Abstract
It is controversial whether type I collagen itself can maintain and improve chondrogenic phenotype of chondrocytes in a three-dimensional (3D) environment. In this study, we examined the effect of type I collagen concentration in hydrogel (0.5, 1, and 2 mg/ml) on the growth and phenotype expression of rat chondrocytes in vitro. All collagen hydrogels showed substantial contractions during culture, in a concentration-dependent manner, which was due to the cell proliferation. The cell viability was shown to be the highest in 2 mg/ml collagen gel. The mRNA expression of chondrogenic phenotypes, including SOX9, type II collagen, and aggrecan, was significantly up-regulated, particularly in 1 mg/ml collagen gel. Furthermore, the production of type II collagen and glycosaminoglycan (GAG) content was also enhanced. The results suggest that type I collagen hydrogel is not detrimental to, but may be useful for, the chondrocyte culture for cartilage tissue engineering.
Keywords: Type I collagen, Chondrocyte, Proliferation, Chondrogenic phenotype
Introduction
Articular cartilage is composed of sparse chondrocytes and a dense extracellular matrix (ECM). Type II collagen and glycosaminoglycan (GAG) are primary compositions of the ECM. In particular, the mechanical function of articular cartilage is dependent on the quantity of type II collagen [1]. Therefore, type II collagen has been widely studied for the cartilage repair and regeneration [2, 3].
Type I collagen is the most abundant collagen type, which is mostly found in bone, tendon, ligament and skin [4]. Hence, it is widely used to prepare scaffolds that mimic natural ECM of those tissues [5–7]. Even though type II collagen might be the most appropriate scaffold for cartilage tissue engineering, attempt has also been made to use the type I collagen for reconstruction of injured cartilage. Yasui et al. [8] previously observed that the chondrocytes proliferated and maintained their cartilage phenotype in type I collagen gel. Negri et al. [9] also demonstrated that human chondrocytes were capable of multiplying in type I collagen to present cartilage ECM. We fabricated previously alginate-hyaluronic acid based hydrogel with the incorporation of type I collagen [10]. The results showed that collagen proportion significantly accelerated chondrocytes proliferation and preserved chondrogenic phenotypes [10]. The type I collagen-assisted autologous chondrocyte transplantation (ACT) has also been applied to clinics. For instance, Nöth et al. [11] used type I collagen-based ACT for the treatment of three young patients. The results showed a good integration into the host articular cartilage tissue 12 months after surgery. Behrens et al. [12] reported also that the collagen I/III-assisted ACT for twenty-five patients improved the function of knee over a period of up to 5 years after operation.
In contrast, Chen et al. [13] revealed that type I collagen incorporated with dexamethasone and TGF-β1 reduced GAG production and mRNA level of SOX9 and aggrecan, with little expression of type II collagen gene in the cultured mesenchymal progenitor cells. Farjanel et al. [14] demonstrated that the chondrocytes exposed to type I collagen could lose their differentiated phenotype. In addition, the expansion of the chondrocytes in monolayer culture results in a rapid cell dedifferentiation. The collagen biosynthesis also switches from type II to type I collagen [15]. The type I collagen expression is indicative of the dedifferentiated status of chondrocytes.
Therefore, there are still some controversies as to the use of type I collagen in the culture of cells for cartilage tissue engineering. In this study we aim to investigate the effect of type I collagen in a hydrogel form on the growth and phenotype expression of the chondrocytes. Among the parameters, different concentrations of collagen gels were used. The cell-associated gel contraction behavior, and the proliferation and chondrocyte-related molecular expression (gene and protein levels) of cells were investigated, which will guide if the type I collagen 3D gel matrix can be useful for cartilage tissue engineering.
Materials and methods
Preparation of collagen hydrogels
The type I collagen solution (3.87 mg/ml, rat tail type I collagen, BD Biosciences, Bedford, MA, USA) was diluted with Dulbecco’s Modified Eagle’s Medium (DMEM; 10×; Gibco), 1% penicillin/streptomycin, and chondrocyte maintenance medium consisting of DMEM, 50 μg/ml ascorbic acid, 1% insulin-transferrin-selenium, 100 nM dexamethasone, 10% fetal bovine serum to yield final concentrations of 0.5, 1, and 2 mg/ml. The solutions were kept on ice less than a minute until neutralizing. A neutralization of the solution was carried out by adding 1 N NaOH aqueous solution: the volume of 1 N NaOH needed was equaled to 0.023 times of the volume of collagen solution, which was predetermined to adjust pH at 7.4.
For culture experiments, rat chondrocytes from articular cartilage of the knees were monolayer-expanded through four passages according to the procedures in a previous study [16], and thoroughly mixed with each collagen hydrogel to reach a final concentration of 3 × 105 cells/ml. The cell-seeded hydrogels were poured into bottomless polydimethylsiloxane molds with a dimension of 8 mm diameter and 2 mm thickness. The hydrogels were allowed to polymerize in a humidified incubator at 37 °C for 30 min. After gelation, the hydrogels were cultured with the chondrocyte maintenance medium or the chondrogenic medium for 14 days. The chondrogenic medium consists of the chondrocyte maintenance medium supplemented with 10 ng/ml transforming growth factor-β1 (PeproTech, USA). The media were changed twice every week.
