Abstract
Human dermal fibroblast is essential in wound healing of the skin through the synthesis of extracellular matrix proteins. With respect to oxidative stress, the effects of remifentanil on human dermal fibroblast have received little attention. Therefore, we investigated the effects of remifentanil on the apoptosis and autophagic reaction of human dermal fibroblasts under oxidative stress. The subjects were divided into the following groups: Control group: cells were incubated at 37°C in a humidified atmosphere with 5% CO2. Hydrogen peroxide (H2O2) group: cells were exposed to H2O2 for 2 h. RPC/H2O2 group: cells were pretreated with remifentanil for 2 h and exposed H2O2 for 2 h. 3-MA/RPC/H2O2 group: cells were pretreated with 3-methyladenine (3-MA) and remifentanil for 1 h and 2 h, respectively. We measured cell viability using MTT assay. Western blot analysis was used to determine the expression levels of proteins associated with apoptosis and autophagy. Quantification of apoptotic cells was performed using flow cytometer analysis, and autophagic vacuoles were observed under a fluorescence microscope. Remifentanil treatment increased the proliferation of human dermal fibroblast and decreased apoptotic cell death, enhancing autophagic activity under oxidative stress. However, 3-MA, the autophagy pathway inhibitor, inhibited the protective effect of remifentanil in oxidative stress. This study demonstrates that remifentanil activated autophagy and decreased apoptotic death of human dermal fibroblasts under oxidative stress. Our results suggest that remifentanil may help in the treatment of oxidative stress.
Keywords: Remifentanil, Dermal fibroblast, Oxidative stress, Autophagy, 3-methyladenine
Introduction
As the integumentary system of the body, the skin acts a natural barrier against the environment and plays a key role in essential protective functions, protecting the body against external factors, insulation, temperature regulation, and sensation [1, 2]. A wound is a disruption of the normal structure and function of the skin, especially during surgery, and mainly arises from surgical stimulation but is also affected by other clinical conditions (diabetes and atherosclerosis) [3, 4].
Reactive oxygen species (ROS) are extensively regarded as a causative factor for aging and some pathological conditions, such as trauma, atherosclerosis, diabetes, carcinogenesis, infection and infarction [5]. They are also indispensable to prevent the organism from invading bacteria and other microorganisms. Bacteria and cell debris are subsequently eliminated from the wound by phagocytosis. Even in the absence of infection, low levels of ROS are necessary for cellular signaling, particularly for angiogenesis. However, immoderate production of ROS or impaired detoxification of these aggressive molecules causes oxidative stress, and this has been identified as significant feature in the pathogenesis of chronic, healing wounds [6, 7].
Human dermal fibroblasts help in maintaining tissue homeostasis of the tissue and improving wound repair through the synthesis of extracellular matrix proteins. In addition, secretion of paracrine-acting growth factors and cytokines that have a direct effect on the proliferation and differentiation of adjacent epithelial tissues contribute to the management of tissue homeostasis and wound repair [8–10]. Oxidative stress causes interruption of cellular metabolism, which negatively affects the function of skins at the cellular and tissue level. Exposure of human dermal fibroblasts to sub-cytotoxic concentrations of hydrogen peroxide (H2O2) also has been observed to push these cells into a non-proliferative, quiescent state, otherwise known as stress-induced premature senescence [11, 12]. Autophagy is a self-eating process, which is important for balancing energy sources at critical times of development and in responses to stress. Autophagy also plays a crucial role in exfoliating damaged intracellular organelles and aggregated proteins as well as eliminating intracellular pathogens [13–15].
Remifentanil, an ultra-short-acting mu-opioid receptor agonist, is unique and exclusive because of its esterase-based metabolism, minimal accumulation, and very rapid onset and offset of clinical action. Furthermore, remifentanil prevents the inflammatory response and suppressed inducible nitric oxide synthase expression in septic mouse models [16].
However, the effects of remifentanil on human dermal fibroblast and autophagy during oxidative stress are not yet sufficiently studied. Thus, we conducted an investigation to determine the protective effect of remifentanil against oxidative stress injury in human dermal fibroblasts and to assess whether this effect would be mediated by autophagy.
