Abstract
Solid freeform techniques are revolutionising technology with great potential to fabricate highly organized biodegradable scaffolds for damaged tissues and organs. Scaffolds fabricated via Solid freeform (SFF) techniques have more pronounced effect in bone tissue engineering. SFF techniques produce various types of scaffolds from different biomaterials with specific pore size, geometries, orientation, interconnectivity and anatomical shapes. Scaffolds needs to be designed from such biomaterials which can attach directly to natural tissues and mimic its properties, so ideally mechanical properties of scaffolds should be same as that of regenerating tissues for best results. The scaffolds designed without optimized mechanical properties would lead to the reduced nutrition diffusion within tissue engineered constructs (TECs) causing tissue necrosis. These scaffolds are mainly processed from ceramics and polymers like calcium phosphate, polydioxane, €-polycaprolactone, polylactic and polyglycolic acids etc. While, hydrogel scaffolds provide bridge for encapsulated cells and tissues to integrate with natural ECM. Likewise, 2D images from radiography were not sufficient for the prediction of the brain structure, cranial nerves, vessel and architecture of base of the skull and bones, which became possible using the 3D prototyping technologies. Any misrepresentation can lead to fatal outcomes. Biomodelling from these techniques for spinal surgery and preoperative planning are making its way toward successful treatment of several spinal deformities and spinal tumor. In this review we explored laser based and printing SFF techniques following its methodologies, principles and most recent areas of application with its achievements and possible challenges faced during its applications.
Keywords: Solid Freeform Techniques, Stereolithography, Selective laser sintering, 3D Printing, Fused Deposition Modelling
Introduction
The limitation of medical devices in terms of biocompatibility, device performance and failure led to advent of technologies for scaffolds manufacturing. These scaffolds grow, develop and can be implanted inside the host body. Prior organs are not easily available and the available one may lead to the immune response within host tissues. To avoid such problems tissue engineering has created its way in repairing the damaged body tissues through fabrication of similar tissue graft without any requirement of immunosuppressant in the host.
Scaffolds are highly porous three dimensional constructs provides surface for cells attachment, multiplication and proliferation. Increase in cell count develops structural and functional proteins and polysaccharides homologous to the ECM of living tissues. Hutmacher took pioneering step in analysing the requirements for suitable and compatible scaffold material and reviewed its manufacturing methodologies [1]. Ideally tissue engineered scaffolds should possess following characteristics (i) biocompatible, biodegradable and bioactive or bioresorbable (ii) highly interconnected porosity (iii) tailored properties and dynamic architecture for specific site application (iv) suitable surface chemistry for cell growth, attachment and proliferation, (v) desired or required mechanical properties for better implants results. The mechanical parameters that should be considered before fabricating any scaffolds for bone tissue engineering are: scaffold’s volume fraction, scaffold bone volume, total bone volume, scaffold bone mineral density and total bone mineral density. Need for mechanical integrity and customized shapes has given rise to laser based technologies, commonly known as Solid freeform technology (SFF).
Solid Freeform technology also referred to as Rapid Prototyping or 3D manufacturing and categorised into laser based technologies and printing technologies. 3D designs are developed using computer software in several slices. These slices were then fabricated in layers using various printing technologies and consolidated into a single 3D structure. For solid object fabrication materials are added in layers on the basis of provided coordinates, thus known as additive manufacturing technology.
Computer aided design (CAD) with additive manufacturing technologies provides better control over the complex geometry fabrication of rationale scaffolds for specific tissues and organs [2]. Anatomical complexity of human being differs from head to toe. Bones from ear and spine are tremendously complicated in terms of operating and placement of scaffolds for its regeneration. Therefore, achieving exact mechanical capabilities and load bearing as natural bone tissues is still one of the challenges to overcome [3].
SFF techniques are capable of fabricating biodegradable scaffolds having greater potency to attach directly to bone tissues. These scaffolds are mainly processed from wide range of biomaterials (Table 1).
Table 1.
SFF techniques used to process different biomaterials to fabricate scaffolds for specific applications
| Biomaterials | Applied SFF Techniques | Advantages | Disadvantages | Potential Applications |
|---|---|---|---|---|
| Poly(propylene fumarate) [38, 39] | SLA | Naturally derived polymer, controllable mechanical properties | It is viscous at room temperature making its handling difficult | Bone Tissue Regeneration |
| Photo cross-linkable polyethylene glycol dimethacrylate [43] | SLA | Provides better cell adhesion and spatial segregation of biological factors | It has low porosity and interconnectivity. Only simple shapes like sheets and tube | Scaffolds fabricated analogous to ECM |
| Polyethylene glycol (PEG) with photo cross-linked hydrogels [44, 49] | SLA | Better spatial patterning of micro-fabricated scaffolds | It lacks significant mechanical strength | Culturing osteocytes and chondrocytes |
| Ceramics or bioglasses | SLA | Have greater potency to attach directly to bone tissues | Synthetic material, cannot be used inside the host | Biomodelling |
| Bioceramics, poly(e-caprolactone), PLLA [61, 62] | SLS | Higher compliance with SLS fabrication technique and are biocompatible Can be blended with other low melting point material to act as an adhesive for making implants |
It cannot be processed in presence of binders such as thermoplastics using commercial laser | Bone implants |
| UHMWPE [73] | SLS | Highly interconnected and porous | Limited availability | Customized implants |
| Polycaprolactone | SLS | Higher compliance with SLS to fabricate 3D scaffold mould In association with Hydroxyapatite provide appropriate mechanical properties No cytotoxic effect |
Complexity of material increases when used in combination with other compound | Bone and cartilage repair |
| Calcium Phosphate like hydroxyapatite, tricalcium phosphate | SLS | Osteoconductive and bioactive Can easily be associated biopolymers such as chitosan and collagen to provide natural bone structure |
Lack of mechanical flexibility | Nano-sized composite |
| Polystyrene [51] | SLS, 3DP | Used for planning surgical process by fabricating corresponding models Prevent unexpected malfunctioning during spinal surgery |
Poor mechanical properties | Spine or vertebral model for surgical process |
| Hydroxyapatite | Direct Writing | Microporousand osteoconductive properties helps in mimicking the natural structure of bone | Cytotoxic and has issue with biocompatibility Poor mechanical properties |
Bone Fillers |
| Ca-P, PLL | FDM | Carry favourable kinetics to provide strength and stiffness with feasibility of incorporation and immunization of growth factors | Limited availability | Bone engineering (provides suitable biochemical and mechanical properties) |
| PolybutaleneTerephthalate | FDM | Strengthens mechanical properties | Do not biomimic the natural bone functionality | Trabecular bone scaffolds |
| Nanohydroxyappatite | FDM | Mechanical properties of natural bone can be evaluated and functionality can be mimicked | Non-biodegradable | Bone repair |
| Calcium Phosphate hemihydrate | Indirect SFF | High resolution porous scaffolds can be fabricated Used in fabrication of 3D porous moulds |
Does no depend on composition and it is mechanically weak | Bone implants |
| Collagen Type I | Indirect SFF with 3DP | Have interlinked internal channels No cytotoxic effect |
Limited donor sites | Attachments for human mesenchymal stem cells |
Laser based SFF techniques
Stereolithography [SLA]
Stereolithography is one of the most popular and highly accurate additive manufacturing process. It is pioneering rapid prototyping industry, used for fabricating complex tissue engineered constructs and scaffolds by selectively adding materials layer by layer. SLA is highly versatile in designing structures at various scales ranging from sub-micron size to decimetre size. It usually involves ultraviolet curable liquid photo-sensitive polymer. This polymer is cured or solidified selectively by a laser beam vector scanned across its surface. This technique has best resolution and accuracy among all SFF techniques and is easily employed in living cells, hydrogels, polymers, ceramics and composites [4].
