Abstract
This study investigated the therapeutic effects of low-level laser irradiation (LLLI) on the recovery of motor function and its underlying mechanisms in rats with spinal cord injury (SCI). The spinal cord was contused at the T11 level using a New York University impactor. Thirty-eight rats were randomly divided into four groups: LLLI with 0.08 J, 0.4 J, 0.8 J, and sham. We transcutaneously applied at the lesion site of the spinal contusive rats 5 min after injury and then daily for 21 days. The Basso, Beattie and Bresnahan (BBB) locomotor scale and combined behavioral score (CBS) were used to evaluate motor function. The spinal segments of rostral and caudal from the lesion site, the epicenter, and L4–5 were collected from normal and the all groups at 7 days after SCI. The expression of tumor necrosis factor-α (TNF-α) and inducible nitric oxide synthase (iNOS) was compared across groups in all regions. In the present study, LLLI with 0.4 J and 0.8 J led to a significant improvement in motor function compared to sham LLLI, which significantly decreased TNF-α expression at the lesion epicenter and reduced iNOS expression in the caudal segment for all LLLI groups and in the L4–5 segments for the 0.4 J and 0.8 J groups when compared to sham LLLI group. Our results demonstrate that transcutaneous LLLI modulate inflammatory mediators to enhance motor function recovery after SCI. Thus, LLLI in acute phase after SCI might have therapeutic potential for neuroprotection and restoration of motor function following SCI.
Keywords: Low-level laser irradiation, Motor recovery, Post-traumatic inflammation, Secondary injury, Spinal cord injury
Introduction
Traumatic spinal cord injury (SCI) typically results in a loss of motor function due to neuronal cell death which can be a consequence of primary and secondary injury [1]. The primary injury to the spinal cord occurs tissue destruction and hemorrhage at the lesion site [2]. Pathological events following primary injury trigger subsequent secondary neuronal injury that can exacerbate spinal cord damage and limit restorative [3]. Experimental evidences suggest that post-traumatic inflammatory response might be a possible mechanism for secondary neuronal injury after SCI [4, 5]. The post-traumatic inflammatory response is a complex biological process of vascular, biochemical, and cellular events [6]. In this process, cytokines such as tumor necrosis factor-alpha (TNF-α) and nitric oxide (NO) play a critical role during the initial phase. Among these cytokines, TNF-α act as an active participant of the inflammatory response in the central nervous system (CNS) [7, 8]. Experimental studies have shown that TNF-α expression rapidly increases, and inhibition of that helps functional recovery after SCI [9, 10]. NO is produced by nitric oxide synthase (NOS) which contributes to the pathogenesis of secondary injury after CNS damage [11, 12]. In particular, the expression of inducible NOS (iNOS) after SCI acts as an indicator of the inflammatory reaction that leads to secondary injury [13]. Previous studies have reported that motor function improves when an iNOS inhibitor is administered after SCI [14, 15]. Taken together, inhibition of TNF-α and iNOS expression can reduce secondary damage after SCI, which may be able to minimize the loss of motor function.
Low-level laser irradiation (LLLI) has been widely used for various pathological conditions, including wound healing [16] and pain control [17]. Previously, in vitro experiments have shown the anti-inflammatory effects of LLLI [18]. Several researches using various animal models have also demonstrated the anti-inflammatory effects of LLLI in and have tried to unravel the underlying mechanisms [19, 20]. They have shown that LLLI reduces the production of pro-inflammatory mediators or cytokines such as TNF-α and iNOS, implying that LLLI has therapeutic potential as an alternative to anti-inflammatory drugs [19, 20]. Recently, experimental studies have revealed that LLLI could have neuroprotective and therapeutic effects on functional recovery following brain injury, reducing the post-traumatic inflammatory response [21–23], but there are still remain controversy. In addition, early studies related to the effects of LLLI on motor recovery have focused on brain injury, and systematic studies of LLLI effects of on SCI are still lacking. Furthermore, most research has been conducted using different laser parameters such as wavelength, power, dose, and duration, and thus, the possible mechanisms behind these effects remain unclear.
Thus, we examined the effects of LLLI applied at the lesion site of SCI on the recovery of motor function in the present study. The expression of TNF-α and iNOS was also assessed in order to investigate mechanisms underlying the therapeutic effects of LLLI.
Materials and methods
Experimental animals
All animal protocols were approved by the Korea University Institutional Animal Care and Use Committee (KUIACUC-2010-170). Sixty-three adult male Sprague-Dawley rats (200 g, Orient Bio Inc., Seoul, Korea) were used in this study. Rats were maintained under a 12-h light/dark cycle with free access to water and food.
Contusive spinal cord injury
Contusive SCI was induced at T11 spinal level, using the NYU impactor under isoflurane anesthesia (3% isoflurane and 97% O2). In brief, a laminectomy was performed at the T10 vertebra and a 10 g weight was dropped onto the spinal cord from a 12.5 mm height. The musculature was sutured, and the wound closed using clips. Ampicillin/sulbactam, (Unasyn, 100 mg/kg, Pfizer, Seoul, Korea) was administered intraperitoneally twice a day to prevent urinary tract infection.
