Abstract
Tissue injury provokes a series of events containing inflammation, new tissue formation and tissue remodeling which are regulated by the spatially and temporally coordinated organization. It is an evolutionarily conserved, multi-cellular, multi-molecular process via complex signalling network. Tissue injury disorders present grievous clinical problems and are likely to increase since they are generally associated with the prevailing diseases such as diabetes, hypertension and obesity. Although these dynamic responses vary not only for the different types of trauma but also for the different organs, a balancing act between the tissue degradation and tissue synthesis is the same. In this process, the degradation of old extracellular matrix (ECM) elements and new ones’ synthesis and deposition play an essential role, especially collagens. Lysyl oxidase (LOX) and four lysyl oxidase-like proteins are a group of enzymes capable of catalyzing cross-linking reaction of collagen and elastin, thus initiating the formation of covalent cross-links that insolubilize ECM proteins. In this way, LOX facilitates ECM stabilization through ECM formation, development, maturation and remodeling. This ability determines its potential role in tissue repair and regeneration. In this review, based on the current in vitro, animal and human in vivo studies which have shown the significant role of the LOXs in tissue repair, e.g., tendon regeneration, ligament healing, cutaneous wound healing, and cartilage remodeling, we focused on the role of the LOXs in inflammation phase, proliferation phase, and tissue remodeling phase of the repair process. By summarizing its healing role, we hope to shed light on the understanding of its potential in tissue repair and provide up to date therapeutic strategies towards related injuries.
Keywords: Lysyl oxidase, Cross-link, Repair, Remodeling, Tissue-engineering
Summary
Tissue injury provokes a series of events containing inflammation, new tissue formation and tissue remodeling which are regulated by the spatially and temporally coordinated organization. It is an evolutionarily conserved, multi-cellular, multi-molecular process via complex signalling network [1]. Tissue injury disorders present grievous clinical problems and are likely to increase since they are generally associated with the prevailing diseases such as diabetes, hypertension and obesity [2]. Although these dynamic responses vary not only for the different types of trauma but also for the different organs, a balancing act between the tissue degradation and tissue synthesis is the same [3]. In this process, the degradation of old extracellular matrix (ECM) elements and new ones’ synthesis and deposition play an essential role, especially collagens [4].
Lysyl oxidase (LOX) and four lysyl oxidase-like proteins are a group of enzymes capable of catalyzing cross-linking reaction of collagen and elastin, thus initiating the formation of covalent cross-links that insolubilize ECM proteins. In this way, LOX facilitates ECM stabilization through ECM formation, development, maturation and remodeling [5, 6]. This ability determines its potential role in tissue repair and regeneration.
In this review, based on the current in vitro, animal and human in vivo studies which have shown the significant role of the LOXs in tissue repair, e.g., tendon regeneration, ligament healing, cutaneous wound healing, and cartilage remodeling, we focused on the role of the LOXs in inflammation phase, proliferation phase, and tissue remodeling phase of the repair process. By summarizing its healing role, we hope to shed light on the understanding of its potential in tissue repair and provide up to date therapeutic strategies towards related injuries.
Introduction
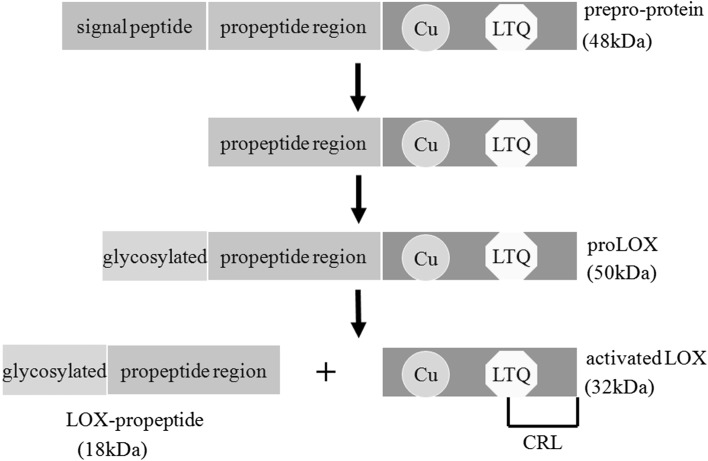
Lysyl oxidase (LOX), a copper-dependent amine oxidase, initiates covalent cross-linking of elastin and collagen by catalyzing oxidative deamination of the ε-amino group of lysine and/or hydroxylysine [7] and ultimately insolubilizes these extracellular proteins resisting to proteolysis. The LOX family (LOXs) contains another four additional members, lysyl oxidase-like protein 1 (LOXL-1) [8], lysyl oxidase-like protein 2 (LOXL-2) [9], lysyl oxidase-like protein 3 (LOXL-3) [10], and lysyl oxidase-like protein 4 (LOXL-4) [11, 12], respectively. Each shows the highly similarity in the carboxyl (C)-terminus, including the copper binding domain, the lysyl-tyrosyl quinone (LTQ) residues, and the cytokine receptor-like (CRL) domain [13]. By comparison, the amino (N)-terminal regions of the LOXs are variable, showing little homology except for LOXL-2, LOXL-3, and LOXL-4, which are characterized by a conservation of four copies of scavenger receptor cysteine-rich (SRCR) domains [14]. Another N-terminal consensus related to cleavage by procollagen C-proteinases also appears among the LOX and LOXL-1 [15]. The distinct sequence of each member may contribute to their individual biological functions. The human LOX [16] is synthesized as a 48-kDa prepro-protein in the ribosome. Following translation, the N-terminal signal peptide sequence is removed in the endoplasmic reticulum and the propeptide domain is N-glycosylated in the golgi [16]. After these intracellular processing, a 50-kDa pro-protein (proLOX) is secreted to the extracellular compartment in which the proLOX is cleaved between residues Gly168 and Asp169 by procollagen C-proteinase, mainly bone morphogenic protein-1 (BMP-1) [5]. Later on, the 32-kDa active LOX is released to the ECM to participate in cross-linking of the extracellular proteins (Fig. 1).
Fig. 1.
The synthesis of activated lysyl oxidases. The output of the process is the removal of the N-terminal signal peptide sequence of human LOX in the endoplasmic reticulum. Then the propeptide domain is N-glycosylated in Golgi and the 50-kDa pro-protein (proLOX) is generated. The proLOX is cleaved by procollagen C-proteinase, mainly bone morphogenic protein-1 (BMP-1), leading to the formation of activated LOX and LOX-propeptide (LOX-PP). In the carboxyl (C)-terminus, the copper binding domain (Cu), the lysyl-tyrosyl quinone (LTQ) residues, and the cytokine receptor-like (CRL) domain are highly conserved in each member of the LOX family
It has been confirmed that LOXs are not only present in the ECM but also in the cytoplasm and nucleus [17]. In human being, LOX gene has been detected in adult human heart, placenta, testis, lung, kidney, and uterus, but only marginally in brain and liver; LOXL-1 is much higher in placenta, kidney, muscle, heart, lung, and pancreas, but much lower in liver or brain [8]; LOX and LOXL-1 are expressed in most tissues, while the expressions of the LOXL-2, LOXL-3, and LOXL-4 genes are more restricted [18]. LOXL-2 is detected in several human tissues and especially high in spleen, testis, uterus, and placenta, but is expressed at much lower levels in brain and liver [19]; LOXL-3 is high in testis, spleen, and prostate, moderately in placenta, but not in liver [20]; LOXL-4 is expressed at the highest levels in skeletal muscle, testis, and pancreas. LOXs are responsible for diverse biological functions, including developmental regulation, tumor suppression, cell motility, gene transcription regulation, signal transduction, and cell adhesion [21, 22].
The first direct evidence of the correlation between the LOX activity and granulation tissue development in the wound healing was observed in 1974 [23]. Later, Fushida-Takemura firstly examined the time course and the localization of LOX mRNA during wound healing, revealing its gene expression pattern in injured tissues [24]. Recently, roles of LOX and other family members are studied extensively in tissue repair and tissue engineering, aiming at providing a promising therapy for abnormality and disability in tissue injuries.
Lysyl oxidases and tissue repair process
Conspectus of tissue injury and repair
With participation of numerous mediators, blood factors, stromal cells and other ECM elements, cellular and tissue responses are instantly activated following injury, leading to the goal of restoring organized matrix accomplished [25]. Necrotic tissues and fibrin clots are degraded by inflammatory components. Subsequently, new tissues are synthesized along with the deposition of collagen and elastin [26]. It is the balance between degradation and synthesis of the ECM components that results in a favorable healing outcome. In other words, the two processes appear to be two sides of the same coin, their opposing activities facilitate the shift of the balance, then repair process is promoted [27]. Pathophysiologic factors, i.e., the age, hormone level, and other diseases, e.g., pulmonary arterial hypertension [28], varicose vein [29] and congenital heart disease [30], have all demonstrated distinct discrepancies in the process, resulting in abnormal healing, which is still a challenging clinical problem.
To provide a promising avenue to refine these problems, several researchers have focused on the variation of the primary structural component of the ECM, collagen. Collagen is a triple helix of polypeptide chains. In general, collagen type I is the fundamental component of the ECM, while other kinds of collagens just comprise a small proportion, for instance, in skin the collagen type III only accounts for 10%. However, this proportional relation converts after wounding. Collagen I decreases and collagen III has a significant increase [31]. And the early increased presence of collagen type III mRNA becomes a typical wounded phenotype [32]. The increase in the expression of collagen type III coordinates closely with the increase of the LOX following skin injury, in which the increased mRNA peak of the LOX precedes that of collagen type III by several days. The result suggestes that LOX is produced before collagen synthesis in preparation for cross-linking in the early phase of wound healing [24]. Similarly, Giampuzzi reported that the promoter activity of the collagen type III gene was increased remarkably in COS-7 cells due to ectopic over-expression of the mature form of the LOX and that this effect was abolished with β-aminopropionitrile (BAPN), the most potent inhibitor of the LOXs. Evidence was also shown in another report that the over-expression of the LOX promoted the activity of the collagen III promoter through LOX-induced binding of Ku antigen, leading to the increased gene expression of collagen type III [33]. An increase in collagen type IV and other subtypes were also observed [34]. Since collagen type III fibers provide less strength for the wound than collagen I fibers, they will be degraded and replaced with collagen I as repair process goes on. The switch from collagen type III to type I provides greater mechanical strength. Most importantly, the ratio of collagen I to collagen III fibers and their degree of matured architecture significantly influence the rapidity of tissue remodeling and the resulting strength of the new-formed wound [35].
