Abstract
Dental pulp stem cells (DPSCs) are mesenchymal stem cells with high self-renewal potential that have the ability to differentiate into several cell types. Thus, DPSCs have become a promising source of cells for several applications in regenerative medicine, tissue engineering, and stem cell therapy. Numerous methods have been reported for the isolation, expansion, and preservation of DPSCs. However, methods are diverse and do not follow specific rules or parameters, which can affect stem cell properties, adding more variation to experimental results. In this review, we compare and analyze current experimental evidence to propose some factors that can be useful to establish better methods or improved protocols to prolong the quality of DPSCs. In addition, we highlight other factors related to biological aspects of dental tissue source (e.g., age, genetic background) that should be considered before tooth selection. Although current methods have reached significant advances, optimization is still required to improve culture stability and its maintenance for an extended period without losing stem cell properties. In addition, there is still much that needs to be done toward clinical application due to the fact that most of DPSCs procedures are not currently following good manufacturing practices. The establishment of optimized general or tailored protocols will allow obtaining well-defined DPSCs cultures with specific properties, which enable more reproducible results that will be the basis to develop effective and safe therapies.
Keywords: Stemness, Cryopreservation, Differentiation, Cell culture, Banking, Enrichment
Introduction
Stem cells are defined as clonogenic, self-renewing, progenitor cells that can generate one or more specialized cell types. These cells have been isolated from many organs and tissues, including bone marrow, blood, heart, intestine, adipose tissue, liver, pancreas and teeth [1]. In particular, the dental tissues have become valuable alternative sources of stem cells because tissue collection for stem cell isolation involves non-invasive procedures, in contrast to conventional sources, such as adipose tissue and bone marrow, nor ethical issues as in the case of embryonic stem cells [2]. To date, several dental stem cells have been isolated, characterized and classified, depending on tissue origin and characteristics, as stem cells from human exfoliated deciduous teeth (SHEDs) [3], periodontal ligament stem cells (PDLSCs) [4], dental follicle progenitor cells (DFPCs) [5], stem cells from apical papilla (SCAPs) [6] and dental pulp stem cells (DPSCs) [7].
Dental pulp is a soft connective tissue contained in the central cavity of each tooth and is originated by the ectomesenchyme, which is derived from migratory neural crest stem cells during early development [8]. Stem cells from dental pulp have been isolated from several sources, including premolars, third molars, supernumerary, permanent and deciduous teeth [3, 7, 9, 10]. These stem cells are termed DPSCs or SHEDs whether they were isolated from adult or deciduous teeth, respectively, and both displayed a typical fibroblast-like morphology in culture [3, 7]. SHEDs exhibit a higher proliferation rate than DPSCs, but both display multidifferentiation potential, especially toward odontogenic lineage, like other dental stem cells [3]. Given the importance of dental stem cells for regenerative and biomedical research, over the last years, numerous methods for the isolation and expansion of DPSCs have been published, but variations, lack of specificity and non-optimal protocols have resulted in the isolation of heterogeneous cell populations, the gradual loss of stem cell properties, spontaneous differentiation, apoptosis and genomic instability mainly during expansion [11–13]. So, there is a growing interest in optimizing and establishing standard procedures to obtain and preserve well-defined dental stem cell cultures for specific applications, as well as increase efficiency, reproducibility and safety, especially when these cells are intended for clinical approaches [14]. In this review, we compare and analyze current experimental evidence to propose some factors that could be potential candidates for culture optimization and cryopreservation with the aim to improve DPSCs growth and storage. In addition, we highlight other factors related to DPSCs biology that should be considered before tooth selection.
Factors that influence DPSCs properties during experimental procedures
Teeth transport and short-term storage
Few rigorous studies have been conducted to improve transport and short-term storage medium for teeth, which are crucial to avoid degradation of tissue between the tooth extraction and DPSCs isolation step, whereby little progress has been achieved on this issue. A previous work demonstrated that DPSCs remained viable up to five days after tooth storage at 4°C in phosphate buffered saline (PBS), but viability was dramatically reduced after 24 h of storage, indicating that teeth should be immediately processed to ensure the highest cell recovery [15]. In addition, Ferro et al. [16] recommended the use of fresh pasteurized milk for teeth transport due to its antimicrobial properties. Other procedures involve teeth or dental pulp storage with cryoprotectant agents (CPA), but this issue is discussed later.
Tissue dissociation and stem cell isolation
For DPSCs isolation, the most widely used methods are (1) enzymatic digestion (ED) of tissues or (2) outgrowth from tissue explants (OG). ED enables isolation of single-cell suspensions from primary tissues by digestion with enzymes such as collagenases type I and II, dispase, trypsin and accutase [7, 17, 18]. On the other hand, the OG method is simpler and faster and consists of placing pulp fragments (1-2 mm3 pieces) directly into the culture plate, so that cells outgrow from the pulp tissue explants [19]. Comparison of two methods showed that DPSCs displayed a higher proliferation rate, differentiation and expressed other surface markers by ED method [20, 21]. In contrast, Hilkens et al. [19] compared both methods and found no significant differences in cell surface marker expression and differentiation potentials. Kerkis et al. [22] isolated a cell population termed dental pulp immature stem cells (IDPSCs) from deciduous teeth using the OG method, and they suggested that the culture of pulp fragments promoted the selective proliferation of IDPSCs preventing premature differentiation. This was supported by the fact that the continuous transfer of explants from deciduous teeth into new cell culture dishes allowed the isolation of IDPSCs at least for six months [23]. Thus, the OG method provides a sufficient amount of stem cells for basic research and transplant models [16, 24, 25], which may be promising for clinical studies. The establishment of primary cell culture from tissue explants spends more time than enzyme-digested tissues, but enables the isolation of a more homogeneous populations [22, 26].
Several digestion protocols have been reported for dental pulp dissociation, but the combination of 3 mg/ml collagenase type I and 4 mg/ml dispase has been used more frequently (Table 1). Nevertheless, digestion protocols have not been compared between them, so the optimal conditions to obtain more viable cells have not been evaluated. In addition, other enzymes or even mechanical dissociation for other stem cells could be useful to be assessed for DPSCs and increase survival during isolation. In this regard, for example, the combination of 0.1% collagenase IV plus 0.25% trypsin EDTA and 5 mg/ml collagenase type II plus 2.5 mg/ml dispase I have been used to isolate DFPCs [38] and PDLSCs [39], respectively. Finally, devices such as gentleMACSTM dissociator (Miltenyi, Biotec) could be adapted for mechanical dissociation of dental pulp tissue, which may be attractive for clinical stem cell isolation.
Table 1.
Enzyme digestion protocols used for DPSCs isolation
| Enzymatic cocktail | Conditions | Teeth | References |
|---|---|---|---|
| 3 mg/ml collagenase I, 4 mg/ml dispase | 1 h at 37°C | Third molars, molars | [7] |
| 3 mg/ml collagenase I, 4 mg/ml dispase | 1-2 h at 37°C | Mouse incisor | [27] |
| 4 mg/ml collagenase I, 4 mg/ml dispase II | 24 h at 4°C | Deciduous teeth | [28] |
| 4 mg/ml collagenase I, 2 mg/ml dispase | 1 h at 37°C | Incisors | [29] |
| 0.2 mg/ml collagenase I, 2 mg/ml dispase | 70 min 37°C | Third molars | [30] |
| 1 mg/ml collagenase I, 2.4 mg/ml dispase | 1 h at 37°C | Deciduous teeth, adult molars | [31] |
| 0.3 mg/ml collagenase I, 0.1% dispase II | 1 h at 37°C | Deciduous teeth, premolars | [10] |
| 1-3 mg/ml collagenase/dispase | 20 min at 37°C | NS | [32] |
| 1 mg/ml collagenase/dispase | 30 min at 37°C | Third molars | [26] |
| 3 mg/ml collagenase type I | 1 h at 37°C | Third molars | [33] |
| 3 mg/ml collagenase type I | 40 min at 37°C | Third molars | [34] |
| 0.2% collagenase type II | 30 min at 37°C | Third molars | [35] |
| 0.2% collagenase type I | 1 h at 37°C | Incisors, canines, molars, deciduous teeth | [36] |
| 1 mg/ml collagenase I | 30 min at 37°C | Third molars | [18] |
| Accutase solution | 30 min at 37°C | Third molars | [18] |
| 0.04 mg/ml Liberase (mix collagenase I and II) | NS | Third molars | [37] |
| 0.2% trypsin pretreated explants | 5 min at 37°C | Third molars | [17] |
NS not specified
Cell attachment
A key step to improve the establishment of primary culture involves the optimal cell attachment in the plastic dish, which can be enhanced through the pre-coating of plastic surfaces with extracellular matrix (ECM) proteins, peptide modified surfaces, synthetic polymeric cations or culture treated surfaces [40–43]. Some works have used fibronectin [17, 44] and Cell+ surfaces [45] to establish primary cultures of DPSCs, but if these conditions enhanced cell recovery were not determined. Besides, Spath et al. [17] showed that poly-D-lysine did not sustain expansion of DPSCs due to most cells remained in suspension, whereas collagen-coated dishes sustained growth but altered morphology. Interestingly, dental pulp stem cell-derived ECM was found to promote the growth, proliferation and expression of stemness markers of induced pluripotent stem cells (iPSCs) generated from DPSCs, in comparison to matrigel [46]. This finding suggests that ECM components may enhance long-term culture of DPSCs, similar to previous reports in other stem cells [47]. In this regard, ECM provides more than a substrate for attachment, but also plays a key role in signaling events that are essential to maintain stem cell niche [48]. Several ECM molecules, such as recombinant vitronectin [49], laminin-511 [50] and laminin-521/E-cadherin [51], have been reported to support long-term culture of pluripotent stem cells. Other options may include the use of synthetic polymers, for example, polyethyleneimine [42] and poly[2-(methacryloyloxy)ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide] (PMEDSAH) [52], which have been shown that promote the attachment of weakly anchoring cells and primary tissues. Considering that recombinant proteins may significantly increase costs, their incorporation into DPSCs methods will depend on research purposes and a cost-benefit balance to make it a feasible option.
