Abstract
Artificial uterus using endometrium implant can be a novel treatment strategy for infertile women with refractory endometrial dysfunction. At early pregnancy, the function of uterine endometrial cells for the communication between the conceptus of pre-implantation period and maternal reproductive system is essential. MicroRNA (miR) expression profile of endometrial cells according to progesterone, a crucial pregnancy-maintaining hormone, provides important data for in vitro endometrial cell culture strategy that is useful for engineering artificial uteri using endometrial implants. The present study aimed to evaluate the miR expression profile of in vitro cultured endometrial cells under hormonal milieu mimicking early pregnancy period in terms of progesterone concentration. We cultured murine uterine endometrial cells, human uterine endometrial carcinoma cells, and immortalized human uterine endometrial cells using different progesterone concentrations, and analyzed the expression of miRs critical for early pregnancy. The expression of miR-20a, -21, -196a, -199a, and -200a was differently regulated according to progesterone concentration in different endometrial cell lines. The analysis of candidate target genes showed that the expression of phosphatase and tensin homolog, mucin 1 (MUC1), progesterone receptor, transforming growth factor β receptor II, matrix metallopeptidase-9 was up-regulated by progesterone treatment in mouse and human endometrial cell lines. These results indicate that physiological concentration range (10−7 and 10−9 M) of progesterone affect the survival and target gene expression via modulating miR expression. Taken together, progesterone can be a crucial factor in regulating miR expression on in vitro cultured endometrial cells.
Keywords: microRNA, Uterine endometrial cell, Progesterone, Infertile women
Introduction
At early pregnancy, the function of uterine endometrial cells for the reciprocal communication between the conceptus of pre-implantation period and maternal reproductive system is essential [1, 2]. The dialogue via endometrium between the two ‘actors’ is a morphological [3–5], immunological [6, 7], and also hormonal process [8–12]. Progesterone is an essential hormone for embryo implantation and pregnancy maintenance by the modulation of maternal immune response [13], reduction of uterine contractility [14], and improvement of utero-placental circulation [15].
MicroRNAs (miRs), endogenously produced non-coding RNA molecules inhibiting complementary target mRNAs, have been known as regulating factors for oocyte maturation and embryogenesis [16–18]. In reproductive system, the expression of miR has been known to be regulated by hormones [19, 20]. Some studies suggested a regulatory role of miRs in endometrial cells regarding endometrial receptivity during early pregnancy period [21, 22]. However, the hormonal regulation of miR expression in endometrial cells has been not fully understood yet.
As an application of tissue engineering in gynecologic field, the production of artificial uterine models using endometrium implant is a progressive strategy for improving implantation rate in patients with refractory endometrial defects [23–28]. Among many hurdles, the establishment of optimal endometrial cell culture conditions is a basic step. Furthermore, the profiles of miR expression in endometrial cells under hormonal status of early pregnancy period may be a main requirement to assure the efficacy of this model.
In this study, we aimed to evaluate the profile of miR expression in endometrial cells during in vitro culture under early pregnancy-mimicking hormonal milieu regarding progesterone concentration. Three types of human and murine endometrial cell lines were used to evaluate the miR profiles.
Materials and methods
Ethics
Entire experimental procedures were approved by Institutional Animal Care Unit Committee (IACUC) of Seoul National University Hospital (No. 16-0005-C1A0(1)). Six-week-old C57BL/6 female mice were maintained in the animal facility and five mice per cage were housed in a room with a 12-h light/dark cycle and unlimited access to food and water.
Mouse endometrial cell preparation and culture
Uterine horn of 6-week-old female mice (C57BL/6) was excised using scissors after cervical dislocation. The excised horn was washed with DPBS (Invitrogen, Waltham, MA, USA) and chopped as 1 × 1 cm2 using surgical blade. The chopped uterine tissue pieces were collected and centrifuged. The pellets were digested with 5 mg/mL of collagenase type I (Invitrogen) for 1 h at 37 °C. And then, they were filtered through 70-µm cell strainer and the digested cells were blocked and centrifuged. The cells were re-suspended in culture medium and plated for incubation. After an initial culture of 30 min, the medium was changed to remove residual epithelial cells and further culture occurred in a fresh medium. The medium was consisted of DMEM/F12 phenol red-free (Invitrogen), supplemented with 10% FBS (Invitrogen), 1× Insulin-Transferrin-Selenium-G supplement (ITS, Invitrogen) and 50 IU/mL penicillin–streptomycin (Invitrogen).
Culture of human endometrial carcinoma cells
Human endometrial carcinoma cell line, SNU-1077, was purchased from Korean Cell Line Bank. Cells were maintained as density of 3 × 105 cells in 100 mm tissue culture dish and medium was exchanged every two other days. The medium was consisted of RPMI 1640 (Invitrogen), supplemented with 10% FBS, 1× ITS and 50 IU/mL penicillin–streptomycin.
Culture of human immortalized endometrial cells
Immortalized human endometrium cell line, CRL-4003, was purchased from ATCC (Manassas, VA, USA). Cells were maintained as density of 3 × 105 cells in 100 mm tissue culture dish and medium was changed every 2 days. The medium was consisted of DMEM/F12 phenol red-free, supplemented with 10% FBS, 1× ITS and 50 IU/mL P/S.
Progesterone treatment
Progesterone (P, Sigma-Aldrich, St. Louis, MO, USA) was purchased as powder and dissolved in absolute ethanol. In vitro grown cells of each cell line were split using 0.25% trypsin–EDTA (Invitrogen) and re-plated as 1 × 104 density. After 24 h, 10−5, 10−7, and 10−9 M of P was treated for 48 h in 5% CO2 environment.
Annexin V staining
Grown cells were dissociated into single cells using 0.25% trypsin–EDTA. And then, cells were washed with 1 × PBS (Invitrogen) by centrifugation at 1500 rpm for 5 min. FITC-conjugated Annexin V antibody (BD Biosciences, San Diego, CA, USA) was applied for 15 min at room temperature. The samples were analyzed using FACS Calibur™ (BD Biosciences).
