Abstract
Injury to podocytes is an early event in diabetic nephropathy leading to proteinuria with possible progression to end-stage renal failure. The podocytes are unique and highly specialized cells that cover the outer layer of kidney ultra-filtration barrier and play an important role in glomerular function. In the past few decades, adult stem cells, such as mesenchymal stem cells (MSCs) with a regenerative and differentiative capacity have been extensively used in cell-based therapies. In addition to their capability for regeneration and differentiation, MSCs contributes to their milieu by paracrine action of a series of growth factors via antiapoptotic, mitogenic and other cytokine actions that actively participate in treatment of podocyte damage through prevention of podocyte effacement, detachment and apoptosis. It is hoped that novel stem cell-based therapies will be developed in the future to prevent podocyte injury, thereby reducing the burden of kidney disease.
Keywords: Mesenchymal stem cells, Podocyte damage, Diabetic nephropathy, Kidney ultrafiltration, Glomerular function
Introduction
Diabetic complications comprise the dysfunction of several major organs, including kidney, heart, blood vessels, nerves and eyes leading to serious health problems such as nephropathy, cardiac dysfunction, atherosclerosis, neuropathy and retinopathy [1, 2]. Diabetic nephropathy (DN) is a complication of diabetes mellitus types 1 and 2 and is caused by the angiopathy of glomerular capillaries [3]. DN is a major cause of progressive kidney disease, about 20–40% of patients with diabetes develops evidence of nephropathy [4]. The earliest clinical hallmark of DN is microalbuminuria followed by thickening of glomerular basement membrane (GBM), glomerular hypertrophy and mesangial expansion leading to proteinuria, renal fibrosis, eventually progressing to irreversible end stage renal disease over years or decades. Currently we are short of effective medications for treatment of DN. However, control of hyperglycemia and hypertension at early stages of kidney damage is effective at retarding disease progression. Dialysis or kidney transplant might be helpful for treatment of renal failure. Unfortunately, clinical application of kidney transplantation is limited due to rejection by the immune system and shortage of kidney donors. An effective and safe method of treatment for DN is required. In recent years, stem cell therapy has become an attractive and novel therapeutic strategy for DN.
Numerous recent studies have indicated that podocyte injury is an early event in diabetes and highlighted the importance of podocytopathy in the pathogenesis of DN [5, 6]. In this review, we focused on the potential role of mesenchymal stem cells (MSCs) in the treatment of DN with particular emphasis on therapeutic effect of MSCs in diabetic podocyte injury.
Podocyte injury in DN
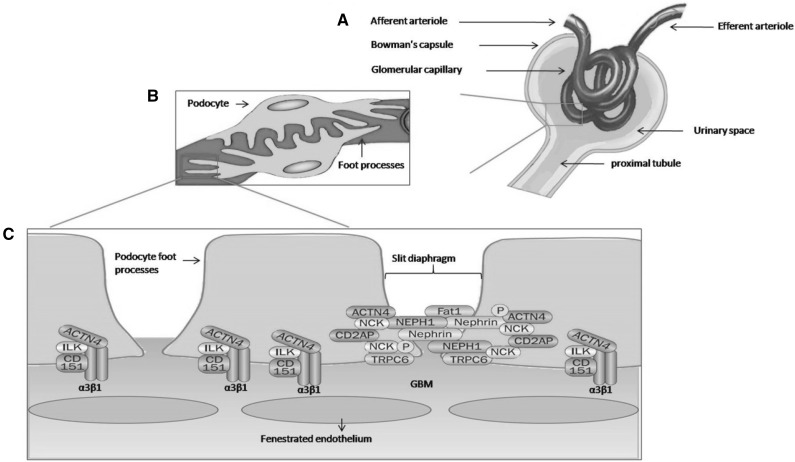
Many different cell types have been reported to undergo cellular changes in diabetes. Major cellular abnormalities were reported in podocytes. Podocytes are glomerular visceral epithelial cells. They are unique and highly specialized cells that cover the outer layer of kidney ultra-filtration barrier. They play an important role in glomerular function. The podocytes consist of three major structural parts: cell body, cell processes and foot processes (Fig. 1). The foot process is the most noticeable feature of podocytes. The interdigitated foot processes leave filtration slits in between, known as slit diaphragm, which establish a size-selective barrier to prevent proteinuria [7–9]. In addition to slit diaphragm proteins, several metabolic and endocrine factors modulated podocyte function, including growth hormones [10, 11], sex hormones [12], components of renin-angiotensin system [13–16], vitamin D [17], insulin [18] and adiponectin [19].
Fig. 1.
Structure of the glomerular filtration barrier. A) Fluids from blood in the glomerulus are collected in the Bowman’s capsule that empties into proximal tubules. B) The glomerular filtration barrier consist of three layers: the innermost fenestrated endothelium, the glomerular basement membrane (GBM), and the podocyte layer. C) The foot process of neighboring podocytes interconnected by several slit diaphragm molecules. Proteins that anchor foot processes to the GBM (α3β1 integrin, α-actinin-4 [ACTN4], integrin linked kinase [ILK], and the tetraspanin CD151) and those associated with the slit diaphragm (nephrin, NEPH1, podocin [P], Fat1, ACTN4, the adaptor protein NCK, CD2-associated protein [CD2AP], and transient receptor potential cation channel 6 [TRPC6]) are important in maintaining of filtration barrier
Numerous studies have been carried out to explore the role of podocytes in pathogenesis of DN. These studies demonstrated a positive correlation between diabetic proteinuria and podocyte injury [5, 20–22]. Foot process effacement, dedifferentiation and apoptosis have been identified as the main types of podocyte damage in DN [23–27].