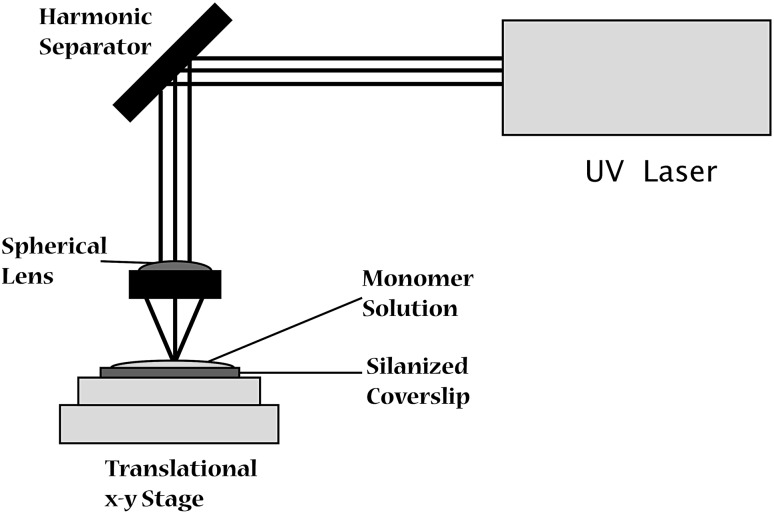
In SLA technique, fabrication of parts begins with CAD file, while geometry of the object to be designed can also be complemented with mathematical models and equations [5]. CAD generates a STL file which constitutes a set of coordinates to create 3D design. These designs are sliced into layers of 25–100 µm thickness for fabrication [4]. Stereolithography apparatus is loaded with this data, consisting of a liquid photo-polymerisable polymer bath. The ultraviolet laser focused and vector scanned over to liquid bath where polymerization initiates (Fig. 1). The polymerization by tracing laser beams leads to curing of material and provides first solid layer. Once the pattern is traced, the SLA’s elevator platform provides room for the next layer by shifting down to equal distance as the thickness of previous layer. A new layer of polymer is spread over previous structure and process is repeated until 3D object is completely designed. Then model is taken out and excess polymer drained off. The model obtained is solid but soft and delicate so cured in ultraviolet oven. Now finishing is done by removing surface irregularities or excess of resin by immersing in chemical bath [4].
Fig. 1.
Schematics of Stereolithography process [4]
SLA combined with mask projection excimer laser has appealing capabilities of fabricating dynamic millimetre size, patterned and biodegradable 3D scaffolds for regenerative health care. Most materials used in stereolithography are epoxy resins, thermoplastic, elastomer and PEG based hydrogel. Poly(propylene fumarate) (PPF) and other fumarate based scaffolds are used mainly in bone tissue regeneration due to their controllable mechanical properties [6, 7]. Recently, PPF/DEF resins with bisacrylphosphorine (photoinitiator) have been used to fabricate 3D prototype using SLA technique to repair critical sized defects [8, 9]. This methodology although convenient for fabrication under optimal laser parameters, but their feasibility compared to traditional SLA not yet investigated.
The fundamental limitation in 3D scaffold designing is developing an environment analogous to living tissues such as ligands patterning, extracellular matrix formation, controlled release of various response factors, etc. [10]. This limitation was sorted out by using photo-cross-linkable polyethylene glycol dimethacrylate in SLA for scaffolds fabrication which provides better cell adhesion and spatial segregation of biological factors [11]. PEG acrylate monomers constitute of soluble and suspended particle also provides better spatial patterning of micro-fabricated scaffolds. PEG in association with photo-cross-linked hydrogels is used to culture osteocytes and chondrocytes in bone tissue engineering [12–14].
Stereolithographic biomodelling is latest technology for designing exact replicas of human anatomical structure from 3D data of CT scans. SLA use for biomodelling complex spine structure have enhanced assistance to surgeons for pre-operative diagnosis, surgical planning, highly resolved knowledge of deformity, morphological assessment of spinal pathology, efficient implant and screw positioning, identifying the hidden hemivertebra, possible surgical interventions and risks (Figs. 2, 3, 4) [15]. The lumber polystyrene model prepared using this technology based on 3D reconstruction digital spine data provided by Mimics 6.3 software for treatment of lumber herniation [16]. Virtual models of spine for disease scoliosis and tortecolis was also prepared for planning surgery and understand clearly any abnormality in patient’s spine [17].
Fig. 2.
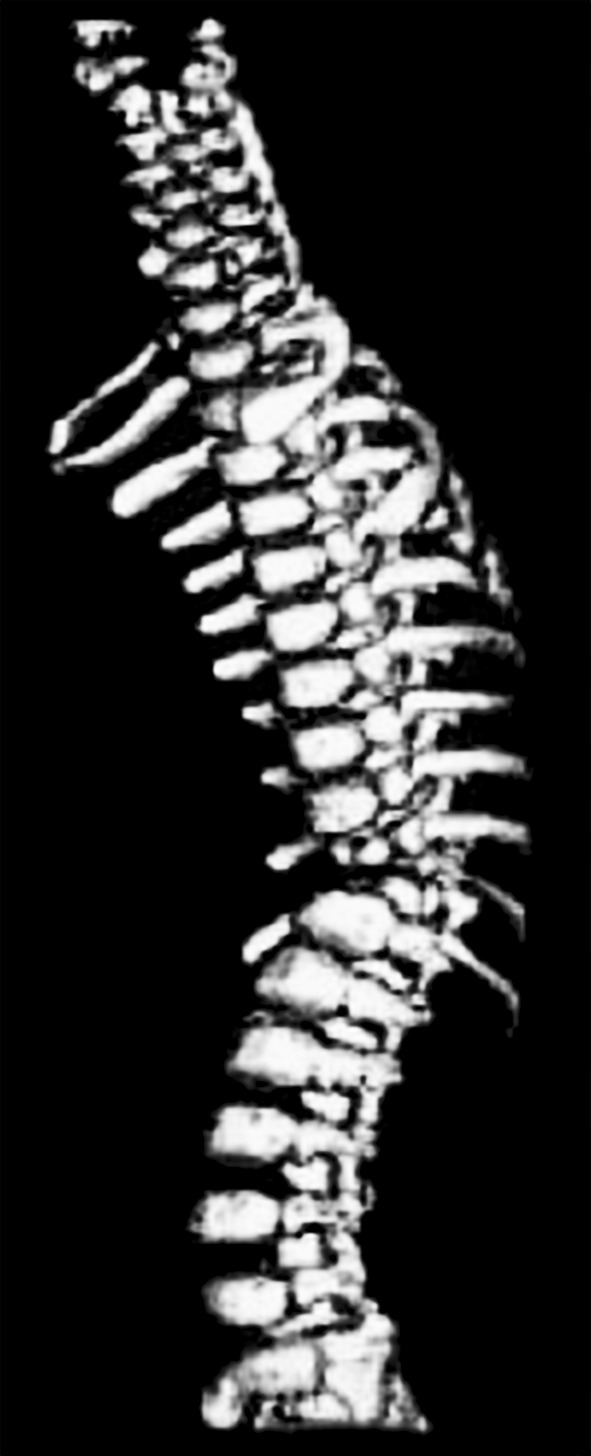
3D model of thoracic vertebra fron CT scan data of nearly 2 years old child having acute thoracic kyphosis at spine T10/T11 [15]
Fig. 3.
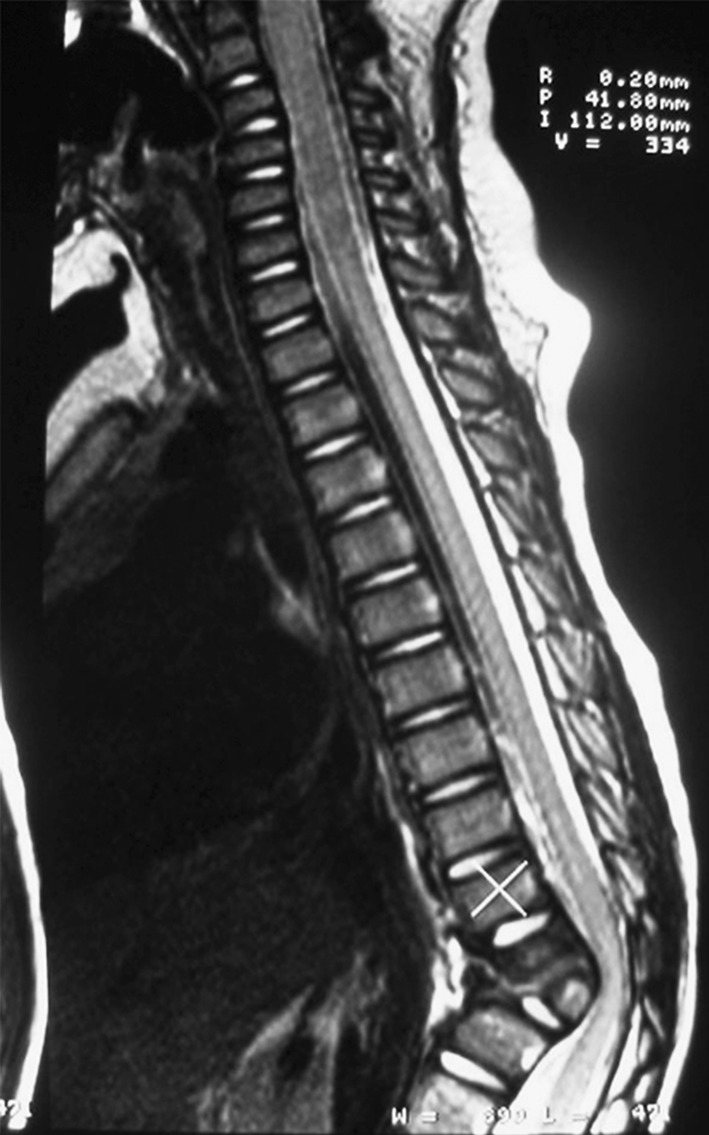
Two hemivertebra obseved at lower thoracic spine from MRI [15]
Fig. 4.