Quantitative real-time polymerase chain reaction (qRT-PCR) for miRs
To evaluate the expression level of microRNA, total RNAs were extracted from the samples using Trizol reagent (Invitrogen). cDNAs were synthesized from 50 ng total RNAs using NCode™ VILO™ miRNA cDNA Synthesis Kit (Invitrogen). And then, NCode™ EXPRESS SYBR® GreenER™ miRNA qRT-PCR premix (Invitrogen) were added to the cDNAs and amplified under the following conditions: incubation for 2 min at 50 °C and following 2 min at 95 °C, and followed by 40 cycles of 15 s at 95 °C, and 60 s at 58 °C. All the reactions were performed in triplicate and the Ct value was calculated based on the U6 expression. The primers used for the reaction are listed as Table 1.
Table 1.
The sequence and accession number of used microRNAs
| miR | Species | Mature sequence | RT-PCR primer sequence(s) | miR base # |
|---|---|---|---|---|
| miR-20a | Human | UAAAGUGCUUAUAGUGCAGGUAG | TAAAGTGCTTATAGTGCAGGTAG | MIMAT0000075 |
| Mouse | UAAAGUGCUUAUAGUGCAGGUAG | MIMAT0000529 | ||
| miR-21 | Human | UAGCUUAUCAGACUGAUGUUGA | TAGCTTATCAGACTGATGTTGA | MIMAT0000076 |
| Mouse | UAGCUUAUCAGACUGAUGUUGA | MIMAT0000530 | ||
| miR-199a | Human | CCCAGUGUUCAGACUACCUGUUC | CCCAGTGTTCAGACTACCTGTTC | MIMAT0000231 |
| Mouse | CCCAGUGUUCAGACUACCUGUUC | MIMAT0000229 | ||
| miR-196a | Human | UAGGUAGUUUCAUGUUGUUGGG | TAGGTAGTTTCATGTTGTTGGG | MIMAT0000226 |
| Mouse | UAGGUAGUUUCAUGUUGUUGGG | MIMAT0000518 | ||
| miR-200a | Human | UAACACUGUCUGGUAACGAUGU | TAACACTGTCTGGTAACGATGT | MIMAT0001620 |
| Mouse | UAACACUGUCUGGUAACGAUGU | MIMAT0004619 |
qRT-PCR for specific genes
The primers used for the reaction are listed as Table 2. The samples were collected and total RNAs were isolated using TRIzol® reagent (Invitrogen). For the evaluation of target genes, 50 ng of the RNAs were reverse-transcribed for the synthesis of cDNA using an Accute RT PreMix (Bioneer, Daejeon, Korea). The amplification of specific genes was performed in RotorGene Q (Qiagen, Valencia, CA, USA) using QuantiTect SYBR green PCR kit (Qiagen). PCR reaction was performed as following condition for 35 cycles: denaturation at 95 °C and annealed at 60 °C. All the reactions were performed in triplicate and the Ct value was calculated based on the GAPDH expression.
Table 2.
The primer sequence of candidate target genes
| Target gene | Species | Forward | Reverse |
|---|---|---|---|
| GAPDH | Human | GAAGGTCGGTGTGAACGAAT | TTTGATGTTAGCGGGGTCTC |
| Mouse | TGTGTCCGTCGTGGATCTGA | GCATCGAAGGTGGAAGAGTGG | |
| TGFBRII | Human | ACGTGTTGAGAGATCGAGG | CCCAGCACTCAGTCAACGTC |
| Mouse | AGCATCACGGCCATCTGTG | TGGCAAACCGTCTCCAGAGT | |
| MMP-9 | Human | CCCGGAGTGAGTTGAACCA | GGATTTACATGGCACTGCC |
| Mouse | TCGCGTGGATAAGGAGTTCTC | AACTCACACGCCAGAAGAATTTG | |
| PTEN | Human | CAAGATGATGTTTGAAACTATTCCAATG | CCTTTAGCTGGCAGACCACAA |
| Mouse | CCCACCACAGCTAGAACTTATCAA | TCCGTCCCTTTCCAGCTTTAC | |
| MUC1 | Human | AGAGAAGTTCAGTGCCCAGC | TGACATCCTGTCCCTGAGTG |
| Mouse | GTCTTCAGGAGCTCTGGTGG | TACCACTCCAGTCCACAGCA | |
| PR | Human | GCCTATACCGATCTCCCTG | TTCCCTATGAGTGGCTTCTAC |
| Mouse | GGTCCCCCTTGCTTGCA | CAGGACCGAGGAAAAAGCAG |
Immunofluorescence staining
The samples of each condition were fixed using 4% paraformaldehyde (PFA, Sigma-Aldrich) for 20 min at room temperature (RT). After washing with PBS, samples were treated with 3% BSA (Bovine serum albumin, Sigma-Aldrich) solution for prevention of non-specific reaction for 12 h at 4 °C. And then, primary antibody, mouse anti-Caspase 3 (Molecular Probes, Eugene, OR, USA) was treated as 1:100 concentration. After washing three times with PBST [PBS containing tween 20 (Sigma-Aldrich)], secondary antibody, goat anti-mouse IgG Alexa Fluor 594 was added to samples and incubated for 1 h at RT. After washing three times with PBST, samples were mounted with anti-fade solution containing DAPI (Invitrogen) and images were captured using EVOS FL microscope.
Statistical analysis
The entire experiments were repeated at least three times. All data were expressed as means and standard deviations and compared using the Student’s t test. The differences were considered statistically significant when they were p < .05. Data were analyzed using the Statistical Package for the Social Sciences for Windows (version 12.0, SPSS Inc., Chicago, IL).
Results
Proliferation of three endometrial cell types
The characteristics of three types of endometrial cell lines used in this study are summarized in Table 3. Figure 1 shows the proliferation of mouse endometrial cells, human endometrial carcinoma and immortalized human endometrial cells in each culture condition for 48 h. Among the three groups, the proportion of apoptotic cells was significantly low in immortalized human endometrial cell group. Independent of progesterone concentration, mouse and immortalized human endometrial cells showed homogeneous proliferation with co-existence of fibroblast and epithelial cells.
Table 3.
