Abstract
Atmospheric (in vitro) oxygen pressure is around 150 mm Hg (20% O2), whereas physiologic (in vivo) oxygen pressure ranges between 5 and 50 mm Hg (0.7–7% O2). The normoxic environment in cell culture does not refer to a physiological stem cell niche. The aim of this study is to investigate the effect of oxygen concentration on cell properties of human mesenchymal stem cells (MSCs). We analyzed cell proliferation rate, senescence, immunophenotype, stemness gene expression and differentiation potency with human urine stem cells (USCs), dental pulp stem cells (DPSCs), amniotic fluid stem cells (AFSCs), and bone marrow stromal cells (BMSCs). USCs, DPSCs, AFSCs and BMSCs were cultured under either 5% O2 hypoxic or 20% O2 normoxic conditions for 5 days. MSCs cultured under hypoxia showed significantly increased proliferation rate and high percentage of S-phase cells, compared to normoxic condition. In real-time PCR assay, the cells cultured under hypoxia expressed higher level of Oct4, C-Myc, Nanog, Nestin and HIF-1α. In immunophenotype analysis, MSCs cultured under hypoxia maintained higher level of the MSC surface markers, and lower hematopoietic markers. Senescence was inhibited under hypoxia. Hypoxia enhances osteogenic differentiation efficiency compared to normoxia. Hypoxia showed enhanced cell proliferation rate, retention of stem cell properties, inhibition of senescence, and increased differentiation ability compared to normoxia.
Keywords: Hypoxia, Mesenchymal stem cells, Stem cell property, Stem cell niche, Normoxia
Introduction
Cell therapy using stem cells has become an important field for the development of tissue engineering and regenerative medicine [1]. Human mesenchymal stem cells (MSCs) have self-renewal and multi-lineage differentiation potential [2, 3]. MSCs have been found in several tissues including chondrocytes, adipocytes, osteocytes [2], bone-marrow [3], tooth [1], amniotic fluid [4] or urine [5]. MSCs are recognized as important cell sources for stem cell therapy, and it has been applied to treat cardiovascular disorders [6], nerve injury [7], or bone regeneration [8].
MSCs from patients exhibit decreased survival, proliferation, and differentiation, therefore they require a strategy to improve their regenerative ability. The suggested strategies were treatment with growth factors [9], antiaging compounds [10], or hypoxic shock [8]. Beneficial effects of hypoxic culture on proliferation and differentiation potentials of MSCs have been suggested by several groups [11, 12]. These high therapeutic effects of stem cells in this hypoxic condition assumed that stem cells’ original niche or preferable microenvironment is low oxygen concentration [13]. Oxygen is an important factor that serves a key substrate for cellular energy production and metabolism [14]. Cellular processes include cell proliferation [15] and differentiation [16] can be affected by oxygen concentration. Cellular responses along the oxygen gradient are particularly revealed in bone marrow. In the deep hypoxic areas, cells trying to keep maintenance of stem cell characters, whereas better oxygenated areas allow increase of more differentiated progenitors [13]. Hypoxia was also reported to improve the efficiency of pluripotent stem cell generation on human somatic cells [17], and prevention of differentiation of stem cells, including neural stem cells [18], hematopoietic stem cells [13], mesenchymal stem cells [19] and embryonic stem cells (ESCs) [20].
The common cell culture in vitro environment does not refer to a physiological stem cell niche. Atmospheric (in vitro) oxygen concentration is around 150 mm Hg (20% O2, normoxia), whereas physiologic (in vivo) oxygen is much lower, ranging up to 5% in cartilage [14], 4–7% in bone marrow, 10–13% in arteries, lungs and liver [21, 22]. The in vitro culture environmental differences can alter the characteristics of the stem cells. For clinical applications, the characteristics of stem cells should be maintained. Therefore, this study investigated the effect of oxygen concentration on various human MSCs including human urine stem cells (USCs), dental pulp stem cells (DPSCs), amniotic fluid stem cells (AFSCs), and bone marrow stem cells (BMSCs) through cell proliferation rate, senescence, immunophenotype, gene expression and osteogenic differentiation.
