Abstract
This study aims to investigate the roles and effects of EGCG (epigallocatechin-3-gallate) during the osteogenic differentiation of human mesenchymal stem cells (hMSCs) in vitro. Recent studies have shown that proper mechanical stimuli can induce osteogenic differentiation of hMSCs apart from biochemical factors. In this study, the hMSC cultures were subjected to: (1) 25 uM EGCG alone or (2) 3% mechanical stretching (0.2 Hz for 4 h/day for 4 days) or (3) in combination with 3% mechanical stretching (0.2 Hz for 4 h/day for 4 days). The two factors were applied to the cell cultures separately and in combination to investigate the individual and synergistic effect of both mechanical stimulation and ECGC in the osteogenic differentiation of hMSCs. Utilizing real time PCR, we measured various osteogenic markers and even those related to intracellular signalings. Further investigation of mitochondria was performed that mitochondria biogenesis, antioxidant capacity, and morphological related markers were measured. hMSCs were to be osteogenic or myogenic differentiated when they were under 3% stretching only. However, when EGCG was applied along with stretching they were to be osteogenic differentiated rather than to be myogenic differentiated. This was supported by evaluating intracellular signalings: BMP-2 and VEGF. Therefore, the synergistical effects of simultaneous employment of stretching and EGCG on osteogenic differentiation were confirmed. Moreover, simultaneous employment was found positive in mitochondria biogenesis, antioxidant capacity, and morphological changes. Through this study, we came into the conclusion that the combination of proper mechanical stretching, 3% in this study, and EGCG promote osteogenic differentiation. Reflecting that EGCG can be obtained from plants not from the chemical syntheses, it is worth to be studied further either by animal tests or long-term experiments for clinical applications.
Keywords: Mesenchymal stem cells, Stem cell differentiation, Mechanical stretch, Epigallocatechin gallate, Osteogenesis
Introduction
Even some types of stem cell lineages are theoretically available for researches and clinical applications, mesenchymal stem cells (MSCs) are still prominent. Apart from easy acquirements and usage, they have other major advantages such as no ethical issues and immune reactions [1, 2]. Also, they are much closer to clinical applications than the other stem cell sources such as myocardial infarction, diabetes, degenerative arthritis and other treatments [3–5]. Especially, they have been widely tried and used in the area of tissue engineering to cure or replace damaged tissues. For successful outcomes in tissue engineering, the primary requirement is to promote differentiation of stem cells adopted, especially in bone tissues [6]. For this, various growth factors have been used such as dexamethasone or beta-glycerophosphate. However, it has been reported that dexamethasone, one of the steroids reagents in glucocorticoid affiliation, can reduce bone mineral density resulting in bone loss [2]. Such concerns are common to most chemical reagents in general.
To overcome or eliminate such concerns, other methods excluding any growth factors in stem cell differentiation have been widely investigated both in vitro experiments. One of them is to control biophyical environment conditions. Recently, it has been proved that controlling of biophysical environments such as substrate stiffness or biomechanical stimulation can modulate stem cell differentiation even without any biochemical reagents. Engler et al. [7] and Lee et al. [8] found and reported that osteogenic differentiation was promoted when the cells were onto the substrate whose stiffness was more than 40 kPa. Huang et al. [9] and Jang et al. [10] found stretching of 3% to mesenchymal stem cells was effective in inducing osteogenic differentiation. In addition, other mechanical stimuli such as compression or flow-induced shear stresses have also been found effective in the expression of osteogenic related markers [11–13].
With regard to reagents, EGCG can be the candidate and has been recently studied. EGCG, a type of catechin, is found in the dried leaves of most types of teas, such as white, green, or even black tea. It is known to have antioxidant effect [14–16]. It also has been found effective in osteogenesis and other bone related diseases such as rheumatoid arthritis [17]. Consequently, it has been emerged as a candidate in cell therapy due to its osteo-induction capability. Some studies showed the increases of ALP activity and osteo-related markers when EGCG was applied to mesenchymal stem cells and proposed EGCG as a pro-osteogenic agent [18, 19]. Chen et al. [20] also found the increase in ALP activity and even mineralization in D1 cell line under EGCG. However, most studies so far have been focused on the effects of EGCG in osteoblasts or osteoclasts [21, 22]. Moreover, they adopted osteogenic media along with EGCG and investigated the inter-reaction of EGCG and biochemical reagents used [18–20].
This study utilized no other reagents but EGCG. All cells were cultured and to be differentiated by mechanical stretching and EGCG in basal culture media. Therefore, the role of EGCG was investigated with or without mechanical stretching. Also, various changes in mitochondria were also studied. Moreover, intracellular signaling was also investigated to confirm the role of EGCG in promoting osteogenic differentiation.
Materials and methods
Cell culture and EGCG treatment
Human mesenchymal stem cells (hMSCs) were purchased from a company (Lonza, Basel, Switzerland). Based on the manufacturer’s protocol, hMSCs were cultivated at 37 °C in a humidified 5% CO2 incubator until the passage #5. hMSCs were cultivated in Dulbecco’s Modified Eagle Medium with low glucose (DMEM-LG; Life Technologies, USA) containing 5% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin and 100 mg/mL streptomycin (Hyclone, Logan, UT, USA). EGCG was added to hMSCs 24 h after cell seeding at a concentration of 25 μM.
