Abstract
Bone provides mechanical support, and flexibility to the body as a structural frame work along with mineral storage, homeostasis, and blood pH regulation. The repair and/or replacement of injured or defective bone with healthy bone or bone substitute is a critical problem in orthopedic treatment. Recent advances in tissue engineering have shown promising results in developing bone material capable of substituting the conventional autogenic or allogenic bone transplants. In the present review, we have discussed natural and synthetic scaffold materials such as metal and metal alloys, ceramics, polymers, etc. which are widely being used along with their cellular counterparts such as stem cells in bone tissue engineering with their pros and cons.
Keywords: Bone, Bone tissue engineering, Scaffolds, Growth factors, Regenerative medicine
Introduction
Bone is a complex living connective tissue that provides structural frame work, mechanical support, and flexibility to the body along with mineral storage, homeostasis and blood pH regulation [1]. Bone structure typically comprises of cortical and cancellous bone [2] (Fig. 1). The unique organic and inorganic material constitution imparts its mechanical properties to the bone.
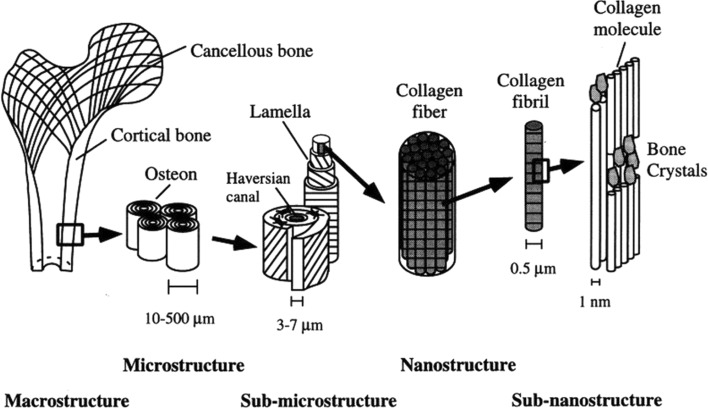
Fig. 1.
Hierarchical structural organization of bone: A cortical and cancellous bone, B osteons with haversian systems, C lamellae, D collagen fibre assemblies of collagen fibris, E bone mineral crystals, collagen molecules, and non-collagenous proteins. Reproduced with permission from Rho et al. [2], ©1998 Elsevier Ltd
Bone defects and their repair is the most common problem worldwide [3] gaining bone as a second most transplanted tissue status followed by blood [4, 5]. In U.S. alone, more than 6.5 million bone defects [6] and more than 3 million facial injuries [7] are recorded every year. Annually, more than 2.2 million bone graft procedures are performed worldwide [8]. Tumor resection, congenital malformation, trauma, fractures, surgery, or diseases like osteoporosis, arthritis [8, 9] are the major cause of bone defects. Some clinical conditions like skeletal reconstruction of large bone defects or compromised regenerative processes such as avascular necrosis, atrophic non-unions and osteoporosis [10] also require bone related transplants. The repair or replacements of such damaged or traumatized bone tissue is achieved by standard approaches like distraction osteogenesis, bone transport [9] or different bone grafting methods like autografts, allografts, bone graft substitutes or by using growth factors [9]. The first commercial bone graft material was introduced in 1993 as Interpore’s coral derived Pro-Osteon® [11]. Autografts have achieved various degrees of success in treating bone defects. However, the donor site morbidity, prolonged rehabilitation, increased risk of deep infection and restricted availability limits its potential applications [12]. Bone allografts have resolved transplantable bone samples limitations to some extents, but with potential risks of transmissible diseases, viral infection, immunological rejections, efficacy and cost effectiveness [13–15]. Due to avascular and porous nature of bone, osteocytes survive by diffusion of nutrients limits their application in case of bone defect size and host viability [16]. Furthermore, there are no heterologous or synthetic bone substitutes available at present which are superior or with similar biological or mechanical properties as of natural bone. Hence, an alternative and effective treatment method for bone regeneration is a necessity.
Recent bone substitute which can replace conventional bone grafts have shown a ray of hope [17]. Use of osteogenic growth factors like bone morphogenic proteins (BMPs), osteoinductive matrix, gene therapy, use of stem cells etc. [18] have demonstrated their potential in bone tissue engineering (Fig. 2). This review is an effort to summarize the different types of available scaffolds and/or biomaterials, stem cells and growth factors used for bone regeneration, either alone or in combination.
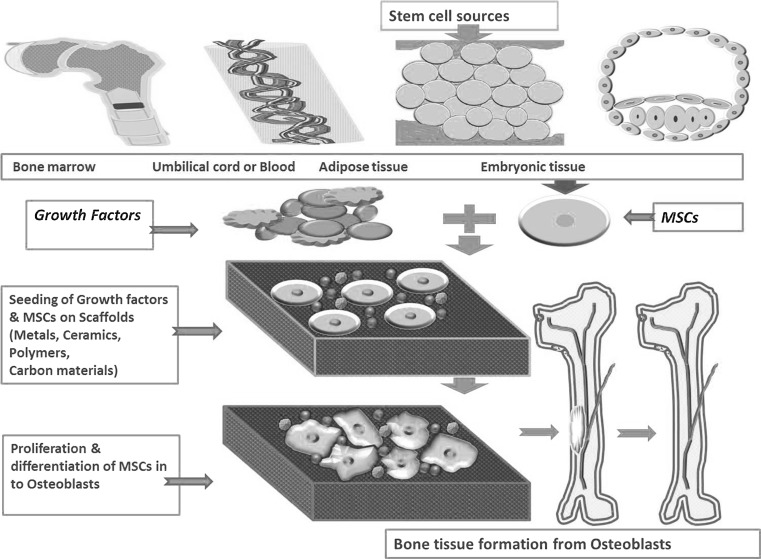
Fig. 2.
Outline of bone tissue engineering: mesenchymal stem cells from bone marrow, umbilical cord, adipose tissue or embryonic tissue can be used along with growth factors on different biomaterials to repair or regenerate bone tissue
Cellular aspect of bone tissue engineering
Bone homeostasisis maintained by osteoblasts, osteocytes, and osteoclasts. Osteoblasts are originated from mesenchymal cells, while osteocytes are mature osteoblasts and osteoclasts are of hematopoietic origin [19]. Among all available cell sources viz. autogenic cells, allogenic cells, embryonic stem cells (ESCs) [20], induced pluripotent stem cells (iPSCs) [21], or mesenchymal stem cells (MSCs) [22]; ESCs are widely studied for bone tissue engineering including differentiation into osteoblasts [20, 23]. Co-culturing of ESCs with fetal fibroblast has showed enhanced formation of bone nodules [24]. However, teratoma formation limits ESCs clinical applications [21]. For example, transplantation of laminin coated 3D poly (l-lactide-co-glycolide) (PLGA) scaffolds with human ESCs into liver lobules of SCID mice resulted in teratoma formation [25].
MSCs are known to differentiate into maturated cells like osteoblasts, chondroblasts and chondrocytes on external chemical stimuli [26]. MSCs isolated from bone marrow [22], peripheral blood [27], adipose tissue [28] have been differentiated to osteoblasts, chondrocytes and healed critical sized bone defects in vivo. The study involving proliferative and osteogenic potential of MSCs from human fetal bone marrow (hfBMSCs), human adult adipose tissue (hADSCs) cultured in to poly(caprolactone) (PCL)-tricalcium phosphate (TCP) scaffolds revealed hfBMSCs possesses highest proliferation and osteogenesis with least immunogenicity [29]. The iPSCs have emerged as an alternative for MSCs and/or ESCs. There are reports available of differentiation of human iPSCs in to osteoblasts in vitro [30] and in vivo without teratoma formation [21, 31]. Murine iPSCs transduced with Special Adenosine-Thymine rich sequence binding protein 2 (SATB2) are known to express the osteoblastic genes [32]. Murine iPSCs overexpressing SATB2 seeded with silk scaffolds [32] and human iPSCs seeded with PCL scaffolds [33] transplanted in to mice model, showed increased mineralization and new bone formation.
Although BMSCs are gold standard in tissue engineering, its clinical use is restricted due to invasive procedures and decreased proliferation and differentiation with increasing age of donor [34]. Although morphologically and phenotypically similar to human umbilical cords, Wharton’s jelly mesenchymal stem cells (hUCMSCs), human dental pulp stem cells (hDPSCs) have demonstrated greater proliferative properties than hBMSCs, hADSCs [35]. The study of hUCMSCs with non-rigid calcium phosphate cement scaffold revealed proliferation and differentiation of hUCMSCs into osteoblast and mineralization in vitro [36]. Cell origin and lineage differentiation conditions have significant effect on stem cells osteogenic differentiation pattern [37]. Many research groups have considered hUCMSCs as an alternative to BMSCs showing comparable expression of osteogenic phenotypes in vitro [34, 35] along with in vivo osteogenic differentiation when transplanted with scaffolds in nude mice model [38].
Human Amniotic fluid derived stem cells (hAFSCs) can be used as alternative for BMSCs in bone tissue engineering [37].The hAFSCs adhered to composite scaffolds of collagen matrix derived from porcine bladder submucosa matrix—PLGA differentiates into osteoblasts expressing osteogenic genes [39]. The hAFSCs and hDPSCs seeded on fibroin scaffolds [40] and on collagen scaffolds [41], support in vivo bone formation in a critical size cranial bone defects in rats. The hDPSCs seeded on collagen-hydroxyapatite (HA)-poly(l-lactide-co-ε-caprolactone) showed cell adhesion, growth, expression of osteogenic genes with mineralization and nodule formation [42]. The hDPSCs with HA-TCP paste transplanted into immunodefecient parietal region cranial defect rats revealed bone formation with increased mineralization and density of bone [43].