Media for cell culture
One of the most challenged and discussed issue is the media composition for DPSCs culture due to the striking impacts on differentiation potential and stability. To date, an optimal culture medium that avoids spontaneous differentiation and changes in stem cell properties has not been reported. In this section, we compare and analyze cell culture media components commonly used for DPSCs culture in order to suggest some factors that should be considered for further optimization, with the aim to maintain self-renewal and differentiation potential, or formulate new and tailored protocols to isolate DPSCs with particular features directed toward specific uses.
Basal media
The most common culture media used for DPSCs include alpha minimal essential medium (α-MEM), Dulbecco’s modified Eagle medium (DMEM), DMEM/Ham’s F12 nutrient medium (F12) (DMEM/F12), DMEM low glucose (DMEM-LG) and DMEM Knock Out (DMEM-KO) media (Table 2). Surprisingly, comparison of cell culture media for DPSCs isolation and expansion in equivalent conditions is scarce, but these studies have provided interesting findings. A previous work showed that α-MEM and DMEM-KO were the most optimal culture media maintaining a higher proliferation rate, differentiation potential and lower levels of senescence, when compared with DMEM-LG and DMEM/F12 [34]. Moreover, α-MEM also enhanced the expression of osteogenic genes during differentiation at early and late DPSCs passages, whereas the other media showed a reduced level of the same genes [34]. IDPSCs also exhibited a better growth in α-MEM during isolation and even after cryopreservation in comparison with DMEM-LG and DMEM/F12 [23]. Another study showed that α-MEM increased proliferation, ALP activity and the number of α-smooth muscle actin positive cells (SMA+), which represent a potential source of progenitors of odontoblastic cells, when compared with RPMI-1640 medium [70]. Based on these findings, α-MEM could be optimized to improve long-term culture of DPSCs by modifying conditions or supplements to prolong self-renewal, as it has been suggested in other studies [44, 45, 72]. Alternatively, taking advantage that several commercial cell culture media have been designed for pluripotent stem cells (e.g., human embryonic stem cells (hESC) or IPSCs [Reviewed in 73]), these developments should be harnessed to evaluate long-term culture and stability or determine if optimization would be possible for DPSCs culture.
Table 2.
Media and supplements commonly used for cell culture of DPSCs
| Basal medium | Supplements | Serum | Tissue origin | Differentiation potential in vitro | References |
|---|---|---|---|---|---|
| α-MEM | Platelet lysate (1-10) used only for expansion | 20% FBS | Third molars | Adipogenic, osteogenic | [53] |
| L-glutamine, ascorbic acid-2-phosphate; 2% UCB-PRP plus 5% FBS only for expansion | 15% FBS | Deciduous teeth, molars | Osteogenic | [31] | |
| GlutaMAX, L-ascorbate-2-phosphate | 10% FBS | Mouse incisors | Neuronal | [27] | |
| L-glutamine, L-ascorbic acid-2-phosphate | 15-20% FBS | Deciduous teeth, third molars | Osteogenic, adipogenic, odontogenic, hepatic, chondrogenic, endothelial, neuronal | [7, 54] | |
| L-glutamine | 10% FBS | Third molars | Neuronal, myogenic, osteogenic, chondrogenic, adipogenic | [19, 55] | |
| GlutaMAX | 10% FBS | Permanent teeth | Osteogenic, adipogenic, chondrogenic | [34] | |
| L-glutamine, ascorbic acid, bovine pituitary extract | 10% FBS | Human/rat molars | Osteogenic (spontaneous) | [11] | |
| L-glutamine, ascorbic acid-2-phosphate, dexamethasone, EGF and PDGF-BB | 2% FCS | Third molars | Osteogenic, chondrogenic, endothelial, myogenic, neuronal | [30] | |
| Dexamethasone, ITS, EGF and PDGF-BB | 2% FCS | Premolar, third molars, deciduous teeth | NA | [45] | |
| Non-essential amino acids | 20% FBS | Third molars | Odontogenic, chondrogenic | [56] | |
| DMEM-KO | GlutaMAX | 10% FBS | Permanent teeth | Osteogenic, adipogenic, chondrogenic | [34] |
| GlutaMax, ascorbic acid, ITS | 10% FBS or PL | Third molars | Osteogenic, adipogenic, chondrogenic | [57] | |
| DMEM | SFM was used for expansion and supplemented with combinations of ITS-X, ETF, FGF-a, sodium pyruvate and ascorbic acid | 10% FBS | Deciduous teeth, third molars | NA | [58] |
| None | 10-15% FBS | Third molars, premolars deciduous teeth | Hepatic, osteogenic, adipogenic, chondrogenic | [25, 28, 59] | |
| Nonessential Amino Acids | 20% FBS | Molars | Osteogenic, adipogenic | [60] | |
| L-ascorbic acid-2-phosphate | 20% FCS | Deciduous incisors | Adipogenic, chondrogenic, myogenic, osteogenic | [61] | |
| L-glutamine, nonessential amino acids, sodium pyruvate, β-mercaptoethanol | 15% FBS | Molars | Osteogenic | [62] | |
| G-CSF was added to 2nd passaged to mobilize DPSCs | 10% HS | Third molars | Osteogenic, odontogenic, neuronal, angiogenic | [63] | |
| MegaCell DMEM | L-glutamine, β-mercaptoethanol | 10% FCS | Third molars | Osteogenic, chondrogenic, myogenic | [17] |
| DMEM/F12 | GlutaMAX | 10% FBS | Permanent teeth | Osteogenic, adipogenic, chondrogenic | [34] |
| GlutaMAX | 15-20% HS or 10% FBS | Third molars | Osteogenic, chondrogenic, adipogenic | [64] | |
| L-glutamine, nonessential amino acids | 15% FBS | Deciduous teeth | Myogenic, chondrogenic | [23] | |
| GlutaMAX, L-ascorbic acid-2-phosphate | 10% FBS | Third molars | Osteogenic, adipogenic, chondrogenic | [65] | |
| Basal medium: Glucose and HEPES. After 12 h, medium was supplemented with N2, EGF, bFGF and heparin, FBS was not added | 10% FBS | Third molars | Neuronal, osteogenic | [66] | |
| 60% DMEM-LG/ 40% MCDB-201 | ITS, LA-BSA, dexamethasone, ascorbic acid-2-phosphate, PDGF-BB, EGF, LIF, chemically defined lipid concentrate, BSA, β-ME | 2% FBS | Third molars | Osteogenic, endothelial, hepatocyte, neuronal | [33, 44] |
| DMEM-LG | GlutaMAX | 10% FBS | Permanent teeth | Chondrogenic, adipogenic, osteogenic, | [34] |
| NH | Medium is similar to DMEM and contains FBS and L-glutamine | FBS | Molars | Osteogenic, endothelial, neuronal, glial, chondrogenic, adipogenic | [67] |
| RPMI-1640 | GlutaMAX with or without dexamethasone | 10% FCS | Third molars | Odontogenic (spontaneous) | [68] |
| L-glutamine, sodium bicarbonate, glucose, HEPES, sodium pyruvate | 10% FBS | Permanent teeth | NA | [69] | |
| L-glutamine | 10% FCS | Third molars | Osteo/odontogenic (spontaneous) | [70] | |
| MesenCult | MSC stimulatory supplements | ND | Third molars | Adipogenic, osteogenic, chondrogenic | [15] |
| MegaCell | L-glutamine, ascorbic acid-2-phosphate | 10% FBS | Permanent teeth | Myogenic, neuronal, adipogenic | [71] |
| FMC | PDGF-ββ, EGF, IGF-1, bFGF, dexamethasone, linoleic acid, ascorbic acid | 0.25-2.5% HS | Deciduous teeth | Osteogenic, myogenic, neural, hepatocyte | [16, 72] |
| EBM2 | IGF-1, EGF | 10% FBS | Third molars | Chondrogenic, adipogenic, odontogenic | [35] |
| SERICIN GIT or STK2 or MF | None | – | Third molars | NA | [18] |
| MSCGM-CD | None | – | Third molars | Osteo/odontogenic, adipogenic | [18] |
FBS fetal bovine serum, FCS fetal calf serum, UCB-PRP platelet rich plasma derived from umbilical cord blood, EGF epidermal growth factor, PDGF ββ platelet-derived growth factor-ββ, ITS insulin-transferrin-selenium, ITS-X insulin-transferrin-selenium-ethanolamine, ETF embryotrophic factor, FGFα fibroblast growth factor α, G-CSF granulocyte-colony stimulating factor, bFGF basic fibroblast growth factor, LIF leukaemia inhibitor factor, BSA bovine albumin serum, IGF-1 insulin growth factor, ND not determined, β-ME β-mercaptoethanol, NA not analyzed, NH non-hematopoietic (NH) stem cell expansion medium, SFM serum free media, FMC F-12 Coon’s and Ambesi’s modified:Medium-199: CMRL-1066, EBM2 endothelial cell basal medium 2
Serum supplementation and humanized substitutes
