Abstract
Both mature and stem cell-derived hepatocytes lost their phenotype and functionality under conventional culture conditions. However, the 3D scaffolds containing the main extracellular matrix constitutions, such as heparin, may provide appropriate microenvironment for hepatocytes to be functional. The current study aimed to investigate the efficacy of the differentiation capability of hepatocytes derived from human Wharton’s jelly mesenchymal stem cells (WJ-MSCs) in 3D heparinized scaffold. In this case, the human WJ-MSCs were cultured on the heparinized and non-heparinized 2D collagen gels or within 3D scaffolds in the presence of hepatogenic medium. Immunostaining was performed for anti-alpha fetoprotein, cytokeratin-18 and -19 antibodies. RT-PCR was performed for detection of hepatic nuclear factor-4 (HNF-4), albumin, cytokeratin-18 and -19, glucose-6-phosphatase (G6P), c-met and Cyp2B. The results indicated that hepatogenic media induced the cells to express early liver-specific markers including HNF4, albumin, cytokeratin-18 and 19 in all conditions. The cells cultured on both heparinized culture conditions expressed late liver-specific markers such as G6P and Cyp2B as well. Besides, the hepatocytes differentiated in 3D heparinized scaffolds stored more glycogen that indicated they were more functional. Non-heparinized 2D gel was the superior condition for cholangiocyte differentiation as indicated by higher levels of cytokeratin 19 expression. In conclusion, the heparinized 3D scaffolds provided a microenvironment to mimic Disse space. Therefore, 3D heparinized collagen scaffold can be suggested as a good vehicle for hepatocyte differentiation.
Keywords: Hepatocytes, Collagen type I, Heparin, Scaffold, Gel
Introduction
Intense research has been conducted to obtain an efficient hepatocyte differentiation protocol for liver therapeutic applications and toxicological examinations. However, in vitro differentiated hepatocytes in traditional 2D condition do not maintain their phenotype and function properly due to the influence of various key factors including the extracellular matrix and proper cell–cell adhesion. Although liver extracellular matrix constitutes a minor fraction of the liver volume, it plays an important role in hepatocyte functions [1]. In vitro mimicking of hepatocyte niche may lead to improvement of the liver cell differentiation and functionality.
Hepatocytes form bilaminar palates with canalicular and sinusoidal surfaces. The sinusoidal surface of the hepatocytes is separated from the endothelial cells by a narrow space called Disse space [2]. Sinusoidal spaces lack typical basement membrane, but it contains fibronectin, discontinuous layer of collagen type III, continuous network of collagen type I [1], and also heparan sulfate [3]. Viability of the hepatocytes is reduced by modification in Disse space constituents. Disse space contents have been demonstrated to prevent the hepatocyte dedifferentiation [4].
Isolated adult primary hepatocytes lose their function and differentiation state when they are grown in 2D conventional culture condition. Hepatosphere formation has been exhibited to keep the adult hepatocyte functions better than their culturing in 2D environment [5]. Gene expression pattern and metabolic activity of a hepatocyte cell line cultured in 3D condition have shown an increase in albumin expression and a reduction in bile duct marker expression compared with 2D environment [6]. The hepatogenic capability of the human mesenchymal stem cells (MSC) also improved by pre-formation of cell base aggregate [7]. 3D culture also improved the expression of hepatic nuclear factor-4, the key regulator of hepatocyte differentiation, in hepatocytes-like cells derived from human Wharton’s jelly MSCs (WJ-MSC) [8]. This evidence revealed that 3D culture can prevent the hepatocyte function loss and improve the hepatocyte differentiation efficiency.
Collagen type I, as a prominent part of Disse space [1], can be used to mimic the naïve hepatocyte niche. Collagen base scaffold has been demonstrated to improve the adult hepatocyte functions [9]. Well-developed bile canaliculi were found to form between primary hepatocytes cultured within collagen sandwich [10]. The culture conditions, 3D versus 2D, also modified the expression of liver-specific markers [11]. Disse space also contains glycosaminoglaycans such as heparan sulfate that influence hepatocyte functions [3]. Heparin and heparan sulfate reduced cell death in the hepatocyte cell line [12]. Increase in liver-specific marker expression in the primary hepatocytes cultured on heparin hydrogel [13] suggested heparin containing hydrogels as an appropriate niche for stem cell development toward hepatogenic linage.
WJ-MSCs are obtained from medical waste products that can be considered as a good, non-invasive source of stem cells for therapeutic purposes without any ethical concern. WJ-MSCs have a high proliferation rate and express a low level of hepatocyte-specific factors [11]. Therefore, these stem cells can be used for hepatocyte differentiation. This study was designated to find the efficiency of 3D collagen/heparin scaffold and 2D gel for WJ-MSC differentiation toward hepatocyte cell lineage.
Materials and methods
Collagen scaffold and gel preparation
The stock containing 3 mg/mL of rat tail collage type I (Gibco, UK) was gently diluted 1:8 with 10× α-minimal essential medium (α-MEM, Gibco, UK) on ice. Then, 125 µL of 10× reconstitution buffer containing 2.2% NaHCO3 and 200 mM HEPES was added to the collagen solution. Changing the color into red indicated the neutralized solution. The solution was centrifuged at 10,000 RPM for 3 min at 4 °C to release the air bubbles. The final concentration of 1 mg/mL of the reconstituted collagen was prepared by adding 540 µL of 1× α-MEM to 460 µL of the solution. For gelation, a based layer (200 µL/well) of the reconstituted collagen was added to each well of a 24 well plate and incubated at 37 °C for 1 h.