Materials and methods
Cell preparation
The Ethics Committee of Kyungpook National University Hospital approved this study and all patients gave informed consent before providing human samples. For USCs preparation, sterile voided urine sample (100 mL) was collected from a healthy male volunteer, and centrifuged. Cell pellets were washed with phosphate buffered saline (PBS). The cell pellets were resuspended and plated in 24-well tissue culture plates at about 500 cells per well with mixed medium composed of keratinocyte-serum free medium and progenitor cell medium (Gibco, Carlsbad, CA, USA) in a 1:1 ratio as previously described [5]. DPSCs were kindly provided by Prof. Eui Kyun Park, Kyung-pook National University, Korea. The DPSCs were maintained in α-minimum essential medium (α-MEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 units/mL penicillin, and 100 mg/mL strep-tomycin (HyClone Labs, Thermo Scientific, Rockford, IL, USA). For AFSCs preparation, amniotic fluid was obtained from consenting by women who underwent a routine amniocentesis at a gestational age 16 weeks. Amniotic fluids (10 mL) were centrifuged, and cell pellets were suspended with Chang Medium with a-MEM, 15% embryonic stem cell-fetal bovine serum (Gibco-Invitrogen, Grand Island, NY, USA) with 18% Chang B and 2% Chang C (Irvine Scientific, Irvine, CA, USA) in a petri dish. BMSCs were purchased from ATCC (PCS-500-012) and cultured in recommended medium (PCS-500-030). All adherent cells were passaged for expansion on reaching 80% confluence, and culture medium was replaced every three days. Cells that had undergone less than five passages were used for this study.
Hypoxic condition
The cells were seeds in well-plates or plates for conduct the various assays. For normoxia studies, cells were cultured at 37 °C with 95% air (20% O2) and 5% CO2 incubator. For hypoxia studies, cells were cultured in the hypoxia chamber (Billups-Rothenberg, Del Mar, CA, USA) that was filled with a gas mixture of composition 5% O2 and 5% CO2, balanced with N2. The chambers were placed into 37 °C incubator. Gas mixture of hypoxia chamber inside also infused every 3 days.
Cell proliferation assay
The cells were cultured at passage 3, and were distributed to 500 cells/cm2 in a plate. Cell proliferation assay were conducted at day 0, 1, 3 and 5 of culture. At the each time point, the culture medium was removed from the wells, and assay medium containing 10% Cell Counting Kit-8 (CCK-8) solution (Dojindo, Gaithersburg, MD, USA) was added to each well. After incubation for 4 h in the incubator, the 96 well plate was measured the absorbance at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA) and OD values were obtained.
Cell cycle analysis
Each stem cell was plated in tissue culture plate and was allowed to be cultured for 5 days in normoxic and hypoxic condition. Stem cells collected on day 5 for cell cycle analysis. Stem cells were pre-fixed with cold ethanol for 1 h followed by stained with PI buffer (50 ug/ml Rnase A, 50 ug/ml Propidium iodine and 0.05% Triton X-100 in PBS). After incubation for 40 min at 37 °C, cells were washed and resuspended in 2% FBS in PBS. Cell cycle analysis was assessed by flow cytometry (Becton–Dickinson, Research Triangle Park, NC, USA).
Gene analysis
After cell cultivation under normoxic and hypoxic condition, each stem cell was harvested at each time point. For gene expression analysis, total RNA was extracted using TRI Reagent® (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. cDNA synthesis was performed using Maxime RT PreMix kit (iNtRON Biotechnology, Inc., Sungnam, Korea). The human primers were designed using primer express (Applied Biosystems), and sequences for pluripotency markers (Oct-4, C-Myc, and Nanong), multipotency marker (Nestin) and hypoxia specific marker (HIF-1α) are listed in Table 1. The assay was carried out by the ABI Prism Sequence Detection System 7000 (PE Biosystems, Carlsbad, CA, USA) with SYBR Green PCR Master Mix (Applied Biosystem). The thermal cycling conditions were followed to inset condition (40 cycles at 94 °C for 5 s, 60 °C for 34 s) and relative quantitation was performed using the 2−∆∆ CT method. Results were normalized as a ratio to β-actin.
Table 1.
