Abstract
Spherical neural mass (SNM) is a mass of neural precursors that have been used to generate neuronal cells with advantages of long-term passaging capability with high yield, easy storage, and thawing. In this study, we differentiated neural retinal progenitor cells (RPCs) from human induced pluripotent stem cells (hiPSC)-derived SNMs. RPCs were differentiated from SNMs with a noggin/fibroblast growth factor-basic/Dickkopf-1/Insulin-like growth factor-1/fibroblast growth factor-9 protocol for three weeks. Human RPCs expressed eye field markers (Paired box 6) and early neural retinal markers (Ceh-10 homeodomain containing homolog), but did not photoreceptor marker (Opsin 1 short-wave-sensitive). Reverse transcription polymerase chain reaction revealed that early neural retinal markers (Mammalian achaete-scute complex homolog 1, mouse atonal homolog 5, neurogenic differentiation 1) and retinal fate markers (brain-specific homeobox/POU domain transcription factor 3B and recoverin) were upregulated, while the marker of retinal pigment epithelium (microphthalmia-associated transcription factor) only showed slight upregulation. Human RPCs were transplanted into mouse (adult 8 weeks old C57BL/6) retina. Cells transplanted into the mouse retina matured and expressed markers of mature retinal cells (Opsin 1 short-wave-sensitive) and human nuclei on immunohistochemistry three months after transplantation. Development of RPCs using SNMs may offer a fast and useful method for neural retinal cell differentiation.
Keywords: Human induced pluripotent stem cells, Retinal photoreceptor, Retinal progenitor cell, Spherical neural mass
Introduction
Retinal degenerative diseases are leading causes of legal blindness worldwide [1]. In retinal degenerative diseases, including age-related macular degeneration, retinitis pigmentosa and Stargardt disease, irreversible visual loss is caused by the loss of neuro-retinal cells [1–4]. Various pharmacological therapies have been developed that attempt to slow visual deterioration, but cannot restore damaged cells and visual function [2, 3]. Due to the limitations of current pharmacologic therapies, alternative approaches including gene therapy and artificial retina are under investigation. Among these, stem cell therapy is an approach that can restore and replace damaged neuro-retinal cells and various stem cells are currently being evaluated for the treatment of retinal injury [2, 3, 5, 6].
Stem cells, which are capable of indefinite self-renewal and can give rise to various cell types, are classified according to their origin [7, 8]. Embryonic stem cells (ESCs) are obtained from the inner cell mass of the blastocyst and induced pluripotent stem cells (iPSCs) are formed from somatic cells [7, 8]. The eye is an organ suitable for studies of stem cell transplantation because of its relatively easy access and immune privileged status [3]. Using these advantages, various stem cell research initiatives on retinal disease are underway and several clinical trials have been completed [9].
Several methods of differentiation of retinal cells from stem cells have been reported, with varying results [10–14]. Spherical neural masses (SNMs) are pure masses of neural precursors and originate from neural rosettes or neural tubes [10, 15]. SNMs have several advantages including long-term passaging with high yield and without the loss of differentiation capability as well as easy storage and thawing [15]. SNMs have been used to generate dopaminergic neurons, retinal progenitor cells (RPCs) and retinal pigment epithelium (RPE) [10, 15, 16]. In this study, we used human iPSC-derived SNMs to differentiate into RPCs and investigated the survival and differentiation potential of these RPCs in the mouse retina after intraocular transplantation.