Low level laser irradiation
A laser in the infrared range (ASA, Italy) was used with a wavelength of 850 nm and continuous output power of 500 mW. The LLLI unit controls the output by modulating the frequency of the cycle to deliver 0.08 J, 0.4 J, or 0.8 J. Rats (n = 38) were randomly divided into one sham group (n = 11), and three experimental groups (n = 9, in each group), each receiving different doses of LLLI: 0.08 J (n = 9), 0.4 J (n = 9), and 0.8 J (n = 9). LLLI was transcutaneously applied to the lesion site for 4 min 10 s, at a time point 5 min after injury and then daily for 21 days. The distance between the laser head and the lesion site was fixed accurately at 30 cm. During laser applications, rats were gently handled and immobilized by an experimenter. Rats in the sham group were handled identically, except they did not receive LLLI.
Behavioral tests for motor function
Behavioral tests were performed before and after SCI at days 1, 4, 7, 10, 14, 21, 28, and 35. The Basso, Beattie, and Bresnahan (BBB) locomotor rating scale [24] and a modified combined behavioral score (CBS) [25] were used for test motor function. The CBS assigns a weighted score to particular tests, which are then combined into one total score. Individual tests comprising the CBS are motor score, toe spread, right reflex, extension withdrawal reflex, and placing reflex. Motor function scores in the right and left hindlimb were averaged.
Western blot
The spinal segments rostral and caudal to the lesion site, at the epicenter, and the L4–5 segments were collected from normal rats and all groups including sham and three experimental groups (n = 5 in each group) 7 days after SCI. Tissues were homogenized in extraction buffer [50 mM Tris-HCl (pH 7.4), 1% Triton X-100, 1% SDS, 10 mM EDTA (pH 8.0), 1% protease inhibitor, 150 mM sodium chloride] and centrifuged. The supernatant was collected, and assayed for protein concentration using the BCA assay (Pierce, Rockford, IL). Samples containing approximately 40 μg protein were separated by 13% SDS-PAGE, and transferred to a PVDF membrane for 2 h at 90 V on ice (Mini-PROTEAN tetra cell; Bio-Rad Laboratories, Richmond, CA, USA). Membranes were blocked in 5% non-fat dried milk for 3 h with agitation. They were then incubated for 12 h at 4 °C in primary antibodies, prepared in 5% blocking solution at the following dilutions: anti-TNF-α (Chemicon, Temecular, CA, USA.) at 1:1000; anti-iNOS (Chemicon, Temecular, CA, USA) at 1:2000; anti-ß-tubulin (Abcam, Cambridge Science Park, UK) at 1:500,000. After washing with TBST, membranes were incubated for 1 h at room temperature with peroxidase-conjugated anti-rabbit IgG secondary antibody in 5% blocking solution at 1:6000 dilution (TNF-α and iNOS) or 1:100,000 (β-tubulin) (Vector Labs, PI-1000, Burlingame, CA, USA). After several washes with TBST, membranes were incubated with ECL (Millipore, catalog no. WBKLS 0100), and then exposed to medical X-ray film (AGFA). The densities of specific bands were analyzed using Scion software.
Statistical analysis