Primer sequences for real-time PCR
| Primer | Sequence |
|---|---|
| β-actin | F-ATCGTCCACCGCAAATGCT |
| R′-AAGCCATGCCAATCTCATCTTG | |
| OCT-4 | F-GGAGAATTTGTTCCTGCAGTGC |
| R-AGAACCACACTCGGACCACATC | |
| C-Myc | F-GGCCCCCAAGGTAGTTATCCTT |
| R-CGTTTCCGCAACAAGTCCTCT | |
| Nanog | F-GCATCCGACTGTAAAGAATCTTCA |
| R-CATCTCAGCAGAAGACATTTGCA | |
| Nestin | F-ACACCTGTGCCAGCCTTTCTT |
| R-TGAACACTCTAGACCCACCGGA | |
| HIF-1α | F-GTAGTGCTGACCCTGCACTCAA |
| R-TCCATCGGAAGGACTAGGTGTC |
Immunophenotype
Cellular properties of cells cultured under normoxia and hypoxia were identified using flow cytometry at day 0 and day 5. For this analysis, cells were incubated for 30 min at 4 °C with the PE-conjugated monoclonal antibodies (mAb) specific for MSC markers (CD44, CD73, CD90, CD105), hematopoietic stem cell markers (CD34, CD45), endothelial cell markers (CD31, CD34), immune related marker (HLA-DR) (all from BD Biosciences, San Jose, CA, USA). An isotype-identical mAb (BD Biosciences) served as a control. The stem cells were subjected using a flow cytometry (BD Biosciences). The results were analyzed using FACS software.
Senescence assay
Senescence assay was performed using β-galactosidase staining kit (Cell signaling Technologies, Danvers, MA, USA) according to the manufacturer’s protocol. Senescent cells were determined by the blue color precipitate over the cells.
In vitro osteogenic differentiation potential
In vitro osteogenic differentiation of cells after expansion was performed with differentiation medium (PromoCell, Heidelberg, Germany). For differentiation, 6 × 104 cells per well in a 24-well tissue culture plate were cultured. Osteogenic differentiation medium was treated for 21 days. Mineralized deposits were visualized by Alizarin red S stain (Sigma, St. Louis, MO, USA). For staining, cells were fixed in 10% formalin for 30 min and stained with 2% Alizarin Red S solution for 30 min. Subsequently, cells were rinsed with PBS. Mineralization is assessed by Alizarin Red S Staining Quantification Assay (ScienCell, Carlsbad, CA, USA).
Statistical Analysis
The t test and one-way analysis of variance were used for statistically analyzing the data. All values were expressed as the mean ± SD. p values <0.05 were considered significant. Results were representative of at least three experiments.
Results
Effect of hypoxia for the proliferation of hMSCs
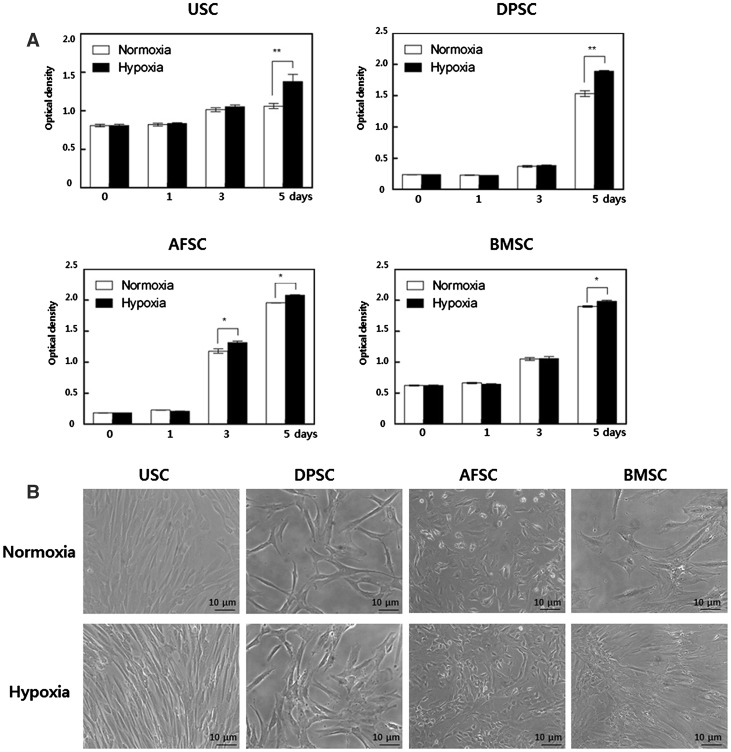
We examined the proliferation rates of human stem cells under 5% hypoxic and 20% normoxic condition through cell proliferation assay. MSC proliferation was assessed by CCK-8 assay on 0, 1, 3, and 5 days. The proliferation rate of cells cultured in 5% hypoxia significantly distinguished at day 5 compared to normoxia (Fig. 1A). Representative cell morphologies at day 5 were shown at Fig. 1B, and there was no difference in cell morphology between hypoxic and normoxic conditions; USCs, DPSCs and BMSCs showed fibroblast-like morphology with bipolar or multipolar and elongated shape, and AFSCs displayed cobble stone-like morphology. All of the cells included granularity around the nucleus.