Another remarkable change of the ECM constituents in tissue repair is the activation of fibroblasts, which play a crucial role in healing. Fibroblasts participate in a range of reparative activities under phenotypic modulation [36], including the initial secretion of matrix metalloproteinases (MMPs), which can lyse fibrin clots produced in the inflammation phase. Besides, cytokines and growth factors generated from fibroblasts also act as an indirect signalling way. Above all, collagens synthesized by fibroblasts deposit after the cross-linking reaction catalyzed by the LOX, which promotes the healing cascade of reparative process in tissues such as cardiovascular, skin and bone [37, 38].
It has been confirmed that four LOX isoforms have the same traditional function of catalyzing cross-linking reaction of collagen and elastin [5]. And each of them plays a significant role in the healing process. The LOXL-1 localizes specifically to the sites of elastogenesis and serves as an element of the scaffold to ensure spatially defined deposition and homeostasis of elastins. Liu [39] reported that mice lacking the LOXL-1 did not deposit normal elastic fibers in the uterine tract post partum and developed pelvic organ prolapse, enlarged airspaces of the lung, loose skin, and vascular abnormalities with concomitant tropoelastin accumulation. The LOXL-2 functions for the formation of lysine-derived cross-links and plays a functional role in maintenance of the structural integrity of connective tissues [40]. Yu [41] revealed that LOXL-3-mediated collagen cross-linking was associated with cardiac ECM remodeling. And the LOXL-4, the latest member of the LOX family, also functions as an amine oxidase like other family members to promote the formation of lysine-derived cross-links [42]. As the healing process goes on, the arrangement of the cross-link varies. In other words, the LOX-related arrangement of cross-link impenetrates the remodeling phase from the disordered collagen network formation to a more ordered collagen fiber arrangement. This transition with unclear mechanism is consistent with the increase in the mechanical strength at the injury site. During bone fracture healing, an examination of the transcriptome demonstrated that all five LOXs were highly expressed [43]. In particular, LOXL-2 expression was regulated in a temporal manner and remarkably increased in the chondrogenic phase of fracture healing in vivo [44]. On the other hand, injury stimulates the release of ligands, such as transforming growth factor-β1 (TGF-β1), tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), interleukin-16 (IL-16), bone morphogenetic protein (BMP-12), insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF-2) etc [45], and some of them exert effects together to regulate the activity or expression of the LOXs within the healing phase (Table 1).
Table 1.
The variation of the LOX mRNA/protein under the modulation of influencing factors
| Influencing factors | Effects/responses | Research objects | Culture type | Study |
|---|---|---|---|---|
| PGE2 | Enzyme↓ | Neonatal rat lung fibroblast | In vitro | [46] |
| PDGF | mRNA↑Enzyme↑ | Adult rat vascular smooth muscle cell | In vitro | [47] |
| BFGF | mRNA↓Enzyme↓ | Osteoblastic MC3T3-E1 cell | In vitro | [48] |
| TGF-β1 | mRNA↑Enzyme↑ | Osteoblastic MC3T3-E1 cell | In vitro | [49] |
| Angiotensin II | Enzyme↑ | Rat neonatal cardiac fibroblast | In vitro | [50] |
| IGF-1 | mRNA↑ | High-growth C57BL/6 J mouce | In vivo | [51] |
| Estrogen | mRNA↑ | Vaginal wall of guinea pig | In vitro | [52] |
| HIF-1 | mRNA↑Enzyme↑ | Human MDA231 breast and SiHa cervical cancer cell | In vitro | [53] |
| IFN-γ | mRNA↓ | Rat aortic smooth muscle cell | In vitro | [54] |
| TNF-α | Enzyme↓ | ACL and MCL fibroblast | In vitro | [55] |
| CTGF | mRNA↑Enzyme↑ | Human gingival fibroblast | In vitro | [50] |
| LDL | mRNA↑ | Vascular wall of hypercholesterolemic pig | In vivo | [56] |
| RA | mRNA↓ | Rabbit retinal pigment epithelium cell | In vitro | [57] |
| IL-6 | mRNA↓ | Pre-osteoblastic MC3T3-E1 cell | In vitro | [58] |
| IL-4 | mRNA↑ | Human ovarian surface epithelium cell | In vitro | [59] |
In conclusion, numerous constituents of the ECM, such as collagens, fibroblasts, and varieties of chemical mediators play significant roles in the homeostasis of tissues. Their reciprocities promote the processes of tissue repair. The multi-cellular and multi-molecular response can be divided into three characteristic phases which overlap in time and space. First step is inflammation, then new tissue formation, the last is tissue remodeling [6]. To the best of our knowledge, the three stages are interconnected and interpenetrative. Here we provide an overview of the cross-linking role of the LOXs in terms of these three phases, which would help to understand the insight of the LOXs in the tissue healing and pathological process.
Up-regulation of LOXs in inflammation
As an important part of the innate immune response, acute inflammation is the initial response towards injury. To some extent, acute inflammation itself serves as a tool for tissues to maintain ECM mechanical integrity and return the impaired tissue to a normal state [60]. Although synthetic activities also participate, degradative activities mediated by inflammatory markers, e.g., cytokines, elastases, and interleukins, are typical of inflammation, especially in the early inflammatory phase in which mature elastin and collagen fibers are degraded into soluble peptides [61]. The acute inflammatory response usually persists for 24 to 48h in some tissues, but the duration could even last for up to two weeks in certain cases of healing [62]. Various inflammatory cytokines, growth factors, adhesion molecules, and cells are in close cooperation with each other, in this way modulating the responses of the LOXs and other constituents of the ECM and consequently prompting ECM regeneration. Thus, the LOXs are regulated by these components and play an essential role in the early period of the healing response, i.e., inflammation phase.
The increase of collagen cross-linking is congruent with the up-regulation of the LOXs after tissue injury [63]. A few investigators have reported some approaches to accelerating healing of injured tissues through up-regulating the LOX in the acute phase after injury. For example, direct injection of mesenchymal stem cells (MSCs) into the damaged anal sphincter tissues isolated from rats increased the expression of the LOX and matrix deposition. 21 days later, twitch tension and maximal field-stimulated force of the MSC-injected sphincters were virtually identical to sphincters from the unoperated controls, while the phosphate-buffered saline (PBS)-injected sphincters didn’t recover. The accelerated recovery of the direct MSC-injected sphincters might be due to the stimulated the LOX expression [64]. Elvira [65] reported that the wounded skin of discoidin domain receptor 2-deficient mice which expressed less LOX had defective dermal wounding responses. Therefore, the LOX could act as an appealing target for a favorable healing outcome. Not only the LOX, the LOXL-2 also shows the same variation. In the study of healing process after glaucoma filtration surgery, Tine [66] observed the up-regulation of the LOX and LOXL-2 in Tenon’s capsule and the conjunctiva in the acute phase. In addition, targeting LOXL-2 with an inhibitory monoclonal antibody inhibited angiogenesis, inflammation and profibrotic components. Similarly, Barry [67] put forward that the inhibition of LOXL-2 resulted in a marked reduction of inflammatory cells and molecules, e.g., activated fibroblasts, endothelial cells, growth factors, and cytokines. Meanwhile, TGF-β1 pathway signalling and collagen deposition both decreased. Further studies demonstrated that all five LOXs were highly expressed in fibroblasts isolated from injured anterior cruciate ligament (ACL) and medial collateral ligament (MCL) [68]. Some investigators referred that the intrinsic difference in the reaction of the LOXs to injuries within different tissues could be partly blamed for their different healing abilities . The activities of the LOXs are stronger in MCL fibroblasts than that in ACL fibroblasts, which could be an explanation for the different healing abilities between the well functionally self-healing MCL and the poorly self-healing ACL [69]. It should be noted that the LOXL-1 doesn’t have significantly higher expressions like other family members in the injured state. And the distinction in the activity of the LOXL-1 is also obsolete between impaired ACL and MCL fibroblasts. Further in vitro studies showed that the expressions of the LOXs were mostly down-regulated in injured posterior cruciate ligament (PCL) fibroblasts after co-culture with synovial cells. The decline was probably due to the interaction of injured PCL fibroblasts and synovial cells [69].
The LOXs were activated in a time-dependent manner and were relatively highly expressed in the late phase of inflammation, which was consistent with their anti-inflammatory effects of promoting the transition from inflammation to proliferation phase and the up-regulation of transforming growth factor-β1 (TGF-β1) in this period. Among all the cytokines, TGF-β1 which appears in the damaged area accompanying the inflammation phase is highly related to the up-regulation expressions of the LOXs [70]. TGF-β1 plays a pivotal role in the healing process of many tissues such as muscle, tendon, ligament, skin, and bone [71]. It has been reported that TGF-β1 induces the expression of collagen type I mRNA in cardiac fibroblasts [72]. The in vitro investigations into injured ACL and MCL fibroblasts showed that TGF-β1 remarkably up-regulated the expressions of the LOXs [73]. TNF-α is another key cytokine in the acute inflammation phase. Several studies have reported the decrease in the expressions of the LOX/LOXs caused by TNF-α in endothelial cells, osteoblasts, and fibroblasts [55, 74, 75]. Showing the similar effect like TNF-α, pro-inflammatory cytokine IL-1 contributed to lower expressions of the LOXs in injured ACL fibroblasts compared with those in injured MCL fibroblasts [76]. Prostaglandin E2 (PGE2), a crucial chemical mediator of the early phase of healing process, is assumed to be a negative regulator of the LOX [46]. A research into the effects of pulsed ultrasound on bone fracture repair showed that high-dose PGE2 induced by pulsed ultrasound may be detrimental to the formation of cross-links which were required for the initiation of mineralization. This healing retardation of pulsed ultrasound is attributed to the decreased activity of the LOX caused by PGE2 [77]. In the inflammation phase of healing, the levels of IL-1, VEGF, BMP-1, IGF-1, and MMP-2 are also elevated and these chemical mediators may further modulate the levels of the LOXs released from the damaged tissues [78].