Employment of mechanical stretch
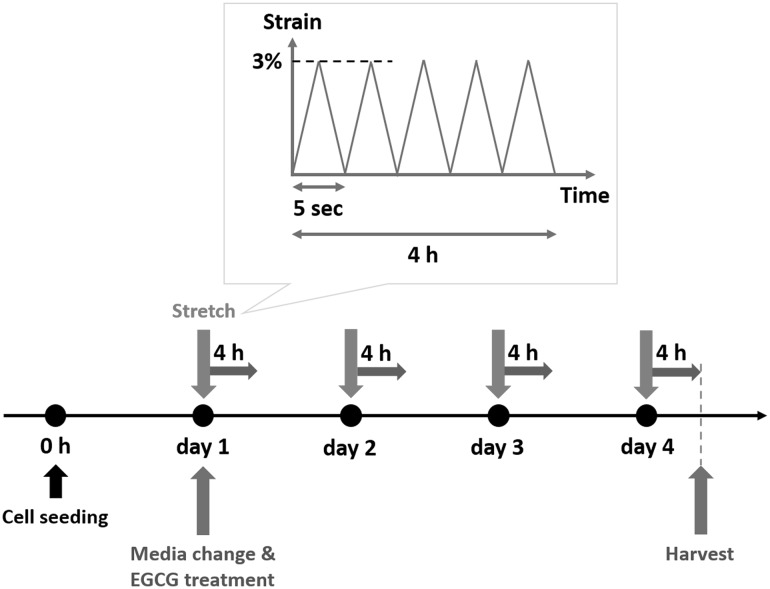
A bioreactor system (ACBT-200, Anycasting Inc., Seoul, Korea) was used to employ mechanical stretching to the cells. To apply mechanical stretch to the cells hMSCs were seeded on the elastomeric substrate. To fabricate elastomeric substrate Polydimethylsiloxane (PDMS, SYLGARD 184 Silicone Elastomer Kit, Dow Corning Corp., USA) were used. Silicone elastomer base and a curing agent were mixed at a ratio of 10:1. Then the mixture was baked at 120 °C for 20 min. Then PDMS membrane was cut into 9 cm × 4 cm × 800 μm and was sterilized using 70% ethanol and deionized water followed by being exposed to ultraviolet (UV) for 30 min. Then, membranes were coated with fibronectin (F0895, Sigma, MO, USA) after treatment of atmospheric plasma (APP, Gyeonggi, Korea). Then hMSCs were seeded on the PDMS membrane at a density of 1 × 104 cells/cm2. The mechanical stretching of 3% was applied: 0.2 Hz, 4 hours/day for 4 days starting 24 hours after seeding (Fig. 1). The cells were harvested right after stimulation. And hMSCs without any treatment were used as a control group.
Fig. 1.
Schematics of experimental procedure
Quantitative real-time PCR
The various expressions of related markers were evaluated by real time PCR. From each sample, total cellular RNA was extracted using RNeasy Mini Kit (#74106, Qiagen, Valencia, CA, USA) and cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (#4374966, Applied Biosystems, USA). Then, cDNA was amplified by using Power SYBR Green PCR Master Mix (Applied Biosystems) and QuantStudio-3 (Applied Biosystems, USA). The primers used in this study were listed in Table 1. The expressions of Runt-related transcription factor 2 (RUNX2) and myocardin were measured to evaluate differentiation direction: osteogenic or myogenic differentiation. And vascular endothelial growth factor (VEGF), transforming growth factor – beta 1 (TGF-beta1) and bone morphogenetic protein 2 (BMP-2) were observed to investigate changes of intracellular signaling due to mechanical stretch and/or EGCG. Also, peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1alpha), mitochondrial transcription factor A (mtTFA), superoxide dismutase 1 (SOD1), superoxide dismutase 1 (SOD2), catalase (CAT), and glutathione peroxidase 1 (GPX1) were analyzed for mitochondrial function. Also, mitochondrial fusion-related genes (mitofusin 1 (MFN1), mitofusin 2 (MFN2), optic atrophy 1 (OPA1)) and mitochondrial fission-related genes (dynamin-related protein 1 (DRP1), mitochondrial fission protein 1 (FIS1), mitochondrial E3 ubiquitin ligase (MARCH5)) were investigated to confirm the effect on mitochondrial morphology. All gene expression levels were normalized by house-keeping gene, beta-actin.
Table 1.
Primers for quantitative real-time PCR used in the experiments
| Primer | Forward (F) and reverse (R) primer (5′–3′) | Genebank accession no. | Product size |
|---|---|---|---|
| RUNX2 | (F) ACCCACGAATGCACTATCCA | AH005498.1 | 154 |
| (R) CTGGTGGGAAGGGTCCACT | |||
| Myocardin | (F) ATTCCCTGAAGCGCAAAGC | NM_001146312 | 350 |