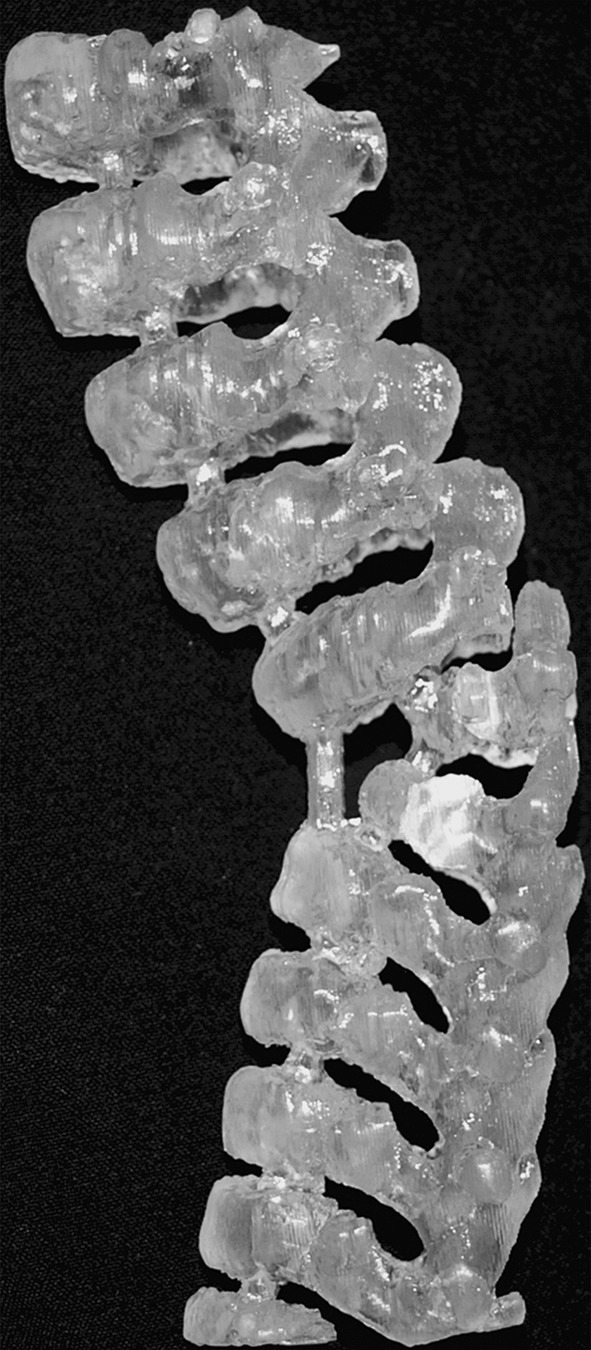
3D model of lower spine from MRI data showing distinctly two hemivertebra [15]
Stereolithography for fabricating components of thoracic lumber sacral orthosis used for the treatment of back injuries, chest injuries, spinal deformities, post-surgical immobilization, hemiated disk, spinal stenosis, spondylolisthesis, compression fracture, degenerative spinal pathologies such as osteoporosis. Stereolithographically fabricated 3D scaffolds from biomaterials such as PPF, DEF, and phenylbis (2, 4, 6-trimethylbenzoyl)-phosphine oxide (BAPO) with controlled microstructure are used in several tissue engineering application [18, 19]. 3D scaffolds from polyethylene oxide and polyethylene glycol (photopolymerizable hydrogels) has already been used commercially using stereolithography technique.
Advantages: (1) One of the biggest advantages of using stereolithography is its speed. Constructs are fabricated very shortly. (2) Relatively smoother surfaces are created compared to other SFF techniques. (3) Medical applications are possible due to creation of low volume water resistant constructs with complex geometries. (4) The prototypes designed have enough mechanical strength to be machined or tailored.
Limitations: (1) SLA is very expensive as photo-curable resins and its tailoring cost is high. (2) Scarcity of biomaterials with application specific elasticity and mechanical strength for biological application.
Selective laser sintering [SLS]
The selective laser sintering process was developed and patented in 1980 by Carl Deckard and Dr. Joe Beaman. It is a rapid prototyping technology in which small particle, ceramics or glass are fused together using high power laser to form 3D structure. The physical 3D objects created from 3D CAD data which provides geometry for tracing laser in SLS. Several biomaterials used in tissue engineering application have been successfully fabricated into scaffolds or TECs using SLS.
SLS is an additive manufacturing technology, employ powdered nylon 11, nylon 12, polyamides, polyetheretherketone (PEEK) and nylon with fillers (glass beads or carbon fibres) to form highly durable light objects. Carbon Dioxide laser is mainly used in this process. A typical pulsed laser is used in SLS, as the density of prototype developed depends on peak laser power while independent of laser duration.
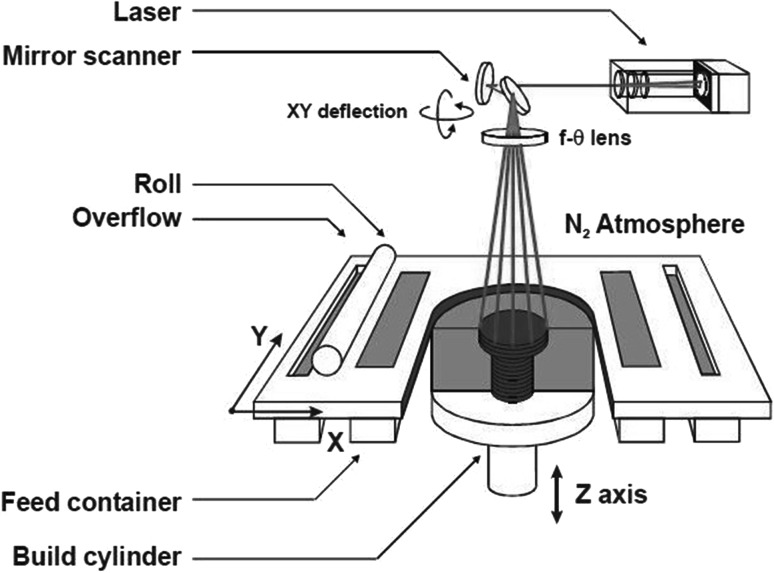
The SLS process begins by splitting 3D CAD data into the layers. The data is then transferred to SLS additive manufacturing machine. It preheats the powdered material spread by a levelling roller on the bed, slightly below their melting point. This eases tracing laser to raise the temperature to melting point of material over selected areas. A CO2 laser traces the cross-section on the material. As the laser scans the surface, material is sintered and first layer is created. Once single layer is complete, the powdered bed is lowered to make space for the next layer. Now more material is introduced from the powdered cartridge and rolled out smoothly, while unused is recycled (Fig. 5). The process is repeated layer by layer until the part is completed. As the parts are built, they are encompassed by unsintered powder which provides supplementary strength and eliminates the need for support structure. The constructs produced possess some defect such as uneven surfaces, lumps, cracks etc. Post processing is required for surface smoothening and enhancing the physical and mechanical properties. Hence high degree of integrity and compactness is achieved [20].
Fig. 5.
Experimental setup of SLS process [20]
Selective laser sintering can be of two types i.e. indirect and direct. In indirect SLS, metal powder is coated with polymer of 5mm thickness. While, in direct SLS two different grain size of same material is used where a low melting point component is melted and taken as matrixes base for higher melting point component. Dewidar et al 2008 stated the limitation of direct SLS for producing composite which are weak and lack mechanical strength [21].
Several physical and mechanical parameters affect the performance, functionality and accuracy of SLS. The physical parameters includes frequency pulsars, energy intensity laser scan size, scanning speed, surface temperature during scanning, laser performance, powder size, density, particulate mixture and volume of material [22, 23]. While, mechanical parameters are porosity, elasticity, strength, young’s modulus, roughness and depth [20].