Bone tissue engineering using growth factors
Growth factors viz. Bone morphogenetic proteins (BMPs) [18], Fibroblast growth factors (FGFs) [44], Platelet derived growth factors (PDGFs) [45], Transforming growth factors (TGF-β) [46], Vesicular endothelial growth factors (VEGFs) [47], Insulin like growth factors (IGFs) [46]; alone or in combination are known to play important role in regulation of bone formation at different level. BMP is involved in skeletal development, adult bone homeostasis, and fracture healing along with differentiation of MSCs in to the cartilage, bone, tendon/ligament [18] with highest in vitro and in vivo osteogenic potential [48]. Three dimensional (3D) bio-printing of BMP-2 in DermaMatrix™ human allograft revealed differentiation of mouse C2C12 progenitor cells in vitro and tissue formation in calvarial defect in vivo [49]. The high doses requirement of BMPs limits its direct use in regenerative medicine [18], but BMPs with combinations of growth factors have been used in bone regeneration. For example, adenovirus based expression of BMP2 in the C3H10T1/2 cell line, osteoblastic differentiations increased 10 fold [50]. The BMP-2 loaded nanoparticles with fibrin scaffolds showed more bone formation in vitro than BMP-2 alone [51]. Silica xerogel-chitosan hybrid coated BMP-2 with porous HA showed in vitro osteoblastic cell response and in vivo bone formation in calvarial defects in rabbits [52]. The study with MG-63 cells seeded on TCP scaffolds showed higher cell seeding efficiency in vitro while alginate gel assisted cell seeding with BMP-2 showed osteocalcin and osteoid deposition in vivo [53].PLGA scaffold coated with BMP-2 and PDGF polyelectrolyte on transplantation in calvarial bone defect rat model induced mechanically competent local bone formation [54].
The osteogenic growth factor bFGF has a potential to accelerate bone regeneration when used with MSCs [44]. Also use of bFGF with gelatin hydrogels have resulted in improved bone regeneration in skull defects of rabbits [55] and monkeys [56]. The mesoporous bioactive glass nanospheres used for the delivery of FGF2 and FGF18. Rat MSCs culture with these growth factors showed cell proliferation, cellular mineralization in vitro and their transplantation into rat calvarial defects revealed bone formation with higher bone volume and bone density [57]. The PDGF stimulate VEGF secretion and contributes to the osteogenic lineage and helps to formation of new bone by differentiation of MSCs in presence of BMP via Wnt signaling [45] and chitosan-TCP [58]. The combination of PDGF and IGF-1 with aqueous gel transplanted to periodontitis affected teeth in beagle dogs’ revealed cementum and new bone formation [59]. The PDGF with deproteinized bovine bone mineral showed higher bone regeneration as compared to β-TCP in calvarial defect rabbits models [60]. The patients with alveolar defects transplanted with PDGF, hMSCs seeded on biphasic scaffolds, three month post-surgery revealed more than 50% bone repair [61]. In a clinical trial patients with one localized periodontal osseous defect treated with PDGF and β-TCP, 36 month follow up revealed filling of potential bone defect [62].
When BMSCs cultured on VEGF-silk-fibroin-chitosan scaffolds showed significant cell attachment, cell proliferation compared to BMSCs cultured on silk-fibroin-chitosan scaffolds [63]. VEGFs incorporated PLGA scaffolds showed proliferation of endothelial cells and apatite formation revealing osteogenic and angiogenic potential [64]. Osteoblasts cultured on AD-VEGF activated chitosan-HA showed attachment, proliferation, differentiation in vitro and in vivo with neo-vessel formation in newly formed ectopic bone [65]. VEGFs, when used synergistically with BMP-4 [47]and BMP-2 [66] enhanced bone formation than VEGFs alone (Table 1).
Table 1.
Cells for bone tissue engineering
| Cells for bone tissue engineering | Tissue repair | References |
|---|---|---|
| ESCs | Osteoblast differentiation but Teratoma formation in SCID mice | [24, 25] |
| BMSCs | Osteoblast differentiation; osteoinduction; osteogenesis; mineralization; in vitro & in vivo bone regeneration | [22, 29, 63, 109, 138, 147, 149, 153] |
| ADSCs | Osteoblast differentiation | [29, 35, 137] |
| DPSCs | Mineralization; in vivo bone regeneration | [40–43] |
| AFSCs | Osteoblast differentiation; in vivo bone regeneration | [39–41] |
| UCMSCs | Osteoblast differentiation; mineralization | [35, 36, 38] |
| iPSCs | Osteoblast differentiation; mineralization; in vitro & in vivo bone regeneration | [30–33] |
| MG63 cells | Osteoblast differentiation | [53] |
| MC3T3-E1 | Osteoblast differentiation | [88] |
| Osteoblast cells | Biocompatibility; mineralization; in vivo bone regeneration | [87, 94, 123, 124, 136] |
IGF-1 is secreted by mature osteoblasts and stimulates in vitro and in vivo proliferation and differentiation of osteoblasts [46]. Human periodontal ligament stem cells treated with exogenous IGF-1 showed the in vitro osteogenic differentiation and in vivo there was mineralization in the tissues [67]. The IGF transplanted with MSCs in the mice models improved the bone fractures through the callus mineralization and autocrine osteogenic effects via IRS-1 signaling [68]. IL-3,induces BMP2 and activate Smad1/5/8, enhancing the differentiation of MSCs in to the osteoblasts and bone regeneration, both in vitro and in vivo [69].
Bone tissue engineering with scaffolds
Scaffolds are porous 3D matrices that act as temporary templates for cell adhesion and proliferation, while providing mechanical support until formation of new tissue at the diseased area [70]. Scaffolds can also mimic the natural extra cellular matrix (ECM) [70] without activating host immune response or secretion of toxic metabolites [71]. A variety of materials such as metals [72], ceramics [73], natural [74] and synthetic polymers and their combinations (Table 2) have been explored for replacement and repair of damaged or traumatized bone tissues.
Table 2.
Types of scaffolds used for bone tissue engineering
| Type of scaffolds | Type of study | References |
|---|---|---|
| Metals | ||
| Lotus type porous nickel free stainless steel | In vivo | [76, 77] |
| Cobalt-Chromium (Co-Cr) & Ti alloys | In vivo | [72, 79, 80] |
| Ti6Al4 V alloy | In vitro | [81, 82] |
| Nitinol (NiTi) alloy | In vitro & In vivo | [81, 83] |
| Ceramic composites | ||
| BDHA scaffolds | In vitro | [22, 85] |
| calcium silicate scaffolds | In vitro & In vivo | [86] |
| calcium phosphate composite | In vitro | [87] |
| Bioglass 45S5 | In vitro & In vivo | [84, 89–91] |
| BCP scaffolds | In vitro | [94] |
| Bioactive glass-Strontium | In vitro | [88] |
| Polymers | ||
| Collagen composites | In vitro & In vivo | [74, 97–99] |
| Chitosan-gelatin-nano silica nanocomposite | In vitro | [102] |
| Chitosan-forsterite composite | In vitro | [95] |
| nHA-chitosan-CMC | In vitro | [105] |
| EDC treated Gelatin scaffolds | In vivo | [106] |
| PGA-PLA scaffolds | In vitro | [109] |
| PLLA-HA nanocomposites | In vivo | [115] |
| PLGA-nHA composite | In vitro | [119] |
| PDLLA-nHA-PPy-Alg scaffolds | In vitro | [117] |
| PCL, PCL-PLGA-HA, PCL-TCP-nHA | In vitro | [26, 99, 120, 121] |
| PCL-HA-CNTs; PCL-MNPs | In vitro & In vivo | [122, 123] |
| PLA, PLA-HA, PLA-HA-GO | In vitro | [124] |
| PHB, PHB-gelatin, PHB-gelatin-nHA | In vitro | [117] |
| Carbon materials | ||
| nHA + SWCNT scaffold | In vitro | [128, 129] |
| SWCNT networks, rGO | In vitro | [130] |
| HA-GN composites | In vitro | [127] |
The metallic materials such as Stainless steel, Co-Cr alloys and Ti alloys etc. [72] are in use over 100 years for bone replacements due to their mechanical properties [75]. However, these materials are corrosive and release cytotoxic ions [75] and often suffer from the wear and stress-shielding effect on transplantation into the human body [76]. Stainless steel is the most common bone implant material because of its combination of properties like mechanical properties, biocompatibility, corrosion resistance and cost effectiveness [77]. Nickel free stainless steel implants are recent focus of metallic bone implants [77].
Biocompatibility and osteogenesis were observed with corrosive resistant implants made from Tantalum (Ta), Hafnium (Hf) Niobium (Nb), Titanium (Ti), Rhenium (Re) [78]. The properties of pure metals can be enhanced by alloying the different types of metals. Co-Cr alloys are wear resistant but possess corrosion properties [79]. The coiled wire and particle form of Co-Cr alloy and Ti implants are found to be devoid of inflammatory response upon transplantation [80].
