Abstract
Mesenchymal stem cells (MSCs) can be obtained from a variety of human tissues. Placenta has become an attractive stem cell source for potential applications in regenerative medicine and tissue engineering. The aim of this study was to localize and characterize MSCs within human chorionic membranes (hCMSCs). For this purpose, immunofluorescence labeling with CD105 and CD90 were used to determine the distribution of MSCs in chorionic membranes tissue. A medium supplemented with a synthetic serum and various concentrations of neurotrophic factors and cytokines was used to induce hCMSCs to neural cells. The results showed that the CD90 positive cells were scattered in the chorionic membranes tissue, and the CD105 positive cells were mostly located around the small blood vessels. hCMSCs expressed typical mesenchymal markers (CD73, CD90, CD105, CD44 and CD166) but not hematopoietic markers (CD45, CD34) and HLA-DR. hCMSCs differentiated into adipocytes, osteocytes, chondrocytes, and neuronal cells, as revealed by morphological changes, cell staining, immunofluorescence analyses, and RT-PCR showing the tissue-specific gene presence for differentiated cell lineages after the treatment with induce medium. Human chorionic membranes may be the source of MSCs for treatment of nervous system injury.
Keywords: Mesenchymal stem cells, Chorionic membranes, Neural differentiation, Hematopoietic markers
Introduction
Stem cell transplantation is one of the most applicable treatment modalities proposed to improve the outcome of patients with neuronal diseases such as Alzheimer and Parkinson. For these neurodegenerative diseases are characterized by irreversible loss of specific groups of neurons, thus leading to tissue and organ dysfunction [1, 2]. In recent years, more and more research suggests that Mesenchymal stem cells (MSCs) are multipotent and are capable of trans differentiating across tissue lineage boundaries into mature cell types, suggesting that it is possible to substitute specific damaged neurons with MSC-derived neuron-like cells and therapies for neurodegenerative diseases [3–5].
MSCs are multipotent stromal cells with the strong capability of proliferation and differentiation potential, which have received extensive attention in the field of regenerative medicine for it can migrate to the injured tissue to promote tissue repair [6]. Although bone marrow has been considered the gold standard source for MSCs isolation and bone marrow mesenchymal stem cells (BMSCs) was commonly used adult stem cells, several limitations exist for their clinical application [7, 8]. Among these are the invasive manipulations associated with bone marrow harvesting, BMSCs content is very low, and BMSCs cannot tolerate the damage to the tissue in the hypoxia ischemia microenvironment [9–11].
In addition, ethical issues need to be resolved in order to make stem cell therapies available to the public. For this reason, a cell type that easy to obtain in routine medical practices, maintain the characteristics of stem cells in vitro, and devoid of the ethical dilemmas surrounding is preferred for cellular therapies [12, 13]. Many recent studies have shown that fibroblast-like adherent cells isolated from various birth-associated tissues including placenta, amnion, umbilical cord and umbilical cord blood are phenotypically similar to BMSCs [14, 15]. The fetal adnexa may prove to be a useful source of human MSCs and a significant advantage of these neonatal tissues is their ready availability, thus avoiding invasive procedures and ethical issues.
Human placenta is composed of a fetal part and a maternal part; within these components different cell subpopulations with mesenchymal characteristics may be isolated. As established by the consensus of the First International Workshop on Placenta-Derived Stem Cells, they are referred to amniotic mesenchymal stem cells (hAMSCs) and chorionic mesenchymal stem cells (hCMSCs) [15, 16]. More than that, placenta mesenchymal stem cells (PMSCs) contain pluripotent cells that can differentiate along multiple cell lineages [17]. Placenta as a new source of mesenchymal stem cells, compared with bone marrow, has the advantages of abundant sources, low immunogenicity, low viral contamination rate, and no social and ethical issues.
Mesenchymal stem cells from bone marrow have been demonstrated to trans differentiate into cells of other lineages other than mesodermal lineages. In vitro studies have indicated that the ability of rodent and human MSCs to acquire a neural crest-like cell phenotype after induction with a specific culture medium [18–20]. In 1999, Kopen GC [21] first discovered that BMSCs can differentiation into neural cells and glial cells after transplantation into newborn rat brain; in 2000, Woodbury D [22] confirmed BMSCs to nerve cell differentiation in vitro for the first time. Now MSCs have been shown to have potential to generate phenotypically different types of neuron-like cells. Neural protein markers, mainly, vimentin, nestin, and glial fibrillary acidic protein (GFAP), were expressed in these neuronal induced cells [23–25]. All these suggest that MSC-derived neuron-like cells are a perfect source of neural precursor cells for clinical usage.
In view of the complexity of the placenta, CMSCs need to be properly characterized. In this study, we have isolated CMSCs from the placentas and characterized their capacity for self-renewal and potential for multipotent differentiation. Also we have induced CMSCs into neural-like cells using a simple and short method, thus making it easy for other neuron-regenerative medicine researchers to obtain neural-like cells from placenta.
Materials and methods
Human tissues
Human placentas (4 placentas; gestational age, 38–40 wks.) were obtained at the Department of Obstetrics and Gynecology of Wuxi No. 3 People’s Hospital from healthy mothers during births by cesarean section. The mothers gave informed consent and were negative for syphilis, HIV, CMV, HBSAg, and HCV, and had no history of infectious diseases or complications during pregnancy. The study was approved by the Ethics Committee of Wuxi No. 3 People’s Hospital (IRB no. 2015 0411023), and the protocols conform to the ethical guidelines of the 1975 Helsinki Declaration.