Materials and methods
Cell culture
Human Dermal Fibroblast, adult (HDFa) were purchased from the Life Technologies (Carlsbad, CA, USA). They were preserved in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 1% penicillin–streptomycin (Hyclone, Logan, UT, USA). Twenty-four hours after cells were subcultured, the original medium was removed. The cells were washed with phosphate-buffered saline (PBS) and then incubated in the same fresh medium. Cells were cultured at 37 °C in a humidified 5% CO2–95% air incubator.
Oxidative stress of cultured human dermal fibroblast and drug treatment
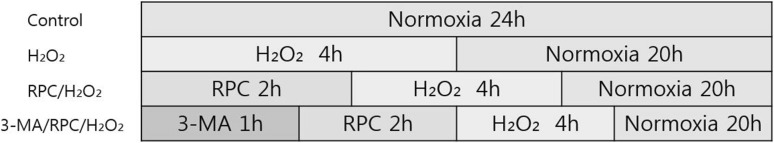
The stock of remifentanil (Ultiva; Glaxo Smith Kline Pharmaceuticals, UK) was kept frozen at −20 °C until use and diluted to their concentration with DMEM, when needed. Prior to remifentanil treatment, cells were grown to about 75% confluence and then exposed to remifentanil at various concentrations (0, 0.1, 0.5, 1, and 2 ng/mL) for 2 h. The subjects were divided into the following groups; Control group: cells were incubated at 37°C in a humidified atmosphere with 5% CO2 without remifentanil treatment. H2O2group: cells were exposed to 400 μM of H2O2 for 2 h. RPC/H2O2group: cells were pretreated with 2 ng/mL of remifentanil for 2 h and exposed to 400 μM of H2O2 for 2 h. 3-MA/RPC/H2O2group: cells were pretreated with 1 mM of 3-methyladenine (3-MA) and 2 ng/mL of remifentanil for 1 hand 2 h, respectively. After treatment of 3-MA and remifentanil, cells were exposed to 400 μM of H2O2 for 2 h (Fig. 1).
Fig. 1.
The experimental protocols followed for all in vitro experiments are represented as follows. Control = normoxia group, H2O2 = H2O2 treatment group, RPC/H2O2 = remifentanil preconditioning + H2O2 treatment group, 3-MA/RPC/H2O2 = both 3-MA and remifentanil preconditioning + H2O2 treatment group
Cell viability assay
The cell viability was determined using a MTT assay. Cells were cultured in 96-well plates (4×103 cells/well). The cells were then treated with indicated concentrations of drug. At the end point of treatment, the 100 µl of MTT (500 mg/mL) was added to each well. The cells were incubated for 4 h at 37 °C. The formazan crystals that formed were then solubilized in DMSO (200 µl/well) by constant shaking for 15 min. The cell viability was measured by an ELISA reader (Tecan, Männedorf, Switzerland) at 620 nm excitatory emission wavelength.
Hoechst staining
Cells were harvested and cytocentrifuged onto a clean, glass slide with a cytocentrifuge. Cells were stained in 1 μg/ml Hoechst 33342 for 15 min at 37 °C in the dark and washed in phosphate buffered saline (PBS). The slides were mounted with glycerol. The samples were observed and photographed under an epifluorescence microscope (Carl Zeiss, Goettingen, Germany).
Flow cytometry analysis
The cells were seeded in 60-mm dishes at 70% confluence and incubated overnight. The shikonin-treated cells were incubated for different periods. The harvested cells were washed with PBS containing 1% BSA and centrifuged at 2500 rpm for 10 min. The cells were then resuspended in ice-cold 95% ethanol with 0.5% Tween 20 to a final concentration of 75% ethanol. After 24 hours, the fixed cells were washed in 1% BSA-PBS solution, resuspended in 1 ml PBS containing 40 μg/ml RNase A (Sigma), incubated at 4 °C for 30 minutes, and resuspended in 10 μg/ml PI solution (Sigma). The DNA contents were examined using a CYTOMICS FC500 flow cytometer (Beckman Coulter) and the data were analyzed using Multi Cycle software, which allowed a simultaneous estimation of cell-cycle parameters and apoptosis.