Materials and methods
Differentiation of human iPSCs into retinal progenitor cells
Human iPSCs were supplied by the Stem Cell Distribution & Education System (SCDES, Seoul National University College of Medicine, Seoul, Korea), and maintained in mTeSR1 medium (Stem Cell Technologies, Vancouver, Canada). Differentiation of human iPSCs to SNMs followed Cho et al. with minor modifications [15]. Human iPSC colonies were detached and cultured in a Petri dish for 7 days to form embryoid bodies (EBs). EBs were cultured in neuro-progenitor (NP) selection medium for 5 days, and the resulting NPs were expanded for another 4 days in an neural expansion medium. To form SNMs, neural rosettes and neural tube-like structures that were observed during neural expansion culture were mechanically isolated and cultured onto a Petri dish containing neural expansion medium. For passaging, SNMs were mechanically fragmented into four or six pieces and expanded for 10 days. SNMs can be expanded for a long period (greater than 4 months) and stored in a freezing medium. Pure SNMs were used for differentiation into retinal progenitor cells. SNMs were dissected into small pieces and transferred to matrigel-coated culture dishes, and cultured in N2B27 medium supplemented for Dickkopf-1 (Dkk-1) (10 ng/ml), noggin (100 ng/ml) and Insulin-like growth factor-1 (IGF-1) (10 ng/ml) for 1 week, followed by the addition of fibroblast growth factor 9 (FGF-9) and fibroblast growth factor-basic (bFGF) (10 ng/ml each) for 2 weeks.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS, pH 7.4) for 20 minutes at room temperature (RT) followed by washes in PBS. The cells were incubated in a blocking solution containing 0.3% Triton X-100 and 3% bovine serum albumin (BSA) for 45 minutes, and then in primary antibody diluted with the same blocking solution overnight at 4°C. After washing in PBS, the cells were incubated in secondary antibodies diluted in the blocking solution for 1 hour at RT. After washing in PBS, cells were stained with 4, 6-diamidino-2-phenylindole (DAPI), and mounted with Fluoromount-G (Southern Biotech, Birmingham, AL). The primary antibodies used were: anti-stage-specific embryonic antigen 4 (SSEA-4), anti-octamer-binding transcription factor 4(OCT4), anti-Tra-1-60 and anti-Tra-1-81 (all above, Chemicon, Billerica, MA, USA), anti-Ceh-10 homeodomain containing homolog (CHX10), anti-paired box 6 (PAX6) (all above, Abcam, Cambridge, MA, USA) and anti-opsin 1 short-wave-sensitive (OPN1SW) (Novus Biologicals, Littleton, CO, USA). The secondary antibodies used were: anti-rabbit Alexa Fluor 488, and anti-mouse Alexa Fluor 594 (Invitrogen, Burlington, ON, Canada). Images were captured by a confocal laser scanning microscope (LSM510; Carl Zeiss, Inc., Germany)
Real-time reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from cells using a ToTALLY RNA™ kit (Ambion, Austin, TX, USA) following manufacturer’s instructions. One microgram of the RNA template was reverse-transcribed by a Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics, Basel, Switzerland) according to manufacturer’s instructions. Real-time PCR was performed using a 2-μl aliquot of the reverse transcribed product for each 20-μl sample of the reaction mixture containing 4 mM MgCl2, 10 pmole of upstream and downstream primers, and 2 μl of 10X Light Cycler Fast Start DNA Master SYBR Green 1 (Roche Diagnostics, Basel, Switzerland). Light Cycler software (Ver. 3.5) was used to analyze the data. The list of primer sets used for RT-PCR were OCT4, forkhead box G1 (FOXG1), LIM homeobox 2 (LHX2), PAX6, SIX3, SIX6, mammalian achaete-scute complex homolog 1 (MASH1), neurogenic differentiation 1 (NEUROD1), mouse atonal homolog 5 (MATH5), brain-specific homeobox/POU domain transcription factor 3B (BRNB3), microphthalmia-associated transcription factor (MITF), Cone-rod homeobox (CRX), recoverin (RCVRN) and these are detailed in Table 1.
Table 1.
Primers used for real-time polymerase chain reaction
| Upper primer | Lower primer | Gene |
|---|---|---|
| TGAGTAGTCCCTTCGCAAGC | GCGAGAAGGCAAAATCTGA | OCT4 |
| TTTGAGTTACAACGGCACCA | TCTGAGTCAACACGGAGCTG | FOXG1 |
| CCAAGGACTTGAAGCAGCTC | AAGAGGTTGCGCCTGAACT | LHX2 |
| TCACCATGGCAAATAACCTG | CAGCATGCAGGAGTATGAGG | PAX6 |
| CTCCTCCCCCACTCCTTC | GGGTATCCTGATTTCGGTTTG | SIX3 |
| GGACACTGCAAGCCCAGTAT | ATGATTCGCGCCCTTTCT | SIX6 |
| CGACTTCACCAACTGGTTCTG | ATGCAGGTTGTGCGATCA | MASH1 |
| CTGCTCAGGACCTACTAACAACAA | GTCCAGCTTGGAGGACCTT | NEUROD1 |
| CAGACCTATGGACGCAATCA | CAACCCATTCACAAGATCCA | MATH5 |
| CCCTTTGAACCCCACCTC | CTTCCTGCAAACAGCCATCT | BRN3B |
| CAGGTGCCGATGGAAGTC | GCTAAAGTGGTAGAAAGGTACTGCTT | MITF |
| CGAGTTGGTACACACCGTCA | TCTCTTCACATCTCGCCTTTC | CRX |
| TAACGGGACCATCAGCAAG | CCTCGGGAGTGATCATTTTG | RCVRN |
OCT4 octamer-binding transcription factor 4, FOXG1 forkhead box G1, LHX2 LIM homeobox 2, PAX6 paired box 6, MASH1 mammalian achaete-scute complex homolog 1, NEUROD1 neurogenic differentiation 1, MATH5 mouse atonal homolog 5, BRNB3 brain-specific homeobox/POU domain transcription factor 3B, MITF microphthalmia-associated transcription factor, CRX cone-rod homeobox, RCVRN recoverin
Histology
Human iPS cells (2 × 106/100 μl) were injected on the back of the severe combined immunodeficiency (SCID) mouse. One month after transplantation, teratoma was dissected, dehydrated in ethanol and embedded in paraffin. For histological analysis, 5-μm-thick paraffin sections were cut and stained with hematoxylin and eosin (Sigma, St Louis, MO).
