Abstract
MSC-based therapy is providing a cure for degenerative diseases with unmet medical need and usually iliac crest bone marrow (ICBM) are being applied in clinics. Alternative sources, including adipose tissue and reamer/irrigator/aspirator hold great potential for isolating MCSs. Here, we compared original MSCs features of adipose tissue (Ad-MSCs) and bone marrow of long-bone (RIA-MSCs) or iliac crest, and the expression of chemokine receptors (including CXCR4, CX3CR1, CXCR6, CXCR2, CCR1 and CCR7) in these three sources, which are important in the context of homing. We further investigated the role of SDF-1/CXCR4 axis as a key player in motility of different population of MSCs using Transwell migration assay. All cells exhibited typical MSCs characteristics. However, different MSCs sources expressed different levels of chemokine receptors. Generally, the expression of these chemokine receptors was decreased with increasing passage (P) number from 2 to 3. Interestingly, it was observed that the CXCR4 expression and migration capacity in Ad-MSCs is significantly higher than ICBM and RIA-MSCs in P2. Although our data showed that CXCR4 had highest expression in P2 Ad-MSCs, but it dramatically declined following sub-culturing in the P3. Hence, to improve homing of MSCs by means of chemokine/their receptors axis, the source of isolation and passage number should be considered for clinical applications.
Keywords: Stem cells, Reamer-irrigator-aspirator, Iliac crest bone marrow, Adipose tissue, Chemokine receptors
Introduction
Stem cell technology is a growing field among researchers around the world which has attracted a lot of attentions and many hopes to treatment of degenerative diseases [1–3]. In cell therapy, tissue engineering and regenerative medicine, mesenchymal Stem Cells (MSCs) have noticed particular interest on themselves as an appropriate candidate for therapy of many injured tissues such as tendon, bone, cartilage, myocardial infarct tissue, injured liver or renal tissue and spinal cord or brain injuries [3–5].
MSCs can obtain from various sources, but mainly from bone marrow and adipose tissue [6, 7]. However, Stem Cells frequency in iliac crest bone marrow (ICBM) is low and typically represented only 0.01–0.0001% of the nucleated cells [8]. Scarcity of MSCs in ICBM aspirate, high cost and time consuming to culture of cells, has prompted researcher to search for alternative harvesting sites. Recently, the reamer-irrigation-aspirator (RIA) device by continuous irrigation and aspiration of bone marrow during reaming of long bones has been provided a better source for derivation of large number of MSCs called RIA-MSCs [9]. An alternative way to achieve high yield of MSCs is digestion of adipose tissue by collagenase, which seems to release large numbers of Adipose derived MSCs (Ad-MSCs) [10]. However, the use of relatively expensive bacterially derived collagenase and the relatively lengthy process of enzymatic digestion are limited factors of such method. Nonetheless, these higher-yield sources of MSCs, with minimal cellular expansion would be required to attain sufficient number of cells at lower passages (typically P2 or P3 for clinical settings) [11, 12].
In most of clinical cell therapy, Stem Cells are being administrated systemically at P2 or P3 [6, 7, 13, 14]. Insufficient homing and retention of transplanted Stem Cells will dramatically decrease therapeutic effects [15]. Therefore, it is crucial to have the chemotaxis and retention of transplanted MSCs in order to maintain long-term and more effective cell-based therapy [9, 16, 17].
In spite of the natural mechanisms of body for guiding MSCs to injury sites to promote regeneration, these processes are not sufficient for tissue repair [7, 9, 18]. Chemoattractants such as chemokines secreted by injury sites enhance the recruitment of Stem Cells toward injured tissues, thus enhancing the natural healing [19]. Therefore, chemokines and their receptors on the membrane of the cell have a crucial role in Stem Cells migration [20]. Several studies have reported a wide set expression of chemokine receptors on the surface of human MSCs [21–26], however, the data are partly contradictory because some surface markers in some reports have expression but in another reports don’t have, such as CXCR4, CX3CR1, CXCR6, CXCR2, CCR1 and CCR7. This difference in reports is likely due to donor-dependent sources, absence of standard protocols for isolation and culturing and also phenotypically changes during in vitro culture [27]. Among the ligand/chemokine receptors signaling pathway, SDF-1/ CXCR4 axis is probably the most important stem cell homing pathway for MSCs migration toward injured tissues [28].
MSCs hold great promises for many clinical applications because they have differentiation ability into a variety of lineages, self-renewal potential without malignant transformation, good paracrine effects and allograft capability without rejection [6, 13, 29, 30]. However, it has not been yet well-defined how different the homing properties of MSCs depend on the tissue sources in which they are resident.
Here, after characterization of MSCs from three different sources concerning surface markers and differentiation potential, we have examined the comparative expression of CXCR4, CXCR2, CXCR6, CX3CR1, CCR1 and CCR7 among those in passage 2 and 3 that are most commonly used passages in clinical settings. To the best of our knowledge, this is the first study to directly compare the chemokine receptors’ expression of ICBM-MSCs, Ad-MSCs, and RIA-MSCs in the context of homing under the same condition to select the best source for cell therapy.
Materials and methods
Isolation of human MSCs from different sources
Iliac crest bone marrow (ICBM) MSCs
Human MSCs were isolated from 10 ml the iliac crest bone marrow of healthy individuals undergoing bone marrow harvest. Each 1 mL of iliac crest bone marrow directly cultured in one 75 cm2 tissue culture treated flask in low glucose DMEM containing 10% fetal bovine serum (FBS) (Gibco), 100 μg/ml streptomycin and 100 U/ml penicillin (Invitrogen). After 48 h the medium was replaced.