However, when inflammatory factors exist continuously, inflammation persists and will transform into chronic disease. Chronic inflammation is characterized by hyperplasia and lasts for a longer time, in which fibroblasts produce excessive ECM proteins, leading to disorders of structure and function. Pathological fibrosis and liver chirrhosis are typical examples. An early study has been described the increase of LOX expression in fibroblasts in different types of fibrosis [79]. Similarly, Zeneca referred to the remarkable increase of LOX in organ fibrosis and pointed out inhibitors of the enzyme could limit fibrogenesis, although side effects of manipulation of collagen metabolism were still unneglectable [80]. Airway fibrosis caused by TGF-β1 was attenuated after the treatment of BAPN [81]. Even in rheumatoid arthritis, an autoimmunological inflammation, an increased LOX-mediated cross-linking activity was observed, conformed with the increased urinary cross-link excretion [82]. It’s worth mentioning that LOXs expression doesn’t always show the same pattern, and different kinds of chronic inflammations have the own features. Reduction of LOX expression was noted in chronic periodontitis, which was probably blamed for the tissue lesions and impaired healing abilities [83]. An animal study into the treatment of abdominal aortic aneurysm provided a therapy in which LOX expression was improved in order to stimulate ECM synthesis [84].
Participation of the LOXs in various biological actions in the proliferation phase
Biomechanical strength of the injured tissues gradually recovers through the formation of granulation tissue in the proliferation phase [85]. Granulation tissue is a resultant structure successfully performed through fibroplasia and angiogenesis. The reciprocities of fibroblasts, keratinocytes, endothelial cells (ECs), and other stromal cells are also involved. More importantly, the significance of the temporary fibrin-fibronectin structure is to act as a scaffold to guide cellular infiltration and differentiation [86].
In response to injury, activated fibroblasts start to proliferate, migrate to the damaged site, secret cytokines, and lay down ECM components [87]. In the migration process, the fibrin clots and necrotic tissues are lysed by MMPs released from fibroblasts while the fibronectin and hyaluronic acid (HA) take their place and then participate in the development of granulation tissue and angiogenesis [88]. At the same time, ECs and keratinocytes start to migrate from the edge of the wound, over a provisional irregular matrix comprised mostly of fibronectin and fibrin, to achieve epithelialization and form granulation tissue together with fibroblasts [89]. Once entering the center of the wound, fibroblasts begin to produce collagen as well as other ECM components to develop a fibrous tissue. Stiffness, integrity, and strength of the granulation tissue all increase accompanying this process. Eventually, the previous granulation tissue is replaced and the transition is successfully achieved along with cross-linking of collagens. The LOX is involved in the maturation of granulation tissue. In human post-burn hypertrophic scars, the activity of the LOX is rather low in the newly formed granulation tissue and it will be promoted after scarring and reach a significantly higher value compared with normal skin [90].
Angiogenesis, a necessary step in the formation of granulation tissue, provides the fibroblasts with sufficient nutrient supply [91]. The LOXs also play a role in angiogenesis, especially the LOXL-2. A previous study showed angiogenesis regulators, such as VEGF, could induce the expression of the LOXL-2 in ischemia-induced angiogenesis of mouce limbs, suggesting its potential in the EC-mediated angiogenic response [92]. Moreover, anti-LOX and anti-LOXL-2 antibody treatment increased the expressions of β 2-microglobulin and intercellular cell adhesion molecule (ICAM)-1, factors known to induce blood vessel formation [93–95]. The LOXL-2 is also expressed as a hypoxia-target in angiogenic ECs and accumulated in the ECM to regulate angiogenesis [96].
When granulation tissue contains sufficient ECM, fibroblasts will differentiate to myofibroblasts which are replete with actin bundles along the cytoplasmic face of the plasma membrane and have great migratory and contractile abilities [97]. Contraction is a normal physiologic phenomenon characterized by a decreased area of a full-thickness wound, leading to tissue distortion as well as cosmetic disfiguration. Contraction even limits joint mobility on certain occasions [98], so researchers are dedicated in finding therapies to reduce contraction. Wound contraction mediated by collagen and elastin cross-links plays an important role in constrictive remodeling [99]. Consequently, contraction is dependent on the LOX and specific inhibition of the LOX with BAPN reduces the collagen cross-link-dependent contraction [90]. Moreover, the number of LOX-dependent cross-links correlates with the degree of contraction [100], so different LOX capacity between the fetal and adult wound healing could be an explanation for the different contraction. As a result, the LOX appears to be a promising potential target for preventing contraction.
Low LOX activity in granulation tissue reaches a notably higher value and results in scarring. Scarring, the culmination of abundant deposition of the ECM components mostly from fibroblasts and myofibroblasts, is not a necessary stage to repair damaged tissues. It is well-known that scar forms not only in dermal wounds, but also in many other injured conditions, e.g., spinal cord ruptures, scarification of hand tendons after injury, corneal abrasions, human vascular restenosis lesions, myocardial infarction, diffuse fasciitis etc [101]. Despite the fact that scarring is unsightly and detrimental to tissue functions, it is able to restore mechanical integrity of the injured region [102].
Collagens are involved in the scarring process. The ratio of collagen type III to type I, the architecture of collagen network, and other aspects of collagen co-modulate scar formation. The role of the LOX in scar formation has been previously identified by Fushida [24], who referred the increased expression of the LOX during scar formation in wounded rat skin. In rat central nerve system (CNS) injury, Gilad [103] detected the high expressions of the LOX and pointed out that the LOX was synthesised and secreted in a spatio-temporal manner by cells attracted to the CNS injury site. These cells participated in scar formation in this way. Colwell [104] demonstrated that compared with early-gestation scarless wounds, fetal scarring wounds had higher expressions of the LOX in the early response of excisional wound healing in mice, which promoted scar formation through LOX-mediated cross-linking. This difference was just correlated with another finding which showed less TGF-β1 was expressed in scarless wounds and contributed to the low expression and activity of the LOX [105]. Excessive scarring is one of the characteristics of surgical failure [106]. It was reported by Tine [66] that the LOXL-2 could be an efficient target for treatment of hypertrophic scar formation after glaucoma filtration surgery. Other fibrotic-related pathologies are also related to the low expression and activity of the LOX at different stages of scar formation [90].
Facilitation role of the LOXs in tissue remodeling
Tissue remodeling can be defined as an orchestrated change of structural components of the ECM, especially collagens and various proteoglycans. Old or damaged proteins are broken down and new proteins take place in this process, which is dependent on the metabolic balance between the proteoglycan molecules and the components of the collagen network [107]. Since the collagen network provides tissues with tensile strength and shear stiffness, when the deposition of collagens outstrips that of proteoglycans, an increase in mechanical integrity will manifest [108]. On the contrary, the maintenance of proteoglycans and the decrease in the concentrations of collagens result in tissue swelling, which is characterized by an increase in resistance to compressive loading and a decrease in tensile strength [109]. The two situations mentioned above are exactly observed in vivo and in vitro, respectively. They represent two different types of remodeling, constrictive remodeling and excessive enlargement remodeling [110]. Cross-linking reaction of collagen and elastin participates in the healing process via promoting constrictive remodeling. However, excessive accumulation of cross-linked collagens probably result in a bad outcome after injury, such as restenosis after balloon angioplasty [111].
To a large extent, the collagen network is stabilized through cross-linking reaction dependent on the LOXs. Hence the LOXs are critical intermediaries responsible for remodeling during the process of healing [112]. Tensile strength of the wounds can be enhanced with an increased level of the LOX activity stimulated by dexamethasone (DEX), a half analog of curcumin [61]. The change of the LOX activity caused by DEX indicates that the up-regulation of the LOX might act as a way to make up for the insufficiency of mature collagen fibers in case of suppression of healing process. A study of wound breaking strength (WBS) demonstrated that ethanol exposure decreased the LOX activity and collagen production, which could be partially blamed for reduced WBS and increased incidence of wound failure [88]. So it’s conceivable that BAPN treatment or the inhibition of the LOX would pose an obstacle for tissue remodeling. Maki observed increased fragmentation of elastin fibers and excessive enlargement remodeling in the LOX-knockout mice which had high morbidity rate of aortic aneurysms [37]. In an atherosclerotic rabbit model after balloon injury, restenosis decreased in the BAPN-treated animals compared with controls. This discrepancy could be explained that the LOX activity was statistically reduced by the addition of BAPN, then collagen fibers became disorganized and the collagen content was decreased. In the end, constrictive remodeling was significantly inhibited [113].
The LOX is regulated by TGF-β1 and many other factors in the repair process, including the remodeling phase. Effects of TGF-β1 on human ocular fibroblasts increase the LOX expression, expediting the pace of ECM remodeling [114]. Likewise, the expression of the LOX in injured Achilles tendons treated with TGF-β1-transfected bone marrow-derived mesenchymal stem cells (BMSCs) was up-regulated, which accelerated collagen synthesis, cross-link formation, and matrix remodeling [115]. Estrogen is another mediator that promotes remodeling probably via the activation of TGF-β1 [116]. The LOX was up-regulated along with induced collagen gene expression after the addition of exogenous estrogen to the injured vaginal wall of guinea pigs, contributing to a remarkable increase in the density of elastin and collagen fibers as well as tensile strength of the tissue [52].
The growth factors are usually applied in the inflammatory or early proliferative phase of healing, while the mechanical loading generally plays a great role in the entire healing process [117]. It has been indicated that physiologic levels of mechanical strain on osteoblasts may regulate the transformation of a calcifiable collagenous matrix into a mature one with a specific molecular packing arrangement through collagen cross-linking as mineralization begins [118]. By contrast, Martinez reported that simulating the effects of weightlessness with the implementation of the rat hindlimb unloading (HLU) model decreased the LOX expression and retarded the repair of connective tissues [119]. A recent research into periodontal ligament (PDL) cells showed that low-level mechanical stretching could promote the formation and stabilization of the ECM via up-regulating the gene transcriptions of collagen and the LOX. Moreover, the LOX activity and total collagen production were also enhanced [120]. Therefore, mechanical stimulation can be appropriately applied to facilitate tissue remodeling.