Fig. 1.
Effect of hypoxia on proliferation of MSCs. A Cell proliferation was measured by CCK-8 assay. At day 5, proliferation rate of cells cultured in 5% hypoxia significantly increased compared to normoxia. B Representative cell morphologic images at day 5. There was no difference in cell morphology between hypoxic and normoxic conditions. C Percent of S-phase cell. In hypoxia, S-phase cell numbers were increased compared to normoxia. The optical density (OD) value was measured at 450 nm absorbance. The data were drawn from three independent experiments and the results were expressed as mean ± SD. *p < 0.05; **p < 0.01. USC urinary stem cells, DPSC dental pulp stem cells, AFSC amniotic fluid stem cells, BMSC bone marrow stem cells. Normoxia 20% oxygen, hypoxia 5% oxygen
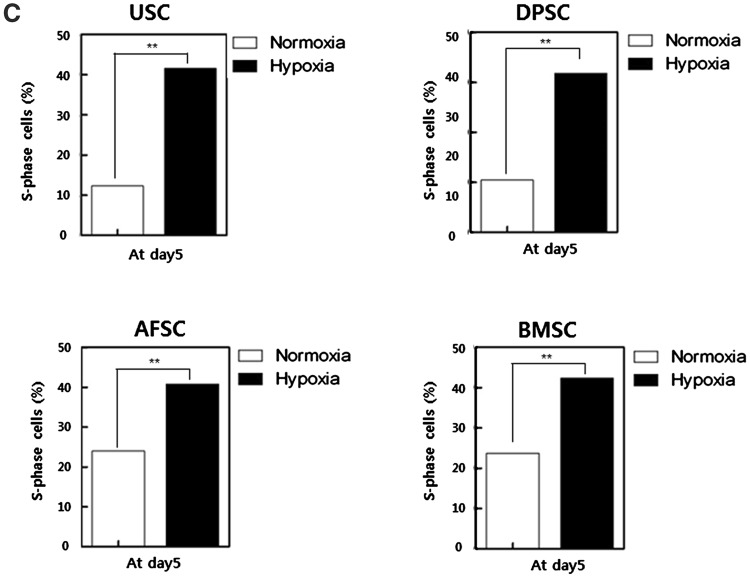
S-phase of cell cycle has close relation with cell proliferation, which is represent increased-proliferation mechanism of MSCs cultured under hypoxia. In hypoxia, S-phase rate of USC, DPSC, AFSC and BMSC showed 3.4, 3.1, 1.7 and 1.9 fold increase compared to normoxia, respectively (Fig. 1C). Obtained results suggested that hypoxic culture increased proliferation of MSC through the upregulation of S-phase cells.
