Abstract
Some gel types have been reported to prevent left ventricular (LV) remodeling in myocardial infarction (MI) animal models. In this study, we tested biodegradable thermoresponsive gels. Poly(L-lactic acid)–poly(ethylene glycol) (PLLA–PEG) and poly(D-lactic acid)–poly(ethylene glycol) (PDLA–PEG) were synthesized by the polycondensation of l- and D-lactic acids in the presence of PEG and succinic acid. Each of these block copolymers was used to prepare particles dispersed in an aqueous medium and mixed together to obtain a PLLA–PEG/PDLA–PEG suspension, which was found to show a sol-to-gel transition around the body temperature by the stereocomplex formation of enantiomeric PLLA and PDLA sequences. In the present study, the G′ of the PLLA–PEG/PDLA–PEG suspension in the rheological measurement remained as low as 1 Pa at 20 °C and increased 2 kPa at 37 °C. The sol–gel systems of PLLA–PEG/PDLA–PEG might be applicable to gel therapy. The effect of the PLLA–PEG/PDLA–PEG gel injection was compared with that of a calcium-crosslinked alginate gel and saline in a rat MI model. The percent fractional shortening improved in the PLLA–PEG/PDLA–PEG (20.8 ± 4.1%) and alginate gel (21.1 ± 4.8%) compared with the saline (14.2 ± 2.8%) with regard to the echocardiograph 4 weeks after the injection (p < 0.05). There were reduced infarct sizes in both PLLA–PEG/PDLA–PEG gel and alginate gel compared with the saline injection (p < 0.05). Moreover, a greater reduction in LV cavity area was observed with the PLLA–PEG/PDLA–PEG gel than with the alginate gel (p = 0.06). These results suggest that the PLLA–PEG/PDLA–PEG gel should have high therapeutic potential in gel therapy for LV remodeling after MI.
Keywords: Myocardial infarction, Poly(L-lactic acid), Thermoresponsive gel, Stereocomplex formation
Introduction
Much attention has recently been paid to the gel injection therapy for myocardial infarction (MI), and alginate gels were originally proposed to have excellent effectiveness for preventing left ventricular (LV) remodeling [1–4]. Clinical trial has been already reported [5]. When the sodium ions involved in alginates are exchanged with divalent calcium ions (Ca2+r), alginates are easily crosslinked and become resistant to enzymatic degradation and hydrolysis in vivo. When implanted in the body, however, ionically crosslinked gels are likely de-crosslinked via an ion-exchange process involving Ca2+ loss and readily excreted with decreased molecular weight. In fact, alginates with a molecular weight <50,000 Da can be secreted through the urine [6, 7]. In addition, even ordinary alginate gels that are highly crosslinked with an appropriate Ca2+ content exhibit poor mechanical properties in wet gel therapy conditions for LV remodeling after MI [3].
Wall et al. [8] previously used finite element model simulation to indicate that the therapeutic effect of the injected gel is mostly related with injection volume, gel stiffness, and injection location. They also pointed out the importance of the gel’s elastic modulus, which is difficult to control with naturally occurring polymers such as alginates. Accordingly, gel injection therapy using synthetic polymer gels with easily controlled physical properties has been attempted [9]. For example, polymer gels consisting of poly(ethylene glycol) (PEG) [10, 11] and poly(N-isopropylacrylamide) (pNIPPAm) [12–14] have been evaluated. It has been revealed, however, that these synthetic polymers are likely to elicit a stronger inflammatory response than natural polymers. In fact, Garbern et al. [12] reported that an inflammatory response was caused upon pNIPPAm gel injection in a rat MI model. Dobner et al. injected a nonbiodegradable and bioinert PEG gel into MI model rats and observed a pack of LV remodeling 4 weeks thereafter compared with the saline group. However, even in the PEG-injected groups, the LV remodeling was worsened 13 weeks after the injection compared to the saline group, probably because the long-term gel implantation induced a chronic inflammatory response. Therefore, it was suggested that gel biodegradability would be beneficial for gel therapy [11]. The implantation of biocompatible and biodegradable gels can avoid both long-term bioreactions and toxic inflammatory reactions due to the degradation products.
Poly(lactic acid) (PLA), poly(glycolic acid), and poly(caprolactone) (PCL) have been used as biodegradable materials for medical devices. The United States Food and Drug Administration and Japanese Ministry of Health, Labour and Welfare approved PLA and its copolymers for various applications such as intravascular stents and surgical sutures. In our previous studies, multiblock copolymers consisting of PLA and PEG were processed into polymer films that are applied as adhesion preventing films with controlled biodegradability [15, 16]. These films were also used to inhibit heart adhesions. It was suggested that such PLA–PEG films showed higher degradability and a milder inflammatory response than a PLA control film [16]. A typical example of PLA copolymers in the clinical stage is a PLA–PEG block copolymer that can form a micellar solution in water. The micelle nanoparticles of this copolymer can encapsulate anticancer drugs in their hydrophobic PLA cores and are effectively transferred to tumor cells through blood flow to exhibit an efficient therapeutic effect. They are utilized in the real clinical application as Genexol-PM® [17]. We previously confirmed the thermoresponsive property of PLA–PEG block copolymer micelles [18]. An enantiomeric mixture of Poly(L-lactic acid)-b-poly(ethylene glycol)-b-Poly(L-lactic acid) and poly(D-lactic acid)-b-poly(ethylene glycol)-b-poly(d-lactic acid) forms a micellar suspension showing a sol–gel transition around the body temperature by stereocomplex (sc) formation of the PLLA and PDLA segments [18–20]. This system was shown to have high potential as a thermosensitive injectable scaffold for tissue engineering because it was characterized by the easy control of elastic modulus and degradation time by tuning of the block composition and molecular weight. Looking at the above gelation behaviors of the PLA–PEG block copolymers, these sol–gel systems can be applicable to gel therapy for LV remodeling after MI. Therefore, we injected the gels consisting of PLA–PEG block copolymer suspension into chronic MI model rats to study their effect on tissue response and cardiac functions. To date, several types of polymer systems have been examined as the gels in the treatment of MI and have shown different therapeutic effects. However, it was difficult to directly compare the effects because the optimal experimental conditions such as injection timing [1, 21, 22], volume [4], end point of treatment [4], and severity of the model illness differed for each material. Therefore, an identical model assay system should be established to directly compare the effects of different gel materials. In this study, we compare the cardiac functions of a PLA–PEG gel with alginate gel, which is most widely studied in gel therapy, as a positive reference and with saline as an experimental control in terms of LV cavity area, infarct size, and CD68 positive immunostaining.