Most of the biocompatible polymers comply with SLS fabrication technique. SLS has been successfully employed to fabricate the complex scaffolds for bone tissue engineering combining bioceramics with biopolymer such as poly(e-caprolactone) [24] and PLLA [25]. But, the major limitation of bioceramics is its inability to fuse in presence of thermoplastic (binder) during fabrication. Other biomaterials used for fabrication of composites are polylactideglycolide, polycaprolactone, PEEK, polyvinyl alcohol, etc. [26–29].
SLS is found to have potential to fabricate scaffolds with biomimetic structure, customized implants of ultrahigh molecular weight polyethylene (UHMWPE) and highly interconnected porous structure of PCL for bone and cartilage repair [24, 30]. Other biomaterials which are already used for scaffolds fabrication are PMMA, HA-PEEK and HA-PVA (polyvinyl alcohol) [31, 32]. Researchers have anticipated developing customized implants and fabricating scaffolds using high density polyethylene (HDPE) mixed with hydroxyapatite (HA). Shuai et al. investigated the effect of various sintering parameter and inspected microstructure of nanohydroxyapatite via X-Ray Diffraction, Fourier Transform infrared spectroscopy, scanning electron microscopy and reported their excellent potential in bone tissue engineering [33].
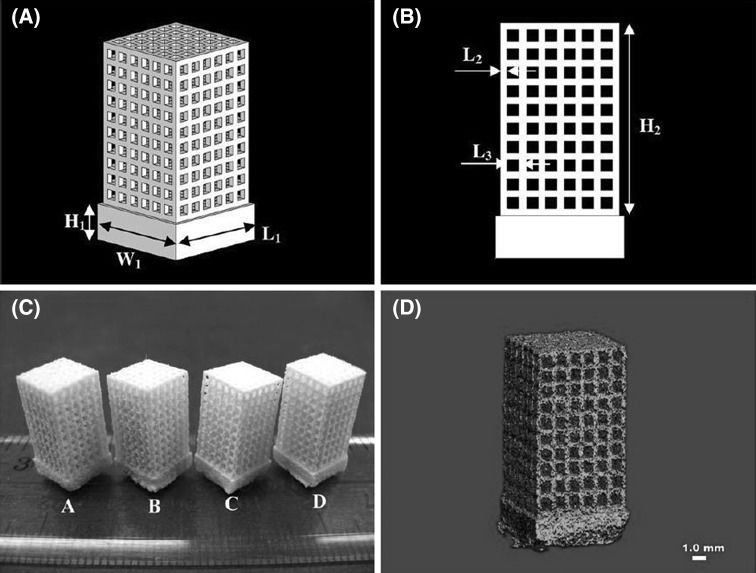
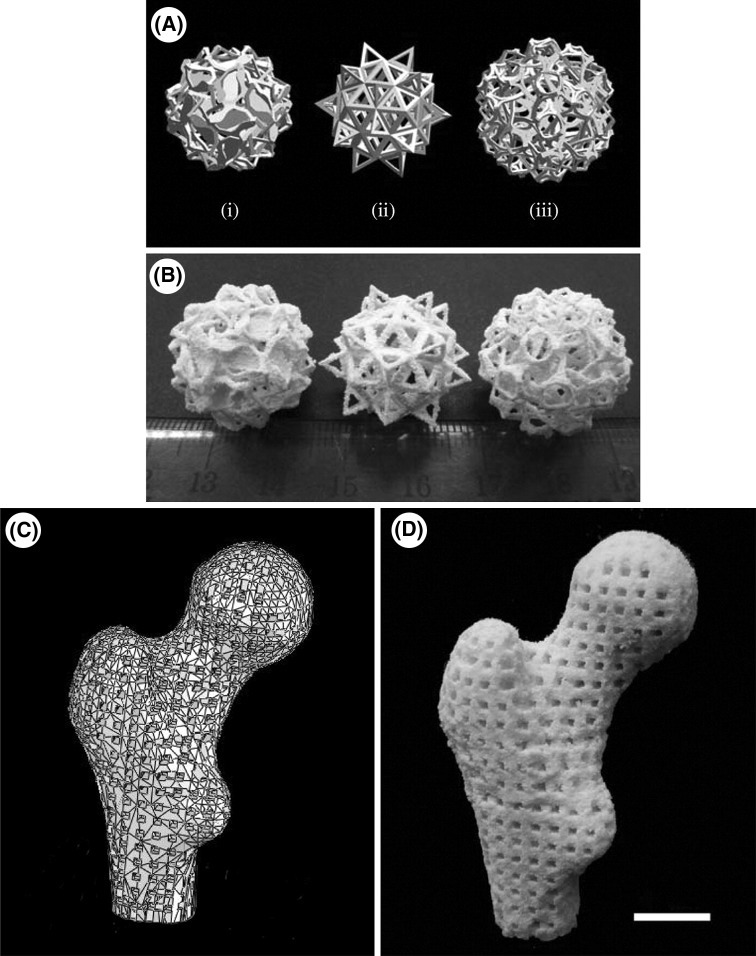
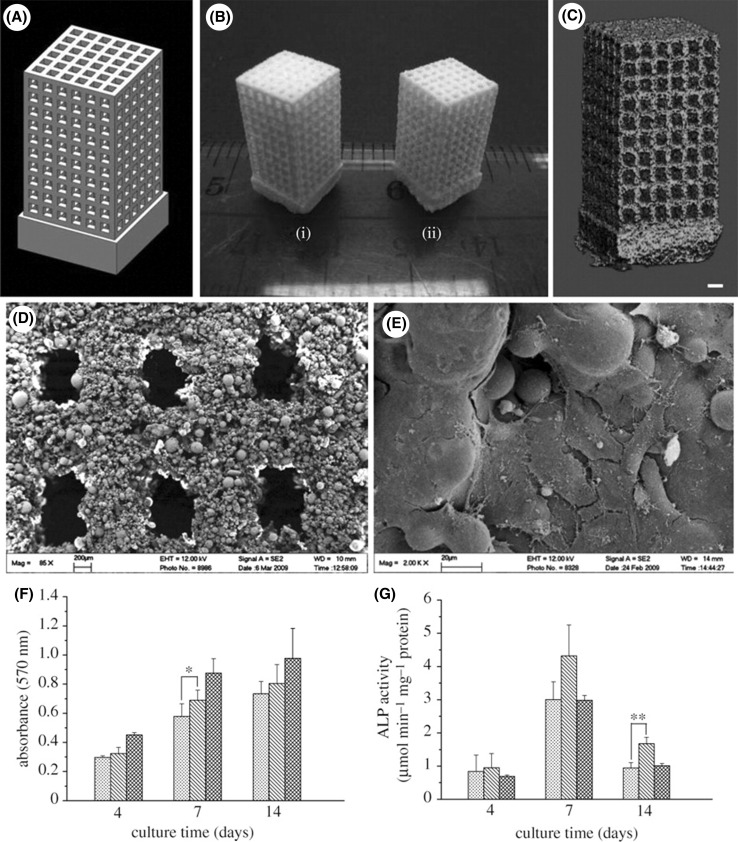
As the technological revolution is taking place, the requirement of biomaterials having small size, high surface area to volume ratio are increasing. The constructs or scaffolds designed via this type of material can mimic the properties, functionality and performance of natural bone tissues much better. Nano-sized composite scaffolds are one such example, made from calcium phosphate (Ca-Ps) like HA, tricalcium phosphate is osteoconductive and in association with biopolymers such as chitosan, collagen and PLLA provides natural bone structure [34, 35]. These scaffolds bears fully integrated porous structure and supports cell proliferation and cell viability. Bin 36 deeply studied the application of selective laser sintering in bone tissue engineering using nanomaterials to fabricate nanocomposite 3D scaffolds [36]. Designing complex nanostructure via SLS based on tissue images shown in Fig. 6. Human proximal condyle 3D scaffold model was build using SLS having pore size of 2mm (Fig. 7). Bin 36 had thoroughly studied the osteogenic differentiation of different bone cells obtained from mouse based on previous works done by MSCs [37, 38]. They anticipated achieving similar structural and functional similarities and found that C3H10T1/2 (Pluripotent stem cells) cells derived from mouse embryo, differentiated into similar primary bone marrow. It’s been inferred that scaffolds fabricated by combining nanocomposite material with sustained release of growth factor is very useful for bone tissue engineering (Fig. 8) [39].
Fig. 6.
A Schematic of scaffold structure. B lateral view of scaffolds. C SLS based scaffolds on scale [1] PHBV, [2] Ca-P/PHBV, [3] PLLA, [4] CHAp/PLLA. D Ca-P/PHBV 1mm scaffold image [36]
Fig. 7.