Comparison of stem cell markers and senescence
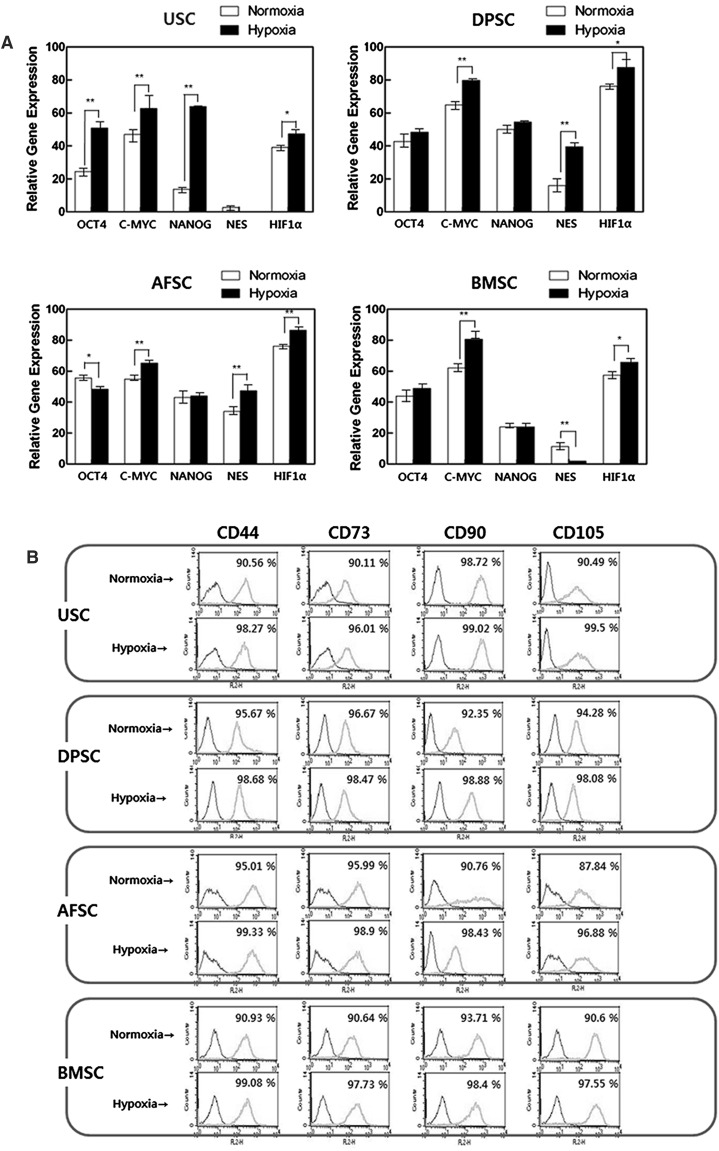
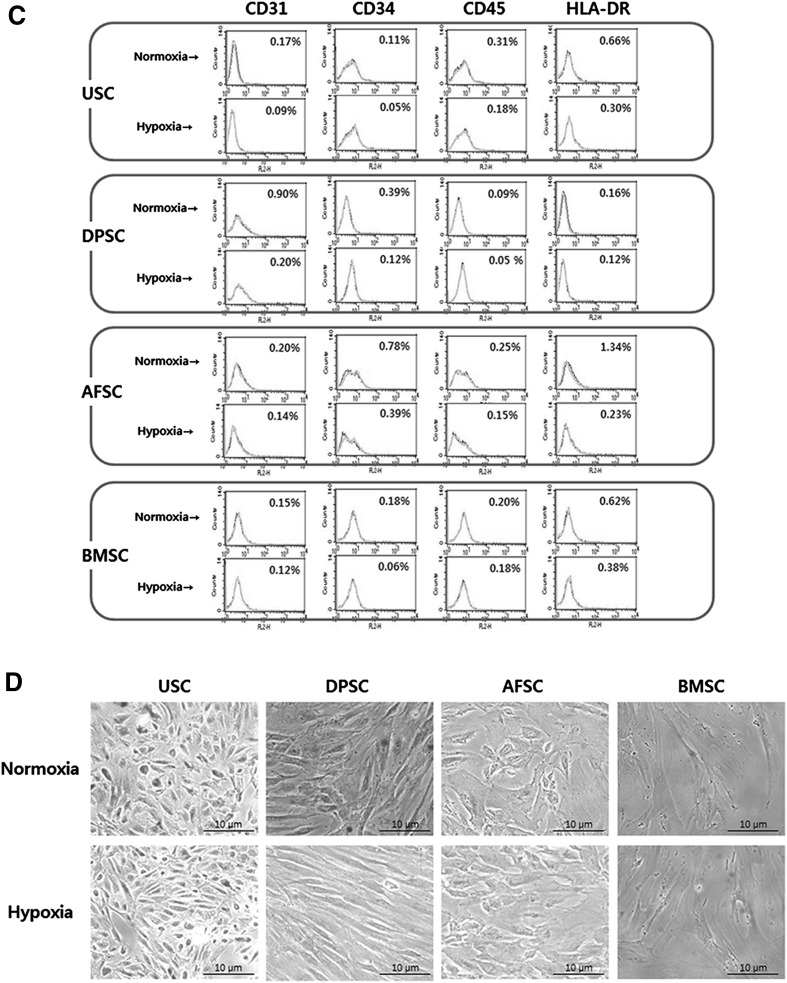
Hypoxia induced increase of mRNA levels of pluripotency markers (Oct4 and C-Myc) in most of hMSCs (except of AFSCs’ Oct4), while, Nanog was only significantly affected by hypoxia in USCs. Nestin, as a multipotent marker, was significantly increased at DPSCs, AFSCs and BMSCs. HIF-1α, oxygen sensing pathway marker, was significantly highly expressed in all of hMSCs (Fig. 2A). The analyses of mesenchymal stem cell markers were carried out with flow-cytometry (Fig. 2B–C). In surface immunophenotype analysis, CD44, CD73, CD90 and CD105 were chosen as MSC positive markers, and CD31, CD34, CD45, and HLA-DR were chosen as negative markers. MSC positive markers on USCs, DPSCs, AFSCs and BMSCs were shown more than 90%, and MSC negative markers were shown less than 1.5%. All of the cells revealed enhanced expression of positive markers and reduced expression of negative markers in hypoxic condition.