Adipose-derived tissue (Ad) MSCs
Adipose-derived Stem Cells were obtained from raw healthy human lipoaspirates (Razavi hospital, Mashhad, Iran) and cultured as published in our previous study [17]. Briefly, lipoaspirates were washed with sterile phosphate-buffered saline (PBS) to remove red blood cells and contaminating debris. Washed adipose tissue were treated with 1 mg collagenase (type I, invitrogen), 10 mg of bovin serum albomin (BSA) (Biowest, Nuaillé–France) and 2 mM CaCl2 in 1 ml PBS per 3 ml lipoaspirate for 45 min at 37 °C with gentle agitation. After centrifugation, the supernatant was discarded and the pellet containing adherent multipotent cells including MSCs, was cultured in DMEM low glucose containing 100 μg/ml streptomycin and 100 U/ml penicillin (Invitrogen), 2 ng/ml basic fibroblast growth factor (bFGF, Royan Institute, Iran) and 10% fetal bovine serum (FBS, Invitrogen). Medium changed after two days.
Reamer-irrigator-aspirator (RIA) MSCs
Technical guidance on MSC isolation of RIA has been previously reported [14]. Briefly, mononuclear cells containing MSCs from RIA were separated by centrifugation over a Ficoll-Hypaque gradient (Cederlan) and suspended in DMEM low glucose (Gibco) containing 10% fetal bovine serum (Gibco), 100 μg/ml streptomycin and 100 U/ml penicillin (Invitrogen). Medium changed after 48 h.
Isolation human dermal fibroblast (HDF)
Skin samples were obtained from consenting patients as redundant tissue from abdominoplasty (age 30–40 years). The skin punches (about 6 mm) was rinsed in DMEM high glucose containing 20% FBS and 2.5 mg collagenase type I in 1 ml DMEM high glucose and was incubated in 37 °C for 10–12 h. Then, after several pipetting the cells cultured in T75 flasks in DMEM High glucose (Gibco) containing 20% fetal bovine serum (Gibco), 100 μg/ml streptomycin and 100 U/ml penicillin (Invitrogen). Medium changed after 72 h.
Adipogenic and osteogenic differentiation
To promote adipogenic differentiation, HDF as negative control and MSCs from ICBM, Ad and RIA (from three different donors) were treated with induction medium supplemented with 1 μM dexamethasone (Sigma-Aldrich), 500 μM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), 5 μg/ml insulin (Sigma-Aldrich) for 21 days. Finally lipid-rich vacuoles stained with Oil Red O reagent.
For osteogenic differentiation, HDF as negative control and MSCs from ICBM, Ad and RIA were cultured in induction medium with 0.1 μM dexamethasone (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich) and ascorbate-2-phosphate (Sigma-Aldrich). Osteogenic differentiaton was verified on day 21 by calcifications produced in extracellular matrix of differentiated cells that stainable with Alizarin Red and confirmed by ALP activity of osteoblasts [31].
Flow cytometry
The MSCs isolated from each source (from three different donors) have cultured on tissue culture-treated flasks were digested with 0.25% trypsin/EDTA and washed with PBS containing 5% FBS. For identification of surface antigens, MSCs (1 × 106) were labeled with antibodies for characterization including anti-CD34 antibody, mouse anti-CD44 antibody (Antibodies-online, Aachen Germany), rabbit anti-CD45 antibody, Rabbit anti-CD105 antibody (Bioss Inc, Woburn, MA, USA), rabbit anti-CD11b antibody, mouse anti-CD73 antibody, and mouse anti-CD90 antibody (Biologicals, Littleton, Colorado, USA) for 1 h. For each antibody, relative isotype control was used.
Population doubling time (PDT)
Previously isolated and characterized three populations of MSCs from different sources (ICBM-, Ad-, RIA-MSCs) were seeded into the T25 cm2 culturing flask at a density of 5000 cells/cm2 in triplicate. Cells were harvested after 5 days on reaching >90% confluence with trypsin/EDTA and stained with trypan blue and were count with hemacytometer for up to 6 passages. The mean PDT for each cell type was calculated after every passage by using the following formula: PDT = T × lg2/(lgNt − lgN0), T = culture time (day), N0 = initial cell number, Nt = harvested cell number [32].
RT-PCR
Total RNA was extracted from MSCs (Trizol reagent, invitrogen), and cDNAs were synthesis by reverse transcription of 1 μg of cellular RNA (M-MULV Reverse Transcriptase; fermentase), according to the manufacturer’s instruction. PCR was performed for 40 cycles, with each cycle consisting of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s, and finally 5 min incubation at 72 °C after the last cycle (the sequences of primers were used according to Table 1). PCR products were separated by electrophoresis on 1.5% agarose gel, stained with gel red (Biotium) and visualized under UV light.
Table 1.
