Abstract
We investigated the use of Polycaprolactone (PCL)/ β-tricalcium phosphate (β-TCP) composites with applied mechanical stimulation as scaffold for bone tissue engineering. PCL-based three-dimensional (3D) structures were fabricated in a solvent-free process using a 3D-printing technique. The mass fraction of β-TCP was varied in the range 0–30%, and the structure and compressive modulus of the specimens was characterized. The shape and interconnectivity of the pores was found to be satisfactory, and the compressive modulus of the specimens was comparable with that of human trabecular bone. Human mesenchymal stem cells were seeded on the composites, and various biological evaluations were performed over 9 days. With a mass fraction of β-TCP of 30%, differentiation began earlier; however, the cell proliferation rate was lower. Through the use of mechanical stimulation, however, the proliferation rate recovered, and was comparable with that of the other groups. This stimulation effect was also observed in ECM generation and other biological assays. With mechanical stimulation, expression of osteogenic markers was lower on samples with a β-TCP content of 10 wt% than without β-TCP; however, with mechanical stimulation, the sample with a β-TCP content of 30 wt% exhibited significantly greater expression of those markers than the other samples. We found that mechanical stimulation and the addition of β-TCP interacted closely, and that a mass fraction of β-TCP of 30% was particularly useful as a bone tissue scaffold when accompanied by mechanical stimulation.
Keywords: Polycaprolactone (PCL), β-tricalcium phosphate (β-TCP), Human mesenchymal stem cell, 3D-printing system, Scaffold
Introduction
Various types of biomaterial have been investigated for use as scaffolds for tissue engineering applications [1]. Tissue engineering for recovery of damaged tissue or part of an organ requires three major factors for a successful outcome: scaffolds, cells and appropriate environmental conditions. One of the most widely used biomaterials for bone tissue scaffolds is polycaprolactone (PCL), which has been shown to be biocompatible and biodegradable [2]. However, its hydrophobic characteristics are a disadvantage for cell adhesion and survival [3, 4]. β-tricalcium phosphate (β-TCP) is a bioceramic material with a similar mineral composition to bone and teeth, as well as good structural stability. In addition, it is readily biodegraded as new bone formation occurs [5, 6], and hence has potential for use in bone tissue engineering [7, 8]; however, it is available only in powder form and does not have sufficient mechanical strength for bone tissue engineering [9]; therefore, an additional process is required to fabricate a scaffold using β-TCP. Consequently, it is advantageous to form composites with other biopolymers.
Lu et al. investigated the efficacy of cell adhesion to composites formed of PCL and β-TCP, and found better cell adhesion as more β-TCP was added [10]. Cao et al. fabricated scaffolds using polyglycolic acid (PGA) and β-TCP with mass ratios of 1:1 and 1:3. They found improved osteoconductivity as more β-TCP was added [11]; however, their scaffolds were formed via salt leaching, which requires the use of a solvent, and leads to poor interconnectivity in the scaffold. Three-dimensional (3D) bioprinting has been used to address these issues with the salt leaching method [12]; however, it is not straightforward to construct solid 3D structures when large amounts of β-TCP are added.
Here we investigate the use of mesenchymal stem cells (MSCs), which are widely used in tissue engineering studies, especially for bone regeneration [13], and the growth factors for osteogenic differentiation of MSCs are well established. Moreover, we used mechanical stimulation to enhance differentiation into bone cells. The efficacy of mechanical stimulation to promote osteogenesis is widely recognized [14, 15], and we investigated the effects of intermittent hydrostatic pressure (IHP) on osteogenic differentiation of MSCs, which has been shown to promote differentiation of MSCs into osteo-related cells and chondrocytes [16–18].
We formed 3D PCL/β-TCP composite scaffolds using bioprinting, and investigated the effects of the β-TCP content on osteogenic differentiation and the strength of the scaffold. Furthermore, we investigated the use of IHP to mimic the in vivo biophysical environment that the bones in human body experience during daily activity.
