Abstract
The aim of this study was to investigate the synergistic effect of cold atmospheric plasma (CAP) treatment and RGD peptide coating for enhancing cellular attachment and proliferation over titanium (Ti) surfaces. The surface structure of CAP-treated and RGD peptide-coated Ti discs were characterized by contact angle goniometer and atomic force microscopy. The effect of such surface modification on human bone marrow derived mesenchymal stem cells (hMSCs) adhesion and proliferation was assessed by cell proliferation and DNA content assays. Besides, hMSCs’ adhesion and morphology on surface modified Ti discs were observed via fluorescent and scanning electron microscopy. RGD peptide coating following CAP treatment significantly enhanced cellular adhesion and proliferation among untreated, CAP-treated and RGD peptide-coated Ti discs. The treatment of Ti surfaces with CAP may contribute to improved RGD peptide coating, which enables increased cellular integrations with the Ti surfaces.
Keywords: RGD peptide, Non-thermal atmospheric plasma, Titanium dental implants, Surface modification, Cell adhesion and proliferation
Introduction
Dental implant applications have been gold standard oral rehabilitation technique for partially or fully edentulous patients [1, 2]. However, due to the insufficient osseointegration, which causes micromotion of the implant, up to 10% of these implants fail at the early stages of integration [3, 4]. Titanium (Ti) and Ti alloys, particularly Ti6Al4 V, are commonly used material to manufacture dental implants, due to their biocompatibility, suitable mechanical properties and resistance to corrosion [5]. Ti implant surface modifications by surface treatment or bioactive material coating have been attractive techniques to improve osseointegration capability that directly enhances stability and reduces failures of the implants [6, 7].
Osseointegration of Ti dental implant is a biological process that involves formation of new peri-implant bone in direct contact between implant surface and adjacent bone tissue [8, 9]. Implant surface is modified to achieve high bioactivity with optimal biological responses of surrounding osteogenic cell environment [10]. Due to the close relationship between implant surface properties and osseointegration time and quality, different surface modification techniques have been applied to dental implants in last two decades [11]. The ultimate goal of such surface modifications is to convert osteoconductive surface of dental implants to bioactive surface that could accelerate the bone tissue formation.
The key factors to achieve optimum surface properties of Ti implants are correlated to roughness, topography, chemical composition and surface energy [12]. Dental implant surface characteristics affect the normal host biological response such as extracellular matrix (ECM) protein adsorption, cell/tissue growth, cell-implant surface interaction at the interface between the implant body and surrounding environment [13]. Interactions at the cell–implant interface play a major role to determine the success of the implant by influencing the processes of cell adhesion, proliferation, and differentiation. Higher bone apposition rate has directly been linked to increased roughness and hydrophilicity of the Ti implant surfaces [14]. Also, in vivo and in vitro studies demonstrated higher affinity of mature osteoblasts to rougher and more hydrophilic Ti surfaces [15–18].
Surface energy and chemical composition of the dental implant surface are gaining interest since those parameters directly affect the hydrophilicity of the Ti surface [19]. As demonstrated previously, higher hydrophilicity of implant surfaces expedites osseointegration compared with hydrophobic surface implants both in animal and human models [20, 21]. Moreover, increasing implant surface wettability presents greater bone-to-implant contact (BIC) and interfacial shear strength over microrough surfaces alone [13, 22, 23].
Methods to get more hydrophilic surface have been made in the form of physicochemical modifications such as anodic oxidation [24], acid treatment [25], and hydrogen peroxide treatment [26, 27]. Cold (non-thermal) atmospheric plasma (CAP) is also reported as another method, that increases the surface hydrophilicity of the various materials including Ti [28]. Plasma is defined as ionized gas, which comprises highly reactive particles such as electrically excited atoms, molecules, and ionic and free radical species. These highly reactive plasma species change the properties of the materials’ surfaces by the way of interacting with clean and etched surfaces, bonding to various materials or gathering to form a thin layer of plasma coating [29]. CAP treatment holds the hydroxyl groups temporarily to cover the material surface that significantly improves surface’s wettability, modifies the oxide layer that reacts with proteins and cells of surrounding environment and can lead to an increased adhesion of osteoblasts [30, 31].
Another approach for physicochemical surface modifications is the conjugation of bioactive peptides, which are found in the ECM proteins, to enhance osteoprogenitor cell adhesion [32]. Peptides are composed of short amino acid sequences that can function as a conjoining unit between integrin receptors of cells and material surface. The RGD (arginine–glycine–aspartic acid) peptide derived from fibronectin and vitronectin is one of the common peptide sequences, which acts as a binding unit for alpha/beta integrin receptors during osteoprogenitor cells adhesion and migration [33]. It was reported that RGD peptide coating significantly enhanced osteoblast adhesion on the dental implant surface and shorten the osseointegration process [34–37]. For instance, Yang et al. investigated the effect of RGD peptide coating on titanium implants for the formation of BIC. The results revealed that RGD-coated implants exhibited significantly greater percentages of BIC compared to neat titanium implants at 4, 8, and 12 weeks after implantation. Furthermore, the RGD-coated implants significantly increased the removal torque values at 8 and 12 weeks after implantation [38].
We hypostasized that CAP treatment modifies the Ti surface for enhanced conjugation of RGD peptide to improve cellular adhesion which subsequently may lead accelerated healing of jaw bone in future possible clinical applications. Despite enhanced cellular adhesion on Ti implants in consequence of individual RGD peptide coating and CAP treatment were reported, the synergistic effect of CAP treatment and RGD peptide coating has yet to be studied. In this study, we examined the attachment and proliferation of human bone marrow derived mesenchymal stem cells (hMSCs) on synergistically modified surface of Ti discs. In addition, we also compared effects of RGD peptide coating, CAP treatment and their combination on the hMSCs adhesion to titanium surface along with cell morphology and proliferation.
Materials and methods
Synthesis and characterization of RGD peptide
The RGD peptide used in the present study refers to Gly-Arg-Gly-Asp-Ser-Lys (GRGDSK) peptide sequence. RGD peptide was synthesized manually on Fmoc-Rink amide 4-methylbenzhydrylamine (MBHA) resin as described previously [39, 40]. All the chemicals used for peptide synthesis were provided from AAPPTech (Louisville, KY, USA). Briefly, the Fmoc-protected amino acid (6 equiv), N,N′-di-isopropylcarbodiimide and hydroxybenzotriazole (12 equiv) were added into reaction tube where 100 mg of Rink amide MBHA resin was swelled in N,N-dimethylformamide (DMF) for 2 h before the reaction. Next, 0.2 ml of 0.05 M N,N-dimethylaminopyridine (DMAP) was added to the mixture and the coupling reaction was carried out for 4–6 h at 30 °C in an orbital shaker. The resin was tested for the presence of unreacted amines using the Kaiser reagent. Fmoc deprotections were performed with 20% Cyclohexylamine/DMF solutions for 15 min and every amino acid were coupled using the same method. Cleavage of peptides from the resin was performed using a mixture of 95% trifluoroacetic acid (TFA)/2.5% triisopropylsilane (TIPS)/2.5% water for 3 h. Rotary evaporator was used to remove TFA. Remaining viscous peptide solution was poured into cold ether and suspension was centrifuged, the supernatant was decanted, and the solid was allowed to dry in the desiccator. Next, peptide was dissolved in DI water and freeze dried. The RGD peptide was purified and characterized by preparative high-pressure liquid chromatography (HPLC, Agilent 1260 Quaternary LC) equipped with mass spectrometry (Agilent 6530 Q-TOF) with an electrospray ionization (ESI) source.