Fig. 2.
Effect of hypoxia on gene expression, immunophenotype and senescence in MSCs. A Expression of Oct4, C-Myc, Nanog, Nestin and HIF-1α in MSCs 5 days after normoxia or hypoxia culture. The multipotent and oxygen sensing pathway markers were significantly increased on hypoxia. B Surface immunophenotype analysis for mesenchymal stem cell markers. These markers were enhanced on hypoxia C Surface immunophenotype expression for hematopoietic/immune markers. These markers were decreased on hypoxia. D Cell senescence assay with β-galactosidase. The percentage of senescence cell was significantly reduced in USCs and DPSCs. The data were drawn from three independent experiments and the results were expressed as mean ± SD. *p < 0.05; **p < 0.01. USC urinary stem cells, DPSC dental pulp stem cells, AFSC amniotic fluid stem cells, BMSC bone marrow stem cells. Normoxia 20% oxygen, hypoxia 5% oxygen. In real-time analysis, β-actin was used as an innate control
β-galactosidase is a biomarker for cellular senescence, and it conveniently identified individual senescent cells in vitro. The inhibited senescent status of the cells in hypoxic condition were confirmed by reduced positive cell numbers of β-galactosidase in all kinds of hMSCs. The percentage of positive cell numbers was significantly reduced on USCs and DPSCs (Fig. 2D).
In vitro osteogenic differentiation potential
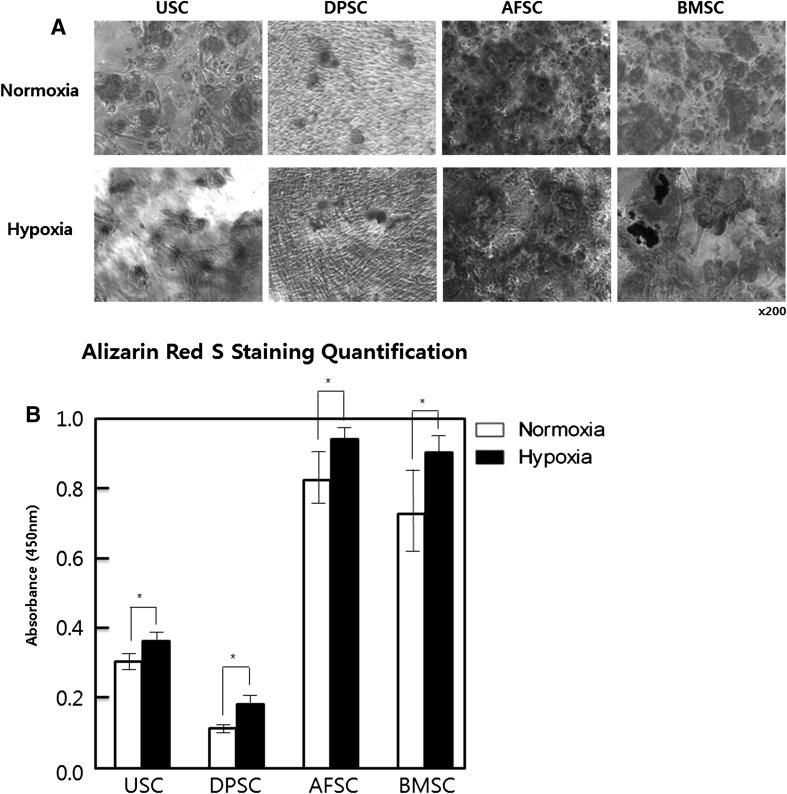
To determine the role of hypoxic culture on osteoblast differentiation of hMSCs, Alizarin Red S staining was performed at day 21 after differentiation. Positive Alizarin Red S stained area was relatively greater in hypoxic cultured cells compared to normoxia in all kinds of the hMSCs (Fig. 3A, B). This result indicated that the cells cultured in hypoxia could maintain and enhance osteogenic differentiation potential of hMSCs.