Materials and methods
Scaffold fabrication
β-TCP and PCL (Mw 45,000) were purchased from Sigma-Aldrich (St. Louis, MO, USA). To fabricate the 3D PCL/β-TCP composite structures, we used a customized bioprinting system. For the homogeneous blending of β-TCP powder (average particle size: 250 μm) and PCL powder (average particle size: 250 μm) a mini ball mill Pulverisette23® (Fritsche, Germany) was used. No solvent was used to dissolve the PCL. Then the mixture was plotted in a layer-by-layer fashion via a heated dispenser to control the structure of the scaffold. Polymer was melted at 110 °C in heated dispenser. The melted polymer was extruded from the nozzle by an air pressure of 450 kPa. Three types of scaffold were fabricated: a PCL scaffold, a β-TCP/PCL(10) scaffold (i.e., PCL with 10 wt% β-TCP) and a β -TCP/PCL(30) scaffold (i.e., PCL with 30 wt% β-TCP).
Characterization of scaffolds
Scanning electron microscopy and energy dispersive spectrometer (EDS) analysis of scaffolds
The porous structure of the scaffolds and the cell adhesion behavior were investigated using a scanning electron microscope (SEM, Hitachi Ltd, Tokyo, Japan). Each sample was fixed in 10% formalin solution (Sigma Aldrich, MO, USA) for 30 min and washed with Dulbecco’s phosphate buffered saline (D-PBS, Sigma-Aldrich, St. Louis, MO, USA) several times. The samples were then dehydrated using a series of ethanol/water mixtures containing 50%, 60%, 70%, 80%, 90% and 99% ethanol, whereby the samples were soaked for 2 min in each solvent, and then air-dried overnight. Each sample was sputter-coated with gold, and the morphology was observed using SEM at an acceleration voltage of 10.0 kV. Also a field emission scanning electron microscope (FE-SEM, Supra 25-Zeiss, Germany) was utilized to examine the changes in composition ratio. For this energy-dispersive X-ray spectroscopy (EDS) was carried out to qualitative and quantitative analysis of the elements in the scaffolds.
Fourier transform infrared spectroscopy (FTIR)
FTIR-ATR was performed using spectrum GX equipment (Perkin-Elmer, USA) to investigate any changes in crystallinity due to heating and pressurizing during 3D bioplotting processes. The samples were analyzed in the wavenumber range of 4000–650 cm−1
Porosity
Liquid displacement method was used to measure porosity of each specimen. Five samples were tested in each group.
Compressive modulus
The compressive mechanical properties of the each scaffold were characterized at room temperature using a micro-load system (R&B Inc., Daejeon, Korea). The displacement rate was 0.1 mm/min, and compressive modulus was calculated based on stress–strain curves from an average of n = 5 measurements.
Cell culturing
The scaffolds were cut into 0.8 × 0.8 × 0.5 cm3 samples and sterilized using ultraviolet (UV) light for 30 min. They were then coated with 10 µl/ml of fibronectin (Sigma, St. Louis, MO, USA) to enhance cell adhesion. Human MSCs (hMSCs, PT-2501; Lonza, Basel, Switzerland) were prepared using Dulbecco’s Modified Eagle’s Medium-Low Glucose (DMEM-LG; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Hyclone, Logan, UT, USA) and 1% penicillin/streptomycin (Hyclone) at 37 °C in a humidified 5% CO2 incubator. The hMSCs were seeded at a density of 1 × 105 cells/scaffold. The culture medium was replaced with osteogenic differentiation medium 4 h after seeding, which consisted of Dulbecco’s Modified Eagle’s Medium-low glucose (DMEM-LG; GibcoBRL) with 10% fetal bovine serum (FBS, invitrogen, Carlsbad, CA, USA), 1% (v/v) penicillin (100 U/ml)/ streptomycin (100 μg/ml) solution, and 10−7 M dexamethasone (Sigma), 10−2 M β-glycerophosphate (Sigma) and 50 μg/ml ascorbate-2-phosphate (Sigma).
Bioreactor system for mechanical stimulation
A bioreactor system (ACBC-100, Anycasting, Korea) was used to apply the IHP to the scaffolds containing the cells. The bioreactor consisted of a controller, a chamber and a pressure pump. The IHP was engaged 4 days after seeding, and operated for 2 h/day for up to 5 days. The magnitude of the applied pressure was 100 kPa, which was applied for 2 min followed by a rest period of 15 min.