Collagen gel contraction assay
At each culturing time, the diameter of collagen gels was measured with a ruler. The extent of contraction of the collagen gels was expressed as the percentage of initial area. Each experiment was performed in triplicate.
Cellular proliferation assay
The cell-seeded hydrogels were placed in 24-well plates and incubated in the chondrocyte maintenance medium for 1, 4 and 7 days, to evaluate the cell proliferation behavior. Cell proliferation was assessed using a CellTiter 96 aqueous one solution cell proliferation kit (MTS assay, Promega, USA). The culture medium was removed and a diluted MTS solution was added to each sample and allowed to react for 3 h at 37 °C. A 200 μl aliquot of the reaction sample was used for a colorimetric measurement at a wavelength of 490 nm using a microplate reader (Molecular Devices, USA). Three replicate samples were tested.
Cell viability
For the cell viability assay, the cell-seeded hydrogels were placed in 24-well plates and incubated in the chondrocyte maintenance medium for 1 and 7 days. At each culturing time, the cell viability in the gels was detected by the Live/Dead assay (Molecular Probes, Reduced Biohazard Viability/Cytotoxicity Kit, USA) according to manufacturer’s instruction. The samples were incubated in Live/Dead assay stain solution for 30 min at room temperature, and subsequently observed under an inverted fluorescence microscope equipped with a DP-72 digital camera (DP2-BSW, Olympus Co., Tokyo, Japan). The viable cells were indicated with green fluorescence signals while dead cells were indicated with red fluorescence signals.
Quantitative real-time polymerase chain reaction (qPCR)
For qPCR assay, the cell-seeded hydrogels were incubated in the chondrogenic medium for 7 and 14 days. At each culturing time, the expression of cartilage-related genes was analyzed using a Rotor-Gene RG-3000A qPCR machine (Australia). The first strand cDNA was synthesized from the total RNA (1 μg) using a SuperScript first strand synthesis system for real-time PCR (Invitrogen, USA) according to the manufacturer’s instruction. The reaction mixture was made up to 50 μl. Real-time PCR was conducted using SYBR GreenER qPCR SuperMix reagents (Invitrogen, USA). The relative transcript quantities were calculated using the 2−ΔΔCt method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous reference gene amplified from the samples. The primer sequences of the genes are summarized in Table 1.
Table 1.
Primer sequences of chondrogenic genes for qPCR
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| SOX9 | 5′-CGTCAACGGCTCCAGCA-3′ | 5′-TGCGCCCACACCATGA-3′ |
| Type II collagen | 5′-GAGTGGAAGAGCGGAGACTACTG-3′ | 5′-CTCCATGTTGCAGAAGACTTTCA-3′ |
| Aggrecan | 5′-CTAGCTGCTTAGCAGGGATAACG-3′ | 5′-TGACCCGCAGAGTCACAAAG-3′ |
| GAPDH | 5′-TGAACGGGAAGCTCACTGG-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ |
Immunofluorescence staining
To detect the expression of type II collagen, the samples were cultured in the chondrogenic medium for 14 days. Thereafter, the culture medium was removed and the harvested samples were fixed with 4% paraformaldehyde (PFA) for 20 min, incubated with 5% normal goat serum (Vector Laboratories, USA) in PBS for 30 min to suppress nonspecific staining, and then incubated with a primary antibody, anti-type II collagen (1:150 dilution, sc-52658; Santa Cruz Biotechnology, USA), for 24 h at 4 °C. The specimens were subsequently incubated with the FITC-conjugated antibody against mouse IgG (1:100 dilution, 115-095-003; Jackson Immunoresearch, USA) for 50 min at room temperature. The nuclei of the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min. The samples were examined with an inverted fluorescence microscope equipped with a DP-72 digital camera (Olympus Co., Tokyo, Japan).
Histological analysis of in vitro samples
For the assessment of GAG content, the cell-seeded hydrogels were incubated in the chondrogenic medium. After 14 days, the samples were harvested and fixed with 4% PFA, and then stained with Safranin O (Sigma-Aldrich, USA) and Alcian blue (Sigma-Aldrich, USA). Images were observed under an optical microscope.
Statistical analysis
Data are shown as the mean ± one standard deviation. Statistical analysis was performed using one-way ANOVA followed by a post hoc LSD test. p < 0.05 was considered to be statistically significant.