However, the normal continuum of wound healing is disrupted in some pathological conditions, e.g., diabetes, in which repair process might enter into a non-healing state characterized by persistent inflammation, enhanced proteolytic activity and impaired ECM remodeling [121]. The vitreous level of the LOX activity is markedly decreased in proliferative diabetic retinopathy, leading to inadequate cross-links and ECM changes notorious in this disease. Aiming at these circumstances, wound healing models have been established both in vitro and in vivo to understand the insight of the LOXs in tissue remodeling. A report of the rat dorsal skin showed that the mechanical strength of the fibroblast-inserted gene activated matrix (GAM) with LOX transgene was much higher than that of the green fluorescence protein (GFP)-transgene controls. The development of mechanical strength was achieved through the increase of collagen cross-links after the treatment with LOX-producing GAM [122]. On the other hand, the increased expression and activity of the LOX could be a mechanism for compromised ECM barrier function. A recent research into the retina of diabetic mice showed that the integrity of the ECM, which was characterized by the decreased collagen fibril diameter and the irregular arrangement of collagens resulting from high glucose levels, could be compromised caused by the overexpression of the LOX [123]. These discoveries mentioned above may provide novel insight in the treatment of poorly healing wounds from the perspective of the LOXs.
Lysyl oxidases and tissue engineering
Conspectus of tissue engineering
Tissue engineering, a new and exciting technique, provides an effective reparative treatment for acute or chronic tissue injury. Unlike the autograft tissue and allograft tissue which are restricted by the inability to successfully integrate with the host tissue, the strategically engineered neotissue mimics the complex structure of native tissue and has promising prospects of application in tissue repair [124], especially for tissues which lack an intrinsic ability to self-repair following disease- or injury-induced degradation, e.g., bone, cartilage, vascular, knee menisci, intervertebral discs, tendons, and ligaments [125, 126]. Although tissue engineering technique holds great potential, the engineered tissues are prone to have a inferior function in integration with the host tissue in vivo and are unable to withstand the complex distribution of mechanical loading. These insufficiencies hinder the widespread application of tissue engineering technique [127]. Various methods such as using biological tissue adhesives at the interface encourage integration to some extent, whereas low levels of collagens synthesized between native-to-engineered tissues don’t change, so the poor integrative ability and low mechanical strength of neotissues are still the same [128]. A desirable collagen network depends on collagen concentration, relative proportion of different types of collagen, the density and diameter of collagen fibers, the spatial alignment, and more importantly, cross-linking [129, 130]. Hence, enhancing the functional properties and integrative ability of the neotissue through increasing collagen cross-linking could be a viable solution to the graft problems mentioned above [131].
The formation of mature cross-links is catalyzed by the LOXs. Lysine residues within elastin and collagen are converted into hydroxy-lysine residues by lysyl hydroxylase, then peptidyl lysine and/or hydroxy-lysine residues both serve as the precursors of cross-links and undergo the oxidative deamination catalyzed by the LOXs, and unstable aldehydes are generated. The resulting aldehydes then react with neighboring lysine residues or with other peptidyl aldehyde residues spontaneously through Schiff’s base formation or aldol condensation to generate intra- and intermolecular covalent cross-links, leading to the formation of immature covalent cross-links which are converted into stable multivalent cross-links. Mature cross-links, the most abundant cross-links in varieties of tissues, result in the formation of large heterodispersed insoluble extracellular proteins that are resistant to proteolysis [132] (Fig. 2).
Fig. 2.
Formation of stable multivalent cross-links in the ECM. Lysine residues within elastin and collagen are firstly converted into hydroxy-lysine residues by lysyl hydroxylase, then peptidyl lysine and/or hydroxy-lysine residues both serve as the precursors of cross-links and undergo the oxidative deamination catalyzed by the LOX family (LOXs), and unstable aldehydes are generated. The resulting aldehydes then react with neighboring lysine residues or with other peptidyl aldehyde residues spontaneously through Schiff’s base formation or aldol condensation to generate intra- and intermolecular covalent cross-links, leading to the formation of immature dehydroxylysinorleucine (DHLNL) cross-links which are converted into stable pyridinoline (PYR) cross-links, the main type of cross-links in the extracellular matrix (ECM) of cartilage
The pivotal role of the mature cross-link has been confirmed in the stabilization and mechanical support of the collagen network [133]. A recent study demonstrated that increasing LOX-mediated collagen cross-links through supplementing the medium with copper sulfate and hydroxylysine would synergistically increase both the tensile and compressive properties of the engineered articular cartilage. Some investigators even pointed out that the cross-link could be useful as a functional marker to access mechanical properties of the engineered tendon and could be used in tissue-engineered scaffold design to regulate regeneration [134]. Saito [135] referred the direct effect of collagen cross-links on the strength of bones, tendons, and ligaments. Regardless of engineered tissues or native tissues, a collagen network with highly favorable biological properties requires sufficient cross-links to stabilize the matrix and to provide it with mechanical support [136], so the activity and expression of the LOX are essential to the maturation of the ECM and properties of the engineered tissue.
Effects of the LOX on tissue-engineered constructs in in vitro culture
Current tissue engineering approaches to generate highly cellularized and metabolically active neotissues in vitro typically involve a combination of cells, signals, and scaffolds. With structure and function being intimately linked, a direct tie between mechanical integrity and both the composition and structure of the ECM has been observed in a wide variety of engineered tissues [137]. And the LOX is an essential intermediary for tissue-engineered constructs to achieve a better mechanical integrity.
When vascular smooth muscle cells (VSMCs) transfected with the LOX gene were seeded in collagen gels, the tensile strength and elastic modulus of the tissue-engineered constructs were greatly enhanced due to the increase of cross-links [138]. Moreover, the LOX-mediated cross-links are independent on the collagen content or cell proliferation rate. This result is consistent with another study focused on the development of embryonic tendon functional properties which showed that the LOX-mediated cross-links altered mechanical properties without affecting matrix content and organization [139]. Eleftherios [140] put forward a novel use of the LOX through a combination of the LOX, chondroitinase-ABC (C-ABC) and TGF-β1 could promote the maturation of the in vitro neofibrocartilage in a manner akin to native morphogenesis, and eventually result in a more biomechanically robust matrix. Not only aiding tissue maturation, the exogenous LOX along with C-ABC and TGF-β1 also facilitated integration in the interface between the engineered implant and native tissue. It’s conceivable that BAPN treatment would inhibit the formation of collagen cross-links and mechanical integrity of the tissue [141]. Since non-cross-linked collagens are more sensitive to the degradation by MMPs and collagen cross-links inhibit the activity of MMP-2, BAPN treatment leads to a reduced collagen content [142]. Although the primary effect of BAPN appears to be on collagen assembly, Marvy [143] reported that the altered state of the ECM caused additional effects on gene expressions of cartilage-specific collagens, i.e., collagen type II and type XI. The expressions of these collagens were up-regulated while the collagen content of the BAPN-treated cultures was actually less than that of controls. Additionally, it has been suggested that the up-regulation of collagen synthesis is related to impaired cell-matrix interactions due to the increased extractability of collagens. From a mechanobiological point of view, the LOX activity decrease caused by BAPN reduced insoluble collagens and mechanical strength, and ultimately provided a negative feedback stimulus for further collagen synthesis [144]. On the other hand, fibrogenic cells synthesized and assembled significantly larger collagen fibrils in the presence of BAPN compared with control cultures. The increase of fibril diameter with BAPN treatment may be caused by the lack of proteins, such as decorin which is on the surface of the fibril that normally limits the fibril diameter [144]. So the rational utilization of BAPN could be a potential therapeutic strategy towards facilitating mechanical properties. And several studies in vitro have referred some therapies with the addition of BAPN to treat the defects in tissues. For example, Ahsan reported that pre-treatment with BAPN caused an accumulation of unmodified hydroxylysines, the precursors of cross-links in the explants. Meanwhile, the formation of collagen cross-links reduced [145]. Upon BAPN withdrawal, these precursors converted rapidly into cross-links catalyzed by the LOXs, leading to an acceleration of subsequent integrative proceedings and an increase in tensile properties of the explants [146].
Not only mechanical integrity, a stable and robust integration interface is another crucial point for tissue engineering technique. Proper integration is the most imperative aspect ensuring that the implant remains stabilized and is able to competently function in vivo. It is generally agreed that the development of adhesive strength between the native tissue and engineered explant tightly correlates with the collagen synthesis, cross-linking, and deposition. Aristos [138] referred that the LOX could promote integration between native-to-implant cartilage surfaces through targeting the collagen assembly and organization. Thus, we can induce the activity/expression of the LOX to promote integration and eventually optimize tissue properties.
Micro-array data showed that the LOX was significantly induced under hypoxic conditions. Definitely, oxygen concentration which is widely investigated for potential application to tissue engineering is critical to regulate the ECM production and cross-linking [147]. To the best of our knowledge, hypoxia has a strong impact on cell biology via the transcription factor hypoxia-inducible factor-1 (HIF-1) and other targets of the hypoxia-inducible factors which are responsible for the exponential increase of gene expressions in the decreasing oxygen concentration conditions [148, 149]. Human vascular-derived myofibroblasts which were cultured at the physiological oxygen concentration of 7% showed an increase in the total amount of collagens and collagen cross-links compared with the myofibroblasts cultured at 21% of oxygen [150]. In addition, hypoxia in which the oxygen level dropped below 4% enhanced the gene expressions and protein production of the ECM components, including the LOX and collagen [147]. Similarly, tenocytes in vivo live commonly in a low O2 environment and are difficult to expand due to the phenotype drift and functional loss [151]. Whereas the in vitro low O2 tension culture, i.e., 2% O2, enhanced the proliferative capacity of the tenocytes derived from the newborn pigs and inhibited the expressions of MMP-1 and IL-6, and eventually lead to decreased collagen degradation and increased collagen deposition [152]. These discoveries might be beneficial to the maturation of engineered tissues. Although the effects of hypoxia in vitro vary due to different cell sources, oxygen concentration and the time of exposure, we can take advantage of the hypoxia-mediated increase of the LOX expression to stimulate matrix production and maturation of the explants and promote their mechanical integrity. For instance, 4% O2-mediated up-regulation of the LOX expression during the 3rd and 4th weeks of the self-assembling process increased the pyridinoline (PYR) cross-links and tensile integrity of the tissue-engineered cartilage [153].