Cell viability
Calcein AM and ethidium homodimer-I (LIVE/DEAD® Viability/Cytotoxicity, Molecular Probes, USA) were used to observe live and dead cells, respectively. Samples were removed from culture media, rinsed in PBS and incubated in calcein-AM (2 μM in PBS) and ethidium homodimer-1 (4 μM in PBS). After incubating for 30 min in the dark, images were acquired using a confocal laser scanning microscope (LSM 510, Zeiss, Oberkochen, Germany).
DNA content
Cell proliferation was assessed by measuring the DNA content using a Quant-iT PicoGreen dsDNA reagent kit (Molecular Probes, Eugene, OR, USA). PicoGreen dye binds to nucleic acids, and the measured fluorescence intensity is indicative of the concentration of DNA. The cells were harvested from each scaffold, the cell membranes were dissolved using detergent (0.1% Triton X-100, USB, Cleveland, OH, USA), and samples were centrifuged at 13,000 rpm for 5 min at 4 °C. PicoGreen (100 µl at 1:200 in a TE buffer) reagent was added to 100 µl of each sample. After incubating for 5 min in the dark, the samples were excited at 480 nm, and the fluorescence emission intensity was measured at 520 nm using a multi-detection microplate reader (Synergy HT BioTek, Winooski, VT, USA).
Immunofluorescence staining
Differentiation of MSCs into osteoblasts was assessed via fluorescence staining and confocal microscopy. The cells on the scaffold were fixed with 4% paraformaldehyde and permeabilized in 0.2% Triton X-100 for 10 min. They were then washed twice with PBS and incubated with 1% BSA in PBS for 30 min at room temperature to block nonspecific binding. Next, the cells were labeled with antibodies, which served as stains for osteogenic markers during immunofluorescence. The presence of the osteogenic biomarker osteopontin (OPN) was probed using mouse anti-osteopontin monoclonal antibodies (1:200, Santa Cruz, USA), followed by an Alexa Fluor 488-labeled goat anti-mouse secondary antibodies (1:200, Molecular Probes). Cell nuclei were stained using Hoechst 33258 (Life Technologies, Eugene, OR, USA) and then viewed under an LSM 510 microscope.
Real-time polymerase chain reaction (RT-PCR)
Gene expression was assessed using RT-PCR. The total cellular RNA was extracted using the RNeasy Mini Kit (Qiagen, Chatsworth, CA, USA). cDNA was synthesized using the High Capacity RNA-to-cDNA Kit (Applied Biosystems, USA). Amplification was carried out using gene specific primers using an Applied Biosystems 7500 RT-PCR system with the SYBR Green Reaction Mix (Applied Biosystems, Foster City, CA, USA). The expression levels of core binding factor alpha 1 (Cbfa-1, 5′-ACCCACGAATGCACTATC CA-3′ and 3′-CTGGTGGGAAGGGTCCACT-5′), osteonectin (ONN, 5′-TTCCTGTTG CCTGTCTCTAA-3′ and 3′-CGCTCTCCGGGCAGTCT-5′), and osteocalcin (OCN, 5′-CAGGAGGGGCAGCGAGGTA-3′ and 3′-GGCTCCCAGCCATTGATACA-5′) were determined and normalized to that of beta-actin (Beta-actin, 5′-CCCAAAGTTCACAATG TGGC-3′ and 3′-GATGGCAAGGGACTTCCTGT-5′).
Statistical analysis
One-way analysis of variance (ANOVA) was carried out using the software package SPSS (version 22.0K; SPSS Inc., Chicago, IL, USA), and when a significant difference among groups was found, it was evaluated using the least-significant difference (LSD) test. All of the data are presented as the means plus/minus the standard deviation (SD) with a significance level of p ≤ 0.05.
Results
Scaffolds
Three types of specimen were prepared using the bioprinting system; i.e., PCL, PCL/β-TCP(10) and PCL/β-TCP(30), where the numerical values denote the mass fraction of β-TCP as a percentage. The overall size of strand was ranged from 400–450 um in all groups. As the pore was shaped to be almost square, the side length of each pore was measured and found to be ranged from 350–400 um, approximately. These were confirmed through at least 10 spots in each group (Fig. 1). Therefore, this size is assumed to be good enough for cells seeded even inside the scaffolds. The measurements of porosity were found to be 59.90 ± 1.30%, 60.47 ± 1.28%, 61.87 ± 1.22% in average for PCL, PCL/β-TCP(10), and PCL/β-TCP(30), respectively. When more β-TCP was used, it tended to show higher porosity as expected. However, no significant difference was found among the groups.
