Abstract
Adipose-derived stromal cells (ASCs) have been investigated as a cell source for tissue regeneration. The purpose of this study was first to confirm if medial meniscectomy induces osteoarthritis (OA) in goats within a relative short period of time, and more importantly, to investigate if systemic treatment with immunosuppressive drugs is necessary in intra-articular ASC xenotransplantation for successful regeneration of articular cartilage and prevention of joint inflammation. Eight Korean native black goats 1–2 years of age underwent medial meniscectomy. To evaluate the gross and histological appearance of articular cartilage, knee joints were re-exposed by a medial parapatellar incision at 8 weeks. After macroscopic scoring of gross appearance, cartilage biopsy specimens 6 mm in diameter were obtained from the femoral condyle in four goats. The goats were injected with single intra-articular dose of 7×106 human ASCs (hASCs) 7 days after the second arthrotomy. Four animals were treated with daily injections of cyclosporin A 10 mg/kg for 7 days, followed by a reduced dose of 5 mg/kg for another 7 days, while other 4 animals did not receive immunosuppressive therapy. All animals were sacrificed for analysis 8 weeks after injection of hASCs. OA was successfully induced 8 weeks after medial meniscectomy. Eight weeks after injection of hASCs, various signs of articular cartilage regeneration were observed. There were no significant macroscopic and histological differences between goats treated with cyclosporine and untreated goats. Interleukin-1ß and tumor necrosis factor-α level from synovial fluid did not differ between cyclosporine-treated and untreated goats. The results indicate that immunosuppressive therapy did not influence the result of ASC xenotransplantation to treat OA.
Keywords: Adipose-derived stromal cell, Osteoarthritis, Xenotransplantation, Articular cartilage, Cyclosporin A
Introduction
Articular cartilage (AC) does not regenerate itself after injury in adults. Although several therapeutic measures have been developed to induce the healing of AC, none of them successfully regenerated hyaline cartilage [1]. Osteoarthritis (OA) is a degenerative disease involving AC and subchondral bone [1, 2]. Progression of OA causes pain and stiffness of joints, and eventually results in long-term disability of the patient [2]. Local therapy by IA injection of anti-inflammatory agents has been effectively used to alleviate symptoms and prevent the progression of OA [3, 4].
Tissue engineering purposed to regenerate damaged tissue or organs using combination of cell, growth factor and scaffolding materials [5]. Cartilage tissue engineering using various cell sources is a rapidly growing area in this field of research. Autologous chondrocytes were first used in clinical practice [6]. However, autologous chondrocyte implantation is fraught with problems, such as dedifferentiation of cells with passaging, as well as donor site morbidity [7–9]. For this reason, alternative cell sources have also been investigated for cartilage tissue engineering [10, 11].
Adipose-derived stromal cells (ASCs) have been investigated as a cell source for tissue regeneration [12]. ASCs can differentiate into multiple tissue lineages including osteogenic and chondrogenic phenotypes [13]. Data from several groups including ours suggests that successful in vitro chondrogenesis and in vivo cartilage regeneration can be achieved using ASCs [13–18]. Eventual commercialization of cell therapy may include use of xenograft as well as allograft. In addition, preclinical trials in cell therapy have used human cells in animal models [19–22]. However, the host immune response against transplanted human ASCs (hASCs) is not well characterized. In fact, whether or not hASCs are immune-privileged cells remains debatable. Since AC is relatively protected from the immune response due to the absence of a blood supply, the necessity for immune suppression in an intra-articular (IA) xeno- or allogenic ASC therapy is even more controversial. Immunosuppressive drugs (ISDs) suppress or reduce the strength of the body’s immune system. In xenotransplantation, ISDs are used to prevent the rejection of implanted foreign tissue or cells [19, 23, 24]. ISD use considerably raises the cost and complexity of an animal model, and lowers the resistance of the animals to infection.
Establishment of proper animal models is a prerequisite for any study that tests the effect of a certain therapeutic measure. While small animal models, such as mouse and rat, have been used to study differences in cartilage structure, discrepant metabolic rate between these species and humans makes it difficult to translate the results into clinical significance. Goats are a manageable large preclinical animal model for human OA [25, 26]. Several studies have shown that medial meniscectomy cause OA on 4 to 8 months post-surgery in sheep [27–29] and goats [19]. In goats, surgical partial or complete meniscectomy is generally used to induce OA, as ACL transection causes only limited cartilage damage [30].