Ti and Ti alloys like Titanium-Aluminum (6%)-Vanadium (4%) alloy (Ti6Al4 V) have excellent tensile strength, resistance to corrosion [81], lower modulus and superior biocompatibility as compared to stainless steel, Co based alloys [82]. Nickel-titanium alloy called Nitinol (NiTi) possesses shape memory effect, biocompatibility, super-plasticity, damping properties [81, 83].
Ceramics such as HA [76], bioactive glasses [84], calcium phosphate [73] are widely used for bone repair. These are similar to the inorganic component of bone and possess chemical and structural similarity to the native bone [74]. Being natural component of bone HA is biocompatible, biodegradable, biomimetic and bioactive in nature has been widely used in different types of scaffolds as major or partial component. For example, HA and its derivatives like nano-HA, bovine derived porous HA (BDHA) [22, 85].
Calcium Phosphate ceramics are biocompatible, safe, cost effective, easily available and show lower morbidity hence widely used as bone substitutes, coatings, cements, drug delivery systems and tissue engineering scaffolds [73]. The mechanically stable 3D printed calcium silicate scaffolds showed in vitro mineralizationand in vivo osteogenesis [86]. Bio-mimetic composites of calcium phosphate and mixtures of chitosan, hyaluronic acid found to have biodegradability and good biocompatibility with osteoblasts cells [87]. The 3D printed bioactive glass-Strontium mesoporous scaffolds showed apatite formation and proliferation and differentiation of MC3T3-E1 cells in vitro [88]. Bioglass 45S5 showed good osteogenic cellular activities, osteocalcin synthesis, and calcified extracellular matrix production along with formation of calcified bone nodule [84, 89], hence proposed for bone tissue engineering [90] alone or in combination [91].
Biphasic calcium phosphate (BCP), which is made up of varying concentration of HA and β-TCP, possesses controllable biological and chemical properties and has become preferred choice for promoting bone ingrowth over other calcium phosphate ceramics [74, 92, 93]. For example, 3D printed BCP scaffolds dynamically cultured with rat osteoblasts and BMSCs showed increased osteoinduction, ALP activity and mineralization [94]. Like metals, ceramics too lacks degradability in a biological environment, and their limited processability [95, 96] can become a hurdle in tissue engineering.
Polymers are widely used in biomaterial applications worldwide. For bone tissue engineering natural polymers such as collagens, glycosaminoglycans(GAG), starch, chitin, and chitosan are used [74] which possess good biocompatibility but have poor mechanical strength [74]. Natural polymers are biocompatible which advantageous for cellular adhesion. In some cases, these polymers may contain pathogenic impurities which can exhibit immunogenicity. Other disadvantages include less control over their mechanical properties, biodegradability, batch-to-batch variability and limited supply can affect the cost efficacy [74].
Collagen is most accepted scaffold among all due to its biocompatibility and availability. Type I collagen which constitutes >90% of the organic mass of the bone [97] promotes proliferation and differentiation of human MSCs in to the osteoblasts in vitro and osteogenesis in vivo [97, 98]. The composite scaffolds of collagen-apatite [13], BSP-collagen composite scaffolds [99] are known to support bone repair. Collagen in combination with ceramics like HA, silk fibroin-HA, GAG exhibits good biocompatibility and bone regeneration properties [74]. A natural polymer chitosan is biocompatible, biodegradable, hydrophilic [100] and stimulate the differentiation of osteoprogenitor cells [101]. It is observed that chitosan-gelatin scaffold, chitosan-gelatin-nano silica nanocomposite scaffolds showed improved bioactivity and cellular behavior [102] as compared to control chitosan. Interconnected porosity and mechanical strength of chitosan scaffolds can be improved by reinforcement with additives like forsterite (FS) nanopowder without altering its biocompatibility [95].
Combinations of natural and synthetic polymers like corn starch with functionalized polycaprolactone are widely used in preparation of composite scaffolds for bone tissue engineering [17]. These biodegradable scaffolds not only promote osteogenic differentiation [103] but also shows adequate mechanical properties with highly interconnected pores and porosity [17]. Natural polymers like Bacterial cellulose derived from Acetobacter xylinum (ATCC 53582) [104], Carboxymethyl cellulose (CMC) incorporated nHA-chitosan (nHA-chitosan-CMC) [105] composite, 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) treated gelatin scaffolds [106] and Modified cellulose-poly (vinyl alcohol) (PVA) [107] are some of the promising scaffold for bone tissue regeneration.
Unlike natural polymers, synthetic polymers have advantage of reproducibility, large scale production with controlled properties of strength, degradation rate and microstructure. Poly (α-hydroxy acids), including poly(galactic acid) (PGA), poly(lactic acid) (PLA), and their copolymer PLGA, are the most popular and widely used synthetic polymeric materials in bone tissue engineering. When degraded, PGA, PLA [108] and PLGA [99] secretions are nontoxic, natural metabolites, and are eventually eliminated from the body in the form of carbon dioxide and water. The 3D printed PGA-PLA scaffolds found to be biocompatible with BMSCs [109]. Also composites viz. PCL-CaCO3 [110], HA-gelatin [111], silk-HA [112], PLA-HA [113] and triphasic HA-collagen-PCL [114] have been used for bone regeneration applications.
A wide range of PLLA based composites like PLLA-HA, PLLA-gel, PLLA-gel-HA, PLLA-apatite have been studied by various groups worldwide. Composite polymers prepared using combination of PLLA with various other materials increased its suitability for bone regeneration compared to the plain PLLA scaffolds [100]. Formation of new bone trabeculae with complete repair of bone was seen in nano-composites scaffold like PLLA-HA [115] or PLLA-Gel-HA with negligible complement activation [116]. The poly-d, l-lactic acid (PDLLA) materials in combination with additives like nHA, polypyrrole-alginate (PPy-Alg), chitosan have demonstrated good cytocompatibility, hydrophilicity, bioavailability and compressive strength [117], along with mineralization and osteogenesity [118]. PLGA-HA composite foams demonstrated comparatively higher density, compressive modulus and compressive yield strength [119]. PCL alone [26] or in combination with other polymers like PLGA-HAcomposite [99], TCP, nHA [120] have been observed to increase porosity, tensile strength and cellular activities than rest of the scaffolds [121]. The porous PCL-HA-CNTs (Carbon Nano Tubes) composites prepared by 3D printing with comparable compressive strength of trabecular bone revealed HA bioactivity, cell adhesion and spreading properties seemly to regenerate bone [122]. Magnetic nanofibrous PCL scaffolds prepared by incorporating magnetic nanoparticles (MNPs) (PCL-MNPs). These PCL-MNPs showed apatite formation with simulated body fluid in vitro. Osteoblasts were adhered and penetrated in to PCL-MNPs and expressed osteogenic genes as compared to pure PCL. Also in vivo there were bone regeneration in segmental bone defects and neo-vessel formation [123].
PLA, PLA-HA and PLA-HA-GO scaffolds have showed osteoblast growth and proliferation on their surface [124]. Another poly (3-hydroxybutyrate) (PHB) based nanofibrous scaffolds namely PHB, PHB-gelatin, PHB-gelatin-nHA and PHB-gelatin have demonstrated similar results along with higher level of ALP activity and matrix bio-mineralization in presence of MSCs [117]. The biomorphic scaffolds like demineralized bone matrixes, calcined animal bone and decellularized ECMs derived from various tissues are known to promote differentiation of ASCs, MSCs, ESCs, iPSCs in to the osteoblasts and supported bone regeneration [125, 126].
Carbon materials and their use in bone tissue engineering
Due to the similar dimensions, carbon nano-materials are considered to be physical analogue of ECM components like collagen fibers [127]. Various forms of carbon materials or their composites like single-walled carbon nanotubes (SWCNTs), multi-walled carbon nanotubes (MWCNTs), and grapheme oxide (GO) have been investigated for their efficacy in tissue engineering in last couple of years. The nHA-SWCNT scaffold in chitosan enhanced the mechanical properties suitable for bone tissue engineering. These scaffolds are found to have osteoblast adhesion and proliferation [128], biocompatible and nontoxic cellular compatibility properties [129].
The SWCNT networks and rGO are chemically similar in nature, but differ by topographical features, with rGO exhibiting higher biocompatibility than the SWCNT [130]. In other hand rough, porous HA-graphene nanosheet (GN) composites contributes to increased fracture properties of HA based scaffolds with post mineralization apatite formation in vitro [127].
Surface modification of scaffolds
Altering the physicochemical surface properties can change biocompatibility, influence cell adhesion and growth; can improve wear resistance and corrosion resistance properties of material to be used as biomaterial. The surface modification can be achieved by various methods (Table 3) such as coating by self-assembled film/electrolyte multilayers, surface gradient, surface activation, and surface chemical reaction. Stainless steel screws when coated with bisphosphonate increased new bone formation around implants [131]. Similarly Co-Cr alloy coated with HA showed superior osteogenesis and integration than uncoated alloy [132].
Table 3.