The characteristics of three types of endometrial cell lines used in this study
| Cell types | Species | Origin | Cellular morphology | Histopathology |
|---|---|---|---|---|
| Endometrial cell | Mouse | Uterus, Endometrium | Fibroblast-like | Normal |
| Endometrial carcinoma | Human | Uterus, Endometrium | Polypoid | Malignant mixed Müllerian tumor (MMMT), primary |
| Immortalized endometrial cell | Human | Uterus, Endometrium, immortalized with human telomerase reverse transcriptase (hTERT) | Fibroblast-like | Non-malignant myoma |
Fig. 1.
Morphological observation of three different endometrial cell tines. The cells were cultured for 48 h in the presence of progesterone. Cellular growth was observed under phase-contrast microscope
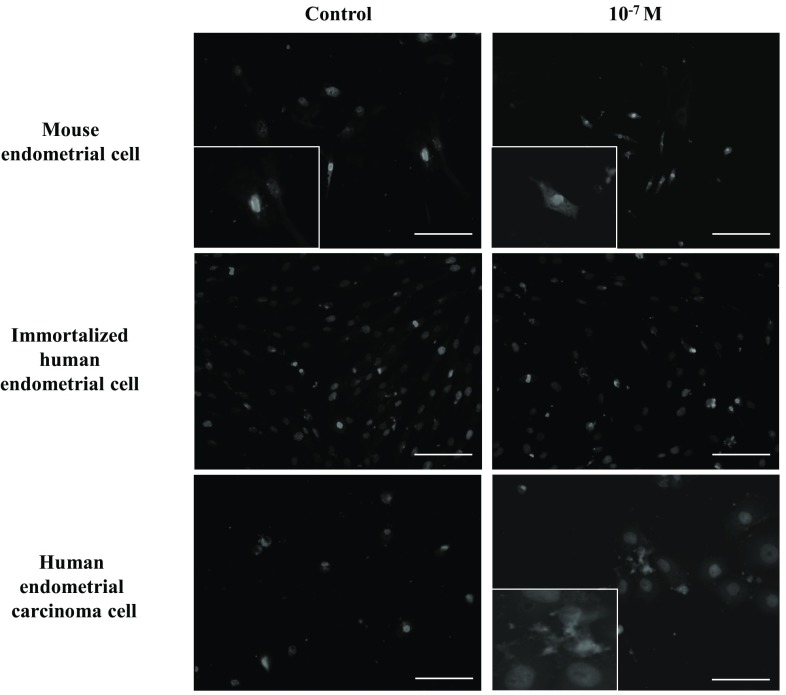
Localization of apoptosis-specific caspase-3 was observed in three cell lines. Treatment of progesterone showed an increase of apoptosis in mouse endometrial cells and human endometrial carcinoma cells, while this expression was minimal in immortalized human endometrial cell line group (Fig. 2).
Fig. 2.
Localization of caspase-3 in three cell lines. Immunostaining results indicate the effects of progesterone on the proliferation of non-malignant and malignant endometrial cells (magnification, ×200). The inset images are taken under lager magnifications (×350)
Figure 3 shows the population of apoptotic cells confirmed by annexin V assay. The population of apoptotic cells increased in a dose-dependent manner according to progesterone concentration (p < .05 in all). Mouse and immortalized human endometrial cells showed the least population of apoptotic cells in control. Progesterone at 10−7 M group showed less population of apoptotic cells than those of progesterone at 10−9 and 10−5 M groups (p < .05).
Fig. 3.
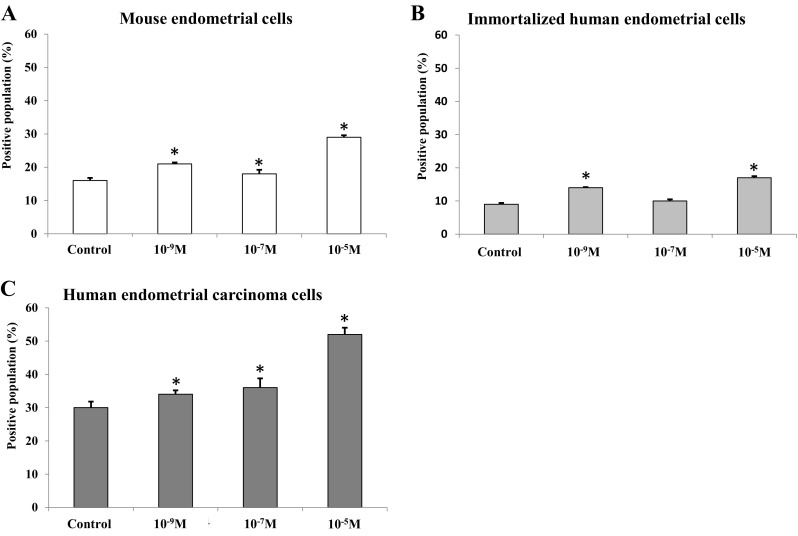
Population of apoptotic cells after progesterone treatment (*p < .05 vs. control). The population of apoptotic cells after progesterone treatment was analyzed using Annexin V staining. The positively stained proportion indicate the cells that are in the process of apoptosis. A Mouse endometrial cells, B Immortalized human endometrial cells and C Human endometrial carcinoma cells
miR expression in three endometrial cell types
Figure 4 shows the miR expression in mouse endometrial cells according to progesterone concentration. The expression of miR-20a, -196a, and -199a was found to be up-regulated with an increase in progesterone concentration compared to control (p < .05). The expression of miR-21 was down-regulated with an increase in progesterone concentration to 10−7 M (p < .05), and was up-regulated in progesterone at 10−5 M group (p < .05). These results suggest that the expression of miR-21 could be regulated within a specific window of progesterone concentration in mouse endometrial cells. The expression of miR-200a was similar among the three progesterone concentration groups. Our results showed that progesterone did not affect the expression of miR-200a in in vitro cultured mouse endometrial cells.
Fig. 4.

Expression of miRs in mouse endometrial cells after progesterone treatment (*p < .05 vs. control). The expression of endometrial cell-related miRs was analyzed in mouse endometrial cells. Progesterone treatment up-regulated the miR expression
The profile of miR expression in immortalized human endometrial cells according to progesterone concentration is shown in Fig. 5. The expression of miR-20a was similar among the three progesterone concentration groups. The expression of miR-21 was up-regulated in progesterone at 10−9 M group (p < .05), and was down-regulated in progesterone at 10−7 and 10−5 M groups (p < .05), compared to control. These results suggested that miR-21 expression was regulated within a specific window of progesterone concentration in immortalized human endometrial cells, but in a different way from that shown in mouse endometrial cells. The expression of miR-200a, -196a, -199a was down-regulated in all progesterone concentration groups compared to control (p < .05).