Elastin, another substrate of the LOXs, is a vital structural protein conferring elasticity and resiliency to the ECM. Tissues rich in elastins include aorta and large blood vessels (28–32% of the dry mass), lung (3–7%), elastic ligaments (50%), tendons (4%), and skin (2%) [21]. The elaboration and homeostasis of elastins are essential for the regeneration of these highly elastic tissues. And the LOX plays a role in the process since the enhanced production/activity of the LOX contributes to the increased cross-linked elastins. Knocking out the LOX gene in mice is perinatally lethal and results in the dysfunction of elastic tissues and fragmented elastic fibers [37]. While in culture of adult rat aortic smooth muscle cells, supplementation with the exogenous bovine LOX can improve elastin synthesis, deposition, and cross-linking in a dose-dependent manner [154]. Targeting the LOX with a mixture of TGF-β1 and HA oligomers is of tremendous utility to restore elastin matrix homeostasis in the tissue-engineered constructs. HA oligomers and TGF-β1 together modestly increased the LOX production and activity, and synergistically improved tropoelastin synthesis and matrix assembly in in vitro adult rat aortic SMCs. Meanwhile, the tropoelastins were also up-regulated likely via the increased the LOX production [155]. Similarly, Chandrasekhar referred that copper nanoparticles (CuNPs), the effective delivery vehicles for Cu2+ ion, together with HA oligomers could facilitate the regeneration of cross-linked elastins via stimulation of the LOX in adult vascular SMCs [156].
Effects of the LOX on tissue-engineered constructs in in vivo culture
The afore-mentioned views were studied in vitro, which only partially mimicked the actual micro-environment in vivo. In the in vitro studies, integration mostly occurs parallel to the surface. While in the typical clinical application, integration is often performed in a direction perpendicular to the tissue surface. Furthermore, when cells or tissues are suspended in collagen gels, there is little constraint counteracting swelling in the thickness direction and no appreciable distortion of the apposed geometry of the tissue samples [140]. In addition, oxygen level in the two-dimensional (2D) cell cultures in vitro is well-distributed. However, there is an oxygen gradient within tis sue-engineered constructs at the three-dimensional (3D) level in vivo due to poor oxygen diffusion in the middle of the tissue [157]. So in order to understand the insight of the LOX in tissue engineering, it’s necessary to perform studies in vivo to describe the actual properties and functions of the engineered tissues.
The LOX has been reported to promote integration and enhance the formation of cross-links between native-to-implant surfaces in an animal model. Neofibrocartilage pre-treated with a combination of the LOX, C-ABC, and TGF-β1 carries over an effect into an in vivo model following implantation, achieving the tensile properties of the integration interface on par with the values of the intact native fibrocartilage [134]. Another recent study provided applications of the exogenous LOX for improving tensile properties of a spectrum of native and engineered tissues both in vitro and in vivo [158]. In vivo subcutaneous implantation of the LOX-treated neocartilage into the nude mice promoted further maturation of the neotissue, enhancing tensile strength and PYR content approximately 3-fold and 14-fold, respectively, compared with in vitro controls [158].
Signal transduction of the LOXs in tissue repair
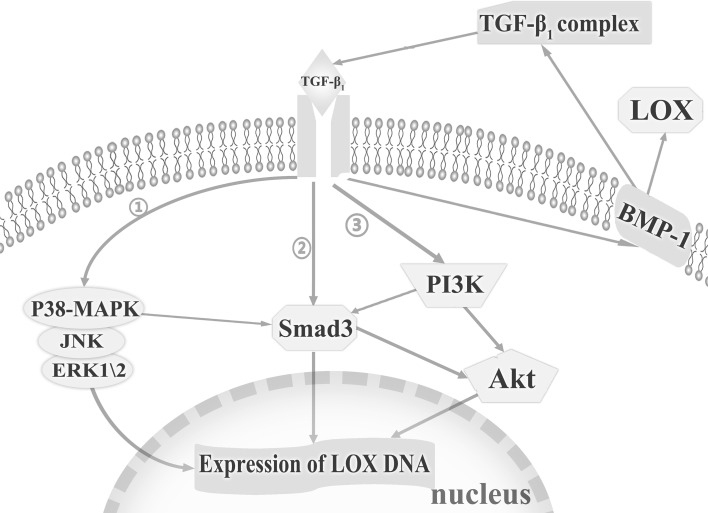
Enhanced TGF-β1 expression is a typical response to injury accompanied by the increase of the LOXs expressions and synthesis of multiple ECM proteins [159]. Elvira reported the increase of the LOX mRNA and activity induced by TGF-β1 in adult cardiac fibroblasts was mediated through the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt), drosophila mothers against decapentaplegic protein 3 (Smad3), and mitogen-activated protein kinases (MAPKs) pathways [154]. The reciprocities of the three signalling pathways contribute to tissue remodeling through the regulation of the LOXs [160–162]. In addition, TGF-β1-mediated increase in the expression of BMP-1, a key activator of the LOX, may activate TGF-β1 and provide a positive feedback to generate more LOX in the formation of the ECM [163]. In MAPK signalling pathway, TGF-β1 induces the phosphorylation of p38-MAPK, c-Jun N-terminal kinase (JNK) and extracellular regulated protein kinases 1/2 (ERK1/2) [149] (Figure 3). The role of the p38-MAPK signalling pathway in anti-inflammation has also been explored by Georgia, who reported that IL-4 and IL-1α induced the expression of the LOX in the post-ovulatory wound healing process via the p38-MAPK signalling pathway [59].
Fig. 3.
Signal transduction initiated by transforming growth factor-β1 (TGF-β1). The increase of the lysyl oxidase (LOX) mRNA and activity induced by TGF-β1 is mediated through the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt), drosophila mothers against decapentaplegic protein 3 (Smad3), and mitogen-activated protein kinases (MAPKs) pathways. The reciprocities among these signalling pathways have been noticed. Akt phosphorylation and the increase in total Akt expression are PI3K-dependent. Moreover, the activation of p38-MAPK is necessary for the induction of TGF-mediated Smad3 signalling. Meanwhile, there is a cross communication between the PI3K and Smad3 signalling pathways in which the inhibition of Smad3 reduces the activation of Akt by TGF-β1 and that PI3K inhibition reduces the activation of Smad3. In MAPK signalling pathway, TGF-β1 induces the phosphorylation of p38-MAPK, c-Jun N-terminal kinase (JNK) and extracellular regulated protein kinases 1/2 (ERK1/2). Moreover, TGF-β1-mediated increase in expression of bone morphogenetic protein-1 (BMP-1) may also activate TGF-β1 and provide positive feedback to generate more LOX in the formation of the extracellular matrix (ECM). ➀ represents the MAPK pathway; ➁ represents the Smad3 pathway; ➂ represents the PI3K pathway [149, 154, 159, 163]
Unlike TGF-β1, PGE2 significantly inhibits the LOX activity in the inflammation phase of healing. Recently, Utako put forward that this inhibition from PGE2 acted as a means to regulate elastogenesis in the ductus arteriosus (DA) [164]. PGE2 signal transduction promoted the lysosomal degradation of the LOX protein through the clathrin-mediated endocytosis. And the negative effect of PGE2 is achieved through the PGE2-EP4-c-Src-PLCγ signal pathway which plays a primary role in the post-translational decrease of the LOX. It is clearly demonstrated that prostaglandin E receptor 4 (EP4), a kind of PGE2 receptor, can directly stimulate the activity of c-Src, a tyrosine kinase acting as a cellular signal transducer that can be activated by EP4 [165]. So the direct association between EP4 and c-Src may activate its downstream signalling in the EP4-mediated degradation of the LOX.
Conclusion and future prospects
Tissue repair is a dynamic response to injury with lots of ambiguous mechanisms. Current researches indicate the essential role of the LOXs on expediting healing proceedings in various phases. The LOXs are differentially regulated by different ligands depending on the different phase of healing process. In this review, we have concluded the experimental results from the in vitro, animal and human in vivo studies and summarized the effects of the LOXs in tissue repair. In addition, the LOX can be a new target for improved mechanical strength and integration in tissue engineering. So the application of the LOX and its activators could act as a new therapeutic approach to desirable grafting and regeneration of tissue-engineered constructs. In general, the role of the LOXs in tissue repair and tissue engineering still deserves further attention and should be investigated in more detail.
Acknowledgements
This work was funded Funding of State Key Laboratory of Oral Diseases (SKLOD201527), the youth start-up fund (2015SCU11013) and the National Undergraduate Training Programs for Innovation and Entrepreneurship (201510610117).
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical Statement
There are no animal experiments carried out for this article.
Contributor Information
Xiangli Kong, Phone: +86-28-85503465, Email: kongxiangli1990@163.com.
Jing Xie, Phone: +86-28-85503465, Email: xiejing2012@scu.edu.cn.