Standard cell culture media are commonly supplemented with fetal bovine serum (FBS; or also called FCS “fetal calf serum”), which provides many components required for cell growth, metabolism and proliferation [74]. Most of the culture protocols use high concentrations of serum (10-20%), because it allows for better cell adhesion and growth, and therefore, the isolation of a larger population of DPSCs (Table 2). However, some concerns have raised by the use of FBS for DPSCs isolation directed toward human cell therapy, because it involves potential risks of infections, severe immune reactions and pathogen contamination [75]. Thus, humanized substitutes, such as human serum (HS) or blood-derived preparations, have been explored as an option to replace FBS supplementation. Isolation and expansion of DPSCs have been successful using DMEM/F12 and StemPro® MSC media supplemented with 15-20% HS, respectively [64]. Besides, culture medium supplemented with growth factors and 2.5% HS was able to sustain proliferation, osteoblastic differentiation and marker expression similar to FBS-containing media [72]. In addition, similar results to FBS have been achieved by addition of human blood-derived preparations such as platelet lysates (PL) and platelet-rich plasma from human umbilical cord blood (UCB-PRP) during DPSCs expansion [31, 53, 57]. In fact, DPSCs expanded in medium containing 10% PL showed acceptable ranges for all standard quality controls for GMP conditions [57]. Despite the benefits offered by the humanized substitutes, the potential risk of disease transmission or viral infection from human origin remains, as well as variation in composition associated to different human samples. Therefore, the development of chemically defined culture media has gained a lot of importance to satisfy the guidelines required for therapeutic use.
Growth factors and signal molecules
The role of growth factors has been widely characterized in stem cells, especially for differentiation toward specific lineages or inhibition of spontaneous differentiation. Some works have evaluated the effect of growth factors on DPSCs culture (Table 3), showing that they can been associated to stimulate proliferation [72], maintain genomic stability for long-term [30, 44, 45] and enhance growth under low levels of serum [30, 44, 45]. Basic fibroblast growth factor (bFGF, also known as FGF-2) and epidermal growth factor (EGF) have been used routinely for MSCs culture because they have a pronounced mitogenic effect and retain differentiation potential toward several lineages [76, 77]. In fact, it has been confirmed that bFGF enhanced proliferation and stemness in DPSCs [78]. These findings underpin that growth factors and signal molecules that regulate signaling pathways represent key candidates to optimize cell culture media; however, determining the ideal growth factor supplementation is not an easy task due to implicate: 1) the selection of growth factors alone or in combination that displayed a positive effect on growth and survival of DPSCs, and 2) determination of the optimal concentrations that stimulate proliferation without compromising differentiation potential and stability. Taking into account that growth factors may trigger multiple biological effects regulating cell fate, specific protocols can be established to increased commitment of stem cells into particular lineages when is required. For example, bone marrow stem cells grown under high concentrations of bFGF enhanced commitment toward osteogenic lineage, but decreased neuronal differentiation [79], whereas addition of vascular endothelial growth factor (VEGF) increased commitment into osteogenic lineage in PDLSCs [80]. Interestingly, Wnt/β-catenin pathway inhibited osteogenic differentiation in DPSCs suggesting that maintained them in an undifferentiated state [81], albeit if Wnt/β-catenin activation increased stemness was not evaluated. Based on this finding, the understanding of signaling pathways involved in stemness and differentiation will provide novel targets to modulate stem cell fate, enabling cell culture to support DPSCs expansion in the undifferentiated state.
Table 3.
Growth factors supplemented to media culture of DPSCs
| Growth factor(s) | Conditions | Results | References |
|---|---|---|---|
| EGF and PDGF-BB (concentration not specified) | α-MEM, 2% FBS, dexamethasone and ITS | Enhanced proliferation of DPSCs maintaining a stable karyotype beyond 65 population doublings without exhibiting signs of spontaneous differentiation | [45] |
| 100 µg/ml ETF | DMEM with 1% ITS-X | DPSCs and SHEDs exhibited higher survival and proliferation rates compared with other combinations of growth factors during expansion, although was not compared with FBS. Surface marker expression was comparable to FBS | [58] |
| 10 ng/ml EGF, 10 ng/ml PDGF-BB | α-MEM, 2% FBS, L-glutamine, ascorbic acid-2-phosphate, dexamethasone | Enhanced initial cell adhesion of primary culture compared to serum free-media, but cell recovery was lower than FBS; DPSCs expressed several stem cell-associated markers similar to FBS medium, except CD146 and α-SMA | [30] |
| 1000 units/ml LIF, 10 ng/ml EGF, 10 ng/ml PDGF-BB | 60% DMEM low glucose, 40% MCDB-201, 2% FBS, ITS, LA-BSA, ascorbic acid-2-phosphate, BSA, β-ME, dexamethasone, chemically defined lipid concentrate | Allowed the isolation of a heterogeneous population containing DPPSCs and favored long-term expansion after 65 passages maintaining a stable karyotype | [33, 44] |
| 10 ng/ml EGF, 25 ng/ml bFGF | DMEM/F12, glucose, Hepes, N2 supplement, heparin | This medium was used for culturing of adherent (ADH)-DPSCs and non-ADH DPSCs; comparison of medium without growth factors was not performed | [66] |
| EGF and IGF-1 (concentration not specified) | EBM2, 10% FBS | Isolation of a highly proliferative DPSCs population, although comparison of medium without growth factors was not performed | [35] |
| 10 µg/ml PDGF-ββ, 100 µg/ml EGF, 100 µg/ml IGF-1 and 100 µg/ml bFGF | F-12 Coon’s and Ambesi’s modified:Medium 199: CMRL166 with 1.25% HS | Enhanced proliferation when combined with low levels of HS displaying a similar expression of cell markers compared to FBS | [72] |
Serum-free chemically defined media
Despite HS or blood-derived substitutes can be used to isolate stem cells for clinical applications, they contained several undefined compounds, which vary depending on each donor [74, 75]. So, the development of chemically defined media is the ultimate goal for therapeutic use due to composition is totally controlled, as well as precise concentrations are known. So far, few reports have documented the isolation and expansion of DPSCs in serum-free chemically defined media. Khanna-Jain et al. [64] reported that StemPro® MSC was unsuccessful to establish primary cell culture and poorly sustained expansion of DPSCs with a low proliferation and differentiation capability. Takeda-Kawaguchi et al. [18] tested several serum-free chemically defined media for isolation and expansion of DPSCs from third molars, but only MSCGM-CD medium supported DPSCs culture comparable to FBS-containing media. So, there is an imminent need to develop new formulations employing defined components that support DPSCs expansion and stemness, guarantying reproducibility and safety.
Additional supplements
Other compounds added to cell culture not only provide the nutritional requirements for sustaining cell growth and proliferation, but also modulate commitment and maintenance. Glucose is an essential source of energy for cells, but higher concentrations have been associated to induce replicative senescence in MSCs, especially under high oxygen concentrations [82]. In PDLSCs, high glucose levels inhibited proliferation and osteogenic differentiation [83], revealing that glucose concentration may be an important factor that modulates DPSCs behavior. Besides, Insulin-Transferrin-Selenium (ITS) was shown to increase cell proliferation during first passages of DPSCs cultures, although induced senescence several days before than DPSCs grown on basal medium without ITS [45]. Interestingly, BiodentineTM, a tricalcium silicate cement formulation that enhance osteogenic differentiation, was shown to increase proliferation, migration and adhesion of DPSCs under normal culture medium [84], although if Biodentine modified differentiation potential was not evaluated. Other compounds such as dexamethasone, ascorbic acid and β-glycerophosphate, have been demonstrated that enhance the commitment of DPSCs toward osteogenic and odontogenic lineages [85, 86], which could be directed for the establishment of specific protocols. On the other hand, Rho-associated coiled coil kinase (ROCK) inhibitor Y-27632 has been reported that prevents dissociation-induced apoptosis [87], delays senescence during passaging [88] and increases survival of cryopreserved stem cells after thawing [89]. Based on these findings, Y-27632 inhibitor could be tailored to DPSCs protocols to improve primary culture, expansion and cryopreservation methods.