Fig. 5.
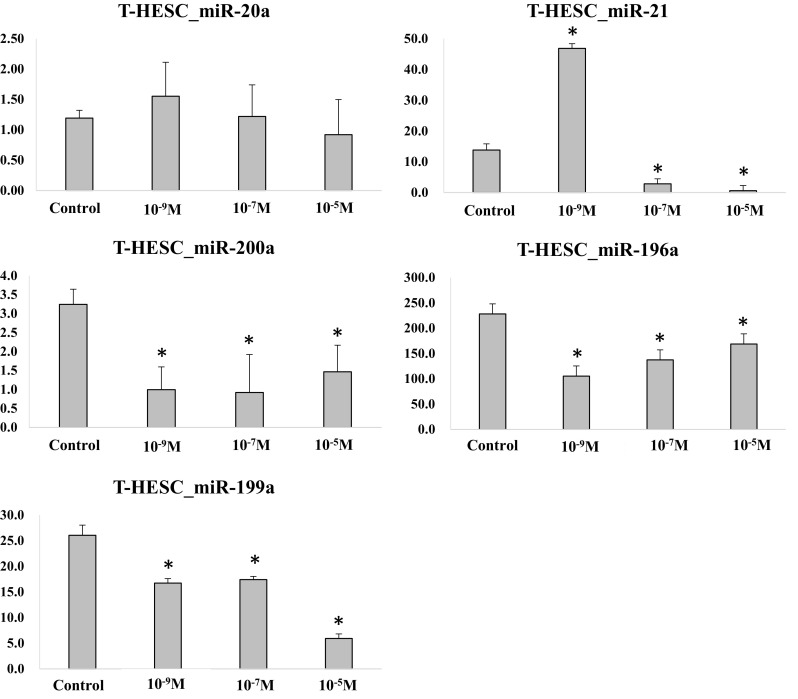
Expression of miRs in immortalized human endometrial cells after progesterone treatment (*p < .05 vs. control). The expression of endometrial cell-related miRs was analyzed in immortalized human endometrial cells. The progesterone treatment significantly affected the expression of miRs
In the human endometrial carcinoma cells, the expression of all analyzed miRs was down-regulated in all progesterone-treated groups compared to control (p < .05) (Fig. 6). Our results suggested that the expression of miRs showed diverse patterns according to progesterone concentration and endometrial cell types.
Fig. 6.
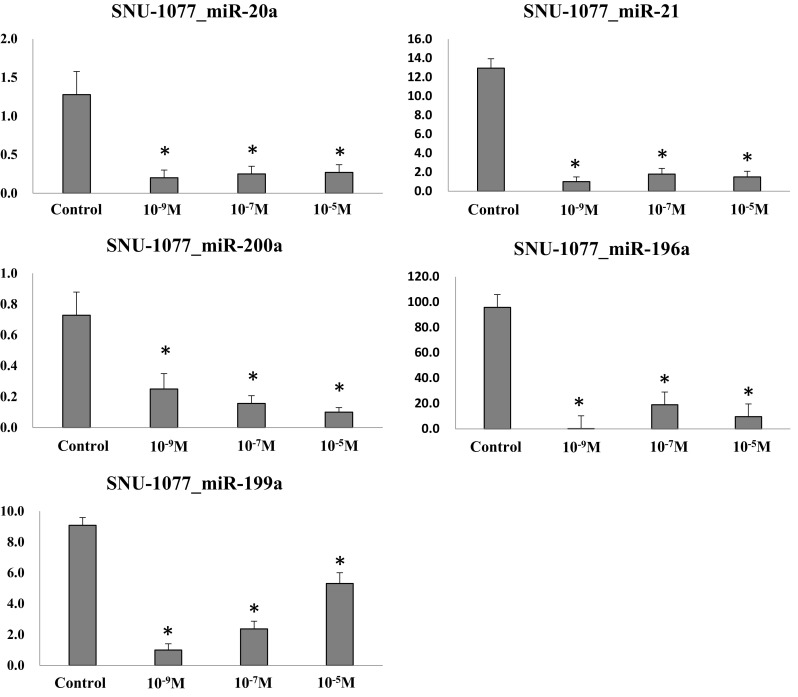
Expression of miRs in human endometrial carcinoma cells after progesterone treatment (*p < .05 vs. control). The expression of endometrial cell-related miRs was analyzed in human endometrial carcinoma cells. The progesterone treatment down-regulated the expression of all the miRs evaluated
Candidate target gene expression in three endometrial cell types
Figure 7 shows the candidate target gene expression in mouse endometrial cells according to progesterone concentration. The expression of phosphatase and tensin homolog (PTEN) and progesterone receptor (PR) was similar between progesterone at 10−7 M group and control group, and was up-regulated in progesterone at 10−9 M and 10−5 M groups compared to control (p < .05). The expression of mucin 1 (MUC1) was up-regulated with an increase in progesterone concentration to 10−9 and 10−7 M (p < .05), and was down-regulated in progesterone at 10−5 M group (p < .05). The expression of TGF-β receptor II (TGFB-R II) was up-regulated in progesterone at 10−9 and 10−7 M groups when compared to control (p < .05), and was similar between progesterone at 10−5 M and control groups. The expression of matrix metallopeptidase-9 (MMP-9) was up-regulated in progesterone at 10−9 M group compared to control (p < .05), and was similar between progesterone at 10−7 and 10−5 M groups and control group.
Fig. 7.