MDC and AO staining
The cells were grown on coverslips. After 48 h, they were stained with 1.25 μM MDC, a selective fluorescent marker for autophagic vacuoles, at 37 °C for 30 min. The cellular fluorescence changes were observed using a fluorescence microscope (Axioskop, Carl Zeiss, Göettingen, Germany). As an autophagy control, cells were starved using EBSS. For further detection of the acidic cellular compartment, we used acridine orange (AO), which emits bright red fluorescence in acidic vesicles but fluoresces green in the cytoplasm and nucleus. The cells were stained with 1 μg/mL AO for 5 min and washed with PBS. The formation of acidic vesicular organelles (AVOs) was observed using an LSM 700 confocal microscope (Carl Zeiss, Göettingen, Germany).
Western blot analysis
The cells (1.5×106) were washed twice in ice-cold PBS, resuspended in 200 μl ice-cold solubilizing buffer (300 mM NaCl, 50 mM Tris-Cl (pH 7.6), 0.5% Triton X-100, 2 mM PMSF, 2 μl/ml aprotinin and 2 μl/ml leupeptin) and incubated at 4 °C for 1 h. The lysates were centrifuged at 13,200 rpm for 30 min at 4 °C. Protein concentrations of cell lysates were determined using a Bradford protein assay (Bio-Rad, Richmond, CA, USA), and 20 μg of proteins were resolved by 10% SDS/PAGE gel. The gel was transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking, the membranes were reacted with appropriate primary antibodies. Immunostaining with secondary antibodies was detected using Super Signal West Femto substrate (Pierce, Rockford, IL, USA).
Statistical analysis
All experiments were repeated five times. Multiple groups were compared using one-way analysis of variance (ANOVA) followed by a post hoc Tukey test. The data were expressed as mean ± standard deviation (SD). p < 0.05 was considered as significant score (SPSS 13.0 Software, SPSS Inc., Chicago, IL, USA).
Results
Effect of remifentanil treatment on cell viability
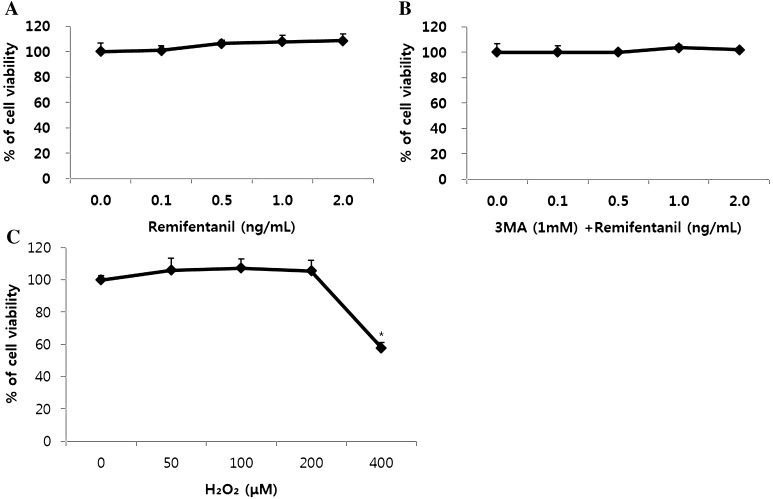
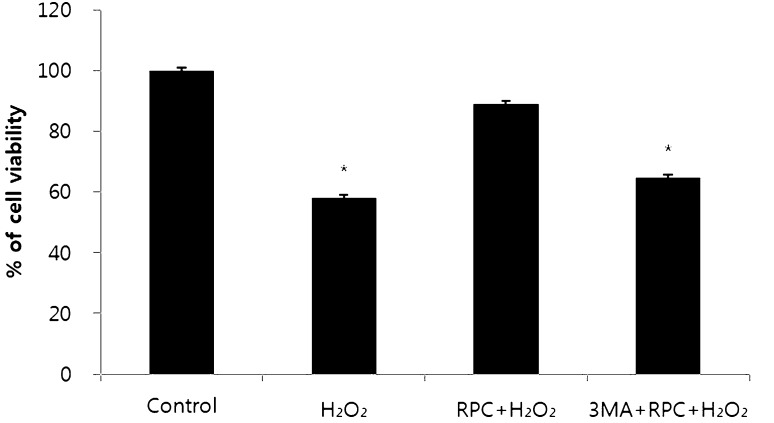
Remifentanil preconditioning and 3-MA treatment had no influence on cell viability at the normoxia state. However, treatment of 400 μM H2O2 decreased cell viability (Fig. 2A–C). The cell viability rate (%) was calculated and compared with the control group. H2O2 group and 3-MA/RPC/H2O2 group had lower cell viability than control group (Fig. 3).