With this in mind, the present study was undertaken first to confirm if medial meniscectomy induces OA in goats within a relative short period of time, and more importantly to investigate if systemic ISD treatment is necessary in intra-articular ASC xenotransplantation for successful regeneration of AC and prevention of joint inflammation.
Material and methods
Induction of OA using medial meniscectomy
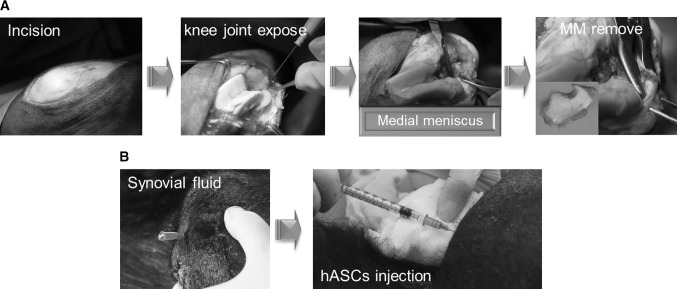
The goat experiments were approved by the Animal Research and Care Committee of Research and Development institution (MCTTIACUC ASP-15-001). Eight Korean native black male goats (Dooyeol Biotech, Seoul, Korea) were used. All goats were aged between 1 and 2 years and weighed 30 to 35 kg (mean, 32.5 kg) at the time of surgery. The goats were kept in a barn and were fed nutrient pellets and hay on a daily basis. The animals were anesthetized using ketamine (Yuhan Corp., Seoul, Korea) 2 mg/kg and xylazine (Bayer Korea Corp., Seoul, Korea) 10 mg/kg. After skin preparation, a knee joint of each goat was exposed using a medial parapatellar approach. The medial meniscus was removed by sharp dissection. Working from caudal to lateral then cranial, the meniscus was excised from its attachments until it was completely removed (Fig. 1a). After recovery, animals were allowed free movement in an unconfined environment.
Fig. 1.
The surgical process of medial meniscectomy to induce OA in goat (A), IA injection of hASCs (B)
Verification of OA induction
To evaluate the gross and histological appearance of AC, the previously altered knee joint of each goat was re-exposed by a medial parapatellar incision 8 weeks after medial meniscectomy. After macroscopic observation of gross appearance, 6-mm diameter cartilage biopsy specimens were obtained from the femoral condyle in four goats. Biopsies were evaluated histologically using hematoxylin (Merck, Darmstadt, Germany)-eosin (Sigma-Aldrich, St. Louis, MO, USA) (HE) and Safranin-O-fast green (Sigma-Aldrich) staining.
Preparation of hASCs
hASCs were isolated from the lipoaspirates generated during elective liposuction of three female patients with a mean age of 52 years. The protocol involving human tissue was approved by the Institutional Review Board (IRB) Of Hallym University Hangang Sacred Heart Hospital (IRB 2015-001). All patients gave informed consent. The hASCs were cultured and expanded as previously described [31]. The 10 ml of Dulbecco’s modified Eagle’s medium(DMEM)/F12 (Gibco, Gaithersburg, MD, USA) containing 10% fetal bovine serum (Gibco) was changed every 48–72 h. Cells passaged 5–7 times were used for implantation.
IA injection of hASCs and ISD treatment
Seven days after second arthrotomy (verification of OA induction), each goats was injected with single IA dose of 7×106 hASCs as a suspension in DMEM/F12. The animals were anesthetized and placed in dorsal recumbency. After aspiration of synovial fluid, 1 ml of the cell suspension was injected into the operated knee joint (Fig. 1b). Four animals were assigned to either group 1 (with ISD treatment) or group 2 (without ISD treatment; control group). Goats in group 1 received daily injections of cyclosporin A (Chongkendang Bio., Seoul, Korea) 10 mg/kg for 7 days, followed by a reduced dose of 5 mg/kg for 7 days. All animals were sacrificed for analysis 8 weeks after hASC injection.