Surface modification of titanium discs
ISO 5832-2, grade 4 titanium discs (8 mm diameter, 2 mm thickness) were kindly donated by Titania Medical Devices (Izmir, Turkey). Surface modifications made by three different groups on control Ti discs, CAP-treated, RGD peptide-coated and CAP-treated + RGD peptide Ti discs.
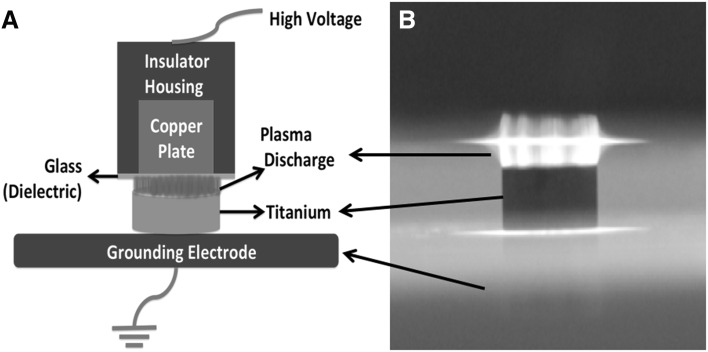
CAP treatment was performed as described previously [28]. A custom made, microsecond-pulsed, alternating current (AC) power supply (Advanced Plasma Solutions, Melvern, PA, USA) was utilized to generate cold, atmospheric pressure air dielectric barrier discharge (DBD) plasma. Titanium discs were placed on 1 mm thick glass slide, which is attached to a grounding electrode. A copper plate whose surface was covered with a 1 mm thick glass slide was used as high voltage electrode to form DBD set-up. The discharge gap in which CAP generated between the surface of high voltage electrode and surface of titanium disc was fixed to 2 mm. The microsecond AC power supply was operated at 1.5 kHz and 31.4 kV for various time points (Fig. 1).
Fig. 1.
A Schematic diagram and B image of plasma treatment of titanium discs
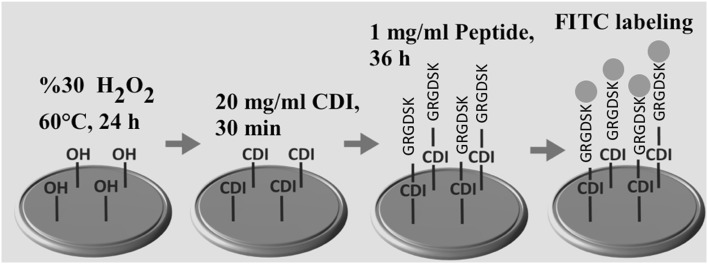
RGD peptide conjugation was carried out by using a previously described procedure [41]. Briefly, 1,1′-carbonyldiimidazole (CDI) (Sigma- Aldrich, St. Louis, MO, USA) was used for activation of Ti samples and for peptide conjugation [42], CAP-treated and untreated Ti discs were kept in the hydrogen peroxide solution (30%) at 60 °C for 24 h. Ti samples were washed with acetone for five times then incubated with CDI solution in acetone (20 mg/ml) for 30 min with gentle stirring. Discs were washed with acetone for five times to remove uncoupled CDI molecules. Synthesized peptide molecules were dissolved in 100 mM NaHCO3 at the concentration of 1 mg/ml. The reaction between CDI activated Ti surface and free amino group on lysine of RGD peptide was carried out for 36 h at room temperature with gentle shaking. Ti samples were sterilized via immersing in ethanol solution (70%) for 60 min and washed by sterile PBS for five times.
Surface characterization of titanium discs
Determination of the presence of RGD peptide on RGD peptide-coated Ti discs were characterized as performed in a previous study [43]. Fluorescein isothiocyanate (FITC) (Sigma Aldrich, St. Louis, MO, USA) was used to label RGD peptide-coated Ti discs. Fluorescently labelling reaction was performed in 5 mg/ml Dulbecco’s phosphate-buffered saline (PBS; Cellgro, Herndon, VA, USA) for 4 h at ambient conditions. FITC were reacted with the free amine group of the lysine on the peptide chain. Labeled peptides were immobilized to the Ti samples as the procedure mentioned before. Fluorescence microscopy (Olympus CKX41, Tokyo, Japan) was used to visualize immobilized FITC-peptide molecules on the titanium surfaces.
Effect of CAP treatment, RGD peptide coating and CAP + RGD application on Ti disc surface hydrophilicity was assessed by contact angle measurements. Contact angle measurements on Ti discs were performed thrice in triplicate using KSV Attension Theta goniometer (Biolin Scientific, Stockholm, Sweden). 4 μL droplet of distilled water was dripped over titanium discs. Contact angle was measured from right and left sides of the droplet over the titanium disc surface. Total 120 measurements were obtained from right and left sides of the droplet and the contact angle was calculated as the mean of all measurements by the system.
Atomic force microscopy (AFM) was used to investigate the surface topography of the surface modified Ti discs. Prior to AFM measurements, control, CAP, RGD, and CAP + RGD groups of Ti discs were dried at room temperature for 30 min. AFM measurements were performed at ambient conditions by using an instrument (Nanosurf Flex, Liestal, Switzerland) in tapping mode with a V-shaped cantilever (frequency = 164 kHz, L = 225 mm, W = 40 mm, tip radius = 10 nm, spring constant k = 0.2 N/m) for the images. Contact mode was used to capture topography images and a 10 × 10 µm2 area was analysed.
Surface potential on control, CAP, RGD, CAP + RGD discs were characterized using the Scanning Kelvin Probe technique on a Kelvin Probe AFM (NTEGRA Solaris, NT-MDT, Moscow, Russia). The measurements were performed for each disc on three random locations on each of the three replicates under dry conditions.