Transplantation of retinal progenitor cells
An adult 8-week-old C57BL/6 mouse was used as a transplant recipient and general anesthesia was performed with intraperitoneal administration of a mixture of zolazepam/tiletamine (80 mg/kg; Zoletil 50®, Virbac, France) and xylazine (20 mg/kg; Rompun®, Bayer HealthCare, Germany). Anesthesia status was checked after 5 minutes. The pupil was dilated with 0.5% tropicamide/phenylephrine HCl (Tropherine, Hanmi, Korea). The right eye was chosen for the transplantation. The mouse was positioned in the lateral decubitus position with the right eye upward under the microscope. Topical anesthesia of the mouse cornea was performed with proparacaine HCl ophthalmic solution (Paracaine, Hanmi, Korea). Periocular drape was performed with 5% povidone iodine solution. Using a 34-gauge needle, a scleral wound was created 1 mm posterior from the limbus. A beveled retinal injection needle (INCYTO needle-RN, Incyto, Korea) was connected to the injector pump. After filling with the prepared retinal progenitor cells (≤1.5 μl, total of about 50,000 cells), the retinal injection needle was inserted through the scleral wound. An assistant grasped a microscope coverslip and placed it on the mouse cornea to evaluate intraocular status. With caution not to touch the crystalline lens, the retinal injection needle was brought to the retina. Avoiding the optic disc and retinal vessels, the needle was advanced into the retina and the cell suspension was slowly deposited over 5 seconds. The mouse was housed in the breeding room for 3 months and analyzed 3 months after transplantation.
Enucleation and tissue sectioning
The mouse was sacrificed 3 months after transplantation. The eyes were enucleated with a fine microdissection forceps and scissors. The enucleated eye was rinsed with PBS and fixed in 4% PFA overnight with a corneal window to permit more efficient fixation. After fixation, the cornea and crystalline lens were removed and the eyes were post fixed in 4% PFA for 1 hour. The eyes were then soaked in 15% and 30% sucrose solution based on PBS for 1 hour in order. They were snap frozen in optimal cutting temperature (OCT) compound for 15 min and the material was serially sectioned at 20 μm.
Immunohistochemistry
For immunohistochemistry of the transplanted eye, frozen sections were mounted on silane-coated slides (Muto Pure Chemicals Co., LTD., Tokyo, Japan). Sections were washed in PBS and incubated in blocking solution containing 0.3% Triton X-100 and 3% bovine serum albumin (BSA) for 45 minutes. The sections were incubated with primary antibodies diluted with the same blocking solution overnight at 4°C. After washing with PBS, the sections were incubated in secondary antibodies diluted with the same blocking solution for 1 hour at RT. After washing with PBS, the sections were stained with DAPI, and mounted with Fluoromount-G. The primary antibodies used were: anti-human nuclear antigen (Millipore, Temecula, CA, USA), (HNu, Chemicon, Billerica, MA, USA) and anti-OPN1SW (Novus Biologicals, Littleton, CO, USA). The secondary antibodies used were: anti-rabbit Alexa Fluor 488, and anti-mouse Alexa Fluor 594 (Invitrogen, Burlington, ON, Canada). Images were captured by a Zeiss LSM 510 confocal laser scanning microscope.