Fig. 2.
The effect of remifentanil on cell viability in human dermal fibroblast assessed by MTT assay. A Effect of remifentanil on human dermal fibroblast. B Effect of 3-MA and remifentanil on human dermal fibroblast. C Cell viability under oxidative stress
Fig. 3.
Cell viability comparison. Control = normoxia group, H2O2 = H2O2 treatment group, RPC/H2O2 = remifentanil preconditioning + H2O2 treatment group, 3-MA/RPC/H2O2 = both 3-MA and remifentanil preconditioning + H2O2 treatment group. *p < 0.05 as compared with the control group
Effects of remifentanil treatment on apoptosis
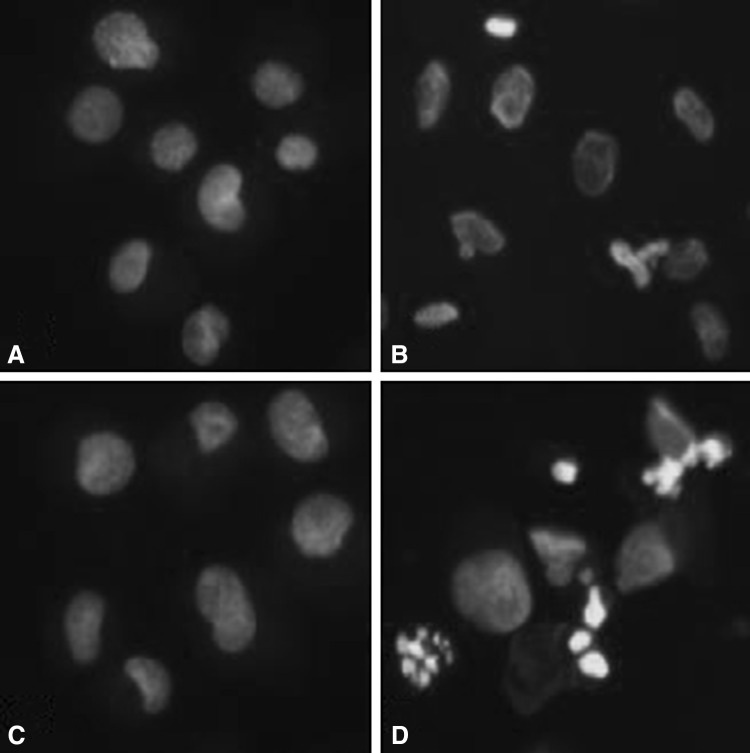
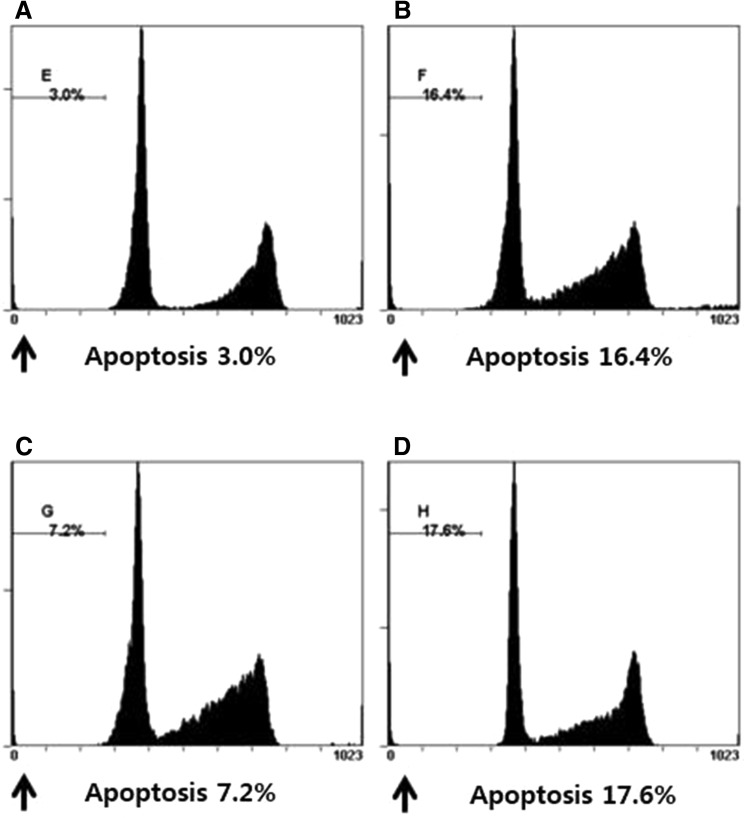
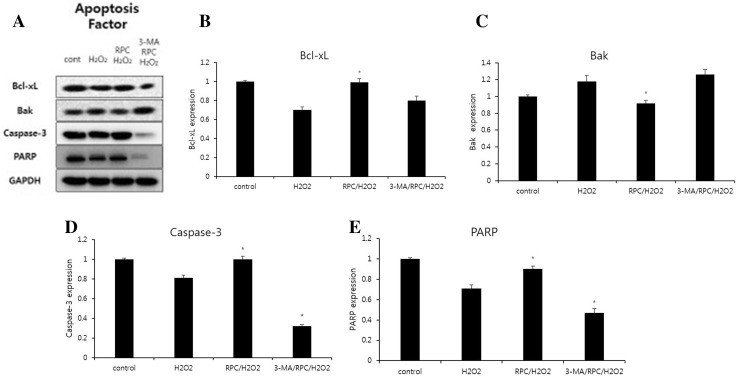
Hoechst 33258 staining was used to detect the cells under a fluorescence microscope (×400, Fig. 4). A majority of the cells in control group and RPC/H2O2 group showed normal morphology with round regular nuclei. In contrast, apoptotic bodies were seen in H2O2 group and 3-MA/RPC/ H2O2 group cells. Remifentanil treatment effectively prevented the apoptosis of cells as indicated by the morphological analysis. Flow cytometry analysis confirmed the anti-apoptotic effects of remifentanil quantitatively (Fig. 5). To ascertain the effect of remifentanil on apoptosis, we also examined the expression of factors associated with apoptosis in the cells subjected to oxidative stress by western blotting analysis. Cellular expression of caspase-3 and PARP were elevated while the ratio of Bak/Bcl-xL were down-regulated in RPC/H2O2 group as compared to the H2O2 group (Fig. 6).
Fig. 4.
Hoechst staining: Morphological changes in human dermal fibroblast treated with remifentanil and 3-MA under oxidative stress. A Morphological changes of the cells in control group. B Morphological changes of the cells in H2O2 group. C Morphological changes of the cells in RPC/H2O2. D Morphological changes of the cells in 3-MA/RPC/H2O2
Fig. 5.
Flow cytometric assay was used to detect the percentage of apoptotic cells. Detection of apoptosis and propidium iodide staining. Every group of cells with propidium iodide staining was measured by flow cytometry. A The proportion of apoptosis cells in control group. B The proportion of apoptosis cells in H2O2 group. C The proportion of apoptosis cells in RPC/H2O2 group. D The proportion of apoptosis cells in 3-MA/RPC/H2O2 group
Fig. 6.