Cell attachment and proliferation analysis
Human bone marrow derived mesenchymal stem cells (hMSCs) (HMSC-AD-500, CLS cell lines Service, Lot#102, Eppelheim, Germany) were used in this study. hMSCs were routinely cultured in 75 cm2 culture flasks at 37 °C and 5% CO2 atmosphere with Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma Aldrich, St. Louis, Missouri, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, Steinheim, Germany), 1% l-glutamine (Genaxxon BioScience, Ulm, Germany), and 0.1% penicillin/streptomycin (Genaxxon BioScience, Ulm, Germany). Cells were kept in exponential phase and used at passage three.
hMSCs were seeded directly on the top of control, CAP, RGD and CAP + RGD group of discs at a density of 40,000 cells/cm2. Cell proliferation analysis was assessed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Invitrogen, Waltham, MA, USA) assay according to the manufacturer’s instructions, after 1, 4, and 7 days of incubation. Briefly, MTT solution was (5 mg/mL) added into culture medium (with 10% concentration) and incubated for 2 h at 37 °C. Next, the medium was replaced with 500 μL DMSO (Sigma Aldrich, St. Louis, MO, USA), and the optical density for each well was measured at 540 nm using a Synergy™ HTX Multi-Mode Microplate Reader (BioTek, Winooski, VT, USA). Measured absorbance values were correlated to the equivalent number of cells by using a calibration curve constructed with reference number of cells. Effect of different surface modifications on initial cell adhesion were analyzed 24 h after cell seeding by MTT assay and cell adhesion study.
DNA content of cell-seeded control, CAP, RGD, CAP + RGD discs were analysed at day 1, 4, and 7 using Quant-it PicoGreen assay (Invitrogen, Grand Island, NY, USA) according to manufacturer’s instructions as previously described [40, 44]. Briefly, 100 µl of cell lysate were mixed with 100 µl of working solution and incubated for 4 min at ambient conditions. DNA quantification was carried out using a plate reader with emission and excitation wavelengths of 485 and 528 nm, respectively. Measured fluorescence intensity values were correlated to the equivalent DNA content by using a calibration curve constructed with serial diluted known concentration of DNA content.
Cell adhesion was also characterized by immunofluorescent staining as previously described [40, 45]. Briefly, cell-seeded (40,000 cells/cm2) control, CAP, RGD, CAP + RGD discs were washed twice in PBS and fixed with 4% paraformaldehyde (Sigma- Aldrich, St. Louis, MO, USA) at 4 °C for 12 h. Then, 0.1% Triton X-100 and 100 mM glycine in PBS were treated for 1 h to permeabilize the cells for 1 h and blocked with 1.5% bovine serum albumin (BSA) and 0.5 mM glycine in PBS for 2 h. Next, discs were incubated with 0.16 μM Alexa Fluor® 594 phalloidin (Invitrogen, Waltham, MA, USA) and 300 nM DAPI in PBS for 30 min to stain actin filaments and nuclei of the cells, respectively. Fluorescence microscopy images of stained discs were taken at same exposure time and light intensity for each group. The percentage of surface area covered by adhered and spread cells on control, CAP, RGD, CAP + RGD discs at day 1 were analyzed by using the ImageJ software (NIH, Bethesda, MD, http://www.rsb.info.nih.gov/ij).
To investigate cell morphology, representative samples from each group at day 7 were fixed using 2.5% glutaraldehyde and 2% osmium tetroxide. Next, samples were dehydrated by incubating the Ti discs for 2 min in 20, 40, 60, 80, and 100% ethanol, sequentially. Dried samples were visualized with SEM at an accelerating voltage of 5 kV (FEI Quanta 250 FEG, FEI Inc., OR, USA).
Statistical analysis
All data were expressed as mean ± standard error and were statistically analysed by one-way ANOVA (SPSS 12.0, SPSS GmbH, Germany) and the Student- Newman-Keuls method as a post hoc test. Significant differences between groups were determined at p values at least less than 0.05. (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Characterization of surface modified Ti discs
The RGD peptide was covalently attached to Ti discs by the reaction between the free amino groups of the lysine residue of RGD peptide and CDI activated Ti surface, as shown in Fig. 2. To examine the presence of RGD peptide, surface modified Ti implants were analyzed by fluorescence microscopy. Fluorescence microscopy images of control, CAP, RGD coated, and CAP + RGD groups are shown in Fig. 3A–D, respectively. The representative fluorescence micrograph clearly showed that RGD and CAP + RGD coated groups showed intense fluorescent emission from FITC conjugation, while control and CAP applied groups showed no presence of FITC. Although the fluorescent microscopy images were taken at the same intensity for all groups, CAP + RGD Ti discs showed brighter surface compared to RGD coated Ti discs.
Fig. 2.
Schematic diagram of RGD peptide coating and FITC labeling on Ti discs
Fig. 3.

Fluorescence microscopy images of FITC labeled. A Control, B CAP, C RGD coated, and D CAP + RGD groups of titanium discs. Scale bar represents 100 µm
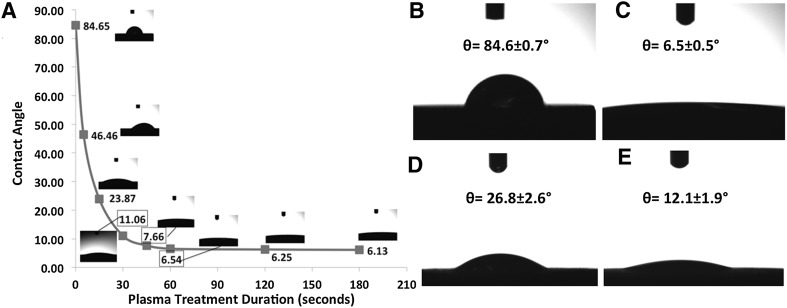
As depicted in Fig. 4A, hydrophilicity of titanium disc surfaces increased significantly with increasing plasma treatment duration. In detail, contact angle was determined as 84.6° for untreated control titanium discs (Fig. 4A, B) whereas 46.4°, 23.8°, 11.06°, 7.66° and 6.54° of contact angle values were determined for 5, 15, 30, 45 and 60 s of CAP respectively (Fig. 4A, C). The measured contact angles on titanium discs reached a plateau in consequence of plasma treatment duration more than 60 s and were determined around 6° for 120 and 180 s of CAP treatment time. Moreover, also RGD peptide coating improves hydrophilicity of titanium disc surfaces compared to control samples significantly. The contact angle on RGD peptide-coated Ti discs was measured as 26.8° (Fig. 4D). Furthermore, 60 s of plasma treatment of Ti disc prior to RGD peptide coating led an increase on the hydrophilicity of Ti disc surface compared to control and only RGD coated samples in which the contact angle on plasma treated-RGD coated titanium discs was determined as 12.1° (Fig. 4E).
Fig. 4.