Harvest of specimens and gross and microscopic findings
Atropine sulfate (Jeil Pharm., Daegu, Korea; 0.1 mg/kg) was given intramuscularly as a pre-anesthetic agent to prevent any respiratory distress. The goats were then anesthetized with an intraperitoneal injection of ketamine 2 mg/kg and xylazine 10 mg/kg. Finally, the goats were euthanized by an intravenous injection of potassium chloride (Sigma-Aldrich) 2 mmol/kg. Femoral and tibial condyles were carefully dissected separately without damaging the cartilage surface and examined to ascertain the location, size and severity of cartilage degeneration. These tissues were fixed in 4% paraformaldehyde (Sigma-Aldrich), pH 7.4, for 3 days, decalcified and embedded in paraffin. HE and Safranin-O staining were performed. The Osteoarthritis Research Society International (OARSI) Scoring System in goats was used to grade the degenerative status of the repaired tissue [25]. The system takes into account the macroscopic findings of cartilage (score: 0–16) and osteophytes (score: 0–12) from 4 site in the knee joint. The microscopic scoring of cartilage is obtained from observing the cartilage structure (score: 0–10), chondrocyte density (score: 0–4), cell cloning (score: 0–4), proteoglycan staining (score: 0–4) and tidemark (score: 0–3).
Enzyme-linked immunosorbent assay (ELISA) of inflammatory cytokines from synovial fluid
Synovial fluid (SF) of each goat was aspirated from the knee joint during sacrifice. The fluid was centrifuged within 4 h at 10,000×g for 10 min and then frozen at −70° to –75 °C. The supernatant was assayed for goat tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β) by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Mybiosource, San Diego, CA, USA). The optical density (OD) of each assay well was read at 450 nm.
Statistical analyses
All quantitative data are expressed as mean ± SEM of at least four independent experiments. Statistical comparisons were made by T-test (SPSS 18.0; SPSS Inc., Chicago, IL) when two groups were involved. Statistical significance was set at p < 0.05.
Results
Gross and histological appearance in surgically-induced OA
Eight weeks after medial meniscectomy, we assessed whether OA was successfully induced in goats. Gross findings from all animals demonstrated broad areas of cartilage destruction with irregular surface fibrillation. Histological findings from biopsy revealed degenerative changes of AC including fibrillation and loss of proteoglycan (Fig. 2). The presence of femoral condyles after 8 weeks demonstrated that OA was successfully induced after medial meniscectomy and that medial meniscectomy, without any additional procedure, was sufficient to cause osteoarthritic changes in all the goats.
Fig. 2.
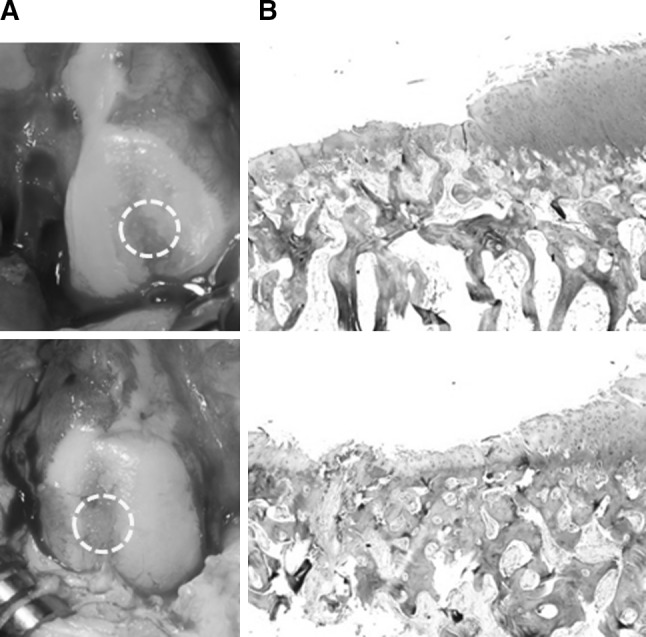
Confirmation of the surgically-induced OA in goats. Gross appearance (A) and histological analysis by Safranin-O staining (B) from osteochondral punch biopsy from femoral condyle in 4 goats demonstrates successful OA induction
Effects of ISD treatment on articular cartilage after hASC transplantation
Animals tolerated the injection of ASCs well. There was no evidence of local inflammation, immobilization or unloading of the joint resulting from the cell treatment. To determine whether ISD treatment is necessary in xenotransplantation of hASCs, we compared the macroscopic observation, histological scoring and inflammatory conditions of goats with and without ISD treatment.