Description of the real time RT-PCR primers
| Gene | Strand | Primer sequence | Product size |
|---|---|---|---|
| β-actin | Sense | 5′-GCGGAAATCCTGCGTGACATT-3′ | 232 |
| Antisense | 5′-GATGGAGTTGAAGGTAGTTTCGTG-3′ | ||
| CXCR4 | Sense | 5′-TCTAGGCAGGACCTGT-3′ | 225 |
| Antisense | 5′-CACTTTGGGCTTTGGTT-3′ | ||
| CXCR6 | Sense | 5′-ATGCCATGACCAGCTTTCACT-3′ | 70 |
| Antisense | 5′-TTAAGGCAGGCCCTCAGGTA-3′ | ||
| CXCR2 | Sense | 5′-CTCAAGACCTCCTGCCTAAG-3′ | 102 |
| Antisense | 5′-ACACTGAGACCAAGAAGAACC-3′ | ||
| CX3CR1 | Sense | 5′-ATAGATTCCCCATTGCCTCCTC-3′ | 120 |
| Antisense | 5′-GGTTTTTCTATTTCCCTTACTGG-3′ | ||
| CCR7 | Sense | 5′-GCACAGCCTTCCTGTGTG-3′ | 112 |
| Antisense | 5′-CGTCCGTGACCTCATCTTG-3′ | ||
| CCR1 | Sense | 5′-CACGGACAAAGTCCCTTGG-3′ | 134 |
| Antisense | 5′-CAAAGGCCCTCTCGTTCAC-3′ |
Real time PCR
To quantify CXCR4, CXCR2, CXCR6, CX3CR1, CCR1 and CCR7 levels, a real time RT-PCR assay was established with a Biorad CFX96 thermal cycler and Syber green Universal Master Mix (Pars tous), according to the manufacturer’s instructions. The thermal profile for Real time PCR (94 °C for 30 s, 60 °C for 40 s and 72 °C for 30 s) was run for 40 cycles after an initial single cycle of 94 °C for 15 min (all experiments were performed from three donors and each test was at least duplicate). The sequences of primers were used according to Table 1.
Migration assays
Chemotactic activity of ICBM-, Ad- and RIA-MSCs (from three different donors and each test was at least triplicate) toward SDF-1 was examined using a 24-well 8 μm pore size polycarbonate memberane (Costar, corning) as described elsewhere [7]. Briefly, 25 × 103 cells/ml of untreated MSCs and AMD3100 (Sigma-Aldrich) treated MSCs (5 μg/ml) in 100 μl medium without FBS were added in the top wells and 100 ng/ml SDF-1α (Peprotech) was loaded in the bottom chamber (10% FBS for positive control). Then, after 18-h incubation at 37 °C and 5% CO2, the migrated cells were stained with DAPI and counted. Also the migration was inhibited by AMD3100 as a CXCR4 inhibitor to prove that the migration depends on CXCR4.
Statistical analysis
Statistical analyses were performed using Graph Pad Prism, version 6.0 software. Results are presented as mean ± SD. Statistical significance was evaluated with one way ANOVA and post hoc test for Tukey’s and Dunett’s multiple comparison tests.
Results
Characterization of MSCs from ICBM, Ad and RIA
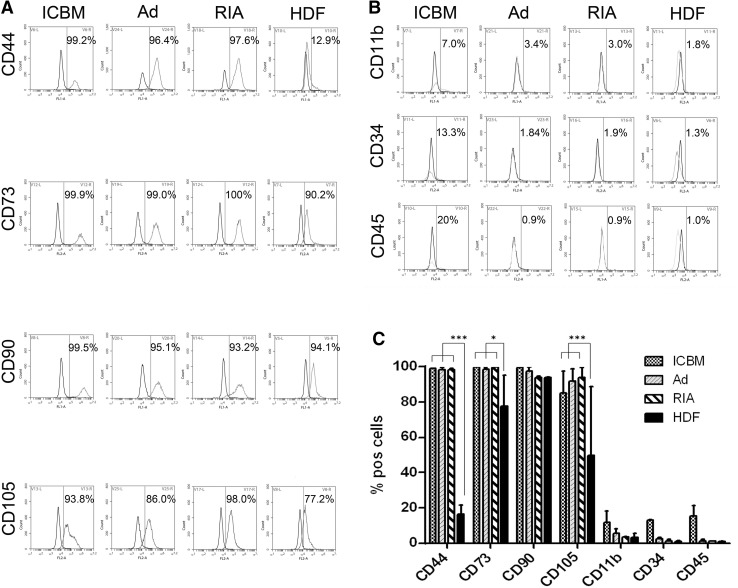
The flow cytometry characterization has shown that the different MSCs contained similar set of surface antigens. ICBM-, Ad- and RIA-MSCs have expressed high level of CD90, CD105, CD73 and CD44 in nearly similar ways (Fig. 1A, C) and all cultures were negative for hematopoietic-associated markers: CD11b, CD34 and CD45 (Fig. 1B). Also, analysis of cell markers has revealed that HDF are positive for CD90 as well as CD105, CD73 and CD44 but significantly less than other groups (Fig. 1B, C).
Fig. 1.