A Plasma treatment time dependent contact angle values. Contact angle measurements of B control, C CAP (60 s treated), D RGD, and E CAP + RGD groups of titanium discs
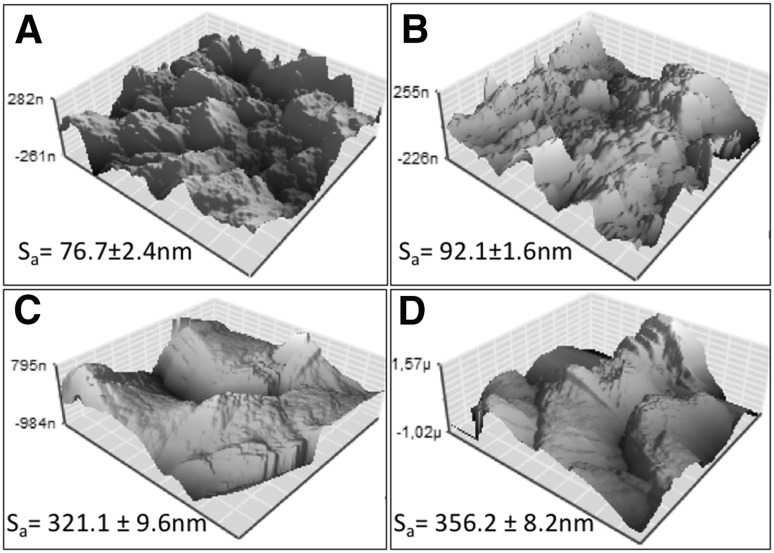
Roughness analysis of the surface modified Ti discs was conducted by using AFM (Fig. 5). For each surface modification, Ti (control), CAP treatment, RGD peptide coating and RGD coating following to CAP treatment Ti discs (areas of 10 µm × 10 µm) were subjected to analysis. The areal average surface roughness (Sa) of the Ti disc (control) was 76.7 ± 2.4 nm (Fig. 5A). After the treatment with CAP, the surfaces showed a small increase in roughness to 92.1 ± 1.6 nm (Fig. 5B). Moreover, direct and after CAP treatment RGD peptide coating on Ti surfaces and increased roughness to 321.1 ± 9.6 and 356.2 ± 8.2 nm, respectively (Fig. 5C, D).
Fig. 5.
Atomic force microscopy (AFM) images of A control, B CAP, C RGD coated, and D CAP + RGD groups of titanium discs
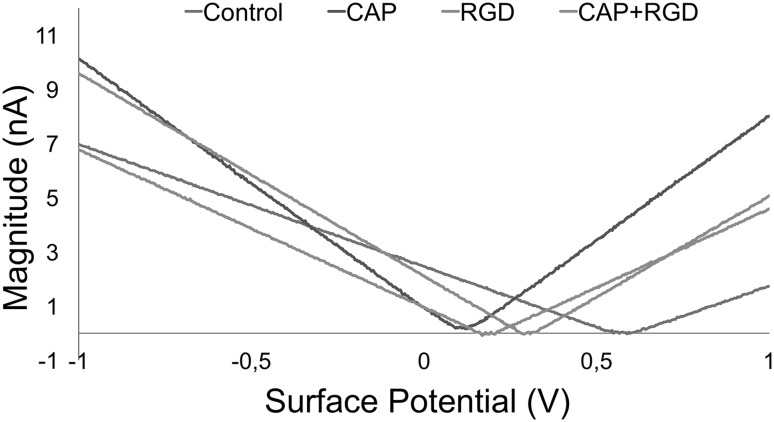
Surface potentials of titanium discs following CAP treatment, RGD conjugation and CAP + RGD conjugation were determined by measuring the local contact potential difference (CPD) between titanium disc surfaces and AFM conductive tip. As depicted in Fig. 6, the surface potential of control titanium discs without any surface modification was measured as 0.587 V whilst surface potentials for CAP treatment, RDG conjugation and CAP + RGD conjugation were determined as 0.115, 0.307 and 0.184 V, respectively.
Fig. 6.
Magnitude–surface potential curves of control (blue), CAP (purple), RGD (orange), and CAP + RGD (green)
Cell adhesion and proliferation on surface modified Ti discs
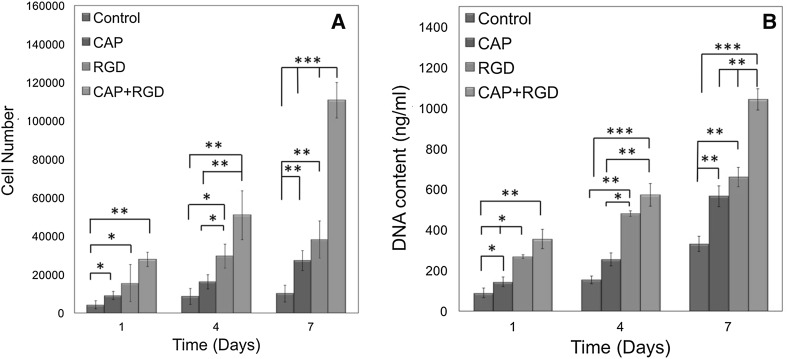
Cell proliferation on surface modified Ti discs was assessed by MTT assay. As shown in Fig. 7A, cell adhesion, which can be correlated with day 1 MTT results, were significantly higher on CAP, RGD and CAP + RGD compared to control group (p < 0.05). For instance, cell number after day 1 on CAP (9083 ± 1186), RGD (15,583 ± 4541), and CAP + RGD (27,917 ± 3767) groups was significantly higher compared to control (4250 ± 1287; p < 0.05). After day 4, cell number was the highest on the CAP + RGD (50,917 ± 12,719) discs followed by RGD (29,583 ± 6194), CAP (16,250 ± 3775) and control (8667 ± 2106) group. CAP + RGD (p < 0.01), RGD (p < 0.05), and CAP (p < 0.05) groups were significantly higher compared to control groups. Furthermore, there was also significant difference between CAP + RDG and CAP groups (p < 0.01). At day 7, cell numbers on CAP (27,250 ± 5131; p < 0.01), RGD (38,182 ± 9569; p < 0.01), and CAP + RGD (110,750 ± 9291; p < 0.001) groups were significantly higher compared to control discs (10,083 ± 4342). Additionally, CAP + RGD had significantly higher cell number compared to CAP and RGD groups (p < 0.001).
Fig. 7.
Results of the A MTT assay and B DNA content of hMSCs cultured on control, CAP, RGD, and CAP + RGD Ti surfaces after 1, 4, and 7 days of incubation. Error bars represent mean ± SE (n = 5) (significant differences were determined by one-way ANOVA [Newman–Keuls multiple comparison test, (*p < 0.05, **p < 0.01, ***p < 0.001)]
The DNA content of hMSCs seeded on control, CAP, RGD and CAP + RGD group of discs with respect to incubation time is shown in Fig. 6B. For all time points, total DNA content on the control group was significantly lower than CAP, RGD, and CAP + RGD groups. In detail, at day 1, the DNA content on CAP (p < 0.05), RGD (p < 0.01), and CAP + RGD (p < 0.001) groups were significantly higher of 1.6-, 2.9- and 3.5-fold compared to control group, respectively. At day 4, the DNA content on CAP + RGD were higher of 1.2-, 2.2-, and 3.6- fold compared to RGD, CAP (p < 0.01), and control groups (p < 0.001), respectively. Similar trend was observed at day 7 where DNA content on CAP + RGD was significantly higher of 1.6-, 1.8-, and 3.1- fold compared to RGD, CAP, and control groups (p < 0.001), respectively. The results of MTT assay and DNA content suggested that CAP treatment followed with RGD peptide coating showed improved surface properties for cell adhesion and proliferation.