Results
Characterization of human induced pluripotent stem cells
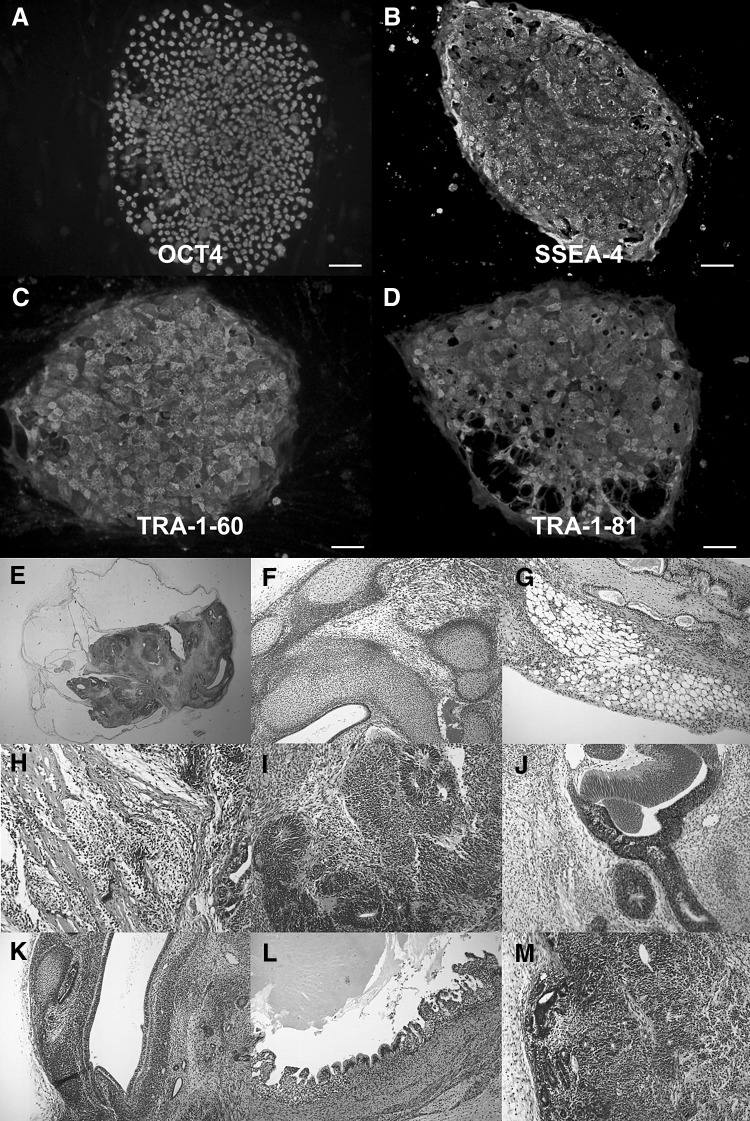
The pluripotency of human iPS cells was evaluated with immunohistochemistry and teratoma formation. Colonies of human iPS cells expressed pluripotent markers including OCT4, SSEA-4, Tra-1-60 and Tra-1-81 on immunocytochemical analysis (Fig. 1). Teratomas made from human iPS cells transplanted onto the back of SCID mice contained cartilage, adipose tissue, smooth muscle (mesoderm), neural rosettes, pigment epithelium (ectoderm), and vascular endothelium, glandular tissue, and gut-like epithelial tissues (endoderm) (Fig. 1).
Fig. 1.
Characterization of human induced pluripotent stem cells (iPSCs). A–D Immunocytochemical analysis revealed iPSC expression of pluripotency markers OCT4, SSEA-4, Tra-1-60 and Tra-1-81. B Histological analysis of an iPSC-generated teratoma (E) revealed tissue-specific cells to ectodermal, mesodermal and endodermal germ layers. F Cartilage, G adipose tissue, H smooth muscle, I neural rosettes, J pigmented epithelium, K vascular endothelium, L gut-like epithelial cells and M glandular tissue. Scale bars 100 μm. iPSCs human induced pluripotent stem cells, OCT4 octamer-binding transcription factor 4, SSEA-4 stage-specific embryonic antigen 4
Differentiation of human induced pluripotent stem cells to retinal progenitor cells
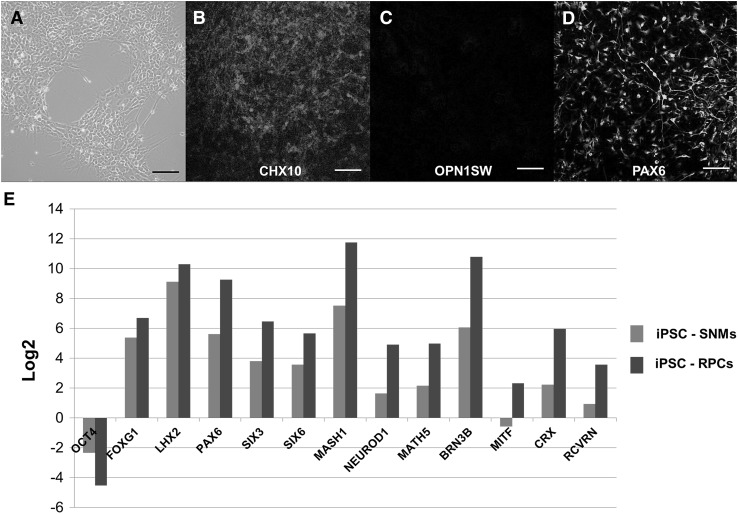