A Effect of remifentanil on apoptosis factors in human dermal fibroblast. Western blot analysis. B Effect of remifentanil on the expression of Bcl-xl. C Effect of remifentanil on the expression of Bak. D Effect of remifentanil on the expression of Caspase-3. E Effect of remifentanil on the expression of PARP. *p < 0.05 as compared with the H2O2 group
Effect of remifentanil treatment on the activation of autophagy
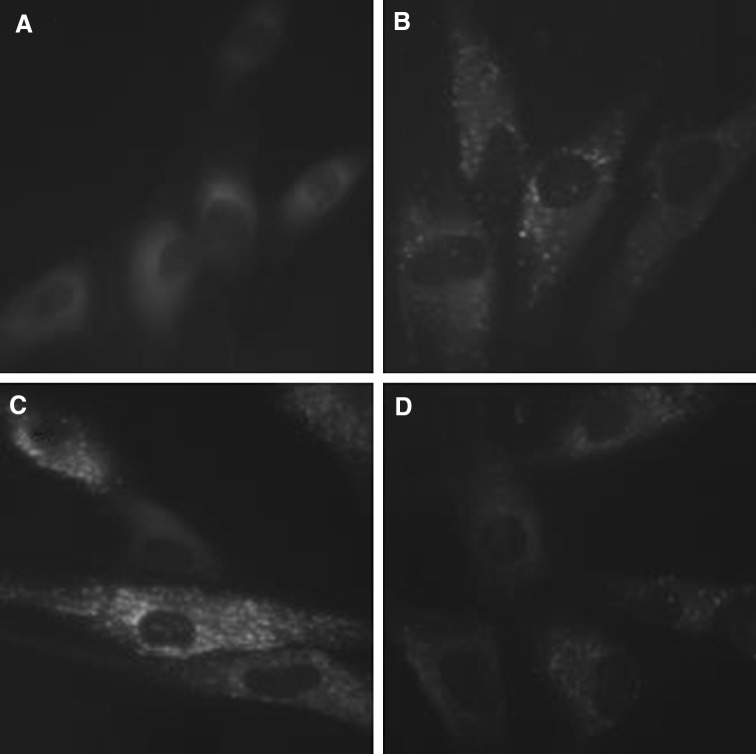
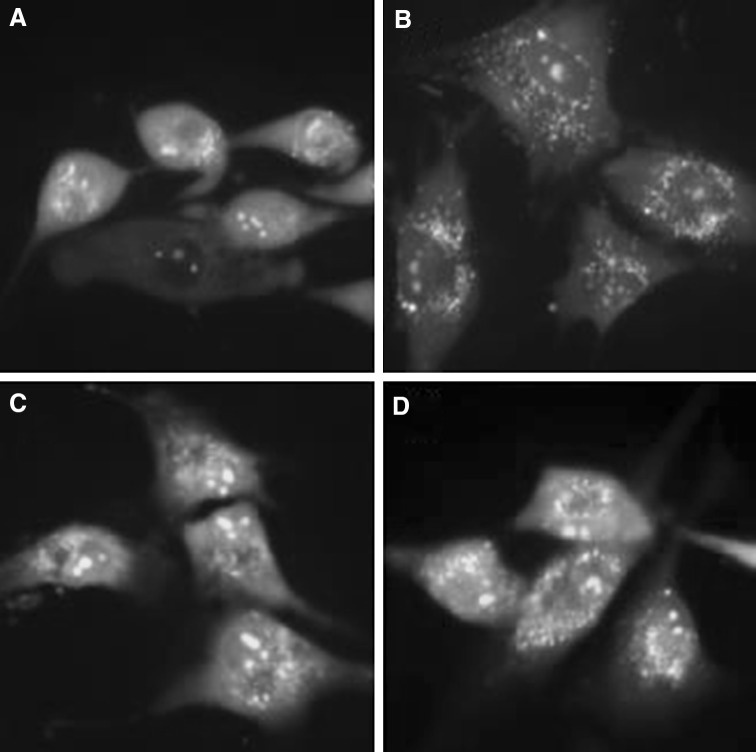
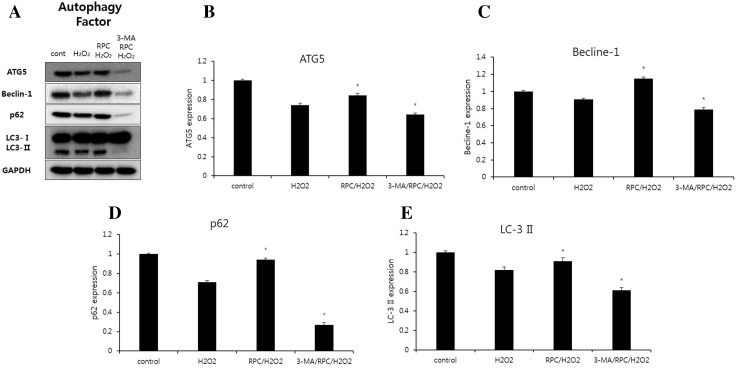
Significant accumulation of autophagic specific staining of MDC was observed around the nuclei in RPC/H2O2 group cells (Fig. 7). Similarly, AO staining, indicated by red fluorescent spots appeared in RPC/H2O2 group cells, while the control, H2O2 and 3-MA/RPC/H2O2 groups showed mainly green cytoplasmic fluorescence (Fig. 8). We examined the activation of autophagy related proteins in cells by western blotting analysis. ATG5, Becline-1, LC-3 II, and P62 were elevated in RPC/H2O2 group as compared to the H2O2 group. However, the protein levels decreased when autophagy was suppressed by 3-MA (Fig. 9).
Fig. 7.
MDC staining of cytoplasmic vacuoles after remifentanil treatment in human dermal fibroblast. Fluorescence microscopic (×400) analysis of autophagosome in human dermal fibroblast. A Autophagosomes of cells in control group. B Autophagosomes of cells in H2O2 group. C Autophagosomes of cells in RPC/H2O2 group. D Autophagosomes of cells in 3-MA/RPC/H2O2 group
Fig. 8.
AO staining of cytoplasmic vacuoles after remifentanil treatment in human dermal fibroblast. Fluorescence microscopic (×400) analysis of autophagosome in human dermal fibroblast. A Autophagosomes of cells in control group. B Autophagosomes of cells in H2O2 group. C Autophagosomes of cells in RPC/H2O2 group. D Autophagosomes of cells in 3-MA/RPC/H2O2 group
Fig. 9.
A Effects of remifentanil on autophagy markers in human dermal fibroblast. Western blot analysis. B Effect of remifentanil on the expression of ATG5. C Effect of remifentanil on the expression of Becline-1. D Effect of remifentanil on the expression of P62. E Effect of remifentanil on the expression of LC-3 II. *p < 0.05 as compared with the H2O2 group