Culture conditions
During cell culture, several environmental conditions (temperature, humidity, acidity among others) but, in particular, the levels of oxygen play a crucial role influencing the growth of cells. The physiological oxygen levels vary from 3% to 6% in adult organs and tissues, in contrast to 18% to 21% reached in conventional cell culture systems [90], causing hyperoxic conditions and oxidative stress. Recently, it has been demonstrated that 21% O2 decreased cell proliferation and promoted the activation of antioxidant defenses in DPSCs when compared to 3% O2 [91]. These effects were reversed through supplementation with trolox, a vitamin E analog, which reduced oxidative effects triggered by 21% O2 and increased proliferation at similar levels found in hypoxic cultures [91], highlighting the importance of oxygen levels in cell culture. In other stem cells, hypoxia induced the expression of stemness genes such as Oct4, nanog, sall4 and Klf4, as well as reduced spontaneous differentiation and maintained chromosome stability for long-term cultures [92, 93]. Another strategy to reduce oxidative stress involves the addition of antioxidants, such as N-acetyl-L-cysteine and L-ascorbic acid 2-phosphate, to change the redox conditions in cell culture [94, 95]. However, antioxidant concentration should be carefully optimized and evaluated due to the possible side effects in stem cell biology at long-term.
Cell selection by sorting methods
The homogeneity of cell culture populations at a defined stage of differentiation is a requirement needed to guarantee reproducibility and safety in basic and clinical research. Cell separation techniques are mainly based on density, adherence or antibody binding, this last one including immunopanning and cell sorting methods [96]. For DPSCs, current cell selection methods involve fluorescence-activated cell sorting (FACS) and magnetic-activated cell sorting (MACS). DPSCs are MSCs, so they are positive for at least CD73, CD90, CD105, and negative for CD11b, CD14, CD19, CD34, CD45, CD79a and HLA-DR surface antigens, according to The Committee of Mesenchymal Stem Cells and Tissues of the International Society for Cellular Therapy (ISCT) [97]. Other groups have reported the expression of CD29, CD44, CD166, STRO-1, CD13, CD146, CD117, SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, NANOG, Oct4 and Sox2 [28, 44, 98]. However, to date, a specific marker that exclusively identifies DPSCs has not been reported. Indeed, it has been suggested that several subpopulations of DPSCs coexist in dental pulp and exhibit different biological functions, which have hampered the identification of unique markers. Moreover, controversy has arisen over the isolation of DPSCs subpopulations that differentially expressed some cell surface markers, for example, CD34 [55, 99] and CD73 [44]. Several attempts have been made to analyze cell surface markers for selection of specific subpopulations of DPSCs (Table 4). Despite these efforts, there is a lack of consensus to define the combinations of relevant cell surface markers to identify specific DPSCs subpopulations, and it is clear that more markers still need to be explored. In addition, it has been reported that DPSCs exhibit the ability to exclude Hoechst33342 dye enabling the isolation of a CD31−/CD146− “side population” (SP) with highly angiogenic potential [102], but the use of Hoechst dye as a selective method is so far for therapeutic use because this dye is a potential carcinogen and affects differentiation [104].
Table 4.
Comparison of cell surface markers used for specific cell enrichment in DPSCs cultures
| Cell surface marker(s) | Method | Enrichment | Tissue | Description | Ref. |
|---|---|---|---|---|---|
| STRO-1+ or CD146+ or 3G5+ | MACS | After tissue dissociation | Third molars | Significant increase in the number of colony-forming cells when compared with unsorted cells | [100] |
| CD73− | MACS | Primary culture | Third molars | DPPSCs expressed TERT, Oct3/4 and Sox2, and displayed a high proliferative potential unlike its CD73+ counterpart. | [44] |
| c-kit+/CD34+/CD45− | FACS | Primary culture | Molars | Subpopulation highly clonogenic with the potential to differentiate into self-renewing osteoblast precursors | [99] |
| STRO-1+/c-Kit+/CD34− | MACS | Primary culture | Third molars | Higher proliferation in long-term cultures, reduced senescence and apoptosis rates compared to STRO-1+/c-Kit+/CD34+ cells; differentiation was similar for mesodermal lineages, but STRO-1+/c-Kit+/CD34+ showed a greater commitment into neurogenic induction. | [55] |
| CD271+ | FACS | Primary culture | Third molars | Higher differentiation potential, especially odontogenic, compared with STRO-1+CD146+ or CD51+/CD140α+ subpopulations isolated in the same study | [56] |
| CD271+ | MACS | Primary culture | Permanent teeth | Cells expressed STRO1, vimentin, CD105 and Notch-2 and differentiated toward mesenchymal lineages | [101] |
| STRO1+ | MACS | Primary culture | Human/rat molars | STRO1+ cells decreased proliferation and increased spontaneous differentiation during subsequent passages. Probably represent a heterogeneous subpopulation | [11] |
| CD117+ | MACS | Primary culture | Deciduous teeth | Homogeneous subpopulation differentiated toward hepatocytes and pancreatic cells | [28] |
| CD117+ | MACS | Primary culture | Molars | Homogeneous subpopulation was enhanced into osteogenic differentiation in novel conditioned medium | [62] |
| CD105+ | FACS | Primary culture | Third molars | Subpopulations used for isolation of mobilized DPSCs (MDPSCs) by G-CSF. CD105+ cells showed greater neovascularization and pulp regeneration in models than DPSCs unsorted, but less then MDPSCs | [63] |
| SSEA4+ | FACS | Primary culture | Third molars | Highly pluripotent stems cells with multilineage differentiation potential | [33] |
| CD117+/CD34+/STRO-1+/ flk1+ | FACS | Primary culture | Adult teeth | Homogeneous subpopulation differentiated toward several lineages, including osteoblasts and endotheliocytes | [71] |
| CD31−/CD146− | FACS | After tissue dissociation | Porcine tooth germ | Side population was sorted with CD31−/CD146− markers; selected cells showed differentiation to several lineages, but showed a high angiogenic potential | [102] |
| CXCR4+ | MACS | Primary culture | Permanent teeth | STRO-1 and CD146 were higher in CXCR4+ cells than CXCR4− or unsorted cells | [103] |
CD271 is the same that LANGFR, low-affinity nerve growth factor receptor; CD117 is c-kit marker
G-CSF growth colony stimulating factor, CXCR4 CXC chemokine receptor type 4, NS not specified
Cryopreservation and banking
The development of reliable methods for cryopreservation and banking is essential for stem cell research and future clinical applications. Two storage methods are commonly used: traditional cryopreservation and magnetic cryopreservation/freezing. A general protocol for traditional cryopreservation consists in the addition of 10% dimethyl sulphoxide (DMSO) (or other CPA) in complete medium or FBS, followed by slow freezing (conventional or programmed) and storage in liquid nitrogen, which has been applied for preservation of DPSCs [15, 16, 22, 105]. Using this method, DPSCs were capable of bone differentiation in vitro and transplantation assays after 2 years of cryopreservation [106]. Viable DPSCs have also been recovered from cryopreserved entire pulp from third molar and deciduous teeth, displaying cell surface markers and differentiation potential similar to fresh cultures [54, 59, 107], although morphological alterations and lower proliferation rates have been reported [59]. Wood et al. [107] isolated DPSCs from cryopreserved third molars and entire or digested pulp tissues, but results were not reproducible between samples. However, they demonstrated that cryopreserved DPSCs isolated from fresh tooth were functional after storage at -85 and -196°C at least for 6 months [107]. In addition, digging micro-channels into the tooth has been suggested to increase DMSO penetration, which may improve cell viability in deciduous teeth banked at -80°C [108].