Results
Contraction of hydrogels
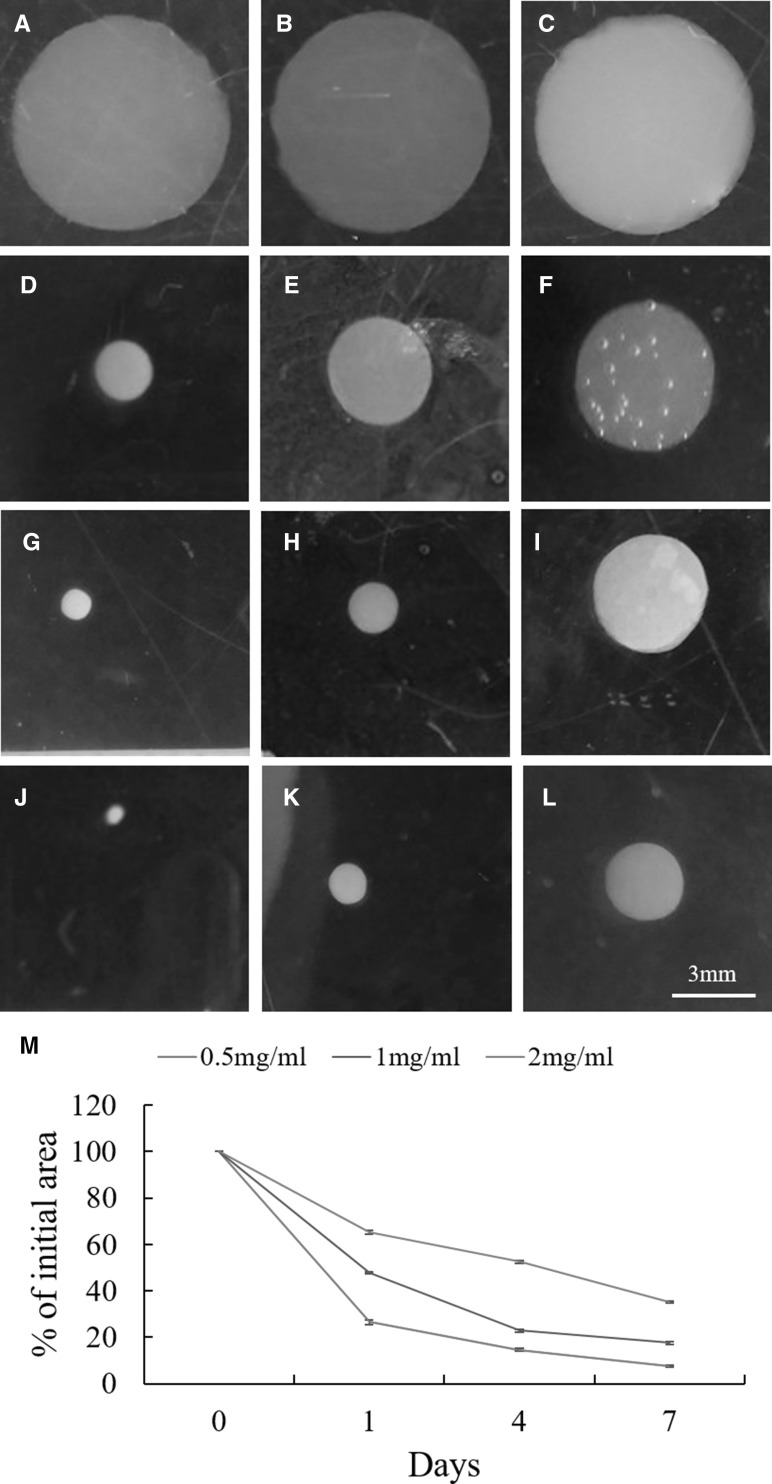
Unfixed hydrogels were observed at each time point. 0.5 mg/ml collagen gel contracted most rapidly compared with other groups. 1.0 mg/ml gel contracted moderately, and 2.0 mg/ml gel contracted most slowly among three groups (Fig. 1). This observation confirmed that the gel contraction was in a collagen concentration-dependent manner.
Fig. 1.
Effect of collagen concentration on the gel contraction by rat chondrocytes. A–L Images of collagen gel contraction. A, D, G, J 0.5 mg/ml collagen gels; B, E, H, K 1 mg/ml collagen gels; C, F, I, L 2 mg/ml collagen gels. A–C culture day 0; D–F culture day 1; G–I culture day 4; J–L culture day 7. M Quantitative analysis of collagen gel contraction
Cellular growth behaviors
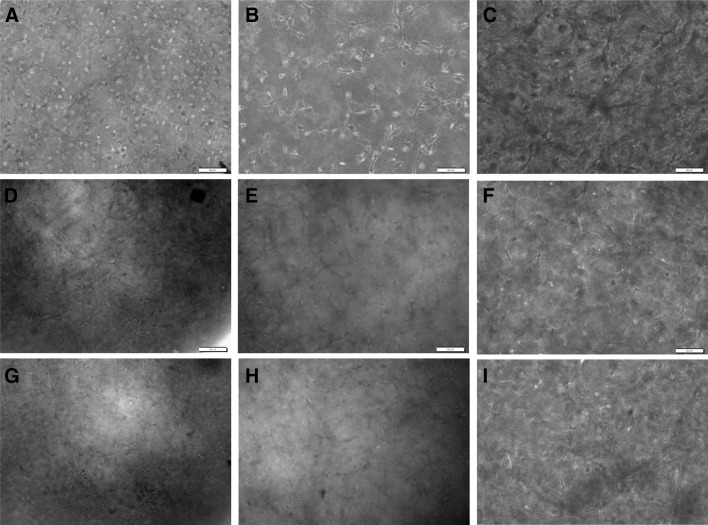
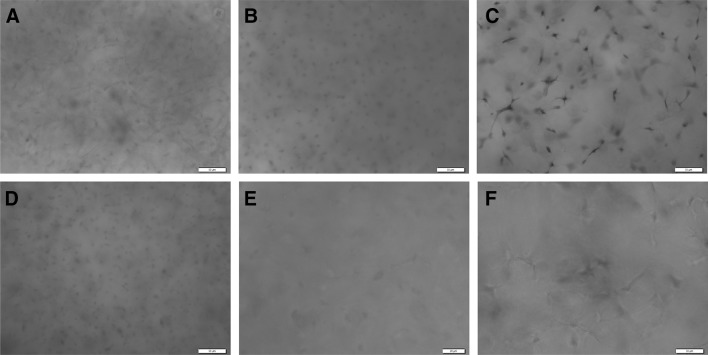
The cell-gel constructs were cultured using the chondrogenic medium for 14 days, and the cell morphology was observed under a phase contrast microscope, as shown in Fig. 2. In all groups, both round- and spindle-shaped cells were observed at day 1. Thereafter, the cells embedded in 2 mg/ml collagen gel presented a more elongated morphology than in 0.5 and 1 mg/ml collagen gels with time.