With a stepwise differentiation protocol, human iPSCs were developed into RPCs (Fig. 2). The differentiated cells expressed CHX10, an early neural retinal progenitor marker and PAX 6, an eye field marker, while they did not express OPN1SW, a mature photoreceptor marker on immunocytochemical analysis (Fig. 3). Analysis of the differentiated RPCs with quantitative RT-PCR revealed that OCT4, an undifferentiated pluripotent cell marker, was greatly downregulated. However, markers of the anterior neuroectoderm (FOXG1, SIX3, SIX6 and LHX2), markers of eye field (PAX6, SIX3 and SIX6), markers of early neural retinal cells (MASH1, MATH5 and NEUROD1), and a marker of retinal ganglion cell (BRN3B) were upregulated (Fig. 3). In addition, markers of neural retinal cell fate markers (BRN3B, CRX and RCVRN) were upregulated while the marker of RPE (MITF only showed slight upregulation. When compared with SNMs, RPCs exhibited upregulated eye field and retinal cell fate markers (PAX6, SIX3 and SIX6, MASH1, MATH5, NEUROD1, BRN3B, CRX and RCVRN).
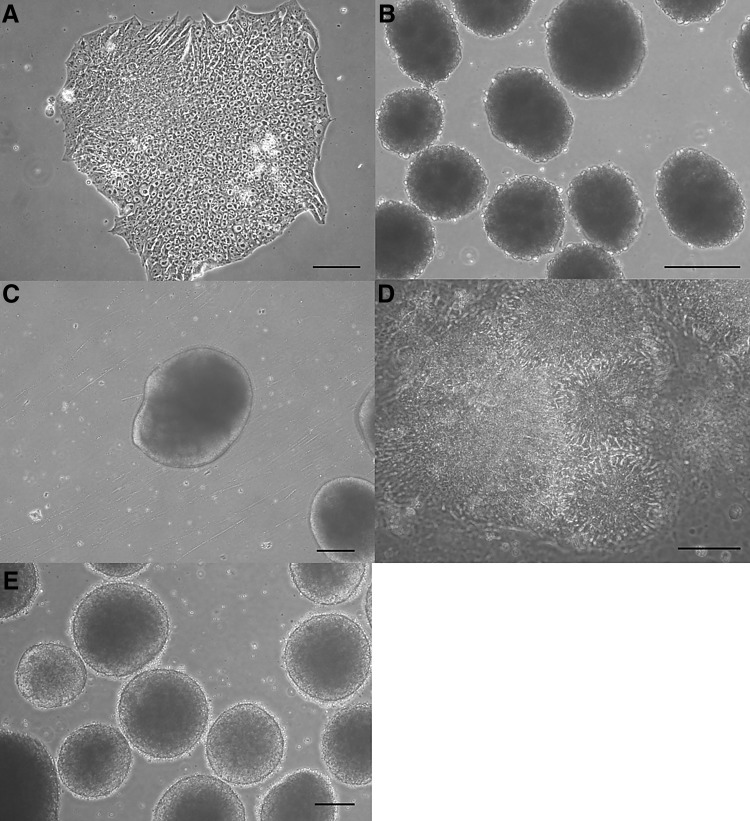
Fig. 2.
Morphologies of human iPSCs, EBs, and neural structures. A Typical morphology of the human iPSC colony. B Morphology of the EBs. C, D Neural structure and neural rosettes. E Spherical neural masses. A, B, C, E Scale bars 200 μm, and D 50 μm. iPSC induced pluripotent stem cell, EB embryoid body
Fig. 3.