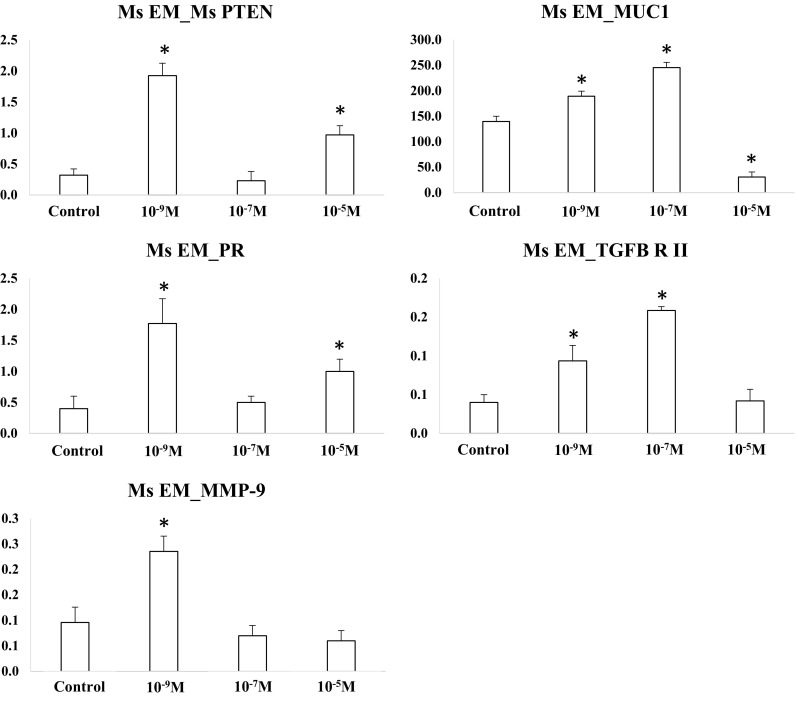
Target gene expression in mouse endometrial cells (*p < .05 vs. control). The expression of specific genes was evaluated by qRT-PCR. Variable expression patterns were observed among different genes
In the immortalized human endometrial cells, the expression of PTEN, MUC1, PR, and MMP-9 were up-regulated in all progesterone concentration groups compared to control (p < .05) (Fig. 8). The expression of TGFB-R II was similar between progesterone at 10−9 and 10−7 M groups and control, and was up-regulated in progesterone at 10−5 M group compared to control (p < .05).
Fig. 8.
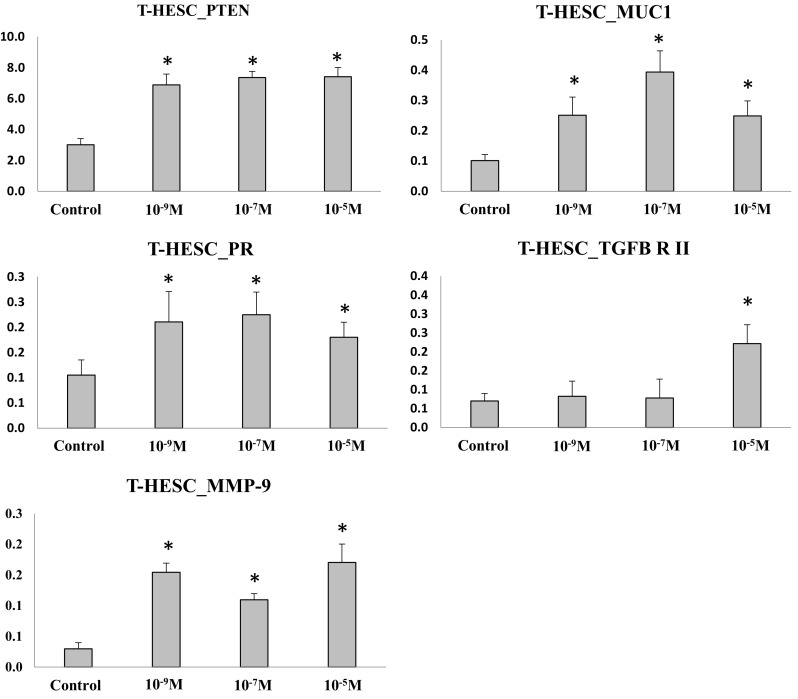
Target gene expression in immortalized human endometrial cells (*p < .05 vs. control). The expression of specific genes was evaluated by qRT-PCR. The expression pattern differed significantly between the genes
The profile of the candidate target gene expression in human endometrial carcinoma cells according to progesterone concentration is shown in Fig. 9. The expression of PTEN and PR was down-regulated in all concentration groups compared to control (p < .05). The expression of MUC1 and TGFB-R II was up-regulated in progesterone at 10−9 M group compared to control (p < .05), and was similar between progesterone at 10−7 and 10−5 M groups and control group. The expression of MMP-9 was down-regulated in progesterone at 10−5 M group compared to control (p < .05).
Fig. 9.