Materials and methods
Materials
Aqueous solutions (90%) of l-lactic acid (l-LA) and d-lactic acid (d-LA) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and Musashino Chemical Laboratory, Ltd. (Tokyo, Japan), respectively. PEG has an average molecular weight (M n) of 3020 Da (determined by proton nuclear magnetic resonance [1H-NMR]). Succinic acid (SA) and p-toluenesulfonic acid (TSA) were purchased from Nacalai Tesque Inc. (Kyoto, Japan). Tin(II) chloride (SnCl2) was purchased from Sigma Aldrich (St. Louis, MO, USA). Sodium alginate (15–25 cps) was purchased from Funakoshi Co., Ltd. (Tokyo, Japan).
Synthesis of PLA–PEG block copolymers
The PLLA–PEG and PDLA–PEG block copolymers were prepared as reported previously [23]. In short, a mixture of 90% l-LA (25.0 g) or 90% d-LA (25.0 g), PEG (22.5 g), and SA (0.8 g) was added to SnCl2 (0.46 g) and TSA (0.46 g) as two-component catalysts and heated to 150 °C for dehydrative polycondensation. The reaction system was heated to 150 °C and gradually evacuated to a pressure of 0.67 kPa over 15–30 h. The resultant polycondensate was dissolved in a dichloromethane and reprecipitated into a cold diethyl ether. The obtained precipitates were isolated by filtration and thoroughly dried under reduced pressure to obtain a PLLA–PEG sample.
Preparation of PLA–PEG gel and alginate gel
Each of the PLLA–PEG and PDLA–PEG block copolymers (150 mg) obtained above was dissolved in tetrahydrofuran (THF; 1 mL). Deionized water (1 mL) was then added dropwise to the solution with an ultrasonic wave applied for 5 min. The resulting THF–H2O solution was evaporated with an evaporator to thoroughly remove the THF. The finally obtained suspensions contained the PLLA–PEG or PDLA–PEG block copolymer as 15 and 20 wt% solute. The PLLA–PEG and PDLA–PEG suspensions were mixed together in equal amounts at 20 °C to obtain a mixed suspension (PLLA–PEG/PDLA–PEG suspension). One milliliter each of the PLLA–PEG, PDLA–PEG, and PLLA–PEG/PDLA–PEG suspensions was separately transferred to a vial (5 mL) and warmed to 37 °C for 1 h. The sol–gel transition was determined by the ordinary vial inverting method.
An alginate gel was also prepared by mixing sodium alginate with an appropriate amount of calcium gluconate in aqueous solution. The final alginate gel consisted of 1 wt% alginate and 0.3 wt% calcium gluconate [1].
Instrumentation
The 1H NMR spectra were recorded on a Varian 300 MHz Instrument (Varian Inc., Tokyo, Japan) in deuterated chloroform (CDCl3) containing 0.03 vol% tetramethylsilane as the internal standard. The number- [M n (gpc)] and weight-average [M w (gpc)] molecular weights and molecular weight distribution (M w/M n) were determined by gel permeation chromatography. The analyzer consisted of a Shimadzu LC-10AD pump and a RID-10A refractive-index detector. A set of Tosoh TSK gel-GMHHR-H columns (mean particle size, 5 µm; internal diameter, 7.8 mm; length, 30 cm) was used with CHCl3 as the eluent at a flow rate of 0.3 mL/min at 35 °C. The molecular weights were calibrated with polystyrene standards. Wide-angle X-ray diffraction (WAXD) was measured on a RINT 2100 FSL refractometer (Rigaku Co., Tokyo, Japan). The scattering intensity was recorded in a Bragg angle range of 5–40° at a scan rate of 2°/min at room temperature. The PLLA–PEG, PDLA–PEG, and PLLA–PEG/PDLA–PEG suspensions (20 wt%) were incubated at 37 °C for 60 min, freeze-dried overnight, and subjected to the WAXD measurement.
Degradation test
A sample of the PLLA–PEG/PDLA–PEG mixed suspension (20 wt%; 200 mg) and the alginate gel (1 wt%; 50 mg) was enclosed in a dialysis membrane tube (Spectra/Por, molecular weight cut-off, 50,000) and soaked in deionized water at 37 °C. The deionized water was replaced every other day. The suspension was taken out of the membrane tube after soaking for 1, 7, 14, or 28 days and subjected to freeze-drying. The freeze-dried samples were weighed and analyzed.