Fig. 2.
Phase contrast images of the chondrocytes cultured in collagen gels at day 1, day 7, and day 14. A, D, G 0.5 mg/ml collagen gels; B, E, H 1 mg/ml collagen gels; C, F, I 2 mg/ml collagen gels. A–C culture day 1; D–F culture day 7; G–I culture day 14
The Live/Dead assay was used to detect the viability of the cells in the gels (Fig. 3). The results showed that the chondrocytes were mostly alive and only few dead cells were observed in all groups at day 1. However, dead cells significantly increased in 0.5 and 1 mg/ml collagen gels after 7 days of culture. At this time point, the viability of cells remained high in 2 mg/ml collagen gel.
Fig. 3.
Fluorescence image of cells cultured in collagen gels at day 1 and day 7 by the live/dead assay. Live cells marked with green-fluorescent calcein, and dead cells labeled with red-fluorescent ethidium homodimer-1. A, D 0.5 mg/ml collagen gels; B, E 1 mg/ml collagen gels; C, F 2 mg/ml collagen gels. A–C culture day 1; D–F culture day 7
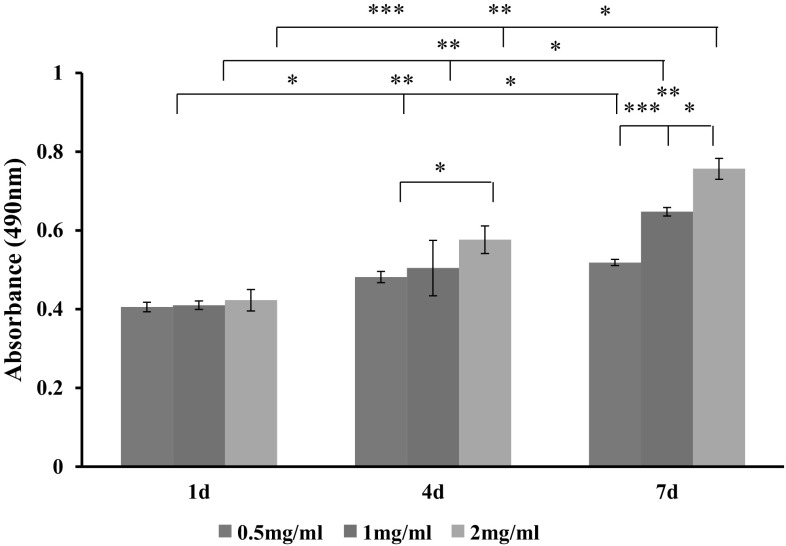
The cell growth level was examined by MTS cell proliferation assay, as shown in Fig. 4. All groups showed similar cell growth behaviors at day 1. Thereafter, the proliferation level of cells increased in a collagen concentration-dependent manner.
Fig. 4.