An initial response of podocytes to any type of injury is a change in podocyte shape also known as podocyte foot effacement. Recent work showed that in diseases such as DN, alterations in the interaction of the podocyte actin cytoskeleton with slit pore proteins resulted in abnormally flattened and ‘spread-out’ podocyte shape with subsequent foot-process effacement and proteinuria [28, 29]. Podocytes lose contact with GBM as consequent to alterations in actin dynamics and podocyte–GBM interactions. Mutations in several genes encoding slit pore proteins, such as nephrin and podocin have been documented to result in nephrotic syndrome and proteinuria [25, 30]. Nephrin plays a major role in the integrity of podocyte actin cytoskeleton, podocyte survival signalling and insulin-stimulated glucose uptake [31]. In response to high glucose concentration, protein kinase C-alpha expression (PKC-α) increases which promotes nephrin endocytosis. This leads to a decline in nephrin surface expression and eventually contributes to proteinuria [32]. The second phenotype of podocyte injury in DN is dedifferentiation of podocytes causing them to lose their specialized feature as a glomerular filtration barrier [33].
Finally, apoptosis of podocytes have been shown to be associated with reduction of podocyte density in DN. Podocyte depletion in diabetic patients is the strongest predictor of progressive nephropathy and many clinical studies have documented reduced podocyte density in renal biopsy of individuals with type 1 or type 2 diabetes [34–39].
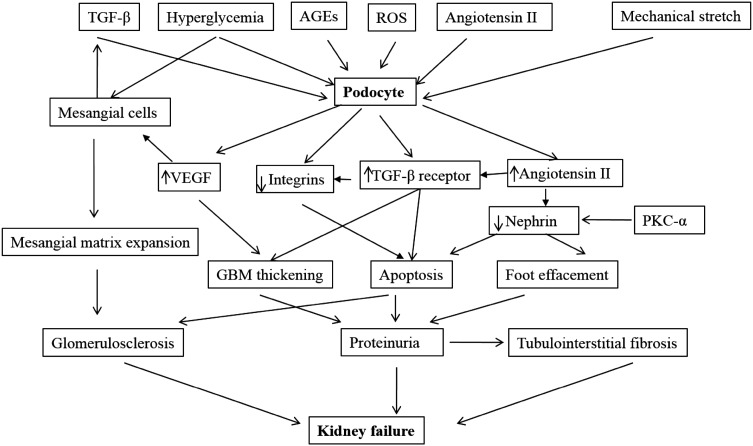
Chronic hyperglycemia and advanced glycation end products (AGEs) stimulate transforming growth factor beta (TGF-β) secretion in mesangial cells and expression of TGF-β receptor (TGF-β R’) in podocytes. Latent TGF-β complex may be stored in mesangial matrix and then localized to podocyte surface when activated by angiotensin II (Ang II) [40]. Over expression of TGF-β1 and mechanical stretch will suppress α3β1 integrin eventually resulting in decreased podocyte adhesion and apoptosis [41]. Another contributing factor to podocyte apoptosis in diabetes is activation of p38MAPK and caspase-3, mediated by reactive oxygen species (ROS). Role of ROS in this process and protective effect of various ROS inhibitors in dampening activation of caspase in vivo and in vitro have been shown by others [42].
Hyperglycemia also resulted in activation of angiotensin receptor type 1 in podocytes leading directly to apoptosis independent of blood pressure effects [43, 44]. Angiotensin-II upregulated cyclin-dependent kinase inhibitor (p27Kip1) culminating in cell cycle arrest and driving cells towards hypertrophy [45]. Vascular endothelial growth factor (VEGF) also contributed to podocytopathy in DN. During diabetes, podocytes induce VEGF up-regulation leading to overproduction of matrix in mesangial cells and diabetic glomerulosclerosis [40, 46]. Mechanisms of podocyte injury in DN are as shown (Fig. 2).
Fig. 2.
Podocyte damage in diabetic nephropathy. In diabetic condition, metabolic factors (TGF-β, glycated proteins, hyperglycemia, ROS, Angiotensin II (Ang II) and hemodynamic factors (via mechanical stretch) lead to increased VEGF and Ang II production by podocytes. Podocyte-derived VEGF leads to overproduction of matrix in mesangial cells and diabetic glomerulosclerosis. Chronic hyperglycemia and advanced glycation end products lead to TGF-β secretion in mesangial cells and expression of TGF-β R’ in podocytes. The latent TGF-β complex may be stored in mesangial matrix and then localized to the podocyte surface via activation by Ang II. The TGF-β type II receptor interaction stimulates the over production of extracellular matrix by podocyte and mesangium (leading to GBM thickening and mesangial matrix expansion). Over expression of TGF-β and mechanical stretch suppress α3β1 integrin and leads to decreased podocyte adhesion and apoptosis. In response to high glucose concentration, protein kinase C-alpha (PKC-α) expression increase which promotes nephrin endocytosis. This leads to a decline in nephrin surface expression and eventually contributes to proteinuria. Worsening proteinuria coupled glomerulosclerosis and tubulointerstitial fibrosis, leads to progressive renal insufficiency
Mesenchymal stem cells
Stem cells (SCs) are undifferentiated cells with remarkable capabilities for self-renewal, they are able to differentiate into specialized cells [47]. According to their source and malleability, stem cells are classified as fetal, adult stem cells (ASCs) and embryonic stem cells (ESCs) [48, 49]. Recently, a new kind of high-potential stem cells called induced pluripotent stem cells (iPSCs) was developed which were neither ‘embryonic’ nor ‘adult’ stem cells. iPSCs were generated from terminally differentiated cells, such as fibroblasts via a process of re-programming [50, 51].