Fig. 1.
The macroscopic and microscopic structure of A PCL, B PCL/β-TCP(10) and C PCL/β-TCP(30) scaffolds (Bar = 400 μm)
Energy-dispersive X-ray spectroscopy (EDS)
FE-SEM images of strand surface and its cross section showed added β-TCP, its distribution and rough surface due to β-TCP (Fig. 2A). Meanwhile EDS was performed to investigate compositions in scaffolds. Scanning of six randomly chosen areas showed the Ca/P ratios to be 0.79 ± 0.06, 1.49 ± 0.05 for PCL/β-TCP(10) and PCL/β-TCP(30), respectively, while only carbon and oxygen were detected in PCL scaffolds. Also, uniform distribution of β-TCP particles was confirmed again through EDS mapping (Fig. 2B).
Fig. 2.
A FE-SEM images of scaffolds, EDS analyses: B SEM-EDS mappings (P, phosphorous; O, oxygen; C, carbone; Ca, calcium) and typical spectrum of C PCL/β-TCP(10) scaffold and D PCL/β-TCP(30) scaffold
Fourier transform infrared spectroscopy (FTIR)
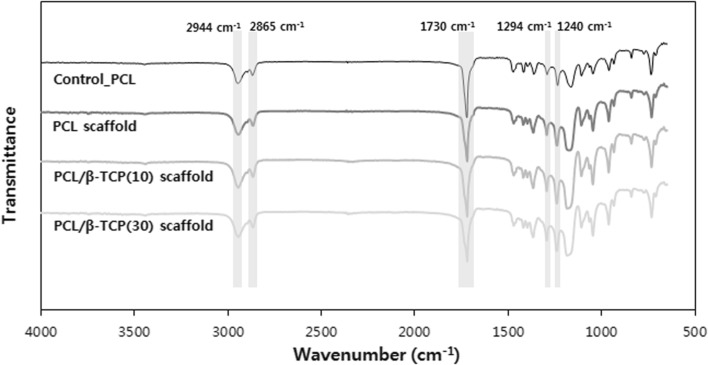
The chemical structure of each specimen including raw PCL was investigated by FTIR-ATR and their results were presented in Fig. 3. They all showed similar patterns. Specifically, they showed five major peaks: 1730 cm−1 (carbonyl stretching), 2944 cm−1 (asymmetric CH2 stretching), 2865 cm−1 (symmetric CH2 stretching), 1294 cm−1 (C–O and C–C stretching in the crystalline phase) and 1240 cm−1 (asymmetric C–O–C stretching). Therefore, we could confirm that no change in chemical structure was found after extrusion even under being heated and pressurized.
Fig. 3.
FTIR spectra of the specimens: control_PCL, PCL scaffold, PCL/β-TCP(10), and PCL/β-TCP(30)
Mechanical tests
Figure 4A shows the typical stress-strain curves of all specimens tested. All three curves show similar trend even the points, which look like yielding, were different each other. The average compressive modulus was calculated based on that point in each specimen and shown in Fig. 4B: 52.61 MPa, 51.23 MPa, and 48.00 MPa for PCL, PCL/β-TCP(10), and PCL/β-TCP(30), respectively. It tended to be deceased when more β-TCP was added. However, no significant difference was found.
Fig. 4.
A The stress-strain curves and B compressive modulus (n = 4)
Cell proliferation
The proliferation of cells was assessed at days 1, 4 and 9 by measuring the DNA content, as shown in Fig. 5. Twenty-four hours after seeding (D1), with the PCL scaffold the number of cells was significantly lower than on the other two scaffolds. This may be due to the hydrophobicity of PCL (even though it was coated with fibronectin), as the samples that contained β-TCP will have had rougher surfaces, which is advantageous for cell adhesion. However, by day 4 the PCL sample no longer exhibited the lowest cell proliferation. Without IHP, the PCL/β-TCP(30) sample exhibited a significantly lower proliferation compared with the two other groups. It follows that the differentiation process began earlier when a larger amount of β-TCP was present, but that the proliferation on the PCL/β-TCP(30) recovered, and eventually was comparable to that on the other two groups when IHP was employed.
Fig. 5.