A Complex structure designed by Hart (i) slammanders, (ii) elevated icosidodecahedron, (iii) snarl. B porous Ca-P/PHBV nanocomposite via SLS based on Hart model. C 3D scaffold of human proximal femoral condyle from cubic cells using CT pictures. D Ca-P/PHBV nanocomposite of proximal femoral condyle post sintering [39]
Fig. 8.
A CAD model scheme B sintered scaffolds (i) PHBV based scaffold (ii) Ca-P/PHBV nanocomposite based scaffold C micro-CT picture of Ca-P/PHBV nanocomposite scaffold D Image of single layer of sintered Ca-P/PBHV obtained from SEM E Morphological structure of SaOS-2 cells cultured on Ca-P/PHBV nanocomposite scaffold for seven days [39]
Scaffolds fabricated from bioactive glasses have promising effect on repair and regeneration in bone tissue engineering. The properties of scaffolds associated with bone are strong, lightweight and highly interconnected porosity [40, 41]. The only limitation of using it as biomaterial for bone scaffold is low compressive strength, fracture toughness, pore interconnection and loadbearing capacity. Although this limitation has become less significant recently, as scaffolds of desired architecture and of comparable strength of human trabecular and cortical bone was fabricated by optimizing composition and sintering conditions [42, 43]. Qiang [42] recently reviewed on current progress in fabrication of bioactive glass scaffolds, their limitations due to mechanical strength, reliability and possible application in bone tissue engineering [42]. Several experiments were conducted on animals like rats and rabbits for investigation and evaluation of mechanical toughness and reliability of bioactive glass scaffolds fabricated using SLS [44].
SLS is also employed for fabrication of polystyrene model to treat severe spinal deformities. These models prevent unexpected malfunctioning during spinal surgery and other complications. Based on CAD data polystyrene spine model prepared for planning surgical process using selective laser sintering. The final model obtained possesses every finer internal and external detail. Since these models are subject specific and are prepared from the CT scan data of particular patient so they aid in inspecting complexity and abnormality such as bone defect and osteoproliferation position [16].
The advantage of using SLS is its speed, rigorous prototyping test, cost effective, no special tooling is required like casting.
Solid ground curing [SGC]
Solid ground curing is an additive manufacturing technology uses photopolymer for designing prototype and models fabrication. The SGC uses digital mask generators (e.g. liquid crystal display or digital mirror devices) to build structure out of polymer and ceramics. Unlike SLA or SLS, in solid ground curing each layer of photosensitive resin is hardened at once, thus provides high throughput production. Convention UV laser based SLA is not as versatile and cheaper in model fabrication as SGC in using digital light processing.
As previous fabrications, SGC too begin with designing CAD models and slicing it into layers. The base of the apparatus is spread with photosensitive resin. For each layer optical mask is generated similar to each cross-section. The photomask is levelled over the base and covered with a thin layer of liquid photopolymer. The photosensitive resin is then exposed to a powerful ultraviolet laser and cured. After curing excess resin removed and melted wax spread over to fill the cavities, followed by solidification to provide continuous support to fabricated structure. The wax on surface is then trimmed to accurate and desired finish using milling disk. Then a layer of liquid photopolymer is spreaded over the previous surface and its repeated until the top most layers are processed. Once all the layers are completed, wax melted down and removed. No post curing is required.
The major application of SGC in fabricating scaffolds based on gelation technology of biomolecules which are chemically altered with photo-dimerizable group. Only few applications of SGC based scaffolds are known, as this technology has relatively more consequential limitation than other SFF techniques [45, 46]. SGC does not require any support structure for model designing, as the voids are filled with wax. Thus provides good accuracy and high throughput. The system of SGC is highly complex, produces too much of wastes and extremely expensive so can’t be used as often as other techniques.
Printing SFF techniques
3D printing [3DP]
3D printing is an additive manufacturing method of creating 3D structures which are made by solidifying or depositing materials layer by layer, one top of another. The materials used are plastic, powder, ceramics, liquid metal or even biological cells. The solid objects are created using inkjet printers. It binds the materials from selective areas which specified by CAD information. A thin layer is created by materials on the surface of powder bed. Then powder bed makes room for the new layer of material along with maintaining proper geometry to avoid any errors when printer head moves along defined coordinates.
3D printing in medical has potential application to treat and diagnose various patient conditions. Number of recent article stated possible and successful application of 3DP in the areas of exoskeleton [47], Ears [48], bones [49], prostheses of jaw bone [50, 51], cell culture, blood vessel and vascular networks [52]. Recently a twelve week old sister joined at hips and lower spine is successfully separated using 3D printing technology. This technology provided two models based on CT and MRI scan of babies, which assisted and shown clear picture of starting and following the surgery.
3DP currently reached to height to print anything and in medical field it has already been used for printing prosthesis, devices and implants. Now researchers and neurosurgeons have been planning to dive into neurosurgery. Based on the two dimensional images obtained from the radiography, prediction of the brain structure, cranial nerves, vessels and architecture of base of the skull is very challenging. Any misrepresentation in the structure can lead to the fatal consequences. The models created by 3DP have not yet been used in neurosurgery but surgeons are planning to go forth, as they already used this technology to plan liver transplantation [53].
Recently an experiment was conducted by Japanese orthopaedic association on patients with lumber disk herniation who needs to undergo revision lumber discectomy. They divided these patients in two groups, first group treated with usual revision lumber discectomy while for second group 3DP is used to fabricate polystyrene vertebral model specific to particular subjects by scanning patient lumber region using CT scan. It was found that the group underwent surgery assisted with 3D printing technology took less operation time, reduced preoperative blood loss and postoperative complications [16].
Advantage of this technique is its higher dynamicity and speedy prototyping due to its layer by layer assembly of material to create scaffold models. 3D printing develops cheap and thousands of different model in almost every discipline. The successful application of 3DP includes titanium pelvis implanted to British patient, titanium lower jaw implanted to Belgium patient [54], plastic tracheal splint for an American infant [55]. 3DP is also used to build the face of the motorcyclist who injured seriously in a road accident [56]. There are several on-going researches to bioprint replacement for the lost tissues due to arthritis and cancer [57]. Prosthetic hands form 3DP is designed for five year old girl based on the plaster cast provided by her parent [58].
The limitation is that it rely on expensive hardware and materials, so designed part is costly and one at a time. Thus, competing with mass production technologies is hard. Removal of unwanted material from porous scaffolds is also difficult.
Future application of 3D Printing: Creating body parts and organs using biological cells as raw material to prevent rejection of developed organs. It can be used for in situ printing in which models or implants are printed inside the host body during operation. 3DP for cell and tissue culture and scaffolding has already been used to repair several types of deformities and lesion with precise digital control over thickness [59]. In situ bioprinting for repairing external organs, skin has already done. And even 3D printers are used for filling the skin lesions with keratinocytes and fibroblast in stratified region throughout the wound area [60, 61].
Cell printing: bio printing
Bio-printing is a 3D printing technology used to build organs and body parts. It is also known as organ printing, body part printing [62], computer aided tissue engineering [63], 3D tissue printing or cell printing. In this process living cells are deposited onto sugar matrix or gel medium in layers and gradually 3D structures including vascular systems are builded [64]. Organ printing is a self-assembly based tissue engineering technique where cellular aggregates are processed using any of the bioprinting or biofabrication technologies. The basic idea of organ printing is to allow cellular aggregates to fuse after deposition and building several small units of specific tissues. Organ printing using bioprinting is still under research purview.
Biofabrication is a technical term for bioprinting tissues and organs based on the techniques like 3D printing, stereolithography, bioplotting etc. Previously this was performed on 2D templates where cells are cultured in favourable environment. Later on laser light are used to guide cell proliferation onto the target. Spinal cord cells guided on glass surface through culture medium and remained viable even after exposed to laser light [65, 66].
Bioprinting can also be applied to cell and tissue patterning, scaffolds printing and organ printing. It provides better way to organize cells and molecules into 3D models with controlled functionality and desired density to mimic biological systems [67].
Extrusion technology/direct writing
Direct writing is layer by layer assembly of biomaterials by depositing a concentrated polyelectrolyte ink through micro capillary nozzle to produce flexible, inexpensive 3D scaffolds. It includes fused deposition modelling, 3D plotting, multi-phase jet solidification and precise extrusion manufacturing techniques. These are basically based on extrusion of materials layer by layer at specific coordinates to fabricate scaffolds. Variety of biomaterials can be used in these techniques to build various types scaffolds either specific or non-specific to host [68].