MI rat model experimental groups
The animal research protocol using male Sprague–Dawley rats (200–250 g) was approved by the Animal Care Committee of the National Cerebral and Cardiovascular Center Research Institute (Suita, Osaka, Japan) (permit number: 13023). The rats were put under anesthesia using isoflurane and pentobarbital sodium (60 mg/kg). The rats were incubated and mechanically ventilated with a volume-controlled mechanical ventilator. A left thoracotomy was performed to expose the heart, and the left anterior descending coronary artery was ligated with a 7-0 polypropylene suture. When stable cardiac rhythm had been recovered, the chest was closed with a 3-0 silk suture. Four weeks later, MI echocardiography was conducted to measure the percent fractional shortening (%FS). The MI model rat was excluded if it showed a %FS >25%. The MI rats were randomly assigned to receive an injection of 100 µL of saline (n = 5), alginate gel (n = 5), and PLLA–PEG/PDLA–PEG gel (n = 4) into the infarct area with a 30-gauge needle. After that, the chest was closed using a 3-0 silk suture.
Echocardiography
Echocardiography was performed under anesthesia by an intravenous injection of pentobarbital sodium (60 mg/kg) and controlled ventilation using a Pro Sound II SSD-6500SV (Hitachi Aloka Medical, Ltd., Tokyo, Japan) installed with a 25-mm high-frequency linear transducer before and 4 weeks after the gel injection. A two-dimensional view of the LV was obtained. The LV dimension end diastolic (LVDd) and LV dimension end systolic (LVDs) values were measured using short-axis M-mode images. The %FS was calculated as %FS = [(LVDd − LVDs)/LVDd] × 100.
Histological analysis
The rat heart was arrested with KCl (1 mEq/mL) 4 weeks after the injection and quickly extirpated. Each slice was fixed with 10% buffered formalin, embedded in paraffin, and sectioned into 4-µm slices. The sections were then stained with hematoxylin-eosin (H&E) or Masson’s trichrome (MT) stain. The infarct size (infarct area/LV total area) and LV cavity area were evaluated using NIH Image J software.
Immunohistochemical staining was performed using antibodies against CD68 (MCA341R, Serotec). The number of CD68 positive cells in the MI area was observed.
Results
Characteristics of PLA–PEG block copolymers
Enantiomeric PLLA–PEG and PDLA–PEG block copolymers were synthesized by direct polycondensation of l-LA and d-LA, respectively, in the presence of PEG and succinic acid with the bicomponent catalyst TSA–SnCl2 (Fig. 1). Table 1 summarizes the characteristics of the obtained block copolymers. The M n and block composition of the resultant block copolymers were evaluated using 1H-NMR. PLLA–PEG: M n = 4300 Da (PLLA:PEG = 1280:3020 in Mn)1.0, PLLA/PEG = 30/70 wt/wt%. PDLA–PEG: M n = 6790 Da (PDLA: PEG = 1460:3020)1.5, PDLA/PEG = 32/68 wt/wt%. The Mn value of the resultant PDLA–PEG block copolymer was almost 1.5 times that of the PLLA–PEG block copolymer under an identical block composition of PLA/PEG = 30/70. The block numbers of PLLA–PEG and PDLA–PEG block copolymer were determined to be 1.0 and 1.5, respectively.
Fig. 1.
Synthesis of the enantiomeric PLA–PEG block copolymers by using l-LA and d-LA as the starting monomers
Table 1.
Characteristics of the PLA–PEG block copolymers
| Block copolymer | PLA/PEG ratioa | Mn a (Da) | Mn b (Da) | Mw b (Da) | Mw/Mn b |
|---|---|---|---|---|---|
| PLLA–PEG | 30:70 | 4300 | 3100 | 4600 | 1.45 |
| PDLA–PEG | 32:68 | 6790 | 5200 | 8300 | 1.61 |
aMeasured by nuclear magnetic resonance
bMeasured by gel permeation chromatography
Formation of PLLA–PEG/PDLA–PEG and alginate gels
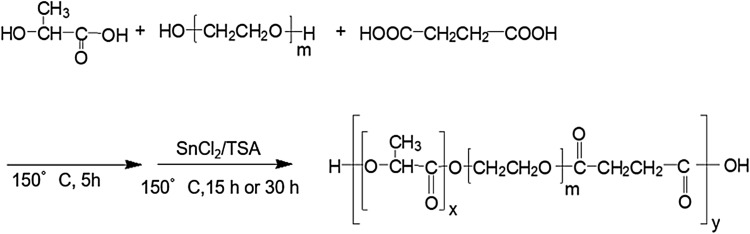
The PLLA–PEG and PDLA–PEG block copolymer suspensions were prepared and mixed together in 1:1 ratio to obtain a PLLA–PEG/PDLA–PEG suspension. The PLLA–PEG suspension was cloudy and the PDLA–PEG suspension was transparent. This results shows that the PLLA–PEG and PDLA–PEG suspensions might have different particle sizes. The mixed suspension became slightly translucent. Each suspension was warmed, and its sol–gel transition was evaluated by the tube inverting method. Both the single PLLA–PEG and PDLA–PEG suspensions maintained the sol state at 20 and 37 °C. The PLLA–PEG/PDLA–PEG suspension maintained the sol state at 20 °C and turned to gel at 37 °C within 5 min (Fig. 2A).
Fig. 2.