Fig. 3.
Effect of hypoxic culture for osteogenic differentiation of MSC. A The mineralization nodes were observed by Alizarin Red S staining 21 days after cells were induced in an osteogenic induction medium. B The mineralization was quantified with Alizarin Red S Staining Quantification Assay. Mineral deposition was increased in hypoxia. The data were drawn from three independent experiments and the results were expressed as mean ± SD. *p < 0.05. USC urinary stem cells, DPSC dental pulp stem cells, AFSC amniotic fluid stem cells, BMSC bone marrow stem cells. Normoxia 20% oxygen, hypoxia 5% oxygen
Discussion
In this study, hypoxic condition enhanced the proliferation and maintenance of stemness and osteogenic differentiation potential of MSCs, while, senescence and expression of hematopoietic/immune related markers were inhibited.
Some diseases are caused by abnormal MSCs, usually due to reduced proliferation or differentiation potency of MSCs. These patients showed reduced cell proliferation rate, stemness, and enhanced randomized differentiation compared to normal MSCs [8]. Therefore, MSCs augmentation and correctly induced differentiation could be a potential strategy for the treatment of these diseases. Among several methods for impaired stem cell proliferation ability and differentiation potential, preconditioning of stem cells with low oxygen represents an effective strategy to enhance cell survival, proliferation and differentiation.
First, the effects of hypoxia on cell proliferation and cell cycle were investigated. For cell proliferation, the cell cycle is controlled by a regulatory network, and transition from G1 phase of the cell cycle to S phase must be controlled precisely [23]. Cell proliferation rate in hypoxic condition was analyzed using CCK-8 assay, the result showed enhanced proliferation rate with an increased in S phase of cell cycle using flow cytometry. In addition, real-time PCR analysis revealed an increased expressions of HIF-1α. HIF-1α is a well-known transcription factor which is robustly induced during hypoxia and an essential factor for adaptation under lower oxygen tension [24]. Even though some reports showed cell cycle arrest in G0/G1 phase by HIF-1α through p27 expression [25], considering different cellular contexts HIF-1 activation can show variable results by impacting various aspects of cell biology [26], we could assume that increased HIF-1α expression on 5% hypoxic condition can stimulate MSCs proliferation. Ryan et al. [27] reported similar suggestion, and Stoeltzing et al. [28] also considered that HIF-1α promotes cell proliferation and induces vascular endothelial growth factor expression and angiogenesis.
The second hypoxic effects were observed with expression of stemness markers and senescence. Recent studies have indicated that low oxygen plays an important role in altering characteristics of various types of stem cells. Yamamoto et al. [29] found that 2% oxygen tension promotes the expression of Oct3/4 and Nanog of adipose tissue-derived stromal cells. Hung et al. [12] reported that BMSCs cultured under the condition of 1% oxygen enhanced expression of stemness genes including OCT4, NANOG, SALL4, and KLF4. Similar results were also observed in our study. We found that 5% oxygen promoted expression of stemness genes including OCT4, C-Myc, Nanog and Nestin in USCs, DPSCs, AFSCs and BMSCs (except for AFSCs’ Oct4 and BMSCs’ Nestin), indicating that hypoxic culture condition could enhance stemness in MSCs. The increased expression of pluripotency markers showed inhibition of senescence of MSCs. Tsai et al. [30] also reported that increased Oct4 and Nanog expression revealed along with inhibition of senescence of MSCs. In our results, MSCs in hypoxia showed lower expression of senescence-associated β-galactosidase compared to normoxic cultured cells.
The third hypoxic effects were observed on osteogenic differentiation. Although MSCs need to show to be differentiated into osteogenic, chondrogenic, adipogenic, myogenic, and neurogenic lineages [31], only the differentiation into osteogenesis was examined in this study. MSCs cultured in hypoxia maintained the ability to differentiate, and in the case of differentiation into osteoblasts, the efficiency of differentiation was rather increased. This result was consistent with previous reports [22, 32]. These results, of course, may vary with oxygen tensions, exposure time, initial cell number and cell source.