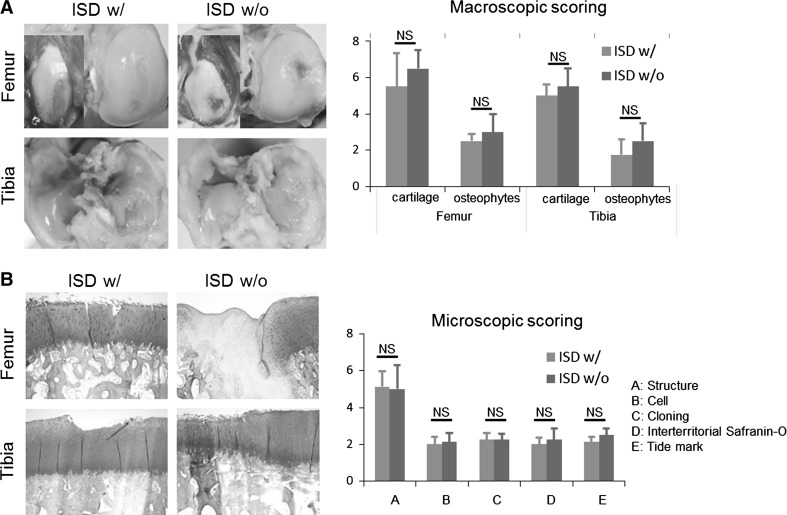
Eight weeks after injection of hASCs, various signs of regeneration were observed from AC. Cartilage of some animals was almost completely covered by the repaired tissues with relatively smooth surface, while other animals showed incomplete coverage of the chondral damage created by previous medial meniscectomy. There was no discernable difference between goats with or without ISD treatment. The macroscopic scores did not significantly differ between the two groups (Fig. 3).
Fig. 3.
The gross observation and macroscopic scoring of articular cartilage after ASC injection with or without immunosuppression. ISD w/: with immunosuppressive drug treatment, ISD w/o: without immunosuppressive drug treatment, NS: not specific
The histological findings also demonstrated various levels of regeneration that corresponded with the gross findings. Some animals showed osteoarthritic changes including the loss of proteoglycan and fibrillation up to the deep zones of the cartilage layers, while relative smooth margin and restoration of full thickness articular cartilage was evident in other animals. As with the macroscopic scores, the microscopic scores were of similar level, and did not show significant differences between the two groups (Fig. 3).
Effect of ISD treatment on IA inflammation
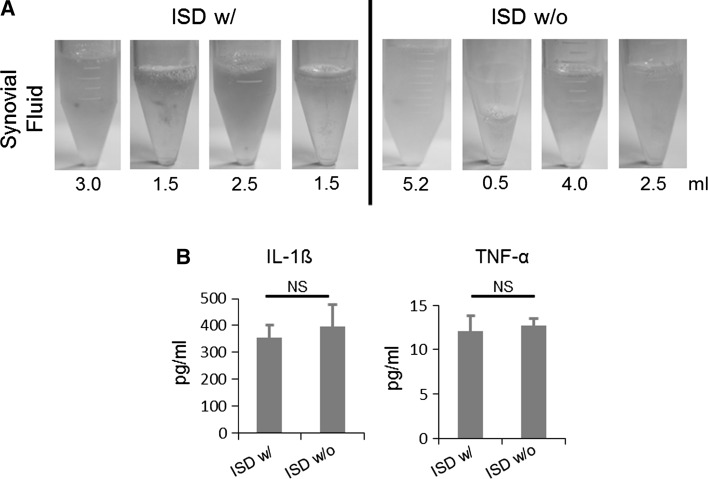
The volume of synovial fluid (SF) was collected and measured. Volume of SF showed great individual variation (Fig. 4). To assess whether ISD treatment suppress inflammation after IA injection of ISD, we measured the IL-1ß and TNF-α levels in SF using ELISA. IL-1β levels ranged from 15.6 to 1000 pg/ml and did not differ according to the presence of ISD (353.4 ± 48.3 pg/ml) or absence of ISD (395.6 ± 81.8 pg/ml). Similarly, IL-6 levels were similar in the presence and absence of ISD (group 1: 12.0 ± 1.8 pg/ml; group 2: 12.7 ± 0.8 pg/ml; Fig. 4). These results suggest that ISD treatment did not influence IA inflammation after xenotransplantation of hASCs in the goat OA model.
Fig. 4.
The determination of inflammatory conditions after ASC injection with or without immunosuppression in the goat medial meniscus model. Measurements of synovial fluid volume (A) and evaluation of IL-6 and TNA-α by ELISA (B). ISD w/: with immunosuppressive drug treatment, ISD w/o: without immunosuppressive drug treatment, NS: not specific
Discussion
This study investigated if OA is induced by medial meniscectomy in a relatively short period (8 weeks) and if systemic ISD treatment is necessary after IA injection of hASCs to successfully regenerate AC and suppress IA inflammation. The overall results showed that OA is consistently induced by medial meniscectomy and that ISD treatment did not influence the results of IA xenoplantation of hASC to treat OA induced in goats.