Fluorescent micrographs representing morphology of hMSCs seeded on control, CAP, RGD, and CAP + RGD groups at day 1 were presented in Fig. 8A–D, respectively (blue for nuclei and red for cytoskeletal actin). The images indicated that each surface of the discs supported cellular adhesion and spreading. The fluorescent micrographs of hMSCs were analysed with ImageJ to determine the effect of surface modification on hMSCs adhesion. It was observed that percentage of the surface area covered by hMSCs increased with surface modifications of CAP, RGD, and CAP + RGD. In detail, surface area occupied by hMSCs covered 20.12 ± 2.2, 27.83 ± 5.7, 36.143 ± 7.5, and 47.35 ± 8.1% of total surface on control, CAP, RGD, and CAP + RGD groups, respectively.
Fig. 8.
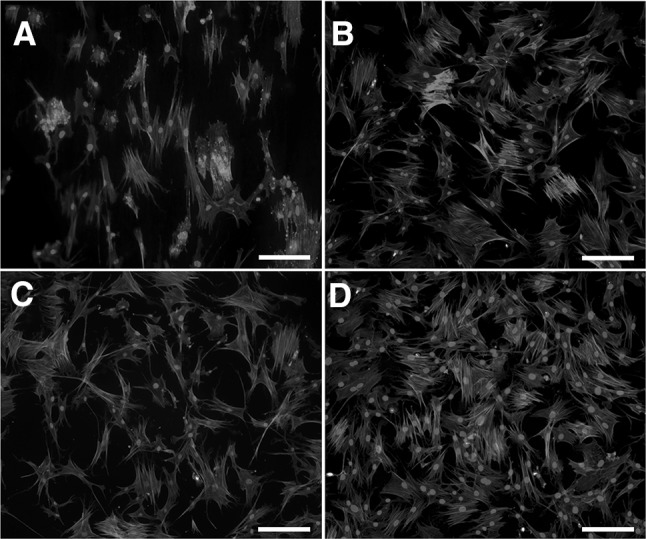
Morphologies of hMSCs seeded on A control, B CAP, C RGD, and D CAP + RGD groups after 1 day of culture. Scale bar represents 50 μm
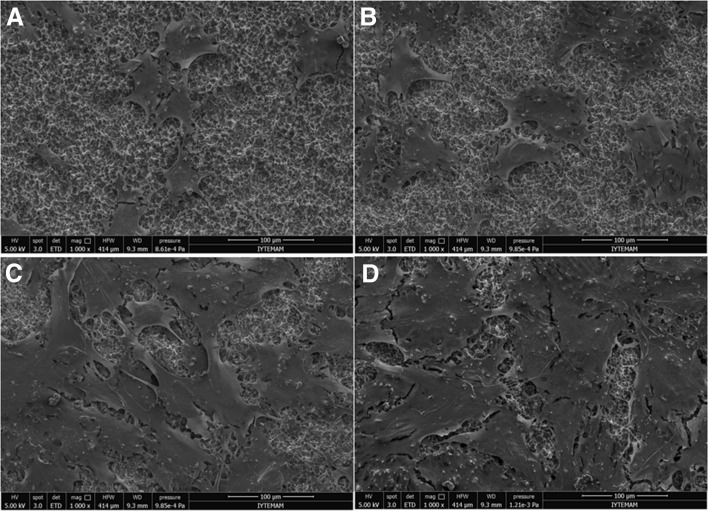
SEM images of control, CAP, RGD, and CAP + RGD groups after 7 days of cell culture were shown in Fig. 9A–D, respectively. Typical hMSCs morphology on the surface of all discs with different spreading capacity was observed. Furthermore, RGD coating not only increased spreading area of hMSCs, but also increased the number of cells.
Fig. 9.
SEM micrographs of hMSCs cultured for 7 days on the surface of A control, B CAP, C RGD, and D CAP + RGD. Scale bar represents 100 µm
Discussion
Developing ideal implant surface induces improved cellular interactions which directly affects the osseointegration process of the dental implants. Implant surface is modified to achieve high bioactivity with optimal biological responses of surrounding osteogenic cell environment [10]. Due to the close relationship between implant surface properties and osseointegration time and quality, different surface modification techniques including ultraviolet (UV) light, laser, plasma treatments and RGD coating have been utilized to alter surface properties of Ti implants in order to achieve enhanced cellular attachment on implant for accelerated healing process [11, 46–48]. The ultimate goal of such surface modifications is to convert osteoconductive surface of dental implants to bioactive surface that could accelerate the bone tissue formation. In the present study, we modified the surface of Ti discs via CAP treatment, RGD peptide coating and CAP treatment followed with RGD peptide coating, and evaluated the in vitro hMSCs adhesion and proliferation.
Ti discs coated with the FITC labeled peptide were detected by fluorescence microscopy to confirm the successful coating of RGD peptide. Micrographs of control and CAP-treated discs were not revealed any fluorescence signal while RGD and CAP + RGD groups demonstrated intense fluorescent emission, which proves RGD peptide completely coated on the surface (Fig. 3). Effect of CAP treatment for increased RGD peptide conjugation on titanium discs were clearly manifested by micrographs obtained following FITC labeling. As depicted in Fig. 3, CAP treatment prior to RGD coating clearly caused increased fluorescence intensity compared to only RGD coated group. Such result might rise in consequence of increased hydroxyl groups after CAP treatment that initiate RGD peptide coating. Similarly, Seo et al. [43] reported increased fluorescent intensity after FITC labeled RGD peptide coating on Ti samples.
Plasma is as an established method for the cleaning of titanium materials to be used medically [49]. Also, plasma treatment increases surface hydrophilicity via affecting oxide layer which interacts with proteins that play role on cell attachment [28, 50, 51]. Increased hydrophilicity of titanium dental implant surfaces improves adhesion of cells and proteins to the surface of implant and thus accelerates healing [47, 50, 52]. Similarly, in the present study, we also observed substantial increase in hydrophilicity of Ti discs. In detail, the contact angle was decreased exponentially with respect to increasing CAP treatment duration up to 60 s (Fig. 4). However, further decrease of contact angle was not observed in consequence of extended treatment durations which could be attributed to achievement of completion of chemical interactions in between Ti disc surface and plasma discharge in 60 s. It could be deduced that 60 s of CAP treatment exposed the maximum number of hydroxyl groups on the surface of the Ti discs. Moreover, 60 s of CAP treatment resulted as 6 degrees of contact angle which is considered as an indicator of superhydrophilic surface that strongly enhances protein adsorption and cell attachment. Plasma is the fourth state of matter and a complex medium that contains free electrons, free radicals, reactive oxygen (ROS) and reactive nitrogen species (RNS) along with UV light [53]. In addition to role of hydroxyl groups for reduced contact angle, UV light generated during formation of plasma discharge may also show a synergistic effect on increased hydrophilicity [28].