Surface modification of scaffolds for bone tissue engineering
| Surface modified material | Coated by material | Study outcomes | References |
|---|---|---|---|
| Stainless steel screws | Bisphosphonate | New bone formation | [131] |
| Co-Cr alloy | HA | Osteogenesis; implant integration | [132] |
| β-TCP-HA scaffolds | Alginate | Osteoblasts adhesion, proliferation | [71] |
| β-TCP scaffolds | Ca-P-polydopamine | Cell attachment, proliferation and mineralization | [133] |
| β-TCP scaffolds | ZnO | Cell attachment, proliferation | [134] |
| 45S5 Bioglass® based scaffolds | PHBV | Improved porosity, mechanical properties | [70] |
| corn starch-ethylene-vinyl alcohol based scaffolds | Ca-P | Normal cellular activity, osteogenic expression | [135] |
| PDLLA foams | Bioglass® particles | Osteoblasts adhesion, proliferation | [136] |
| PLA composite | Polydopamine | Normal cellular activity, osteogenic expression | [137] |
| PVF sponges | Dextran | Cell attachment, proliferation, osteogenic expression calcium deposition | [138] |
Osteoblasts were able to adhere and proliferate on composites of β-TCP-HA scaffolds coated with alginate [71]. The uniform Ca-P-polydopamine composite nanolayer on β-TCP bio-ceramics results in improved surface roughness and hydrophilicity of β-TCP bio-ceramics. These composites when seeded with hBMSCs showed cell attachment, proliferation and alkaline phosphatase activity and expression of bone related genes (ALP, OCN, COL1 and Runx2) [133]. The interconnected porous β-TCP scaffolds improved by ZnO showed good mechanical properties like compressive strength, stiffness, fracture toughness and micro hardness. These scaffolds showed bioactivity, biodegradability in vitro and cell attachment, proliferation [134]. Porous 45S5 Bioglass® based scaffolds fabricated and coated with poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) revealed higher porosity with increased interconnected pore structure and high mechanical properties [70] hence ideal candidate for bone tissue engineering (Tables 4, 5).
Table 4.
Growth factors for bone tissue engineering
| Growth factors | Tissue repair | References |
|---|---|---|
| BMPs | Osteoblastic differentiation; in vivo bone formation | [18, 50–54] |
| FGFs | Mineralization; in vivo bone regeneration | [44, 55–57] |
| PDGFs | Stimulate VEGF secretion; osteogenic lineage differentiation; in vivo bone regeneration | [45, 58–62] |
| VEGFs | Osteogenic and angiogenic potential; bone formation | [47, 63–66] |
| IGFs | Osteogenic differentiation; mineralization | [46, 59, 67, 68] |
Table 5.
Bioreactor systems for bone tissue engineering
| Sl. no. | Bioreactor systems | Culturing of under bioreactor | Aftermaths | References |
|---|---|---|---|---|
| 1 | Biaxial bioreactor | Umbilical cord blood endothelial progenitor cells & hBMSCs + PCL-TCP | Mineralization, ectopic bone formation | [145] |
| 2 | Perfusion bioreactors | hBMSCs + collagen/silk | In vitro bone formation | [147] |
| 3 | Flow Perfusion bioreactors | Goat bone marrow stromal cells seeded with biphasic calcium phosphate | In vivo bone formation | [148] |
| 4 | Multiplate Xpansion bioreactor | Human periosteum derived stem cells | In vivo bone formation | [152] |
| 5 | Hollow fibre bioreactors | hBMSCs + semipermeable polyethersulphone | Osteoblastic differentiation of hBMSCs | [153] |
The corn starch-ethylene-vinyl alcohol (50/50 wt %) based scaffolds when coated with Ca-P showed compressive modulus of 224.6 and compressive strength of 24 without affecting normal cellular activity, expression of osteopontin, collagen type I and alkaline phosphatase activity (ALP) [135]. PDLLA foams and PDLLA foams coated with Bioglass® particles showed complete covering with HA in 28 days of incubation in SBF. Osteoblasts were attached and spread on both PDLLA uncoated and coated foams [136]. While in another in vitro study with SBF, the HA formation was slower in uncoated composites than coated composites of PDLLA [108]. The 3D printed polydopamine coated PLA composite showed cell adhesion, cell cycle progression, increased ALP activity, osteocalcin on culturing with hADSCs [137]. Dextran coated polyvinyl formal (PVF) sponges with water holding capacity showed more adhesion, proliferation, and differentiation of BMSCs in vitro along with increased DNA content, ALP activity, osteocalcin content, and calcium deposition [138].
Bioreactors for bone tissue engineering
A bioreactor is a culture system to proliferate the cells through dynamic culture and restrained environment [139]. The limitation of nutrient transfer in the 3D tissue engineering scaffolds can be overcome by continuously mixing media and by convectively transporting nutrients to cells through bioreactor [140]. Various studies revealed potential role of bioreactors in the cell seeding [141], cell proliferation [142] and differentiation of MSCs in to osteoblasts [143] with mineralization and calcium deposition [144]. The umbilical cord blood endothelial progenitor cells and hBMSCs seeded with PCL-TCP scaffolds dynamically cultured into biaxial bioreactor showed mineralization as well as calcium deposition and subcutaneous implantation in to NOD/SCID mice showed ectopic bone formation as compared to static culture [145].
Among different bioreactors, for example, spinner flasks, rotating wall systems, and a perfusion system (Fig. 3), the latter has potential applications in bone tissue engineering [139]. Perfusion bioreactors increase mass transfer, removes waste and seed scaffolds dynamically by controlled distribution of cells compared to static culture [146].For example, the study with hBMSCs cultured on collagen/silk scaffolds in three different environments viz. static dish, spinner flask and perfusion system showed highest in vitro bone formation in perfusion system [147]. The goat bone marrow stromal cells seeded with biphasic calcium phosphate cultured in perfusion system proliferated homogeneously on scaffolds and after implantation in to nude mice showed bone formation [148]. In comparison with static culture, hBMSCs cultured on the PLGA-PCL scaffolds in perfusion systems, when implanted into femoral condyle defects in rat, showed rapid bone regeneration [149].
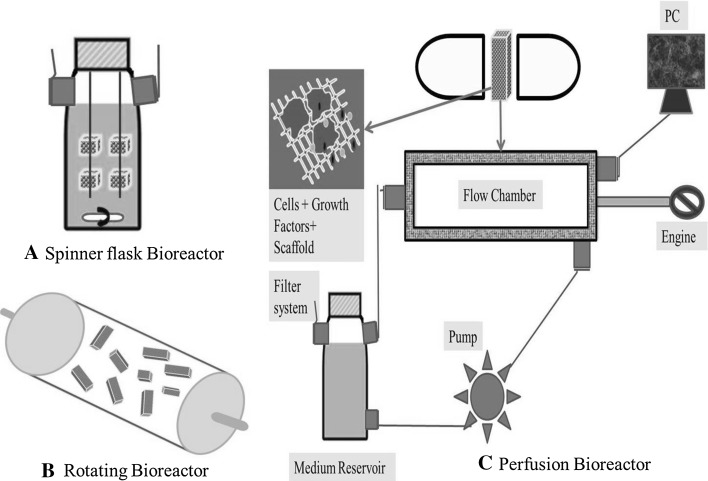
Fig. 3.
Schematic diagram of bioreactors: A spinner flask bioreactor, B rotating bioreactor, C perfusion bioreactor. Cells, growth factors filled 3D constructs cultured in bioreactors can be used to regenerate bone tissues
Rat MSCs seeded on PCL scaffolds cultured under engineered flow perfusion bioreactor demonstrated cell adhesive, remodeling, structural proteins as well as HA [150]. The hMSCs seeded with Poly (l-lactide-co-caprolactone) cultured in the dynamic conditions showed calcification, expression of osteogenic genes and induction of osteogenic lineage [151]. Human periosteum derived stem cells cultured in the multiplate Xpansion bioreactor showed proliferation of cells and in vivo bone formation [152]. The hBMSCs separated by semipermeable polyethersulphone cultured in hollow fibre bioreactors maintained their immunophenotype and osteoblastic differentiation capacity [153]. Flow perfusion culture of rat MSCs seeded on PLA scaffolds increased the growth and proliferation of MSCs with higher ALP Activity [154].
Bone tissue engineering and future perspectives
From the first attempt of bone regeneration by Urist [155], the field of bone tissue engineering has grown rapidly to develop bone substitute which is more close to natural bone or to regenerate bone using different approaches. Advanced studies in bone tissue engineering in recent past both in vitro and in vivo have explained the potential of variety of cells to differentiate into osteoblasts and the supporting role of growth factors and/or biomaterials. Most of these studies have revealed the biocompatibility, biodegradability, osteoinductivity, osteoconductivity, osteogenicity and/or physico-mechanical properties. Some in vivo studies showed repair of bone defects or bone regeneration. However, complete replacement of defective bone using biomaterials is still not achieved. Creation of functional bone in laboratory condition using cell therapy is still a challenge, although different types of stem cells have shown osteogenic lineage differentiation. Because of many functional problems like mechanical strength, host immune integration, vascularization, etc. in development of bone or bone substitute that can mimic natural bone, clinical trials in human are still at bay. So far, researchers have shown successful use of biomaterials or scaffolds growth factors, and cells for bone tissue engineering, alone or in combination. However, when it comes to clinical application of these materials as bone substitute, it is difficult to obtain approval from regulatory bodies for clinical trials. The future direction should focus on establishing an ethical threshold that is effective and obtainable for future researchers to partake in more high-level studies within the clinical setting. Another reason for only few approved bone substitute for clinical trials, is the difficulties in performing pre-clinical large animal trials. High research and development costs, in combination with the current regulatory environment, present a challenge to high-quality evidence-based study.