| (R) GGATCCCGCTGAGTTTTGG | |||
| VEGF | (F) CCTGGTGGACATCTTCCAGG | KT581010.1 | 196 |
| (R) GAAGCTCATCTCTCCTATGT | |||
| TGF-beta1 | (F) ATTGAGGGCTTTCGCCTTAG | NM_000660.5 | 78 |
| (R) GAACCCGTTGATGTCCACTT | |||
| BMP-2 | (F) GGGGTCACAGATAAGGCCA | AF040249 | 310 |
| (R) CAGCATCGAGATAGCACTGA | |||
| PGC-1alpha | (F) GGACAGAACTGAGGGACCGT | NM_013261.3 | 124 |
| (R) GCAGCAAAAGCATCACAGGT | |||
| mtTFA | (F) TTAAAGCTCAGAACCCAGATGCA | BC126366 | 280 |
| (R) GCTGAACGAGGTCTTTTTGGTTT | |||
| SOD1 | (F) ACAGCAGGCTGTACCAGTGC | NM_000454.4 | 107 |
| (R) GCAGTCACATTGCCCAAGTC | |||
| SOD2 | (F) GCATCTGTTGGTGTCCAAGG | NM_000636.2 | 106 |
| (R) TTCCTTGCAGTGGATCCTGA | |||
| CAT | (F) ACCACTGGAGCTGGTAACCC | NM_001752.3 | 120 |
| (R) CTCTCGGTCAAAATGAGCCA | |||
| GPX1 | (F) CAACGATGTTGCCTGGAACT | NM_000581.2 | 102 |
| (R) TCAGGCTCGATGTCAATGGT | |||
| MFN1 | (F) TTTTGGGCCCTAGAAATGCT | NM_033540.2 | 107 |
| (R) TGCTGGAGTGGTAGGAGCAG | |||
| MFN2 | (F) GCTGGAGGAGTGGGAGTAGC | NM_014874.3 | 100 |
| (R) GAGAGGCAAGTCCCTCCTTG | |||
| OPA1 | (F) CATGGCTCCTGACACAAAGG | NM_015560.2 | 108 |
| (R) CGTTCAGCATCCACAGATCC | |||
| DRP1 | (F) TGATCCACTTGGTGGCCTTA | NM_005690.3 | 116 |
| (R) GCCGCTTCACCAGTAACTCA | |||
| FIS1 | (F) ATCGGACTTGCTGTGTCCAA | NM_016068.2 | 142 |
| (R) AGCTGAAGGCCACAGAGGAT | |||
| MARCH5 | (F) TAGGCAAGATGATTCGCTGG | NM_017824.4 | 123 |
| (R) CAGCTGGAATTCGAGGAACA | |||
| Beta-actin | (F) CCCAAAGTTCACAATGTGGC | NM_001101.3 | 103 |
| (R) GATGGCAAGGGACTTCCTGT |
Statistical analysis
All data are presented as mean ± standard deviation. And One-way Analysis of Variance (ANOVA) test was performed using SPSS (PASW Statistics 18; SPSS Inc. USA) at a significance level of p < 0.05 (n = 3). When ANOVA indicated a significant difference among groups, the difference was evaluated using the least significant different (LSD). * denotes significant difference from the other group.
Results
The goal of this research is to determine the individual and synergistic effect of both EGCG and mechanical stimulation in osteogenic differentiation of the hMSCs. In addition, we investigate the role of EGCG when stem cells are being differentiated by mechanical stretching. For this four groups were set: EGCG, Stretched, EGCG+Stretched (E+S), and Control group. No other biochemical reagent was used for differentiation.
Effects of EGCG and/or mechanical stretch on osteogenic and myogenic differentiation
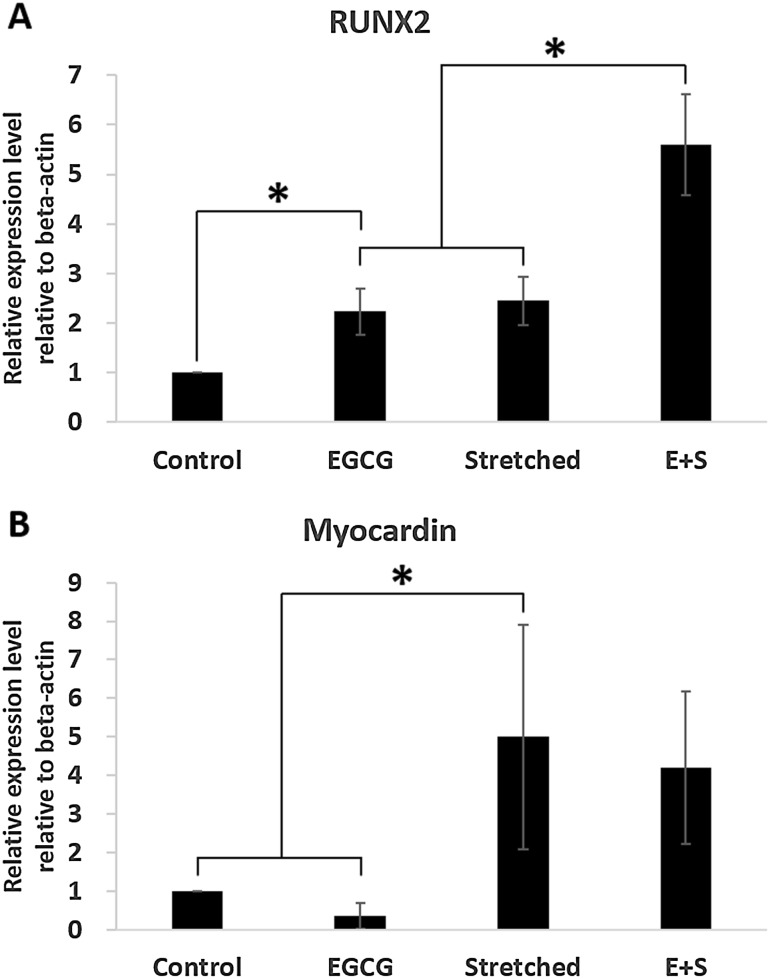
The expressions of typical markers, RUNX2 and myocardin indicating early stage of osteogenesis and myogenesis, respectively under 3% strain were investigated as shown in Fig. 2. The group EGCG showed significantly high expression of RUNX2 than control group. However, the expression of myocardin was lower in group EGCG than that in control group. When strain was applied alone both of RUNX2 and myocardin were significantly expressed compared with those in control group. And much higher expression of RUNX2 was observed when EGCG and 3% stretching were simultaneously applied (group E+S). However, the expression of myocardin in group E+S was comparable with that in group Stretched.
Fig. 2.