RGD peptide coating also boosted the hydrophilicity of the Ti discs. The contact angle was measured as 26° for RGD group whereas CAP + RGD resulted in a further decrease on contact angle to 12°. The reduction of contact angle could be related with the increased RGD peptide coating on the Ti discs. Such result also indicates that plasma treatment caused augmented interaction with RGD peptide and Ti surface. The decrease of the contact angle after RGD peptide-coated on the pre-CAP-treated Ti discs could be correlated with the hydrophilicity of six amino acid containing RGD peptide. Consistently, we have observed superior hMSCs adhesion and proliferation on CAP + RGD groups compared to only CAP and only RGD group. Thus, in the present study we reported enhanced conjugation of RGD peptide to Ti surface (which increases cellular viability and attachment) in consequence of plasma treatment rather than increased cellular attachment via only plasma treatment as opposed to literature [40]. In conclusion in the present study plasma treatment is defined not only as a method for enhancing hydrophilicity and cellular attachment but also an intermediate step for increasing RGD peptide conjugation to improve cellular attachment and viability. Despite various studies reporting no remarkable improvement of cellular attachment to surfaces with contact angle values less than 60°, there are also reports stating improved cellular attachment in consequence of decreasing contact angle below 60°. Increased contact angle of metallic implants was correlated to enhanced cell adhesion, proliferation differentiation and mineralization particularly on titanium implants [22, 54]. Positive influence of the superhydrophilicity on enhanced cellular attachment was attributed to increased interaction of blood components with implant surface which is needed for protein adhesion on implant—a crucial process for cellular attachment [55–57].
AFM images of the surface topography of control, CAP, RGD, and CAP + RGD are shown in Fig. 5A–D, respectively. It was demonstrated that CAP treatment and RGD peptide coating increased the surface roughness (Sa) of Ti discs. It was also observed that the surface roughness increased at same rate for control to RGD and CAP to CAP + RGD groups which could be considered as indication of an efficient RGD peptide coating on RGD and CAP + RGD group. Kook et al. and Sechi et al. also demonstrated that RGD peptide coating on Ti surfaces resulted with greater surface roughness [43, 58].
Cell adhesion, spreading and proliferation are directly related with the surface properties of biomaterials including roughness and wettability [59]. Huang et al. [60] demonstrated that increased surface roughness on Ti surfaces significantly enhanced osteoblast-like U2 OS cells. Cell adhesion process on biomaterials starts with adsorption of serum proteins on the substrate. Protein adsorption directly influences the biocompatibility of the Ti surfaces and increases the cell adhesion [61]. In addition, rough surfaces also provide higher surface area which increases the interaction between Ti surface and integrin binding points of the cells [62, 63]. Previous studies compared the effect of different surface roughness values on cell adhesion. For instance, Mustafa et al. investigated the effect of different surface roughness of titanium surface on cellular adhesion. It was observed that cellular adhesion significantly increased when Ti surface roughness (Sa) increased from 0.2 to 1.3 µm. However, further increase in surface roughness did not influence cell adhesion [64]. However, in another study, Ponsennet et al. [65] stated that cell proliferation was negatively affected when surface roughness of Ti surface reached over 1 µm of Sa value. Therefore, it could be speculated that optimal Sa of Ti surfaces should be lower than 1 µm in terms of both cellular adhesion and proliferation. In our study, surface modifications of CAP treatment, RGD and CAP + RGD coating did not exceed the Sa value over the reported suitable range. Peptide coating on Ti surfaces also affects the roughness. For instance, Mante et al. demonstrated that RGD coating on Ti discs significantly increased the surface roughness. It was also noted that higher quantity of the RGD peptide attachment obtained when coating conducted on rougher surfaces because of the increased surface area for attachment [66]. It is consistent with our findings of increased peptide coating after CAP treatment of Ti discs, where surface roughness was enhanced. Similar to our findings, Secchi et al. [67] reported increased roughness after RGD peptide coating on Ti alloy surface.
Plasma treatment might cause chemical and physical modifications on various surfaces such as etching [68]. Increase of wettability is closely related with increased roughness [69]. However, plasma related increased wettability doesn’t necessarily show direct correlation with roughness. Our results indicate that, in the present study chemical effects of CAP treatment seems dominant over physical effects of plasma such as etching. Similarly, Ito et al. [70] has reported increased wettability with no remarkable influence on surface roughness following CAP treatment. In another study, it was obtained that RGD peptide coating decreased the contact angle of neat Ti alloy surfaces without changing the roughness [67]. Although slight increase observed in contact angle values on RGD and CAP + RGD groups, cell adhesion and proliferation were significantly higher compared to CAP and control groups. Similar to our findings, slightly increased contact angle values after chemically coating of RGD peptide was obtained previously [67]. It is considered that for the RGD and CAP + RGD groups, RGD peptide coating was the major factor on enhanced cellular adhesion compared to surface roughness and wettability.
Surface potential of titanium discs was decreased in CAP-treated, RGD coated and CAP + RGD groups. Previous studies have correlated decreased surface potential with increased negative charge on the surface which could be considered as a sign of increased number of hydroxyl groups on the surface of titanium discs treated with CAP, coated with RGD and their combination [71]. Such findings show consistency with cellular adhesion results as shown in Fig. 8, which could be the result of increased RGD conjugation on titanium discs via increased hydroxyl groups.
Cell proliferation and total DNA content were obtained at 1, 4 and 7 days after hMSCs seeded on the surface of control, CAP, RGD, and CAP + RGD groups (Fig. 7A, B). Cell numbers depicted from MTT study showed consistency with DNA content results at corresponding time points of the cell culture. It was shown that enhanced RGD coating by CAP treatment led increased cell adhesion which could be observed from day 1 MTT and DNA content study. Such results could be linked to increased surface roughness and biomimetic peptide coating on the Ti surface. It was previously reported that surface roughness is an important parameter that influences cellular adhesion and the rougher Ti surfaces enables better cell adhesion [64]. Similar to our result, Secchi et al. observed enhanced osteoblast adhesion on RGD peptide-coated Ti implants compared to neat Ti surface [58]. Moreover, cell proliferation and DNA content assays showed that the fastest cell proliferation after 7 days of hMSCs culture was seen on the CAP + RGD group. These data support the hypothesis that the enhanced RGD peptide coating via intermediate plasma treatment led increased hMSCs proliferation.
Fluorescent micrographs of hMSCs after 1 day of culture on control, CAP, RGD, and CAP + RGD groups were presented in Fig. 8. The micrographs were analyzed and surface coverage percentage were calculated by ImageJ. These results revealed that RGD peptide coating on Ti discs enhanced the adhesion percentage of hMSCs. Furthermore, improved RGD coating by CAP treatment also enhanced cellular adhesion compared to RGD group. Similar to our findings, Vidal et al. [72] demonstrated that Ti surface functionalization with RGD peptide showed significantly increased fibroblast adhesion.