Heparin was crosslinked to the collagen by adding 50 mM 2-(N-morpholino) ethanesulfonic acid buffer (MES, Sigma, USA) with dilution of 3:7 in 70% ethanol at pH 5. 5 mM 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC, Sigma, USA) and 2 mM N-hydroxysulfosuccinimide (NHS, Sigma, USA) were prepared freshly in MES/Ethanol solution. To activate the carboxyl group, we added the crosslink solution to 0.25 mg/mL of heparin (Hepalink, China) for 30 min, and then, the mixture was added to the collagen at 4 °C for 24 h. The crosslink solution was then removed and the collagen gel was washed with distilled water for several times and then sterilized by 70% ethanol. The collagen gel was then exposed to medium containing 1% gentamycin (Gibco, UK) for 24 h before use in cell culture.
The 3D scaffold was prepared by mixing 10 mg/mL of rat tail collagen in 1% acetic acid. Then, 1 mL of the collagen solution was added to each well of a 24 well plate and lyophilized by freeze dryer (Christ, Alpha 1-2 LD, Germany) overnight. Heparin was cross-linked to the collagen scaffolds by the same procedure performed for 2D gels. To prepare the scaffold for culturing the cells, we exposed it to the culture medium for 24 h at 37 °C [14]. The non-heparinized control scaffolds and gels were prepared in the same way but without any heparin.
The presence of heparin in collagen scaffolds and gels was shown by adding 0.0005% toluidine blue in 0.001 N hydrochloric acid with 0.02% (w/v) sodium chloride for 1 min [15].
To quantify the heparin crosslinking, safranin O was used as a metachromatic dye. After crosslinking, 30 µL of the supernatant solution was removed and added to 240 µL of 0.04 mg/mL safranin O in 50 mM sodium acetate buffer at pH 7.4. The optical density was evaluated at 510 nm [16] to measure the amount of the uncrosslinked heparin molecules remaining free within the supernatant solution. Optical density correlated with the amount of glycosaminoglycan on the solution [17].
Cell isolation and characterization
Wharton’s jelly-derived mesenchymal stem cells were obtained from umbilical cord blood at cesarean section delivery. The parents’ gave their informed consent and the umbilical cords were transported to the lab on ice. The umbilical cords were flashed by phosphate buffer saline (PBS) through the umbilical vein. The vein was cut longitudinally and the endothelial cells were scratched, the umbilical arteries were removed and the rest was cut into small pieces. The pieces were placed in culture dish and α-MEM containing 10% Fetal Bovine Serum (FBS, Gibco, UK), 1% l-glutamine (Bioidea, Iran) and 1% penicillin/streptomycin (Gibco, UK) was added; then, the explants were incubated at 37 °C and 5% CO2 for 8–10 days. Upon confluency, the cells were passaged.
To characterize the cells, we harvested and centrifuged them at 1200 RPM for 5 min. The cell palate was suspended and aliquoted to 106 cell/mL. They were washed with PBS and permeabilized with PBS containing 1% tween 20 (Sigma, USA) and 1% Bovine Fetal Albumin (BSA). FITC-conjugated anti-CD90, CD44, CD144, PE-conjugated anti-CD34, CD106, CD73 and PerCP-conjugated anti-CD105 antibodies (all from Abcam, UK, Cambrige) were added to the cell aliquots for 30 min. The cells were washed and fixed by 4% paraformaldehyde. The corresponding isotype controls were used to omit the background staining. The cells were resuspended in PBS and the frequencies of the stained cells were evaluated by flow cytometer (BD FACSCalibur™, BD Biosciences). The data were analyzed by FlowJo software.
The pluripotecy was checked by treating the cells with osteogenic medium (MACS, Germany) for 3 weeks and adipogenic medium (Technologies, Inc., Canada) for 4 weeks. The differentiated osteoblasts and adipocytes were then stained by alizarin red S and oil red O, respectively.
To evaluate the cell viability and proliferation rate, we performed MTT assay. The WJ-MSCs were seeded on the heparinized and non-heparinized gels, and also on the culture dishes at a density of 3000 cell/each well of a 24 well plate. After 3, 7 and 14 days, the culture media were replaced with 1 mg/mL (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) at 37 °C, 5% CO2 for 3 h. Then the MTT replaced with Dimethyl sulfoxide and the optical density of the eluted MTT was evaluated at 595 nm.
Hepatocyte differentiation protocol
The cells were seeded on both 3D collagen scaffolds and 2D collagen gels with or without heparin at a density of 4 × 105 and 2 × 104 per mL; respectively. All cultures were treated with hepatogenic media constituting of low glucose Dulbecco’s minimum essential medium supplemented with 10% FBS, 20 ng/mL insulin-like growth factor 1 (IGF-1, Peprotech, UK), 20 ng/mL hepatocyte growth factor (HGF, Peprotech, UK), 100 nM dexamethasone (Sigma, USA), 100U/mL penicillin and 100 mg/mL streptomycin for 7 days. Thereafter, 10 ng/mL oncostatin M (Peprotech, UK) was added to the culture media for additional 2 weeks to induce hepatocyte maturation.
Immunostaining
The differentiated cells in the 3D scaffolds were fixed with buffer formalin, dehydrated in gradually increased ethanol, cleared in xylene and embedded in paraffin. The samples were sectioned at 5 µm thickness. 2D gels were fixed with paraformaldehyde.
The differentiated cells on the 2D gels, along with paraffin-embedded sections of 3D scaffolds were washed with PBS. Endogenous enzymes were blocked by 1% H2O2 in methanol. The non-specific binding sites were blocked by PBS containing 10% goat serum. The specimens were incubated in anti-alpha-fetoprotein (AFP), -cytokeratin 18 (CK18) (ready to used) and 19 (CK19) (at a dilution of 1:200) primary antibodies (all from DAKO, Denmark) for 45 min. The specimens were treated with super enhancer envision for 15 min and then exposed to polymer HRP label for 30 min. The color was developed by adding 3,3′-diaminobenzidine containing H2O2 for 3 min. Finally, the samples were counterstained with hematoxylin.