The measured cell proliferation based on DNA contents (n = 5). Here SX refers to without IHP and SO refers to with IHP
Cell viability
Live/dead staining was carried out to evaluate the viability of cells at days 4 and 9. As shown in Fig. 6A, most cells were found to be alive among all groups. We could see denser green color corresponding to live cells in the IHP group than in the non-stimulated group. Overall no damage to the cells was found in any group.
Fig. 6.
A Fluorescence images showing the cell viability. Live cells are shown in green and dead cells in red. The scale bar is 100 μm. B SEM images showing the ECM that was generated in the different samples
Generation of extracellular matrix (ECM)
Figure 6B shows SEM images that reveal the effects of IHP and the addition of β-TCP. The effect of IHP on the ECM was most marked in the PCL/β-TCP(30) sample.
Immunofluorescence staining
The cell nuclei were stained using Hoechst 33235, and we found that cells were well seeded. Immunofluorescence staining was used to investigate the expression of osteopontin (OPN), which is a biomarker for cell differentiation, as shown in Fig. 7. Until day 4, significant quantities of OPN were not observed; however, at day 9 it OPN was clearly observed, and furthermore was found in greater quantities with IHP, and in greater quantities on the PCL/β-TCP(30) sample.
Fig. 7.
Immunofluorescence images showing osteopontin (OPN) at A day 1, 4 and B day 9. The scale bar is 100 μm
Expression of Osteo-differentiation related markers
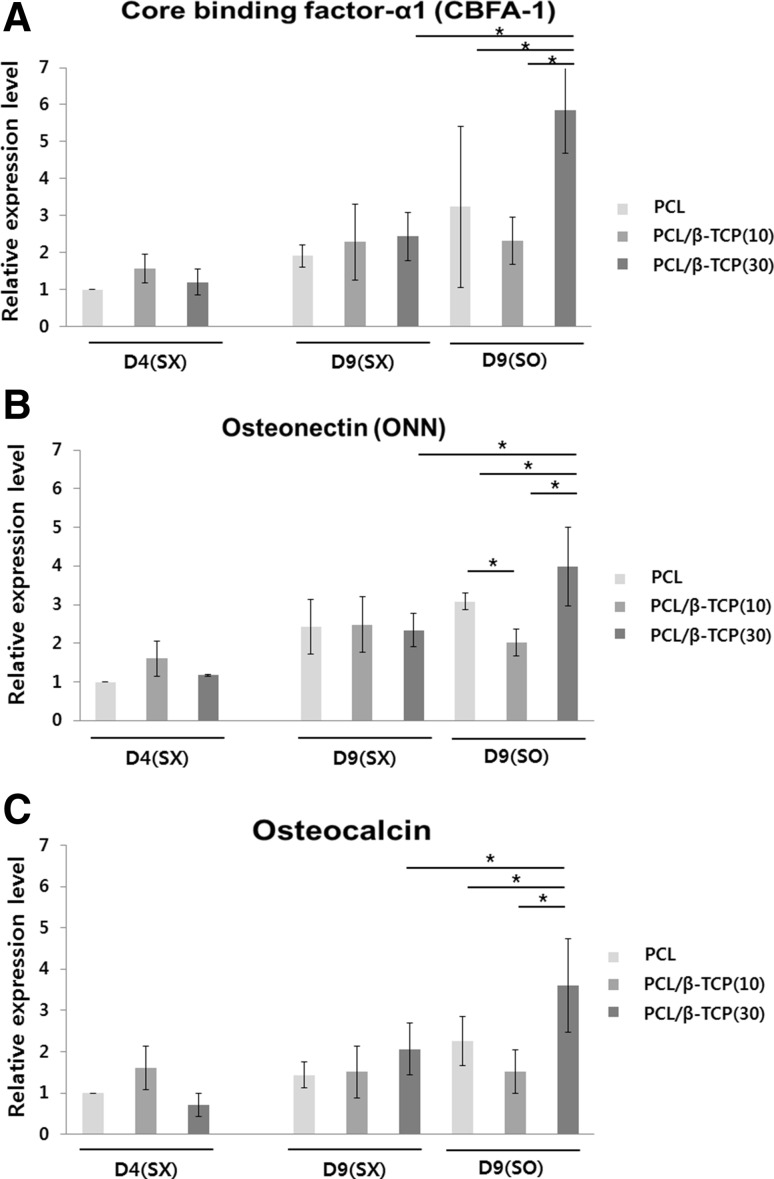
The expressions of three major markers were evaluated in real time using PCR, as shown in Fig. 8. The expression levels of each marker increased with time, as expected. At day 9, there were no clear differences among the three groups without IHP (SX), which suggests that the effects of β-TCP were not significant without stimulation. When IHP was employed (SO), a clear difference in the expression of the biomarkers could be seen between the different scaffolds. The expression levels of all three markers were lower on the PCL/β-TCP(10) sample than the PCL sample; however, the expression levels of all three biomarkers were higher on the PCL/β-TCP(10) sample when IHP was employed.