SEM images of hMSCs morphology after 7 days culturing on control, CAP, RGD, and CAP + RGD groups also demonstrated higher cellular proliferation on CAP + RGD group (Fig. 9). Moreover, almost complete coverage of the surface by hMSCs was observed on the CAP + RGD group. In line with previous studies which also confirm that cell–matrix interactions are crucial in order to provide enhanced cell proliferation as well as osseointegration, the current investigation demonstrates increased cell adhesion and proliferation when RGD peptide coating enhanced by CAP treatment. Consequently, these results suggest that enhanced coating of RGD peptide to Ti discs caused increased cellular adhesion and proliferation due to increased cell matrix interaction.
In this study, synergistic effect of CAP treatment and RGD peptide coating on cellular adhesion and proliferation over Ti surfaces was investigated. Fluorescence microscopy and AFM images confirmed RGD peptide coating on Ti discs. The in vitro biological assay and SEM images of hMSCs showed that RGD peptide coating to the CAP-treated Ti discs significantly increased on cell adhesion and proliferation. Consequently, herein described data clearly underlines the benefit of increased RGD peptide coating via CAP treatment on cell adhesion and proliferation. Improved surface characteristics of Ti discs via CAP treatment and RGD coating might lead development of novel dental implants that could enhance osseointegration and be a future possible clinical practice. However, further studies including in vivo tests are needed for better understanding of surface cell interactions that will cause enhanced osseointegration. Thus, CAP treatment following with RGD peptide coating could potentially be a promising surface modification technique for dental implant surface modifications.
Acknowledgements
Authors acknowledge funding from TÜBİTAK (The Scientific and Technological Research Council of Turkey) through the Research Project 214M268 and BAP (Scientific Research Projects Fund of Izmir Katip Çelebi University) through the Research Projects 2015-TDR-SABE-0012 and 2016-ONP-MUMF-0022. Authors also acknowledge Dr. Mustafa Can, Eyup Yalçın and Dr. Nesrin Horzum Polat (Department of Engineering Sciences, Izmir Katip Çelebi University) for their assistance on AFM and contact angle measurements, respectively. Finally authors would like to acknowledge Titania Medical Devices (Izmir, Turkey) and Bonegraft Biological Materials A.Ş. (Izmir, Turkey) for providing the Ti discs and DNA quantification kit, respectively.
Conflict of interest
Authors have declared that there is no conflict of interest. The authors alone are responsible for the content and writing of this article.
Ethical approval
There are no animal experiments carried out for this article.
Contributor Information
Ozan Karaman, Phone: +90 (232) 329-3535/3765, Email: ozan.karaman@ikc.edu.tr.
Utku Kürşat Ercan, Phone: +90 (232) 329-3535/3747, Email: utkuk.ercan@ikc.edu.tr.
References
- 1.Raphel J, Karlsson J, Galli S, Wennerberg A, Lindsay C, Haugh MG, et al. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials. 2016;83:269–282. doi: 10.1016/j.biomaterials.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafar MS, Khurshid Z, Almas K. Oral tissue engineering progress and challenges. Tissue Eng Regen Med. 2015;12:387–397. doi: 10.1007/s13770-015-0030-6. [DOI] [Google Scholar]
- 3.Schwartz-Arad D, Kidron N, Dolev E. A long-term study of implants supporting overdentures as a model for implant success. J Periodontol. 2005;76:1431–1435. doi: 10.1902/jop.2005.76.9.1431. [DOI] [PubMed] [Google Scholar]
- 4.Karoussis IK, Brägger U, Salvi GE, Bürgin W, Lang NP. Effect of implant design on survival and success rates of titanium oral implants: a 10-year prospective cohort study of the ITI dental implant system. Clin Oral Implants Res. 2004;15:8–17. doi: 10.1111/j.1600-0501.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 5.Geetha M, Singh AK, Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Prog Mater Sci. 2009;54:397–425. doi: 10.1016/j.pmatsci.2008.06.004. [DOI] [Google Scholar]
- 6.Schliephake H, Scharnweber D. Chemical and biological functionalization of titanium for dental implants. J Mater Chem. 2008;18:2404–2414. doi: 10.1039/b715355b. [DOI] [Google Scholar]
- 7.Goodman SB, Yao Z, Keeney M, Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34:3174–3183. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brånemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3:81–100. doi: 10.3109/02844316909036699. [DOI] [PubMed] [Google Scholar]
- 9.Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 10.Klein MO, Bijelic A, Ziebart T, Koch F, Kämmerer PW, Wieland M, et al. Submicron scale-structured hydrophilic titanium surfaces promote early osteogenic gene response for cell adhesion and cell differentiation. Clin Implant Dent Relat Res. 2013;15:166–175. doi: 10.1111/j.1708-8208.2011.00339.x. [DOI] [PubMed] [Google Scholar]
- 11.Le Guehennec L, Lopez-Heredia MA, Enkel B, Weiss P, Amouriq Y, Layrolle P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008;4:535–543. doi: 10.1016/j.actbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Puleo D, Nanci A. Understanding and controlling the bone–implant interface. Biomaterials. 1999;20:2311–2321. doi: 10.1016/S0142-9612(99)00160-X. [DOI] [PubMed] [Google Scholar]
- 13.Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–533. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- 14.Buser D. Titanium for dental applications (II): implants with roughened surfaces. In: Brunette DM, Tengvall P, Textor M, Thomsen P, editors. Titanium in medicine. Berlin: Springer; 2001. p. 875–88.
- 15.Boyan BD, Dean DD, Lohmann CH, Cochran DL, Sylvia VL, Schwartz Z. The titanium-bone cell interface in vitro: the role of the surface in promoting osteointegration. In: Brunette DM, Tengvall P, Textor M, Thomsen P, editors. Titanium in medicine. Berlin: Springer; 2001. p. 561–85.