MTS assay of the cell viability in collagen gels (*p < 0.05; **p < 0.01; ***p < 0.001, n = 3)
Chondrogenic phenotype expressions
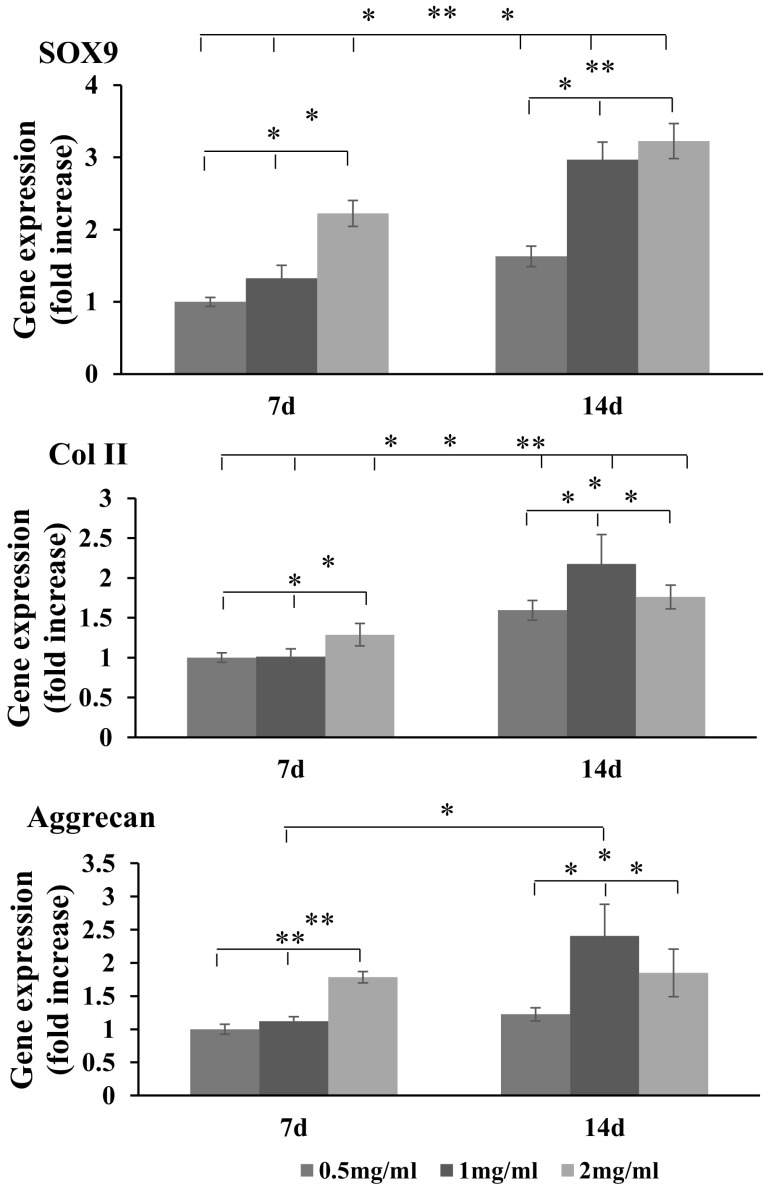
The expression of chondrocyte-related genes was analyzed at day 7 and 14 (Fig. 5). The SOX9, considered an early marker of chondrogenesis, increased in all groups with time. At day 7, the expression of SOX9 was higher in 2 mg/ml gel group than in other groups. At day 14, the gene expression was significantly higher in 1 and 2 mg/ml gel groups than in 0.5 mg/ml gel group. The type II collagen and aggrecan—relatively late stage markers of chondrogenesis—were expressed slightly higher in 2 mg/ml gel group than in other gel groups at day 7. These gene expressions significantly increased in 1 mg/ml gel group compared with those in 2 and 0.5 mg/ml gel groups at day 14.
Fig. 5.
Quantitative PCR chondrogenic gene expressions of the cells in collagen gels (*p < 0.05; **p < 0.01; n = 3)
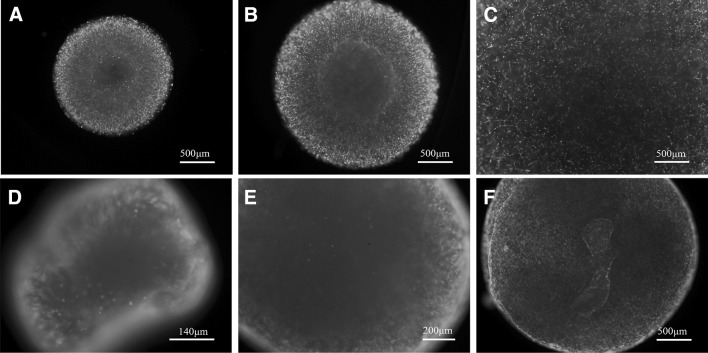
After culturing for 14 days the production of type II collagen was further examined by the immunofluorescence staining. As shown in Fig. 6, the green fluorescence signals were found mainly in the peripheral area of the gels in 0.5 and 2 mg/ml groups. Interestingly, the signals were observed in both peripheral and center regions of the gels in 1 mg/ml group. More positive staining was found in 1 mg/ml gel than in 0.5 and 2 mg/ml gels. In order to support the immunofluorescence staining result, the production of cartilaginous matrix of the cell-gel constructs at 14 days was then investigated by histological staining. Safranin O and Alcian blue were widely used for the histochemical detection of cartilaginous matrices, glycosaminoglycan (GAG). As shown in Fig. 7, all gel groups were positive for Safranin O and Alcian blue stainings at day 14. Both stainings were observed to be more intense and homogeneous in 1 mg/ml gel group than in the other groups.
Fig. 6.
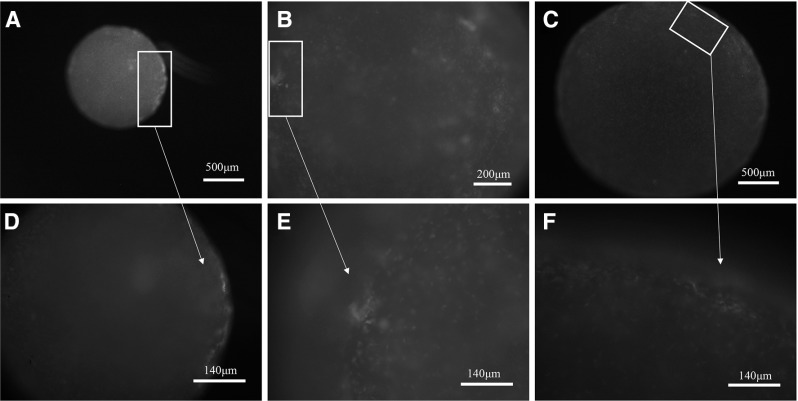
Type II collagen immunofluorescence staining of cells in collagen gels at day 14. A, D 0.5 mg/ml collagen gels; B, E 1 mg/ml collagen gels; C, F 2 mg/ml collagen gels
Fig. 7.