Goats are a large animal species commonly used to study pathogenesis and treatment of OA. OA can be induced by cutting of the anterior cruciate ligament, medial meniscectomy or both [25, 30, 32–36]. Various periods are also reported as the period of time needed for OA induction [25, 30, 32–36]. In this study, we confirmed that the removal of the medial meniscus was sufficient for the surgical induction of OA in goats within 8 weeks. Also, the results suggest that ISD treatment is not necessary in IA xenotransplantation of hASCs to regenerate cartilage. IA injection of hMSCs contributed to regeneration of AC in this traumatic OA model. While the mechanism of cartilage regeneration is beyond the scope of this study, the injected ASCs probably worked by a paracrine effect, secreting cytokines that have a demonstrated anabolic effect on the synthesis of matrix or recruiting stem cells. A small portion of injected hASCs may have been incorporated into host tissue.
Cell therapy in OA aims to overcome the limitations of current treatment modalities. While symptomatic treatment to alleviate pain and subside IA inflammation are indicated for early OA and joint replacements are used for advanced disease, there is a lack of a treatment modality that acts to regenerate the damaged tissue. Implantation of culture-expanded autologous chondrocytes or autologous mesenchymal stem cells has been clinically applied to treat focal AC defects. Because OA involves the entire AC, a different strategy should be considered for treatment. IA injection of cells provides a better and less invasive option than focal treatment by arthroscopic or open arthrotomy. If proven to be effective, IA cell injection can be a minimally invasive regenerative therapeutic modality in the treatment of OA. ASCs have drawn great attention due to their abundance and minimal morbidity during their collection. Previous studies have demonstrated successful results of ASCs for OA treatment in various animal models [37, 38] and in a clinical study. Considering that various human cell therapy products will be developed for clinical applications, an animal model that can reliability and reproducibly test the effectiveness of the product are necessary. We think that our study provides a model that will serve the needs in preclinical testing. While the current study has limitations including relatively small number of animals which was not enough to provide statistical power to the results and lack of detailed immunological investigation, we believe that this study still offers a reference on the posed question of proper animal model for the evaluation of IA xenograft for the treatment of OA.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D project, Ministry for Health & Welfare Affairs, Republic of Korea (HI14C0310). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
All authors have no conflict of interest in the presented work.
Ethical Statement
This study was approved by the Animal Research and Care Committee of Research and Development institution (MCTTIACUC ASP-15-001).
References
- 1.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann NY Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 3.Derendorf H, Möllmann H, Grüner A, Haack D, Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther. 1986;39:313–317. doi: 10.1038/clpt.1986.45. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7:850–856. [PubMed] [Google Scholar]
- 5.Nerem R, Sage H, Kelley CA, McNicol LA. Symposium summary. Ann NY Acad Sci. 2002;961:386–391. doi: 10.1111/j.1749-6632.2002.tb03128.x. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 7.Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre V, Peeters-Joris C, Vaes G. Production of collagens, collagenase and collagenase inhibitor during the dedifferentiation of articular chondrocytes by serial subcultures. Biochim Biophys Acta. 1990;1051:266–275. doi: 10.1016/0167-4889(90)90132-W. [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 10.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 11.Deasy BM, Jankowski RJ, Huard J. Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis. 2001;27:924–933. doi: 10.1006/bcmd.2001.0463. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Liu L, Wang FK, Zhao DP, Dai XM, Han XS. Coculture of vascular endothelial cells and adipose-derived stem cells as a source for bone engineering. Ann Plast Surg. 2012;69:91–98. doi: 10.1097/SAP.0b013e3182583eb9. [DOI] [PubMed] [Google Scholar]
- 13.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr Cartil. 2005;13:845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Im GI, Lee JH. Repair of osteochondral defects with adipose stem cells and a dual growth factor-releasing scaffold in rabbits. J Biomed Mater Res B Appl Biomater. 2010;95:552–560. doi: 10.1002/jbm.b.31552. [DOI] [PubMed] [Google Scholar]