A Sol–gel states of PLLA–PEG, PDLA–PEG, and PLLA–PEG/PDLA–PEG suspensions warmed up to 37 °C. B WAXD profiles of the freeze-dried samples from the PLLA–PEG, PDLA–PEG, and PLLA–PEG/PDLA–PEG suspensions warmed up to 37 °C. The labels sc and hc represent the stereocomplex and homo-chiral crystals, respectively. C Temperature-dependent changes in G′ and G″ for the PLLA–PEG, PDLAPEG, and PLLA–PEG/PDLA–PEG suspension. The heating rate was 0.5 °C/min
The WAXD profile of the freeze-dried sample from the PLLA–PEG/PDLA–PEG gel compared with the profiles of the freeze-dried samples of PLLA–PEG and PDLA–PEG suspensions is shown in Fig. 2B. The diffractions from the PEG crystals appeared at 2θ = 23.5° and 19°. The latter diffraction overlapped with that from the homo chiral (hc) crystals of PLLA and PDLA. A diffraction peak from the PLLA hc crystals was detected at 2θ = 17°. However, no diffraction peak was detected in the WAXD profile of PDLA–PEG. The PLA of the PLLA–PEG and PDLA–PEG block copolymer was crystal and amorphous, respectively. The crystallinity of the block copolymer might affect its block number and molecular weight [23]. The diffraction peaks from the sc crystals appeared at 2θ = 12° and 21° in the WAXD profiles of PLLA–PEG/PDLA–PEG, while no diffraction from the sc crystals was observed in the single PLLA–PEG and PDLA–PEG copolymers. These findings indicated that hydrogelation of the PLLA–PEG/PDLA–PEG suspension was induced by the sc formation between the enantiomeric PLLA and PDLA blocks [18, 19].
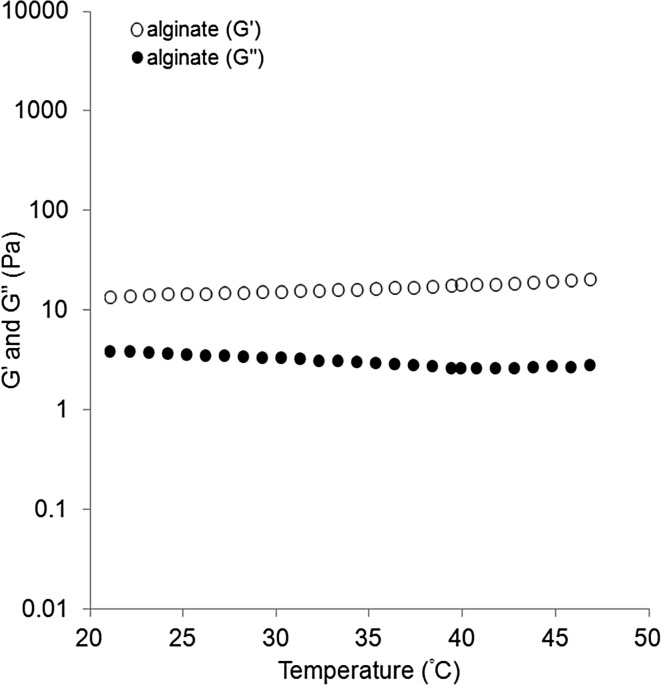
The temperature-dependent changes in the storage (G′) and loss (G″) moduli of the PLLA–PEG, PDLA–PEG, and PLLA–PEG/PDLA–PEG suspensions were evaluated (Fig. 2C). The G′ and G″ of the PLLA–PEG/PDLA–PEG suspension remained as low as 1 Pa at 20 °C and increased to 10 kPa with increase in the temperature. The gelation point determined from the crossover of the G′ and G″ curves was 29 °C. Above this temperature, the G′ and G″ increased and reached to about 2 and 0.2 kPa, respectively, at 37 °C. On the other hand, the G′ and G″ values of the alginate gel were constant around 15 and 4 Pa, respectively, irrespective of the temperature change (Fig. 3), suggesting that the gelation had occurred at the initial gel preparation stage. The G′ and G″ values were significantly higher in the PLLA–PEG/PDLA–PEG gel than in the alginate gel at 37 °C.
Fig. 3.
Temperature-dependent changes in G′ and G″ for an aqueous solution of sodium alginate (1 wt%) and calcium gluconate (0.3 wt%). The heating rate was 0.5 °C/min
Degradability
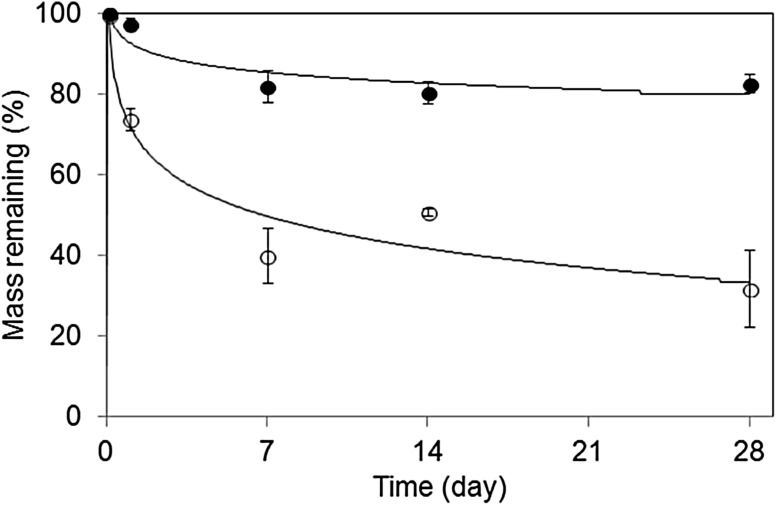
The degradation profiles of the PLLA–PEG/PDLA–PEG and alginate gels were evaluated in deionized water at 37 °C (Fig. 4). The weight of the PLLA–PEG/PDLA–PEG gel quickly decreased to 30% within 1 day and then slowly to 70% in 4 weeks relative to the initial mass. In contrast, the weight of the alginate gel decreased to 20% of the initial mass in 1 week and leveled off thereafter. Since the alginate gel consisted of broad molecular weight fractions, the low-molecular-weight fraction was dialyzed to leave a high-molecular-weight fraction with little degradation. Therefore, this gel could survive for at least 21 days because of its least degradation at the physiological conditions, which is well known in alginates [6]. These results revealed that the PLLA–PEG/PDLA–PEG gel can more easily biodegrade than the alginate gel.