Expressions of RUNX2 and myocardin indicating the direction of stem cell differentiation induced by EGCG and mechanical stretching: A RUNX2 showing osteogenic differentiation, B Myocardin showing myogenic differentiation
Expression of intracellular genes: VEGF, TGF-beta1, BMP-2
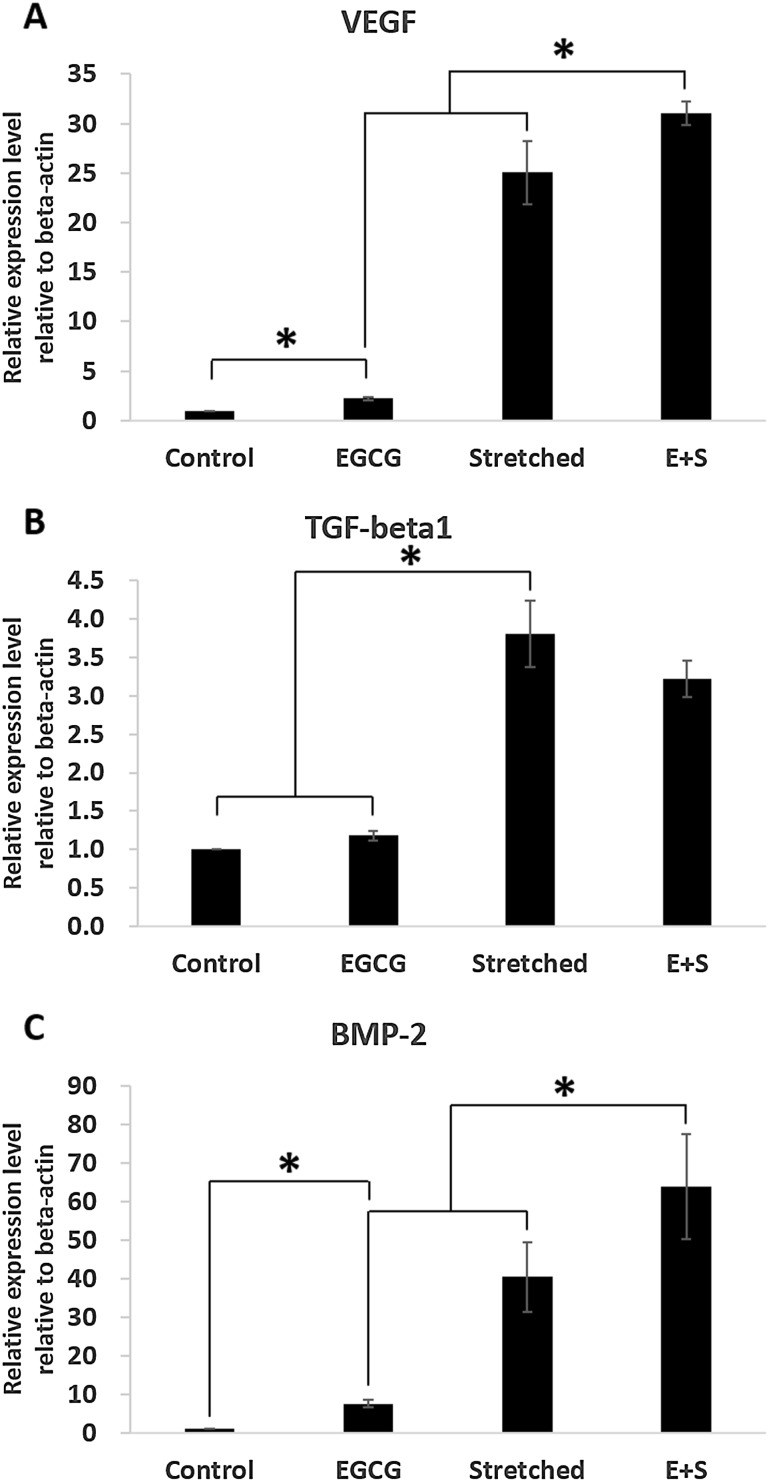
As shown in Fig. 3 synergistic effect on expressions of VEGF, TGF-beta1 and BMP-2 was observed when EGCG and stretching were simultaneously applied. Those three expressions were upregulated due to 3% stretching significantly. Even the expression of TGF-beta1 in group E+S was lower than that in group Stretched it was still significantly high than in control group. Moreover, the synergistic effect was observed when EGCG was added to 3% stretching in expression of VEGF and BMP-2.
Fig. 3.