- 15.Im GI, Kim HJ. Electroporation-mediated gene transfer of SOX trio to enhance chondrogenesis in adipose stem cells. Osteoarthr Cartil. 2011;19:449–457. doi: 10.1016/j.joca.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373:104–108. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res. 2009;27:612–619. doi: 10.1002/jor.20766. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Im GI. The effects of ERK1/2 inhibitor on the chondrogenesis of bone marrow- and adipose tissue-derived multipotent mesenchymal stromal cells. Tissue Eng Part A. 2010;16:851–860. doi: 10.1089/ten.tea.2009.0070. [DOI] [PubMed] [Google Scholar]
- 19.Ko JY, Kim KI, Park S, Im GI. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials. 2014;35:3571–3581. doi: 10.1016/j.biomaterials.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Kim BS, Lee H, Im GI. In vivo tracking of mesechymal stem cells using fluorescent nanoparticles in an osteochondral repair model. Mol Ther. 2012;20:1434–1442. doi: 10.1038/mt.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liau MT, Amini F, Ramasamy TS, Williams L, Tuch BE. The therapeutic potential of stem cells and progenitor cells for the treatment of parkinson’s disease. Tissue Eng Regen Med. 2016;13:455–464. doi: 10.1007/s13770-016-9093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yun JW, Ahn JB, Kwon E, Ahn JH, Park HW, Heo H, et al. Behavior, PET and histology in novel regimen of MPTP marmoset model of parkinson’s disease for long-term stem cell therapy. Tissue Eng Regen Med. 2016;13:100–109. doi: 10.1007/s13770-015-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaratunga A, Khoury P, Wang L, Williams L, Tuch BE. Porcine pancreatic icosapeptide as a marker of graft survival and rejection in xenotransplantation. Xenotransplantation. 2003;10:622–627. doi: 10.1034/j.1399-3089.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 24.Emborg ME, Zhang Z, Joers V, Brunner K, Bondarenko V, Ohshima S, et al. Intracerebral transplantation of differentiated human embryonic stem cells to hemiparkinsonian monkeys. Cell Transpl. 2013;22:831–838. doi: 10.3727/096368912X647144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little CB, Smith MM, Cake MA, Read RA, Murphy MJ, Barry FP. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthr Cartil. 2010;18:S80–S92. doi: 10.1016/j.joca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong SJ, Read RA, Ghosh P, Wilson DM. Moderate exercise exacerbates the osteoarthritic lesions produced in cartilage by meniscectomy: a morphological study. Osteoarthr Cartil. 1993;1:89–96. doi: 10.1016/S1063-4584(05)80023-8. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh P, Burkhardt D, Read R, Bellenger C. Recent advances in animal models for evaluating chondroprotective drugs. J Rheumatol Suppl. 1991;27:143–146. [PubMed] [Google Scholar]
- 29.Ghosh P, Sutherland J, Bellenger C, Read R, Darvodelsky A. The influence of weight-bearing exercise on articular cartilage of meniscectomized joints. An experimental study in sheep. Clin Orthop Relat Res. 1990;252:101–113. [PubMed] [Google Scholar]
- 30.Rørvik AM, Teige J. Unstable stifles without clinical or radiographic osteoarthritis in young goats: an experimental study. Acta Vet Scand. 1996;37:265–272. doi: 10.1186/BF03548093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dragoo JL, Samimi B, Zhu M, Hame SL, Thomas BJ, Lieberman JR, et al. Tissue engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Jt Surg Br. 2003;85:740–747. doi: 10.1302/0301-620X.85B5.13587. [DOI] [PubMed] [Google Scholar]
- 32.Bylski-Austrow DI, Malumed J, Meade T, Grood ES. Knee joint contact pressure decreases after chronic meniscectomy relative to the acutely meniscectomized joint: a mechanical study in the goat. J Orthop Res. 1993;11:796–804. doi: 10.1002/jor.1100110604. [DOI] [PubMed] [Google Scholar]
- 33.Ho C, Cervilla V, Kjellin I, Haghigi P, Amiel D, Trudell D, et al. Magnetic resonance imaging in assessing cartilage changes in experimental osteoarthrosis of the knee. Investig Radiol. 1992;27:84–90. doi: 10.1097/00004424-199201000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Laurent D, O’Byrne E, Wasvary J, Pellas TC. In vivo MRI of cartilage pathogenesis in surgical models of osteoarthritis. Skelet Radiol. 2006;35:555–564. doi: 10.1007/s00256-006-0133-1. [DOI] [PubMed] [Google Scholar]
- 35.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 36.Saw KY, Hussin P, Loke SC, Azam M, Chen HC, Tay YG, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy. 2009;25:1391–1400. doi: 10.1016/j.arthro.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 37.ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604–3613. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 38.Toghraie F, Razmkhah M, Gholipour MA, Faghih Z, Chenari N, Torabi Nezhad S, et al. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012;15:495–499. [PubMed] [Google Scholar]