On the other hand, magnetic cryopreservation, also called Cell Alive System (CAS), is a new technology in which a weak magnetic field is applied to cells and tissues in order to low the freezing point by up to 6-7°C, enhancing vitrification without inducing cell membrane damage caused by ice expansion and nutrient drainage [109]. Magnetic cryopreserved premolars retained 73% DPSCs viability with no visible changes in the morphology, expression of stem cell markers, osteogenic and adipogenic potential when compared to cells isolated from fresh teeth [110]. Another study showed that magnetic cryopreserved DPSCs exhibited a post-thawing cell viability, proliferation, expression of surface markers and differentiation ability similar to fresh DPSCs and better than traditional cryopreserved cells [29]. Importantly, this study evidenced that magnetic cryopreservation preserved cells using a reduced DMSO concentration (3%) in serum-free cryopreservation solution, make it promising for banking cells directed toward stem cell-based therapies [29]. In addition, tooth banking for tooth autotransplantation has also been successfully performed using this method, showing satisfactory implantation outcomes in patients [111, 112]. At histological level, magnetic cryopreservation retained tissue architecture of tooth under storage, maintaining viable cells of odontoblastic region and cell-rich zone, where MSCs from dental pulp reside, while traditional cryopreservation disrupted and damaged the tissue [113, 114].
These methods undoubtedly offer promising options for preserving cells, tissues and organs. However, optimization is still ongoing and involves the determination of the optimal magnetic field, slow-freezing programs and composition of cryopreservation solution. In this regard, DMSO is the most common CPA used for cryopreservation, but is toxic to tissues and cells rising some concerns for clinical applications [115–117]. However, little work has been conducted to test other CPAs or supplements that improve DPSCs storage, and alternatively, the development of methods or devices for CPA removal. It has been confirmed that DMSO preserved DPSCs with higher viability upon ethylene glycol or propylene glycol under traditional cryopreservation [107]. Promising results have been obtained using trehalose/DMSO for umbilical cord blood cells [118], glucose/sucrose/ethylene glycol cocktail for DFSCs [119] and polyvinylpyrrolidone polymer for adipose derived stem cells (ASCs) [120], which could be evaluated for DPSCs cryopreservation. Other additives, such as ROCK inhibitor Y-27632, which enhances viability of cryopreserved pluripotent stem cells and stimulates colony growth when added to culture medium after thawing [89], should be evaluated for preservation of DPSCs.
Other factors influencing DPSCs properties
In addition to experimental procedures, several works have pointed out other factors that should be considered for tooth selection. It has been demonstrated that SHEDs possess higher proliferation rates and differentiation potential even after freezing and storage than DPSCs [10, 54]. A possible explanation may be associated with the loss of a more immature state due to aging, which may lead to reduced viability, proliferation and differentiation potential [67, 121]. Some studies suggest a maximum donor age of 25 years old to isolate high-quality stem cells from permanent teeth, because DPSCs obtained from older tooth displayed a low level of telomerase expression [98] and proliferation rate [67], although differentiation potential is slightly reduced [67]. Moreover, tooth aging may increase the risk of acquired mutations throughout donor life, compromising the quality and safety of DPSCs [122]. On the other hand, genetic background also must be considered in tooth selection and interpretation of results of further studies based on DPSCs cell lines. Particular mutations or polymorphisms present in the donor genome can have a profound effect on cellular processes, such as differentiation toward certain lineages or represent a risk factor for developing a disease [123, 124]. Despite these conditions are not desirable for transplants, it is important to underline that patient-derived DPSCs could be tremendously useful for modeling human disorders and drug screening, broadening their applications [123, 125].
Future challenges
Notwithstanding significant advances have been conducted to improve DPSCs manufacturing, there is still a need to develop better methods and optimized protocols to maintain stem cell properties for long-term, making them available for future research. In the case of therapeutic use, many improvements need to satisfy International GMP guidelines to generate established and accepted protocols for human applications. This issue has been reviewed by Ducret et al. [14], where they proposed several modifications for a more GMP-compliant approach, emphasizing the criteria for tooth selection and the use of serum- and xeno-free components at clinical grade to process tooth and expand cell culture, which represent the major goals to produce cell-based products for cell therapy [14]. With the growing interest in clinical application, allogenic banking of DPSCs could provide a platform for the development of therapeutic products, however, immune rejection still remains as a major concern for cell transplantation. A potential strategy to minimize the risk of rejection may imply the use of the human leukocyte antigen (HLA) typing, which could ensure the HLA compatibility between patient and DPSCs cell lines [126]. In addition to HLA typing, DPSCs should be assessed for the presence of pathogens, virus, toxins as well as other immunological risks as a pre-requisite for banking. Finally, new strategies or the modification of current policies need to be developed to explore the translational potential of DPSCs toward effective clinical therapies, which would be important to feedback current experimental protocols allowing their optimization. These actions involve researchers/clinicians and regulatory institutions to develop new approaches to solve these limitations, encouraging to evaluate the therapeutic potential of DPSCs through clinical trials or procedures.
Conclusions
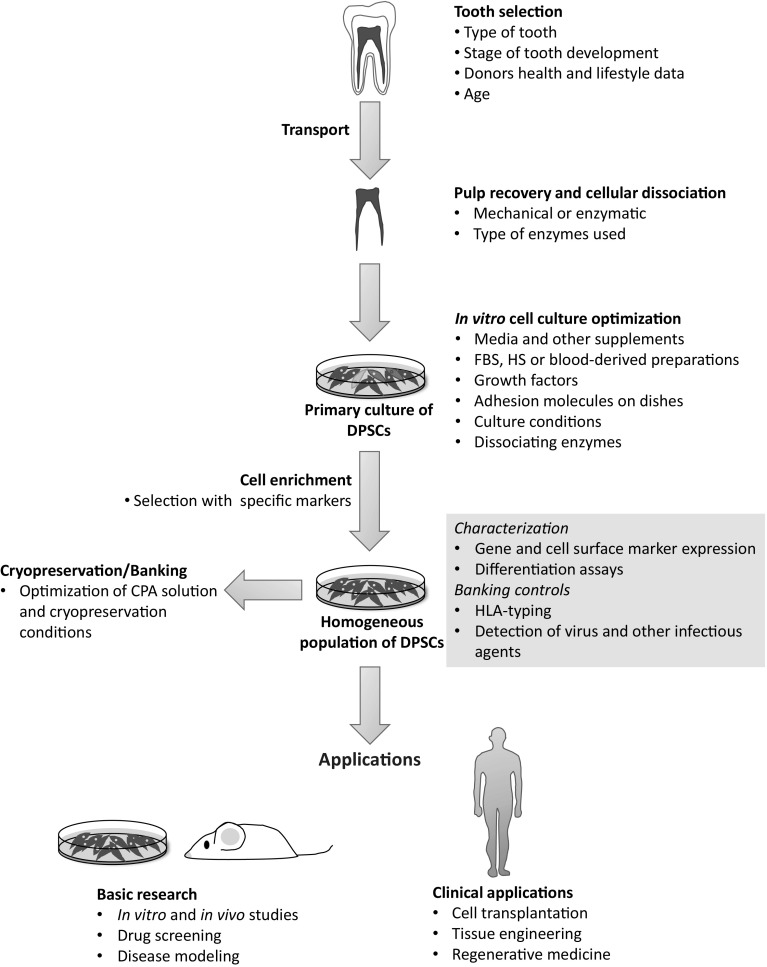
Current methods for the isolation, expansion and preservation of DPSCs have reached significant improvements, but undoubtedly, optimization is still required, especially to follow more homogeneous rules to reduce phenotypic variation and address safety issues to generate DPSCs for cellular therapy. The detailed analysis of the steps from tooth selection to cryopreservation highlighted some factors that can be essential to maintain stem cell properties, becoming potential candidates for further optimization, either general or tailored protocols (Fig. 1). One of the most challenging issue is the cell culture media optimization due to the large number of components, but importantly represents the cornerstone to maintain well-characterized DPSCs in vitro. In this regard, key media ingredients (e.g. growth factors, nutrients and other additional supplements) should be optimized by trial and error until satisfactory results are reached. On the other hand, biological factors associated with dental source should be considered to establish specific criteria for tooth selection according to research needs. Nevertheless, we noticed that research focused on solving the clinical limitations associated with the use of animal-derived or xenobiotic compounds, has received less attention. Thus, more studies should be directed for the development of new strategies and methods to promote the progress of DPSCs as stem cell-based therapies in clinical trials.
Fig. 1.
Schematic representation of critical steps followed from tooth selection to DPSCs cryopreservation and applications. Each step summarizes critical factors that should be considered for optimization in order to improve DPSCs culture and stability during manufacturing. For tooth selection, several factors related to donor characteristics must be considered to establish specific criteria according to research interests. As we can see, many key factors fall into cell culture formulation suggesting that this issue is one of the most important to establish general or tailored protocols and obtain DPSCs with specific properties, as well as the long-term maintenance of cultures. Homogeneous populations of DPSCs may be cryopreserved or directly applied to basic or clinical research, either previous or simultaneous molecular and biochemical characterization. A quality control assessment (blue box) should be required not only for banking, but also to ensure the safety of these stem cells before application. (Color figure online)
Acknowledgements
The authors gratefully acknowledge to the CONACYT Fellow (Project 1882) from National Council of Science and Technology (CONACYT), Facultad de Ingeniería Química of Universidad Autónoma de Yucatán and Red Temática Células Troncales y Medicina Regenerativa.