Glycosaminoglycan (GAG) production of the cells in collagen gels visualized by Safranin O and Alcian blue staining at day 14. A, D 0.5 mg/ml collagen gels; B, E 1 mg/ml collagen gels; C, F 2 mg/ml collagen gels. A–C Safranin O staining; D–F Alcian blue staining
Discussion
Type I collagen, a major extracellular component of connective tissues, has proven to be one of the most promising biomaterials for tissue engineering because of its excellent biocompatibility, biodegradability, rich source and easy processing [17]. It has been widely applied to bone [6, 7], nerve [18, 19] and tendon/ligament tissue engineering [20, 21]. Although type II collagen is the main protein of a cartilage ECM, type I collagen has also been demonstrated to be beneficial for supporting cartilage regeneration [22, 23]. In this study, we observed that the gel contraction and cell growth were collagen concentration-dependent; while the collagen gel contracted the most in the lowest concentration (0.5 mg/ml), the chondrocytes proliferated the most rapidly in the highest concentration (2 mg/ml). In fact, type I collagen gel has been widely used to encapsulate cells of different origins, having proven the cell and tissue compatibility. Therefore, the slower cell growth in lower collagen concentration groups (0.5 and 1 mg/ml) compared with that in 2 mg/ml is not from the toxicity of collagen, but maybe due to the low density of collagen fibrils that are actively engaged in the cellular adhesion, spreading and division.
The contraction of collagen gels during cultures has been reported by Zhou et al. [24]. They demonstrated that the gels with lower collagen concentrations contracted more than the gels with higher concentrations, in good agreement with our results (Fig. 1). The contraction of the collagen hydrogel is considered as a major obstacle for the applications in tissue engineering, and high cell density and low collagen concentration cause uncontrollable contraction of the gels [24, 25]. Therefore, many studies have been carried out to solve the collagen shrinkage issue. Visscher et al. [26] showed that collagen hydrogel contraction could be prevented by a 3D-printed polye-ε-caprolactone cage. Sheu et al. [27] revealed that cross-linking of collagen hydrogel using glutaraldehyde enhanced the strength and inhibited the contraction of the gel, which however, showed substantial cytotoxicity. Fibrin was incorporated into the collagen matrix to improve the mechanical property and to resist the gel contraction [28].
Here we thus hypothesized that the collagen concentration might affect the hydrogel contraction level. Initially, the collagen concentrations of 0.5–3 mg/ml were tried, however, the 3 mg/ml collagen solution produced some bubbles due to a high viscosity during the mixing with cells; thus the range of 0.5–2 mg/ml collagen concentrations was selected for this study. The gel contraction result demonstrated well that increasing the collagen concentration reduced the gel contraction significantly, supporting the merit of 2 mg/ml group.
However, the chondrocyte phenotype expression was not dependent on the collagen concentration, which was different from the cell growth and gel contraction behaviors. The chondrogenic genes and the cartilage ECM appeared to be the most significantly up-regulated in 1 mg/ml collagen gel (Figs. 5, 6, 7). We consider that the cells may need to aggregate for the proper maintenance of chondrogenic phenotypes, thus the gel contraction to a certain level can help this cellular contacts and aggregation. In fact, low cell seeding density results in very little cartilaginous ECM [29], while high cell density can form clinically relevant level of cartilage tissue [30, 31]. Therefore, the collagen gel concentration and cell density are considered to be properly used in order to gain optimal cellular functions for cartilage.
Type I collagen has significant advantages for tissue engineering applications because of its ability to promote cell adhesion and proliferation through collagen-binding integrins [32, 33]. In particular, sufficient number of cells is required for cartilage repair, thus the use of type I collagen is biologically relevant to increase the cell number for cartilage tissue engineering. However, a long-term expansion of cells often leads to dedifferentiation of the chondrocytes. Thus the maintenance of cartilage phenotype is another important consideration in cartilage tissue engineering [34–36]. Within hydrogels composed of some natural materials including alginate and agarose, the chondrocytes were shown to maintain in part their chondrogenic phenotype, suggesting the importance of 3D gel matrix environment for chondrocytes culture [37–39].
In this study the chondrocytes cultured in 3D collagen gels exhibited a mixture of round and spindle-shaped cells. In fact, the round shape is indicative of chondrocyte phenotype maintenance, and the dedifferentiated phenotype is often characterized by a change from the round to the spindle-shaped cells [40], therefore, the chondrocytes are considered to preserve in part their chondrogenic phenotype within type I collagen gels. Our previous work also demonstrated that the combination of type I collagen with other natural polymers, such as alginate and hyaluronic acid, increased the cell proliferation and chondrogenic gene expression [10]; therefore, the role of type I collagen to be played in chondrocyte cultures is considered beneficial, suggesting its potential use as a 3D gel matrix for cartilage regeneration. In this case, the gel contraction and the cell seeding density need to be carefully considered to enable proper cell growth and cell–cell contact induced aggregation, and ultimately to preserve the chondrogenic phenotypes and cartilage formation in vivo, which remains as further study.