All values were expressed as mean ± standard error of mean (SEM). Statistical significance was set at the 0.05 level. The Kruskal and Wallis test followed by post hoc analysis was used to compare differences in the behavioral results obtained for the three groups. TNF-α and iNOS expression among groups was compared using one-way analysis of variance (ANOVA), followed by the least squares difference (LSD) post hoc test.
Results
Effect of LLLI on functional motor recovery after SCI
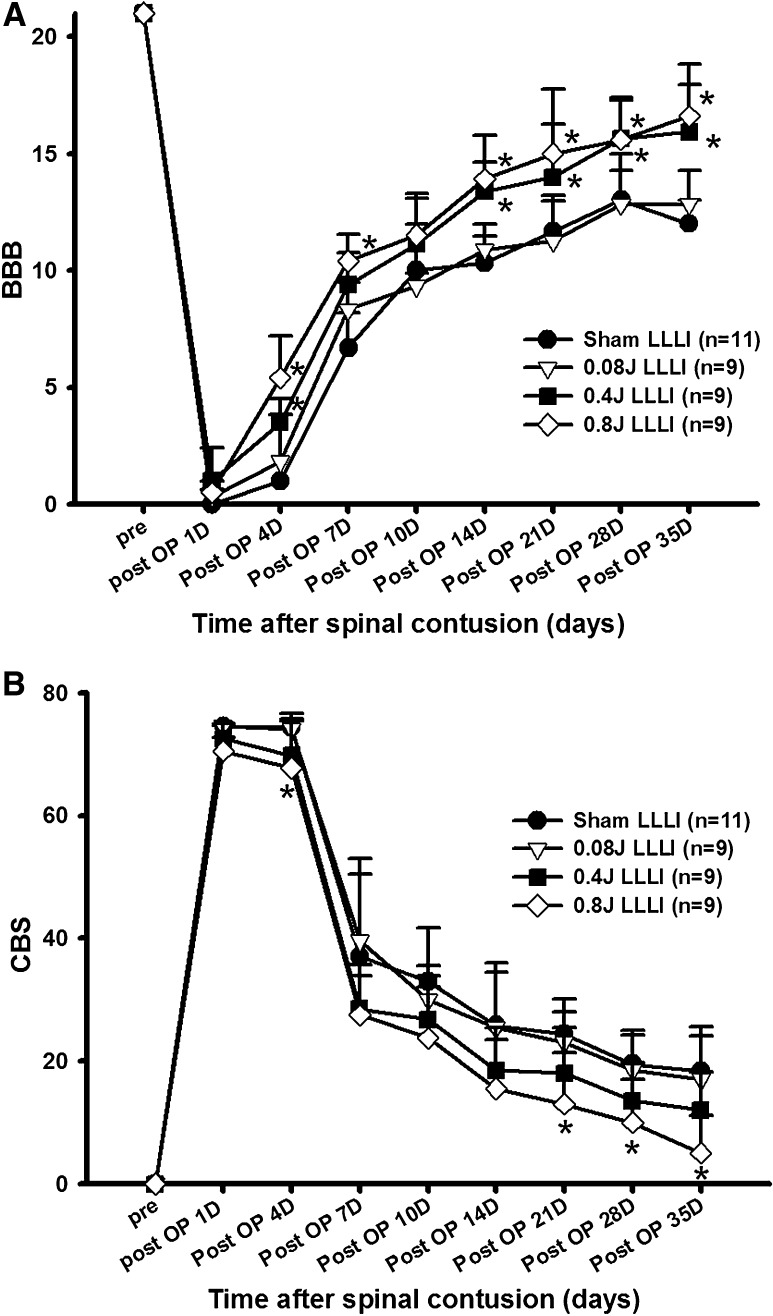
Prior to spinal contusion, all rats showed normal gaits with BBB scores of 21. Immediately after injury, rats showed paralysis in both hindlimbs, corresponding to a BBB score of zero. There was no significant difference in the BBB scores among groups one day after SCI. Progressive motor recovery was observed with hindlimb joint movement four days after injury, and continued until days 35, as in our previous reports [26]. At that time point, most of the rats could step with their weight supported by their hindpaws. Throughout the entire recovery period, LLLI-treated rats showed more pronounced motor recovery compared to the sham-treated rats (Fig. 1A). The BBB score of rats in the 0.8 J group was significantly higher than that of rats in the sham group at days 4 to 35, after SCI, except on days 10 (P < 0.05). Rats in the 0.4 J group also showed significant recovery of locomotor function compared to the sham group at days 4 to 35, except on days 7 and 10 (P < 0.05). There was no significant difference in the BBB score between sham-treated and 0.08 J groups throughout the study (P > 0.05). Marked differences in locomotor function between the LLLI and sham groups were noticed at days 21, even though statistically significant differences existed as early as 14 days after SCI. At 21 days after SCI, the groups receiving higher doses of LLLI scored 15.0 ± 2.76 and 14.00 ± 2.24 on the BBB scale (for the 0.8 J and 0.4 J groups, respectively), indicating consistently coordinated forelimb and hindlimb stepping, whereas the 0.08 J and sham groups scored 11.25 ± 1.73 and 11.67 ± 1.53, respectively, indicating uncoordinated stepping (Fig. 1A). At this time point, although differences in the BBB scores did not seem very large, the differences in terms of functional gain were significant.
Fig. 1.
Effects of LLLI on motor function. A Changes in locomotor function after LLLI (BBB score). In the 0.8 J group, locomotor function recovered significantly from days 4 to 35 after SCI, except on days 10. BBB scores for the 0.4 J group significantly increased from days 4 to 35 after SCI, except from days 7 to 10. *P < 0.05. B Changes in combined behavioral score after LLLI. CBS in the 0.8 J group was significantly lower on days 4, 21, 28, and 35 after SCI than the sham group. *P < 0.05. All data are expressed as mean ± SEM
Simultaneously, the CBS was also assessed with and without LLLI. Rats in all groups showed a decrease in CBS over time during the experimental period. However, the rats in 0.8 J group showed significantly lower CBS than those in the sham group at days 4, 21, 28, and 35 after SCI (Fig. 1B) (P < 0.05). Prominent differences in CBS was also observed on days 21, 28, and 35 after SCI in the 0.8 J group with accompanying coordinated stepping (Fig.1B) (p < 0.05).