Discussion
The purpose of this study was to determine the significance of the protective effect of remifentanil on human dermal fibroblast in oxidative stress, and to investigate whether autophagy is associated with the protective mechanism or not. Our study shows that remifentanil preconditioning increased the proliferation of human dermal fibroblast in oxidative stress via MTT assay (Fig. 3). Hoechst staining distinguishes dead cells from live cells by indicating fragmentation and condensation of chromatin, which cause the cells to brightly fluorescence. We explored this process in more detail by Annexin V-FITC/PI double staining. One event of the earlier stages of apoptosis is the translocation of phosphatidylserine (PS) from the inside of the cell membrane to the surface. Annexin V has high affinity for PS, and fluorochrome-labeled Annexin V can be used for the detection of PS translocation using flow cytometry. PI is frequently used for nuclear staining dye. The ability of PI to enter a cell is dependent on the membrane permeability; PI cannot get into live or early apoptotic cells because of an intact cell membrane [17, 18]. In our results, control and RPC/H2O2 groups had lower portion of Annexin-V(+)/PI(+) cells than H2O2 and 3-MA/RPC/H2O2 groups. Therefore, we suggest that remifentanil preconditioning protected human dermal fibroblast against oxidative stress-induced apoptosis through flow cytometry (Figs. 4, 5). 3-MA inhibits autophagy by blocking autophagosome formation via the inhibition of type III Phosphatidylinositol 3-kinase [19]. 3-MA treatment also blocked this protective effect of remifentanil preconditioning in this study. Among those factors associated with apoptosis, caspae-3 and PARP play an important role in caspase-dependent pathway and Bcl-xl protein family, such as the anti-apoptotic protein Bcl-xl and pro-apoptotic protein Bak, are related with mitochondria-dependent apoptotic pathway [20–22]. In western blot analysis, we determined that remifentanil preconditioning increased cellular expression of caspase-3 and PARP, and decreased Bak/Bcl-xl ratio.