Effects of mechanical stretch and EGCG on intracellular signaling: A VEGF, B TGF-beta1, C BMP-2
Functional and morphological changes of mitochondria
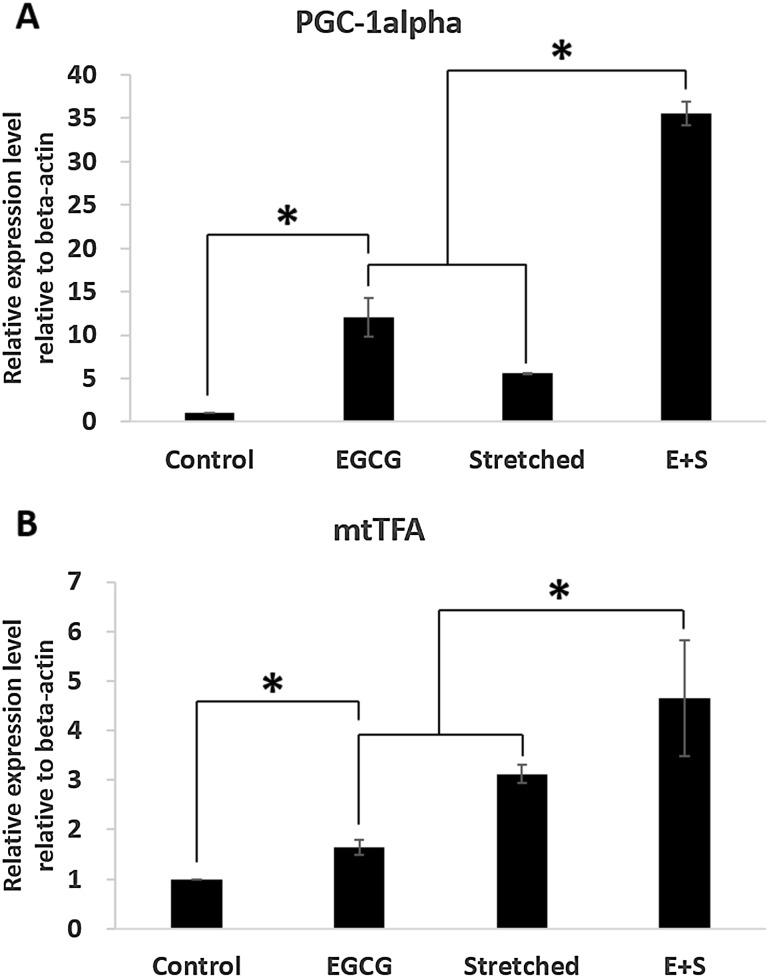
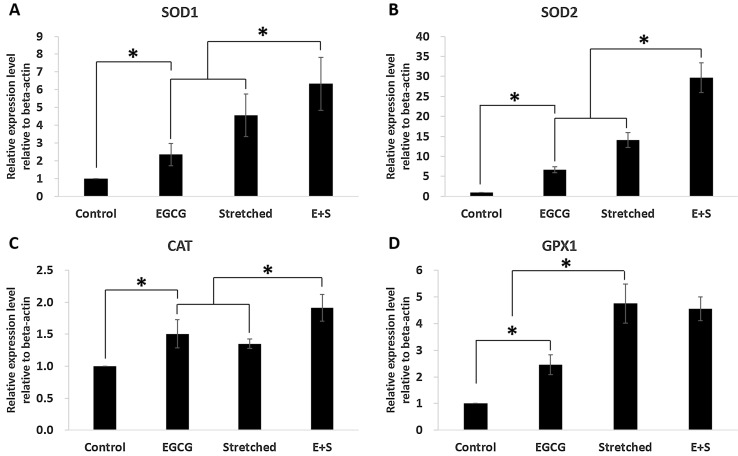
PGC-1alpha and mtTFA expression were significantly increased due to EGCG or mechanical stretch (Fig. 4). Also, simultaneous employment of EGCG and stretching showed synergistic effect in expression of PGC-1alpha and mtTFA as shown in group E+S. Other markers, which are related to anti-oxidant enzyme, were also investigated. They were SOD1, SOD2, GPX1, and CAT. All those were significantly expressed when EGCG or stretching was applied. Also, expressions of SOD1, SOD2 and CAT were significantly increased when the EGCG and stretching were applied simultaneously. However, the expression of GPX1 was comparable between the groups of Stretched and E+S as shown in Fig. 5.
Fig. 4.
Effects of mechanical stretch and EGCG on mitochondrial biogenesis: A PGC-1alpha, B GPX1
Fig. 5.