Hydroxyapatite (HA) based scaffolds are commonly used as bone fillers due to their osteoconductivity and possibility to mimic natural bone structure like trabecular bone. Recent studies emphasized mainly on direct write assembly of 3D structures for bone repair and regeneration. This methodology creates microporous HA array within scaffold to produce macro porous structure matching with trabecular bone. Thus, beneficial for bone replacement. Joshu et al. observed that scaffolds as HA rods when coated with new trabecular bone cells before filling the scaffolds radially within tissues gives better growth and differentiations of cells [69].
Fused deposition modelling [FDM]
Fused deposition modelling is a direct method of prototyping model parts or scaffolds by extruding small beads of thermoplastic material which solidifies instantly after extrusion to form layers. This process was developed in late 1980s by S. Scott and within few years it became supporting and complementary to other printing technologies. Several polymers in the form of filament are wound on coil and unreeled for material supply to extrusion nozzle head. Nozzle head gets heated to change polymer filament into molten state, allowing the flow on surface in both horizontal and vertical directions and hardens over it. The nozzle head movement coordinates is represented by CAM software and controlled by microcontroller. The polymers used are acrylonitrile butadiene styrene, polycarbonates, PLA, nylon, plasticine, RTV silicone, high density polyethylene, PC/ABS, polyphenylsulfone and high impact polystyrene. FDM can only be used for thermoplastics and encapsulation of living cells cannot be possible during fabrication of scaffolds.
FDM has been used in bone tissue engineering via utilizing scaffolds made from polymer like PCL and Ca-P composites. Ca-P provides suitable biochemical and mechanical properties while ceramics and polymers provide strength, physical stiffness and favourable kinetics. Another advantage is feasibility of incorporation and immunization of growth factors. Scaffolds of PCL and Ca-P for bone tissue engineering reported well anticipated results [70]. Trabecular bone scaffolds are fabricated from FDM using unfilled polybutylene terephthalate, although this scaffolds does not mimic the natural bone functionality and chemical composition but mechanical properties and microstructure are close enough to trabecular bone [70].
Like other SFF techniques FDM had also been used for making anatomical replicas for surgical planning based on computer tomography data. CT data provide precise anatomical geometry with highly resolved defects, determines bone shape and volume for 3D replicas, thus assist in further treatment [71]. Nanohydroxyapatite based 3D synthetic scaffolds for bone repair are prepared via CT guided FDM. They focused mainly on evaluating mechanical properties to mimic functionality of natural bone [72].
The FDM being used so frequently, as this technique of fabrication is cheaper since it uses plastic. The use of thermoplastic material is its major limitation which allows limited testing.
Indirect SFF
Indirect SFF is a method of creating negative mould and cast the scaffolds using desired polymers, ceramics or any other biomaterials. It can be used to create 3D structure with desired porosity. Moulds using collagen are dissolved in liquid ethanol and dried with liquid carbon dioxide to fabricate the collagen based scaffolds. Ceramic scaffold with porous 3D architecture shows bone formation and cell constructs.
Scaffolds designed using indirect SFF techniques are highly versatile and enhances functionality. The problem of mould inability to produce complex shapes and geometries during casting was also resolved with this technique. It allows use of various types of biomaterials along with different combination.
Indirect SFF employed for production of topologically ordered porous Mg (TOPM) structures which are biologically safe and relatively cheap for bone implants application [73–75]. Collagen type I composites constructed with or without hydroxyapatite fabricated by using indirect SFF based 3D printing, have interlinked internal channels [76]. These channels are shown to have good properties for cell attachment, so human mesenchymal stem cell (hMSCs) can proliferate and form new extracellular matrix without any cytotoxic affect [77].
Three dimensional scaffolds engineered microstructure of ceramics developed from indirect SFF based on stereolithography is said to be novel fabrication method for bone tissue engineering. These scaffolds are highly porous and interconnected with strong interfacial bonding among ceramic particles and sufficient mechanical strength [78, 79]. During cast preparation there may be some defects like cracks, errors in geometry or misalignment. So there should also be a proper way to remove mould without disturbing scaffold cast.
Gaps in research and major areas of debate
Solid freeform techniques have wide application in scaffolds fabrication for bone tissue engineering. It has evolved significantly in past few decades. Most researches were conducted to cure several deformities of human body. These deformities take longer time to heal using conventional medical techniques or when left untreated becomes fatal. Bone tissue engineering covers the major proportion of researches from scaffolds fabrication to its application. Despite of such a rigorous in these explorations, most researches seems to focus on specific section of body. Considering the stereolithography technique, its greatest achievement is to fabricate scaffolds for critical sized defects. But the focus on the treatment of major defects or major accidental defects seems to be relinquished. Anatomical models are prepared to treat such defects. It is obvious that fabrication of large and suitable scaffolds and cell culturing over is time taking process, so cannot be used in case of emergencies. This area needs to be reinforced to fill the gaps in such a field of extensive research. SLA, SLS, FDM and 3D Printing are used predominantly and frequently among all SFF Techniques. SLS is used mainly in making customized implants and 3D printing for designing surgical models. Developing analogous environment by pattering ligand and extracellular matrix for scaffolds fabrication is still not very reliable. It’s mostly been in controversies with the compatibility of biomaterials used in its long term application. In spinal bone tissue engineering polystyrene anatomical models are prepared to perform surgery. Treatment using scaffolds over these tissues are not yet successfully implemented.
Feasibility of fusing biopolymers, effect of laser light and post application possible side effects are major areas of debate in this field. Nanomaterials being small in size and properties entirely different from bulk materials can give new direction to SFF Techniques. Desired properties like toughness porosity for bones can easily be achieved.
Conclusion
The scaffolds prepared using SFF techniques are known to be analogous to normal tissues thus assist in wound healing and preparing the replicas for tissues or bony structures. It has capability to fabricate scaffolds using various kinds of biomaterials possessing different mechanical, physical and biochemical properties. Laser based SFF techniques provide best resolution and highly accurate scaffolds with least or no surface irregularities compared to printing technologies. The constructs produced are flexible and can be of any range from centimetre to nano scale thus having broad applications. Laser based technologies has advantage of fabricating complex geometries with least effort. So techniques like SLA and SLS has been widely used for same reason in almost every area.
Acknowledgements
The authors are thankful to Dr. T. Lahiri, Head of the Department of Applied Science, IIIT, Allahabad for encouragements.