Fig. 8.
The results of the RT-PCR analysis (n = 3). A Core binding factor-α1 (CBFA-1), B osteonectin (ONN), and C osteocalcin (OCN)
Discussion
We have investigated the potential of PCL/β-TCP composites for applications as scaffolds for bone tissue engineering. The composites were extruded using a 3D bioprinting system, and the mass fraction of β-TCP was varied. The upper limit of the mass fraction of β-TCP of 30 wt% was used in consideration of the mechanical strength when implanted in human body, and because when more than 30 wt% of β-TCP was added, it was very difficult to form a solid 3D scaffold with uniform and controllable pore distributions without additional treatments such as laser sintering.
With larger mass fractions of β-TCP, we may expect greater differentiation of stem cells into osteo-lineages [19]; however, an additional process, such as laser sintering, is required for solid formation.
The mechanical strength of a scaffold decreased as the β-TCP content increased. However, within the range of mass fractions investigated here, we found a relatively small decrease in the compressive modulus from 52.61 MPa to 48 MPa as the β-TCP content increased from 0 wt% to 30 wt%. It has been reported that the strength of human trabecular bones is 50 MPa [20, 21], which is similar to the strength of the scaffolds investigated here, and smaller than the compressive modulus of the PCL/β-TCP(10) sample. However, expression of osteo-lineage related markers was not observed in the PCL/β-TCP(10) sample.
Regarding the proliferation of MSCs, the PCL sample did not provide a favorable environment for cell adhesion at day 1; however, this recovered during the course of cell culturing, and cell adhesion was similar in the different scaffolds at days 4 and 9 regardless of the β-TCP content, although the addition of 10 wt% of β-TCP provided favorable cell adhesion and proliferation. At day 4, i.e,, until IHP was engaged, the proliferation rate in PCL/β-TCP(30) was lower than that in other group. It can be suggested that differentiation process seemed to start earlier than the other groups. This observation is consistent with other previous reports saying that proliferation rate becomes lower when differentiation is ready to start [22, 23]. Looking at the results of PCR in Fig. 8, we can see relatively higher expression of osteogenic related markers at day 9 even without IHP. Moreover, significantly higher expression of the markers was observed at day 9 when IHP was engaged. Therefore, the lower proliferation from day 4 to 9 in PCL/β-TCP(30) can be explained that the cells are ready to differentiate. The comparable proliferation in PCL/β-TCP(30) at day 9 was observed when IHP was engaged. Similar result was reported that observable proliferation was obtained when a bioreactor was utilized in culturing human mesenchymal stem cells due to supply of nutrition and exchange of gases [24].
Therefore, we may conclude that the mass fraction of β-TCP and mechanical stimulation conditions should both be considered in the fabrication and use of scaffolds.
With IHP, the optimal magnitude of the pressure and the cycle pattern differ depending on the cell and/or tissue type. The objective is to mimic the in vivo conditions. Zhang et al. reported that the hydraulic pressure that osteocytes experience in vivo is at most 270 kPa, and is typically 100 kPa during physiological activity, due the lacunar-canalicular system [25]. Here we used a magnitude of the IHP of 100 kPa. We have previously shown [26] that a cycle of pressure applied for 2 min followed by a rest of 15 min led to enhanced adhesion and cellular activity.
More ECM was observed in PCL/β-TCP(30) at day 9 with IHP. ECM is known to play important roles in growth, differentiation, and other various activities via integrines. From this we can say that mechanical stimulation promoted ECM generation, consequently differentiation was accelerated as shown in PCR results (Fig. 8).