The expression pattern for cell surface markers of MSCs from ICBM, Ad, RIA and HDF cells. A Flow cytometry analysis was done to profile characteristic markers of MSCs. ICBM-, Ad-, RIA-MSCs have shown the same immunophenotype but not with HDF as a control. B Flow cytometry results for negative surface markers of MSCs were negative similar to HDF. C Cumulative data of FACS analysis of MSCs from ICBM, Ad, RIA and HDF cells for several surface markers. ICBM Iliac crest bone marrow, Ad Adipose derived, RIA Reamer-irrigator-aspirator, HDF Human dermal fibroblast
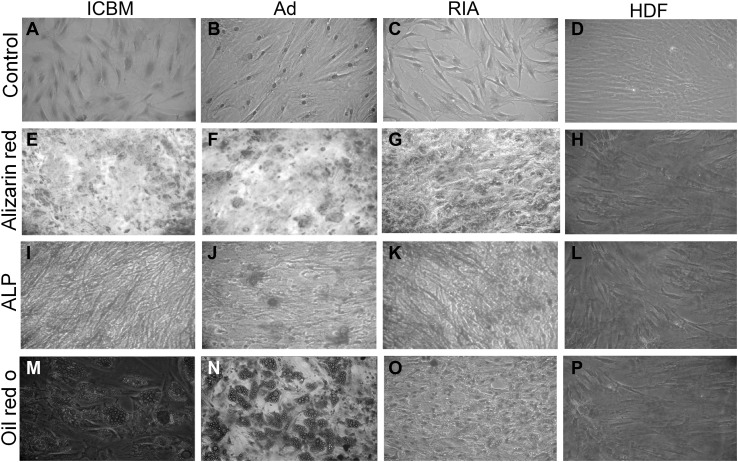
To determine that MSCs from ICBM, Ad and RIA, are capable for differentiate into multiple cell types, the cells were cultured in induction media specific for differentiate to adipocytes and osteoblasts. Our results showed that all three types of MSCs can differentiate into osteoblasts and adipocytes but not HDF and there was no difference in the differentiation capacity except the osteogenesis capacity of RIA-MSCs that was higher than other groups (Fig. 2).
Fig. 2.
Differentiation capacities of MSCs populations toward osteoblast and adipocyte. A–D Spindle shaped fibroblastic-like cells in the primary culture of human MSCs derived from ICBM, Ad, and RIA. Human dermal fibroblast (HDF) is as control. E–H Osteogenic medium was used to MSCs and HDF cells induction at P2 toward osteoblasts for 21 days. MSCs after differentiation have produced calcified extracellular matrix that stained by Alizarin Red S reagent as evident for osteogenesis in comparison to HDF. I–L Additionally, osteogensis was confirmed by ALP assay of osteoblasts compared with HDF cells. M–P Also, Oil Red O staining was performed to staining of lipid droplets in order to prove adipogenic differentiation of MSCs, but not HDF, that were cultured in adipogenic induction medium. ICBM iliac crest bone marrow, Ad Adipose, RIA Reamer-irrigator-aspirator
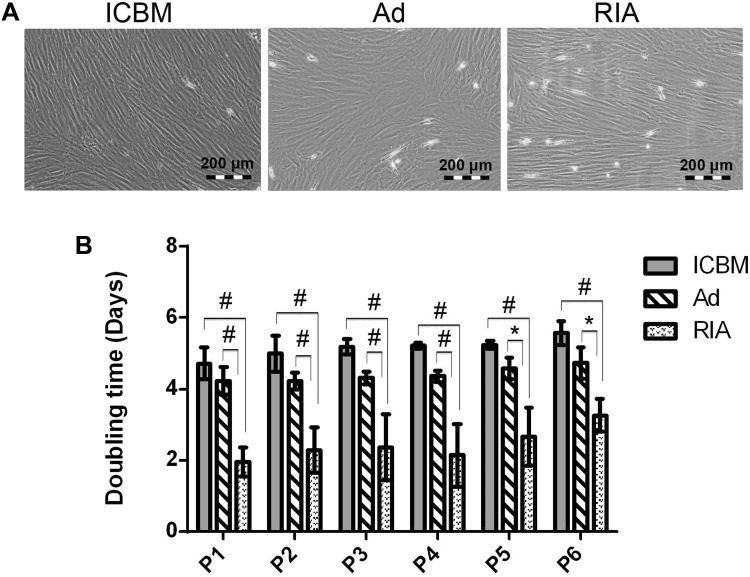
Morphology and population doubling times in three sources of MSCs
The MSCs in P2 and P3 were photographed and their morphology was studied. Spindle fibroblast-like form was observed in all three sources (Fig. 3A). To study PDT of the cells, the MSCs were cultured up to passage 6. In all passages, RIA-MSCs showed the significantly highest cumulative cell population than Ad- and ICBM-MSCs (Fig. 3B). The population doubling time for ICBM-, Ad- and RIA-MSCs were approximately 5.1, 4.4 and 2.4 days, respectively. The PDT among the 6 passages had no significant differences in all three sources (Fig. 3B).
Fig. 3.
Morphology and population doubling times in three sources of MSCs. A Spindle shape fibroblast-like form was observed in all three sources at P0. B The population doubling time (PDT) of the ICBM-, Ad- and RIA-MSCs were approximately 5.1, 4.4 and 2.4 days, respectively. The PDT among the 6 passages had no significant change
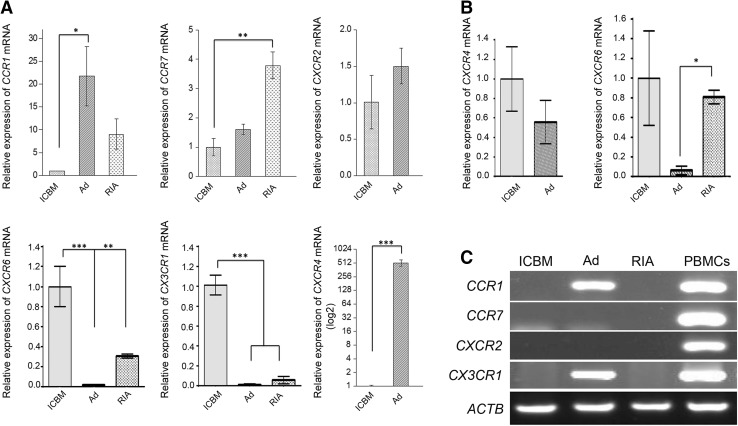
The chemokine receptor expression profile in MSCs
In this study, the mRNA expression of six chemokine receptors (CXCR4, CXCR2, CXCR6, CX3CR1, CCR1 and CCR7) in MSCs from ICBM, Ad and RIA were analyzed. The expression of each chemokine receptor in ICBM was considered as basal expression and quantified. As shown in Fig. 4A, Ad-MSCs in P2, have shown significantly highest level in the mRNA expression of CXCR4 (mean 515.2 ± 152.6) compared with ICBM, whereas CXCR4 in RIA-MSCs had no expression. Comparing the cells in P2, very low expression of CX3CR1 (mean 0.010 ± 0.008 for Ad and 0.057 ± 0.036 for RIA) and CXCR6 (mean 0.017 ± 0.006 for Ad and 0.306 ± 0.02 for RIA) was observed, whereas CCR7 with mean 3.8 ± 0.8 fold change in RIA-MSCs and CCR1 with mean 21.7 ± 11.3 fold change in Ad-MSCs had higher expression rather than ICBM (Fig. 4A).