Periodic acid-Schiff staining
The glycogen storage in differentiated cells on the 2D gels and in the sections prepared from 3D scaffolds was evaluated by Periodic Acid Schiff (PAS) staining. The cells on 2D gels were fixed by 4% paraformaldehyde and along with the rehydrated sections from 3D scaffolds, exposed to 0.5% periodic acid for 5 min to form aldehyde groups. The cells and sections were then incubated in Schiff reagent for 15 min to stain aldehyde groups. The sections were counterstained with hematoxylin.
Reverse transcription-polymerase chain reaction (RT-PCR)
The harvested cells were spun down at 110 g for 5 min. The cell palate was resuspended in 1 mL Biozol (Bioflux) and the total RNA was isolated by chloroform and precipitated by cold isopropanol. The precipitated RNA was centrifuged at 12,000g for 5 min at 4 °C and then washed with ethanol, air dried and resuspended in DEPC-water. The RNA/protein ratio was evaluated by the assessment of the absorbency at 260/280 nm.
cDNA was synthesized by cDNA synthesis kit (Fermentas, USA) according to the company’s instructions; followed by incubating the mixture at 70 °C for 5 min, 25 °C for 5 min and 42 °C for 60 min. PCR was performed using cDNA template and the corresponding primers (Table 1). The initial denaturation was performed at 94 °C for 5 min followed by denaturation at 94 °C for 10 s. The annealing was carried out at temperatures as referred to Table 1 for 45 s followed by 1 min extension at 72 °C and 10 min final extension at the same temperature. The cycles for all genes were 35. The PCR products were run on 2% agar gels and visualized using a UV transilluminator (Uvidoc, Cambridge, UK). HepG2 cell line (Pasteur Institute, Tehran, Iran) and undifferentiated WJ-MSCs were used as positive and negative control, respectively. The DNA bonds were semi-quantified by syngene software (version 3.06, UK).
Table 1.
The primer sequences used for RT-PCR
| Primer | Sequence | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| ALB |
F 5′ACAGAGACTCAAGTGTGCCAGT-3′
R 5′-GCAAGGTCCGCCCTGTCATC-3′ |
60 | 198 |
| CK-18 | 5′-AAATCCGGGAGCACTTGGAG-3′ 5′-CAATCTGCAGAACGATGCGG-3′ |
60 | 132 |
| CK-19 |
F 5′-ACTACACGACCATCCAGGAC-3′
R 5′-CCGTCTCAAACTTGGTTCGGA-3 |
60 | 126 |
| CYP2B6 |
F 5′-TTCTTCCGGGGATATGGTGT-3′
R 5′-TCCCGAAGTCCCTCATAGTG-3′ |
55 | 91 |
| G-6-P |
F 5′-CGACGAAGCGCAGACAG-3′
R 5′-GTATCCGACTGATGGAAGGC-3′ |
60 | 113 |
| HNF4 |
F 5′-AAGAAATGCTTCCGGGCTGG-3′
R 5′-GACGGGGGAGGTGATCTGTC-3′ |
60 | 156 |
| C met |
F 5′TAGGAAGAGGGCATTTTGGTTGTG-3′ R 5′-GCGAGAGGACATTGGGATGACTA-3′ |
60 | 174 |
| GAPDH |
F 5′-CTCTCTGCTCCTCCTGTTCG-3′
R 5′-ACGACCAAATCCGTTGACTC-3′ |
60 | 114 |
Italic letters indicate the sequences of primers
Results
Scaffold features
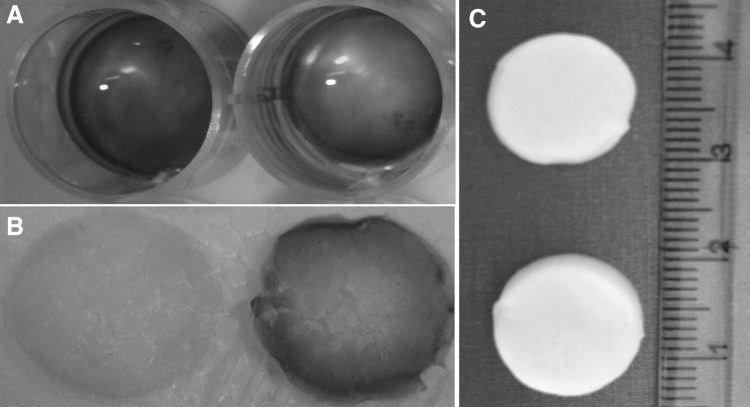
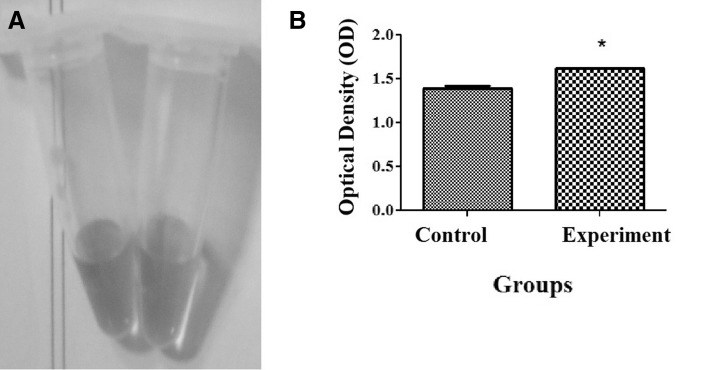
The 3D scaffolds formed a highly porous discoid structure while 2D gels formed a white opaque construct. The presence of heparin in the gels and scaffolds was confirmed by toluidine blue test. The heparinized gels and scaffolds were stained metachromatic while non-heparinized one was stained orthochromatic (Fig. 1). Safranin O reactions with the supernatant solutions from crosslinked and non-crosslinked heparin containing scaffolds confirmed the occurrence of the crosslinking (Fig. 2). The cells could attach to the surface of both 2D gel and 3D scaffold, and the cell migration to the deep on the 2D gel was very limited.