Localization of mesenchymal stem cells in the chorionic membrane
Immunofluorescence was performed on chorionic membranes to identify the distribution of cells that had positive markers of mesenchymal stem cells. Cryosections of human placenta were prepared and immunostained as described previously [26]. The human chorionic membranes trimmed to create a square sheet (5 mm × 5 mm) near the center, and stored at −80°C until thick cryosections were prepared (thickness, 5 μm) (Leica Microsystems). Antibody permeability was increased by incubation with a detergent (0.1% Triton X-100, Sigma-Aldrich) in phosphate buffered saline (30 min). Nonspecific binding of immunoglobulins was blocked by incubation with a protein-blocking agent (20 min) (Millipore). Sections were incubated with primary antibodies against CD90 (1:500) (BD Biosciences) and CD105 (1:500) (BD Biosciences) (16 h, 4°C). Sections were incubated with secondary goat anti-rat immunoglobulin M (1:200) conjugated with fluorescein isothiocyanate (FITC) (eBioscience) and viewed on a laser-scanning microscope (Olympus IX-73) equipped with an image analysis system (Olympus DP-73).
Isolation and culture of human chorionic mesenchymal stem cells
Human chorionic mesenchymal stem cells were isolated and cultured with a modification of a previously described method [27, 28]. To isolate hCMSCs, the chorionic membranes were separated by blunt dissection from the placental body and thoroughly washed with Dulbecco’s phosphate buffered saline (DPBS; Gibco BRL) and cut into small pieces. Then chorion fragments were digested with 0.1% Collagenase IV (Sigma-Aldrich) for 20 min at 37°C. The cell suspension was then passed through a cell strainer (70 µm, BD Falcon). The filtrate containing cell suspension was subjected to centrifugation at 400g for 5 min. The collected cells were re-suspended in RBC lysis buffer and centrifuged at 400×g for 5 min. Finally, the cell pellet was re-suspended in cell expansion medium composed by DMEM low glucose (Gibco BRL) supplemented with 20% FBS (Gibco BRL) and 1% penicillin/streptomycin (Gibco BRL). Cells were plated at 1 × 106 cells/cm2 and the medium was changed every 48 hours.
Growth kinetic analysis
As a direct cell growth assay, 5 × 103 cells were seeded per well in 24-well plates, in triplicates. The media was changed twice weekly and the cells from each well were harvested and counted by hemocytometer for two consecutive weeks, and the mean of the counts was calculated.
Cell surface marker analysis
Flow cytometry analysis was performed on hCMSCs culture at third passage. Cells were treated with trypsin (Gibco BRL) and harvested cells were centrifuged at 1200 rpm for 10 min and washed with 1 × DPBS with added 0.5% bovine serum albumin (Sigma-Aldrich). The cells were filtered through a 70 μm nylon membrane. Viable cell density was determined by hemacytometer and trypan blue dye (Gibco-Invitrogen). A minimum of 1 × 105 cells was incubated with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) conjugated mouse antihuman monoclonal antibodies for 30 min in the dark: CD34-FITC, CD44-PE, CD90-PE, CD73-FITC, human leukocyte antigen DR FITC (BD Bioscience), CD45-PE, CD105-FITC, CD166-PE, and CD29-FITC (Biolegend). Nonspecific background was evaluated by parallel staining with isotype-matched immunoglobulin G conjugates (IgG1-FITC and IgG1-PE) (BD Bioscience). All samples were analyzed using flow cytometer (FACSCalibur, Becton–Dickinson) and software (CellQuest, Becton–Dickinson).
Mesodermal differentiation assays
Cells were seeded in 6-well plate at a density of 1 × 105 cells/cm2 and grown until 80% confluence. Osteogenic differentiation was induced in confluent cells with osteogenic medium (StemPro® Osteogenesis Differentiation Kit, Gibco BRL) for 3 weeks. Osteogenesis was demonstrated by accumulation of mineralized calcium phosphate assessed by Alizarin red staining.
Adipogenic differentiation was induced in confluent cells with adipogenic medium (StemPro® Adipogenesis Differentiation Kit, Gibco BRL) for 3 weeks. Oil Red O (Sigma-Aldrich) staining was used to visualize cytoplasmic inclusions of neutral lipids.
Chondrogenic differentiation was induced in confluent cells with chondrogenic medium (StemPro® Chondrogenesis Differentiation Kit, Gibco BRL,) for 3 weeks. Chondrogenic differentiation was evaluated with Alcian blue staining.
Neural differentiation of the hCMSCs
Human chorionic mesenchymal stem cells were differentiated with a modification of a previously described method [29]. CMSCs were cultured in 0.1% gelatin-coated (Sigma–Aldrich) tissue culture plates with a density of 5 × 104 cell/ml at third passage. Neural differentiation was induced in confluent cells by changing the expansion medium to DMEM with Glutamax supplemented and 1% penicillin/streptomycin, 10% ES-FBS, 10 μM forskolin (Sigma–Aldrich), 5 mM KCl, 2 mM valproic acid (Sigma–Aldrich), 1 μM hydrocortisone (Sigma–Aldrich) and 5 μg/ml insulin (Sigma–Aldrich). Cells were analyzed for protein expression by immunofluorescence after 2 weeks in this differentiation media.