Although both ASCs and ESCs share the capability for self-renewal and differentiation to specialized cell types, they differ in other attributes. ESCs are pluripotent and can differentiate into any cell type. They have an infinite proliferative potential and are able to grow in culture to provide sufficient cell numbers for stem cell transplantation and tissue engineering. Their clinical application is however limited due to their tumorigenic potential and the ethical problems associated with their use [52]. In contrast, ASCs are multipotent meaning that their ability for differentiation is limited. ASCs are easy to access and are considered safe as they are harvested from adult tissue [53]. A number of tissue specific stem cells have been isolated from organs including skin, brain, gastrointestinal tract and kidney [54–58]. Renal stem cells exist in adult human kidney and are capable of differentiating into different kidney cell types. Many studies have reported the successful isolation of kidney-derived stem cells [59]. Stem cells isolated from papilla of adult mice and rats expressed epithelial and mesenchymal markers when grown in culture and under appropriate conditions, these displayed evidence of plasticity by differentiation into myofibroblasts and into cells which expressed neuronal markers [60]. Stem cells isolated from adult human kidney were differentiated into epithelial and endothelial cells and showed formation of tubular structures and functional vessels both in vivo and in vitro [61]. A population of stem cells was harvested from microdissected proximal tubules and was identified by expression of stem cell markers such as Sca-1 and Musahi-1, these cells were differentiated into mature tubular cells in appropriate culture medium [62]. Mesenchymal stem cells isolated from kidneys of adult mice displayed the capability of differentiating into erythropoietin producing fibroblasts [63].
Adult stem cells that have the highest therapeutic potential are MSCs [51]. MSCs represent a class of adult progenitor cells that possesses the ability to differentiate into chondrocytes, osteoblasts and adipocytes. The role of MSCs in regulating the proliferation and function of immune cells, such as B and T lymphocytes, NK cells, dendritic cells and neutrophils have been demonstrated [64]. In addition, antifibrotic, anti-apoptotic, bactericidal and pro-angiogenic factors have been shown to be secreted by MSCs [65].
The enormous expansion potential, ease of harvest, low immunogenicity and lesser ethical concerns rendered MSCs attractive candidates in cell-based therapy for treatment of a variety of inflammation related diseases and subsequent tissue regeneration and repair of damaged tissue [66]. MSCs may promote endogenous repair of the kidney via chemotaxins with homing to injured tissue in vivo, and paracrine action of different cytokines [67–70]. In humans, MSCs were extracted from several sources including bone marrow (bone marrow mesenchymal stem cells; BM-MSCs), umbilical cord (umbilical cord mesenchymal stem cells; UC-MSCs), adipose tissue (adipose-derived mesenchymal stem cells; AD-MSCs), and fetus (fetal membrane mesenchymal stem cells; FM-MSCs) [71, 72]. Of these, BM-MSCs are the most used in cell-based therapy and tissue engineering despite the low numbers of MSCs derived from bone marrow which limit their application. Both BM-MSCs and AD-MSCs are equally capable of differentiating into cells and tissues of mesodermal origin. Compared with BM-MSCs, AD-MSCs are readily available and can be easily harvested in large quantities with little patient discomfort and therefore have been extensively used in preclinical and clinical studies [73, 74].
MSCs as treatment for diabetic nephropathy
In the last few decades, there has been much interest in the potential therapeutic effects of MSCs particularly BM-MSCs and AD-MSCs on DN and podocyte injury. Numerous studies have been carried out to explore the potential role of MSCs in the treatment of DN (Table 1). MSCs can significantly reduce hyperglycemia and proteinuria and improve renal pathological changes, including glomerular sclerosis, tubule dilatation, mesangial proliferation and protein casts [75–78]. MSCs were isolated from subcutaneous adipose tissue of SD rats and transplanted autogenously four week after induction of diabetes. The successful homing of MSCs was confirmed by double stain of CM-Dil and 4′, 6-diamidino-2-phenylindole (DAPI). The MSCs transplantation resulted in restoration of mesangial matrix expansion, inhibition of oxidative stress and reduction of proinflammatory cytokines. The decrease in proinflammatory cytokines was shown to be due to the effect of MSCs on MAPK signaling pathway [75]. It has been well documented that the expression of pro-inflammatory cytokines is regulated by activation of the MAPK pathway. MSC implementation attenuated the increased phosphorylation of p38, extracellular signal-regulated kinase (ERK) and c-Jun amino-terminal kinase (JNK) in diabetic animals [75]. Human BM-MSCs were injected into the cardiac ventricle of NOD/scid mice after induction of diabetes [76]. This animal model is immune-deficient that lacks functional B and T cells which can facilitate transplantation without host immune rejection. The human DNA infused as human BM-MSCs was detected in pancreas and kidney but not in liver, lung or spleen of the experimental animals suggesting the successful and selective homing of MSCs. Intracardiac infusion of MSCs decreased trapping of the cells in the capillary beds of the lung, however the highest level were observed in pancreas and kidneys which might be due to specific signals from injured tissues. Infusion of MSCs resulted in lower blood glucose and increased blood insulin. In addition, MSCs infusion improved glomerular morphology of diabetic animals. However, it is not clear whether it was a direct effect on the kidney or the effect was secondary to lower blood glucose levels. In the study human skin fibroblasts were used as control where the infusion of these cells did not show any effect in blood glucose level [76]. In a similar study, BM-MSCs were injected into the cardiac ventricle of SD rats four weeks after induction of diabetes [78]. BrdU labeling confirmed the localization of MSCs in heart, pancreas and kidneys. MSCs transplantation resulted in decreased blood glucose, urine albumin/creatinine and kidney/body weight ratios especially at the early phase of the treatment. Administration of cyclosporine strengthened these effects by suppressing the host immune response. However, proliferating cell nuclear antigen (PCNA) immunostaining showed MSCs did not proliferate in the kidney which suggest the renoprotective effects of MSCs through paracrine mechanisms [78]. Endovenous injection of MSCs prevented renal failure in diabetic mice as characterized by maintenance of basal levels of albuminuria and improvement of tubular dilation. However, blood glucose and insulin levels were unchanged [79]. Similarly, MSC treatment of diabetic mice did not result in a reduction in blood glucose and there was no regeneration of pancreatic β-cells although high glucose-induced alterations in kidney structure was reversed [3].