Fig. 1.
The gross structure of the gel and scaffold. The heparin immobilization was shown by toluidine blue staining. The heparinized gel A and scaffold B were stained purple (metachromatic) while the non-heparinized one stained blue (orthochromatic). The gross structure of the lyophilized scaffolds was also shown C
Fig. 2.
The supernatant solution was assessed by safranin O after crosslinking processes. A safranin O is red (left) and after adding heparin, it turns to yellow (right) due to metachromatic property of the dye in the presence of heparin. B The optical density of the control (non-crosslinked scaffold with the same heparin concentration) had the significant lower optical density than the experiment samples. This indicated the presence of a less amount of heparin in the supernatant solution and as a result, crosslinking of heparin with the scaffold. * Significant difference with control (p < 0.05). (Color figure online)
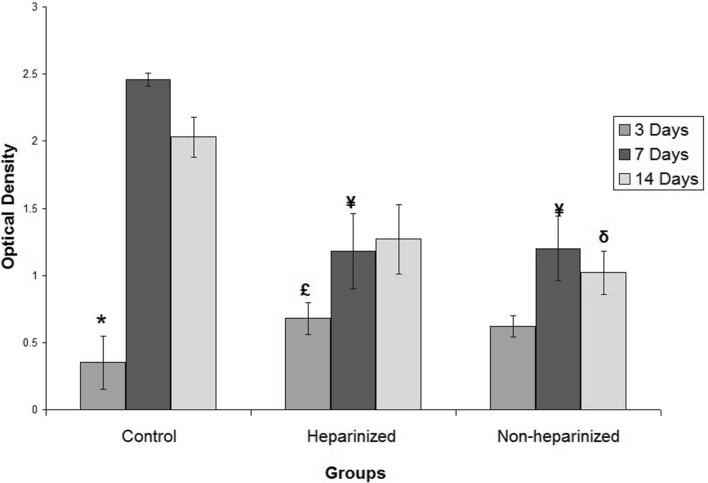
The MTT assay showed that the cell viability was the same for all culture conditions after 3 days. However; for long term, the cells on heparinized and non-heparinized culture conditions showed a significant lower proliferation rate than control cells (p = 0.001 for both 7 and 14 days). The cells on heparinized gels also proliferated and produced a significant higher number of viable cells up to day 14 (p = 0.005) (Fig. 3).
Fig. 3.
MTT test showed the cells cultured on heparinized, non-heparinized gels and culture dishes were viable and the various culture conditions had no significant effect on the number of viable cells. The cells also could proliferate in all conditions. However, in long term, the proliferation rate in conventional culture condition was higher than on the gels. * Significant difference with control cultured for 14 days (p < 0.05); ¥ Significant difference with control cultured for 7 days (p < 0.05); δ Significant difference with control cultured for 14 days (p < 0.05); £ Significant difference with cells cultured on heparinized gels for 14 days (p < 0.05)
Cell morphology and characterization
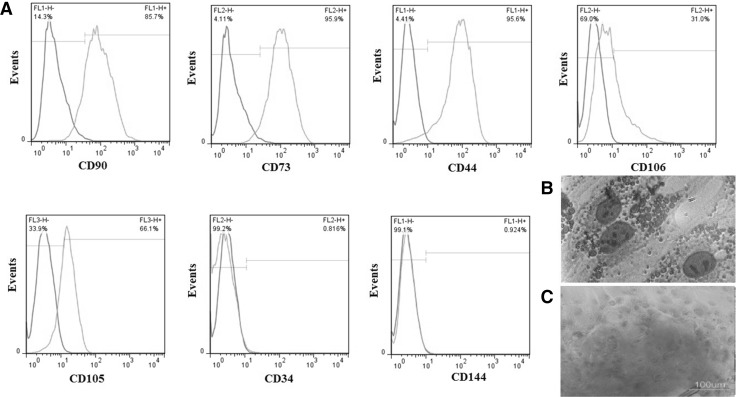
WJ-MSCs showed a fibroblast-like phenotype with many slender processes in 2D cultures. The paraffin-embedded sections were used to observe cell phenotype and location within 3D cultures. It was found that the cells were mostly located close to the thick collagen fibers and attached to them while the middle parts of the pores usually lacked the cells. The isolated cells can be considered as MSCs due to the expression of a high level of CD90 (85.7%), CD73 (95.9%), CD44 (95.6%) and, a moderate level of CD106 (31%) and CD105 (66.1%). The expression of CD34, hematopoietic cell marker (0.816%), and CD144, endothelial marker (0.924%) was negligible (Fig. 4A). The isolated cell also showed differentiation potential toward osteoblast and adipocytes as indicated by alizarin red S and oil red O staining, respectively (Fig. 4B, C).
Fig. 4.