Fig. 4.
The expression pattern for different chemokine receptors from ICBM, Ad- and RIA-MSCs using RT-PCR and Real time RT-PCR in P2 and P3. A The expression of CCR1, CCR7, CXCR2, CXCR4, CXCR6 and CX3CR1 in MSCs at P2. B The expression of CXCR4 and CXCR6 in MSCs at P3. C The expression of CCR1, CCR7, CXCR2, and CX3CR1 in MSCs at P3. A–C CXCR4 and CXCR2 mRNA levels in RIA MSCs was undetectable in P2 and P3. The β-actin (ACTB) housekeeping gene product was used to as an endogenous reference. Human peripheral blood mononuclear cells (PBMCs) were used to a positive control. *p < 0.05, **p < 0.01 and ***p < 0.001 was considered significant as compared to untreated control. ICBM Iliac crest bone marrow, Ad Adipose, RIA Reamer-irrigator-aspirator
Despite the highest expression levels of CXCR4 in the P2 Ad-MSCs (Fig. 4A), its expression was dramatically reduced in P3 (0.043 ± 0.015; p < 0.01) so that no significant difference was observed between the Ad and ICBM cells (Fig. 4B). Moreover, it was observed that the expression of CCR1 and CX3CR1 in the P3 were detectable only in Ad-MSCs without significant variation as compared with P2 (1.5 ± 0.7 and 0.8 ± 0.4 respectively) and the expression of CCR7 and CXCR2 was not observed in all three sources of cells at P3 (Fig. 3C). The lowest level of expression of CXCR6 (mean 0.061 ± 0.043) was observed in Ad-MSCs at P3. CXCR6 was significantly reduced in Ad-MSCs at P3 0.19 ± 0.057 and in RIA-MSCs, there was no significant changes observed between P2 and P3 (0.8 ± 0.13) (Fig. 4B).
Migration behavior of ICBM, Ad and RIA MSCs in P2 and P3
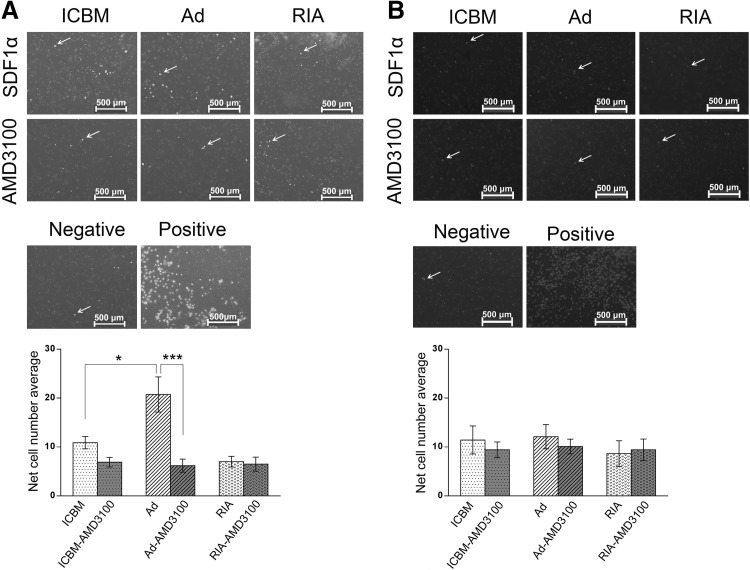
As CXCR4 is key player in chemotaxis, we further investigated the migration potential of human MSCs in each source in P2 and P3 in response to SDF-1α using Boyden chamber assay. It was found that the migration of Ad-MSCs was much higher (20.75 ± 6.2) compared with that of ICBM (10.9 ± 2.2) and RIA-MSCs (7 ± 1.9) in P2. However, in P3 no differences were found among three sources. For showing that SDF-1/CXCR4 axis is the cause of cellular migration and homing capabilities of Ad-MSCs, the cells were treated with specific CXCR4 inhibitor, AMD3100, that resulted in the impairment of SDF-1/CXCR4 axis (Fig. 5).
Fig. 5.