Acknowledgements
This study was supported by grants from the Priority Research Centers Program (2009-0093829) through the National Research Foundation (NRF) founded by the Korea Ministry of Education, Science and Technology, Republic of Korea.
Conflict of interest
The authors have no financial conflict of interest.
Ethical statement
The chondrocyte isolation from Sprague–Dawley rats was according to the consent from Dankook University Institutional Animal Care and Use Committee (DKU-IRB-2014-039).
References
- 1.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43:128–136. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulkkinen HJ, Tiitu V, Valonen P, Jurvelin JS, Lammi MJ, Kiviranta I. Engineering of cartilage in recombinant human type II collagen gel in nude mouse model in vivo. Osteoarthritis Cartilage. 2010;18:1077–1087. doi: 10.1016/j.joca.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Pulkkinen HJ, Tiitu V, Valonen P, Hamalainen ER, Lammi MJ, Kiviranta I. Recombinant human type II collagen as a material for cartilage tissue engineering. Int J Artif Organs. 2008;31:960–969. doi: 10.1177/039139880803101106. [DOI] [PubMed] [Google Scholar]
- 4.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ignatius A, Blessing H, Liedert A, Schmidt C, Neidlinger-Wilke C, Kaspar D, et al. Tissue engineering of bone: effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials. 2005;26:311–318. doi: 10.1016/j.biomaterials.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 6.Oh SA, Lee HY, Lee JH, Kim TH, Jang JH, Kim HW, et al. Collagen three-dimensional hydrogel matrix carrying basic fibroblast growth factor for the cultivation of mesenchymal stem cells and osteogenic differentiation. Tissue Eng Part A. 2012;18:1087–1100. doi: 10.1089/ten.tea.2011.0360. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, El-Fiqi A, Han CM, Kim HW. Physically-strengthened collagen bioactive nanocomposite gels for bone: a feasibility study. Tissue Eng Regen Med. 2015;12:90–97. doi: 10.1007/s13770-015-0102-7. [DOI] [Google Scholar]
- 8.Yasui N, Osawa S, Ochi T, Nakashima H, Ono K. Primary culture of chondrocytes embedded in collagen gels. Exp Cell Biol. 1982;50:92–100. doi: 10.1159/000163133. [DOI] [PubMed] [Google Scholar]
- 9.Negri S, Fila C, Farinato S, Bellomi A, Pagliaro PP. Tissue engineering: chondrocyte culture on type 1 collagen support. Cytohistological and immunohistochemical study. J Tissue Eng Regen Med. 2007;1:158–159. doi: 10.1002/term.15. [DOI] [PubMed] [Google Scholar]
- 10.Mahapatra C, Jin GZ, Kim HW. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng Regen Med. 2016;13:538–546. doi: 10.1007/s13770-016-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nöth U, Siebenlist S, Rackwitz L, Schreiber B, Steinert A, Barthel T, Eulert J. Matrix-based autologous chondrocyte transplantation for the treatment of large osteochondral defects. London: Touch Briefings; 2006. pp. 62–64. [Google Scholar]
- 12.Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13:194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Chen CW, Tsai YH, Deng WP, Shih SN, Fang CL, Burch JG, et al. Type I and II collagen regulation of chondrogenic differentiation by mesenchymal progenitor cells. J Orthop Res. 2005;23:446–453. doi: 10.1016/j.orthres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Farjanel J, Schürmann G, Bruckner P. Contacts with fibrils containing collagen I, but not collagens II, IX, and XI, can destabilize the cartilage phenotype of chondrocytes. Osteoarthritis Cartilage. 2001;9(Suppl A):S55–S63. doi: 10.1053/joca.2001.0445. [DOI] [PubMed] [Google Scholar]
- 15.Mayne R, Vail MS, Mayne PM, Miller EJ. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci USA. 1976;73:1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin GZ, Kim HW. Porous microcarrier-enabled three-dimensional culture of chondrocytes for cartilage engineering: a feasibility study. Tissue Eng Regen Med. 2016;13:235–241. doi: 10.1007/s13770-016-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nöth U, Rackwitz L, Heymer A, Weber M, Baumann B, Steinert A, et al. Chondrogenic differentiation of human mesenchymal stem cells in collagen type I hydrogels. J Biomed Mater Res A. 2007;83:626–635. doi: 10.1002/jbm.a.31254. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Yu HS, Lee GS, Ji A, Hyun JK, Kim HW. Collagen gel three-dimensional matrices combined with adhesive proteins stimulate neuronal differentiation of mesenchymal stem cells. J R Soc Interface. 2011;8:998–1010. doi: 10.1098/rsif.2010.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozkurt A, Boecker A, Tank J, Altinova H, Deumens R, Dabhi C, et al. Efficient bridging of 20 mm rat sciatic nerve lesions with a longitudinally micro-structured collagen scaffold. Biomaterials. 2016;75:112–122. doi: 10.1016/j.biomaterials.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Gigante A, Busilacchi A, Lonzi B, Cecconi S, Manzotti S, Renghini C, et al. Purified collagen I oriented membrane for tendon repair: an ex vivo morphological study. J Orthop Res. 2013;31:738–745. doi: 10.1002/jor.22270. [DOI] [PubMed] [Google Scholar]