The flow cytometry showed that the frequencies of the Wharton’s jelly mesenchymal stem cells that expressed CD90, CD73 and CD44 were high, the frequencies of the cells expressed CD106 and CD109 were moderate and those expressing CD34 and CD144 was very low A. Wharton’s jelly mesenchymal stem cells also showed the differentiation potential toward adipocytes B and osteocytes C
Hepatogenic differentiation
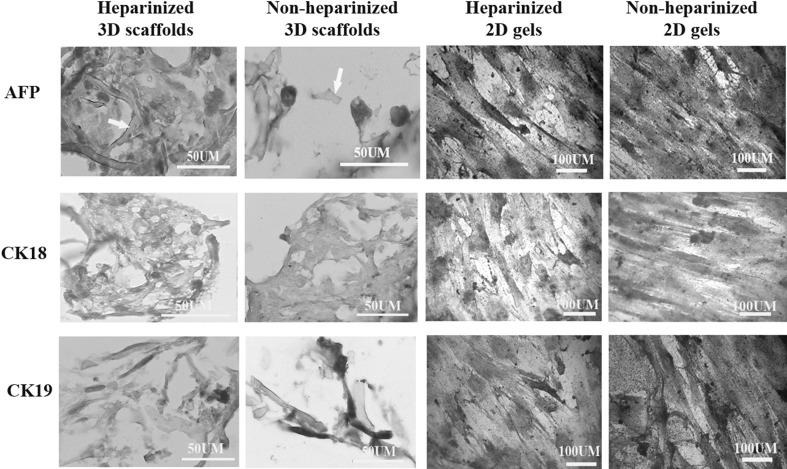
The cells cultured on the surface of collagen gels and sections from the cells within the 3D collagen scaffolds were prepared for immunocytochemistry and immunohistochemistry. In the presence of hepatogenic medium, the cells on both heparinized and non-heparinized 2D collagen gels and 3D scaffolds expressed alpha-fetoprotein, cytokeratin 18 and 19 (Fig. 5) that indicated the differentiation of the cells into hepatocyte-like cells; however, some undifferentiated cells could be found in all culture conditions.
Fig. 5.
Immunohistochemistry and cytochemisrty of the cells cultured in 3D scaffolds and 2D gels in heparinized and non-heparinized conditions for alpha-fetoprotein (AFP, at the top), cytokeratin 18 (CL18, at the middle) and cytokeratin 19 (CK19, at the bottom). The cytoplasm of the differentiated cells was stained brown and the nuclei of undifferentiated cells stained with hematoxylin but the cytoplasm remained unstained. The arrows point collagen fibers in the scaffolds
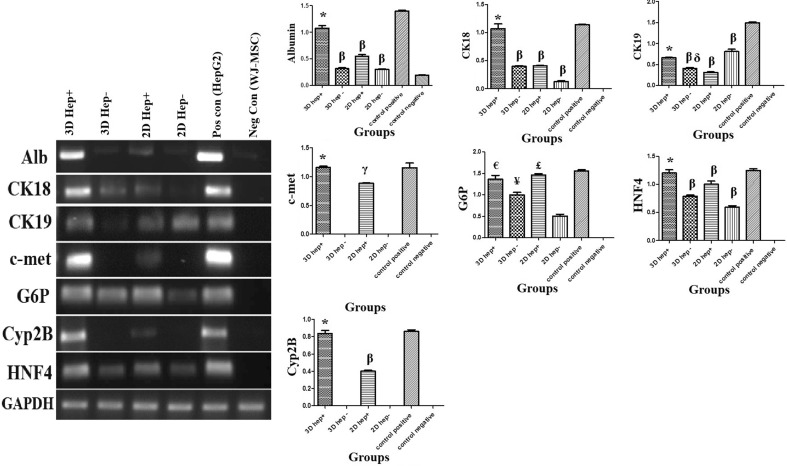
RT-PCR revealed that the cells cultured in all conditions expressed a significant higher amount of HNF4, as a key regulator of hepatogenic fate, in the presence of hepatogenic medium compared with negative control (undifferentiated WJ-MSC); however, those cultured within heparinized 3D scaffolds expressed HNF4 as high as positive control (HepG2 cell line). Although, the cells grown in all conditions expressed G6P, the highest level was expressed by the cells cultured in both heparinized environments. The highest level of CK 18 and albumin expression belonged to the cells differentiated within 3D heparinized scaffolds. The naïve WJ-MSCs also expressed a low amount of albumin. The data showed that the expression of the c-met and Cyp-2B were improved in the presence of heparin. The RT-PCR showed that heparin was important for expression of these two genes. While both heparinized conditions induced the cells to express c-met and Cyp-2B, the 3D condition was more appropriate for hepatogenic differentiation compared with 2D. Non-heparinized 2D gels provided a more efficient condition for expression of cytokeratin19 (Fig. 6). At a glance, the RT-PCR showed that the cells grown within heparinized collagen 3D scaffolds expressed a higher level of the hepatocyte-specific markers than those cultured in non-heparinized 3D scaffolds or heparinized and non-heparinized 2D gels. Also, regardless of the dimensions of culture condition, heparin provided a better condition for hepatocyte differentiation.
Fig. 6.
