was downregulated in type 2 diabetic (db/db) mice-derived dermal fibroblasts, compared with that in the control (Fig. 4D; 0.30 ± 0.13, diabetic dermal fibroblasts s versus 0.90 ± 0.22, the control). Moreover, expression of YAP was also reduced in type 2 diabetic (db/db) mice-derived dermal fibroblasts, compared to that in the control (Fig. 4E; 0.66 ± 0.23, diabetic dermal fibroblasts s versus 1.26 ± 0.43, the control). Therefore, these results indicate that the axis of YAP, TGF-β, and CTGF is compromised in diabetic dermal fibroblasts.
Fig. 4.
Impaired expression of important wound healing mediators in diabetic db/db dermal fibroblasts. The relative mRNA expression of IGF-1 (A), SDF-1 (B), CTGF (C), TGF-β (D), and YAP (E) in the control (CON) and diabetic db/db dermal fibroblasts (db/db) mice. Mouse ribosomal protein 36B4 (36B4) was used as the endogenous control. Data are expressed as the mean ± SD
Discussion
Dysfunctions of dermal fibroblasts are associated with chronic non-healing wounds such as diabetic wounds. Diabetic wound healing is characterized by deficiencies both in the expression of wound healing promoting factors and in wound contraction. In this study, we demonstrated the defect of diabetic dermal fibroblasts in matrix contraction through in vitro collagen gel contraction assay. Moreover, we also demonstrated that the expression levels of SDF-1, IGF-1, CTGF, and TGF-β were decreased in diabetic dermal fibroblasts, compared with that in the control. Additionally, we demonstrated that YAP expression was reduced in diabetic dermal fibroblasts that are associated with defects in wound contraction.
Cutaneous wound healing is impaired in diabetics [4]. Defects in various steps of wound healing, including re-epithelialization, angiogenesis, the ECM synthesis, inflammation response, and contraction, are associated with impaired wound healing [32]. Synthesis of various growth factors and cytokines is also impaired in diabetic wounds [4]. The axis of TGF-β and CTGF is essential in inducing wound healing [10, 22]. There are three isoforms of TGF-β: TGF-β1, TGF-β2, and TGF-β3 [33]. All of the isoforms are expressed in wounds [22] and play roles in various steps of wound healing, such as inflammation, fibroblasts proliferation, and collagen synthesis and deposition [34, 35]. Importantly, it is known that TGF-β signal is impaired in chronic wounds [36] and venous ulcer fibroblasts decrease TGF-β signal following decrease in the expression of TGF-β receptor [37]. CTGF, which is downstream of TGF-β, also plays important roles in wound healing [21, 22]. Dermal fibroblasts express CTGF [38], which enhances angiogenesis through increasing migration, survival, and proliferation of endothelial cells [39]. Our current study demonstrated that expression of both TGF-β and CTGF was also downregulated in diabetic dermal fibroblasts, compared with that in the control. It is likely that the decreased expression of TGF-β and CTGF in dermal fibroblasts is associated with impaired wound healing in diabetes.
The important axis of TGF-β and CTGF functionally interacts with YAP during wound healing [13]. Knockdown of YAP results in delay of wound healing and decreased expression of TGF-β in wounds [21]. Moreover, YAP knockdown also reduces CTGF expression [13, 25] as well as the expression of Smad2 that is an essential mediator of TGF-β signaling [13]. We demonstrated that YAP expression was downregulated in diabetic dermal fibroblasts that expressed lower levels of TGF-β and CTGF than the control. Therefore, it is possible that the reduced expression of YAP induced the decreased expression of wound healing stimulators such as TGF-β and CTGF in diabetic dermal fibroblasts.
Previously, we demonstrated that YAP expression is downregulated in wounds of type 2 diabetic db/db mice (in press). Interestingly, substance P accelerates wound healing in type 2 diabetic db/db mice and the acceleration of wound healing is associated with the increased expression of YAP (in press). Therefore, our previous and current results collectively indicate that YAP would be a novel target of treatment in diabetes with chronic non-healing wounds.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A09057839), the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1479), and a grant from Kyung Hee University in 2016 (KHU-20160701).