Table 1.
MSCs as treatment for DN
| Source of MSCs | Experimental model | Route of injection | In vivo localization of MSCs | Mechanism of action | Findings |
|---|---|---|---|---|---|
| AD-MSCs | SD rats + STZ n = 8/group |
Intravenous | Kidney | Effect on MAPK signaling pathway, suppression of oxidative stress and inflammatory response. | AD-MSCs treatment reduced oxidative damage and expression of pro-inflammatory cytokines, p-p38, p-ERK and p-JNK [75] |
| BM-MSCs | Wistar rats + STZ n = 16/group |
Intravenous | Kidney | Suppression of inflammatory response and inhibition of MCP-1 expression by secreting HGF. | AD-MSCs treatment reduced hyperglycemia and albuminuria, decreased the expression of fibronectin, Collagen I, MCP-1 and pro-inflammatory cytokines while the expression of HGF was up-regulated [77] |
| NOD/scid mice + STZ n = 9/group |
Intracardiac | Pancreas and kidney | Increased insulin secretion and improved renal lesions. | Successful homing of human BM-MSCs in the kidney of diabetic mice resulted in improvement of mesangial thickening and macrophage infiltration [76] | |
| SD rats + STZ n = 16/group |
Intracardiac | Heart, Pancreas and kidney | Decreased blood glucose | BM-MSCs treatment ameliorated DN characterized by decreased blood glucose, albumin/creatinine ratio and renal hypertrophy [78] | |
| C57BL/6 mice + STZ n = 8/group |
Intravenous | Pancreas and kidney | β-pancreatic islets regeneration | BM-MSCs treatment resulted in regeneration of pancreatic islets and prevented kidney damage [3] | |
| C57BL/6 mice + STZ n = 20/group |
Intravenous | Kidney and bone marrow | Produced renotrophic factors or anti-inflammatory cytokines | MSC treated mice maintained basal levels of albuminuria and only showed slight tubular dilatation [79] | |
| Wistar rats + STZ n = 12/group |
Intravenous | Kidney | Inhibited oxidative stress and reduced cellular glucose uptake mediated by GLUT1 | BM-MSCs treatment reduced hyperglycemia, albuminuria and renal mass index. Glomerulosclerosis, expression of collagen I and fibronectin was significantly reduced. Oxidative stress was also markedly reduced. The expression of TGF-β and membrane localization of GLUT1 was also down-regulated by MSCs [80] | |
| Albino rats + STZ n = 20/group |
Intravenous | Kidney | Paracrine action via different growth factors such as VEGF, TGFβ & TNFα and antiapoptotic action via bcl2 & Bax genes | BM-MSCs treatment decreased albuminuria, normalized serum urea and creatinine levels, increased VEGF, and bcl2 while decreasing TNF-α, fibrogenic growth factor TGF β, and Bax [95] | |
| UC-MSCs | NRK-52E SD rats + STZ n = 7/group |
Intravenous | Kidney | Secretion of humoral factors | UC-MSCs treatment prevented DN. However, renal hypertrophy was observed. There was no effect on blood glucose level [90]. |
MSCs secreted hepatocyte growth factor (HGF) which may play an important role in the treatment of DN by reducing macrophage infiltration and down-regulation of interleukin-1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor-α (TNFα) expression in renal tissue [77, 80].
Administration of MSCs prevented glomerular hyperfiltration as indicated by decreased creatinine clearance. Even though the creatinine clearance level normalized after MSCs treatment, the long term effect of lowered GFR need to be investigated [75].
The HGF secreted by MSCs has also been reported to down regulate the expression of high glucose induced TGF-β, thus down-regulating the expression of GLUT1 [80]. It has been reported that TGF-β stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells [81]. A similar study reported paracrine action of MSCs as therapeutic mechanism in DN. Engraftment of UC-MSCs in kidneys and glomeruli of STZ-diabetic rats effectively prevented proteinuria, fractional mesangial area, and excessive accumulation of extracellular matrix without affecting hyperglycemia and renal hypertrophy. Renoprotective effect of MSCs was reported to be through secretion of growth factors such as VEGF and insulin-like growth factor (IGF)-1 [82]. Administration of BM-MSCs in diabetic rats improved kidney function and regenerated kidney tissue by increasing expression of VEGF and anti-apoptotic protein BCL2 while decreasing the expression of TNF-α, TGF-β, and pro-apoptotic protein, Bax [83].
It has been reported that infused MSCs were detected predominately in the glomeruli early after administration [70]. BM-MSCs may home to injured glomerular endothelium and differentiate into endothelial cells [84], or replace injured mesangial cells [85]. Bone marrow transplant–derived podocytes has been reported to be found in wild-type mice [86], genetic mouse models with diffuse mesangial sclerosis [87] and Alport’s syndrome [88]. In contrast, podocyte replacement by BM-MSCs was not observed in aminoglycoside-induced nephropathy and renal ablation models [89]. The discrepancy may have been caused by animal model differences or by predominant podocyte regeneration from a parietal epithelial cell niche. However, the ability of MSCs to replace damaged podocyetes need to be more explored in DN models. In addition to regenerative and differentiative capability, MSCs have been shown beneficial effects against DN by secretion of a number of factors, including HGF, basic fibroblast growth factor (bFGF) and IGF-I, which may have contributed to the amelioration of kidney damage via their antiapoptotic, mitogenic, and other cytokine actions [90].