RT-PCR shows the liver–specific marker expression by the cells in various conditions. The best microenvironment for cells to express liver specific markers was 3D heparinized condition. Semi-quantifications of the RT-PCR products of each liver-specific marker were depicted in the graphs. HepG2 cell line was used as positive control and undifferentiated Wharton’s jelly mesenchymal stem cells were used as negative control. * Significant difference with non-heparinized scaffold, gel, heparinized gel and negative control (undifferentiated WJ-MSCs) (p < 0.05); β Significant difference with both negative (undifferentiated WJ-MSCs) and positive controls (HepG2 cells) (p < 0.05); δ Significant difference with non-heparinized gel (p < 0.05); γ Significant difference with non-heparinized scaffold, gel, negative (undifferentiated WJ-MSCs) and positive controls (HepG2 cells) (p < 0.05); € Significant difference with non-heparinized scaffold, gel and negative control (undifferentiated WJ-MSCs) (p < 0.05); ¥ Significant difference with non-heparinized gel, negative (undifferentiated WJ-MSCs) and positive controls (HepG2 cells) (p < 0.05); £ Significant difference with non-heparinized gel and negative control (undifferentiated WJ-MSCs) (p < 0.05)
Functional tests
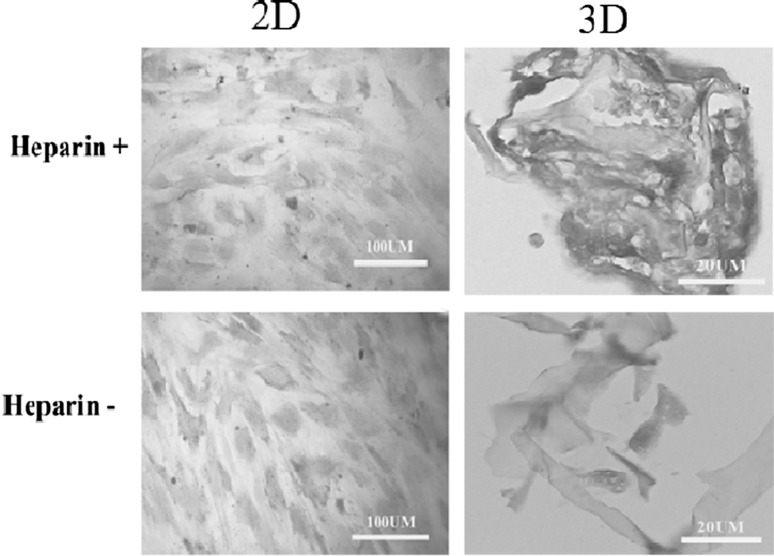
Both heparinized and non-heparinized conditions led to differentiation of the cells with the ability to store glycogen after exposure to hepatogenic media as indicated by PAS staining; however, the cells cultured within heparinized 3D conditions stained more intensely with PAS staining method (Fig. 7).
Fig. 7.
PAS staining showed all cells exposed to hepatogenic media were stored glycogen. The best condition for glycogen storage was heparinized 3D condition
Discussion
Designating a simulated scaffold is a necessary issue for creating liver surrogate and functional engineered construct to be used in regenerative approaches or toxicology tests. Heparinized collagen scaffold can mimic the natural environment of the hepatocytes that is an essential element in maintaining the hepatocyte functions [12, 13]. An improvement in hepatocyte viability and angiogenesis was reported by culturing the primary hepatocytes in heparinized collagen gel within polyurethane foam [18]. Besides, heparin has been shown to improve the biocompatibility of the decellularized scaffolds and hepatocyte viability [19]. The results of the current study showed that both heparinized culture conditions accelerated the differentiation processes of the WJ-MSCs toward hepatocytes as well.
The hepatocyte phenotype can be influenced by the stiffness of the heparinized gels. Softer heparin gel has been shown to provide better niche for the hepatocytes to be functional [20], which may be due to recapitulating Disse space. Micro-molding printing technique provided a 3D environment for the hepatocytes. Micro-molding and micro-contact printing techniques were previously used to construct a collagen/heparin niche for primary hepatocyte culture and the results indicated that the existence of heparin in both floor and wall of each well improved the hepatocyte functions including albumin production [21]. These reports demonstrated the beneficial effects of both heparin and 3D environment in improvement of primary hepatocyte functions [21]. Our data also showed a higher expression pattern of liver-specific markers by WJ-MSC-derived hepatocytes in the presence of heparin and in 3D condition.
The previous report also demonstrated the effects of various heparin concentrations on adipose-derived MSC behaviors such as their stemness, proliferation and differentiation capability toward osteoblasts, adipocytes [22] and chondrocytes [23]. The results of the present study showed that heparinized 3D scaffolds were appropriate for hepatocyte differentiation as well. Among liver-specific markers, HNF4 plays a pivotal role in hepatoblast development [24]. Although, the cells cultured on all conditions could express HNF4 in the presence of hepatogenic medium, only those cultured on 3D heparinized scaffolds could express both early and late liver-specific markers. In contrast, traditional 2D condition mainly induced the expression of the early liver-markers even in the presence of heparin.
The data also showed that some hepatocyte functions such as albumin and G6P production or glycogen storage were influenced by heparin presentation. The enzymatic digestion of heparin in the extracellular matrix of the liver has been reported to reduce G6P production in adult hepatocytes [25]. This evidence along with our data indicated the beneficial role of heparin in the niche of the differentiating stem cells toward hepatogenic fate. Besides, non-heparinized 2D gel might induce the cells to the cholangiocytes rather than hepatocytes as indicated by higher levels of CK-19 expression in this condition. Cytokeratin 19 can be considered as cholangiocyte and hepatoblast markers [21], but not mature hepatocytes [26].
The molecular structure of heparin is responsible for the effects that it exerts on cell phenotype and behaviors. The negative charges on the molecule make it hydrophilic. Hydrophilic nature of heparin containing scaffolds made them a good matrix for cell attachment [27], but its negative charges limited the cell proliferation [28]. In contrast, heparin can sequestrate the growth factors and cytokines and in this way, it influences the cell proliferation and differentiation [29]. Our MTT data showed that cells were viable in heparinized condition and could proliferate as the time progressed. Besides, glycosaminoglycans such as heparin amplify the local signals from the neighboring cells and as a consequence, they influence the cell behavior within the matrix [30]. Both heparinized 3D scaffold and 2D gel improved the differentiation capability of WJ-MSCs toward hepatocytes with the ability to express late liver-markers compared with non-heparinized counterparts; however, heparinized 3D scaffold was the superior choice. This may be due to the growth factor entrapment by heparin contents [29]. Hepatogenic medium contained growth factors such as HGF and IGF that can bind to heparin [31] and modify the differentiation capability of stem cells toward hepatogenic cell lineages.