Immunofluorescence staining
Mature neuronal marker (NF-200) and glial marker (GFAP) were used to analyze the neural differentiation potential of hCMSCs [30, 31]. Following inducement and culture, cells were fixed with 4% paraformaldehyde. Fixed cells were treated with 3% hydrogen peroxide for 6 min and followed by incubation in 1% bovine serum albumin (Sigma-Aldrich) solution for 1 h at room temperature. Cells were stained with individual primary antibody NF-200(1:500, Abcam) and GFAP (1:1000, Abcam) overnight at 4°C and washed with Tris-buffered saline (Sigma- Aldrich). Then cells were incubated with secondary goat antirat immunoglobulin M (1:200) conjugated with fluorescein isothiocyanate (FITC) (eBioscience). Cells were mounted with medium for fluorescence (Vector)-containing DAPI (eBioscience) and viewed on a laser-scanning microscope (Olympus IX-73) equipped with an image analysis system (Olympus DP-73).
Total RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR) analysis
Two weeks after mesodermal differentiated and neural differentiated, total RNA isolation was carried out from hCMSCs using miRN easy micro kit (Qiagen). cDNA was synthesized from the RNA using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies) according to the manufacturer’s protocol. RT- PCR was performed according to the Taq PCR Core Kit (Qiagen) using the primers listed in Table 1, according to the manufacturer’s instructions.
Table 1.
The primers used for RT-PCR
| Gene | Primer sequence |
|---|---|
| Runx2 | F TTACTTACACCCCGCCAGTC RCAGCAGTCAACACCATCATTC |
| FABP4 | F CATCAGTGTGAATGGGGATG R GTGGAAGTACGCCTTTCAT |
| SOX9 | F GAGGAAGTCGGTGAAGAACG R AAGTCGATAGGGGGCTGTCT |
| GFAP | F GCTTCCTGGAACAGCAAAAC R GGCTTCATCTGCTTCCTGTC |
| NF-200 | F GAGGAACACCAAGTGCGAGA R CTTTGCTTCCTCCTTCGTTG |
| GAPDH | F ACCACAGTCCATGCCATCAC R TCCACCACCCTGTTGCTGTA |
Result
Immunofluorescence staining of MSCs in the chorionic membranes