Effects of mechanical stretch and EGCG on mitochondrial antioxidants: A SOD1, B SOD2, C CAT, D GPX1
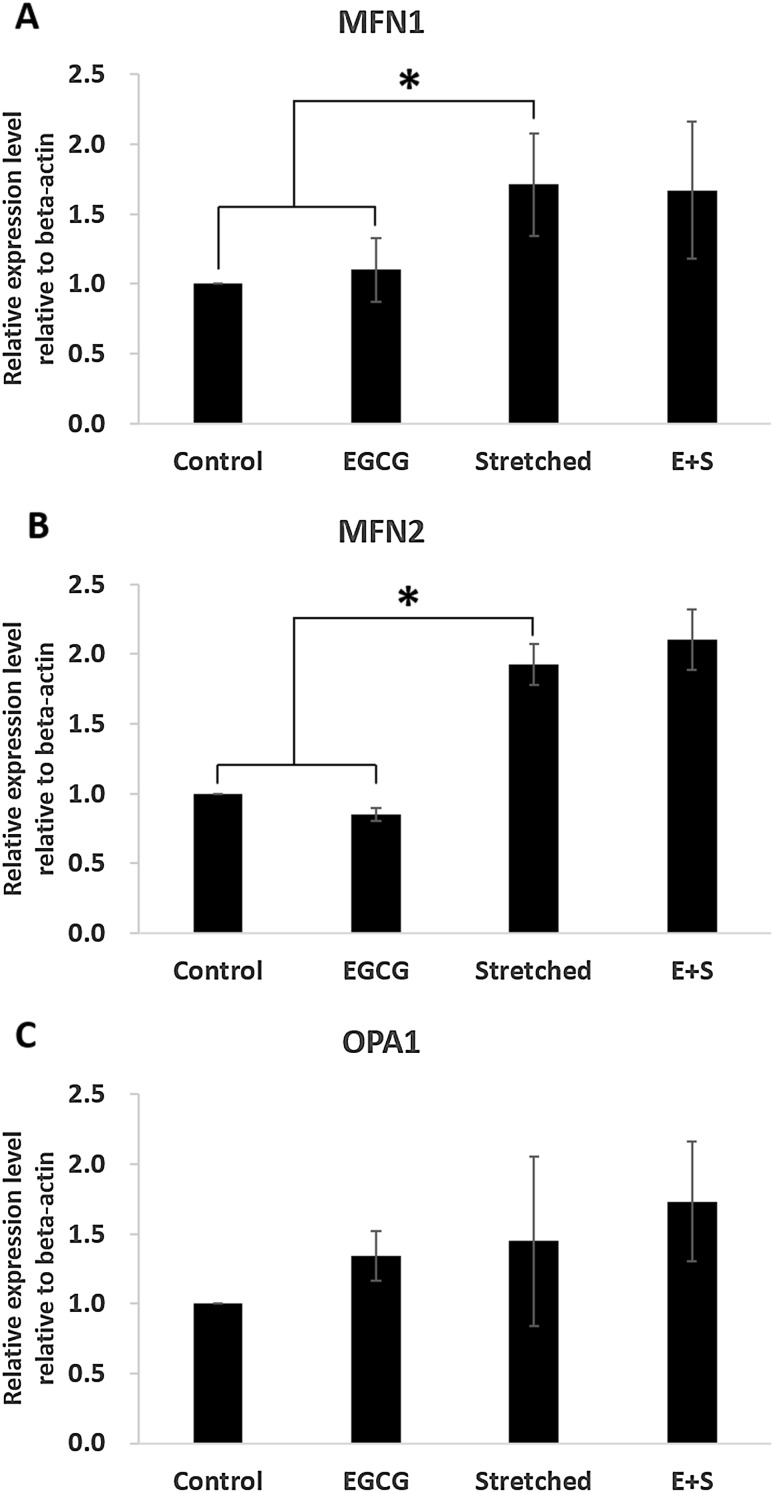
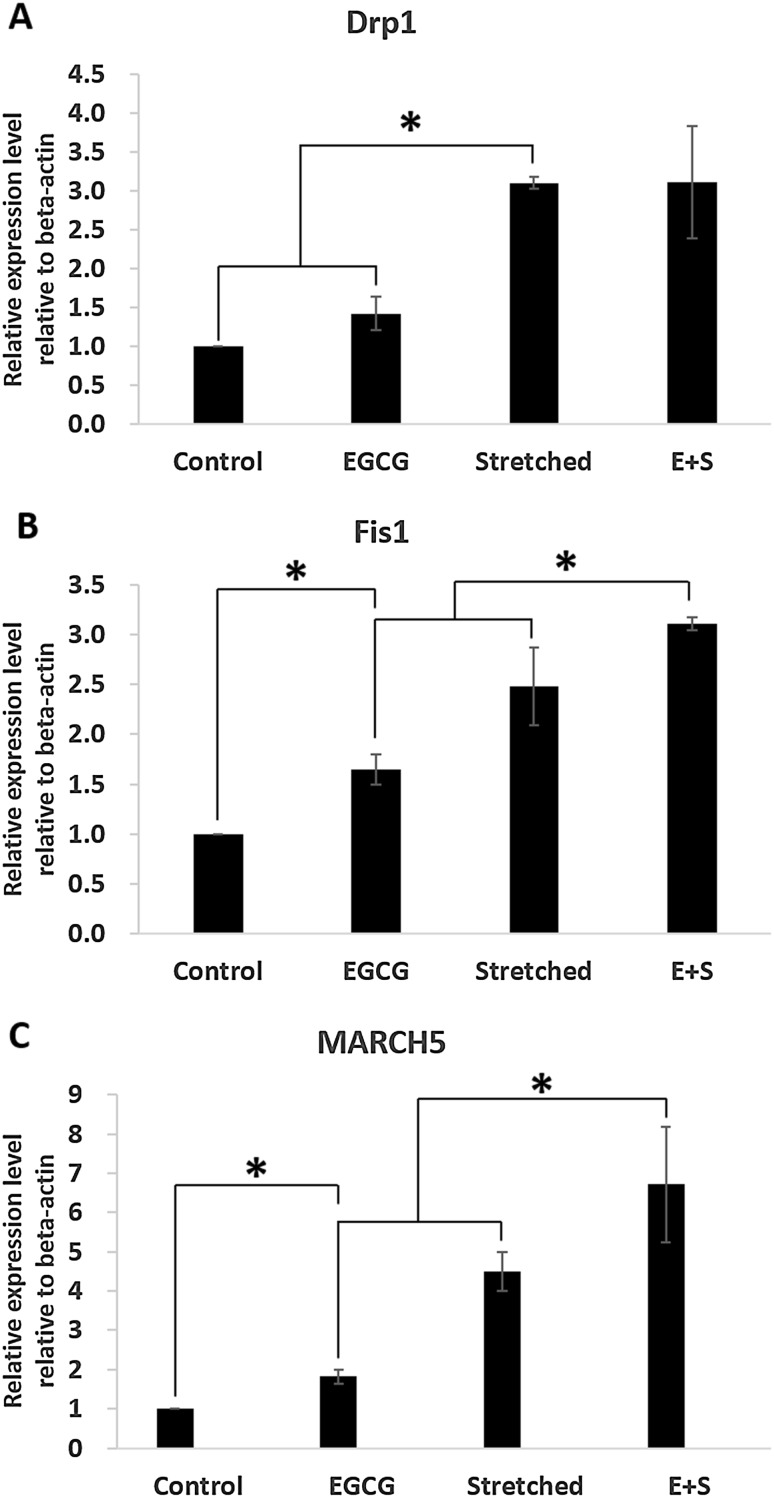
Mitochondrial morphology changes were investigated by measuring mitochondrial morphology-related gene expression (Fig. 6, 7). Mitochondrial fusion-related genes, MFN1 and MFN2, were significantly increased even by stretching only. EGCG did not contribute to the expression of MFN1 or MFN2 (Fig. 6). The expression of OPA1 tended to be increased due to EGCG and/or stretching even there was no significant difference. Mitochondrial fission-related genes were dominantly affected by mechanical stretching, 3% in this study, rather than by EGCG (Fig. 7). However, FIS1 and MARCH5 were significantly expressed by even EGCG alone. And their expression was more observable when EGCG and stretching were simultaneously applied.
Fig. 6.
Effects of mechanical stretch and EGCG on mitochondrial fusion-related gene expression: A MFN1, B MFN2, C OPA1
Fig. 7.