Biomaterials for orthopedic implants have great financial impact all over the world. In U.S. alone it was predicted that the biomaterials for orthopedic implants will costs as much as $3.5 billion by the end of 2017 [156]. Patient specific manufacturing of bone substitute also adds in to the cost of therapy. Hence, further efforts are required to develop cost effective, bio-mimicking constructs which can replace defective bone in reality. Such bone tissue engineering constructs will surely bring fruitful treatments in curing bone defects via bone replacement or by regeneration. As research at the cellular level continues to expand, the opportunity for growth is limitless, with stem cell-based applications and tissue engineering potentially setting the stage for how more effective and cheap bone substitute/regeneration treatments are carried out both today and in the future.
Acknowledgements
Author would like to acknowledge University Grant Commission (UGC), Government of India, New Delhi for doctoral fellowship to Mr. Shivaji Kashte.
Conflicts of interest
Authors have no potential conflicts of interest.
Ethical Statement
There are no animal experiments carried out for this article.
References
- 1.Sowjanya JA, Singh J, Mohita T, Sarvanan S, Moorthi A, Srinivasan N, et al. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf B Biointerfaces. 2013;109:294–300. doi: 10.1016/j.colsurfb.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20:92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 3.Venkatesan J, Bhatnagar I, Kim S-K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar Drugs. 2014;12:300–316. doi: 10.3390/md12010300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fröhlich M, Grayson WL, Marolt D, Gimble JM, Kregar-Velikonja N, Vunjak-Novakovic G. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A. 2010;16:179–189. doi: 10.1089/ten.tea.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng M, James R, Laurencin CT, Kumbar SG. Nanostructured polymeric scaffolds for orthopaedic regenerative engineering. IEEE Trans Nanobiosci. 2012;11:3–14. doi: 10.1109/TNB.2011.2179554. [DOI] [PubMed] [Google Scholar]
- 7.Initial Evaluation and Management of Maxillofacial Injuries: Overview, Clinical Presentation and Approach for Patients with Facial Trauma, Relevant Anatomy and Contraindications. http://emedicine.medscape.com/article/434875-overview. (Accessed 9 Dec 2015).
- 8.Jimi E, Hirata S, Osawa K, Terashita M, Kitamura C, Fukushima H. The current and future therapies of bone regeneration to repair bone defects. Int J Dent. 2012;2012:1–7. doi: 10.1155/2012/148261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smrke D, Rozman P, Borut GM. Treatment of bone defects-allogenic platelet gel and autologous bone technique. In: Andrades JA, editor. Regenerative Medicine and Tissue Engineering. InTech, 2013. doi:10.5772/55987.
- 10.Gamble M, Pope J. Musculoskeletal complications of systemic lupus erythematosus: risk factors and prevalence for avascular necrosis and osteoporosis. J Rheumatol. 2015;42:1341–1342. [Google Scholar]
- 11.Kenley R, Yim K, Abrams J, Ron E. Biotechnology and bone graft substitutes. Pharm Res. 1993;10:1393–1401. doi: 10.1023/a:1018902720816. [DOI] [PubMed] [Google Scholar]
- 12.Euler SA, Hengg C, Wambacher M, Spiegl UJ, Kralinger F. Allogenic bone grafting for augmentation in two-part proximal humeral fracture fixation in a high-risk patient population. Arch Orthop Trauma Surg. 2015;135:79–87. doi: 10.1007/s00402-014-2128-z. [DOI] [PubMed] [Google Scholar]
- 13.Xia Z, Yu X, Jiang X, Brody HD, Rowe DW, Wei M. Fabrication and characterization of biomimetic collagen-apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013;9:7308–7319. doi: 10.1016/j.actbio.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Hu J, Ran J, Shen X, Tong H. Preparation and evaluation of collagen-silk fibroin/hydroxyapatite nanocomposites for bone tissue engineering. Int J Biol Macromol. 2014;65:1–7. doi: 10.1016/j.ijbiomac.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Tan A, Pastorin G, Ho HK. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol Adv. 2013;31:654–668. doi: 10.1016/j.biotechadv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Tägil M, Johnsson R. Incomplete incorporation of morselized and impacted autologous bone graft: a histological study in 4 intracorporally grafted lumbar fractures. Acta Orthop. 1999;70:555–558. doi: 10.3109/17453679908997841. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues AI, Gomes ME, Leonor IB, Reis RL. Bioactive starch-based scaffolds and human adipose stem cells are a good combination for bone tissue engineering. Acta Biomater. 2012;8:3765–3776. doi: 10.1016/j.actbio.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 18.De Gorter DJJ, Van Dinther M, Korchynskyi O, Ten Dijke P. Biphasic effects of transforming growth factor? On bone morphogenetic protein-induced osteoblast differentiation. J Bone Miner Res. 2011;26:1178–1187. doi: 10.1002/jbmr.313. [DOI] [PubMed] [Google Scholar]
- 19.Buckwalter JA, Glimcher MJ, Becker RR. Bone biology. J Bone Joint Surg Instr Course Lect. 1995;77:1256–1275. [Google Scholar]
- 20.Sottile V, Thomson A, McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells. 2003;5:149–155. doi: 10.1089/153623003322234759. [DOI] [PubMed] [Google Scholar]
- 21.Levi B, Hyun JS, Montoro DT, Lo DD, Chan CKF, Hu S, et al. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc Natl Acad Sci USA. 2012;109:20379–20384. doi: 10.1073/pnas.1218052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnamurithy G, Murali MR, Hamdi M, Abbas AA, Raghavendran HB, Kamarul T. Characterization of bovine-derived porous hydroxyapatite scaffold and its potential to support osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Ceram Int. 2014;40:771–777. [Google Scholar]
- 23.Bielby RC, Boccaccini AR, Polak JM, Buttery LDK. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 24.Buttery LD, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SP, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 25.Lees JG, Lim SA, Croll T, Williams G, Lui S, Cooper-White J, et al. Transplantation of 3D scaffolds seeded with human embryonic stem cells: biological features of surrogate tissue and teratoma-forming potential. Regen Med. 2007;2:289–300. doi: 10.2217/17460751.2.3.289. [DOI] [PubMed] [Google Scholar]
- 26.Shin M, Yoshimoto H, Vacanti JP. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng. 2004;10:33–41. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- 27.Wan C, He Q, Li G. Allogenic peripheral blood derived mesenchymal stem cells (MSCs) enhance bone regeneration in rabbit ulna critical-sized bone defect model. J Orthop Res. 2006;24:610–618. doi: 10.1002/jor.20119. [DOI] [PubMed] [Google Scholar]
- 28.Lu W, Ji K, Kirkham J, Yan Y, Boccaccini AR, Kellett M, et al. Bone tissue engineering by using a combination of polymer/bioglass composites with human adipose-derived stem cells. Cell Tissue Res. 2014;356:97–107. doi: 10.1007/s00441-013-1770-z. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z-Y, Teoh S-H, Chong MSK, Schantz JT, Fisk NM, Choolani MA, et al. Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126–137. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 30.Ardeshirylajimi A, Hosseinkhani S, Parivar K, Yaghmaie P, Soleimani M. Nanofiber-based polyethersulfone scaffold and efficient differentiation of human induced pluripotent stem cells into osteoblastic lineage. Mol Biol Rep. 2013;40:4287–4294. doi: 10.1007/s11033-013-2515-5. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Niyibizi C. Cells derived from murine induced pluripotent stem cells (iPSC) by treatment with members of TGF-beta family give rise to osteoblasts differentiation and form bone in vivo. BMC Cell Biol. 2012;13:35. doi: 10.1186/1471-2121-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye J-H, Xu Y-J, Gao J, Yan S-G, Zhao J, Tu Q, et al. Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials. 2011;32:5065–5076. doi: 10.1016/j.biomaterials.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin G-Z, Kim T-H, Kim J-H, Won J-E, Yoo S-Y, Choi S-J, et al. Bone tissue engineering of induced pluripotent stem cells cultured with macrochanneled polymer scaffold. J Biomed Mater Res A. 2013;101:1283–1291. doi: 10.1002/jbm.a.34425. [DOI] [PubMed] [Google Scholar]
- 34.Hou T, Xu J, Wu X, Xie Z, Luo F, Zhang Z, et al. Umbilical cord Wharton’s Jelly: a new potential cell source of mesenchymal stromal cells for bone tissue engineering. Tissue Eng Part A. 2009;15:2325–2334. doi: 10.1089/ten.tea.2008.0402. [DOI] [PubMed] [Google Scholar]
- 35.Stanko P, Kaiserova K, Altanerova V, Altaner C. Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression. Biomed Pap Med Fac Univ Palacký Olomouc Czechoslov. 2014;158:373–377. doi: 10.5507/bp.2013.078. [DOI] [PubMed] [Google Scholar]