Changes in TNF-α and iNOS expression in the spinal cord by LLLI
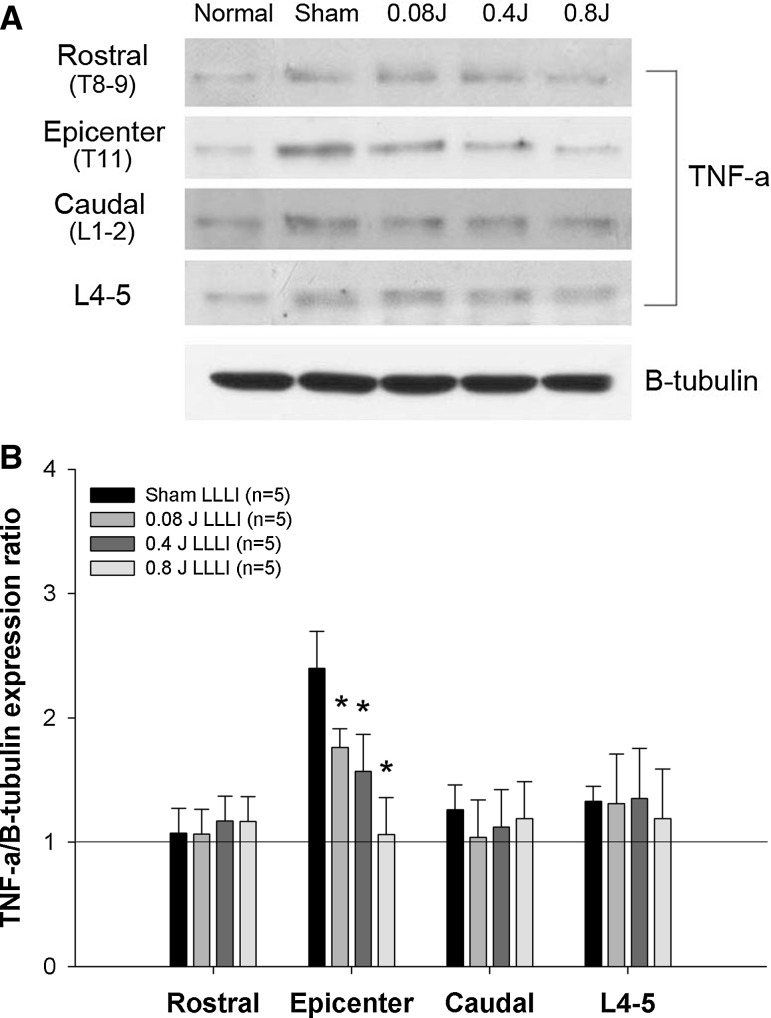
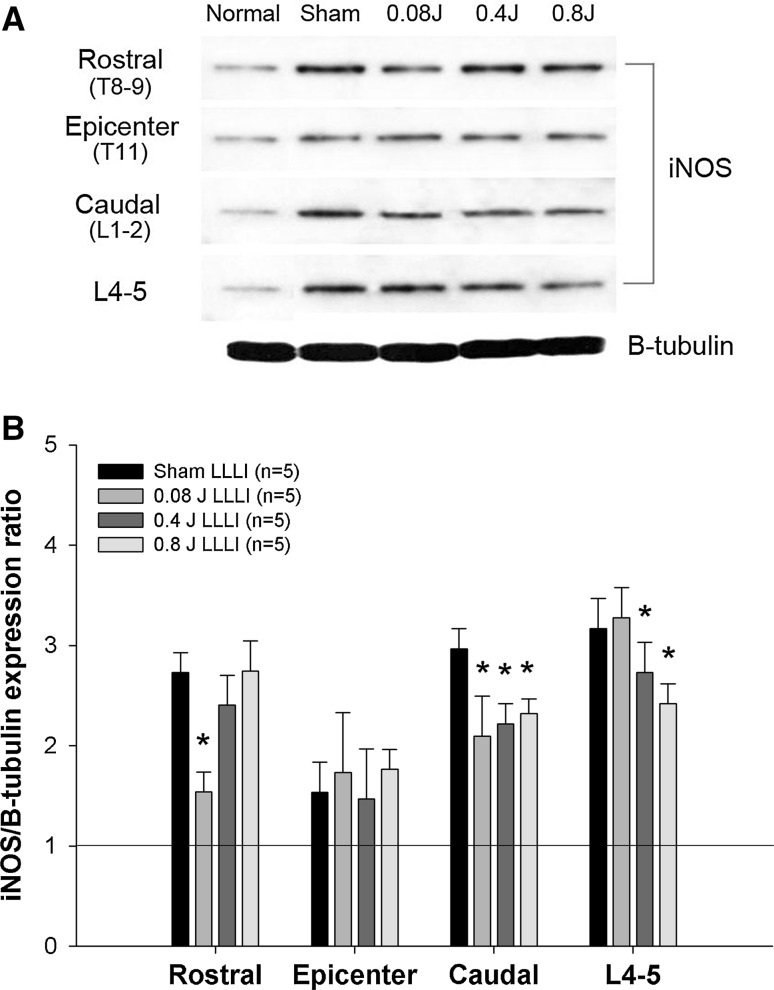
To investigate the effects of LLLI on the post-traumatic inflammatory response after SCI, TNF-α and iNOS expression levels were assessed. Basal levels of TNF-α and iNOS were detected in normal rats. After SCI, TNF-α expression was markedly increased at the epicenter of the spinal tissue, but did not show a significant changes in expression in the rostral, caudal, and L4–5 spinal segments compared to normal rats. Transcutaneously applied LLLI at the lesion site (0.4, 0.8, and 0.08 J) resulted in dose-dependent decreases in the expression levels of TNF-α at the epicenter in all LLLI groups (Fig. 2), and there were significant differences compared to the sham group (P < 0.05). In the rostral, caudal, and L4–5 segments, the expression levels of TNF-α were not significantly different among groups. In contrast to TNF-α expression, iNOS expression was significantly increased in rostral, caudal, and L4–5 spinal segments after SCI compared to normal rats, whereas there was slight increase in iNOS expression at the epicenter. LLLI at the lesion site (0.4, 0.8, and 0.08 J) significantly decreased iNOS expression in rostral, caudal, and L4–5 spinal segments (Fig. 3) (P < 0.05). Particularly, iNOS expression was significantly decreased in the caudal spinal segments of all LLLI groups. In the L4–5 spinal segment, LLLI with 0.4 J and 0.8 J significantly inhibited iNOS expression compared to the sham group. iNOS expression levels in the rostral segments were significantly decreased only in the 0.08 J group compared to the sham group. There were no significant differences at the epicenter among groups.