Effects of mechanical stretch and EGCG on mitochondrial fission-related gene expression: A DRP1, B FIS1, C MARCH5
Discussion
This is the first study to investigate the roles of EGCG in osteogenic differentiation with or without mechanical stimulation followed by measuring various markers in detail. Previous studies [9, 10, 23] showed that mechanical stretching can modulate stem cell differentiation even without any biochemical reagents. Specifically, mechanical stretching is known to induce osteogenic or myogenic differentiation of adult stem cells depending on its strain magnitude. Relatively large strain (~10%) is known to be preferable to myogenic differentiation while small strain (~3%) is to osteogenic differentiation.
Based on other previous studies, we evaluated the expressions of typical markers indicating early stage in differentiation as shown in Fig. 2, first. Interestingly, expression of RUNX2 was significantly increased when EGCG and 3% stretching were simultaneously applied (group E+S), which showed the synergistical effect of EGCG and stretching. However, EGCG did not contribute to myogenic differentiation when it was applied alone. These results enable us to confirm that EGCG plays a positive role in osteogenic differentiation rather than in myogenic differentiation when stem cells were to be differentiated by mechanical stretching. Parker et al. [24] also reported that RUNX2 inhibits expression of myocardin and eventually suppress myogenic differentiation. It has not been clarified whether EGCG affects the expression of myocardin. Our results showed EGCG with mechanical stretching significantly increased the expression of RUNX2, while it significantly decreased the expression of myocardin. Therefore, we can infer that the simultaneous employment of EGCG and mechanical stretching could suppress myocardin expression. However, further investigation is needed to confirm this scenario.
In addition, more osteogenic-related markers were evaluated to confirm the roles of EGCG in osteogenic differentiation. They were BMP-2, VEGF, and TGF-beta1. The importance of BMP-2 in osteogenic differentiation is widely recognized. VEGF, which can be modulated by BMP-2, also plays importance roles in osteogenic differentiation [25]. Moreover, it is known to be essential in angiogenesis [26]. TGF-beta1 is also important in osteogenesis along with BMP-2 [27]. Specifically, Zelzer et al. [28] reported that the intracellular expression of VEGF upregulates RUNX2 expression then osteoblastogenesis is promoted. Yamamoto et al. [29] stipulated that intracellular expression of BMP-2 in C2C12 myoblast suppresses myogenic differentiation while it promotes osteoblast differentiation. Our results in Fig. 3 showed higher expression of BMP-2 and VEGF. Therefore, these expressions are expected to induce RUNX2 expression followed by accelerated osteogenic differentiation, especially when EGCG and stretching were applied simultaneously as in group Stretched (E+S).
Meanwhile, many recent reports on mitochondria in relation to osteogenesis have been released. Obviously mitochondria, one of the major energy sources in a cell, produce ATP. Apart from its well-known functions, mitochondria are known to be closely related to proliferation and cell fate, also [30]. In addition, recent studies revealed that they also play important roles in keeping function of differentiation and/or directing differentiation [24, 31, 32]. Shin et al. [23] and Wilkerson et al. [33] found and reported increasing in mitochondria biogenesis as osteogenic differentiation process goes on. Chen et al. [34] found increase of mitochondria biogenesis and anti-oxidant enzyme when hMSCs were under osteogenic process. Our investigation also showed that biogenesis and anti-oxidant capacity of mitochondria were increased especially when EGCG and mechanical stretching were simultaneously applied.
Apart from physiological changes in mitochondria during differentiation, we also investigated morphologically related markers as morphology and physiological function are known to be closely related each other [35]. Moreover, recent studies have shown the morphological changes and their importance during various differentiation directions by measuring fusion- or fission-related markers [23] or by observing morphological changes through image analyses [36]. The selected fusion-related markers in this study were MFN1, MFN2, and OPA1 while fission-related ones were DRP1, FIS1, and MARCH5. Shin et al. [23] reported changes in expression of fusion- and fission-related markers when differentiation direction of adult stem cells was modulated depending on strain magnitude. They found increased expression of fission- and fusion-related markers under 3% strain without any biochemical reagents. This study also showed the same trends. Most markers, regardless of being fission- or fusion-related, were significantly expressed in group Stretched than in control group but OPA1. This trend was more observable when EGCG and stretching were simultaneously applied. This implies that the mitochondrial morphological activities were more observable when EGCG treatment was accompanied with stretching rather than when EGCG was applied alone.
From the study, here we could confirm the role of EGCG in osteogenic differentiation more in detail by measuring various markers related. We first came into the conclusion that EGCG induces osteogenic gene expression synergetically with mechanical stretch. Specifically, EGCG with mechanical stretching upregulated BMP-2 and VEGF followed by high expression of RUNX2. Also, EGCG was found to be closely related to mitochondria physiology. It increased mitochondria antioxidant function and biogenesis. It was also confirmed that fission and fusion activity of mitochondria was increased.
Although it was confirmed that EGCG has synergistic role in osteogenic differentiation induced by mechanical stretch, this in vitro study still has left much for safe clinical applications. Through long-term and animal experiments the evaluation and validation of EGCG roles in osteogenic differentiation can be clarified, especially in bone tissue regeneration. Also, other unexpected side effects should be examined through further investigation.
Acknowledgements
This work was supported by the Human Resource Training Program for Regional Innovation and Creativity through the Ministry of Education and National Research Foundation of Korea (NRF-2014H1C1A1073148) and by the National Research Foundation of Korea (NRF) Grant (NRF-2014K2A2A7066637).
Conflicts of interest
The authors have no financial conflicts of interest.
Ethical Statement
There are no animal experiments carried out for this article.