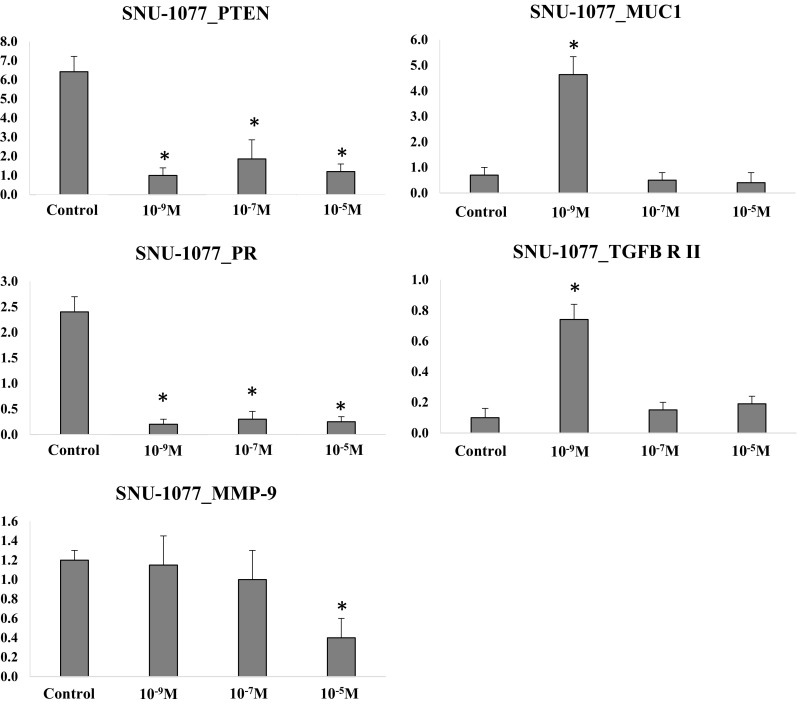
Target gene expression in human endometrial carcinoma cells (*p < .05 vs. control). Dose-dependent effects of progesterone treatment were observed in this cell line
Discussion
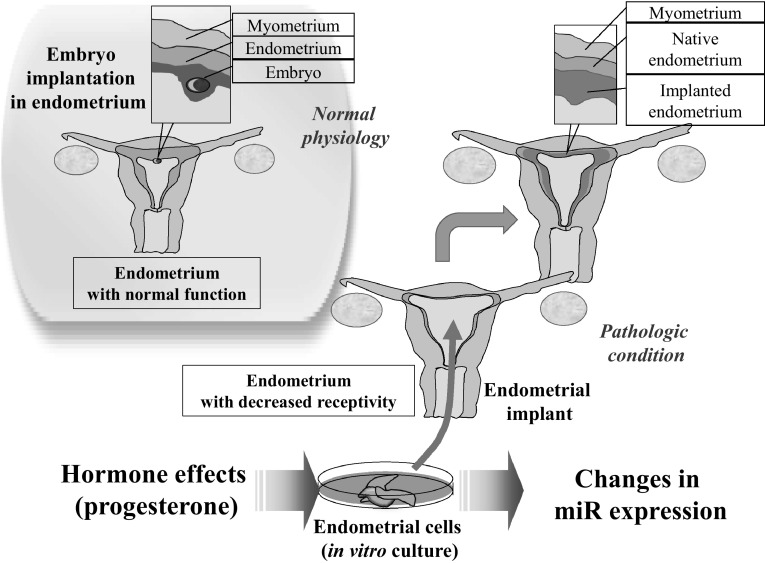
As a novel treatment strategy for women with infertility of refractory endometrial dysfunction, the establishment of optimal in vitro endometrial cell culture condition is an important step to engineer artificial uteri using endometrial cell implant. Our study suggests that miRs in endometrial cells may have a pivot role in the communication between endometrial cells and embryonic cells, and thus they should be regulated for optimal efficacy (Fig. 10).
Fig. 10.
Diagram showing the implication of miR expression pattern regulated by progesterone. Functional uterine endometrium has normal receptivity to embryos. Delivery of in vitro cultured endometrial cells may improve endometrial receptivity when transferred into a uterus with decreased receptivity. This study reveals the changes in miR expression according to hormone concentration
Our experimental model mimics micro-environment of endometrial cells implanted in uterine cavity during early pregnancy period. To fulfill the ideal hormone status, we supplied progesterone to our endometrial cell implant model. Progesterone is a main hormone of this period, with modulation of maternal immune response [13], reduction of uterine contractility [14], and improvement of utero-placental circulation [15], in accordance with human chorionic gonadotropin (hCG) secreted by syncytiotrophoblasts and placenta(e) of the implanted embryo(s) [29, 30]. Progesterone is mainly produced by ovarian corpus luteum, extra-embryonic endometrial cluster at early pregnancy [31], until placental progesterone production is commenced (e.g. 6–8 weeks after embryonic implantation in human). Therefore, progesterone level is an important factor for implantation and maintenance of embryos in artificial endometrial cell implant models.
Our results showed that the expression of miR-20a, -21, -196a, -199a, and -200a was differently regulated according to progesterone concentration in different endometrial cell lines. Regarding the analysis of candidate target genes, the expression of PTEN, MUC1, PR, TGFB-R II, MMP-9 was up-regulated by progesterone treatment in mouse and immortalized human endometrial cells. Higher progesterone concentration during culture induced divergent expression of miRs. Mir-20a and -21 were known as the regulatory modulators of endometrial decidualization and implantation via regulation of candidate target genes including TGFB-R II, RECK, and BMF [32–34]. The expression of these candidate genes was reported to be down-regulated during the period of embryonic implantation [35]. The expression of miR-196a is known to be down-regulated in ectopic endometrial cells, and up-regulated in eutopic endometrial cells to mediate the expression of progesterone receptor via regulating the MEK/ERK signal in women with endometriosis, a disease caused by endometrial-like cell growth outside the uterus [36, 37]. The expression of miR-200a, a regulating factor of cell proliferation and apoptosis via PTEN pathway [38], is reported to be down-regulated in endometrial cells during embryonic implantation period [39]. Our study showed the expression of these miRs was diverse according to progesterone concentration and cell lines. Our results imply that the regulation of miRs may be an important requirement for inducing optimal function of transferred in vitro cultured endometrial cells to improve the receptivity of endometrium. Furthermore, these results indicate that investigating optimal hormonal level for embryonic implantation is important for the maintenance of endometrial cell implant.
Our study showed that optimal progesterone concentration may regulate endometrial receptivity to embryo(s), via miR modulation. These results suggest that progesterone supplementation over the threshold level could modify the expression profiles of miRs affecting the integration of in vitro cultured endometrial cells. A previous clinical study suggests that the optimal progesterone level should be over 25.2 ng/mL [9]. This suggested hormonal environment may be an important requirement in maintaining implantation of transferred embryo(s) via utilizing in vitro cultured endometrial cell implant. Many previous studies revealed that progesterone at 10−7 M be the optimal concentration for in vitro endometrial cell culture [41, 42]. Our results imply that this optimal condition may induce far various miR expression profiles than the lower or higher concentrations of progesterone.
There are other factors to consider such as applicability in cancer patients and the variables related to maintenance of pregnancy. A case report showed a successful live birth with in vitro fertilization and thawed embryo transfer after conservative treatment of recurrent endometrial cancer consistent with endometrial carcinoma in this study [40]. Maintenance of pregnancy may depend on multi-factorial causes including maternal [43], paternal [44], and fetal factors [45]. Endometrial cell could be a crucial platform of crosstalk between these factors. For developing an endometrial implant to improve uterine receptivity, the response of in vitro cultured endometrial cells should have an important role.
Taken together, progesterone concentration may play a crucial role for the maintenance of embryo implantation, by regulating miR expression, in in vitro culture model of artificial endometrial implant. Further studies are necessary to sophisticate the fine conditions for manufacturing artificial uteri using endometrial implant.