A Retinal progenitor cells for transplantation. B–D These cells demonstrated early neural retinal progenitor marker (CHX10) and eye field marker (PAX6), but photoreceptor marker (OPN1SW) was not present. E Quantitative RT-PCR results of retinal progenitor cells (RPCs) for transplantation. The results are shown as binary logarithm (log2) as the expression levels of the RPCs and spherical neural masses (SNMs) compared to the undifferentiated human iPSCs. A value of zero represents no change in gene expression between two types of cells. Red bar comparison between iPSCs and SNMs, blue bar comparison between iPSCs and RPCS. Scale bars 50 μm. CHX10 Ceh-10 homeodomain containing homolog, PAX6 paired box 6, OPN1SW opsin 1 short-wave-sensitive, RT-PCR reverse transcription polymerase chain reaction. iPSC induced pluripotent stem cell
Evaluation of survival and integration of retinal progenitor cells in the mouse retina
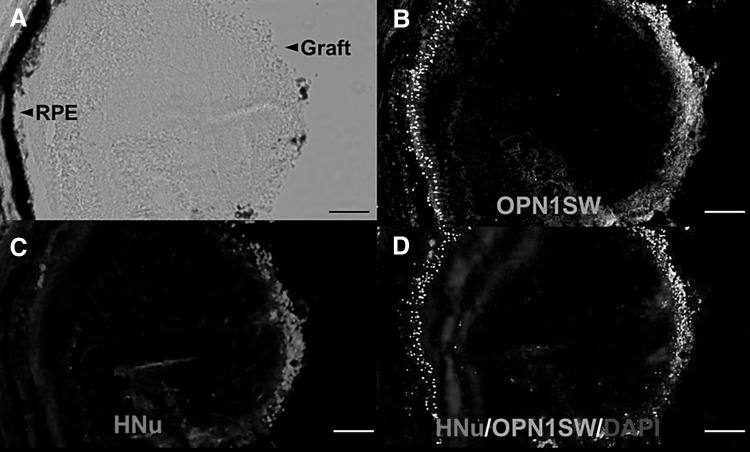
Three months after transplantation, transplanted cells were presented on superficial retinal layers. Immunohistochemistry revealed that transplanted cells were of human origin and differentiated to photoreceptor cells, as established by positive human nuclei (HNu) and OPN1SW (Fig. 4).
Fig. 4.
Photograph of a mouse retina and the results of immunohistochemistry. A Transplanted retinal progenitor cells survived and migrated into the inner retina. B, C Immunohistochemistry showed HNu- and OPN1SW-positive cells in the inner retina. Mouse photoreceptors in the outer retina expressed OPN1SW, but not HNu. D Merged image with DAPI nuclear staining. Scale bars 50 μm. HNu human nuclei, OPN1SW opsin 1 short-wave-sensitive, DAPI 4, 6-diamidino-2-phenylindole
Discussion
We obtained RPCs from human iPSC–derived SNMs and demonstrated that transplanted RPCs survived and matured in mouse retina. In this study, we cultured human iPSCs and used a protocol generating SNMs, which are spherical structures originating from neural tubes or rosettes for differentiation [15]. This has been reported to be an efficient method to generate neuronal cells from stem cells [15]. In addition, using the noggin/bFGF/Dkk-1/IGF-1/FGF-9 protocol, we differentiated SNMs to RPCs [17].
The formation of SNMs is a unique step which is known to offer a high yield of neuronal cells [10, 15]. Cell differentiation with high efficiency is needed because it can improve therapy efficacy and minimize the potential for teratoma development [10, 15]. This can be a critical issue, especially in the eye, because subretinal space for transplantation is limited. A small volume of differentiated cells with higher efficiency will be important in transplantation and will improve the efficacy of cell therapy in the eye. In addition, as previously reported, SNMs have advantages for efficient differentiation of neuronal cells on a large scale as well as easy storage and thawing [10, 15]. The time for development of retinal cells can be shortened relative to protocols that start from the culture of embryonic stem cell or iPSCs [17, 18]. In this study, derivation of neural RPCs from SNMs took about three weeks. Thus, methods with a SNM step might be a rapid and useful approach for both experimental and clinical purposes.
We have shown that RPCs exhibited a positive early neural retinal progenitor marker (CHX10) and eye field marker (PAX6) on immunocytochemistry, while they did not exhibit a photoreceptor marker (OPN1SW). RT-PCR also revealed that markers of retinal progenitors and photoreceptors were upregulated while a marker of RPE showed only slight upregulation. This suggests that RPCs from SNMs were differentiated to neural retina rather than to RPE with the protocols used in this study.
In transplantation, immunological comparability between the host and recipient is an important barrier. Immunosuppression is usually mandatory for transplantation. Compared with other organs, the eyes are immune privileged and this might be associated with the reduced rejection of the transplanted cells [19]. There are concerns about immunosuppression after transplantation because immunosuppression can have adverse effects on the regenerative process and is associated with tumorigenesis [20, 21]. Recently, Hambright et al. reported that human ES cell induced retinal progenitor cells transplanted into a non-immunosuppressed mouse retina survived and differentiated into retinal cells [17].