The impact of heparin in sequestration of growth factors such as HGF on adult hepatocytes was compared in 2D and 3D culture conditions and it has been shown that albumin synthesis was two folds higher in the hepatocytes differentiated within 3D gels [32]. The data of the present study also confirmed a two fold increase in albumin gene expression when WJ-MSCs were cultured in heparinized 3D culture condition compared with the same condition in heparinized 2D gels. Administration of 10 µL heparin has also been shown to lead to two times increase in c-met expression in the hepatocyte cell line through a HGF independent pathway [33]. Our data confirmed that the presence of heparin in culture media was necessary for c-met expression. HNF expression was also shown to increase in 3D compared with 2D culture conditions [8] and our results also confirmed the increase in HNF4 expression in 3D heparinized culture conditions. Despite the presence of HGF in all culture conditions, cells in 3D culture system produced the highest level of albumin, c-met and HNF4. This was also true for CK-18 and Cyp-2B.
In conclusion, although the presence of heparin in collagen constructs improved the hepatocyte differentiation from WJ-MSCs in both 2 and 3D culture conditions; heparinized 3D scaffold was the best surrogate niche for hepatogenesis. Despite the induction of the hepatocyte differentiation by culturing the stem cells in non-heparinized conditions, the differentiated cells could mostly express early hepatocyte-specific markers.
Acknowledgements
The authors wish to thank the Iran National Science Foundation: INSF for offering the Grant No. 91003812 and also Ms. M. Sani and M. Rozitalab for technical supports and Dr. N. Shokrpour for English improvement. All experiments were performed in the laboratory of stem cell research and tissue engineering lab of Anatomy department, Shiraz University of Medical Sciences.
Conflict of interest
There is no conflict of interest.
Ethical statement
The experimental design was approved by ethic committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1395.S803).
References
- 1.Caralt M, Velasco E, Lanas A, Baptista PM. Liver bioengineering: from the stage of liver decellularized matrix to the multiple cellular actors and bioreactor special effects. Organogenesis. 2014;10:250–259. doi: 10.4161/org.29892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohashi K, Okano T. Functional tissue engineering of the liver and islets. Anat Rec. 2014;297:73–82. doi: 10.1002/ar.22810. [DOI] [PubMed] [Google Scholar]
- 3.Foley EM, Esko JD. Hepatic heparan sulfate proteoglycans and endocytic clearance of triglyceride-rich lipoproteins. Prog Mol Biol Transl Sci. 2010;93:213–233. doi: 10.1016/S1877-1173(10)93010-X. [DOI] [PubMed] [Google Scholar]
- 4.Zeisberg M, Kramer K, Sindhi N, Sarkar P, Upton M, Kalluri R. De-differentiation of primary human hepatocytes depends on the composition of specialized liver basement membrane. Mol Cell Biochem. 2006;283:181–189. doi: 10.1007/s11010-006-2677-8. [DOI] [PubMed] [Google Scholar]
- 5.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higuchi Y, Kawai K, Kanaki T, Yamazaki H, Chesné C, Guguen-Guillouzo C, Suemizu H. Functional polymer-dependent 3D culture accelerates the differentiation of HepaRG cells into mature hepatocytes. Hepatol Res. 2016;46:1045–1057. doi: 10.1111/hepr.12644. [DOI] [PubMed] [Google Scholar]
- 7.Talaei-Khozani T, Borhani-Haghighi M, Ayatollahi M, Vojdani Z. An in vitro model for hepatocyte-like cell differentiation from Wharton’s jelly derived-mesenchymal stem cells by cell-base aggregates. Gastroenterol Hepatol Bed Bench. 2015;8:188–199. [PMC free article] [PubMed] [Google Scholar]
- 8.Talaei-Khozani T, Khodabandeh Z, Jaberipour M, Hosseini A, Bahmanpour S, Vojdani Z. Comparison of hepatic nuclear factor-4 expression in two-and three-dimensional culture of Wharton’s jelly-derived cells exposed to hepatogenic medium. Rom J Morphol Embryol. 2015;56:1365–1370. [PubMed] [Google Scholar]
- 9.Nishida Y, Taniguchi A. A three-dimensional collagen-sponge-based culture system coated with simplified recombinant fibronectin improves the function of a hepatocyte cell line. Vitro Cell Dev Biol Anim. 2016;52:271–277. doi: 10.1007/s11626-015-9973-0. [DOI] [PubMed] [Google Scholar]
- 10.Reif R, Karlsson J, Günther G, Beattie L, Wrangborg D, Hammad S, et al. Bile canalicular dynamics in hepatocyte sandwich cultures. Arch Toxicol. 2015;89:1861–1870. doi: 10.1007/s00204-015-1575-9. [DOI] [PubMed] [Google Scholar]
- 11.Khodabandeh Z, Vojdani Z, Talaei-Khozani T, Jaberipour M, Hosseini A, Bahmanpour S. Comparison of the expression of hepatic genes by human Wharton’s Jelly mesenchymal stem cells cultured in 2D and 3D collagen culture systems. Iran J Med Sci. 2016;41:28–36. [PMC free article] [PubMed] [Google Scholar]