Fig. 2.
Change of spinal TNF-a protein expression in the spinal cord after LLLI treatment following SCI. A Representative western blots: a comparison of TNF-α expression in rostral, epicenter, caudal, and L4–5 segments in each group (n = 5/each group). B A quantitative graph for TNF-α protein expression. A line presents the basal level of TNF-α detected in the equivalent segment of the spinal cord of a normal rat. LLLI significantly reduced TNF-α expression in the lesion epicenter compared with the sham group. *P < 0.05. All data are expressed as mean ± SEM
Fig. 3.
Change of spinal iNOS protein expression in the spinal cord after LLLI treatment following SCI. A Representative western blots: a comparison of iNOS expression in rostral, epicenter, caudal, and L4–5 segments in each group (n = 5/each group). B A quantitative graph for iNOS protein expression. A dot line presents the basal level of iNOS detected in the equivalent segment of the spinal cord of a normal rat. LLLI significantly reduced the expression of iNOS in the rostral, caudal, and L4–5 segments compared with the sham group. *P < 0.05. All data are expressed as mean ± SEM
Discussion
The present study demonstrated that transcutaneously applied LLLI to the lesion site after SCI effectively improved recovery of motor function and also decreased the protein expression of TNF-α and iNOS in the lesion or surrounding areas following SCI. Considering that TNF-α and NO are active participants in the inflammatory response to CNS damage, which is a possible mechanism behind further tissue damage and functional impairment after SCI [7, 27]. Beneficial effects of LLLI on inflammation are well known in various pathological conditions [19, 20]. Recently, experimental studies have demonstrated the neuroprotective effects of LLLI after CNS injury, but the therapeutic effects of LLLI on CNS injury are still controversial [21, 22, 28]. Detaboada et al. has reported that LLLI applied at 24 h minimized neurological deficits and improved functional recovery after stroke [21]. However, another study has showed that LLLI failed to improve behavioral performance when transcranial LLLI was applied at same time point [22]. Most previous studies related to LLLI effects on motor recovery after CNS damage have focused on brain damage. Only a few reports show any evidence for significant LLLI effects on motor recovery after SCI [28], and they only considered the effects of a single application of LLLI.
In our study, LLLI was transcutaneously applied at the lesion site. The doses of LLLI (0.08 J, 0.4 J and 0.8 J) were chosen based on previous reports that LLLI was clinically efficacious at doses 0.001–10 J/cm2 [29]. LLLI had applied at the lesion site 5 min post-injury and daily for 21 days after spinal contusion. Motor function was examined for up to 35 days after SCI to investigate whether LLLI can further improve motor recovery, based on previous reports showing that functional motor recovery plateaued three weeks after SCI [26, 30]. The present results showed that LLLI beginning 5 min post-injury improved motor function depending on the dose of LLLI after SCI in rats. Higher doses of LLLI (0.4 J and 0.8 J groups) enhanced recovery of locomotor function compared to the sham group from days 4 to 35 after SCI (Fig. 1A). Interestingly, rats receiving higher doses of LLLI showed improvement of motor function till 35 days after SCI (the last day of this study), even though LLLI had only been applied daily until 21 days after spinal contusion. Our findings are consistent with a previous study showing that transcutaneous LLLI promotes functional recovery after hemisected SCI in rats [28]. They demonstrated that transcutaneous LLLI with the wavelengths between 770 and 850 nm at the lesion site penetrated to the depth of the spinal cord, and LLLI applied 15 min after injury and daily for 14 days restored the angle of hindlimb rotation after SCI. However, this group investigated a single dose (1,589 J per day) of LLLI effects on specific and limited motor functions such as footfalls, stride length, angle of rotation, and time taken to cross a ladder.
Protective effects of LLLI have been demonstrated previously, for instance, by the upregulation of heat shock protein by LLLI in ischemic muscle injury [31]. However, the exact mechanisms associated with the therapeutic effect of LLLI on functional recovery after SCI are poorly understood. Post-traumatic inflammation occurs within minutes of SCI and persists for days, contributing to progressive tissue loss and becoming a major cause of motor dysfunction [2, 3]. The result that LLLI during the acute phase after SCI could induce a significant long-term motor recovery suggests that LLLI has neuroprotective effects of after SCI, and may work by modulating the acute inflammatory response at the lesion site. This is strongly supported by previous reports in which transcranial LLLI during acute phase after brain injury provide long-term functional recovery in rats [22, 23, 32]. Lapchak et al., showed that LLLI within 6 hour after embolic stroke provided long-term functional neurological benefits in rabbit, but not LLLI 24 hours after stroke [22].