To localize MSCs in chorionic membranes, immunofluorescence staining was carried out. The MSCs surface markers, mouse anti-human CD90 and CD105 antibody were used with a goat anti-mouse FITC-conjugated secondary antibody and a blue DAPI counter stain to show cell nuclei. Composite images showed immunoreactivity with CD90 and CD105 were localized to the vascular regions of the chorionic membranes (Fig. 1).
Fig. 1.
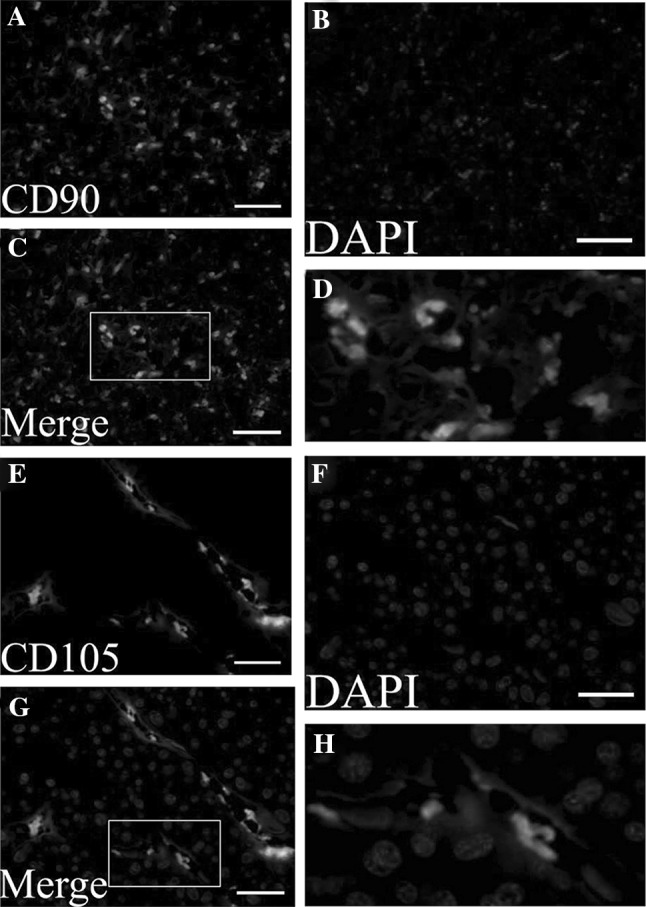
Result of mesenchymal stem cell surface markers positive in chorionic membranes with immunofluorescence staining. A and E show MSCs were stained with anti-CD90 (green) and anti-CD105 (green) antibodies. B and F show nuclei were stained with DAPI (blue). C and G show the result of merge with blue and green. D and H show the magnification display of selected rectangular region within C and G. Scale bar 100 μm. MSCs: mesenchymal stem cells. (Color figure online)
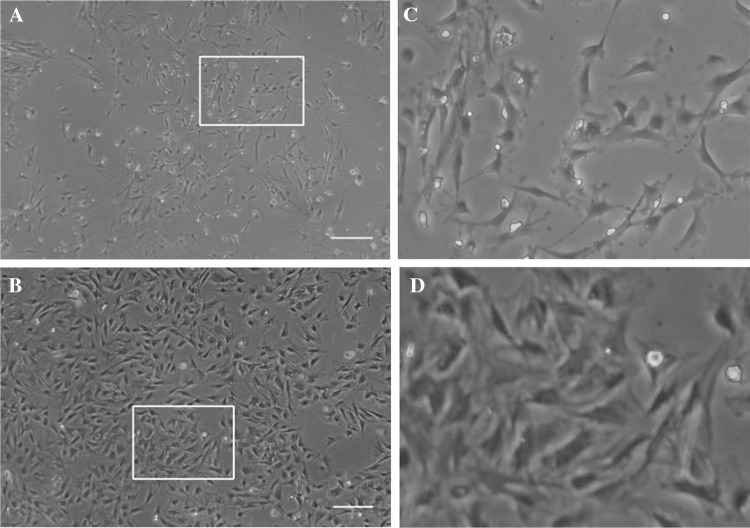
Isolation and culture the MSCs from chorionic membranes
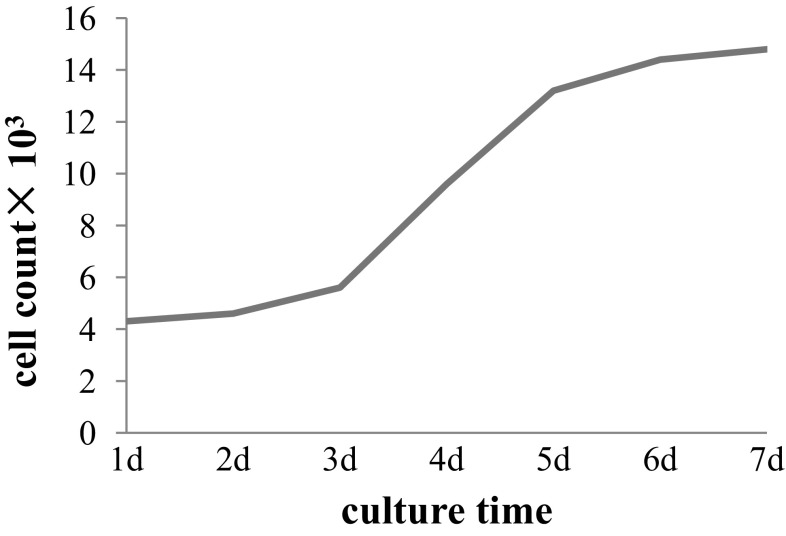
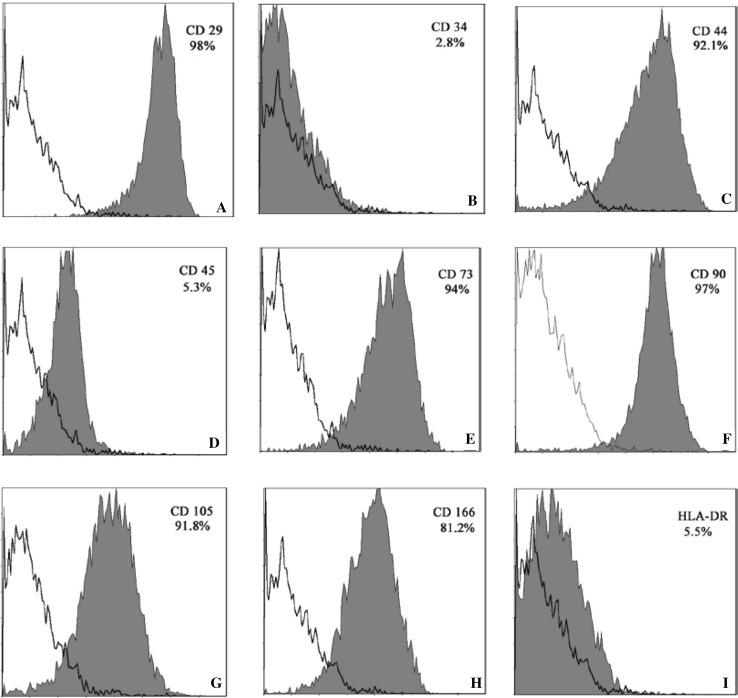
In the early stages of MSCs culture, cells adhered to the surface of tissue culture plates as a small population of polygonal or spindle-shaped cells (Fig. 2A). The hCMSCs primary culture reached a 90% confluence at 10–14 days. After passaged with trypsin, cells formed a monolayer of homogenous bipolar spindle like cells with a whirlpool-like array (Fig. 2B). The hCMSCs showed high capacity of proliferation growth curves from second passages obtained by cell counting with the trypan blue exclusion method displayed an initial lag phase of 3 d, followed by a log phase at an exponential rate from 3 to 5 days and then a plateau phase (Fig. 3). To characterize the isolated cells, flow cytometry was performed for cell-surface markers. Results showed that MSCs were consistently positive for CD90, CD105, CD29, CD44, CD166 and CD73, but negative for hematopoietic surface markers CD34, CD45 and HLA-DR (Fig. 4).
Fig. 2.