Several studies explored the transplantation of stem cells in normal, damaged or degenerative retina and reported various results [17, 22–24]. These studies suggested that the anatomical barrier of the retina and immune reaction were factors affecting the results after transplantation. In this study, transplanted cells expanded along the retinal surface while no human cells were detected in the sub-retinal space. This might be caused by several factors that affect the integration of transplanted cells in the retina. During transplantation, we caused damage in the retina and transplanted RPCs. When the retina is injured, glial cells in the retina tend to recover the damaged area. Glial scars made in the process might affect the integration of the transplanted cell [25, 26]. In addition, the outer limiting membrane is a barrier to the integration of the transplanted cells. These factors might affect the integration of transplanted cells [25, 27–29].
Immune rejection might also contribute to the results of xenogenic human graft transplantation [23]. In the retina, an immune privileged area, there is a blood-retinal barrier [3, 30]. Survival of a xenogenic human graft in the mouse retina without immunosuppression might be possible if transplantation is performed without damage to the blood-retinal barrier. We therefore tried to transplant the progenitor cells into the retina without damage to the retinal vessels and RPE. Because RPE provides a barrier between the neural retina and choroid, RPE layer damage during transplantation or intraocular bleeding present during transplantation signifies that the blood-retinal barrier has been broken [29]. In this study, RPCs derived from xenogenic human iPSCs survived and differentiated after transplantation in the mouse retina without immunosuppression. Previously, Hambright et al. also reported that RPCs transplanted into the retina without breach of the blood-retinal barrier survived and differentiated into photoreceptors without immunosuppression [17].
We noted that transplanted RPCs along the superficial retina showed the OPN1SW photoreceptor marker. Previous studies showed that transplanted cells, which were located in the subretinal area, inner retina or epiretinal area, had matured and transplanted cells are suggested to be affected by various extrinsic signals from host retina, causing maturation [17, 22, 31, 32]. The cellular and molecular mechanisms behind the development of retinal cells are not completely understood. Previous studies suggest that a genetic network of transcription factors is present in the retina of vertebrates and this might play an important role in retinal cell development [33–35].
In conclusion, human iPSCs were differentiated to RPCs in vitro using SNMs and a noggin/bFGF/Dkk-1/IGF-1/FGF-9 protocol. These cells survived and differentiated into cells with specific photoreceptor markers after intraocular transplantation into the mouse retina. Our results suggest that RPC development using SNMs may be a faster and more useful method for differentiation of neural retinal cells.
Acknowledgements
Korea University provided financial support in the form of research grant (Grant Number K1421461). The sponsor had no role in the design or conduct of this research.
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
This study was approved by the Research Ethics board of Korea University (KUIACUC-2015-10) and experiments were performed according to the principles expressed in the Declaration of Helsinki and the association for research in vision and ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research.
References
- 1.Chakravarthy U, Evans J, Rosenfeld PJ. Age related macular degeneration. BMJ. 2010;340:c981. doi: 10.1136/bmj.c981. [DOI] [PubMed] [Google Scholar]
- 2.Bi YY, Feng DF, Pan DC. Stem/progenitor cells: a potential source of retina-specific cells for retinal repair. Neurosci Res. 2009;65:215–221. doi: 10.1016/j.neures.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Borooah S, Phillips MJ, Bilican B, Wright AF, Wilmut I, Chandran S, et al. Using human induced pluripotent stem cells to treat retinal disease. Prog Retin Eye Res. 2013;37:163–181. doi: 10.1016/j.preteyeres.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin OR, Kim YH. Age-related macular degeneration (AMD): current concepts in pathogenesis and prospects for treatment. Tissue Eng Regen Med. 2013;10:164–175. doi: 10.1007/s13770-012-0374-0. [DOI] [Google Scholar]
- 5.Rao M. Stem cells for therapy. Tissue Eng Regen Med. 2013;10:223–229. doi: 10.1007/s13770-013-1081-1. [DOI] [Google Scholar]