To investigate the underlying mechanism of LLLI therapeutic effect, we analyzed the protein expression of proinflammatory mediators after LLLI applied in spinal contusive rats. Many studies show a more complicated picture of secondary pathology which is characterized by neuronal and glial apoptosis, increased blood-CNS barrier permeability and neuro-inflammatory responses. Among these, neuro-inflammatory responses are of central importance in the pathogenesis after SCI and can persist for month or years after the initial trauma. Previously, the protein levels of TNF-α continue to increase till first week after SCI [33], even though mRNA levels of TNF-α and interleukin (IL)-1 are rapidly increased in most cellular components of CNS within 15 min [34] and are reached their peak after several hours after SCI [35]. Nitric oxide (NO) is physiologically produced in small amount by the vascular endothelium and neurons, however, NO concentrations which is mainly controlled by the pro-inflammatory enzyme inducible nitric oxide synthase (iNOS) immediately increase and then gradually decrease between 1 and 12 days after contusive SCI [36]. Immoderate NO causes loss of spinal neuron and motor function, suggesting that excessive NO production is the causative mechanism underlying neurotoxicity [37]. Anti-inflammatory treatment promotes axonal regeneration and functional recovery after SCI [38]. Consequently, the pro-inflammatory cytokines such as TNF-α, IL-1, IL-6 and NO dominates the injured environment during the acute phase (within first 7 days) after SCI. This is the reason we specifically assessed the protein expression of TNF-α and iNOS at 7 days after contusive SCI to determine effects of LLLI on post-traumatic neuroinflammatory response, which may be the possible mechanisms underlying the functional recovery.
A main finding in this study is that protein expression of tumor necrosis factor α (TNF-α) was markedly increased at the epicenter (Fig. 2), while inducible nitric oxide synthase (iNOS) expression was significantly increased in the surrounding areas (rostral, caudal, and L4–5 spinal segments) after SCI (Fig. 3). It suggests that neuroinflammatory response is undergoing to remote site from injured area. In addition, LLLI did change protein level of TNF-α and iNOS at the spinal segments at 1 week and motor function until 5 weeks after SCI in the present study. Previously, microglia activity is appeared at an earlier time point (1 day) in the epicenter, while that increases in the surrounding area from epicenter during later stage (7 days) and reaches their plateaus 2–4 weeks after SCI [39]. Activated macrophages and microglia was inhibited by photo-biomodulation at the lesion site on the spinal cord [28]. TNF-α activates macrophage and microglia [40], which produce inflammatory mediators such as TNF-α, IL-1, ROS, prostaglandin and iNOS through the nuclear factor-κB (NF-κB) pathway after injury [6, 41–43]. Furthermore, TNF-α inhibitor administration after SCI enhance functional motor recovery [9]. The results suggest that a decrease in TNF-α expression at the lesion epicenter in response to LLLI reflects the modulation of the inflammatory response, including the activation of microglia, and this can contribute to the recovery of motor function after SCI.
On the other hand, upregulation of iNOS expression in the tissues surrounding the lesion epicenter after SCI that are not directly damaged by SCI, but could be susceptible to secondary injury [14]. This results suggest that a rapid upregulation of proinflammatory cytokines TNF-α and iNOS at the primary injured site after injury, which extends to rostral, caudal and L4–5 spinal segments as a remote site are undergoing delayed secondary injury after SCI. Interestingly, transcutaneously applied LLLI at the lesion site inhibited the increased iNOS expression in surrounding the lesion epicenter, especially below the level of injury in both caudal and L4–5 segments, compared with the sham group. Several studies showed that the motor function can be improved after SCI by modulating iNOS [14, 44] including our current results. Segmental administration of aminoguanidine, an inhibitor of iNOS activity, improved locomotor recovery and reduced the tissue damage after SCI [14]. Following SCI, phagocytic stimuli boost the generation of reactive oxygen species (ROS) [45], causing oxidative stress in tissues, which could contribute to locomotor dysfunction [46]. ROS activate NF-κB and increase iNOS expression, which directly damages vital cell components [47]. Recent studies showed that long-term repeated LLLI is associated with a ROS reduction [18, 48]. In addition, Yune et al. reported that inhibition of TNF-α reduces the expression of iNOS gene after SCI [49], suggesting that the decreased TNF-α expression at the epicenter by LLLI, as seen in this study, might affect iNOS expression in more remote sites. One possible explanation is that decreased iNOS expression in caudal and L4–5 segments, distant site to the primary injury by LLLI may affect lower limb motor function by reducing the neuroinflammatory-induced further tissue damage after SCI, thereby contributing to improvements in motor function.