Abbreviations
- SFF
Solid Freeform
- CAD
Computer Aided Design
- TECs
Tissue Engineered Constructs
- 3D
Three Dimensional
- 2D
Two Dimensional
- SLA
Stereolithography
- SLS
Selective laser sintering
- SGC
Solid ground curing
- 3DP
Three Dimensional Printing
- FDM
Fused Deposition Modelling
- CT
Computed Tomography
- ECM
Extracellular Matrix
- MRI
Magnetic Resonance Imaging
- PCL
Polycaprolactone
- PLA or PL
Polylactic Acid
- PEG
Polyethylene Glycol
- PLLA
Poly L Lactic Acid
- UHMWPE
Ultrahigh Molecular Weight Polyethylene
- HA
Hydroxyapatite
- PPF
Poly(propylene fumarate)
- DEF
Dilutent Diethyl Fumarate
- PEEK
Polyarylether-etherketone
- SEM
Scanning Electron Microscopy
- Ca-P/PHBV
Calcium Phosphate (Ca–P)/Poly(Hydroxybutyrate-Co-Hydroxyvalerate)
Conflict of interest
All related authors have asserted that there is no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/S0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 2.Melchels FPW, Domingos MAN, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012;37:1079–1104. doi: 10.1016/j.progpolymsci.2011.11.007. [DOI] [Google Scholar]
- 3.Bondy M, Altenhof W, Chen X, Snowdon A, Vrkljan B. Development of a finite element/multi-body model of anewborn infant for restraint analysis and design. Comput Methods Biomech Biomed Eng. 2014;17:149–162. doi: 10.1080/10255842.2012.672563. [DOI] [PubMed] [Google Scholar]
- 4.Melchels FPW, Feijen J, Grijpma DW. A review on stereolithography and its applications. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 5.Gabbrielli R, Turner IG, Bowen CR. Development of modelling methods for materials to be used as bone substitutes. Key Eng Mater. 2008;361–363:901–906. [Google Scholar]
- 6.Lee JW, Kang KS, Lee SH, Kim JY, Lee BK, Cho DW. Bone regeneration using a microstereolithography-produced customized poly (propylene fumarate)/diethyl fumarate photopolymer 3-D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials. 2011;32:744–752. doi: 10.1016/j.biomaterials.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 7.Wei C, Cai L, Sonawane B, Wang S. DongJ. High-precision flexible fabrication of tissue engineering scaffolds using distinct polymers. Biofabrication. 2012;4:025009. doi: 10.1088/1758-5082/4/2/025009. [DOI] [PubMed] [Google Scholar]
- 8.Cooke MN, Fisher JP, Dean D, Rimnac C, Mikos AG. Use of stereolithography to manufacture critical- sized 3D Biodegradable scaffolds for bone ingrowth. J Biomed Mater Res B. 2002;64:65–69. doi: 10.1002/jbm.b.10485. [DOI] [PubMed] [Google Scholar]
- 9.Fisher JP, Dean D, Mikos AG. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials. 2002;23:4333–43. doi: 10.1016/S0142-9612(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 10.Derby B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res. 2010;40:395–414. doi: 10.1146/annurev-matsci-070909-104502. [DOI] [Google Scholar]
- 11.Mapili G, Lu Y, Chen S, Roy K. Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds. J Biomed Mater Res Part B Appl Biomater. 2005;75B:414–424. doi: 10.1002/jbm.b.30325. [DOI] [PubMed] [Google Scholar]
- 12.Elisseeff J, McIntosh W, Anseth K, Riley S. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(SICI)1097-4636(200008)51:2<164::AID-JBM4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 14.Hedberg EL. Controlled release of an osteogenic peptide from injectable biodegradable polymeric composites. J Control Release. 2002;84:137–150. doi: 10.1016/S0168-3659(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 15.Izatt MT, Thorpe PLPJ, Thompson RG, D’Urso PS, Adam CJ, Earwaker JWS, Labrom RD, Askin GN. The use of physical biomodelling in complex spinal surgery. Eur Spine J. 2007;16:1507–1518. doi: 10.1007/s00586-006-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Yang M, Xie Y, Chen Z, Wang C, Bai Y, Zhu X, Li M. Application of the polystyrene model made by 3-D printing rapid prototyping technology for operation planning in revision lumbar discectomy. J OrthopSci. 2015;20:475–480. doi: 10.1007/s00776-015-0706-8. [DOI] [PubMed] [Google Scholar]
- 17.Ahlqvist A, Harrysson O, Conway T, Nayfeh J. Using stereolithography models in planning spinal surgery. In ASME 2002 International Mechanical Engineering Congress and Exposition 2002 Jan 1 (pp. 403–404). American Society of Mechanical Engineers.
- 18.Vlierberghe SV, Dubruel P, Schacht E. Biopolymer-based hydrogels as Scaffolds for tissue engineering application: a review. Biomacromolecules. 2011;12:1387–1408. doi: 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- 19.Lee KW, Wang S, Fox BC, Ritman EL, Yaszemski MJ, Lu L. Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: effects of resin formulations and laser parameters. Biomacromolecules. 2007;8:1077–1084. doi: 10.1021/bm060834v. [DOI] [PubMed] [Google Scholar]
- 20.Despa V, Gheorghe IG. Study of selective laser sintering: a qualitative and objective approach. Sci Bull Valahia Univ Mater Mech. 2011;6:150–155. [Google Scholar]
- 21.Dewidar MM, Lim JK. Properties of solid core and porous surface Ti–6Al–4V implants manufactured by powder metallurgy. J Alloys Comp. 2008;454:442–446. doi: 10.1016/j.jallcom.2006.12.143. [DOI] [Google Scholar]
- 22.Bourell DW, Marcus HL, Barlow JW, Beaman JJ. Selective laser sintering of metals and ceramics. Int J Powder Metall. 1992;28:369–381. [Google Scholar]
- 23.Seunarine K, Gadegaard N, Tormen M, Meredith DO, Riehle MO, Wilkinson CD. 3D polymer scaffolds for tissue engineering. Nanomedicine. 2006;1:281–296. doi: 10.2217/17435889.1.3.281. [DOI] [PubMed] [Google Scholar]
- 24.Williams JM, Adebisi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 25.Tan KH, Chua CK, Leong KF, Cheah CM, Gui WS, Tan WS, et al. Selective laser sintering of biocompatible polymers for applications in tissue engineering. Bio Med Mater Eng. 2005;15:113–124. [PubMed] [Google Scholar]
- 26.Wiria F, Chua C, Leong K, Quah Z, Chandrasekaran M, Lee M. Improved biocomposite development of poly(vinyl alcohol) and hydroxyapatite for tissue engineering scaffold fabrication using selective laser sintering. J Mater Sci Mater Med. 2008;19:989–996. doi: 10.1007/s10856-007-3176-5. [DOI] [PubMed] [Google Scholar]
- 27.Simpson RL, Wiria FE, Amis AA, Chua CK, Leong KF, Hansen UN, et al. Development of a 95/5 poly (L-lactide-co-glycolide)/hydroxyapatite and beta-tricalcium phosphate scaffold as bone replacement material via selective laser sintering. J Biomed Mater Res B Appl Biomater. 2008;84:17–25. doi: 10.1002/jbm.b.30839. [DOI] [PubMed] [Google Scholar]
- 28.Tan KH, Chua CK, Leong KF, Naing MW, Cheah CM. Fabrication and characterization of three-dimensional poly (ether-ether-ketone)/hydroxyapatite biocomposite scaffolds using laser sintering. ProcInstMechEng H J Eng Med. 2005;219:183–194. doi: 10.1243/095441105X9345. [DOI] [PubMed] [Google Scholar]
- 29.Savalani MM, Hao L, Zhang Y, Tanner KE, Harris RA, et al. Fabrication of porous bioactive structures using the selective laser sintering technique. Proc Inst Mech Eng H. 2007;221:873–886. doi: 10.1243/09544119JEIM232. [DOI] [PubMed] [Google Scholar]
- 30.Mao K, Wang Y, Xiao S, Liu Z, Zhang Y, Zhang X, et al. Clinical application of computer-designed polystyrene models in complex severe spinal deformities: a pilot study. Eur Spine J. 2010;19:797–802. doi: 10.1007/s00586-010-1359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen glycosaminoglycan scaffolds for tissue engineering. Biomaterials. 2010;31:461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 32.Chua CK, Leong KF, Tan KH, Wiria FE, Cheah CM. Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J Mater Sci Mater Med. 2004;15:1113–1121. doi: 10.1023/B:JMSM.0000046393.81449.a5. [DOI] [PubMed] [Google Scholar]
- 33.Shuai C, Gao C, Nie Y, Hu H, ZhouY Peng S. Structure and properties of nanohydroxypatite scaffolds for bone tissue engineering with a selective laser sintering system. Nanotechnology. 2011;22:285703. doi: 10.1088/0957-4484/22/28/285703. [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Han Z, Czernuszka JT. Gradient collagen/nanohydroxyapatite composite scaffold: development and characterization. Acta Biomater. 2009;5:661–669. doi: 10.1016/j.actbio.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 35.Xianmiao C, Yubao L, Yi Z, Li Z, Jidong L, Huanan W. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater Sci Eng C Biomimetic Supramol Syst. 2009;29:29–35. doi: 10.1016/j.msec.2008.05.008. [DOI] [Google Scholar]
- 36.Duan B, Wang M, Zhou WY, Cheung WL, Li ZY, Lu WW. Three-dimensional nanocomposites scaffolds fabricated via selected laser sintering for bone tissue engineering. Acta Biomater. 2010;6:4495–4505. doi: 10.1016/j.actbio.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Zhao L, Li G, Chan KM, Wang Y, Tang PF. Comparison of multipotent differentiation potentials of murineprimary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2. Calcif Tissue Int. 2009;84:56–64. doi: 10.1007/s00223-008-9189-3. [DOI] [PubMed] [Google Scholar]
- 38.Shea CM, Edgar C, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem. 2003;90:1112–1127. doi: 10.1002/jcb.10734. [DOI] [PubMed] [Google Scholar]
- 39.Duan B, Min W. Customized Ca–P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor. J R Soc Interface. 2010;7(S615):2. doi: 10.1098/rsif.2010.0127.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tampieri A, Celotti G, Sprio S, Delcogliano A, Franzese S. Porosity graded hydroxyapatite ceramics to replace natural bone. Biomaterials. 2001;22:1365–1370. doi: 10.1016/S0142-9612(00)00290-8. [DOI] [PubMed] [Google Scholar]
- 41.Werner J, Linner-Krcmar B, Friess W, Greil P. Mechanical properties and in-vitro cell compatibility of hydroxyapatite ceramics with graded post structure. Biomaterials. 2002;23:4285–4294. doi: 10.1016/S0142-9612(02)00191-6. [DOI] [PubMed] [Google Scholar]
- 42.Fu Q, Saiz E, Rahaman MN, Tomsia AP. Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives. Mater Sci Eng C Bio Appl. 2011;31:1245–1256. doi: 10.1016/j.msec.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Rahaman MN, Fu Q, Tomsia AP. Porous and strong bioactive glass (13–93) scaffolds prepared by unidirectional freezing of camphene-based suspensions. Acta Biomater. 2012;8:415–423. doi: 10.1016/j.actbio.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.HULL CW. Apparatus for production of three-dimensional objects by stereolithography. U.S. Patent No 4,575,330, 1986.