- 36.TheinHan W, Weir MD, Simon CG, Xu HHK. Non-rigid calcium phosphate cement containing hydrogel microbeads and absorbable fibres seeded with umbilical cord stem cells for bone engineering. J Tissue Eng Regen Med. 2013;7:777–787. doi: 10.1002/term.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues MT, Lee SJ, Gomes ME, Reis RL, Atala A, Yoo JJ. Amniotic fluid-derived stem cells as a cell source for bone tissue engineering. Tissue Eng Part A. 2012;18:2518–2527. doi: 10.1089/ten.tea.2011.0672. [DOI] [PubMed] [Google Scholar]
- 38.Diao Y, Ma Q, Cui F, Zhong Y. Human umbilical cord mesenchymal stem cells: osteogenesis in vivo as seed cells for bone tissue engineering. J Biomed Mater Res A. 2009;91:123–131. doi: 10.1002/jbm.a.32186. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Jeong SY, Ju YM, Yoo JJ, Smith TL, Khang G, et al. In vitro osteogenic differentiation of human amniotic fluid-derived stem cells on a poly(lactide-co-glycolide) (PLGA)-bladder submucosa matrix (BSM) composite scaffold for bone tissue engineering. Biomed Mater. 2013;8:014107. doi: 10.1088/1748-6041/8/1/014107. [DOI] [PubMed] [Google Scholar]
- 40.Riccio M, Maraldi T, Pisciotta A, La Sala GB, Ferrari A, Bruzzesi G, et al. Fibroin scaffold repairs critical-size bone defects in vivo supported by human amniotic fluid and dental pulp stem cells. Tissue Eng Part A. 2012;18:1006–1013. doi: 10.1089/ten.TEA.2011.0542. [DOI] [PubMed] [Google Scholar]
- 41.Maraldi T, Riccio M, Pisciotta A, Zavatti M, Carnevale G, Beretti F, et al. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther. 2013;4:53. doi: 10.1186/scrt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akkouch A, Zhang Z, Rouabhia M. Engineering bone tissue using human dental pulp stem cells and an osteogenic collagen-hydroxyapatite-poly (l-lactide-co-ε-caprolactone) scaffold. J Biomater Appl. 2014;28:922–936. doi: 10.1177/0885328213486705. [DOI] [PubMed] [Google Scholar]
- 43.Asutay F, Polat S, Gül M, Subaşı C, Kahraman SA, Karaöz E. The effects of dental pulp stem cells on bone regeneration in rat calvarial defect model: Micro-computed tomography and histomorphometric analysis. Arch Oral Biol. 2015;60:1729–1735. doi: 10.1016/j.archoralbio.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Song K, Rao N-J, Chen M-L, Huang Z-J, Cao Y-G. Enhanced bone regeneration with sequential delivery of basic fibroblast growth factor and sonic hedgehog. Injury. 2011;42:796–802. doi: 10.1016/j.injury.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29:1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 46.Ochiai H, Okada S, Saito A, Hoshi K, Yamashita H, Takato T, et al. Inhibition of insulin-like growth factor-1 (IGF-1) expression by prolonged transforming growth factor-β1 (TGF-β1) administration suppresses osteoblast differentiation. J Biol Chem. 2012;287:22654–22661. doi: 10.1074/jbc.M111.279091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luu HH, Song W-X, Luo X, Manning D, Luo J, Deng Z-L, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 49.Cooper GM, Miller ED, Decesare GE, Usas A, Lensie EL, Bykowski MR, et al. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue Eng Part A. 2010;16:1749–1759. doi: 10.1089/ten.tea.2009.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang S, Wei D, Wang D, Phimphilai M, Krebsbach PH, Franceschi RT. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 2003;18:705–715. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park K-H, Kim H, Moon S, Na K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108:530–537. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Jun S-H, Lee E-J, Jang T-S, Kim H-E, Jang J-H, Koh Y-H. Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2013;24:773–782. doi: 10.1007/s10856-012-4822-0. [DOI] [PubMed] [Google Scholar]
- 53.Florczyk SJ, Leung M, Jana S, Li Z, Bhattarai N, Huang JI, et al. Enhanced bone tissue formation by alginate gel-assisted cell seeding in porous ceramic scaffolds and sustained release of growth factor. J Biomed Mater Res A. 2012;100:3408–3415. doi: 10.1002/jbm.a.34288. [DOI] [PubMed] [Google Scholar]
- 54.Shah NJ, Hyder MN, Quadir MA, Dorval Courchesne N-M, Seeherman HJ, Nevins M, et al. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc Natl Acad Sci USA. 2014;111:12847–12852. doi: 10.1073/pnas.1408035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tabata Y, Yamada K, Miyamoto S, Nagata I, Kikuchi H, Aoyama I, et al. Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials. 1998;19:807–815. doi: 10.1016/s0142-9612(98)00233-6. [DOI] [PubMed] [Google Scholar]
- 56.Tabata Y, Yamada K, Hong L, Miyamoto S, Hashimoto N, Ikada Y. Skull bone regeneration in primates in response to basic fibroblast growth factor. J Neurosurg. 1999;91:851–856. doi: 10.3171/jns.1999.91.5.0851. [DOI] [PubMed] [Google Scholar]
- 57.Kang MS, Kim J-H, Singh RK, Jang J-H, Kim H-W. Therapeutic-designed electrospun bone scaffolds: mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater. 2015;16:103–116. doi: 10.1016/j.actbio.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 58.Lee YM, Park YJ, Lee SJ, Ku Y, Han SB, Klokkevold PR, et al. The bone regenerative effect of platelet-derived growth factor-BB delivered with a chitosan/tricalcium phosphate sponge carrier. J Periodontol. 2000;71:418–424. doi: 10.1902/jop.2000.71.3.418. [DOI] [PubMed] [Google Scholar]
- 59.Lynch SE, Williams RC, Poison AM, Howell TH, Reddy MS, Zappa UE, et al. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 1989;16:545–548. doi: 10.1111/j.1600-051x.1989.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 60.Thoma DS, Jung RE, Hänseler P, Hämmerle CHF, Cochran DL, Weber FE. Impact of recombinant platelet-derived growth factor BB on bone regeneration: a study in rabbits. Int J Periodontics Restor Dent. 2012;32:195–202. [PubMed] [Google Scholar]
- 61.Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg. 2012;40:2–7. doi: 10.1016/j.jcms.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Nevins M, Kao RT, McGuire MK, McClain PK, Hinrichs JE, McAllister BS, et al. Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial. J Periodontol. 2013;84:456–464. doi: 10.1902/jop.2012.120141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong S, Xue L, Xu D, Liu Z, Wang X. In vitro culture of BMSCs on VEGF-SF-CS three-dimensional scaffolds for bone tissue engineering. J Hard Tissue Biol. 2015;24:123–133. [Google Scholar]
- 64.Jabbarzadeh E, Deng M, Lv Q, Jiang T, Khan YM, Nair LS, et al. VEGF-incorporated biomimetic poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2012;100:2187–2196. doi: 10.1002/jbm.b.32787. [DOI] [PubMed] [Google Scholar]
- 65.Koç A, Finkenzeller G, Elçin AE, Stark GB, Elçin YM. Evaluation of adenoviral vascular endothelial growth factor-activated chitosan/hydroxyapatite scaffold for engineering vascularized bone tissue using human osteoblasts: in vitro and in vivo studies. J Biomater Appl. 2014;29:748–760. doi: 10.1177/0885328214544769. [DOI] [PubMed] [Google Scholar]
- 66.Samee M, Kasugai S, Kondo H, Ohya K, Shimokawa H, Kuroda S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J Pharmacol Sci. 2008;108:18–31. doi: 10.1254/jphs.08036fp. [DOI] [PubMed] [Google Scholar]
- 67.Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S, et al. Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol. 2012;137:513–525. doi: 10.1007/s00418-011-0908-x. [DOI] [PubMed] [Google Scholar]
- 68.Granero-Moltó F, Myers TJ, Weis JA, Longobardi L, Li T, Yan Y, et al. Mesenchymal stem cells expressing insulin-like growth factor-I (MSCIGF) promote fracture healing and restore new bone formation in Irs1 knockout mice: analyses of MSCIGF autocrine and paracrine regenerative effects. Stem Cells. 2011;29:1537–1548. doi: 10.1002/stem.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barhanpurkar AP, Gupta N, Srivastava RK, Tomar GB, Naik SP, Joshi SR, et al. IL-3 promotes osteoblast differentiation and bone formation in human mesenchymal stem cells. Biochem Biophys Res Commun. 2012;418:669–675. doi: 10.1016/j.bbrc.2012.01.074. [DOI] [PubMed] [Google Scholar]
- 70.Li W, Nooeaid P, Roether JA, Schubert DW, Boccaccini AR. Preparation and characterization of vancomycin releasing PHBV coated 45S5 Bioglass?-based glass-ceramic scaffolds for bone tissue engineering. J Eur Ceram Soc. 2014;34:505–514. [Google Scholar]
- 71.Torres L, Gaspar VM, Serra IR, Diogo GS, Fradique R, Silva AP, et al. Bioactive polymeric-ceramic hybrid 3D scaffold for application in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2013;33:4460–4469. doi: 10.1016/j.msec.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Okazaki Y, Gotoh E. Metal release from stainless steel, Co–Cr–Mo–Ni–Fe and Ni–Ti alloys in vascular implants. Corros Sci. 2008;50:3429–3438. [Google Scholar]
- 73.Lobo SE, Arinzeh TL. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials (Basel) 2010;3:815–826. [Google Scholar]
- 74.Sanosh KP, Gervaso F, Sannino A, Licciulli A. Preparation and characterization of Collagen/hydroxyapatite microsphere composite scaffold for bone regeneration. Key Eng Mater. 2013;587:239–244. [Google Scholar]
- 75.Vagaska B, Bacakova L, Filová E, Balik K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol Res. 2010;59:309–322. doi: 10.33549/physiolres.931776. [DOI] [PubMed] [Google Scholar]