Migration ability of MSCs from three different sources toward SDF-1α in P2 and P3. A Cell migration of ICBM-, Ad- and RIA-MSCs was analyzed using a Boyden chamber assay in P2. B Cell migration of ICBM-, Ad- and RIA-MSCs was analyzed using a Boyden chamber assay in P3. Fluorescent images show representative of DAPI-stained cells (upper panel). Statistical analysis revealed that the migration capacity of Ad-MSCs was significantly higher than ICBM and RIA-MSCs in P2, but not in P3 (lower panel). *p < 0.05 and ***p < 0.001 was considered as significant. ICBM iliac crest bone marrow, Ad Adipose, RIA Reamer-irrigator-aspirator
Discussion
MSCs originating from bone marrow or fat tissues are the most widely used in clinical trials for a wide range of diseases [33]. Recently, there has been evidence that RIA-MSCs hold great potential for stem cell based therapy [9, 14]. To characterize MSCs from different sources, we compared human ICBM-MSCs, RIA-MSCs and Ad-MSCs for cell expansion, surface antigen marker, differentiation ability, proliferation capacity, and expression of chemokine receptors each one from three different donors under the same conditions of culture. Flow cytometric analysis showed that all three cell types exhibit nearly similar phenotypes and capable of osteogenic and adipogenic differentiation. However, in consistent with other reports [34], RIA-MSCs possessed higher capacity toward osteogenic differentiation compared with Ad-MSCs, while similar adipogenic differentiation potential was observed among the three types of cells. The first user of isolated RIA-MSCs can be the same patient in case of suffering from non-union or any other need. However, as MSCs can be used allogeneic without immune-rejection [29], the second user can be other patients with degenerative disease. There are extensive literature already about Ad-MSCs and ICBM-MSCs [35, 36], but we were curious about RIA-MSCs as we observed that these cells proliferate much better than other sources (Fig. 3B). In consistent with our results, a previous study showed that RIA-MSCs produce large yields of MSCs and exhibited characteristics similar to ICBM-MSCs and Ad-MSCs, including fibroblast-like morphology, and surface markers expression [37]. These data suggest that the tissue source from which MSCs are isolated should be considered for specific clinical therapeutic application [3] and such information may be crucial to identify the most suitable cell source for a given clinical application [18].
Since homing action is important for achieving better therapeutic results in clinical setting [30], we further examined the expression of CXCR4, CXCR2, CXCR6, CX3CR1, CCR1 and CCR7 for each MSCs of different sources under same condition. There has been evidence that the activation of SDF1/CXCR4 axis might be helpful to improve the effectiveness of MSCs-based stem cell therapy [2, 38]. Our results demonstrated that Ad-MSCs have biological advantages regarding expression of CXCR4 in P2 compared to other sources, making Ad-MSCs a useful model for clinical applications of cell therapy. The migration of MSCs to the injured tissues is driven by chemokine receptors in response to chemokine that are immediately produce in injured tissues [39]. It has been shown that different chemokine receptors are belong to homing groups and facilitate this process [21, 23, 24]. Chemokine receptors which are involved in chemotaxis and organ-specific homing of MSCs show different expression levels in various studies [40–43] that may be due to a variety of cell isolation sources. Therefore, selection of suitable MSCs source with high homing capacity is crucial in cell therapy and regenerative medicine [3, 44]. We have compared the mesenchymal Stem Cells from P2 and P3. To fulfill good manufacturing practice (GMP) criteria in cell therapy, most of clinical trials are applying Stem Cells often from P2 or P3 via systemic administration [6, 7, 13, 14]. In clinical trials, the lack of more definitive results often attributed to poor homing and retention of transplanted Stem Cells [34, 45–47]. Increasing in cell chemotaxis and transendothelial migration ability is expected to enhance therapeutic benefit [48, 49].
In present study, relative expression profile of CXCR4, CXCR2, CXCR6, CX3CR1, CCR1 and CCR7 chemokine receptors among ICBM-MSCs, Ad-MSCs and RIA-MSCs were compared. The results showed that the chemokine receptors expression decreased with increasing passage number from 2 to 3 in all cell types to the extent that the expression of some chemokine receptors was undetectable. More interestingly, although the expression of CXCR4 in P2 Ad-MSCs was higher (565.2 times) than ICBM-MSCs and RIA-MSCs, but dramatically reduced in P3 so that differences to ICBM and RIA was not significant. We further investigated the role of SDF-1/CXCR4 axis in MSCs motility by Transwell migration assay. As expected, migration through Transwell chambers was significantly increased in response to SDF-1 only in P2 Ad-MSCs, compared to Ad-MSCs and ICBM-MSCs in P3 or RIA-MSCs in any passage. Such chemotactic response significantly impaired when SDF-1/CXCR4 axis was blocked by AMD3100, an anti-CXCR4 agent. Therefore, CXCR4 may play a significant role in migration and homing of Ad-MSCs, thus improve the efficacy of Ad-MSCs-based therapy.
It has been suggested that selection of appropriate MSCs source and passage number with high homing capacity is important in regenerative medicine [50–52]. SDF-1/CXCR4 axis is probably the most important stem cell homing pathway for MSCs migration toward injured tissues [28, 53]. Although, our data showed that CXCR4 were highly expressed (mean 565.2) in P2 Ad-MSCs, the expression of this receptor declined following a passage in the culture. Similarly, several study have indicated that CXCR4 is highly expressed on MSCs, but is disappear over several passages [51, 52]. This would influence the homing and repairing potentials of MSCs, leading to the impair effect of MSCs-based therapy in injured tissue. In order to overcome this limitation, MSCs genetically modified by liposome and viral vector system for overexpressing CXCR4 [54–56]. Moreover, preconditioning or pretreating with hypoxic or hypoxia-mimicking chemical agents [2, 57–59] also promote expression of CXCR4 in MSCs [57, 60], consequently enhancing their homing and therapeutic effects for various tissue injury. This would be helpful for improving engraftment of repopulating MSCs in clinical transplantation before systemically administration, thus lead to a long-term, more effective MSCs-based therapy. However, these approaches also have limitation for clinical use.