The characteristics of the cultured hCMSCs. A Mixed population of epithelial cells and fibroblast-like cells were seen in MSCs in early stage (7 days). B Fibroblast-like morphology of MSCs was seen in late stage. C and D show the magnification display of selected rectangular region within A and B. Scale bar 50 µm. hCMSCs: human chorionic membranes mesenchymal stem cells, MSCs: mesenchymal stem cells
Fig. 3.
Growth kinetics of hCMSCs from the 2nd passage. hCMSCs: human chorionic membranes mesenchymal stem cells
Fig. 4.
Flow cytometry of undifferentiated MSCs of the third passage for CD29(A), CD34(B), CD44(C), CD45(D), CD73(E), CD90(F), CD105(G), CD166(H) and HLA-DR(I). Red curves indicate positive staining in the sorted population. Empty curves indicate negative background staining. MSCs: mesenchymal stem cells. (Color figure online)
Multi-lineage differentiation studies
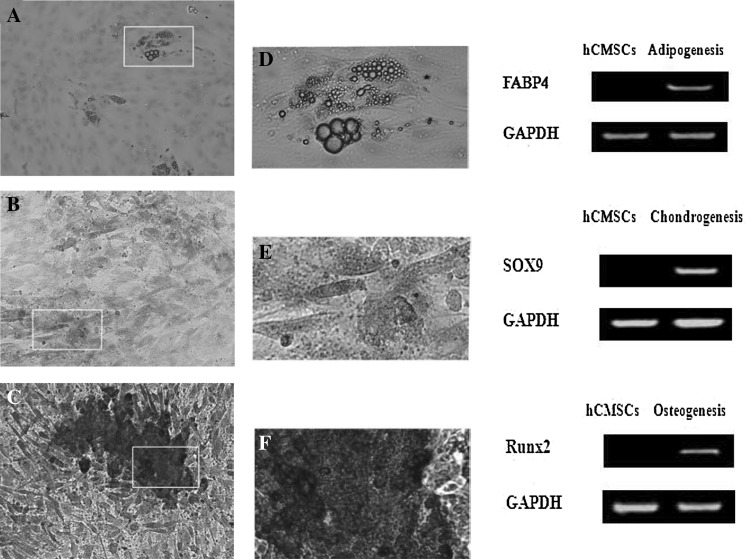
Human CMSCs were differentiated into an adipogenic cell type after approximately 21 days of incubation in adipogenic induction medium. The adipocytic phenotype in induced hCMSCs was signaled by the change in cell morphology from spindle-shaped through round to oval shaped cells, and by the appearance of numerous large, rounded intracytoplasmic lipid droplets. These lipid droplets were staining positive by Oil Red O. RT-PCR results showed that the cells expressed adipocyte-related genefatty acid- binding protein 4 (FABP4) after induction, whereas these genes were not expressed in the control group (Fig. 5).
Fig. 5.
Differentiation capacity of MSCs derived from the human chorionic membranes. A Adipogenic differentiation stimulated cells stained with Oil Red O. Scale bar 100 μm. D show the magnification display of selected rectangular region within A. B Chondrogenic differentiation stimulated cells stained with Alcian blue. Scale bar 200 μm. E show the magnification display of selected rectangular region within B. C Osteogenic differentiation stimulated cells stained with von Kossa. Scale bar 200 μm. F show the magnification display of selected rectangular region within C. Expression of adipocyte-related genes FABP4, osteoblast-related genes Runx2 and chondrocyte-related genes Sox9 was detected by RT-PCR in induced hCMSCs and hCMSCs. GAPDH served as the internal control. MSCs: mesenchymal stem cells, hCMSCs: human chorionic membranes mesenchymal stem cells, RT-PCR: reverse transcription-polymerase chain reaction, FABP4: fatty acid binding protein 4, Runx2: Runt-related transcription factor 2
Subsequently, hCMSCs differentiated into a chondrogenic cell lineage after 3 weeks of incubation in chondrogenic medium. The chondrogenic phenotype in induced hCMSCs was signaled by the changes in cell morphology, from spindles shaped to larger and rounded cell aggregates, and by the accumulation of sulfated proteoglycans that were present in cartilage. These proteoglycans in the matrix stained positive with Alcian blue staining. Also, the expression of chondrocyte-related gene Sox9 was detected by RT-PCR (Fig. 5).
Upon exposure to osteogenic differentiation medium for three weeks, hCMSCs showed changes in cell morphology. They changed from fibroblast-like to cuboidal-shaped, as they differentiated and mineralized. Calcium phosphate mineralization, which stained positive by Alizarin red staining, indicated direct evidence of calcium deposits as an amorphous accumulation between cells. Osteogenic related gene runt-related transcription factor 2 (Runx2) was increased in the induced group analyzed (Fig. 5).
Neural differentiation of the hCMSCs
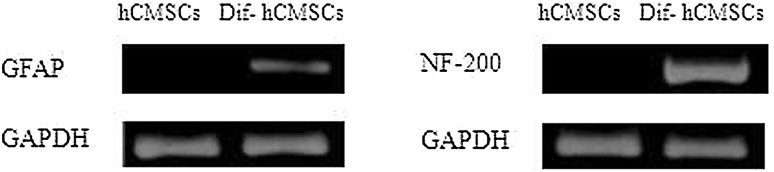
MSCs derived from the human chorionic membranes had spindle shaped morphology, similar to the typical shapes of mesenchymal stem cells. When exposed to a neuronal differentiation cocktail medium within the first 48 h of neural induction, neuron-like morphological changes appeared in the hCMSCs. With progression of differentiation, the majority of the hCMSCs exhibited multipolar morphologies with numerous neuronal processes (Fig. 6). Mature neuronal marker (NF-200) and glial marker (GFAP) were used to analyze the neural differentiation potential of hCMSCs. These cells stained positive for the neuronal markers (Fig. 7). Two weeks after the initiation of differentiation, hCMSCs expressed GFAP and NF-200 mRNAs (Fig. 8).