Acknowledgements
This study was supported by grants of Ministry of Science, ICT and Future Planning (2016R1E1A1A01943455, 2016R1D1A1A02937287).
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical statements
Entire experimental procedures were approved by the institutional animal care unit committee (IACUC) of Seoul National University Hospital (No. 16-0005-C1A0(1)).
References
- 1.Klein C, Scoggin KE, Ealy AD, Troedsson MH. Transcriptional profiling of equine endometrium during the time of maternal recognition of pregnancy. Biol Reprod. 2010;83:102–113. doi: 10.1095/biolreprod.109.081612. [DOI] [PubMed] [Google Scholar]
- 2.Groebner AE, Rubio-Aliaga I, Schulke K, Reichenbach HD, Daniel H, Wolf E, et al. Increase of essential amino acids in the bovine uterine lumen during preimplantation development. Reproduction. 2011;141:685–695. doi: 10.1530/REP-10-0533. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Berga SL, Johnston-MacAnanny EB, Sidell N, Bagchi IC, Bagchi MK, et al. Endometrial stromal decidualization responds reversibly to hormone stimulation and withdrawal. Endocrinology. 2016;157:2432–2446. doi: 10.1210/en.2015-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajihara T, Tanaka K, Oguro T, Tochigi H, Prechapanich J, Uchino S, et al. Androgens modulate the morphological characteristics of human endometrial stromal cells decidualized in vitro. Reprod Sci. 2014;21:372–380. doi: 10.1177/1933719113497280. [DOI] [PubMed] [Google Scholar]
- 5.Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. A comparative study on the outcomes of in vitro fertilization between women with polycystic ovary syndrome and those with sonographic polycystic ovary-only in GnRH antagonist cycles. Arch Gynecol Obstet. 2010;282:199–205. doi: 10.1007/s00404-010-1401-9. [DOI] [PubMed] [Google Scholar]
- 6.Gnecco JS, Pensabene V, Li DJ, Ding T, Hui EE, Bruner-Tran KL, et al. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann Biomed Eng. 2017;45:1758–1769. doi: 10.1007/s10439-017-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima PD, Zhang J, Dunk C, Lye SJ, Croy BA. Leukocyte driven-decidual angiogenesis in early pregnancy. Cell Mol Immunol. 2014;11:522–537. doi: 10.1038/cmi.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piltonen TT, Chen JC, Khatun M, Kangasniemi M, Liakka A, Spitzer T, et al. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum Reprod. 2015;30:1203–1215. doi: 10.1093/humrep/dev055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YJ, Shin JH, Hur JY, Kim H, Ku SY, Suh CS. Predictive value of serum progesterone level on beta-hCG check day in women with previous repeated miscarriages after in vitro fertilization. PLoS One. 2017;12:e0181229. doi: 10.1371/journal.pone.0181229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi YS, Ku SY, Jee BC, Suh CS, Choi YM, Kim JG, et al. Comparison of follicular fluid IGF-I, IGF-II, IGFBP-3, IGFBP-4 and PAPP-A concentrations and their ratios between GnRH agonist and GnRH antagonist protocols for controlled ovarian stimulation in IVF-embryo transfer patients. Hum Reprod. 2006;21:2015–2021. doi: 10.1093/humrep/del091. [DOI] [PubMed] [Google Scholar]
- 11.Ku SY, Choi YM, Suh CS, Kim SH, Kim JG, Moon SY, et al. Effect of gonadotropins on human endometrial stromal cell proliferation in vitro. Arch Gynecol Obstet. 2002;266:223–228. doi: 10.1007/s00404-002-0292-9. [DOI] [PubMed] [Google Scholar]
- 12.Ku SY, Suh CS, Kim SH, Choi YM, Kim JG, Moon SY. A pilot study of the use of low dose human menopausal gonadotropin in ovulation induction. Eur J Obstet Gynecol Reprod Biol. 2003;109:55–59. doi: 10.1016/S0301-2115(02)00476-1. [DOI] [PubMed] [Google Scholar]
- 13.Canellada A, Alvarez I, Berod L, Gentile T. Estrogen and progesterone regulate the IL-6 signal transduction pathway in antibody secreting cells. J Steroid Biochem Mol Biol. 2008;111:255–261. doi: 10.1016/j.jsbmb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Patil AS, Swamy GK, Murtha AP, Heine RP, Zheng X, Grotegut CA. Progesterone metabolites produced by cytochrome P450 3A modulate uterine contractility in a murine model. Reprod Sci. 2015;22:1577–1586. doi: 10.1177/1933719115589414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang Y, Li W, Tran V, Khalil RA. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem Pharmacol. 2013;86:734–747. doi: 10.1016/j.bcp.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YJ, Ku SY, Kim YY, Liu HC, Chi SW, Kim SH, et al. MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum Reprod. 2013;28:3050–3061. doi: 10.1093/humrep/det338. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Li X, Cao L, Huang S, Li H, Zhang Y, et al. MicroRNA-148a overexpression improves the early development of porcine somatic cell nuclear transfer embryos. PLoS One. 2017;12:e0180535. doi: 10.1371/journal.pone.0180535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YJ, Ku SY, Kim YY, Suh CS, Kim SH, Choi YM. MicroRNA profile of granulosa cells after ovarian stimulation differs according to maturity of retrieved oocytes. Geburtshilfe Frauenheilkd. 2016;76:704–708. doi: 10.1055/s-0041-111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang X, Zhang X, Lu Y, Li L, Cui S. MiRNA-143 mediates the proliferative signaling pathway of FSH and regulates estradiol production. J Endocrinol. 2017;234:1–14. doi: 10.1530/JOE-16-0488. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Ku SY, Rosenwaks Z, Liu HC, Chi SW, Kang JS, et al. MicroRNA expression profiles are altered by gonadotropins and vitamin C status during in vitro follicular growth. Reprod Sci. 2010;17:1081–1089. doi: 10.1177/1933719110377663. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Xie Y, Wu M, Geng Y, Li R, Xu L, et al. Expression of mmu-miR-96 in the endometrium during early pregnancy and its regulatory effects on stromal cell apoptosis via Bcl2. Mol Med Rep. 2017;15:1547–1554. doi: 10.3892/mmr.2017.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schjenken JE, Zhang B, Chan HY, Sharkey DJ, Fullston T, Robertson SA. miRNA regulation of immune tolerance in early pregnancy. Am J Reprod Immunol. 2016;75:272–280. doi: 10.1111/aji.12490. [DOI] [PubMed] [Google Scholar]