Fig. 4.
Degradation profiles of PLLA–PEG/PDLA–PEG gel (open circle) and alginate gel (filled circle) in deionized water at 37 °C
Cardiac function
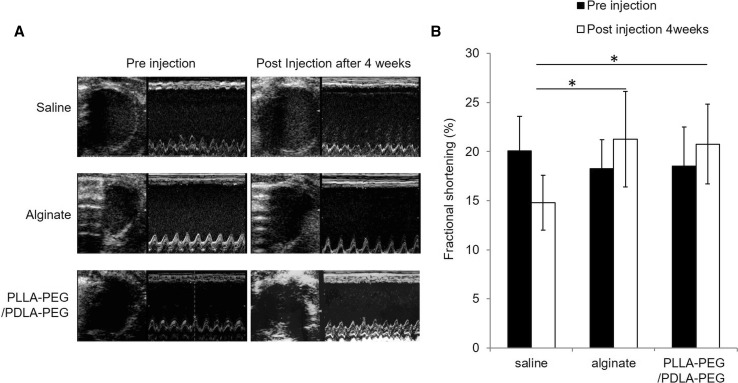
Representative echocardiographic M-mode images obtained before and 4 weeks after the injection of saline, alginate gel, and PLLA–PEG/PDLA–PEG gel are shown in Fig. 5A. The regional LV wall motion was evaluated in these images. The anterior wall motion showed little difference between the pre- and post-injection of saline, alginate gel, and PLLA–PEG/PDLA–PEG gel. However, the LV global wall motion was preserved and improved after the injection of the alginate gel and the PLLA–PEG/PDLA–PEG gel, respectively.
Fig. 5.
A Representative echocardiographs of LV M-mode before and 4 weeks after the injection of saline, alginate gel, and PLLAPEG/PDLA–PEG gel. B Comparisons of %FS before and 4 weeks after the injection of saline, alginate gel, and PLLA–PEG/PDLA–PEG gel. *p < 0.05
The %FS value was assessed by comparing the echocardiographic M-mode before and 4 weeks after the injection. The injection of both the alginate gel and the PLLA–PEG/PDLA–PEG gels improved %FS compared with the saline injection (p < 0.05; saline vs. alginate gel and PLLA–PEG/PDLA–PEG gel) (Fig. 5B). The %FS values became 18.5 ± 4.0% before the injection of PLLA–PEG/PDLA–PEG gel and 20.8 ± 4.1% 4 weeks after the injection. The %FS value was 18.3 ± 2.9% before the injection of alginate gel and in 21.1 ± 4.8% 4 weeks after the injection. On the other hand, the %FS was 20.1 ± 3.5% before the injection of saline but decreased to 14.2 ± 2.8% 4 weeks after the injection. Therefore, it was evident that the injection of PLLA–PEG/PDLA–PEG or alginate gel can improve %FS and preserve cardiac function.
Infarct size and morphology changes in the cardiac structure
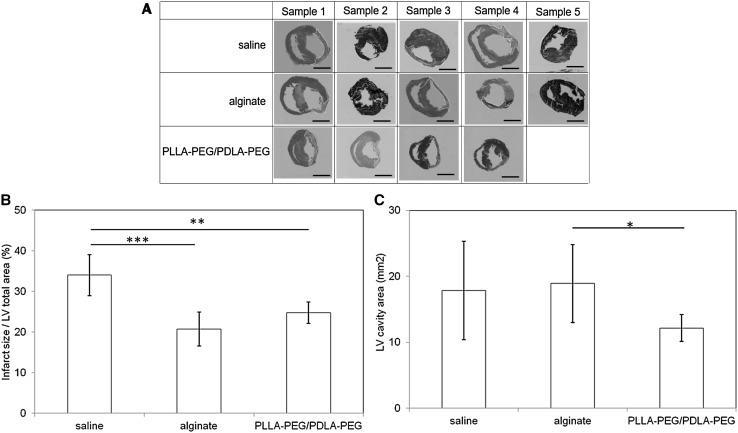
The results of MT staining of the histological sections obtained 4 weeks after the injection showed that the fibrous tissue was stained blue while the cardiac muscle was colored red (Fig. 6A). The infarct ratio was defined as the percentage of infarct area relative to the entire LV cardiac myocyte area (Fig. 6B). The infarct sizes after the injection of saline, alginate gel, and PLLA–PEG/PDLA–PEG gels were 34.0 ± 5.0, 20.8 ± 4.1, and 24.8 ± 2.7%, respectively. The injection of the alginate gel significantly decreased the infarct size compared with the saline injection (p < 0.01). The injection of the PLLA–PEG/PDLA–PEG gel also decreased the infarct size more than the saline injection (p < 0.05). There was no significant difference in infarct size between the PLLA–PEG/PDLA–PEG and alginate gel injections.
Fig. 6.