MSCs as treatment of podocyte injury in diabetic nephropathy
Recently, scientists have focused on MSCs as attractive prospect for stem cell-based therapies as treatment of podocyte injury in DN. A few studies have been conducted to investigate the use MSCs in the treatment of podocyte injury (Table 2). In a recent study, the protective effects of MSCs derived from human adipose tissue (hAD-MSCs) against high glucose-induced podocytic apoptosis and injury was investigated [83]. Mouse podocyte clone 5 (MPC5) were exposed to high glucose to establish a model of high glucose podocytic apoptosis for 24, 48 and 72 h. Podocytic apoptosis was reduced by hAD-MSCs via a decline in caspase-3 protein expression at all time points. The study also showed MSCs maintained integrity of glomerular filtration barrier by increasing podocytic synaptopodin and nephrin expression. It was suggested that high levels of epithelial growth factor (EGF) in MSCs conditioned medium (MSCs-CM) was the key factor in prevention of apoptosis and injury of podocytes where anti-EGF in MSCs-CM completely blocked the effect [83].
Table 2.
MSCs as Treatment of Podocyte Injury in DN
| Source | Experimental model | Route of injection | In vivo localization of MSCs | Mechanism of action | Findings |
|---|---|---|---|---|---|
| AD-MSCs | Mouse podocyte clone 5 cells (MPC5) | _ | _ | Secretion of soluble epithelial growth factor | AD-MSCs reduced podocytic apoptosis reduced the expression of podocytic cleaved caspase-3, maintained integrity of glomerular filtration barriers by increasing podocytic synaptopodin and nephrin expression [83] |
| SD rats + STZ and MPC5, n = 11 | Intravenous | Lung, spleen and a small number in kidney and pancreas | Secretion of GDNF | AD-MSCs attenuated proteinuria, prevented high glucose-induced podocyte injury and maintained the integrity of the podocyte actin cytoskeleton by increasing the expression of WT1 and synaptopodin proteins [92] | |
| BM-MSCs | SD rats + STZ, n = 14 |
Intra-arterial | Kidney | Increased BMP-7, nephrin and podocin secretion | BM-MSCs ameliorated loss of podocytes, GBM thickening and podocyte foot process effacement. By restoration of expression of nephrin and podocin [94] |
| UC-MSCs | Mouse podocyte clone 5 cell (MPC5) | _ | _ | Secretion of soluble epithelial growth factor | UC-MSCs decreased the podocytic apoptosis rate and the expression of PARP, increased the expression of Bcl-2, normalized the expression and arrangement of podocytic podoplanin [91] |
MSCs Mesenchymal stem cells; AD-MSCs Adipose-derived mesenchymal stem cells; BM-MSCs Bone marrow mesenchymal stem cells; AD-MSCs Adipose-derived mesenchymal stem cells; UC-MSCs Umbilical cord mesenchymal stem cells; Sprague–Dawley (SD) rats; STZ Streptozotocin; GBM Glomerular basement membrane; GDNF Glial derived neurotrophic factor; WT1 Wilms tumor protein 1; Bcl-2 B cell lymphoma-2; PARP Poly ADP ribose polymerase
It is possible that harvesting and injecting MSCs-CM containing EGF may be sufficient to prevent podocyte apoptosis without the need for injection of MSCs Ali, Brazil [82]. UC-MSCs protected podocytes from apoptosis induced by high glucose via secretion of HGF [91]. Co-culture of podocytes with UC-MSCs increased the expression of Bcl-2 and normalized the arrangement of podocytic podoplanin. The beneficial effects of UC-MSCs was reversed in the absence of HGF [91].
In a similar study, repeated intravenous injection of AD-MSCs attenuated proteinuria in STZ-diabetic rats even at the overt nephropathy stage [92]. Apparently, AD-MSCs treatment prevented high glucose-induced podocyte injury and maintained the integrity of the podocyte actin cytoskeleton by increasing the expression of Wilm’s tumour (WT1) and synaptopodin proteins. A series of growth factors, including fibroblast growth factor 2 (FGF2), epidermal growth factor and glial cell-line derived neurotrophic factor (GDNF) were shown to be secreted by AD-MSCs in culture media. GDNF is a podocyte survival factor which is secreted by AD-MSCs, it may also play a major role in the amelioration of podocyte injury. The study did not support the theory that stem cell differentiation was responsible for the prevention of DN, rather, the authors highlighted the involvement of endocrine mechanisms in the protection of the diabetic podocyte injury [92].
Intravenous injection of MSCs may not be appropriate due to low uptake of MSCs at the site of injury [91]. Instead, direct intra-arterial injection of MSCs should provide adequate homing of MSCs as confirmed by presence of florescent labelled cells in kidneys of experimental animals. The study showed that treatment with BM-MSCs improved physical and biochemical markers viz proteinuria and creatinine clearance rate [91]. In addition, MSCs ameliorated ultra-structural abnormalities, such as loss of podocytes, GBM thickening and podocyte foot process effacement. VEGF and bone morphogenetic protein-7 (BMP-7) are known as podocyte survival factors. High amounts of VEGF and BMP-7 released by MSCs has shown to exert beneficial effects in chronic kidney disease and acute kidney injury models [68, 94]. Beneficial effects of MSCs at maintenance of podocyte integrity and improvement of proteinuria were associated with restoration of expression patterns for nephrin and podocin. Level of bone morphogenetic protein-7 (BMP-7), the survival and differential factor of podocytes, increased after MSC treatment [91].
In addition, MSCs secreted a number of factors, including HGF, basic fibroblast growth factor (bFGF) and IGF-I, which may have contributed to the amelioration of kidney damage via their antiapoptotic, mitogenic, and other cytokine actions [93]. Therapeutic effect of MSCs against DN may primarily be mediated through paracrine actions [94].