References
- 1.Sun Q, Zhang Q, Sun Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014;1:113–119. doi: 10.1016/j.gendis.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin Y, Guan J, Zhang C. Mesenchymal stem cells: mechanisms and role in bone regeneration. Phostgrad Med. 2014;90:643–647. doi: 10.1136/postgradmedj-2013-132387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antebi B, Pelled G, Gazit D. Stem cell therapy for osteoporosis. Curr Osteoporos Rep. 2014;12:41–47. doi: 10.1007/s11914-013-0184-x. [DOI] [PubMed] [Google Scholar]
- 7.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Abdeen AA, Huang TH, Kilian KA. Controlling cell geometry on substrates of variable stiffness can tune the degree of osteogenesis in human mesenchymal stem cells. J Mech Behav Biomed Mater. 2014;38:209–2018. doi: 10.1016/j.jmbbm.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Huang CH, Chen MH, Young TH, Jeng JH, Chen YJ. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem. 2009;108:1263–1273. doi: 10.1002/jcb.22356. [DOI] [PubMed] [Google Scholar]
- 10.Jang JY, Lee SW, Park SH, Shin JW, Mun C, Kim SH, et al. Combined effects of surface morphology and mechanical straining magnitudes on the differentiation of mesenchymal stem cells without using biochemical reagents. J Biomed Biotechnol. 2011;2011:860652. doi: 10.1155/2011/860652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaine-Smith RM, Reilly GC. Mesenchymal stem cell responses to mechanical stimuli. Muscles Ligament Tendons J. 2012;2:169–180. [PMC free article] [PubMed] [Google Scholar]
- 12.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5:713–724. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JC, Jacobs CR. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res Ther. 2013;4:107–724. doi: 10.1186/scrt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du GJ, Zhang Z, Wen XD, Yu C, Calway T, Yuan CS, et al. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4:1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mähler A, Mandel S, Lorenz M, Ruegg U, Wanker EE, Boschmann M, et al. Epigallocatechin-3-gallate: a useful, effective and safe clinical approach for targeted prevention and individualized treatment of neurological diseases? EPMA J. 2013;4:5. doi: 10.1186/1878-5085-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen CL, Yeh JK, Cao JJ, Wang JS. Green tea and bone metabolism. Nutr Res. 2009;29:437–456. doi: 10.1016/j.nutres.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin P, Li M, Xu G, Zhang K, Zheng L, Zhao J. Role of (−)-epigallocatechin-3-gallate in the osteogenic differentiation of human bone marrow mesenchymal stem cells: an enhancer or an inducer? Exp Ther Med. 2015;10:828–834. doi: 10.3892/etm.2015.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin P, Wu H, Xu G, Zheng L, Zhao J. Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: an in vitro study. Cell Tissue Res. 2014;356:381–390. doi: 10.1007/s00441-014-1797-9. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005;16:2039–2045. doi: 10.1007/s00198-005-1995-0. [DOI] [PubMed] [Google Scholar]
- 21.Mah YJ, Song JS, Kim SO, Lee JH, Jeon M, Jung UW, et al. The effect of epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both in vitro and in vivo. Arch Oral Biol. 2014;59:539–549. doi: 10.1016/j.archoralbio.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Kamon M, Zhao R, Sakmoto K. Green tea polyphenol (−)-epigallocatechin gallate suppressed the differentiation of murine osteoblastic MC3T3-E1 cells. Cell Biol Int. 2009;34:109–116. doi: 10.1042/CBI20090011. [DOI] [PubMed] [Google Scholar]
- 23.Shin JW, Kim HL, Kang YG, Park SH, Kim YM, Shin JW. Changes in mitochondrial properties during stem cell differentiation induced by mechanical stretching. Tissue Eng Regen Med. 2015;12:60–65. doi: 10.1007/s13770-015-0432-5. [DOI] [Google Scholar]
- 24.Parker GC, Acsadi G, Brenner CA. Mitochondria: determinants of stem cell fate? Stem Cells Dev. 2009;18:803–806. doi: 10.1089/scd.2009.1806.edi. [DOI] [PubMed] [Google Scholar]
- 25.Lin Z, Wang JS, Lin L, Zhang J, Liu Y, Shuai M, et al. Effects of BMP2 and VEGF165 on the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Exp Ther Med. 2014;7:625–629. doi: 10.3892/etm.2013.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tachi K, Takami M, Sato H, Mochizuki A, Zhao B, Miyamoto Y, et al. Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-β1. Tissue Eng A. 2011;17:597–606. doi: 10.1089/ten.tea.2010.0094. [DOI] [PubMed] [Google Scholar]
- 27.Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, et al. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech Dev. 2001;106:97–106. doi: 10.1016/S0925-4773(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Zhu C, Wu Y, Ye D, Wang S, Zou D, et al. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur Cells Mater. 2014;27:1–11. doi: 10.22203/eCM.v027a01. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T. Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238:574–580. doi: 10.1006/bbrc.1997.7325. [DOI] [PubMed] [Google Scholar]
- 30.Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res. 2012;111:1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- 31.Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. J Mol Med (Berl). 2010;88:981–986. doi: 10.1007/s00109-010-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schieke SM, Ma M, Cao L, McCoy JP, Jr, Liu C, Hensel NF, et al. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem. 2008;283:28506–28512. doi: 10.1074/jbc.M802763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkerson DC, Sankar U. Mitochondria: a sulfhydryl oxidase and fission GTPase connect mitochondrial dynamics with pluripotency in embryonic stem cells. Int J Biochem Cell Biol. 2011;43:1252–1256. doi: 10.1016/j.biocel.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 35.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–874. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 36.Shin JW, Park SH, Kang YG, Wu Y, Choi HJ, Shin JW. Changes, and the relevance thereof, in mitochondrial morphology during differentiation into endothelial cells. PLoS ONE. 2016;11:e0161015. doi: 10.1371/journal.pone.0161015. [DOI] [PMC free article] [PubMed] [Google Scholar]