A Histological evaluation of the section of heart by staining with MT 4 weeks after the injection of saline, alginate gel, and PLLAPEG/PDLA–PEG gel for 4 weeks. Scale bars correspond to 5 mm. B Comparison of infarct area/LV total area after the injection of saline, alginate gel, and PLLA–PEG/PDLA–PEG gel. C Comparison of LV cavity area after injection of saline, alginate gel, and PLLA–PEG/PDLA–PEG gel. *p = 0.06; **p < 0.05; ***p < 0.01
Quantitative analysis of the LV cavity area evaluated from the histological sections stained with MT (Fig. 6C). The LV cavity area obtained after injection of the PLLA–PEG/PDLA–PEG gel decreased further than that after the alginate gel injection (p = 0.06). This result suggested that the PLLA–PEG/PDLA–PEG gel could effectively prevent cardiac dilatation.
Histological evaluation
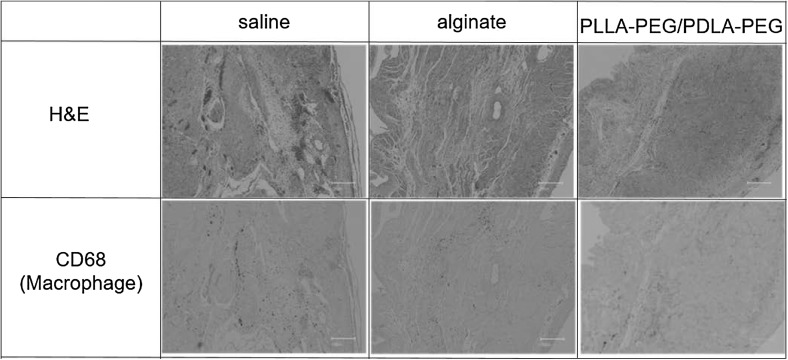
Sections of representative areas of the infarct area stained with H&E and CD68 4 weeks after the injection are shown in Fig. 7. The injections of alginate gel and PLLA–PEG/PDLA–PEG gel were not observed in the sections because they had not been labeled. Therefore, it was unclear whether the gel was present in the injected infarct region.
Fig. 7.
Histology of the sections of heart after injection of saline, alginate gel, and PLLA–PEG/PDLAPEG gel. The slices were stained with H&E and CD68. Scale bars correspond to 200 μm
CD68-positive immunostaining showed the presence of a small number of macrophages in the MI area after the injection of saline, alginate gel, or PLLA–PEG/PDLA–PEG gel. The inflammatory responses to saline, alginate gel, and PLLA–PEG/PDLA–PEG gel were the same.
Discussion
We successfully prepared the PLLA–PEG and PDLA–PEG block copolymers by direct polycondensation of l-LA and d-LA acids in the presence of PEG and SA, respectively. The addition of an equimolar amount of SA relative to PEG adjusted the carboxyl-to-hydroxyl ratio in the polymerization system to 1:1. The M n value of the resultant PDLA–PEG block copolymer was almost 1.5 times that of PLLA–PEG block copolymer under an identical block composition of PLA/PEG = 30/70. The PLLA–PEG and PDLA–PEG block copolymers had block numbers of 1.0 and 1.5, respectively. The polymerization times of the PLLA–PEG and PDLA–PEG block copolymers were 15 and 30 h, respectively. The block number might have been influenced by the polymerization time [23]. Accordingly, the block number of the copolymer chains was higher in the former. With these copolymers, a PLLA–PEG/PDLA–PEG suspension was prepared to demonstrate its sol-to-gel transition by warming to body temperature. The gelation time was within 5 min by the inverting methods at 37 °C, and the gelation temperature was >29 °C by rheological measurement. Therefore, it will quickly become a gel even within the body. Thus, it is evident that this PLLA–PEG/PDLA–PEG suspension can readily be injected into tissue and can turn to gel at the injection site.
The turbidities of the PLLA–PEG and PDLA–PEG suspensions were slightly different from each other, probably because of the size difference of the particles that might be determined by the block numbers of the copolymers and the crystallinities of the PLLA and PDLA cores. In the PLLA–PEG with a block number of 1.0, the PLLA segments can aggregate to form the particle cores with the neighboring PEG segments segregated into the shells, whereas in the PDLA–PEG with the block number of 1.5, the aggregation of the PDLA segments in the cores is likely to involve the PEG segments to a considerable degree to make the segment segregation incomplete. Accordingly, the PLLA–PEG particles showed hc crystallinity, while the PDLA–PEG particles were in an amorphous state as observed by their WAXD profiles (Fig. 2B). It has also been reported that the crystallinity of PLA copolymers shows a decreasing tendency as block number increases [23]. This tendency was also observed in the present suspensions, which caused the different turbidities.
The temperature-dependent rheological changes of the present mixed suspension of the enantiomeric PLA–PEG multiblock copolymers showed an increased storage modulus as temperature increased. Compared with the mixed gel of the corresponding enantiomeric PLA–PEG–PLA triblock copolymers, the enantiomeric PLA–PEG–PEG multiblock copolymer gel showed a much higher storage modulus [24]. However, the solution viscosity of the present mixed suspension of multiblock copolymers was higher than that of the mixed suspension of the triblock copolymers because of the higher molecular weight of the former. Furthermore, the present mixed micelle suspension immediately formed a gel near body temperature with sc formation between the PLLA and PDLA blocks, which was supported by the WAXD profiles. The sc crystallites formed in the suspension provides the cross-linking points for its gelation. Previously, Fujiwara showed the temperature-dependent WAXD for an aqueous suspension of enantiomeric triblock copolymers (PLLA–PEG–PLLA and PDLA–PEG–PDLA) and indicated that the sc crystallinity increased as temperature increased, making the storage modulus increase with it [25]. This feature coincides with the aforementioned increased storage modulus of the present mixed suspension as temperature increased. The present gel system consists of a low-molecular-weight PLLA–PEG block copolymer (block number of 1.0) and a high-molecular-weight PDLA–PEG (block number of 1.5), and the viscosity of the mixed micelle solution can be adjusted for easy injection. The fast gelation rate of the present mixed suspension prevents the migration of the sol from the gel at the injection site. In addition, no chemical cross-linking agent is necessary for gelation, and the biological safety of the gel is guaranteed. This characteristic sol–gel system was subjected to evaluation for gel therapy of MI using an alginate gel as a positive control.