Conflict of interest
All of the authors of this manuscript declare that they have no conflict of interest.
Ethical statement
All animal experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use of Kyung Hee University (KHMC-IACUC 15-020).
References
- 1.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122(Pt 18):3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levinson H. A paradigm of fibroblast activation and dermal wound contraction to guide the development of therapies for chronic wounds and pathologic scars. Adv Wound Care. 2013;2(4):149–159. doi: 10.1089/wound.2012.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23(6):594–608. doi: 10.1111/j.1464-5491.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 4.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolte SV, Xu W, Rennekampff HO, Rodemann HP. Diversity of fibroblasts—a review on implications for skin tissue engineering. Cells Tissues Organs. 2008;187(3):165–176. doi: 10.1159/000111805. [DOI] [PubMed] [Google Scholar]
- 6.Thangapazham RL, Darling TN, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15(5):8407–8427. doi: 10.3390/ijms15058407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roh SS, et al. Stimulation of the extracellular matrix production in dermal fibroblasts by velvet antler extract. Ann Dermatol. 2010;22(2):173–179. doi: 10.5021/ad.2010.22.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan C, et al. Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell. 2015;6(12):890–903. doi: 10.1007/s13238-015-0198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261(9):4337–4345. [PubMed] [Google Scholar]
- 10.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 11.Fuentes-Calvo I, et al. H-Ras isoform modulates extracellular matrix synthesis, proliferation, and migration in fibroblasts. Am J Physiol Cell Physiol. 2012;302(4):C686–C697. doi: 10.1152/ajpcell.00103.2011. [DOI] [PubMed] [Google Scholar]
- 12.Poon R, Nik SA, Ahn J, Slade L, Alman BA. Beta-catenin and transforming growth factor beta have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC Cell Biol. 2009;10:38. doi: 10.1186/1471-2121-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MJ, Ran Byun M, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ regulate skin wound healing. J Invest Dermatol. 2014;134(2):518–525. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- 14.Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25(9):499–513. doi: 10.1016/j.tcb.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27(4):355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163(4):811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 20.Azzolin L, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 22.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2(1):18–28. [PMC free article] [PubMed] [Google Scholar]
- 23.Um J, et al. Substance P enhances EPC mobilization for accelerated wound healing. Wound Repair Regen. 2016;24(2):402–410. doi: 10.1111/wrr.12403. [DOI] [PubMed] [Google Scholar]
- 24.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115(2):245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodson WH, III, Hunt TK. Wound healing and the diabetic patient. Surg Gynecol Obstet. 1979;149(4):600–608. [PubMed] [Google Scholar]
- 26.Coleman DL. Diabetes-obesity syndromes in mice. Diabetes. 1982;31(Suppl 1 Pt 2):1–6. doi: 10.2337/diab.31.1.S1. [DOI] [PubMed] [Google Scholar]
- 27.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 28.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(2):92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162(1):303–312. doi: 10.1016/S0002-9440(10)63821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang DJ et al. Gallic acid promotes wound healing in normal and hyperglucidic conditions. Molecules. 2016; 21(7). [DOI] [PMC free article] [PubMed]
- 32.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-beta—an excellent servant but a bad master. J Transl Med. 2012;10:183. doi: 10.1186/1479-5876-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts AB, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark RA, McCoy GA, Folkvord JM, McPherson JM. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol. 1997;170(1):69–80. doi: 10.1002/(SICI)1097-4652(199701)170:1<69::AID-JCP8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 36.Pastar I, et al. Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol Med. 2010;16(3–4):92–101. doi: 10.2119/molmed.2009.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim BC, et al. Fibroblasts from chronic wounds show altered TGF-beta-signaling and decreased TGF-beta Type II receptor expression. J Cell Physiol. 2003;195(3):331–336. doi: 10.1002/jcp.10301. [DOI] [PubMed] [Google Scholar]
- 38.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8(3):171–179. doi: 10.1016/S1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 39.Shimo T, et al. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126(1):137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]