- 23.Takagi S, Shimizu T, Kuramoto G, Ishitani K, Matsui H, Yamato M, et al. Reconstruction of functional endometrium-like tissue in vitro and in vivo using cell sheet engineering. Biochem Biophys Res Commun. 2014;446:335–340. doi: 10.1016/j.bbrc.2014.02.107. [DOI] [PubMed] [Google Scholar]
- 24.Kuramoto G, Takagi S, Ishitani K, Shimizu T, Okano T, Matsui H. Preventive effect of oral mucosal epithelial cell sheets on intrauterine adhesions. Hum Reprod. 2015;30(2):406–416. doi: 10.1093/humrep/deu326. [DOI] [PubMed] [Google Scholar]
- 25.Yun JW, Kim YY, Ahn JH, Kang BC, Ku SY. Use of nonhuman primates for the development of bioengineered female reproductive organs. Tissue Eng Regen Med. 2016;13:323–334. doi: 10.1007/s13770-016-9091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YJ, Kim YY, Kang BC, Kim MS, Ko IK, Liu HC, et al. Induction of multiple ovulation via modulation of angiotensin II receptors in in vitro ovarian follicle culture models. J Tissue Eng Regen Med. 2016. doi:10.1002/term.2214. [DOI] [PubMed]
- 27.Kim YJ, Park KE, Kim YY, Kim H, Ku SY, Suh CS, et al. Effects of estradiol on the paracrine regulator expression of in vitro maturated murine ovarian follicles. Tissue Eng Regen Med. 2017;14:31–38. doi: 10.1007/s13770-016-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamadon A, Park KH, Kim YY, Kang BC, Ku SY. Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng Regen Med. 2016;13:447–454. doi: 10.1007/s13770-016-9107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M, Lei ZM, Rao ChV. The central role of human chorionic gonadotropin in the formation of human placental syncytium. Endocrinology. 2003;144:1108–1120. doi: 10.1210/en.2002-220922. [DOI] [PubMed] [Google Scholar]
- 31.Choi Y, Wilson K, Hannon PR, Rosewell KL, Brannstrom M, Akin JW, et al. Coordinated regulation among progesterone, prostaglandins, and EGF-like factors in human ovulatory follicles. J Clin Endocrinol Metab. 2017;102:1971–82. [DOI] [PMC free article] [PubMed]
- 32.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20:1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su RW, et al. MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem. 2008;283:23473–23484. doi: 10.1074/jbc.M800406200. [DOI] [PubMed] [Google Scholar]
- 34.Xia HF, Jin XH, Cao ZF, Hu Y, Ma X. MicroRNA expression and regulation in the uterus during embryo implantation in rat. FEBS J. 2014;281:1872–1891. doi: 10.1111/febs.12751. [DOI] [PubMed] [Google Scholar]
- 35.Lin HY, Qian D, Zhang X, Liu GY, Wang HM, Zhu C. Gene expression of transforming growth factor-beta receptors types I and II in rat endometrium during the estrous cycle and early pregnancy. Life Sci. 2006;78:2669–2675. doi: 10.1016/j.lfs.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, et al. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23:265–275. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou M, Fu J, Xiao L, Yang S, Song Y, Zhang X, et al. miR-196a overexpression activates the MEK/ERK signal and represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Hum Reprod. 2016;31:2598–2608. doi: 10.1093/humrep/dew223. [DOI] [PubMed] [Google Scholar]
- 38.Shen LJ, He JL, Yang DH, Ding YB, Chen XM, Geng YQ, et al. Mmu-microRNA-200a overexpression leads to implantation defect by targeting phosphatase and tensin homolog in mouse uterus. Reprod Sci. 2013;20:1518–1528. doi: 10.1177/1933719113488453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haraguchi H, Saito-Fujita T, Hirota Y, Egashira M, Matsumoto L, Matsuo M, et al. MicroRNA-200a locally attenuates progesterone signaling in the cervix, preventing embryo implantation. Mol Endocrinol. 2014;28:1108–1117. doi: 10.1210/me.2014-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SW, Kim H, Ku SY, Suh CS, Kim SH, Choi YM. A successful live birth with in vitro fertilization and thawed embryo transfer after conservative treatment of recurrent endometrial cancer. Gynecol Endocrinol. 2017. doi:10.1080/09513590.2017.1342239. [DOI] [PubMed]
- 41.Rink E, Kuhl J, Aurich C, French H, Nino-Fong R, Watson E, et al. 195 expression of mesenchymal stromal cell (MSC) markers in the equine endometrium and in vitro influence of steroid hormones on endometrial-derived MSC. Reprod Fertil Dev. 2017;29:206. doi: 10.1071/RDv29n1Ab195. [DOI] [Google Scholar]
- 42.Jursza-Piotrowska E, Socha P, Skarzynski DJ, Siemieniuch MJ. Prostaglandin release by cultured endometrial tissues after challenge with lipopolysaccharide and tumor necrosis factor alpha, in relation to the estrous cycle, treatment with medroxyprogesterone acetate, and pyometra. Theriogenology. 2016;85:1177–1185. doi: 10.1016/j.theriogenology.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 43.Lee HJ, Lee JE, Ku SY, Kim SH, Kim JG, Moon SY, et al. Natural conception rate following laparoscopic surgery in infertile women with endometriosis. Clin Exp Reprod Med. 2013;40:29–32. doi: 10.5653/cerm.2013.40.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HS, Kang MJ, Kim SA, Oh SK, Kim H, Ku SY, et al. The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clin Exp Reprod Med. 2013;40:23–28. doi: 10.5653/cerm.2013.40.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svensson-Arvelund J, Ernerudh J. The role of macrophages in promoting and maintaining homeostasis at the fetal–maternal interface. Am J Reprod Immunol. 2015;74:100–109. doi: 10.1111/aji.12357. [DOI] [PubMed] [Google Scholar]