Mesenchymal Stem Cells derived from various sources is considered for cell therapy and tissue engineering of incurable diseases. Three different sources, i.e. human bone marrow derived MSCs from iliac crest (ICBM) and from long bones by RIA device (RIA-MSCs), and adipose tissue (Ad), were compared for differentiation ability, surface antigen markers, and chemokine receptors’ expression. Irrespective of MSCs yields, MSCs from different sources had similar levels of surface antigen markers. RIA-MSCs also showed preferentially differentiation into osteoblast that making them a useful source for cell therapy of bone injury and nonunion fractures. Additionally, we found that Ad-MSCs have biological advantages in the homing capacity in P2. These biological advantages should be considered systematically when choosing a MSCs source for a specific clinical application.
Acknowledgements
This work was financially supported by Iranian Council of Stem cell Technology (ICST) and Iranian Academic Center for Education, Culture and Research (ACECR).
Conflict of interest
Authors declared no conflict of interest.
Ethical statement
This work has been approved by the Ethical Committee of ACECR-Khorasan Razavi Branch and all patients included in this study were informed regarding the usage of their samples for experimental analysis. (IRB No. 910750).
References
- 1.Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2013;9:32–43. doi: 10.1007/s12015-012-9365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreas K, Sittinger M, Ringe J. Toward in situ tissue engineering: chemokine-guided stem cell recruitment. Trends Biotechnol. 2014;32:483–492. doi: 10.1016/j.tibtech.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 4.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Heirani-Tabasi A, Hassanzadeh M, Hemmati-Sadeghi S, Shahriyari M, Raeesolmohaddesin M. Mesenchymal stem cells; defining the future of regenerative medicine. J Genes Cells. 2015;1:34–39. doi: 10.15562/gnc.15. [DOI] [Google Scholar]
- 6.Naderi H, Matin MM, Bahrami AR. Review article: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl. 2011;26:383–417. doi: 10.1177/0885328211408946. [DOI] [PubMed] [Google Scholar]
- 7.Naderi-Meshkin H, Matin MM, Heirani-Tabasi A, Mirahmadi M, Irfan-Maqsood M, Edalatmanesh MA, et al. Injectable hydrogel delivery plus preconditioning of mesenchymal stem cells: exploitation of SDF-1/CXCR4 axis towards enhancing the efficacy of stem cells’ homing. Cell Biol Int. 2015;40:730–741. doi: 10.1002/cbin.10474. [DOI] [PubMed] [Google Scholar]
- 8.Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Cox G, McGonagle D, Boxall S, Buckley C, Jones E, Giannoudis P. The use of the reamer-irrigator-aspirator to harvest mesenchymal stem cells. J Bone Joint Surg Br. 2011;93:517–524. doi: 10.1302/0301-620X.93B4.25506. [DOI] [PubMed] [Google Scholar]
- 10.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 11.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 12.Stolzing A, Colley H, Scutt A. Effect of age and diabetes on the response of mesenchymal progenitor cells to fibrin matrices. Int J Biomater. 2011;2011:1–9. doi: 10.1155/2011/378034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues: cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Porter RM, Liu F, Pilapil C, Betz OB, Vrahas MS, Harris MB, et al. Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J Orthop Res. 2009;27:42–49. doi: 10.1002/jor.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karp JM, Teo GSL. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385–4402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naderi-Meshkin H, Bahrami AR, Bidkhori HR, Mirahmadi M, Ahmadiankia N. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol Int. 2015;39:23–34. doi: 10.1002/cbin.10378. [DOI] [PubMed] [Google Scholar]
- 18.Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transplant. 2014;19:41–46. doi: 10.1097/MOT.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 19.Shi C. Recent progress toward understanding the physiological function of bone marrow mesenchymal stem cells. Immunology. 2012;136:133–138. doi: 10.1111/j.1365-2567.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/S0301-472X(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 21.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 22.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 23.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 24.Lüttichau IV, Notohamiprodjo M, Wechselberger A, Peters C, Henger A, Seliger C, et al. Human adult CD34-progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]
- 25.Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 26.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 27.Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32:1713–1723. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- 28.Rankin SM. Chemokines and adult bone marrow stem cells. Immunol Lett. 2012;145:47–54. doi: 10.1016/j.imlet.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kallmeyer K, Pepper MS. Homing properties of mesenchymal stromal cells. Expert Opin Biol Ther. 2015;15:477–479. doi: 10.1517/14712598.2015.997204. [DOI] [PubMed] [Google Scholar]
- 31.Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MDF, et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146–150. doi: 10.1634/stemcells.2007-0381. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34:695–704. doi: 10.3892/ijmm.2014.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irfan-Maqsood M, Bahrami M, Mirahmadi M, Naderi-Meshkin H. Commercialization of stem cell therapeutic research: bridging a big gap. Genes Cells. 2015;1:40–45. doi: 10.15562/gnc.13. [DOI] [Google Scholar]