Overall, we found that the PCL/β-TCP(30) sample with IHP exhibited the best ECM generation and highest levels of expression of osteo-related biomarkers, as shown in immunofluorescence staining (Fig. 7) and PCR results (Fig. 8). It is interesting to note that the expressions of CBFA-1, osteonectin and osteocalcin on the PCL/β-TCP(10) sample were lower than on the PCL sample, even with IHP, but that PCL/β-TCP(30) exhibited significantly higher expressions of these biomarkers with IHP. This indicates that just adding β-TCP to PCL does not necessarily provide favorable conditions for cell differentiation, and that both the mass fraction of β-TCP and the use of IHP are important.
This can be explained by Ca/P ratios measured through EDS in Fig. 2B. Ca/P ratio in PCL/β-TCP(10) group was measured to be 0.79 ± 0.06, while that in PCL/β-TCP(30) was 1.49 ± 0.05, which was closer to the value in human bone (1.5–1.7) [27]. Moreover, the mechanical stimulation, which is usually adopted to mimic in vivo mechanical environment, was added. Therefore, the synergic effect was believed to be obtained due to preferable combination of composition and mechanical factor.
We investigated the effects of β-TCP and IHP on the proliferation and differentiation in vitro for only 9 days. Further investigation of differentiation and ECM generation is therefore required, and the extended duration of the experiments may be needed. Animal studies should also be considered, as well as varying the IHP patterns.
Acknowledgements
This work was supported by the grants of the Korea Health Technology R&D Project through the KHIDI (HI16C0362, the Ministry of Health & Welfare, ROK) and the National Research Foundation of Korea (NRF) Grant (NRF-2014K2A2A7066637).
Conflicts of interest
The authors have no financial conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.O’Brien FJ. Biomaterials and scaffolds for tissue engineering. Materialstoday. 2011;14:88–95. [Google Scholar]
- 2.Park SA, Lee JB, Kim YE, Kim JE, Lee JH, Shin J, et al. Fabrication of biomimetic PCL scaffold using rapid prototyping for bone tissue engineering. Macromol Res. 2014;22:882–887. doi: 10.1007/s13233-014-2119-5. [DOI] [Google Scholar]
- 3.Liao H, Chen J, Lee M. Bone tissue engineering with adipose-derived stem cells in bioactive composites of laser-sintered porous polycaprolactone scaffolds and platelet-rich plasma. Materials. 2013;6:4911–4929. doi: 10.3390/ma6114911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su J, Chen L, Li L. Characterization of polycaprolactone and starch blends for potential application within the biomaterials field. Afr J Biotechnol. 2012;11:694–701. [Google Scholar]
- 5.Aunoble S, Clément D, Frayssinet P, Harmand MF, Le Huec JC. Biological performance of a new beta–TCP/PLLA composite material for applications in spine surgery: in vitro and in vivo studies. J Biomed Mater Res A. 2006;78:416–422. doi: 10.1002/jbm.a.30749. [DOI] [PubMed] [Google Scholar]
- 6.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Gao C, Deng Y, Feng P, Mao Z, Li P, Yang B, et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci. 2014;15:4714–4732. doi: 10.3390/ijms15034714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripathi G, Basu B. A porous hydroxyapatite scaffold for bone tissue engineering: physico-mechanical and biological evaluations. Ceram Int. 2012;38:341–349. doi: 10.1016/j.ceramint.2011.07.012. [DOI] [Google Scholar]
- 9.Yeo MG, Kim GH. Preparation and characterization of 3D composite Scaffolds based on rapid-prototyped PCL/β-TCP struts and electrospun PCL coated with collagen and HA for bone regeneration. Chem Mater. 2012;24:903–913. doi: 10.1021/cm201119q. [DOI] [Google Scholar]
- 10.Lu L, Zhang Q, Wootton D, Chiou R, Li D, Lu B, et al. Biocompatibility and biodegradation studies of PCL/β-TCP bone tissue scaffold fabricated by structural porogen method. J Mater Sci Mater Med. 2012;23:2217–2226. doi: 10.1007/s10856-012-4695-2. [DOI] [PubMed] [Google Scholar]