The storage modulus of the present PLLA–PEG/PDLA–PEG gel is significantly higher than that of the alginate gel at 37 °C. It is known that the storage modulus of alginate gel can be controlled by the Ca2+ concentration and molecular weight, although tuning it is not easy. However, the properties of the present PLLA–PEG/PDLA–PEG gel can readily be controlled in the range of 1–6 kPa by the PLA/PEG composition, molecular weight, and block sequences [18, 26]. Plotkin et al. compared the cardiac function of several PEG gels with different moduli with a rat MI model and suggested that a greater therapeutic effect was obtained by a gel with a modulus of 1800 Pa versus one with a modulus of 434 Pa [27]. In this study, it was difficult to evaluate the influence of G′ on the therapeutic effect in our comparison of PLLA–PEG/PDLA–PEG gel and alginate gel, which have different polymer structures.
A gel’s degradation rate depends on the molar mass, crystallinity, and morphology of implanted gel devices. Physical stress and force to the devices must also affect the in vivo decomposition rate. In particular, a beating heart stimulates the decomposition rate of polymeric materials. Landa et al. reported that only a small amount of alginate gel implanted around the heart remained at the implantation site after 4 and 6 weeks. Since 80% of the alginate gel remained even after 4 weeks in an vitro degradation test, the in vivo degradation must be enhanced in the body fluids within a dynamic environment. The PLLA–PEG/PDLA–PEG gel showed a similar degradation rate in vivo, and it might be absent from the implantation site around the heart within 4 weeks. We believe that the PLLA–PEG/PDLA–PEG gel did not completely degrade but instead disappeared from the implantation site around the heart.
Both PLLA and PLGA implants are known to cause inflammation since they are considered foreign bodies in vivo [28]. Moreover, the inflammatory response is strongly caused by the oligomers and monomers liberated from the implants [29]. On the contrary, in our previous reports, the PLLA–PEG multiblock copolymer film showed a weaker tissue response than the PLLA film [15, 16]. Macrophage expression peaks at 3–4 days after infarction and then continuously decreases. Macrophages are reportedly hardly seen beyond 4 weeks after infarction [20]. Therefore, the influence of infarct formation and injection on macrophages would be minor in this study and strongly reflect only the influence of the gel. The PLLA–PEG/PDLA–PEG gel caused a weak inflammatory reaction at a level identical to that caused by the control alginate gel and saline in the myocardium 4 weeks after the injection. This mild inflammatory reaction of the PLLA–PEG/PDLA–PEG gel suggested that it was mostly decomposed into less harmful substances 4 weeks after the injection into the myocardium.
Landa et al. [1] showed that the injection of alginate gel could prevent LV remodeling after MI. In the present study, we confirmed that the alginate gel and PLLA–PEG/PDLA–PEG effectively improved cardiac function and reduced infarct size compared with saline. The PLLA–PEG/PDLA–PEG gel reduced the LV cavity area to a higher degree than alginate gel injection. This reduction in LV cavity area with PLLA–PEG/PDLA–PEG gel use may be related with a long-term inhibitory effect on LV remodeling. Therefore, the present PLLA–PEG/PDLA–PEG gel should have high potential for clinical application in the gel treatment of MI, but further investigations are needed to determine the optimal conditions.
Acknowledgements
This work was partly supported by Intramural Research Fund of National Cerebral and Cardiovascular Center (22-2-4) and the S-innovation Research Program for the “Development of the biofunctional materials for realization of innovative medicine”, Japan Science and Technology Agent (JST).
Conflict of interest
The authors declare no conflicts of interest.
Ethical standard
All animal protocols in this study were approved by the Animal Care Committee of the National Cerebral and Cardiovascular Center Research Institute (Permit Number 13023).