- 45.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/S0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 46.Liu VA, Jastromb WE, Bhatia SN. Engineering protein and cell adhesivity using PEO-terminated triblock polymers. J Biomed Mater Res. 2002;60:126–134. doi: 10.1002/jbm.10005. [DOI] [PubMed] [Google Scholar]
- 47.Haumont T, Rahman T, Sample W, King MM, Church C, Henley J, et al. Wilmington robotic exoskeleton: a novel device to maintain arm improvement in muscular disease. J Pediatr Orthop B. 2011;31:44–49. doi: 10.1097/BPO.0b013e31821f50b5. [DOI] [PubMed] [Google Scholar]
- 48.Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, et al. 3D printed bionic ears. Nano Lett. 2013;13:2634–2639. doi: 10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leukers B, GülkanH Irsen SH, Milz S, Tille C, Schieker M, et al. Hydroxyapatite scaffold for bone tissue engineering made by 3D printing. J Mater Sci Makter Med. 2005;16:1121–1124. doi: 10.1007/s10856-005-4716-5. [DOI] [PubMed] [Google Scholar]
- 50.Fielding GA, Bandyopadhyay A, Bose S. Effects of silica and zinc oxide doping on mechanical and biologicalpropertiesof 3D printed tricalcium phosphate tissue engineering scaffolds. Dent Mater. 2012;28:113–122. doi: 10.1016/j.dental.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller JS, Stevans KR, Yang MT, Baker BM, Nguyen DT, Cohen DM, Toro E, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geraldine TK, Yi L, Wang MY. 3D printing and neurosurgery—ready for prime time? World Neurosurg. 2013;80:233–235. doi: 10.1016/j.wneu.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Hart GW. Rapid prototyping web page. http://www.georgehart.com/rp/rp.html. Seen on 20-July-2015; 2015.
- 54.Transplant jaw made by 3D printer claimed as first (http://www.bbc.com/news/technology-16907104). BBC. 2012-02-06. Seen on 20-July-2015.
- 55.Stein R. Doctors use 3-D printing to help a baby breathe. (http://www.npr.org/blogs/health/2014/03/17/289042381/doctors-use-3-d-printing-to-help-a-baby-breathe). Seen on 20-July-2015; 2013.
- 56.Perry K. Man makes surgical history after having his shattered face rebuilt using 3Dprinted parts (http://www.telegraph.co.uk/health/10691753/Man-makes-surgical-history-after-having-his-shattered-face-rebuilt-using-3D-printed-parts.html/2014-03-12). Seen on 20-July-2015; 2014.
- 57.Research into Julie Williams, 3D-Bioprinting may soon produce transplantable human tissues. (http://www.3ders.org/articles/20140306-research-into-3d-bioprinting-may-soon-produce-transplantable-human-tissues.html) Seen on 20-July-2015; 2014.
- 58.BBC News. Inverness girl Hayley Fraser gets 3D-printed hand (http://www.bbc.co.uk/news/uk-scotland-highlands-islands-29441115). Seen on 20-July-2015; 2014.
- 59.Cui X, Boland T, D’Lima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul. 2012;6:149–155. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60:691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 61.Ventola CL. Medical applications for 3D printing: current and projected uses. P T. 2014;39:704–711. [PMC free article] [PubMed] [Google Scholar]
- 62.Building body parts with 3D printing”, The Engineer. (http://www.theengineer.co.uk/in-depth/analysis/building-body-parts-with-3d-printing/1002542.article) Seen on 20-July-2015; 2010.
- 63. Silverstein J. ‘Organ Printing’ Could Drastically Change Medicine (ABC News, 2006) (http://abcnews.go.com/Technology/story?id=1603783&page=1). Retrieved 2012-01-31. Seen on 20-July-2015; 2006.
- 64.D-printed sugar network to help grow artificial liver”, BBC. (http://www.bbc.com/news/technology-18677627) Seen on 20-July-2015; 2012.
- 65.Odde DJ, Renn MJ. Laser guided direct writing for applications in biotechnology. Trends Biotechnol. 1999;17:385–389. doi: 10.1016/S0167-7799(99)01355-4. [DOI] [PubMed] [Google Scholar]
- 66.Odde DJ, Renn MJ. Laser guided direct writing of living cells. Biotechnol Bioeng. 2000;67:312–318. doi: 10.1002/(SICI)1097-0290(20000205)67:3<312::AID-BIT7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 67.Mironov V, Reis N, Derby B. Bioprinting: a beginning. Tissue Eng. 2006;12:631–634. doi: 10.1089/ten.2006.12.631. [DOI] [PubMed] [Google Scholar]
- 68.Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biomtechnol. 2004;22:354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Simon JL, Michna S, Lewis JA, Rekow ED, Thompson VP, Smay JE. In vivo bone response to 3D periodic hydroxyapatites caffolds assembled by direct ink writing. Wiley InterScience. 2007;83:747–758. doi: 10.1002/jbm.a.31329. [DOI] [PubMed] [Google Scholar]
- 70.Endres M, Hutmacher DW, Salgado AJ, Kaps C, Ringe J, Reis RL, et al. Osteogenic induction of human bone marrow-derived mesenchymal progenitor cells in novel synthetic polymer-hydrogel matrices. Tissue Eng. 2003;9:689–702. doi: 10.1089/107632703768247386. [DOI] [PubMed] [Google Scholar]
- 71.Tellis BC, Szivek JA, Bliss CL, Margolis DS, Vaidyanathan RK, Calvert P. Trabecular scaffolds using miro CT guided fused deposition modelling. Mater SciEng C Mater BiolAppl. 2009;10:171–178. doi: 10.1016/j.msec.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sah MK, Sadanand J, Pramanik K. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair. ACS Appl Mater Interfaces. 2012;27:13–20. doi: 10.1021/am502716t. [DOI] [PubMed] [Google Scholar]
- 73.Xu N, Ye X, Wei D, Zhong J, Chen Y, Xu G, He D. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair. ACS Appl Mater Interfaces. 2014;6:14952–14963. doi: 10.1021/am502716t. [DOI] [PubMed] [Google Scholar]
- 74.Lipowiecki M, Brabazon D. Design of bone scaffolds structures for rapid prototyping with increased strength and osteoconductivity. Adv Mater Res. 2010;83–68:914–922. [Google Scholar]
- 75.Staiger MP, Kolbeinsson I, Kirkland NT, Nguyen T, Dias G, Woodfield TBF. Synthesis of topological ordered open cell porous magnesium. Mater Lett. 2010;64:2572–2574. doi: 10.1016/j.matlet.2010.08.049. [DOI] [Google Scholar]
- 76.Kang HW, Seol YJ, Cho DW. Development of an indirect solid freeform fabrication process based on microstereolithography for 3D porous scaffolds. Int J Stem Cells. 2010;3:85–95. doi: 10.15283/ijsc.2010.3.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wahl DA, Sachlos E, Liu C, Czernuszka JT. Controlling the processing of collagen hydroxyapatite scaffolds for bone tissue engineering. J Micromech Microeng. 2007;19:015011. doi: 10.1007/s10856-006-0682-9. [DOI] [PubMed] [Google Scholar]
- 78.Liu CZ, Xia ZD, Han ZW, Hulley PA, Triffitt JT, Czernuszka JT. Novel 3D collagen scaffolds fabricated by indirect printing technique for tissue engineering. J Biomed Mater Res B Appl Biomater. 2008;85:519–528. doi: 10.1002/jbm.b.30975. [DOI] [PubMed] [Google Scholar]
- 79.Yin L, Peng HX, Yang L, Su B. Fabrication of 3D inter-connective porous ceramics via ceramic green machining and bonding. J Europ Ceram Soc. 2008;28:531–537. doi: 10.1016/j.jeurceramsoc.2007.07.006. [DOI] [Google Scholar]