- 21.Yunoki S, Hatayama H, Ebisawa M, Kondo E, Yasuda K. A novel fabrication method to create a thick collagen bundle composed of uniaxially aligned fibrils: an essential technology for the development of artificial tendon/ligament matrices. J Biomed Mater Res A. 2015;103:3054–3065. doi: 10.1002/jbm.a.35440. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 23.Andereya S, Maus U, Gavenis K, Müller-Rath R, Miltner O, Mumme T, et al. First clinical experiences with a novel 3D-collagen gel (CaReS) for the treatment of focal cartilage defects in the knee. Z Orthop Ihre Grenzgeb. 2006;144:272–280. doi: 10.1055/s-2006-933445. [DOI] [PubMed] [Google Scholar]
- 24.Zhu YK, Umino T, Liu XD, Wang HJ, Romberger DJ, Spurzem JR, et al. Contraction of fibroblast-containing collagen gels: initial collagen concentration regulates the degree of contraction and cell survival. In Vitro Cell Dev Biol Anim. 2001;37:10–16. doi: 10.1290/1071-2690(2001)037<0010:COFCCG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg BM, Smith K, Colozzo M, Pollack R. Establishment and transformation diminish the ability of fibroblasts to contract a native collagen gel. J Cell Biol. 1980;87:304–308. doi: 10.1083/jcb.87.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visscher DO, Bos EJ, Peeters M, Kuzmin NV, Groot ML, Helder MN, et al. Cartilage tissue engineering: preventing tissue scaffold contraction using a 3D-printed polymeric cage. Tissue Eng Part C Methods. 2016;22:573–584. doi: 10.1089/ten.tec.2016.0073. [DOI] [PubMed] [Google Scholar]
- 27.Sheu MT, Huang JC, Yeh GC, Ho HO. Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials. 2001;22:1713–1719. doi: 10.1016/S0142-9612(00)00315-X. [DOI] [PubMed] [Google Scholar]
- 28.Brougham CM, Levingstone TJ, Jockenhoevel S, Flanagan TC, O’Brien FJ. Incorporation of fibrin into a collagen-glycosaminoglycan matrix results in a scaffold with improved mechanical properties and enhanced capacity to resist cell-mediated contraction. Acta Biomater. 2015;26:205–214. doi: 10.1016/j.actbio.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 29.LeBaron RG, Athanasiou KA. Ex vivo synthesis of articular cartilage. Biomaterials. 2000;21:2575–2587. doi: 10.1016/S0142-9612(00)00125-3. [DOI] [PubMed] [Google Scholar]
- 30.Puelacher WC, Kim SW, Vacanti JP, Schloo B, Mooney D, Vacanti CA. Tissue-engineered growth of cartilage: the effect of varying the concentration of chondrocytes seeded onto synthetic polymer matrices. Int J Oral Maxillofac Surg. 1994;23:49–53. doi: 10.1016/S0901-5027(05)80328-5. [DOI] [PubMed] [Google Scholar]
- 31.Park DY, Min BH, Lee HJ, Kim YJ, Choi BH. Repair of partial thickness cartilage defects using cartilage extracellular matrix membrane-based chondrocyte delivery system in human ex vivo model. Tissue Eng Regen Med. 2016;13:182–190. doi: 10.1007/s13770-016-9043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loeser RF. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 2014;39:11–16. doi: 10.1016/j.matbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guha Thakurta S, Budhiraja G, Subramanian A. Growth factor and ultrasound-assisted bioreactor synergism for human mesenchymal stem cell chondrogenesis. J Tissue Eng. 2015;6:2041731414566529. doi: 10.1177/2041731414566529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.do Amaral RJ, Matsiko A, Tomazette MR, Rocha WK, Cordeiro-Spinetti E, Levingstone TJ, et al. Platelet-rich plasma releasate differently stimulates cellular commitment toward the chondrogenic lineage according to concentration. J Tissue Eng. 2015;6:2041731415594127. doi: 10.1177/2041731415594127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdul Rahman R, Ahmad Radzi MA, Mohamad Sukri N, Md Nazir N, Sha’ban M. Tissue engineering of articular cartilage: from bench to bed-side. Tissue Eng Regen Med. 2015;12:1–11. doi: 10.1007/s13770-014-9044-8. [DOI] [Google Scholar]
- 37.van Susante JL, Buma P, van Osch GJ, Versleyen D, van der Kraan PM, van der Berg WB, et al. Culture of chondrocytes in alginate and collagen carrier gels. Acta Orthop Scand. 1995;66:549–556. doi: 10.3109/17453679509002314. [DOI] [PubMed] [Google Scholar]
- 38.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 39.Häuselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, et al. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107:17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 40.Benya PD. Modulation and reexpression of the chondrocyte phenotype; mediation by cell shape and microfilament modification. Pathol Immunopathol Res. 1988;7:51–54. doi: 10.1159/000157093. [DOI] [PubMed] [Google Scholar]