- 11.Cao H, Kuboyama N. biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone. 2010;46:386–395. doi: 10.1016/j.bone.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Liu F. Synthesis of bioceramic scaffolds for bone tissue engineering by rapid prototyping technique. Key Eng Mater. 2012;64:704–710. [Google Scholar]
- 13.Yamachika E, Iidab S. Bone regeneration from mesenchymal stem cells (MSCs) and compact bone-derived MSCs as an animal model. Jpn Dent Sci Rev. 2013;49:35–44. doi: 10.1016/j.jdsr.2012.11.003. [DOI] [Google Scholar]
- 14.Huang C, Ogawa R. Effect of hydrostatic pressure on bone regeneration using human mesenchymal stem cells. Tissue Eng Part A. 2012;18:2106–2113. doi: 10.1089/ten.tea.2012.0064. [DOI] [PubMed] [Google Scholar]
- 15.Hess R, Douglas T, Myers KA, Rentsch B, Rentsch C, Worch H, et al. Hydrostatic pressure stimulation of human mesenchymal stem cells seeded on collagen-based artificial extracellular matrices. J Biomech Eng. 2010;132:021001. doi: 10.1115/1.4000194. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Lv X, Liu Y, Zhao Y, Li Q, Chen Y, et al. Hydrostatic pressure promotes the proliferation and osteogenic/chondrogenic differentiation of mesenchymal stem cells: the roles of RhoA and Rac1. Stem Cell Res. 2015;14:283–296. doi: 10.1016/j.scr.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Zhao Z, Li J, Zou L, Shuler C, Zou Y, et al. Hydrostatic pressures promote initial osteodifferentiation with ERK1/2 Not p38 MAPK signaling involved. J Cell Biochem. 2009;107:224–232. doi: 10.1002/jcb.22118. [DOI] [PubMed] [Google Scholar]
- 18.Jeong J, Park S, Shin J, Kang Y, Han K, Shin J. Effects of intermittent hydrostatic pressure magnitude on the chondrogenesis of MSCs without biochemical agents under 3D co-culture. J Mater Sci Mater Med. 2012;23:2773–2781. doi: 10.1007/s10856-012-4718-z. [DOI] [PubMed] [Google Scholar]
- 19.Sharaf B, Faris CB, Abukawa H, Susarla SM, Vacanti JP, Kaban LB, et al. Three-dimensionally printed polycaprolactone and β–Tricalcium phosphate scaffolds for bone tissue engineering: an in vitro study. J Oral Maxillofac Surg. 2012;70:647–656. doi: 10.1016/j.joms.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Jayabalan M, Shalumon KT, Mitha MK. Injectable biomaterials for minimally invasive orthopedic treatments. J Mater Sci Mater Med. 2009;20:1379–1387. doi: 10.1007/s10856-008-3683-z. [DOI] [PubMed] [Google Scholar]
- 21.Shea LD, Wang D, Franceschi RT, Mooney DJ. Engineered bone development from a pre-osteoblast cell line on three-dimensional scaffolds. Tissue Eng. 2000;6:605–617. doi: 10.1089/10763270050199550. [DOI] [PubMed] [Google Scholar]
- 22.Erisken C, Kalyon DM, Wang H. Functionally graded electrospun polycaprolactone and b-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials. 2008;29:4065–4073. doi: 10.1016/j.biomaterials.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura I, Hisanaga R, Sato T, Arano T, Nomoto S, Ikada Y, et al. Effect of osteogenic differentiation medium on proliferation and differentiation of human mesenchymal stem cells in three-dimensional culture with radial flow bioreactor. Regen Ther. 2015;2:24–31. doi: 10.1016/j.reth.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Weinbaum S, Cowin SC. Estimates of the peak pressures in bone pore water. J Biomech Eng. 1998;120:697–703. doi: 10.1115/1.2834881. [DOI] [PubMed] [Google Scholar]
- 26.Kim YJ, Park S, Lee YJ, Shin JW, Kim DH, Heo SJ, et al. Effects of intermittent hydrostatic pressure on cell adhesive forces and other related parameters under various resting periods. J Biomed Mater Res B Appl Biomater. 2008;85:353–360. doi: 10.1002/jbm.b.30953. [DOI] [PubMed] [Google Scholar]
- 27.Shim JH, Huh JB, Park JY, Jeon YC, Kang SS, Kim JY, et al. Fabrication of blended polycaprolactone/poly (lactic-co-glycolic acid)/β-tricalcium phosphate thin membrane using solid freeform fabrication technology for guided bone regeneration. Tissue Eng Part A. 2013;19:317–328. doi: 10.1089/ten.tea.2011.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]