In conclusion, we have shown that the proliferation, stemness, and osteogenic differentiation potential of MSCs from human MSCs were enhanced in 5% hypoxic condition. Hypoxic (5%) culture may serve as a useful strategy to maintain the function of human MSCs.
Acknowledgements
This work was supported by Biomedical Research Institute Grant, Kyungpook National University Hospital (2014).
Conflict of interests
The authors declare that they have no Conflict of interest.
Ethical statement
This study was approved by the Ethics Committee of the Kyungpook National University Hospital (IRB No. KNUH 2012-10-018).
Contributor Information
Bum Soo Kim, Email: urokbs@knu.ac.kr.
Tae Gyun Kwon, Email: tgkwon@knu.ac.kr.
References
- 1.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 3.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 4.Kaviani A, Guleserian K, Perry TE, Jennings RW, Ziegler MM, Fauza DO. Fetal tissue engineering from amniotic fluid. J Am Coll Surg. 2003;196:592–597. doi: 10.1016/S1072-7515(02)01834-3. [DOI] [PubMed] [Google Scholar]
- 5.Chun SY, Kim HT, Lee JS, Kim MJ, Kim BS, Kim BW, et al. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology. 2012;79(1186):e1181–e1187. doi: 10.1016/j.urology.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Thakker R, Yang P. Mesenchymal stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16:323. doi: 10.1007/s11936-014-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadon-Nachum M, Melamed E, Offen D. Stem cells treatment for sciatic nerve injury. Expert Opin Biol Ther. 2011;11:1591–1597. doi: 10.1517/14712598.2011.628933. [DOI] [PubMed] [Google Scholar]
- 8.Fan L, Liu R, Li J, Shi Z, Dang X, Wang K. Low oxygen tension enhances osteogenic potential of bone marrow-derived mesenchymal stem cells with osteonecrosis-related functional impairment. Stem Cells Int. 2015;2015:950312. doi: 10.1155/2015/950312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M, Akhtar S, Mohsin S, Khan N, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 2011;20:67–75. doi: 10.1089/scd.2009.0397. [DOI] [PubMed] [Google Scholar]
- 10.De Barros S, Dehez S, Arnaud E, Barreau C, Cazavet A, Perez G, et al. Aging-related decrease of human ASC angiogenic potential is reversed by hypoxia preconditioning through ROS production. Mol Ther. 2013;21:399–408. doi: 10.1038/mt.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 12.Hung SP, Ho JH, Shih YR, Lo T, Lee OK. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res. 2012;30:260–266. doi: 10.1002/jor.21517. [DOI] [PubMed] [Google Scholar]
- 13.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- 14.Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 15.Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267:423–425. doi: 10.1038/267423a0. [DOI] [PubMed] [Google Scholar]
- 16.Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–C1146. doi: 10.1152/ajpcell.00415.2005. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 19.Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 20.Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kofoed H, Sjontoft E, Siemssen SO, Olesen HP. Bone marrow circulation after osteotomy. Blood flow, pO2, pCO2, and pressure studied in dogs. Acta Orthop Scand. 1985;56:400–403. doi: 10.3109/17453678508994357. [DOI] [PubMed] [Google Scholar]
- 22.D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 23.Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–528. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Vaidya M. Hypoxia inhibits mesenchymal stem cell proliferation through HIF1alpha-dependent regulation of P27. Mol Cell Biochem. 2016;415:29–38. doi: 10.1007/s11010-016-2674-5. [DOI] [PubMed] [Google Scholar]
- 26.Palomaki S, Pietila M, Laitinen S, Pesala J, Sormunen R, Lehenkari P, et al. HIF-1alpha is upregulated in human mesenchymal stem cells. Stem Cells. 2013;31:1902–1909. doi: 10.1002/stem.1435. [DOI] [PubMed] [Google Scholar]
- 27.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 28.Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, et al. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946–956. doi: 10.1093/jnci/djh168. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto Y, Fujita M, Tanaka Y, Kojima I, Kanatani Y, Ishihara M, et al. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. Biores Open Access. 2013;2:199–205. doi: 10.1089/biores.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai CC, Su PF, Huang YF, Yew TL, Hung SC. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169–182. doi: 10.1016/j.molcel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 32.Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One. 2011;6:e23965. doi: 10.1371/journal.pone.0023965. [DOI] [PMC free article] [PubMed] [Google Scholar]