Conflict of interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Beatriz A. Rodas-Junco and Claudia Villicaña have contributed equally to this work.
References
- 1.Bissels U, Eckardt D, Bosio A. Characterization and classification of stem cells. In: Steinhoff G, editor. Regenerative medicine. Dordrecht: Springer; 2013. pp. 155–176. [Google Scholar]
- 2.Hyun I. The bioethics of stem cell research and therapy. J Clin Invest. 2010;120:71–75. doi: 10.1172/JCI40435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5810. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 5.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yildirim S. Dental pulp stem cells. Springer briefs in stem cells. New York: Springer; 2013. pp. 5–16. [Google Scholar]
- 9.Huang AH, Chen YK, Lin LM, Shieh TY, Chan AW. Isolation and characterization of dental pulp stem cells from a supernumerary tooth. J Oral Pathol Med. 2008;9:571–574. doi: 10.1111/j.1600-0714.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Xha XJ, Li GH, Yang FS, Ji K, Wen LY, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch Oral Biol. 2012;57:1231–1240. doi: 10.1016/j.archoralbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, He H, Tang C, Zhang G, Li Y, Wang R, et al. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010;11:32. doi: 10.1186/1471-2121-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Piedra MA, Garzon I, Oliveira AC, Alfonso-Rodríguez CA, Carriel V, et al. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy. 2014;16:266–277. doi: 10.1016/j.jcyt.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Duailibi MT, Kulikowski LD, Duailibi SE, Lipay MV, Melaragno MI, et al. Cytogenetic instability of dental pulp stem cell lines. J Mol Histol. 2012;43:89–94. doi: 10.1007/s10735-011-9373-z. [DOI] [PubMed] [Google Scholar]
- 14.Ducret M, Fabre H, Degoul O, Atzeni G, McGuckin C, Forraz N, et al. Manufacturing of dental pulp cell-based products from human third molars: current strategies and future investigations. Front Physiol. 2015;6:213. doi: 10.3389/fphys.2015.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, et al. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14:149–156. doi: 10.1089/ten.tec.2008.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization and differentiation. In: Kioussi C, editor. Stem cell and tissue repair. Methods and protocols. Methods in molecular biology. New York: Springer; 2014. pp. 91–115. [DOI] [PubMed] [Google Scholar]
- 17.Spath L, Rotilio V, Alessandrini M, Gambara G, De Angelis L, Mancini M, et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J Cell Mol Med. 2010;14:1635–1644. doi: 10.1111/j.1582-4934.2009.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda-Kawaguchi T, Sugiyama T, Chikusa S, Iida K, Aoki H, Tamaoki N, et al. Derivation of iPSCs after culture of human dental pulp stem cells under defined conditions. PLoS ONE. 2014;18:e115392. doi: 10.1371/journal.pone.0115392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, et al. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013;353:65–78. doi: 10.1007/s00441-013-1630-x. [DOI] [PubMed] [Google Scholar]
- 20.Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225–236. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- 21.Karamzadeh R, Eslaminejad MB, Aflatoonian R. Isolation, characterization and comparative differentiation of human dental pulp stem cells derived from permanent teeth by using two different methods. J Vis Exp. 2012;24:e4372. doi: 10.3791/4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–116. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 23.Lizier NF, Kerkis A, Gomes CM, Hebling J, Oliveira CF, Caplan AI. Scaling-up of dental pulp stem cells isolated from multiple niches. PLoS ONE. 2012;7:e39885. doi: 10.1371/journal.pone.0039885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Zucconi E, Fonseca SAS, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to Golden retriever muscular dystrophy (GRMD) dogs: local or systemic? J Transl Med. 2008;6:35. doi: 10.1186/1479-5876-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raoof M, Yaghoobi MM, Derakhshani A, Kamal-Abadi AM, Ebrahimi B, Abbasnejad M, et al. A modified efficient method for dental pulp stem cell isolation. Dent Res J (Isfahan) 2014;11:244–250. [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis KM, O’Carroll DC, Lewis MD, Rychkov GY, Koblar SA. Neurogenic potential of dental pulp stem cells isolated from murine incisors. Stem Cell Res Ther. 2014;5:30. doi: 10.1186/scrt419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishkitiev N, Calenic B, Aoyama I, Il H, Yaegaki K, Imai T. Hydrogen sulfide increases hepatic differentiation in tooth-pulp stem cells. J Breath Res. 2012;6:017103. doi: 10.1088/1752-7155/6/1/017103. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Huang GW, Shiung JN, Huang YH, Jeng JH, Kuo TF, et al. Magnetic cryopreservation for dental pulp stem cells. Cells Tissues Organs. 2012;196:23–33. doi: 10.1159/000331247. [DOI] [PubMed] [Google Scholar]
- 30.Karbanová J, Soukup T, Suchánek J, Pytlík R, Corbeil D, Mokrý J. Characterization of dental pulp stem cells from impacted third molars cultured in low serum-containing medium. Cell Tissues Organs. 2011;193:344–365. doi: 10.1159/000321160. [DOI] [PubMed] [Google Scholar]
- 31.Lee JY, Nam H, Park YJ, Lee SJ, Chung CP, Han SB, et al. The effects of platelet-rich plasma derived from human umbilical cord blood on the osteogenic differentiation of human dental stem cells. In Vitro Cell Dev Biol Anim. 2011;47:157–164. doi: 10.1007/s11626-010-9364-5. [DOI] [PubMed] [Google Scholar]
- 32.Derakhshani A, Raoof M, Dabiri S, Farsinejad AR, Gorjestani H, Yaghoobi MM, et al. Isolation and evaluation of dental pulp stem cells from teeth with advanced periodontal disease. Arch Iran Med. 2015;18:211–217. [PubMed] [Google Scholar]
- 33.Atari M, Barajas M, Hernández-Alfaro F, Gil C, Fabregat M, Ferrés-Padró E, et al. Isolation of pluripotent stem cells from human third molar dental pulp. Histol Histopathol. 2011;26:1057–1070. doi: 10.14670/HH-26.1057. [DOI] [PubMed] [Google Scholar]
- 34.Govindasamy V, Ronald VS, Totey S, Din SB, Mustafa WM, Totey S, et al. Micromanipulation of culture niche permits long-term expansion of dental pulp stem cells-an economic and commercial angle. In Vitro Cell Dev Biol Anim. 2010;46:764–773. doi: 10.1007/s11626-010-9332-0. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed NEB, Aboul-Ezz EHA, Zakhary SY, El Badry TH, Ramzy MI. Isolation of dental pulp stem cells and their in vitro differentiation into odontoblast-like cells. MJMS. 2011;4:253–260. doi: 10.3889/MJMS.1857-5773.2011.0142. [DOI] [Google Scholar]
- 36.Werle SB, Lindemann D, Steffens D, Demarco FF, de Araujo FB, Pranke P. Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Invest. 2016;20:75–81. doi: 10.1007/s00784-015-1477-5. [DOI] [PubMed] [Google Scholar]
- 37.Horibe H, Murakami M, Iohara K, Hayashi Y, Takeuchi N, Takei Y, et al. Isolation of a stable subpopulation of mobilized dental pulp stem cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS ONE. 2014;9:e98553. doi: 10.1371/journal.pone.0098553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucaciu O, Soritau O, Gheban D, Ciuca DR, Virtic O, Vulpoi A, et al. Dental follicle stem cells in bone regeneration on titanium implants. BMC Biotechnol. 2015;15:114. doi: 10.1186/s12896-015-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawanabe N, Murata S, Murakami K, Ishihara Y, Hayano S, Kurosaka H, et al. Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen 4. Differentiation. 2010;79:74–83. doi: 10.1016/j.diff.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Kleinman HK, Lucckenbill-Edds L, Cannon FW, Sephel GC. Use of extracellular matrix components for cell culture. Anal Biochem. 1987;166:1–13. doi: 10.1016/0003-2697(87)90538-0. [DOI] [PubMed] [Google Scholar]
- 41.Schaffner P, Meyer J, Dard M, Wenz R, Nies B, Verrier S, et al. Induced tissue integration of bone implants by coating with bone selective RGD-peptides in vitro and in vivo studies. J Mater Sci Mater Med. 1999;10:837–839. doi: 10.1023/A:1008904513304. [DOI] [PubMed] [Google Scholar]
- 42.Vancha AR, Govindaraju S, Parsa KV, Jasti M, González-García M, Ballestero RP. Use of polyethyleneimine polymer in cell culture as attachment factor and lipofection enhancer. BMC Biotechnol. 2004;4:23. [DOI] [PMC free article] [PubMed]