It is well known that overactivation of macrophage and microglia at the site distant to primary injury in chronic stage of SCI can persist neuroinflammatory responses for month or years after the initial trauma. Thus, the effect of LLLI on the neuron or glial cells proliferation, in chronic phase of SCI are an interesting question to elucidate in further study.
In summary, this study demonstrates that transcutaneous LLLI to the lesion site improved motor recovery and suppressed TNF-α and iNOS expression involved in the post-traumatic inflammatory response, in a dose-dependent manner. This study suggests that LLLI application during the acute SCI could help promote motor recovery by modulating the inflammatory responses. These finding support the idea that LLLI has clinical potential as a noninvasive treatment tool for SCI.
Acknowledgements
This work was supported by the convergence technology development program for bionic arm and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014M3C1B2048632) and by the Ministry of Education 2016R1D1A1B03933986. It was also partially supported by a grant of the Korea University College of Health Science (K1508371).
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical statement
All animal protocols in this study were approved by the Korea University Institutional Animal Care and Use Committee (KUIACUC-2010-170).
Reference
- 1.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 2.Taoka Y, Okajima K. Spinal cord injury in the rat. Prog Neurobiol. 1998;56:341–358. doi: 10.1016/S0301-0082(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 3.Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Bartholdi D, Schwab ME. Methylprednisolone inhibits early inflammatory processes but not ischemic cell death after experimental spinal cord lesion in the rat. Brain Res. 1995;672:177–186. doi: 10.1016/0006-8993(94)01410-J. [DOI] [PubMed] [Google Scholar]
- 5.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 6.Hausmann O. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 7.Bethea JR, Dietrich DW. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002;15:355–360. doi: 10.1097/00019052-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Renno T, Krakowski M, Piccirillo C, Lin J, Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- 9.Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muià C, Bramanti P, et al. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Ther. 2006;316:1006–1016. doi: 10.1124/jpet.105.097188. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-α expression and NF-kB activation after spinal cord injury in rats. Mol Brain Res. 1998;59:135–142. doi: 10.1016/S0169-328X(98)00142-9. [DOI] [PubMed] [Google Scholar]
- 11.Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, et al. Roles of nitric oxide in compression injury of rat spinal cord. Free Radic Biol Med. 1996;20:1–9. doi: 10.1016/0891-5849(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 12.Q-w Xie. Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Kim G-M, Chen S, Yan P, Ahmed SH, Ku G, et al. iNOS and nitrotyrosine expression after spinal cord injury. J Neurotrauma. 2001;18:523–532. doi: 10.1089/089771501300227323. [DOI] [PubMed] [Google Scholar]
- 14.Chatzipanteli K, Garcia R, Marcillo AE, Loor KE, Kraydieh S, Dietrich WD. Temporal and segmental distribution of constitutive and inducible nitric oxide synthases after traumatic spinal cord injury: effect of aminoguanidine treatment. J Neurotrauma. 2002;19:639–651. doi: 10.1089/089771502753754109. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, Matsuyama Y, Nakashima S, Yanase M, Kiuchi K, Ishiguro N. Effects of MPSS and a potent iNOS inhibitor on traumatic spinal cord injury. Neuroreport. 2004;15:2103–2107. doi: 10.1097/00001756-200409150-00021. [DOI] [PubMed] [Google Scholar]
- 16.Whelan HT, Smits RL, Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305–314. doi: 10.1089/104454701753342758. [DOI] [PubMed] [Google Scholar]
- 17.Turhani D, Scheriau M, Kapral D, Benesch T, Jonke E, Bantleon HP. Pain relief by single low-level laser irradiation in orthodontic patients undergoing fixed appliance therapy. Am J Orthod. 2006;130:371–377. doi: 10.1016/j.ajodo.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Fujimaki Y, Shimoyama T, Liu Q, Umeda T, Nakaji S, Sugawara K. Low-level laser irradiation attenuates production of reactive oxygen species by human neutrophils. J Clin Laser Med Surg. 2003;21:165–170. doi: 10.1089/104454703321895635. [DOI] [PubMed] [Google Scholar]
- 19.Moriyama Y, Nguyen J, Akens M, Moriyama EH, Lilge L. In vivo effects of low level laser therapy on inducible nitric oxide synthase. Lasers Surg Med. 2009;41:227–231. doi: 10.1002/lsm.20745. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira RG, Ferreira AP, Côrtes AJ, Aarestrup BJV, Andrade LC, Aarestrup FM. Low-level laser reduces the production of TNF-α, IFN-γ, and IL-10 induced by OVA. Lasers Med Sci. 2013;28:1519–1525. doi: 10.1007/s10103-012-1262-5. [DOI] [PubMed] [Google Scholar]
- 21.DeTaboada L, Ilic S, Leichliter-Martha S, Oron U, Oron A, Streeter J. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg Med. 2006;38:70–73. doi: 10.1002/lsm.20256. [DOI] [PubMed] [Google Scholar]