References
- 1.Andrew Chan KL, et al. A coordinated approach to cutaneous wound healing: vibrational microscopy and molecular biology. J Cell Mol Med. 2008;12(5B):2145–2154. doi: 10.1111/j.1582-4934.2008.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank S, Kampfer H. Excisional wound healing. An experimental approach. Methods Mol Med. 2003;78:3–15. doi: 10.1385/1-59259-332-1:003. [DOI] [PubMed] [Google Scholar]
- 3.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 4.Tang Z, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243–248. doi: 10.1002/jor.20763. [DOI] [PubMed] [Google Scholar]
- 5.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19–20):2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48(3):504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akagawa M, Suyama K. Characterization of a model compound for the lysine tyrosylquinone cofactor of lysyl oxidase. Biochem Biophys Res Commun. 2001;281(1):193–199. doi: 10.1006/bbrc.2001.4315. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y, Boyd CD, Csiszar K. A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase. J Biol Chem. 1995;270(13):7176–7182. doi: 10.1074/jbc.270.13.7176. [DOI] [PubMed] [Google Scholar]
- 9.Saito H, et al. Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J Biol Chem. 1997;272(13):8157–8160. doi: 10.1074/jbc.272.13.8157. [DOI] [PubMed] [Google Scholar]
- 10.Jang W, et al. Comparative sequence of human and mouse BAC clones from the mnd2 region of chromosome 2p13. Genome Res. 1999;9(1):53–61. [PMC free article] [PubMed] [Google Scholar]
- 11.Maki JM, Kivirikko KI. Cloning and characterization of a fourth human lysyl oxidase isoenzyme. Biochem J. 2001;355(Pt 2):381–387. doi: 10.1042/0264-6021:3550381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asuncion L, et al. A novel human lysyl oxidase-like gene (LOXL4) on chromosome 10q24 has an altered scavenger receptor cysteine rich domain. Matrix Biol. 2001;20(7):487–491. doi: 10.1016/s0945-053x(01)00161-5. [DOI] [PubMed] [Google Scholar]
- 13.Jourdan-Le Saux C, et al. The mouse lysyl oxidase-like 2 gene (mLOXL2) maps to chromosome 14 and is highly expressed in skin, lung and thymus. Matrix Biol. 2000;19(2):179–183. doi: 10.1016/s0945-053x(00)00061-5. [DOI] [PubMed] [Google Scholar]
- 14.Maki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24(5):651–660. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- 15.Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- 16.Jung ST, et al. Purification of enzymatically active human lysyl oxidase and lysyl oxidase-like protein from Escherichia coli inclusion bodies. Protein Expr Purif. 2003;31(2):240–246. doi: 10.1016/s1046-5928(03)00217-1. [DOI] [PubMed] [Google Scholar]
- 17.Seve S, et al. Expression analysis of recombinant lysyl oxidase (LOX) in myofibroblastlike cells. Connect Tissue Res. 2002;43(4):613–619. [PubMed] [Google Scholar]
- 18.Molnar J, et al. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta (BBA) Proteins Proteom. 2003;1647(1–2):220–224. doi: 10.1016/s1570-9639(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 19.Jourdan-Le Saux C, et al. The LOXL2 gene encodes a new lysyl oxidase-like protein and is expressed at high levels in reproductive tissues. J Biol Chem. 1999;274(18):12939–12944. doi: 10.1074/jbc.274.18.12939. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, et al. Cloning and characterization of a human lysyl oxidase-like 3 gene (hLOXL3) Matrix Biol. 2001;20(2):153–157. doi: 10.1016/s0945-053x(01)00124-x. [DOI] [PubMed] [Google Scholar]
- 21.Grace VMB, Guruvayoorappan C. Lysyl oxidase: a potential target for cancer therapy. Inflammopharmacology. 2011;19(3):117–129. doi: 10.1007/s10787-010-0073-1. [DOI] [PubMed] [Google Scholar]
- 22.Teppo S, et al. The hypoxic tumor microenvironment regulates invasion of aggressive oral carcinoma cells. Exp Cell Res. 2013;319(4):376–389. doi: 10.1016/j.yexcr.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Chvapil M, et al. Activity and extractability of lysyl oxidase and collagen proteins in developing granuloma tissue. Proc Soc Exp Biol Med. 1974;146(3):688–693. doi: 10.3181/00379727-146-38173. [DOI] [PubMed] [Google Scholar]
- 24.Fushida-Takemura H, et al. Detection of lysyl oxidase gene expression in rat skin during wound healing. Arch Dermatol Res. 1996;288(1):7–10. doi: 10.1007/BF02505035. [DOI] [PubMed] [Google Scholar]
- 25.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121(2):219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhou D, et al. Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res. 2005;23(4):949–957. doi: 10.1016/j.orthres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Brines M. Discovery of a master regulator of injury and healing: tipping the outcome from damage toward repair. Mol Med. 2014;20(Suppl 1):S10–S16. doi: 10.2119/molmed.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogaard HJ, et al. Copper dependence of angioproliferation in pulmonary arterial hypertension in rats and humans. Am J Respir Cell Mol Biol. 2012;46(5):582–591. doi: 10.1165/rcmb.2011-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascual G, et al. Down-regulation of lysyl oxydase-like in aging and venous insufficiency. Histol Histopathol. 2008;23(2):179–186. doi: 10.14670/HH-23.179. [DOI] [PubMed] [Google Scholar]
- 30.Urashima T, et al. Molecular and physiological characterization of RV remodeling in a murine model of pulmonary stenosis. Am J Physiol Heart Circ Physiol. 2008;295(3):H1351–H1368. doi: 10.1152/ajpheart.91526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Mankus C, et al. The P2X(7) receptor regulates proteoglycan expression in the corneal stroma. Mol Vis. 2012;18:128–138. [PMC free article] [PubMed] [Google Scholar]
- 33.Giampuzzi M, et al. Lysyl oxidase activates the transcription activity of human collagene III promoter. Possible involvement of Ku antigen. J Biol Chem. 2000;275(46):36341–36349. doi: 10.1074/jbc.M003362200. [DOI] [PubMed] [Google Scholar]
- 34.Laplante AF, et al. Mechanisms of wound reepithelialization: hints from a tissue-engineered reconstructed skin to long-standing questions. FASEB J. 2001;15(13):2377–2389. doi: 10.1096/fj.01-0250com. [DOI] [PubMed] [Google Scholar]
- 35.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31(6):674–686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 36.Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993;306(1):42–48. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Maki JM. Inactivation of the lysyl oxidase gene lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106(19):2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 38.Hong HH, et al. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J Cell Physiol. 2004;200(1):53–62. doi: 10.1002/jcp.10476. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 40.Kim YM, Kim EC, Kim Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep. 2011;38(1):145–149. doi: 10.1007/s11033-010-0088-0. [DOI] [PubMed] [Google Scholar]
- 41.Yu Q, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension. 2006;48(1):98–104. doi: 10.1161/01.HYP.0000227247.27111.b2. [DOI] [PubMed] [Google Scholar]
- 42.Kim MS, et al. Expression and purification of enzymatically active forms of the human lysyl oxidase-like protein 4. J Biol Chem. 2003;278(52):52071–52074. doi: 10.1074/jbc.M308856200. [DOI] [PubMed] [Google Scholar]
- 43.Bais M, et al. Transcriptional analysis of fracture healing and the induction of embryonic stem cell-related genes. PLoS ONE. 2009;4(5):e5393. doi: 10.1371/journal.pone.0005393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iftikhar M, et al. Lysyl oxidase-like-2 (LOXL2) is a major isoform in chondrocytes and is critically required for differentiation. J Biol Chem. 2011;286(2):909–918. doi: 10.1074/jbc.M110.155622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majewski M, et al. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med. 2009;37(11):2117–2125. doi: 10.1177/0363546509348047. [DOI] [PubMed] [Google Scholar]
- 46.Boak AM, et al. Regulation of lysyl oxidase expression in lung fibroblasts by transforming growth factor-beta 1 and prostaglandin E2. Am J Respir Cell Mol Biol. 1994;11(6):751–755. doi: 10.1165/ajrcmb.11.6.7946403. [DOI] [PubMed] [Google Scholar]
- 47.Green RS, et al. Identification of lysyl oxidase and other platelet-derived growth factor-inducible genes in vascular smooth muscle cells by differential screening. Lab Invest. 1995;73(4):476–482. [PubMed] [Google Scholar]
- 48.Feres-Filho EJ, Menassa GB, Trackman PC. Regulation of lysyl oxidase by basic fibroblast growth factor in osteoblastic MC3T3-E1 cells. J Biol Chem. 1996;271(11):6411–6416. doi: 10.1074/jbc.271.11.6411. [DOI] [PubMed] [Google Scholar]
- 49.Feres-Filho EJ, et al. Pre- and post-translational regulation of lysyl oxidase by transforming growth factor-β 1 in osteoblastic MC3T3-E1 cells. J Biol Chem. 1995;270(51):30797–30803. doi: 10.1074/jbc.270.51.30797. [DOI] [PubMed] [Google Scholar]
- 50.Adam O, et al. Increased lysyl oxidase expression and collagen cross-linking during atrial fibrillation. J Mol Cell Cardiol. 2011;50(4):678–685. doi: 10.1016/j.yjmcc.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 51.Reiser K, et al. Effects of elevated circulating IGF-1 on the extracellular matrix in “high-growth” C57BL/6J mice. Am J Physiol. 1996;271(3 Pt 2):R696–R703. doi: 10.1152/ajpregu.1996.271.3.R696. [DOI] [PubMed] [Google Scholar]
- 52.Balgobin S, et al. Estrogen alters remodeling of the vaginal wall after surgical injury in guinea pigs. Biol Reprod. 2013;89(6):138. doi: 10.1095/biolreprod.113.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 54.Song YL, et al. Regulation of lysyl oxidase by interferon-γ in rat aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20(4):982–988. doi: 10.1161/01.atv.20.4.982. [DOI] [PubMed] [Google Scholar]
- 55.Xie J, et al. TNF-α induced down-regulation of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts. Knee. 2014;21(1):47–53. doi: 10.1016/j.knee.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez C, et al. Low density lipoproteins downregulate lysyl oxidase in vascular endothelial cells and the arterial wall. Arterioscler Thromb Vasc Biol. 2002;22(9):1409–1414. doi: 10.1161/01.atv.0000033818.21748.99. [DOI] [PubMed] [Google Scholar]
- 57.Omori K, et al. Regulation of the expression of lysyl oxidase mRNA in cultured rabbit retinal pigment epithelium cells. Matrix Biol. 2002;21(4):337–348. doi: 10.1016/s0945-053x(02)00013-6. [DOI] [PubMed] [Google Scholar]
- 58.Thaler R, et al. Homocysteine suppresses the expression of the collagen cross-linker lysyl oxidase involving IL-6, Fli1, and epigenetic DNA methylation. J Biol Chem. 2011;286(7):5578–5588. doi: 10.1074/jbc.M110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papacleovoulou G, et al. IL1α and IL4 signalling in human ovarian surface epithelial cells. J Endocrinol. 2011;211(3):273–283. doi: 10.1530/JOE-11-0081. [DOI] [PubMed] [Google Scholar]