References
- 1.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 2.Ruvinov E, Cohen S. Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv Drug Deliv Rev. 2016;96:54–76. doi: 10.1016/j.addr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Tsur-Gang O, Ruvinov E, Landa N, Holbova R, Feinberg MS, Leor J, et al. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials. 2009;30:189–195. doi: 10.1016/j.biomaterials.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in swine. J Am Coll Cardiol. 2009;54:1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Frey N, Linke A, Süselbeck T, Müller-Ehmsen J, Vermeersch P, Schoors D, et al. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ Cardiovasc Interv. 2014;7:806–812. doi: 10.1161/CIRCINTERVENTIONS.114.001478. [DOI] [PubMed] [Google Scholar]
- 6.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Shamkhani A, Duncan R. Radioiodination of alginate via covalently-bound tyrosinamide allows monitoring of its fate in vivo. J Bioact Compat Polym. 1995;10:4–13. doi: 10.1177/088391159501000102. [DOI] [Google Scholar]
- 8.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 9.Radhakrishnan J, Krishnan UM, Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv. 2014;32:449–461. doi: 10.1016/j.biotechadv.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Wang T, Jiang XJ, Tang QZ, Li XY, Lin T, Wu DQ, et al. Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG–PCL–MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater. 2009;5:2939–2944. doi: 10.1016/j.actbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. J Card Fail. 2009;15:629–636. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Garbern JC, Minami E, Stayton PS, Murry CE. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011;32:2407–2416. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, Wu DQ, Jiang XJ, Zhang XZ, Li XY, Zhang JF, et al. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. Eur J Heart Fail. 2009;11:14–19. doi: 10.1093/eurjhf/hfn009. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaoka T, Njatawidjaja E, Kasai A, Agudelo CA, Ehashi T, Kakinoki S, et al. Elastic/adhesive double-layered PLA–PEG multiblock copolymer membranes for postoperative adhesion prevention. Polym Degrad Stab. 2013;98:2168–2176. doi: 10.1016/j.polymdegradstab.2013.08.026. [DOI] [Google Scholar]
- 16.Ehashi T, Kakinoki S, Yamaoka T. Water absorbing and quick degradable PLLA/PEG multiblock copolymers reduce the encapsulation and inflammatory cytokine production. J Artif Organs. 2014;17:321–328. doi: 10.1007/s10047-014-0791-z. [DOI] [PubMed] [Google Scholar]
- 17.Park IH, Sohn JH, Kim SB, Lee KS, Chung JS, Lee SH, et al. An open-label, randomized, parallel, Phase III trial evaluating the efficacy and safety of polymeric micelle-formulated paclitaxel compared to conventional cremophor EL-based paclitaxel for recurrent or metastatic HER2-negative breast cancer. Cancer Res Treat. 2017;49:569–577. doi: 10.4143/crt.2016.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujiwara T, Mukose T, Yamaoka T, Yamane H, Sakurai S, Kimura Y. Novel thermo-responsive formation of a hydrogel by stereo-complexation between PLLA–PEG–PLLA and PDLA–PEG–PDLA block copolymers. Macromol Biosci. 2001;1:204–208. doi: 10.1002/1616-5195(20010701)1:5<204::AID-MABI204>3.0.CO;2-H. [DOI] [Google Scholar]
- 19.Hsu YI, Masutani K, Kimura Y, Yamaoka T. A novel bioabsorbable gel formed from a mixed micelle solution of poly(oxyethylene)-block-poly(L-lactide) and poly(oxyethylene)-block-poly(D-lactide) by concomitant stereocomplexation and chain extension. Macromol Chem Phys. 2013;214:1559–1568. doi: 10.1002/macp.201300230. [DOI] [Google Scholar]
- 20.Lee CW, Manoshiro T, Hsu YI, Kimura Y. Gelation behavior of bioabsorbable hydrogels consisting of enantiomeric mixtures of A–B—a tri-block copolymers of polylactides (A) and poly(ethylene glycol) (B) Macromol Chem Phys. 2012;213:2174–2180. doi: 10.1002/macp.201200375. [DOI] [Google Scholar]
- 21.Kadner K, Dobner S, Franz T, Bezuidenhout D, Sirry MS, Zilla P, et al. The beneficial effects of deferred delivery on the efficiency of hydrogel therapy post myocardial infarction. Biomaterials. 2012;33:2060–2066. doi: 10.1016/j.biomaterials.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Blackburn NJR, Sofrenovic T, Kuraitis D, Ahmadi A, McNeill B, Deng C, et al. Timing underpins the benefits associated with injectable collagen biomaterial therapy for the treatment of myocardial infarction. Biomaterials. 2015;39:182–192. doi: 10.1016/j.biomaterials.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Somekawa S, Masutani K, Hsu YI, Mahara A, Kimura Y, Yamaoka T. Size-controlled nanomicelles of poly(lactic acid)–poly(ethylene glycol) copolymers with a multiblock configuration. Polymers. 2015;7:1177–1191. doi: 10.3390/polym7061177. [DOI] [Google Scholar]
- 24.Hiemstra C, Zhong ZY, Jiang X, Hennink WE, Dijkstra PJ, Feijen J. PEG–PLLA and PEG–PDLA multiblock copolymers: synthesis and in situ hydrogel formation by stereocomplexation. J Control Release. 2006;116:e17–e19. doi: 10.1016/j.jconrel.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara T. Thermo-responsive gels: biodegradable hydrogels from enantiomeric copolymers of poly(lactide) and poly(ethylene glycol). In: Kishan K, Carmen S, editors. Degradable polymers and materials: principles and practice, ACS symposium series, 2nd edn; 2012. p. 287–311.
- 26.Abebe DG, Fujiwara T. Controlled thermoresponsive hydrogels by stereocomplexed PLA–PEG–PLA prepared via hybrid micelles of pre-mixed copolymers with different PEG lengths. Biomacromol. 2012;13:1828–1836. doi: 10.1021/bm300325v. [DOI] [PubMed] [Google Scholar]
- 27.Plotkin M, Vaibavi SR, Rufaihah AJ, Nithya V, Wang J, Shachaf Y, et al. The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack. Biomaterials. 2014;35:1429–1438. doi: 10.1016/j.biomaterials.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 28.Ceonzo K, Gaynor A, Shaffer L, Kojima K, Vacanti CA, Stahl GL. Polyglycolic acid induced inflammation. Tissue Eng. 2006;12:301–308. doi: 10.1089/ten.2006.12.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam KH, Schakenraad JM, Groen H, Esselbrugge H, Dijkstra PJ, Feijen J, et al. The influence of surface morphology and wettability on the inflammatory response against poly(l-lactic acid): a semi-quantitative study with monoclonal antibodies. J Biomed Mater Res. 1995;29:929–942. doi: 10.1002/jbm.820290804. [DOI] [PubMed] [Google Scholar]