- 6.Hong K. Cellular reprogramming and its application in regenerative medicine. Tissue Eng Regen Med. 2015;12:80–89. doi: 10.1007/s13770-014-0099-3. [DOI] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 9.Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ. Stem cells in retinal regeneration: past, present and future. Development. 2013;140:2576–2585. doi: 10.1242/dev.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho MS, Kim SJ, Ku SY, Park JH, Lee H, Yoo DH, et al. Generation of retinal pigment epithelial cells from human embryonic stem cell-derived spherical neural masses. Stem Cell Res. 2012;9:101–109. doi: 10.1016/j.scr.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31:741–747. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakowski J, Gonzalez-Cordero A, West EL, Han YT, Welby E, Naeem A, et al. Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell-derived self-forming retina. Stem Cells. 2015;33:2469–2482. doi: 10.1002/stem.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohlemacher SK, Iglesias CL, Sridhar A, Gamm DM, Meyer JS. Generation of highly enriched populations of optic vesicle-like retinal cells from human pluripotent stem cells. Curr Protoc Stem Cell Biol. 2015;32:1H 8 1–1H 8 20. doi: 10.1002/9780470151808.sc01h08s32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai Y, Lu B, Bakondi B, Girman S, Sahabian A, Sareen D, et al. Human iPSC-derived neural progenitors preserve vision in an AMD-like model. Stem Cells. 2015;33:2537–2549. doi: 10.1002/stem.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho MS, Hwang DY, Kim DW. Efficient derivation of functional dopaminergic neurons from human embryonic stem cells on a large scale. Nat Protoc. 2008;3:1888–1894. doi: 10.1038/nprot.2008.188. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shamekh S, Goldberg JL. Retinal repair with induced pluripotent stem cells. Transl Res. 2014;163:377–386. doi: 10.1016/j.trsl.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–936. [PMC free article] [PubMed] [Google Scholar]
- 18.Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 19.Bull ND, Martin KR. Concise review: toward stem cell-based therapies for retinal neurodegenerative diseases. Stem Cells. 2011;29:1170–1175. doi: 10.1002/stem.676. [DOI] [PubMed] [Google Scholar]
- 20.Abraham JM, Thompson JA. Immunosuppression, cancer, and the long-term outcomes after liver transplantation: can we do better? Liver Transpl. 2010;16:809–811. doi: 10.1002/lt.22114. [DOI] [PubMed] [Google Scholar]
- 21.Hunt J, Cheng A, Hoyles A, Jervis E, Morshead CM. Cyclosporin A has direct effects on adult neural precursor cells. J Neurosci. 2010;30:2888–2896. doi: 10.1523/JNEUROSCI.5991-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banin E, Obolensky A, Idelson M, Hemo I, Reinhardtz E, Pikarsky E, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24:246–257. doi: 10.1634/stemcells.2005-0009. [DOI] [PubMed] [Google Scholar]
- 23.Lund RD, Ono SJ, Keegan DJ, Lawrence JM. Retinal transplantation: progress and problems in clinical application. J Leukoc Biol. 2003;74:151–160. doi: 10.1189/jlb.0103041. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Shin JM, Yeum CE, Chae GT, Chun MH, Oh SJ. Intravitreal delivery of mesenchymal stem cells loaded onto hydrogel affects the regulatory expression of endogenous NGF and BDNF in ischemic rat retina. Tissue Eng Regen Med. 2012;9:249–258. doi: 10.1007/s13770-012-0355-3. [DOI] [Google Scholar]
- 25.Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell. 2008;2:538–549. doi: 10.1016/j.stem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singhal S, Lawrence JM, Bhatia B, Ellis JS, Kwan AS, Macneil A, et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Müller stem cells into degenerating retina. Stem Cells. 2008;26:1074–1082. doi: 10.1634/stemcells.2007-0898. [DOI] [PubMed] [Google Scholar]
- 27.West EL, Pearson RA, Tschernutter M, Sowden JC, MacLaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86:601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16:197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Arner K, Ehinger B, Perez MT. Limitation of anatomical integration between subretinal transplants and the host retina. Invest Ophthalmol Vis Sci. 2003;44:324–331. doi: 10.1167/iovs.02-0132. [DOI] [PubMed] [Google Scholar]
- 30.Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- 31.Castro G, Navajas E, Farah ME, Maia M, Rodrigues EB. Migration, integration, survival, and differentiation of stem cell-derived neural progenitors in the retina in a pharmacological model of retinal degeneration. ISRN Ophthalmol. 2013;2013:752161. doi: 10.1155/2013/752161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu G, Seiler MJ, Mui C, Arai S, Aramant RB, de Juan E, Jr, et al. Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats. Exp Eye Res. 2005;80:515–525. doi: 10.1016/j.exer.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/S0168-9525(99)01776-X. [DOI] [PubMed] [Google Scholar]
- 34.Livesey FJ, Young TL, Cepko CL. An analysis of the gene expression program of mammalian neural progenitor cells. Proc Natl Acad Sci USA. 2004;101:1374–1379. doi: 10.1073/pnas.0307014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver G, Gruss P. Current views on eye development. Trends Neurosci. 1997;20:415–421. doi: 10.1016/S0166-2236(97)01082-5. [DOI] [PubMed] [Google Scholar]