- 76.Liao CZ, Li K, Wong HM, Tong WY, Yeung KWK, Tjong SC. Novel polypropylene biocomposites reinforced with carbon nanotubes and hydroxyapatite nanorods for bone replacements. Mater Sci Eng C. 2013;33:1380–1388. doi: 10.1016/j.msec.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 77.Alvarez K, Hyun S, Nakano T. In vivo osteocompatibility of lotus-type porous nickel-free stainless steel in rats. Mater Sci Eng C. 2009;29:1182–1190. [Google Scholar]
- 78.Matsuno H. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials. 2001;22:1253–1262. doi: 10.1016/s0142-9612(00)00275-1. [DOI] [PubMed] [Google Scholar]
- 79.Michel R, Nolte M, Reich M, Löer F. Systemic effects of implanted prostheses made of cobalt-chromium alloys. Arch Orthop Trauma Surg. 1991;110:61–74. doi: 10.1007/BF00393876. [DOI] [PubMed] [Google Scholar]
- 80.Goodman S, Fornasier V. The effects of bulk versus particulate titanium and cobalt chrome alloy implanted into the rabbit tibia. J Biomed Mater Res A. 1990;24:1539–1549. doi: 10.1002/jbm.820241109. [DOI] [PubMed] [Google Scholar]
- 81.Kapanen A, Ryhänen J, Danilov A, Tuukkanen J. Effect of nickel–titanium shape memory metal alloy on bone formation. Biomaterials. 2001;22:2475–2480. doi: 10.1016/s0142-9612(00)00435-x. [DOI] [PubMed] [Google Scholar]
- 82.Long M, Rack H. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials. 1998;19:1621–1639. doi: 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 83.Alvarez K, Nakajima H. Metallic scaffolds for bone regeneration. Materials (Basel) 2009;2:790–832. [Google Scholar]
- 84.Fan JP, Kalia P, Di Silvio L, Huang J. In vitro response of human osteoblasts to multi-step sol–gel derived bioactive glass nanoparticles for bone tissue engineering. Mater Sci Eng C. 2014;36:206–214. doi: 10.1016/j.msec.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 85.Paşcu EI, Stokes J, McGuinness GB. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater Sci Eng C. 2013;33:4905–4916. doi: 10.1016/j.msec.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 86.Wu C, Fan W, Zhou Y, Luo Y, Gelinsky M, Chang J, et al. 3D-printing of highly uniform CaSiO3 ceramic scaffolds: preparation, characterization and in vivo osteogenesis. J Mater Chem. 2012;22:12288. [Google Scholar]
- 87.Ivan FD, Marian A, Tanase CE, Butnaru M, Vereştiuc L. Biomimetic composites based on calcium phosphates and chitosan-hyaluronic acid with potential application in bone tissue engineering. Key Eng Mater. 2013;587:191–196. [Google Scholar]
- 88.Zhang J, Zhao S, Zhu Y, Huang Y, Zhu M, Tao C, et al. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014;10:2269–2281. doi: 10.1016/j.actbio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Xynos ID, Hukkanen MVJ, Batten JJ, Buttery LD, Hench LL, Polak JM. Bioglass ®45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67:321–329. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 90.Izadi S, Hesaraki S, Hafezi-Ardakani M. Evaluation nanostructure properties of bioactive glass scaffolds for bone tissue engineering. Adv Mater Res. 2013;829:289–293. [Google Scholar]
- 91.Ravichandran R, Sundaramurthi D, Gandhi S, Sethuraman S, Krishnan UM. Bioinspired hybrid mesoporous silica-gelatin sandwich construct for bone tissue engineering. Microporous Mesoporous Mater. 2014;187:53–62. [Google Scholar]
- 92.Le Nihouannen D, Duval L, Lecomte A. Interactions of total bone marrow cells with increasing quantities of macroporous calcium phosphate ceramic granules. J Mater Sci Mater Med. 2007;18:1983–1990. doi: 10.1007/s10856-007-3098-2. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz C, Liss P, Jacquemaire B. Biphasic synthetic bone substitute use in orthopaedic and trauma surgery: clinical, radiological and histological results. J Mater Sci Mater Med. 1999;10:821–825. doi: 10.1023/a:1008944227417. [DOI] [PubMed] [Google Scholar]
- 94.Rath SN, Strobel LA, Arkudas A, Beier JP, Maier A-K, Greil P, et al. Osteoinduction and survival of osteoblasts and bone-marrow stromal cells in 3D biphasic calcium phosphate scaffolds under static and dynamic culture conditions. J Cell Mol Med. 2012;16:2350–2361. doi: 10.1111/j.1582-4934.2012.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scalera F, Gervaso F, Sanosh KP, Palamà IE, Dimida S, Sannino A. Development of a novel hybrid porous scaffold for bone tissue engineering: forsterite nanopowder reinforced chitosan. Key Eng Mater. 2014;587:249–254. [Google Scholar]
- 96.Maquet V, Jerome R. Design of macroporous biodegradable polymer scaffolds for cell transplantation. Mater Sci Forum. 1997;250:15–42. [Google Scholar]
- 97.Tsai K, Kao S, Wang C. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. … Res Part A. 2010;94:673–682. doi: 10.1002/jbm.a.32693. [DOI] [PubMed] [Google Scholar]
- 98.Kruger TE, Miller AH, Wang J. Collagen scaffolds in bone sialoprotein-mediated bone regeneration. Sci World J. 2013;2013:812718. doi: 10.1155/2013/812718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Yu X. Preparation, characterization and in vitro analysis of novel structured nanofibrous scaffolds for bone tissue engineering. Acta Biomater. 2010;6:3004–3012. doi: 10.1016/j.actbio.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 100.Duarte A, Mano J, Reis R. Novel 3D scaffolds of chitosan–PLLA blends for tissue engineering applications: Preparation and characterization. J Supercrit Fluids. 2010;54:282–289. [Google Scholar]
- 101.Muzzarelli R, Zucchini C, Ilari P. Osteoconductive properties of methylpyrrolidinone chitosan in an animal model. Biomaterials. 1993;14:925–929. doi: 10.1016/0142-9612(93)90134-n. [DOI] [PubMed] [Google Scholar]
- 102.Kavya KC, Jayakumar R, Nair S, Chennazhi KP. Fabrication and characterization of chitosan/gelatin/nSiO2 composite scaffold for bone tissue engineering. Int J Biol Macromol. 2013;59:255–263. doi: 10.1016/j.ijbiomac.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 103.Correia SI, Pereira H, Silva-Correia J, Van Dijk CN, Espregueira-Mendes J, Oliveira JM, et al. Current concepts: tissue engineering and regenerative medicine applications in the ankle joint. J R Soc Interface. 2014;11:20130784. doi: 10.1098/rsif.2013.0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zang S, Zhuo Q, Chang X, Qiu G, Wu Z, Yang G. Study of osteogenic differentiation of human adipose-derived stem cells (HASCs) on bacterial cellulose. Carbohydr Polym. 2014;104:158–165. doi: 10.1016/j.carbpol.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 105.Liuyun J, Yubao L, Chengdong X. Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J Biomed Sci. 2009;16:65. doi: 10.1186/1423-0127-16-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun H, Zhu F, Hu Q, Krebsbach PH. Controlling stem cell-mediated bone regeneration through tailored mechanical properties of collagen scaffolds. Biomaterials. 2014;35:1176–1184. doi: 10.1016/j.biomaterials.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chahala S, Hussain FSJ, Yusoff MM. Characterization of modified cellulose (MC)/poly (vinyl alcohol) electrospun nanofibers for bone tissue engineering. Procedia Eng. 2013;53:683–688. [Google Scholar]
- 108.Boccaccini AR, Notingher I, Maquet V, Jerome R. Bioresorbable and bioactive composite materials based on polylactide foams filled with and coated by Bioglass® particles for tissue engineering applications. J mater sci. Mater med. 2003;14:443–50. [DOI] [PubMed]
- 109.Xu H, Han D, Dong J-S, Shen G-X, Chai G, Yu Z-Y, et al. Rapid prototyped PGA/PLA scaffolds in the reconstruction of mandibular condyle bone defects. Int J Med Robot. 2010;6:66–72. doi: 10.1002/rcs.290. [DOI] [PubMed] [Google Scholar]
- 110.Fujihara K, Kotaki M, Ramakrishna S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials. 2005;26:4139–4147. doi: 10.1016/j.biomaterials.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 111.Kim H, Song J, Kim H. Nanofiber generation of gelatin–hydroxyapatite biomimetics for guided tissue regeneration. Adv Funct Mater. 2005;15:1988–1994. [Google Scholar]
- 112.Li C, Vepari C, Jin H, Kim H, Kaplan D. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 113.Sui G, Yang X, Mei F, Hu X. Poly-L-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J Biomed Mater Res A. 2007;82:445–454. doi: 10.1002/jbm.a.31166. [DOI] [PubMed] [Google Scholar]
- 114.Catledge S, Clem W. An electrospun triphasic nanofibrous scaffold for bone tissue engineering. Biomed Mater. 2007;2:142. doi: 10.1088/1748-6041/2/2/013. [DOI] [PubMed] [Google Scholar]
- 115.Rainer A, Spadaccio C, Sedati P, De Marco F, Carotti S, Lusini M, et al. Electrospun hydroxyapatite-functionalized PLLA scaffold: Potential applications in sternal bone healing. Ann Biomed Eng. 2011;39:1882–1890. doi: 10.1007/s10439-011-0289-2. [DOI] [PubMed] [Google Scholar]
- 116.Jaiswal AK, Kadam SS, Soni VP, Bellare JR. Improved functionalization of electrospun PLLA/gelatin scaffold by alternate soaking method for bone tissue engineering. Appl Surf Sci. 2013;268:477–488. [Google Scholar]
- 117.Alves A, Duarte ARC, Mano JF, Sousa RA, Reis RL. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J Supercrit Fluids. 2012;65:32–38. [Google Scholar]