- 60.Jakab L. Connective tissue and inflammation. Orv Hetil. 2014;155(12):453–460. doi: 10.1556/OH.2014.29848. [DOI] [PubMed] [Google Scholar]
- 61.Rao MC, et al. Effect of dehydrozingerone, a half analog of curcumin on dexamethasone-delayed wound healing in albino rats. Mol Cell Biochem. 2011;355(1–2):249–256. doi: 10.1007/s11010-011-0861-y. [DOI] [PubMed] [Google Scholar]
- 62.Holzheimer RG, Steinmetz W. Local and systemic concentrations of pro- and anti-inflammatory cytokines in human wounds. Eur J Med Res. 2000;5(8):347–355. [PubMed] [Google Scholar]
- 63.Nuthakki VK, et al. Lysyl oxidase expression in a rat model of arterial balloon injury. J Vasc Surg. 2004;40(1):123–129. doi: 10.1016/j.jvs.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 64.Pathi SD, et al. Recovery of the injured external anal sphincter after injection of local or intravenous mesenchymal stem cells. Obstet Gynecol. 2012;119(1):134–144. doi: 10.1097/AOG.0b013e3182397009. [DOI] [PubMed] [Google Scholar]
- 65.Olaso E, et al. Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenes Tissue Repair. 2011;4(1):5. doi: 10.1186/1755-1536-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Bergen T, et al. The role of LOX and LOXL2 in scar formation after glaucoma surgery. Invest Ophthalmol Vis Sci. 2013;54(8):5788–5796. doi: 10.1167/iovs.13-11696. [DOI] [PubMed] [Google Scholar]
- 67.Barry-Hamilton V, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 68.Xie J, et al. Differential expressions of lysyl oxidase family in ACL and MCL fibroblasts after mechanical injury. Injury. 2013;44(7):893–900. doi: 10.1016/j.injury.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 69.Wang C, et al. Differential expressions of the lysyl oxidase family and matrix metalloproteinases-1, 2, 3 in posterior cruciate ligament fibroblasts after being co-cultured with synovial cells. Int Orthop. 2015;39(1):183–191. doi: 10.1007/s00264-014-2573-x. [DOI] [PubMed] [Google Scholar]
- 70.Tang Z, et al. Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after a mechanical injury: involvement of the p65 subunit of NF-κB. Wound Repair Regen. 2009;17(5):709–716. doi: 10.1111/j.1524-475X.2009.00529.x. [DOI] [PubMed] [Google Scholar]
- 71.Poniatowski LA, et al. Transforming growth factor Beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediat Inflamm. 2015;2015:137823. doi: 10.1155/2015/137823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan X, et al. Transforming growth factor β1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts. PLoS ONE. 2013;8(4):e60335. doi: 10.1371/journal.pone.0060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie J, et al. Up-regulation expressions of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts induced by transforming growth factor-β 1. Int Orthop. 2012;36(1):207–213. doi: 10.1007/s00264-011-1261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alcudia JF, et al. Lysyl oxidase and endothelial dysfunction: mechanisms of lysyl oxidase down-regulation by pro-inflammatory cytokines. Front Biosci. 2008;13:2721–2727. doi: 10.2741/2879. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, et al. Influence of TNF-IL1α and IL4 signalling in human ovarian surface epithelial cells and biomechanical stress on matrix metalloproteinases and lysyl oxidases expressions in human knee synovial fibroblasts. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1997–2006. doi: 10.1007/s00167-013-2425-z. [DOI] [PubMed] [Google Scholar]
- 76.Xie J, et al. Interleukin-1 beta influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts. Int Orthop. 2013;37(3):495–505. doi: 10.1007/s00264-012-1549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito M, et al. Effect of low- and high-intensity pulsed ultrasound on collagen post-translational modifications in MC3T3-E1 osteoblasts. Calcif Tissue Int. 2004;75(5):384–395. doi: 10.1007/s00223-004-0292-9. [DOI] [PubMed] [Google Scholar]
- 78.Aydin S, et al. Influence of microvascular endothelial cells on transcriptional regulation of proximal tubular epithelial cells. Am J Physiol Cell Physiol. 2008;294(2):C543–C554. doi: 10.1152/ajpcell.00307.2007. [DOI] [PubMed] [Google Scholar]
- 79.Kagan HM. Lysyl oxidase: mechanism, regulation and relationship to liver fibrosis. Pathol Res Pract. 1994;190(9–10):910–919. doi: 10.1016/S0344-0338(11)80995-7. [DOI] [PubMed] [Google Scholar]
- 80.Franklin TJ. Therapeutic approaches to organ fibrosis. Int J Biochem Cell Biol. 1997;29(1):79–89. doi: 10.1016/s1357-2725(96)00121-5. [DOI] [PubMed] [Google Scholar]
- 81.Kenyon NJ, et al. TGF-β1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax. 2003;58(9):772–777. doi: 10.1136/thorax.58.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaufmann J, et al. Hydroxypyridinium collagen crosslinks in serum, urine, synovial fluid and synovial tissue in patients with rheumatoid arthritis compared with osteoarthritis. Rheumatol (Oxf). 2003;42(2):314–320. doi: 10.1093/rheumatology/keg102. [DOI] [PubMed] [Google Scholar]
- 83.Milward MR, et al. Micronutrient modulation of NF-κB in oral keratinocytes exposed to periodontal bacteria. Innate Immun. 2013;19(2):140–151. doi: 10.1177/1753425912454761. [DOI] [PubMed] [Google Scholar]
- 84.Chen F, Zhang Z, Zhu X. Inhibition of development of experimental abdominal aortic aneurysm by c-jun N-terminal protein kinase inhibitor combined with lysyl oxidase gene modified smooth muscle progenitor cells. Eur J Pharmacol. 2015;766:114–121. doi: 10.1016/j.ejphar.2015.09.046. [DOI] [PubMed] [Google Scholar]
- 85.Paul RG, et al. Biomechanical and biochemical study of a standardized wound healing model. Int J Biochem Cell Biol. 1997;29(1):211–220. doi: 10.1016/s1357-2725(96)00134-3. [DOI] [PubMed] [Google Scholar]
- 86.Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J. 2015;12(3):313–316. doi: 10.1111/iwj.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 88.Ranzer MJ, Chen L, DiPietro LA. Fibroblast function and wound breaking strength is impaired by acute ethanol intoxication. Alcohol Clin Exp Res. 2011;35(1):83–90. doi: 10.1111/j.1530-0277.2010.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kessides MC, Khachemoune A. A review of epidermal maturation arrest: a unique entity or another description of persistent granulation tissue? J Clin Aesthet Dermatol. 2014;7(12):46–50. [PMC free article] [PubMed] [Google Scholar]
- 90.Szauter KM, et al. Lysyl oxidase in development, aging and pathologies of the skin. Pathol Biol (Paris) 2005;53(7):448–456. doi: 10.1016/j.patbio.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 91.Brown LF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176(5):1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Couffinhal T, et al. Mouse model of angiogenesis. Am J Pathol. 1998;152(6):1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 93.Nomura T, et al. β2-microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-responsive element-binding protein, and vascular endothelial growth factor axis. Clin Cancer Res. 2006;12(24):7294–7305. doi: 10.1158/1078-0432.CCR-06-2060. [DOI] [PubMed] [Google Scholar]
- 94.Byeseda SE, et al. ICAM-1 is necessary for epithelial recruitment of γδ T cells and efficient corneal wound healing. Am J Pathol. 2009;175(2):571–579. doi: 10.2353/ajpath.2009.090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhatwadekar AD, et al. Retinal endothelial cell apoptosis stimulates recruitment of endothelial progenitor cells. Invest Ophthalmol Vis Sci. 2009;50(10):4967–4973. doi: 10.1167/iovs.09-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bignon M, et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood. 2011;118(14):3979–3989. doi: 10.1182/blood-2010-10-313296. [DOI] [PubMed] [Google Scholar]
- 97.Darby IA, et al. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harrison CA, et al. Use of an in vitro model of tissue-engineered skin to investigate the mechanism of skin graft contraction. Tissue Eng. 2006;12(11):3119–3133. doi: 10.1089/ten.2006.12.3119. [DOI] [PubMed] [Google Scholar]
- 99.Brinckmann J, et al. Different pattern of collagen cross-links in two sclerotic skin diseases: lipodermatosclerosis and circumscribed scleroderma. J Invest Dermatol. 2001;117(2):269–273. doi: 10.1046/j.0022-202x.2001.01414.x. [DOI] [PubMed] [Google Scholar]
- 100.Woodley DT, et al. Collagen telopeptides (cross-linking sites) play a role in collagen gel lattice contraction. J Invest Dermatol. 1991;97(3):580–585. doi: 10.1111/1523-1747.ep12481920. [DOI] [PubMed] [Google Scholar]
- 101.Namazi MR, Fallahzadeh MK, Schwartz RA. Strategies for prevention of scars: what can we learn from fetal skin? Int J Dermatol. 2011;50(1):85–93. doi: 10.1111/j.1365-4632.2010.04678.x. [DOI] [PubMed] [Google Scholar]
- 102.Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3(3):20. doi: 10.1186/scrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gilad GM, Kagan HM, Gilad VH. Lysyl oxidase, the extracellular matrix-forming enzyme, in rat brain injury sites. Neurosci Lett. 2001;310(1):45–48. doi: 10.1016/s0304-3940(01)02089-4. [DOI] [PubMed] [Google Scholar]
- 104.Colwell AS, et al. Early-gestation fetal scarless wounds have less lysyl oxidase expression. Plast Reconstr Surg. 2006;118(5):1125–1129. doi: 10.1097/01.prs.0000221056.27536.db. [DOI] [PubMed] [Google Scholar]
- 105.Soo C, et al. Ontogenetic transition in fetal wound transforming growth factor-β regulation correlates with collagen organization. Am J Pathol. 2003;163(6):2459–2476. doi: 10.1016/s0002-9440(10)63601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003;48(3):314–346. doi: 10.1016/s0039-6257(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 107.Karsdal MA, et al. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol. 2013;11(2):70–92. doi: 10.1089/adt.2012.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williamson AK, et al. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21(5):872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 109.Williamson AK, et al. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9(4):625–634. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 110.Asanbaeva A, et al. Cartilage growth and remodeling: modulation of balance between proteoglycan and collagen network in vitro with β-aminopropionitrile. Osteoarth Cartil. 2008;16(1):1–11. doi: 10.1016/j.joca.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 111.Lafont A, et al. Endothelial dysfunction and collagen accumulation: two independent factors for restenosis and constrictive remodeling after experimental angioplasty. Circulation. 1999;100(10):1109–1115. doi: 10.1161/01.cir.100.10.1109. [DOI] [PubMed] [Google Scholar]