- 34.Henrich D, Seebach C, Sterlepper E, Tauchmann C, Marzi I, Frank J. RIA reamings and hip aspirate: a comparative evaluation of osteoprogenitor and endothelial progenitor cells. Injury. 2010;41:S62–S68. doi: 10.1016/S0020-1383(10)70012-7. [DOI] [PubMed] [Google Scholar]
- 35.Maijenburg MW, Noort WA, Kleijer M, Kompier CJ, Weijer K, Van Buul JD, et al. Cell cycle and tissue of origin contribute to the migratory behaviour of human fetal and adult mesenchymal stromal cells. Br J Haematol. 2010;148:428–440. doi: 10.1111/j.1365-2141.2009.07960.x. [DOI] [PubMed] [Google Scholar]
- 36.Burk J, Ribitsch I, Gittel C, Juelke H, Kasper C, Staszyk C, et al. Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Vet J. 2013;195:98–106. doi: 10.1016/j.tvjl.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Wenisch S, Trinkaus K, Hild A, Hose D, Herde K, Heiss C, et al. Human reaming debris: a source of multipotent stem cells. Bone. 2005;36:74–83. doi: 10.1016/j.bone.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Shi M, Li J, Liao L, Chen B, Li B, Chen L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 39.Balasubramanian S, Venugopal P, Sundarraj S, Zakaria Z, Majumdar AS, Ta M. Comparison of chemokine and receptor gene expression between Wharton’s jelly and bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2012;14:26–33. doi: 10.3109/14653249.2011.605119. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain G, Wright K, Rot A, Ashton B, Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS One. 2008;3:e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croitoru-Lamoury J, Lamoury FM, Zaunders JJ, Veas LA, Brew BJ. Human mesenchymal stem cells constitutively express chemokines and chemokine receptors that can be upregulated by cytokines, IFN-β, and copaxone. J Interferon Cytokine Res. 2007;27:53–64. doi: 10.1089/jir.2006.0037. [DOI] [PubMed] [Google Scholar]
- 42.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- 43.Bahrami AR, Ebrahimi M, Matin MM, Neshati Z, Almohaddesin MR, Aghdami N, et al. Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J Mol Neurosci. 2011;44:178–185. doi: 10.1007/s12031-010-9446-6. [DOI] [PubMed] [Google Scholar]
- 44.Park JS, Suryaprakash S, Lao YH, Leong KW. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods. 2015;84:3–16. doi: 10.1016/j.ymeth.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kavanagh DP, Suresh S, Newsome PN, Frampton J, Kalia N. Pretreatment of mesenchymal stem cells manipulates their vasculoprotective potential while not altering their homing within the injured gut. Stem Cells. 2015;33:2785–2797. doi: 10.1002/stem.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen B, Burks S, Kim S, Bresler M, Tebebi P, Frank J. Pulsed focused ultrasound enhances mesenchymal stem cell homing to skeletal muscle in a murine model of muscular dystrophy and homing was suppressed by Ibuprofen. J Ther Ultrasound. 2015;3:P69. doi: 10.1186/2050-5736-3-S1-P69. [DOI] [Google Scholar]
- 47.Wu S, Li L, Wang G, Shen W, Xu Y, Liu Z, et al. Ultrasound-targeted stromal cell-derived factor-1-loaded microbubble destruction promotes mesenchymal stem cell homing to kidneys in diabetic nephropathy rats. Int J Nanomed. 2014;9:5639. doi: 10.2147/IJN.S73950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirzamohammadi S, Aali E, Najafi R, Kamarul T, Mehrabani M, Aminzadeh A, et al. Effect of 17β-estradiol on mediators involved in mesenchymal stromal cell trafficking in cell therapy of diabetes. Cytotherapy. 2015;17:46–57. doi: 10.1016/j.jcyt.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 49.He H, Zhao ZH, Han FS, Wang XF, Zeng YJ. Activation of protein kinase C ε enhanced movement ability and paracrine function of rat bone marrow mesenchymal stem cells partly at least independent of SDF-1/CXCR4 axis and PI3 K/AKT pathway. Int J Clin Exp Med. 2015;8:188. [PMC free article] [PubMed] [Google Scholar]
- 50.Bortolotti F, Ukovich L, Razban V, Martinelli V, Ruozi G, Pelos B, et al. In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Rep. 2015;4:332–339. doi: 10.1016/j.stemcr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 52.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 53.Naderi-Meshkin H, Andreas K, Matin MM, Sittinger M, Bidkhori HR, Ahmadiankia N, et al. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int. 2014;38:72–84. doi: 10.1002/cbin.10181. [DOI] [PubMed] [Google Scholar]
- 54.Bobis-Wozowicz S, Miekus K, Wybieralska E, Jarocha D, Zawisz A, Madeja Z, et al. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp Hematol. 2011;39:e684. doi: 10.1016/j.exphem.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, Zhuo Z, Zhang Q, Xu Y, Wu S, Li L, et al. Transfection of CXCR-4 using microbubble-mediated ultrasound irradiation and liposomes improves the migratory ability of bone marrow stromal cells. Curr Gene Ther. 2015;15:21–31. doi: 10.2174/1566523214666141121111220. [DOI] [PubMed] [Google Scholar]
- 56.Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol: WJG. 2014;20:14884. doi: 10.3748/wjg.v20.i40.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Najafi R, Sharifi AM. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin Biol Ther. 2013;13:959–972. doi: 10.1517/14712598.2013.782390. [DOI] [PubMed] [Google Scholar]
- 59.Mirahmadi M, Heirani-Tabasi A, Hassanzadeh H, Pishbin M, Bidkhori HR, Naderi-Meshkin H. Therapeutic potential of Stem Cell Preconditioning for Ischemic Heart Diseases/Letter to the Editor. J Cell Mol Res. 2013;5:92–93. [Google Scholar]
- 60.Kim SW, Kim HY, Lee HJ, Yun HJ, Kim S, Jo DY. Dexamethasone and hypoxia upregulate CXCR4 expression in myeloma cells. Leukemia Lymphoma. 2009;50:1163–1173. doi: 10.1080/10428190902893801. [DOI] [PubMed] [Google Scholar]