Although these data are encouraging, further studies need to be done using non-MSC cell controls to confirm these beneficial effects were unique to stem cells. In addition, MSCs have shown to secrete high levels of a number of growth factors which may contribute in amelioration of DN. However it is not clear whether the effect is specific to these growth factor. Blockage of these factors may help to address the issue. The studies reviewed in this paper either used immunosuppressants or autogenic stem-cell transplantation to minimize host immune response. However, administration of an irrelevant cell as a negative control may help to address the issue. In addition, in some of the studies reviewed in this paper nonparametric statistics would be more appropriate due to small sample size. Collectively, the possible mechanisms of action of MSCs against DN are paracrine action and fusion or transdifferentiation.
Conclusion
Mesenchymal stem cells have become an attractive prospect for stem cell-based therapies aimed at treatment of podocyte injury in DN. MSCs are multipotent stromal cells which are capable of differentiating into multiple cellular lineages. MSCs demonstrated therapeutic effect in treatment of podocyte injury not only by their regenerative and differentiative capability, but also by secretion of a series of growth factors via antiapoptotic, mitogenic and other cytokine actions. Although recent studies demonstrated successful treatment of diabetic podocyte injury with MSCs, the challenge for future studies is to identify and confirm key molecular mechanisms involved. In addition, clinical trials are required to confirm MSCs as new and specific therapies for diabetic podocyte injury. Successful treatment of diabetic podocyte injury with MSCs holds great promise for the development of novel, MSC-based therapy that can alter the deleterious effects of podocyte injury in DN.
Abbreviations
- AD-MSCs
Adipose-derived mesenchymal stem cells
- Ang II
Angiotensin II
- ASCs
Adult stem cells
- bFGF
Basic fibroblast growth factor
- BM-MSCs
Bone marrow mesenchymal stem cells
- BMP-7
Bone morphogenetic protein-7
- DN
Diabetic nephropathy
- EGF
Epidermal growth factor
- ERK
Extracellular signal-regulated kinase
- ESCs
Embryonic stem cells
- FGF2
Fibroblast growth factor 2
- FM-MSCs
Fetal membranes mesenchymal stem cells
- GBM
Glomerular basement membrane
- GDNF
Glial cell-line derived neurotrophic factor
- HGF
Hepatocyte growth factor
- IGF-I
Insulin-like Growth Factor-I
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin-6
- iPSCs
Induced pluripotent stem cells
- JNK:c
Jun amino-terminal kinase
- MAPK
Mitogen-activated protein kinase
- MSCs
Mesenchymal stem cells
- PKC-α
Protein kinase C-alpha
- ROS
Reactive oxygen species
- SCs
Stem cells
- TGF-β R′
Transforming growth factor beta receptor
- TGF-β
Transforming growth factor beta
- TNFα
Tumor necrosis factor alpha
- UC-MSCs
Umbilical cord mesenchymal stem cells
- VEGF
Vascular endothelial growth factor
Conflict of interest
The authors have declared that no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Manizheh Khalilpourfarshbafi and Fatemeh Hajiaghaalipour had equal contribution in this work.
References
- 1.Sarje SK, Ghiware NB, Kawade RM, Gunjkar VN, Vadvalkar SM. Association of chronic complications of type 2 diabetes with the biochemical and physical estimations in subjects attending single visit screening for complications. Int J Res Pharm Chem. 2013;3:842–845. [Google Scholar]
- 2.Hajiaghaalipour F, Khalilpourfarshbafi M, Arya A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int J Biol Sci. 2015;11:508–524. doi: 10.7150/ijbs.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J kidney Dis Off J Natl Kidney Found. 2000;36:646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 5.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54:1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 6.Fioretto P, Caramori ML, Mauer M. The kidney in diabetes: dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia. 2008;51:1347–1355. doi: 10.1007/s00125-008-1051-7. [DOI] [PubMed] [Google Scholar]
- 7.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 8.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 9.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 10.Flyvbjerg A, Bennett WF, Rasch R, Kopchick JJ, Scarlett JA. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes. 1999;48:377–382. doi: 10.2337/diabetes.48.2.377. [DOI] [PubMed] [Google Scholar]
- 11.Thirone AC, Scarlett JA, Gasparetti AL, Araujo EP, Lima MH, Carvalho CR, et al. Modulation of growth hormone signal transduction in kidneys of streptozotocin-induced diabetic animals: effect of a growth hormone receptor antagonist. Diabetes. 2002;51:2270–2281. doi: 10.2337/diabetes.51.7.2270. [DOI] [PubMed] [Google Scholar]
- 12.Catanuto P, Doublier S, Lupia E, Fornoni A, Berho M, Karl M, et al. 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009;75:1194–1201. doi: 10.1038/ki.2009.69. [DOI] [PubMed] [Google Scholar]
- 13.Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294:F830–F839. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol JASN. 2004;15:1475–1487. doi: 10.1097/01.ASN.0000127988.42710.A7. [DOI] [PubMed] [Google Scholar]
- 15.Reiser J, Mundel P. Dual effects of RAS blockade on blood pressure and podocyte function. Curr Hypertens Rep. 2007;9:403–408. doi: 10.1007/s11906-007-0074-7. [DOI] [PubMed] [Google Scholar]
- 16.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhou J, Minto AW, Hack BK, Alexander JJ, Haas M, Li YC, Heilig CW, Quigg RJ. Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int. 2006;70:882–891. doi: 10.1038/sj.ki.5001624. [DOI] [PubMed] [Google Scholar]
- 18.Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 19.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Investig. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross ML, Dikow R, Ritz E. Diabetic nephropathy: recent insights into the pathophysiology and the progression of diabetic nephropathy. Kidney Int Suppl. 2005;94:S50–S53. doi: 10.1111/j.1523-1755.2005.09412.x. [DOI] [PubMed] [Google Scholar]