- 118.Sajesh KM, Jayakumar R, Nair SV, Chennazhi KP. Biocompatible conducting chitosan/polypyrrole-alginate composite scaffold for bone tissue engineering. Int J Biol Macromol. 2013;62:465–471. doi: 10.1016/j.ijbiomac.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 119.Zhang RY, Ma PX. Poly(alpha-hydroxyl acids) hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–455. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 120.Polini A, Pisignano D, Parodi M, Quarto R, Scaglione S. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS One. 2011;6:1–8. doi: 10.1371/journal.pone.0026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marra KG, Szem JW, Kumta PN, DiMilla PA, Weiss LE. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J Biomed Mater Res. 1999;47:324–335. doi: 10.1002/(sici)1097-4636(19991205)47:3<324::aid-jbm6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 122.Gonçalves EM, Oliveira FJ, Silva RF, Neto MA, Fernandes MH, Amaral M, et al. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J Biomed Mater Res B Appl Biomater. 2015 doi: 10.1002/jbm.b.33432. [DOI] [PubMed] [Google Scholar]
- 123.Singh RK, Patel KD, Lee JH, Lee E-J, Kim J-H, Kim T-H, et al. Potential of magnetic nanofiber scaffolds with mechanical and biological properties applicable for bone regeneration. PLoS One. 2014;9:e91584. doi: 10.1371/journal.pone.0091584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma H, Su W, Tai Z, Sun D, Yan X, Liu B, et al. Preparation and cytocompatibility of polylactic acid/hydroxyapatite/graphene oxide nanocomposite fibrous membrane. Chin Sci Bull. 2012;57:3051–3058. [Google Scholar]
- 125.Qian J, Xu W, Yong X, Jin X, Zhang W. Fabrication and in vitro biocompatibility of biomorphic PLGA/nHA composite scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2014;36:95–101. doi: 10.1016/j.msec.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 126.Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32:462–484. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y, Dang Z, Wang Y, Huang J, Li H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: Inherited nanostructures and enhanced properties. Carbon N Y. 2014;67:250–259. [Google Scholar]
- 128.Wang X, Li Y. Biomimetic modification of porous TiNbZr alloy scaffold for bone tissue engineering. Tissue Eng Part A. 2009;16:309–316. doi: 10.1089/ten.TEA.2009.0074. [DOI] [PubMed] [Google Scholar]
- 129.Chowdhury S, Lalwani G, Zhang K. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials. 2013;34:283–293. doi: 10.1016/j.biomaterials.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Agarwal S, Zhou X, Ye F, He Q, Chen GCK, Soo J, et al. Interfacing live cells with nanocarbon substrates. Langmuir. 2010;26:2244–2247. doi: 10.1021/la9048743. [DOI] [PubMed] [Google Scholar]
- 131.Wermelin K, Suska F, Tengvall P, Thomsen P, Aspenberg P. Stainless steel screws coated with bisphosphonates gave stronger fixation and more surrounding bone. Histomorphometry in rats. Bone. 2008;42:365–371. doi: 10.1016/j.bone.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 132.Hunt JA, Callaghan JT, Sutcliffe CJ, Morgan RH, Halford B, Black RA. The design and production of Co-Cr alloy implants with controlled surface topography by CAD-CAM method and their effects on osseointegration. Biomaterials. 2005;26:5890–5897. doi: 10.1016/j.biomaterials.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 133.Wu C, Han P, Liu X, Xu M, Tian T, Chang J, et al. Mussel-inspired bioceramics with self-assembled Ca-P/polydopamine composite nanolayer: preparation, formation mechanism, improved cellular bioactivity and osteogenic differentiation of bone marrow stromal cells. Acta Biomater. 2014;10:428–438. doi: 10.1016/j.actbio.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 134.Feng P, Wei P, Shuai C, Peng S. Characterization of mechanical and biological properties of 3-D scaffolds reinforced with zinc oxide for bone tissue engineering. PLoS One. 2014;9:e87755. doi: 10.1371/journal.pone.0087755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Salgado AJ, Figueiredo JE, Coutinho OP, Reis RL. Biological response to pre-mineralized starch based scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2005;16:267–275. doi: 10.1007/s10856-005-6689-9. [DOI] [PubMed] [Google Scholar]
- 136.Roether JA, Gough JE, Boccaccini AR, Hench LL, Maquet V, Jérôme R. Novel bioresorbable and bioactive composites based on bioactive glass and polylactide foams for bone tissue engineering. J Mater Sci Mater Med. 2002;13:1207–1214. doi: 10.1023/a:1021166726914. [DOI] [PubMed] [Google Scholar]
- 137.Kao C-T, Lin C-C, Chen Y-W, Yeh C-H, Fang H-Y, Shie M-Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater Sci Eng C. 2015;56:165–173. doi: 10.1016/j.msec.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 138.Togami W, Sei A, Okada T, Taniwaki T, Fujimoto T, Nakamura T et al. Effects of water-holding capability of the PVF sponge on the adhesion and differentiation of rat bone marrow stem cell culture. J Biomed Mater Res A. 2013;102(1):1–33. [DOI] [PubMed]
- 139.Oliveira AL, Costa SA, Sousa RA, Reis RL. Nucleation and growth of biomimetic apatite layers on 3D plotted biodegradable polymeric scaffolds: effect of static and dynamic coating conditions. Acta Biomater. 2009;5:1626–1638. doi: 10.1016/j.actbio.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 140.Pittenger MF. Multilineage potential of adult human mesenchymal stem cells. Science (80). 1999;284:143–47. [DOI] [PubMed]
- 141.Janssen FW, Oorschot A Van, Oostra J, Bruijn JD De. Flow perfusion improves seeding of tissue engineering scaffolds with different architectures. Ann biomed eng. 2007;35:429–442. [DOI] [PubMed]
- 142.Mygind T, Stiehler M, Baatrup A, Li H, Zou X, Flyvbjerg A, et al. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28:1036–1047. doi: 10.1016/j.biomaterials.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 143.Janssen FW, van Dijkhuizen-Radersma R, Van Oorschot A, Oostra J, De Bruijn JD. CAVB. Human tissue -engineered bone produced in clinically relevant amounts using a semi-automated perfusion bioreactor system: a preliminary study. J Tissue Eng Regen Med. 2010;4:12–24. doi: 10.1002/term.197. [DOI] [PubMed] [Google Scholar]
- 144.Grayson WL, Marolt D, Bhumiratana S, Fröhlich M, Guo XE, Vunjak-Novakovic G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;108:1159–1170. doi: 10.1002/bit.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu Y, Teoh SH, Chong MS, Yeow CH, Kamm RD, Choolani MCJ. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in 3d scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng Part A. 2013;7:893–904. doi: 10.1089/ten.TEA.2012.0187. [DOI] [PubMed] [Google Scholar]
- 146.Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48:171–181. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- 147.Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 148.Janssen FW, Oostra J, Oorschot A Van, Blitterswijk CA Van. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: in vivo bone formation showing proof of concept. 2006; 27:315–23. [DOI] [PubMed]
- 149.Yeatts AB, Both SK, Yang W, Alghamdi HS, Yang F. et al. In vivo bone regeneration using tubular perfusion system bioreactor cultured nanofibrous scaffolds. Tissue Eng Part A 2013. http://online.liebertpub.com/doi/abs/10.1089/ten.tea.2013.0168. (Accessed 15 Dec 2015). [DOI] [PMC free article] [PubMed]
- 150.Thibault RA, Mikos AG, Kasper FK. Protein and mineral composition of osteogenic extracellular matrix constructs generated with a flow perfusion bioreactor. Biomacromolecules. 2011;12:4204–4212. doi: 10.1021/bm200975a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kleinhans C, Mohan RR, Vacun G, Schwarz T, Haller B, Sun Y, et al. A perfusion bioreactor system efficiently generates cell-loaded bone substitute materials for addressing critical size bone defects. Biotechnol J. 2015 doi: 10.1002/biot.201400813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lambrechts T, Papantoniou I, Viazzi S, Bovy T, Schrooten J, Luyten FP, et al. Evaluation of a monitored multiplate bioreactor for large-scale expansion of human periosteum derived stem cells for bone tissue engineering applications. Biochem Eng J. 2015 doi: 10.1016/j.bej.2015.07.015. [DOI] [Google Scholar]
- 153.Li M, Tilles AW, Milwid JM, Hammad M, Lee J, Yarmush ML, et al. Phenotypic and functional characterization of human bone marrow stromal cells in hollow-fibre bioreactors. J Tissue Eng Regen Med. 2012;6:369–377. doi: 10.1002/term.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.VanGordon SB, Voronov RS, Blue TB, Shambaugh RL, Papavassiliou DV, Sikavitsas VI. Effects of scaffold architecture on preosteoblastic cultures under continuous fluid shear. Ind Eng Chem Res. 2011;50:620–629. [Google Scholar]
- 155.Urist M. Bone formation by autoinduction. Science (80). 1965; 150:893–899. [DOI] [PubMed]
- 156.Report #A322, “U.S. Markets for Orthopedic Biomaterials for Viscosupplementation and Cartilage, Ligament, and Tendon Repair and Regeneration. 2015. http://www.medtechinsight.com/ReportA321.html#orderinfoA420. (Accessed 21 Nov 2015).