Fig. 6.
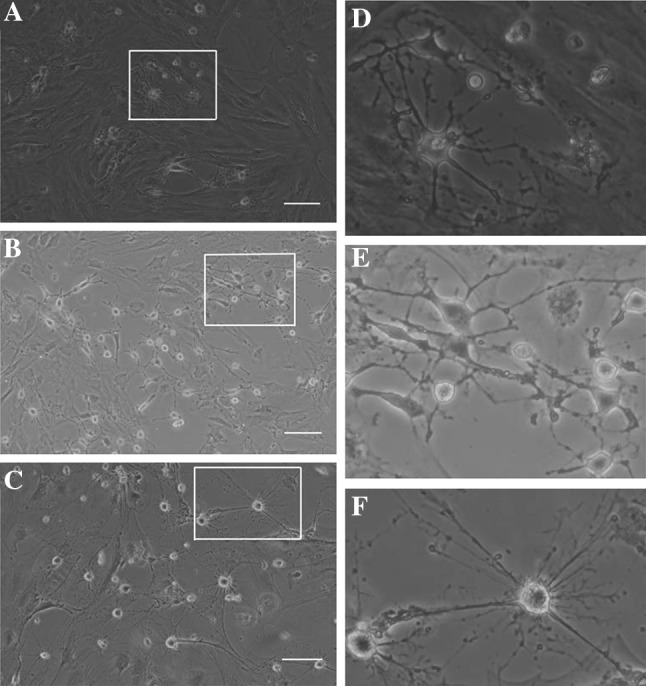
Morphological changes of the hCMCS were noticeable during induction of neuronal differentiation. Upon stimulation by neuronal differentiation medium 3 days (A), 7 days (B) and 14 days (C), spindle-shaped cells changed into neuron-like cells, as identified via microscopy. D, E and F show the magnification display of selected rectangular region within A, B and C. Scale bars 100 μm. hCMSCs: human chorionic membranes mesenchymal stem cells
Fig. 7.
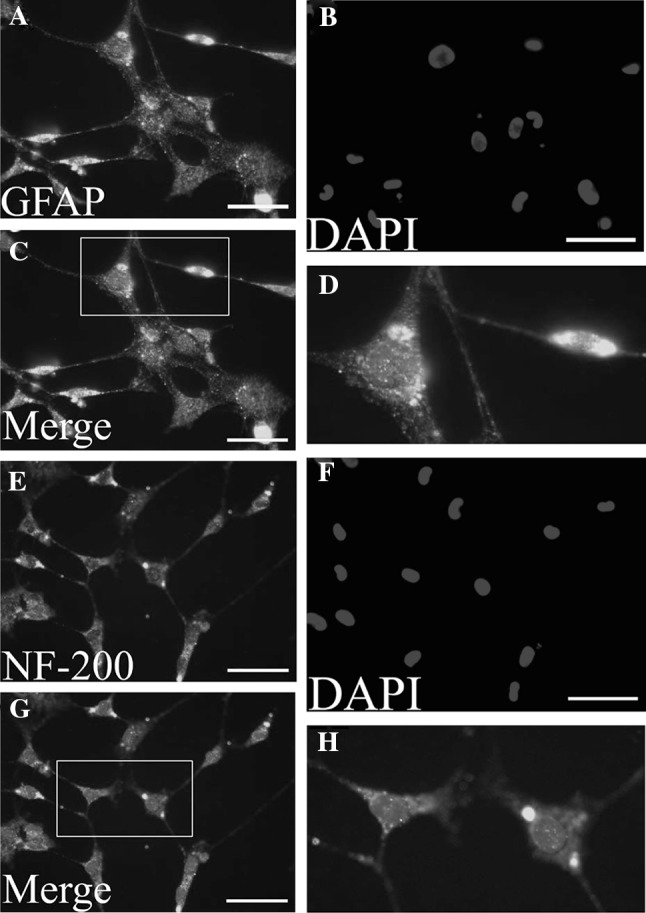
The hCMSCs were fixed and stained with antibodies against the GFAP (up panel) and NF-200 (down panel), and visualized under fluorescent microscopy. A and E show MSCs were stained with anti-GFAP (green) and anti-NF-200 (green) antibodies. B and F show nuclei were stained with DAPI (blue). C and G show the result of merge with blue and green. D and H show the magnification display of selected rectangular region within C and G. Scale bars 100 μm. hCMSCs: human chorionic membranes mesenchymal stem cells, GFAP: glial fibrillary acidic protein, NF-200: Neurofilament 200
Fig. 8.