- 43.Saha K, Mei Y, Reisterer CM, Pyzocha NK, Yang J, Muffat J, et al. Surface-engineered substrates for improved human pluripotent stem cell culture under fully defined conditions. Proc Natl Acad Sci USA. 2011;108:18714–18719. doi: 10.1073/pnas.1114854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atari M, Gil-Recio C, Fabregat M, García-Fernández D, Barajas M, Carrasco MA, et al. Dental pulp of the third molar: a new source of pluripotent-like stem cells. J Cell Sci. 2012;125:3343–3356. doi: 10.1242/jcs.096537. [DOI] [PubMed] [Google Scholar]
- 45.Suchánek J, Soukup T, Ivancaková R, Ahmeanová J, Hubková V, Kucerová L. Human dental pulp stem cells-isolation and long term cultivation. Acta Med (Hradec Kralove) 2007;50:195–201. [PubMed] [Google Scholar]
- 46.Chen Y, Zheng YL, Qiu DB, Sun YP, Kuang SJ, Xu Y, et al. An extracellular matrix culture system for induced pluripotent stem cells derived from human dental pulp cells. Eur Rev Med Pharmacol Sci. 2015;19:4035–4046. [PubMed] [Google Scholar]
- 47.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 48.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 49.Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via αVβ5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 50.Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 51.Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 2014;5:3195. doi: 10.1038/ncomms4195. [DOI] [PubMed] [Google Scholar]
- 52.Villa-Díaz LG, Nandivada H, Ding J, Nogueira-de-Souza NC, Krebsbach PH, O’Shea KS, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol. 2010;28:581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen B, Sun HH, Wang HG, Kong H, Chen FM, Yu Q. The effects of human platelet lysate on dental pulp stem cells derived from impacted human third molars. Biomaterials. 2012;33:5023–5035. doi: 10.1016/j.biomaterials.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 54.Ma L, Makino Y, Yamaza H, Akiyama K, Hoshino Y, Song G, et al. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS ONE. 2012;7:e51777. doi: 10.1371/journal.pone.0051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pisciotta A, Carnevale G, Meloni S, Riccio M, De Biasi S, Gibellini L, et al. Human dental pulp stem cells (hDPSCs): isolation, enrichment and comparative differentiation of two sub-populations. BMC Dev Biol. 2015;15:14. doi: 10.1186/s12861-015-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez R, Lee HL, Hong C, Wang CY. Single CD271 marker isolates mesenchymal stem cells from human dental pulp. Int J Oral Sci. 2015;7:205–212. doi: 10.1038/ijos.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Govindasamy V, Ronald VS, Abdullah AN, Nathan KR, Aziz ZA, Abdullah M, et al. Human platelet lysate permits scale-up dental pulp stromal cells for clinical applications. Cytotherapy. 2011;13:1221–1233. doi: 10.3109/14653249.2011.602337. [DOI] [PubMed] [Google Scholar]
- 58.Hirata TM, Ishkitiev N, Yaegaki K, Calenic B, Ishikawa H, Nakahara T, et al. Expression of multiple stem cell markers in dental pulp cells cultured in serum-free media. J Endod. 2010;36:1139–1144. doi: 10.1016/j.joen.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Lindemann D, Werle SB, Steffens D, Garcia-Godoy F, Pranke P, Casagrande L. Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch Oral Biol. 2014;59:970–976. doi: 10.1016/j.archoralbio.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Ajlan SA, Ashri NY, Aldahmash AM, Alnbaheen MS. Osteogenic differentiation of dental pulp stem cells under the influence of three different materials. BMC Oral Health. 2015;15:132. doi: 10.1186/s12903-015-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikolić N, Krstić A, Trivanović D, Mojsilović S, Kocić J, Santibanez JF, et al. Mesenchymal stem cell properties of dental pulp cells from deciduous teeth. Arch Biol Sci. 2011;63:933–942. doi: 10.2298/ABS1104933N. [DOI] [Google Scholar]
- 62.Maioli M, Basoli V, Santaniello S, Cruciani S, Delitala AP, Pinna R, et al. Osteogenesis form dental pulp derived stem cells: a novel conditioned medium including melatonin within a mixture of hyaluronic, butyric, and retinoic acids. Stem Cells Int. 2016;2016:2056416. doi: 10.1155/2016/2056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murakami M, Horibe H, Iohara K, Hayashi Y, Osako Y, Takei Y, et al. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials. 2013;34:9036–9047. doi: 10.1016/j.biomaterials.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 64.Khanna-Jain R, Vanhatupa S, Vuorinen A, Sándor GKB, Suuronen R, Mannerström B, et al. Growth and differentiation of human dental pulp stem cells maintained in fetal bovine serum, human serum and serum-free/xeno-free culture media. J Stem Cell Res Ther. 2012;2:126. doi: 10.4172/2157-7633.1000126. [DOI] [Google Scholar]
- 65.Lindroos B, Mäenpää K, Ylikomi T, Oja H, Suuronen R, Miettinen S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun. 2008;368:329–335. doi: 10.1016/j.bbrc.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 66.Bonnamain V, Thinard R, Sergent-Tanguy S, Huet P, Bienvenu F, Naveilhan P, et al. Human dental pulp stem cells cultures in serum-free supplemented medium. Front Physiol. 2013;4:357. doi: 10.3389/fphys.2013.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bressan E, Ferroni L, Gardin C, Pinton P, Stellini E, Botticelli D, et al. Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS ONE. 2012;7:e49146. doi: 10.1371/journal.pone.0049146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alliot-Litch B, Bluteau G, Magne D, Lopez-Cazaux S, Lieubeau B, Daculsi G, et al. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005;326:391–400. doi: 10.1007/s00441-005-1115-7. [DOI] [PubMed] [Google Scholar]
- 69.Alleman M, Low E, Truong K, Huang E, Hill CK, Chen TY, et al. Dental pulp-derived stem cells (DPSC) differentiation in vitro into odontoblast and neuronal progenitors during cell passaging is associated with alterations in cell survival and viability. Int J Med Biomed Res. 2013;2:133–141. doi: 10.14194/ijmbr.226. [DOI] [Google Scholar]
- 70.Lopes-Cazaux S, Bluteau G, Magne D, Lieubeau B, Guicheux J, Alliot-Licht B. Culture medium modulates the behavior of human dental pulp-derived cells: technical notes. Eur Cells Mater. 2006;11:35–42. doi: 10.22203/eCM.v011a05. [DOI] [PubMed] [Google Scholar]
- 71.D’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De-Rosa A, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 72.Ferro F, Spelat R, Beltrami AP, Cesselli D, Curcio F. Isolation and characterization of human dental pulp derived stem cells by using media containing low human serum percentage as clinical grade substitutes for bovine serum. PLoS ONE. 2012;7:e48945. doi: 10.1371/journal.pone.0048945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desai N, Rambhia P, Gishto A. Human embryonic stem cell cultivation: historical perspective and evolution of xeno-free culture systems. Reprod Biol Endocrinol. 2015;13:9. doi: 10.1186/s12958-015-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27:53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- 75.Tekkatte C, Gunasingh GP, Cherian KM, Sankaranarayanan K. “Humanized” stem cell culture techniques: the animal serum controversy. Stem Cells Int. 2011;2011:504723. doi: 10.4061/2011/504723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 77.Hu F, Wang X, Liang G, Lv L, Zhu Y, Sun B, et al. Effects of epidermal growth factor and basic fibroblast growth factor on the proliferation and osteogenic and neural differentiation of adipose-derived stem cells. Cell Reprogram. 2013;15:224–232. doi: 10.1089/cell.2012.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morito A, Kida Y, Susuki K, Inoue K, Kuroda N, Gomi K, et al. Effects of fibroblast growth factor on the development of the stem cell properties of human dental pulp stem cells. Arch Histol Cytol. 2009;72:51–64. doi: 10.1679/aohc.72.51. [DOI] [PubMed] [Google Scholar]
- 79.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 80.Lee JH, Um S, Jang JH, Seo BM. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012;348:475–484. doi: 10.1007/s00441-012-1392-x. [DOI] [PubMed] [Google Scholar]
- 81.Scheller EL, Chang J, Wang CY. Wnt/β-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87:126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006;9:31–35. doi: 10.1089/rej.2006.9.31. [DOI] [PubMed] [Google Scholar]
- 83.Kato H, Taguchi Y, Tominaga K, Kimura D, Yamawaki I, Noguchi M, et al. High glucose concentrations suppress the proliferation of human periodontal ligament stem cells and their differentiation into osteoblast. J Periodontol. 2016;87:e44–e51. doi: 10.1902/jop.2015.150474. [DOI] [PubMed] [Google Scholar]
- 84.Luo Z, Li D, Kohli MR, Yu Q, Kim S, He WX. Effect of BiodentineTM on the proliferation, migration and adhesion of human dental pulp stem cells. J Dent. 2014;42:490–497. doi: 10.1016/j.jdent.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 85.Alliot-Litch B, Bluteau G, Magne D, Lopez-Cazaux S, Lieubeau B, Daculsi G, et al. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005;326:391–400. doi: 10.1007/s00441-005-1115-7. [DOI] [PubMed] [Google Scholar]