- 112.Ricard-Blum S, et al. Mechanism of collagen network stabilization in human irreversible granulomatous liver fibrosis. Gastroenterology. 1996;111(1):172–182. doi: 10.1053/gast.1996.v111.pm8698196. [DOI] [PubMed] [Google Scholar]
- 113.Brasselet C, et al. Collagen and elastin cross-linking: a mechanism of constrictive remodeling after arterial injury. Am J Physiol Heart Circ Physiol. 2005;289(5):H2228–H2233. doi: 10.1152/ajpheart.00410.2005. [DOI] [PubMed] [Google Scholar]
- 114.Schultz G, et al. Effects of growth factors on corneal wound healing. Acta Ophthalmol Suppl. 1992;202:60–66. doi: 10.1111/j.1755-3768.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 115.Hou Y, et al. The roles of TGF-β1 gene transfer on collagen formation during Achilles tendon healing. Biochem Biophys Res Commun. 2009;383(2):235–239. doi: 10.1016/j.bbrc.2009.03.159. [DOI] [PubMed] [Google Scholar]
- 116.Ashcroft GS, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-β1 levels. Nat Med. 1997;3(11):1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 117.Eliasson P, Andersson T, Aspenberg P. Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol. 2009;107(2):399–407. doi: 10.1152/japplphysiol.91563.2008. [DOI] [PubMed] [Google Scholar]
- 118.Saito M, Soshi S, Fujii K. Effect of hyper- and microgravity on collagen post-translational controls of MC3T3-E1 osteoblasts. J Bone Miner Res. 2003;18(9):1695–1705. doi: 10.1359/jbmr.2003.18.9.1695. [DOI] [PubMed] [Google Scholar]
- 119.Martinez DA, et al. Temporal extracellular matrix adaptations in ligament during wound healing and hindlimb unloading. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1552–R1560. doi: 10.1152/ajpregu.00423.2007. [DOI] [PubMed] [Google Scholar]
- 120.Chen YJ, et al. Differential regulation of collagen, lysyl oxidase and MMP-2 in human periodontal ligament cells by low- and high-level mechanical stretching. J Periodontal Res. 2013;48(4):466–474. doi: 10.1111/jre.12028. [DOI] [PubMed] [Google Scholar]
- 121.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17(2):153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 122.Lau Y, West J. The effect of overexpression of lysyl oxidase on dermal wound healing. Mol Ther. 2005;11:303. [Google Scholar]
- 123.Chronopoulos A, et al. High glucose increases lysyl oxidase expression and activity in retinal endothelial cells: mechanism for compromised extracellular matrix barrier function. Diabetes. 2010;59(12):3159–3166. doi: 10.2337/db10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kawamura S, Lotito K, Rodeo SA. Biomechanics and healing response of the meniscus. Oper Tech Sports Med. 2003;11(2):68–76. [Google Scholar]
- 126.Gebremariam L, et al. Evaluation of treatment effectiveness for the herniated cervical disc: a systematic review. Spine (Phila Pa 1976) 2012;37(2):E109–E118. doi: 10.1097/BRS.0b013e318221b5af. [DOI] [PubMed] [Google Scholar]
- 127.Guilak F, et al. Biomechanics and mechanobiology in functional tissue engineering. J Biomech. 2014;47(9):1933–1940. doi: 10.1016/j.jbiomech.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scotti C, et al. Healing of meniscal tissue by cellular fibrin glue: an in vivo study. Knee Surg Sports Traumatol Arthrosc. 2009;17(6):645–651. doi: 10.1007/s00167-009-0745-9. [DOI] [PubMed] [Google Scholar]
- 129.Fomovsky GM, Rouillard AD, Holmes JW. Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J Mol Cell Cardiol. 2012;52(5):1083–1090. doi: 10.1016/j.yjmcc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rouillard AD, Holmes JW. Mechanical regulation of fibroblast migration and collagen remodelling in healing myocardial infarcts. J Physiol. 2012;590(Pt 18):4585–4602. doi: 10.1113/jphysiol.2012.229484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MacBarb RF, et al. Engineering functional anisotropy in fibrocartilage neotissues. Biomaterials. 2013;34(38):9980–9989. doi: 10.1016/j.biomaterials.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palamakumbura AH, Trackman PC. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal Biochem. 2002;300(2):245–251. doi: 10.1006/abio.2001.5464. [DOI] [PubMed] [Google Scholar]
- 133.Eleswarapu SV, Responte DJ, Athanasiou KA. Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint. PLoS ONE. 2011;6(10):e26178. doi: 10.1371/journal.pone.0026178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marturano JE, et al. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci USA. 2013;110(16):6370–6375. doi: 10.1073/pnas.1300135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21(2):195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 136.Bastiaansen-Jenniskens YM, et al. Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthr Cartil. 2008;16(3):359–366. doi: 10.1016/j.joca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 137.Balguid A, et al. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets–relevance for tissue engineering. Tissue Eng. 2007;13(7):1501–1511. doi: 10.1089/ten.2006.0279. [DOI] [PubMed] [Google Scholar]
- 138.Athens AA, Makris EA, Hu JC. Induced collagen cross-links enhance cartilage integration. PLoS ONE. 2013;8(4):e60719. doi: 10.1371/journal.pone.0060719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Elbjeirami WM, et al. Enhancing mechanical properties of tissue-engineered constructs via lysyl oxidase crosslinking activity. J Biomed Mater Res A. 2003;66(3):513–521. doi: 10.1002/jbm.a.10021. [DOI] [PubMed] [Google Scholar]
- 140.Makris EA, et al. Combined use of chondroitinase-ABC, TGF-β1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair. Biomaterials. 2014;35(25):6787–6796. doi: 10.1016/j.biomaterials.2014.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DiMicco MA, et al. Integrative articular cartilage repair: dependence on developmental stage and collagen metabolism. Osteoarthr Cartil. 2002;10(3):218–225. doi: 10.1053/joca.2001.0502. [DOI] [PubMed] [Google Scholar]
- 142.Kuzuya M, et al. Glycation cross-links inhibit matrix metalloproteinase-2 activation in vascular smooth muscle cells cultured on collagen lattice. Diabetologia. 2001;44(4):433–436. doi: 10.1007/s001250051640. [DOI] [PubMed] [Google Scholar]
- 143.Beekman B, et al. Synthesis of collagen by bovine chondrocytes cultured in alginate; posttranslational modifications and cell-matrix interaction. Exp Cell Res. 1997;237(1):135–141. doi: 10.1006/excr.1997.3771. [DOI] [PubMed] [Google Scholar]
- 144.Wong M, et al. Collagen fibrillogenesis by chondrocytes in alginate. Tissue Eng. 2002;8(6):979–987. doi: 10.1089/107632702320934074. [DOI] [PubMed] [Google Scholar]
- 145.Ahsan T, et al. Integrative cartilage repair: inhibition by β-aminopropionitrile. J Orthop Res. 1999;17(6):850–857. doi: 10.1002/jor.1100170610. [DOI] [PubMed] [Google Scholar]
- 146.McGowan KB, Sah RL. Treatment of cartilage with β-aminopropionitrile accelerates subsequent collagen maturation and modulates integrative repair. J Orthop Res. 2005;23(3):594–601. doi: 10.1016/j.orthres.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 147.van Vlimmeren MA, et al. Controlling matrix formation and cross-linking by hypoxia in cardiovascular tissue engineering. J Appl Physiol. 2010;109(5):1483–1491. doi: 10.1152/japplphysiol.00571.2010. [DOI] [PubMed] [Google Scholar]
- 148.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70(5):1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 149.Pak O, et al. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J. 2007;30(2):364–372. doi: 10.1183/09031936.00128706. [DOI] [PubMed] [Google Scholar]
- 150.Balguid A, et al. Hypoxia induces near-native mechanical properties in engineered heart valve tissue. Circulation. 2009;119(2):290–297. doi: 10.1161/CIRCULATIONAHA.107.749853. [DOI] [PubMed] [Google Scholar]
- 151.Shukunami C, et al. Chondromodulin-I and tenomodulin are differentially expressed in the avascular mesenchyme during mouse and chick development. Cell Tissue Res. 2008;332(1):111–122. doi: 10.1007/s00441-007-0570-8. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y, et al. Enhanced proliferation capacity of porcine tenocytes in low O2 tension culture. Biotechnol Lett. 2010;32(2):181–187. doi: 10.1007/s10529-009-0137-8. [DOI] [PubMed] [Google Scholar]
- 153.Makris EA, Hu JC, Athanasiou KA. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthr Cartil. 2013;21(4):634–641. doi: 10.1016/j.joca.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kothapalli CR, Ramamurthi A. Lysyl oxidase enhances elastin synthesis and matrix formation by vascular smooth muscle cells. J Tissue Eng Regen Med. 2009;3(8):655–661. doi: 10.1002/term.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kothapalli CR, et al. Transforming growth factor β 1 and hyaluronan oligomers synergistically enhance elastin matrix regeneration by vascular smooth muscle cells. Tissue Eng A. 2009;15(3):501–511. doi: 10.1089/ten.tea.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kothapalli CR, Ramamurthi A. Copper nanoparticle cues for biomimetic cellular assembly of crosslinked elastin fibers. Acta Biomater. 2009;5(2):541–553. doi: 10.1016/j.actbio.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brown DA, et al. Analysis of oxygen transport in a diffusion-limited model of engineered heart tissue. Biotechnol Bioeng. 2007;97(4):962–975. doi: 10.1002/bit.21295. [DOI] [PubMed] [Google Scholar]
- 158.Makris EA, et al. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci USA. 2014;111(45):E4832–E4841. doi: 10.1073/pnas.1414271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dean RG, et al. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53(10):1245–1256. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 160.Voloshenyuk TG, et al. Induction of cardiac fibroblast lysyl oxidase by TGF-β1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine. 2011;55(1):90–97. doi: 10.1016/j.cyto.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 161.Shyu KG, et al. Mechanism of the inhibitory effect of atorvastatin on endoglin expression induced by transforming growth factor-β1 in cultured cardiac fibroblasts. Eur J Heart Fail. 2010;12(3):219–226. doi: 10.1093/eurjhf/hfq011. [DOI] [PubMed] [Google Scholar]
- 162.Fu Y, et al. The p38 MAPK inhibitor, PD169316, inhibits transforming growth factor β-induced Smad signaling in human ovarian cancer cells. Biochem Biophys Res Commun. 2003;310(2):391–397. doi: 10.1016/j.bbrc.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 163.Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF-β signaling pathway in hepatic fibrosis. Liver Int. 2006;26(1):8–22. doi: 10.1111/j.1478-3231.2005.01192.x. [DOI] [PubMed] [Google Scholar]
- 164.Yokoyama U, et al. Prostaglandin E2 inhibits elastogenesis in the ductus arteriosus via EP4 signaling. Circulation. 2014;129(4):487–496. doi: 10.1161/CIRCULATIONAHA.113.004726. [DOI] [PubMed] [Google Scholar]
- 165.Ma YC, et al. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102(5):635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]