Expression of neuronal-related genes GFAP and NF-200 was detected by RT-PCR in induced hCMSCs and hCMSCs. GAPDH served as the internal control. hCMSCs: human chorionic membranes mesenchymal stem cells, GFAP: glial fibrillary acidic protein, RT-PCR: reverse transcription-polymerase chain reaction, NF-200: Neurofilament 200, GAPDH: glyceraldehyde phosphate dehydrogenase
Discussion
In the present study, we localized MSCs in chorionic membranes, then isolated and cultured hCMSCs and characterized the phenotype, and differentiation potential. In addition, the expression of some neuronal and glial markers was investigated in the differentiated hCMSCs. Our results agree with previous in vitro studies performed in human BMSCs, which are chemically induced into cells that display morphologic, and protein characteristics of neural progenitors [30].
To search a cell model that meets the needs of the patient’s treatment, the feasibility and safety of stem cell transplants have been tested, which further promotes the use in clinical practice, and the use of mesenchymal stem cells in particular has been extensively studied [32–35]. One of the main challenges in any cellular therapy approach is the expansion of progenitor cells in order to achieve clinically relevant cell numbers for transplantation. Though bone marrow mesenchymal stem cells have a variety of advantages such as being widely available, can be expanded and induced to differentiate in vitro. But it has been estimated that MSCs represent only a minute fraction (0.01–0.001%) of the nucleated cell population in adult human bone marrow [36, 37]. Isolated from biologic waste, mesenchymal stem cells from full-term placenta are easily available and may present an attractive completion to other classically established stem cells in different clinical approaches [38, 39].
MSCs primary location is presumed to be the perivascular area of small vessels in placenta throughout pregnancy and play a significant role in the structural and functional development of the placenta particularly during vasculogenesis, angiogenesis and the villus branching, from which they can be migrated into the circulation when needed [40, 41].With immunofluorescence analysis, we identified cells positive for CD90 and CD105 dispersed in placenta. Our results also showed that the CD90 positive cells were scattered in the chorionic membranes tissue, and the CD105 positive cells were mostly located around the small blood vessels.
Defined human mesenchymal stem cells according to International Society for Cellular Therapy, which should be adhere to plastic; express CD73, CD90, and CD105; lack express CD45, CD34, and human leukocyte antigen DR; and can differentiate to adipocytes, chondroblasts and osteoblasts in vitro [42]. Here the results showed that phenotypes of culture cells were positive for CD90, CD29, CD166, CD44, CD105, and CD73 and negative for CD34, CD45, and human leukocyte antigen DR. We also showed that human CMSCs differentiate into bone, adipose cells, and cartilage.
Currently, the research of mesenchymal stem cells differentiation to neurons is still in its early stages. Neuronal differentiation can be achieved in three ways: use of neurotrophic factors or cytokines, exposure to chemical inducers, and gene transfection or modification of the induced differentiation. Neuronal specific protein expression and MSCs morphological changed after the induction with chemical inducer, while morphology and function cannot maintain for a long time, it is may be cytotoxic rather than cellular differentiation process. Gene transfection or modification of the induced differentiation method also has some problems, such as poor stability, low expression rate, cell damage and host cell gene may change and so on [43, 44]. Here we used neurotrophic factors and cytokines to induce the hCMSCs and our experiments have demonstrated that induced cells expressed GFAP and NF-200. GFAP is used as an astrocytic marker and can also be expressed in immature neural progenitor cells. NF-200 is used in dendritic cells, granulocytes, and microglia. They are used to identify progenitor or mature neuronal cells.
In summary, the chorionic membranes are more safe and reliable mesenchymal stem cell source. It’s feasibility to isolate and culture MSCs from chorionic membranes and differentiate into neuron like cells, provide experimental basis for CMSCs transplantation for treatment of nervous system injury.
Acknowledgements
The authors thank Dr. Jun Jin for English grammar revision. We especially thank Dr. Lei Cheng for valuable suggestions and the critical review of this manuscript.
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical statement
The study was approved by the Ethics Committee of Wuxi No. 3 People’s Hospital (IRB No. 2015 0411023), and the protocols conform to the ethical guidelines of the 1975 Helsinki Declaration.