- 86.Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther. 2013;4:117. doi: 10.1186/scrt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 88.Lee J, Park S, Roh S. Y-27632, a ROCK inhibitor, delays senescence of putative murine salivary gland stem cells in culture. Arch Oral Biol. 2015;360:875–882. doi: 10.1016/j.archoralbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 91.El-Alami M, Viña-Almunia J, Gambini J, Mas-Bargues C, Siouw RC, Peñarrocha M, et al. Activation of p38, p21, and NRF-2 mediates decreased proliferation of human dental pulp stem cells cultured under 21% O2. Stem Cell Rep. 2014;3:566–573. doi: 10.1016/j.stemcr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hung SO, Ho JH, Shih YR, Lo T, Lee OK. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res. 2012;30:260–266. doi: 10.1002/jor.21517. [DOI] [PubMed] [Google Scholar]
- 93.Busuttil RA, Rubio M, Dollé ME, Campisi J, Vijg J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell. 2003;2:287–294. doi: 10.1046/j.1474-9728.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- 94.Lin TM, Tsai JL, Lin SD, Lai CS, Chang CC. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005;14:92–102. doi: 10.1089/scd.2005.14.92. [DOI] [PubMed] [Google Scholar]
- 95.Debeljak-Martacic J, Borozan S, Radovanovic A, Popadic D, Mojsilovic S, Vucic V, et al. N-acetyl-l-cysteine enhances ex-vivo amplification of deciduous teeth dental pulp stem cells. Arch Oral Biol. 2016;70:32–38. doi: 10.1016/j.archoralbio.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 96.Tomlinson MJ, Tomlinson S, Yang XB, Kirkham J. Cell separation: terminology and practical considerations. J Tissue Eng. 2013;4:2041731412472690. doi: 10.1177/2041731412472690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ledesma-Martínez E, Mendonza-Núñez VM, Santiago-Osorio E. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int. 2016;2016:4709572. doi: 10.1155/2016/4709572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akpinar G, Kasap M, Aksoy A, Duruksu G, Gacar G, Karaoz E. Phenotypic and proteomic characteristics of human d-ental pulp derived mesenchymal stem cells of a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int. 2014;2014:457059. doi: 10.1155/2014/457059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20:1394–1400. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 100.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 101.Waddington RJ, Youde SJ, Lee CP, Sloan AJ. Isolation of distinct progenitor cell populations from dental pulp. Cell Tissues Organs. 2009;189:268–274. doi: 10.1159/000151447. [DOI] [PubMed] [Google Scholar]
- 102.Iohara K, Zheng L, Wake H, Ito M, Nakebura J, Wakita H, et al. A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells. 2008;26:2408–2418. doi: 10.1634/stemcells.2008-0393. [DOI] [PubMed] [Google Scholar]
- 103.Jiang L, Peng WW, Li LF, Yang Y, Zhu YQ. Isolation and identification of CXCR4-positive cells from human dental pulp cells. J Endod. 2012;38:791–795. doi: 10.1016/j.joen.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 104.Kawashima N. Characterisation of dental pulp stem cells: a new horizon for tissue regenerations? Arch Oral Biol. 2012;57:1439–1458. doi: 10.1016/j.archoralbio.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 105.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 106.Papaccio G, Graziano A, D’Aquino R, Graziano MF, Pirozzi G, Menditti D, et al. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–325. doi: 10.1002/jcp.20667. [DOI] [PubMed] [Google Scholar]
- 107.Wood EJ, Perru BC, Hockema JJ, Larson L, Zhou D, Goebel WS. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology. 2009;59:150–157. doi: 10.1016/j.cryobiol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gioventù S, Andriolo G, Bonino F, Frasca S, Lazzari L, Montelatici E, et al. A novel method for banking dental pulp stem cells. Transfus Apher Sci. 2012;47:199–206. doi: 10.1016/j.transci.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 109.Arora V, Arora P, Munshi AK. Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent. 2009;33:289–294. doi: 10.17796/jcpd.33.4.y887672r0j703654. [DOI] [PubMed] [Google Scholar]
- 110.Lee SY, Chiang PC, Tsai YH, Tsai SY, Jeng JH, Kawata T, et al. Effects of cryopreservation of intact teeth on the isolated dental pulp stem cells. J Endod. 2010;36:1336–1340. doi: 10.1016/j.joen.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 111.Kaku M, Kamada H, Kawata T, Koseki H, Abedini S, Kojima S, et al. Cryopreservation of periodontal ligament stem cells with magnetic field for tooth banking. Cryobiology. 2010;61:73–78. doi: 10.1016/j.cryobiol.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 112.Kaku M, Shimasue H, Ohtani J, Kojima S, Sumi H, Shikata H, et al. A case of tooth autotransplantation after long-term cryopreservation using a programmed freezer with a magnetic field. Angle Orthod. 2015;85:518–524. doi: 10.2319/030314-148.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin SL, Lee SY, Lin YC, Huang YH, Yang JC, Huang HM. Evaluation of mechanical and histological properties of cryopreserved human premolars under short-term preservation: a preliminary study. J Dent Sci. 2014;9:244–248. doi: 10.1016/j.jds.2013.04.010. [DOI] [Google Scholar]
- 114.Huang MS, Chang WJ, Huang HM, Lin YC, Huang YH, Yang JC, et al. Effects of transportation time after extraction on magnetic cryopreservation of pulp cells or rat dental pulp. J Dent Sci. 2011;6:48–52. doi: 10.1016/j.jds.2011.02.008. [DOI] [Google Scholar]
- 115.Shu Z, Heimfeld S, Gao D. Hematopoietic stem cell transplantation with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal prior to infusion. Bone Marrow Transplant. 2014;49:469–476. doi: 10.1038/bmt.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benekli M, Anderson B, Wentling D, Bernstein S, Czuczman M, McCarthy P. Severe respiratory depression after dimethylsulphoxide-containing autologous stem cell infusion in a patient with AL amyloidosis. Bone Marrow Transplant. 2000;25:1299–1301. doi: 10.1038/sj.bmt.1702452. [DOI] [PubMed] [Google Scholar]
- 117.Windrum P, Morris TCM. Severe neurotoxicity because of dimethyl sulphoxide following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31:315. doi: 10.1038/sj.bmt.1703848. [DOI] [PubMed] [Google Scholar]
- 118.Motta JP, Paraguassú-Braga FH, Bouzas LF, Porto LC. Evaluation of intracellular and extracellular trehalose as a cryoprotectant of stem cells obtained from umbilical cord blood. Cryobiology. 2014;68:343–348. doi: 10.1016/j.cryobiol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 119.Park BW, Jang SJ, Byun JH, Kang YH, Choi MJ, Park WU, et al. Cryopreservation of human dental follicle tissue for use as a resource of autologous mesenchymal stem cells. J Tissue Eng Regen Med. 2017;11:489–500. doi: 10.1002/term.1945. [DOI] [PubMed] [Google Scholar]
- 120.Thirumala S, Wu X, Gimble JM, Devireddy RV. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng Part C Methods. 2010;16:783–792. doi: 10.1089/ten.tec.2009.0552. [DOI] [PubMed] [Google Scholar]
- 121.Wu W, Zhou J, Xu CT, Zhang J, Jin YJ, Sun GL. Derivation and growth characteristics of dental pulp stem cells from patients of different ages. Mol Med Rep. 2015;12:5127–5134. doi: 10.3892/mmr.2015.4106. [DOI] [PubMed] [Google Scholar]
- 122.Kang E, Wang X, Tippner-Hedges R, Ma H, Folmes CDL, Marti-Gutierrez N, et al. Age-related accumulation of somatic mitochondrial DNA mutations in adult-derived human iPSCs. Cell Stem Cell. 2016;18:625–636. doi: 10.1016/j.stem.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 123.Rennert RC, Sorkin M, Januszyk M, Duscher D, Kosaraju R, Chung MT, et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther. 2014;5:79. doi: 10.1186/scrt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Piao Z, Kim HJ, Choi YZ, Hong CR, Lee JW, Kang HJ, et al. Effect of FOXP3 polymorphism on the clinical outcomes after allogeneic hematopoietic stem cell transplantation in pediatric acute leukemia patients. Int Immunopharmacol. 2016;31:132–139. doi: 10.1016/j.intimp.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 125.Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat Rev Mol Cell Biol. 2016;17:170–182. doi: 10.1038/nrm.2015.27. [DOI] [PubMed] [Google Scholar]
- 126.Taylor CJ, Bolton EM, Bradley JA. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc B Boil Sci. 2011;366:2312–2322. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]