- 21.Saleem MA. Biology of the human podocyte. Nephron Exp Nephrol. 2003;95:e87–e92. doi: 10.1159/000074324. [DOI] [PubMed] [Google Scholar]
- 22.Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Choi HY, Chang JH, et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol CJASN. 2007;2:529–542. doi: 10.2215/CJN.04121206. [DOI] [PubMed] [Google Scholar]
- 24.Chuang PY, He JC. Signaling in regulation of podocyte phenotypes. Nephron Physiol. 2009;111:9–15. doi: 10.1159/000191075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 26.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 27.Anil Kumar P, Welsh GI, Saleem MA, Menon RK. Molecular and cellular events mediating glomerular podocyte dysfunction and depletion in diabetes mellitus. Front Endocrinol. 2014;5:151. doi: 10.3389/fendo.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mundel P. Podocyte biology and response to injury. J Am Soc Nephrol JASN. 2002;13:3005–3015. doi: 10.1097/01.ASN.0000039661.06947.FD. [DOI] [PubMed] [Google Scholar]
- 29.Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–468. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- 30.Salmon AH, Neal CR, Harper SJ. New aspects of glomerular filtration barrier structure and function: five layers (at least) not three. Curr Opin Nephrol Hypertens. 2009;18:197–205. doi: 10.1097/MNH.0b013e328329f837. [DOI] [PubMed] [Google Scholar]
- 31.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, et al. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56:1127–1135. doi: 10.2337/db06-0693. [DOI] [PubMed] [Google Scholar]
- 32.Tossidou I, Starker G, Kruger J, Meier M, Leitges M, Haller H, et al. PKC-alpha modulates TGF-beta signaling and impairs podocyte survival. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2009;24:627–634. doi: 10.1159/000257518. [DOI] [PubMed] [Google Scholar]
- 33.Herman-Edelstein M, Thomas MC, Thallas-Bonke V, Saleem M, Cooper ME, Kantharidis P. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: a model for diabetic podocytopathy. Diabetes. 2011;60:1779–1788. doi: 10.2337/db10-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51:1580–1587. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 35.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 36.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Investig. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 38.Verzola D, Gandolfo MT, Ferrario F, Rastaldi MP, Villaggio B, Gianiorio F, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72:1262–1272. doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 39.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 40.Lee HS. Pathogenic role of TGF-β in diabetic nephropathy. J Diabetes Metab. 2013;59:1–7. [Google Scholar]
- 41.Dessapt C, Baradez MO, Hayward A, Dei Cas A, Thomas SM, Viberti G, et al. Mechanical forces and TGFbeta1 reduce podocyte adhesion through alpha3beta1 integrin downregulation. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2009;24:2645–2655. doi: 10.1093/ndt/gfp204. [DOI] [PubMed] [Google Scholar]
- 42.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. doi: 10.2337/diabetes.55.01.06.db05-0894. [DOI] [PubMed] [Google Scholar]
- 43.Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, et al. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int. 2007;71:1019–1027. doi: 10.1038/sj.ki.5002195. [DOI] [PubMed] [Google Scholar]
- 44.Stieger N, Worthmann K, Schiffer M. The role of metabolic and haemodynamic factors in podocyte injury in diabetes. Diabetes Metabol Res Rev. 2011;27:207–215. doi: 10.1002/dmrr.1164. [DOI] [PubMed] [Google Scholar]
- 45.Terada Y, Inoshita S, Nakashima O, Tamamori M, Ito H, Kuwahara M, et al. Cell cycle inhibitors (p27Kip1 and p21CIP1) cause hypertrophy in LLC-PK1 cells. Kidney Int. 1999;56:494–501. doi: 10.1046/j.1523-1755.1999.00568.x. [DOI] [PubMed] [Google Scholar]
- 46.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090–3094. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 47.Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, et al. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2010;25:3874–3884. doi: 10.1093/ndt/gfq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ilic D, Polak JM. Stem cells in regenerative medicine: introduction. Br Med Bull. 2011;98:117–126. doi: 10.1093/bmb/ldr012. [DOI] [PubMed] [Google Scholar]
- 49.Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respir Int Rev Thoracic Dis. 2013;85:3–10. doi: 10.1159/000345615. [DOI] [PubMed] [Google Scholar]
- 50.Das BC, Tyagi A. Chapter 23—Stem cells: a trek from laboratory to clinic to industry. In: Singh ASV, editor. Animal biotechnology. San Diego: Academic Press; 2014. pp. 425–450. [Google Scholar]
- 51.Whitworth DJ, Banks TA. Stem cell therapies for treating osteoarthritis: prescient or premature? Vet J. 2014;202:416–424. doi: 10.1016/j.tvjl.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 52.Romano G. Stem cell transplantation therapy: controversy over ethical issues and clinical relevance. Drug News Perzspect. 2004;17:637–645. doi: 10.1358/dnp.2004.17.10.873915. [DOI] [PubMed] [Google Scholar]
- 53.Obokata H, Vacanti CA. Chapter 31—Stem cells in tissue engineering. In: Vacanti RLL, editor. Principles of tissue engineering. 4. Boston: Academic Press; 2014. pp. 595–608. [Google Scholar]
- 54.Alison MR, Poulsom R, Forbes SJ. Update on hepatic stem cells. Liver. 2001;21:367–373. doi: 10.1034/j.1600-0676.2001.210601.x. [DOI] [PubMed] [Google Scholar]
- 55.Bernard-Kargar C, Ktorza A. Endocrine pancreas plasticity under physiological and pathological conditions. Diabetes. 2001;50:S30–S35. doi: 10.2337/diabetes.50.2007.S30. [DOI] [PubMed] [Google Scholar]
- 56.Forbes S, Poulsom R, Wright N. Hepatic and renal differentiation from blood-borne stem cells. Gene Ther. 2002;9:625–630. doi: 10.1038/sj.gt.3301720. [DOI] [PubMed] [Google Scholar]