- 22.Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- 23.Oron A, Oron U, Streeter J, De Taboada L, Alexandrovich A, Trembovler V, et al. Near infrared transcranial laser therapy applied at various modes to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2012;29:401–407. doi: 10.1089/neu.2011.2062. [DOI] [PubMed] [Google Scholar]
- 24.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 25.Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985;88:123–134. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- 26.Jung J-I, Kim J, Hong SK, Yoon YW. Long-term follow-up of cutaneous hypersensitivity in rats with a spinal cord contusion. Korean J Physiol Pharmacol. 2008;12:299–306. doi: 10.4196/kjpp.2008.12.6.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100:639–649. doi: 10.1111/j.1471-4159.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 28.Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, et al. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med. 2005;36:171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 29.Rochkind S, Ouaknine G. New trend in neuroscience: low-power laser effect on peripheral and central nervous system (basic science, preclinical and clinical studies) Neurol Res. 1992;14:2–11. doi: 10.1080/01616412.1992.11740003. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe M, Ono K, Honda M, Ono H. Gabapentin and pregabalin ameliorate mechanical hypersensitivity after spinal cord injury in mice. Eur J Pharmacol. 2009;609:65–68. doi: 10.1016/j.ejphar.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Avni D, Levkovitz S, Maltz L, Oron U. Protection of skeletal muscle from ischemia/reperfusion injury by low energy laser irradiation. Photomed Laser Surg. 2005;23:273–277. doi: 10.1089/pho.2005.23.273. [DOI] [PubMed] [Google Scholar]
- 32.Xuan W, Vatansever F, Huang L, Wu Q, Xuan Y, Dai T, et al. Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS One. 2013;8:e53454. doi: 10.1371/journal.pone.0053454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyor WR, Avgeropoulos N, Ohlandt G, Hogan EL. Treatment of spinal cord impact injury in the rat with transforming growth factor-β. J Neurol Sci. 2002;200:33–41. doi: 10.1016/S0022-510X(02)00113-2. [DOI] [PubMed] [Google Scholar]
- 34.Yan P, Li Q, Kim GM, Xu J, Hsu CY, Xu XM. Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J Neurotrauma. 2001;18:563–568. doi: 10.1089/089771501300227369. [DOI] [PubMed] [Google Scholar]
- 35.Klusman I, Schwab ME. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res. 1997;762:173–184. doi: 10.1016/S0006-8993(97)00381-8. [DOI] [PubMed] [Google Scholar]
- 36.Nakahara S, Yone K, Setoguchi T, Yamaura I, Arishima Y, Yoshino S, et al. Changes in nitric oxide and expression of nitric oxide synthase in spinal cord after acute traumatic injury in rats. J Neurotrauma. 2002;19:1467–1474. doi: 10.1089/089771502320914697. [DOI] [PubMed] [Google Scholar]
- 37.Bao F, Liu D. Peroxynitrite generated in the rat spinal cord induces neuron death and neurological deficits. Neuroscience. 2002;115:839–849. doi: 10.1016/S0306-4522(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 38.Nash HH, Borke RC, Anders JJ. Ensheathing cells and methylprednisolone promote axonal regeneration and functional recovery in the lesioned adult rat spinal cord. J Neurosci. 2002;22:7111–7120. doi: 10.1523/JNEUROSCI.22-16-07111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(SICI)1096-9861(19970120)377:3<443::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 42.Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, et al. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res. 2000;85:114–122. doi: 10.1016/S0169-328X(00)00253-9. [DOI] [PubMed] [Google Scholar]
- 43.Leskovar A, Moriarty LJ, Turek JJ, Schoenlein IA, Borgens RB. The macrophage in acute neural injury: changes in cell numbers over time and levels of cytokine production in mammalian central and peripheral nervous systems. J Exp Biol. 2000;203:1783–1795. doi: 10.1242/jeb.203.12.1783. [DOI] [PubMed] [Google Scholar]
- 44.Sharma H, Westman J, Olsson Y, Alm P. Involvement of nitric oxide in acute spinal cord injury: an immunocytochemical study using light and electron microscopy in the rat. Neurosci Res. 1996;24:373–384. doi: 10.1016/0168-0102(95)01015-7. [DOI] [PubMed] [Google Scholar]
- 45.Cuzzocrea S, Thiemermann C, Salvemini D. Potential therapeutic effect of antioxidant therapy in shock and inflammation. Curr Med Chem. 2004;11:1147–1162. doi: 10.2174/0929867043365396. [DOI] [PubMed] [Google Scholar]
- 46.Gwak YS, Hassler SE, Hulsebosch CE. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain. 2013;154:1699–1708. doi: 10.1016/j.pain.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Conti A, Miscusi M, Cardali S, Germanò A, Suzuki H, Cuzzocrea S, et al. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev. 2007;54:205–218. doi: 10.1016/j.brainresrev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Enwemeka CS. Therapeutic light. Rehab Manag. 2004;17:20–25. [PubMed] [Google Scholar]
- 49.Yune TY, Chang MJ, Kim SJ, Lee YB, Shin SW, Rhim H, et al. Increased production of tumor necrosis factor-α induces apoptosis after traumatic spinal cord injury in rats. J Neurotrauma. 2003;20:207–219. doi: 10.1089/08977150360547116. [DOI] [PubMed] [Google Scholar]