- 12.Dudás J, Bocsi J, Fullár A, Baghy K, Füle T, Kudaibergenova S, Kovalszky I. Heparin and liver heparan sulfate can rescue hepatoma cells from topotecan action. Biomed Res Int. 2014;2014:765794. doi: 10.1155/2014/765794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You J, Shin DS, Patel D, Gao Y, Revzin A. Multilayered heparin hydrogel microwells for cultivation of primary hepatocytes. Adv Healthc Mater. 2014;3:126–132. doi: 10.1002/adhm.201300054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajabi-Zeleti S, Jalili-Firoozinezhad S, Azarnia M, Khayyatan F, Vahdat S, Nikeghbalian S, et al. The behavior of cardiac progenitor cells on macroporous pericardium-derived scaffolds. Biomaterials. 2014;35:970–982. doi: 10.1016/j.biomaterials.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Kurpinski KT, Stephenson JT, Janairo RRR, Lee H, Li S. The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous PLLA scaffolds. Biomaterials. 2010;31:3536–3542. doi: 10.1016/j.biomaterials.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uygun BE, Stojsih SE, Matthew HW. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng Part A. 2009;15:3499–3512. doi: 10.1089/ten.tea.2008.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieppo L, Rieppo J, Jurvelin JS, Saarakkala S. Fourier transform infrared spectroscopic imaging and multivariate regression for prediction of proteoglycan content of articular cartilage. PLoS One. 2012;7:e32344. doi: 10.1371/journal.pone.0032344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou Y-T, Ijima H, Takei T, Kawakami K. Growth factor/heparin-immobilized collagen gel system enhances viability of transplanted hepatocytes and induces angiogenesis. J Biosci Bioeng. 2011;112:265–272. doi: 10.1016/j.jbiosc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Bao J, Wu Q, Sun J, Zhou Y, Wang Y, Jiang X, et al. Hemocompatibility improvement of perfusion-decellularized clinical-scale liver scaffold through heparin immobilization. Sci Rep. 2015;5:10756. doi: 10.1038/srep10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You J, Park S-A, Shin D-S, Patel D, Raghunathan VK, Kim M, et al. Characterizing the effects of heparin gel stiffness on function of primary hepatocytes. Tissue Eng Part A. 2013;19:2655–2663. doi: 10.1089/ten.tea.2012.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tee LB, Kirilak Y, Huang W-H, Smith PG, Morgan RH, Yeoh GC. Dual phenotypic expression of hepatocytes and bile ductular markers in developing and preneoplastic rat liver. Carcinogenesis. 1996;17:251–259. doi: 10.1093/carcin/17.2.251. [DOI] [PubMed] [Google Scholar]
- 22.Kim M, Kim YH, Tae G. Human mesenchymal stem cell culture on heparin-based hydrogels and the modulation of interactions by gel elasticity and heparin amount. Acta Biomater. 2013;9:7833–7844. doi: 10.1016/j.actbio.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Zhao S, Wang Z, Chen J, Chen J. Preparation of heparan sulfate-like polysaccharide and application in stem cell chondrogenic differentiation. Carbohydr Res. 2015;401:32–38. doi: 10.1016/j.carres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Nagy P, Bisgaard HC, Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation. J Cell Biol. 1994;126:223–233. doi: 10.1083/jcb.126.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brill S, Zvibel I, Halpern Z, Oren R. The role of fetal and adult hepatocyte extracellular matrix in the regulation of tissue-specific gene expression in fetal and adult hepatocytes. Eur J Cell Biol. 2002;81:43–50. doi: 10.1078/0171-9335-00200. [DOI] [PubMed] [Google Scholar]
- 26.Stosiek P, Kasper M, Karsten U. Expression of cytokeratin 19 during human liver organogenesis. Liver. 1990;10:59–63. doi: 10.1111/j.1600-0676.1990.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 27.Park H, Choi B, Nguyen J, Fan J, Shafi S, Klokkevold P, et al. Anionic carbohydrate-containing chitosan scaffolds for bone regeneration. Carbohydr Polym. 2013;97:587–596. doi: 10.1016/j.carbpol.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikitovic D, Zafiropoulos A, Tzanakakis G, Karamanos N, Tsatsakis A. Effects of glycosaminoglycans on cell proliferation of normal osteoblasts and human osteosarcoma cells depend on their type and fine chemical compositions. Anticancer Res. 2005;25:2851–2856. [PubMed] [Google Scholar]
- 29.Choi WI, Kim M, Tae G, Kim YH. Sustained release of human growth hormone from heparin-based hydrogel. Biomacromolecules. 2008;9:1698–1704. doi: 10.1021/bm701391b. [DOI] [PubMed] [Google Scholar]
- 30.Hortensius RA, Harley BA. The use of bioinspired alterations in the glycosaminoglycan content of collagen–GAG scaffolds to regulate cell activity. Biomaterials. 2013;34:7645–7652. doi: 10.1016/j.biomaterials.2013.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salbach PB, Brückmann M, Turovets O, Kreuzer J, Kübler W, Walter-Sack I. Heparin-mediated selective release of hepatocyte growth factor in humans. Br J Clin Pharmacol. 2000;50:221–226. doi: 10.1111/j.1365-2125.2000.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou Y-T, Ijima H, Matsumoto S, Kubo T, Takei T, Sakai S, et al. Effect of a hepatocyte growth factor/heparin-immobilized collagen system on albumin synthesis and spheroid formation by hepatocytes. J Biosci Bioeng. 2010;110:208–216. doi: 10.1016/j.jbiosc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 33.İşcan E, Güneş A, Korhan P, Yılmaz Y, Erdal E, Atabey N. The regulatory role of heparin on c-Met signaling in hepatocellular carcinoma cells. J Cell Commun Signal. 2016 December 14 [Epub]. [DOI] [PMC free article] [PubMed]