References
- 1.Khanabdali R, Saadat A, Fazilah M, et al. Promoting effect of small molecules in cardiomyogenic and neurogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Drug Des Devel Ther. 2015;10:81–91. doi: 10.2147/DDDT.S89658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar BM, Maeng GH, Lee YM, et al. Neurogenic and cardiomyogenic differentiation of mesenchymal stem cells isolated from minipig bone marrow. Res Vet Sci. 2012;93:749–757. doi: 10.1016/j.rvsc.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Kondo T, Matsuoka AJ, Shimomura A, et al. Wnt signaling promotes neuronal differentiation from mesenchymal stem cells through activation of Tlx3. Stem Cells. 2011;29:836–846. doi: 10.1002/stem.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park HW, Cho JS, Park CK, et al. Directed induction of functional motor neuron-like cells from genetically engineered human mesenchymal stem cells. PLoS ONE. 2012;7:e35244. doi: 10.1371/journal.pone.0035244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzilay R, Kan I, Ben-Zur T, Bulvik S, et al. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008;17:547–554. doi: 10.1089/scd.2007.0172. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 7.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepperdinger G, Brunauer R, Jamnig A, et al. Controversial issue: Is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol. 2008;43:1018–1023. doi: 10.1016/j.exger.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 10.Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev. 2010;16:445–453. doi: 10.1089/ten.teb.2009.0825. [DOI] [PubMed] [Google Scholar]
- 11.Hou M, Cui J, Liu J, et al. Angiopoietin-like 4 confers resistance to hypoxia/serum deprivation-induced apoptosis through PI3K/Akt and ERK1/2 signaling pathways in mesenchymal stem cells. PLoS ONE. 2014;9:e85808. doi: 10.1371/journal.pone.0085808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Jaramillo-Ferrada PA, Wolvetang EJ, Cooper-White JJ. Differential mesengenic potential and expression of stem cell-fate modulators in mesenchymal stromal cells from human-term placenta and bone marrow. J Cell Physiol. 2012;227:3234–3242. doi: 10.1002/jcp.24014. [DOI] [PubMed] [Google Scholar]
- 13.Fortino VR, Chen RS, Pelaez D, et al. Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J Cell Physiol. 2014;229:479–488. doi: 10.1002/jcp.24468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 15.Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 16.Kmiecik G, Spoldi V, Silini A, et al. Current view on osteogenic differentiation potential of mesenchymal stromal cells derived from placental tissues. Stem Cell Rev. 2015;11:570–585. doi: 10.1007/s12015-014-9569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Y, Cai S. Current state of the development of mesenchymal stem cells into clinically applicable Schwann cell transplants. Mol Cell Biochem. 2012;368:127–135. doi: 10.1007/s11010-012-1351-6. [DOI] [PubMed] [Google Scholar]
- 19.Schaakxs D, Kalbermatten DF, Raffoul W, et al. Regenerative cell injection in denervated muscle reduces atrophy and enhances recovery following nerve repair. Muscle Nerve. 2013;47:691–701. doi: 10.1002/mus.23662. [DOI] [PubMed] [Google Scholar]
- 20.Keilhoff G, Goihl A, Langnäse K, et al. Trans differentiation of mesenchymal stem cells into Schwann cell-like myelinating cells. Eur J Cell Biol. 2006;85:11–24. doi: 10.1016/j.ejcb.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Spejo AB, Carvalho JL, Goes AM, et al. Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: synapse stability and axonal regeneration. Neuroscience. 2013;250:715–732. doi: 10.1016/j.neuroscience.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Bossio C, Mastrangelo R, Morini R, et al. A simple method to generate adipose stem cell-derived neurons for screening purposes. J Mol Neurosci. 2013;51:274–281. doi: 10.1007/s12031-013-9985-8. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz-Elías G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells. 2003;21:437–448. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- 26.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 29.Huang T, He D, Kleiner G, Kuluz J. Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J Spinal Cord Med. 2007;30:S35–S40. doi: 10.1080/10790268.2007.11753967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scuteri A, Miloso M, Foudah D, et al. Mesenchymal stem cells neuronal differentiation ability: A real perspective for nervous system repair? Curr Stem Cell Res Ther. 2011;6:82–92. doi: 10.2174/157488811795495486. [DOI] [PubMed] [Google Scholar]
- 31.Drela K, Lech W, Figiel-Dabrowska A, et al. Enhanced neuro-therapeutic potential of Wharton’s Jelly-derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy. 2016;18:497–509. doi: 10.1016/j.jcyt.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Kumar AA, Kumar SR, Narayanan R, et al. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: a phase I/II clinical safety and primary efficacy data. Exp Clin Transpl. 2009;7:241–248. [PubMed] [Google Scholar]
- 33.Sharma A, Gokulchandran N, Chopra G, et al. Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transpl. 2012;21:S79–S90. doi: 10.3727/096368912X633798. [DOI] [PubMed] [Google Scholar]
- 34.Syková E, Jendelová P, Urdzíková L, et al. Bone marrow stem cells and polymer hydrogels—two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26:1113–1129. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell JH, Haycock JW. Next generation nerve guides: materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B Rev. 2012;18:116–128. doi: 10.1089/ten.teb.2011.0498. [DOI] [PubMed] [Google Scholar]
- 36.Kørbling M, Estrov Z. Adult stem cells for tissue repair—A new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 37.Körbling M, Estrov Z, Champlin R. Adult stem cells and tissue repair. Bone Marrow Transpl. 2003;32:S23–S24. doi: 10.1038/sj.bmt.1703939. [DOI] [PubMed] [Google Scholar]
- 38.Barlow S, Brooke G, Chatterjee K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–1107. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 39.Castrechini NM, Murthi P, Gude NM, et al. Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta. 2010;31:203–212. doi: 10.1016/j.placenta.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 40.James JL, Srinivasan S, Alexander M, et al. Can we fix it? Evaluating the potential of placental stem cells for the treatment of pregnancy disorders. Placenta. 2014;35:77–84. doi: 10.1016/j.placenta.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Abedin M, Tintut Y, Demer LL. Mesenchymal stem cells and the artery wall. Circ Res. 2004;95:671–676. doi: 10.1161/01.RES.0000143421.27684.12. [DOI] [PubMed] [Google Scholar]
- 42.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 43.Caddick J, Kingham PJ, Gardiner NJ, et al. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006;54:840–849. doi: 10.1002/glia.20421. [DOI] [PubMed] [Google Scholar]
- 44.Yang JD, Cheng-Huang WJ, et al. The isolation and cultivation of bone marrow stem cells and evaluation of differences for neural-like cells differentiation under the induction with neurotrophic factors. Cytotechnology. 2014;66:1007–1019. doi: 10.1007/s10616-013-9654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]