- 57.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/S0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 58.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol JASN. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 59.Ito T. Stem cells of the adult kidney: where are you from? Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2003;18:641–644. doi: 10.1093/ndt/gfg028. [DOI] [PubMed] [Google Scholar]
- 60.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Investig. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitamura S, Yamasaki Y, Kinomura M, Sugaya T, Sugiyama H, Maeshima Y, et al. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19:1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Park H, Addabbo F, Ni J, Pelger E, Li H, et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–889. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21:1641–1655. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- 65.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells (Dayton, Ohio) 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 67.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol JASN. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burst V, Putsch F, Kubacki T, Volker LA, Bartram MP, Muller RU, et al. Survival and distribution of injected haematopoietic stem cells in acute kidney injury. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2013;28:1131–1139. doi: 10.1093/ndt/gfs513. [DOI] [PubMed] [Google Scholar]
- 69.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Investig. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 71.Gazit Z, Pelled G, Sheyn D, Kimelman N, Gazit D. Chapter 19—Mesenchymal stem cells. In: Lanza R, Atala A, editors. Essentials of stem cell biology. 3. Boston: Academic Press; 2014. pp. 255–266. [Google Scholar]
- 72.Prochazkova M, Chavez MG, Prochazka J, Felfy H, Mushegyan V, Klein OD. Chapter 18—Embryonic versus adult stem cells. In: Ramalingam AVSS, editor. Stem cell biology and tissue engineering in dental sciences. Boston: Academic Press; 2015. pp. 249–262. [Google Scholar]
- 73.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11:160–170. [PubMed] [Google Scholar]
- 75.Fang Y, Tian X, Bai S, Fan J, Hou W, Tong H, et al. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med. 2012;30:85–92. doi: 10.3892/ijmm.2012.977. [DOI] [PubMed] [Google Scholar]
- 76.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lv S, Liu G, Wang J, Wang W, Cheng J, Sun A, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol. 2013;17:275–282. doi: 10.1016/j.intimp.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 78.Zhou H, Tian HM, Long Y, Zhang XX, Zhong L, Deng L, et al. Mesenchymal stem cells transplantation mildly ameliorates experimental diabetic nephropathy in rats. Chin Med J. 2009;122:2573–2579. [PubMed] [Google Scholar]
- 79.Ezquer F, Ezquer M, Simon V, Pardo F, Yanez A, Carpio D, et al. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:1354–1365. doi: 10.1016/j.bbmt.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 80.Lv S, Cheng J, Sun A, Li J, Wang W, Guan G, et al. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting oxidative stress. Diabetes Res Clin Pract. 2014;104:143–154. doi: 10.1016/j.diabres.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 81.Inoki K, Haneda M, Maeda S, Koya D, Kikkawa R. TGF-beta 1 stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells. Kidney Int. 1999;55:1704–1712. doi: 10.1046/j.1523-1755.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- 82.Ali IHA, Brazil DP. Under the right conditions: protecting podocytes from diabetes-induced damage. Stem Cell Res Ther. 2013;4:119/111–119/112. doi: 10.1186/scrt330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li D, Wang N, Zhang L, Hanyu Z, Xueyuan B, Fu B, et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res Ther. 2013;4:103. doi: 10.1186/scrt314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Truong P, Igarashi P, Lin F. Renal and bone marrow cells fuse after renal ischemic injury. J Am Soc Nephrol. 2007;18:3067–3077. doi: 10.1681/ASN.2007030284. [DOI] [PubMed] [Google Scholar]
- 85.Ito T, Suzuki A, Okabe M, Imai E, Hori M. Application of bone marrow-derived stem cells in experimental nephrology. Exp Nephrol. 2001;9:444–450. doi: 10.1159/000052644. [DOI] [PubMed] [Google Scholar]
- 86.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 87.Guo JK, Schedl A, Krause DS. Bone marrow transplantation can attenuate the progression of mesangial sclerosis. Stem Cells. 2006;24:406–415. doi: 10.1634/stemcells.2005-0139. [DOI] [PubMed] [Google Scholar]
- 88.Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, et al. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells. 2006;24:2448–2455. doi: 10.1634/stemcells.2006-0201. [DOI] [PubMed] [Google Scholar]
- 89.Meyer-Schwesinger C, Lange C, Brocker V, Agustian P, Lehmann U, Raabe A, et al. Bone marrow-derived progenitor cells do not contribute to podocyte turnover in the puromycin aminoglycoside and renal ablation models in rats. Am J Pathol. 2011;178:494–499. doi: 10.1016/j.ajpath.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012;98:465–473. doi: 10.1016/j.diabres.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 91.Qi W, Lu S, Liu G, Cheng J, Song Y, Ming T, et al. Human umbilical cord mesenchymal stem cells eo-culture ameliorates podocytic apoptosis: a possible role of HGF. Zhonghua Shenzangbing Zazhi. 2014;30:933–938. [Google Scholar]
- 92.Zhang L, Li K, Liu X, Li D, Luo C, Fu B, et al. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem Cells Dev. 2013;22:3074–3086. doi: 10.1089/scd.2013.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 94.Wang S, Li Y, Zhao J, Zhang J, Huang Y. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. J Am Soc Blood Marrow Transpl. 2013;19:538–546. doi: 10.1016/j.bbmt.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 95.Abdel Aziz MT, Wassef MA, Ahmed HH, Rashed L, Mahfouz S, Aly MI, et al. The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy. Diabetol Metabol Syndr. 2014;6:34. doi: 10.1186/1758-5996-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]