In autophagy-specific staining of MDC and AO, the RPC/H2O2 group demonstrated more autophagic expression than other groups. In the western blot analysis, our study demonstrated that remifentanil preconditioning increased the expression of ATG5, Beclin-1, LC-3 II, and P62 proteins.
In conformity with results of this study, we assert that remifentanil preconditioning stimulates the endogenous cellular protective effect in human dermal fibroblasts against oxidative stress through the activation of signaling pathways associated with autophagy. However, these positive effects of remifentanil were only observed in human dermal fibroblast thus for, so in vivo and clinical trials would be needed to ensure a meaningful effect that there is a viable therapeutic role of remifentanil. No practical study regarding the effect of remifentanil on autophagy in human dermal fibroblast with oxidative stress was performed. We hope that our findings are useful in the study surrounding the recovery of wounds caused by oxidative stress.
Acknowledgements
This study was supported by Research institute for Convergence of biomedical science and technology (30-2013-002), Pusan National University Yangsan Hospital.
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 3.Kuehn BM. Chronic wound care guidelines issued. JAMA. 2007;297:938–939. doi: 10.1001/jama.297.9.938. [DOI] [PubMed] [Google Scholar]
- 4.Meier K, Nanney LB. Emerging new drugs for scar reduction. Expert Opin Emerg Drugs. 2006;11:39–47. doi: 10.1517/14728214.11.1.39. [DOI] [PubMed] [Google Scholar]
- 5.Nohl H. Involvement of free radicals in ageing: a consequence or cause of senescence. Br Med Bull. 1993;49:653–667. doi: 10.1093/oxfordjournals.bmb.a072638. [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 7.Cerutti PA, Trump BF. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991;3:1–7. [PubMed] [Google Scholar]
- 8.Andriani F, Margulis A, Lin N, Griffey S, Garlick JA. Analysis of microenvironmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J Invest Dermatol. 2003;120:923–931. doi: 10.1046/j.1523-1747.2003.12235.x. [DOI] [PubMed] [Google Scholar]
- 9.Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, et al. c-Met is essential for wound healing in the skin. J Cell Biol. 2007;177:151–162. doi: 10.1083/jcb.200701086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smola H, Thiekotter G, Fusenig NE. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol. 1993;122:417–429. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci USA. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumont P, Burton M, Chen QM, Gonos ES, Frippiat C, Mazarati JB, et al. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med. 2000;28:361–373. doi: 10.1016/S0891-5849(99)00249-X. [DOI] [PubMed] [Google Scholar]
- 13.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 15.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 16.Zongze Z, Jia Z, Chang C, Kai C, Yanlin W. Protective effects of remifentanil on septic mice. Mol Biol Rep. 2010;37:2803–2808. doi: 10.1007/s11033-009-9828-4. [DOI] [PubMed] [Google Scholar]
- 17.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(SICI)1097-0320(19980101)31:1<1::AID-CYTO1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 2011 doi: 10.3791/2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cepero E, King AM, Coffey LM, Perez RG, Boise LH. Caspase-9 and effector caspases have sequential and distinct effects on mitochondria. Oncogene. 2005;24:6354–6366. doi: 10.1038/sj.onc.1208793. [DOI] [PubMed] [Google Scholar]
- 21.Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, et al. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]