- 16.Boyan BD, Lohmann CH, Dean DD, Sylvia VL, Cochran DL, Schwartz Z. Mechanisms involved in osteoblast response to implant surface morphology. Annu Rev Mater Res. 2001;31:357–371. doi: 10.1146/annurev.matsci.31.1.357. [DOI] [Google Scholar]
- 17.Cochran DL, Buser D, Ten Bruggenkate CM, Weingart D, Taylor TM, Bernard JP, et al. The use of reduced healing times on ITI® implants with a sandblasted and acid-etched (SLA) surface. Clin Oral Implants Res. 2002;13:144–153. doi: 10.1034/j.1600-0501.2002.130204.x. [DOI] [PubMed] [Google Scholar]
- 18.Roccuzzo M, Bunino M, Prioglio F, Bianchi SD. Early loading of sandblasted and acid-etched (SLA) implants: a prospective split-mouth comparative study. Clin Oral Implants Res. 2001;12:572–578. doi: 10.1034/j.1600-0501.2001.120604.x. [DOI] [PubMed] [Google Scholar]
- 19.Rupp F, Scheideler L, Eichler M, Geis-Gerstorfer J. Wetting behavior of dental implants. Int J Oral Maxillofac Implants. 2011;26:1256–1266. [PubMed] [Google Scholar]
- 20.Hirakawa Y, Jimbo R, Shibata Y, Watanabe I, Wennerberg A, Sawase T. Accelerated bone formation on photo-induced hydrophilic titanium implants: an experimental study in the dog mandible. Clin Oral Implants Res. 2013;24:139–144. doi: 10.1111/j.1600-0501.2011.02401.x. [DOI] [PubMed] [Google Scholar]
- 21.Lang NP, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Bosshardt DD. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res. 2011;22:349–356. doi: 10.1111/j.1600-0501.2011.02172.x. [DOI] [PubMed] [Google Scholar]
- 22.Bornstein MM, Valderrama P, Jones AA, Wilson TG, Seibl R, Cochran DL. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: a histomorphometric study in canine mandibles. Clin Oral Implants Res. 2008;19:233–241. doi: 10.1111/j.1600-0501.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson SJ, Broggini N, Wieland M, de Wild M, Rupp F, Geis-Gerstorfer J, et al. Biomechanical evaluation of the interfacial strength of a chemically modified sandblasted and acid-etched titanium surface. J Biomed Mater Res A. 2006;78:291–297. doi: 10.1002/jbm.a.30678. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan R, Kim SY, Kwon TY, Kim KH. Nanocrystalline hydroxyapatite coatings from ultrasonated electrolyte: preparation, characterization, and osteoblast responses. J Biomed Mater Res A. 2008;87:1053–1060. doi: 10.1002/jbm.a.31852. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi M, Abe Y, Yoshida Y, Nakayama Y, Okazaki M, Akagawa Y. Acid pretreatment of titanium implants. Biomaterials. 2003;24:1821–1827. doi: 10.1016/S0142-9612(02)00576-8. [DOI] [PubMed] [Google Scholar]
- 26.Kim MH, Lee SY, Kim MJ, Kim SK, Heo SJ, Koak JY. Effect of biomimetic deposition on anodized titanium surfaces. J Dent Res. 2011;90:711–716. doi: 10.1177/0022034511400074. [DOI] [PubMed] [Google Scholar]
- 27.Tavares MG, de Oliveira PT, Nanci A, Hawthorne AC, Rosa AL, Xavier SP. Treatment of a commercial, machined surface titanium implant with H2SO4/H2O2 enhances contact osteogenesis. Clin Oral Implants Res. 2007;18:452–458. doi: 10.1111/j.1600-0501.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- 28.Ibis F, Oflaz H, Ercan UK. Biofilm Inactivation and prevention on common implant material surfaces by nonthermal DBD plasma treatment. Plasma Med. 2016;6:33–45. doi: 10.1615/PlasmaMed.2016015846. [DOI] [Google Scholar]
- 29.Shohet JL. Plasma-aided manufacturing. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 1991;19:725–733. doi: 10.1109/27.108405. [DOI] [Google Scholar]
- 30.Germanier Y, Tosatti S, Broggini N, Textor M, Buser D. Enhanced bone apposition around biofunctionalized sandblasted and acid-etched titanium implant surfaces. Clin Oral Implants Res. 2006;17:251–257. doi: 10.1111/j.1600-0501.2005.01222.x. [DOI] [PubMed] [Google Scholar]
- 31.Ritts AC, Li H, Yu Q, Xu C, Yao X, Hong L, et al. Dentin surface treatment using a non-thermal argon plasma brush for interfacial bonding improvement in composite restoration. Eur J Oral Sci. 2010;118:510–516. doi: 10.1111/j.1600-0722.2010.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezania A, Healy KE. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog. 1999;15:19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 33.Smeets R, Stadlinger B, Schwarz F, Beck-Broichsitter B, Jung O, Precht C, et al. Impact of dental implant surface modifications on osseointegration. Biomed Res Int. 2016;2016:6285620. doi: 10.1155/2016/6285620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porté-Durrieu MC, Labrugère C, Villars F, Lefebvre F, Dutoya S, Guette A, et al. Development of RGD peptides grafted onto silica surfaces: XPS characterization and human endothelial cell interactions. J Biomed Mater Res. 1999;46:368–375. doi: 10.1002/(SICI)1097-4636(19990905)46:3<368::AID-JBM9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Olivieri MP, Tweden KS. Human serum albumin and fibrinogen interactions with an adsorbed RGD-containing peptide. J Biomed Mater Res. 1999;46:355–359. doi: 10.1002/(SICI)1097-4636(19990905)46:3<355::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 36.Schliephake H, Scharnweber D, Dard M, Rössler S, Sewing A, Meyer J, et al. Effect of RGD peptide coating of titanium implants on periimplant bone formation in the alveolar crest. An experimental pilot study in dogs. Clin Oral Implants Res. 2002;13:312–319. doi: 10.1034/j.1600-0501.2002.130312.x. [DOI] [PubMed] [Google Scholar]
- 37.Park SY, Kim HS, Kim JH, Shim JH, Yun MJ, Jeon YC, et al. Effects of anodized titanium implant coated with RGD peptides via chemical fixation on osseointegration and bone regeneration. Tissue Eng Regen Med. 2012;9:194–202. doi: 10.1007/s13770-012-0340-x. [DOI] [Google Scholar]
- 38.Yang GL, He FM, Yang XF, Wang XX, Zhao SF. In vivo evaluation of bone-bonding ability of RGD-coated porous implant using layer-by-layer electrostatic self-assembly. J Biomed Mater Res A. 2009;90:175–185. doi: 10.1002/jbm.a.32055. [DOI] [PubMed] [Google Scholar]
- 39.He X, Jabbari E. Solid-phase synthesis of reactive peptide crosslinker by selective deprotection. Protein Pept Lett. 2006;13:715–718. doi: 10.2174/092986606777790610. [DOI] [PubMed] [Google Scholar]
- 40.Karaman O, Kumar A, Moeinzadeh S, He X, Cui T, Jabbari E. Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J Tissue Eng Regen Med. 2016;10:E132–E146. doi: 10.1002/term.1775. [DOI] [PubMed] [Google Scholar]
- 41.Zhao BH, Tian WM, Feng HL, Lee IS, Cui FZ. Effects of RGD peptide grafting to titanium dental implants on the adhesion of human gingival fibroblasts and epithelial cells. Curr Appl Phys. 2005;5:407–410. doi: 10.1016/j.cap.2005.01.002. [DOI] [Google Scholar]
- 42.Hearn MT. 1, 1′-Carbonyldiimidazole-mediated immobilization of enzymes and affinity ligands. Methods Enzymol. 1987;135:102–117. doi: 10.1016/0076-6879(87)35068-2. [DOI] [PubMed] [Google Scholar]
- 43.Seo HS, Ko YM, Shim JW, Lim YK, Kook JK, Cho DL, et al. Characterization of bioactive RGD peptide immobilized onto poly(acrylic acid) thin films by plasma polymerization. Appl Surf Sci. 2010;257:596–602. doi: 10.1016/j.apsusc.2010.07.040. [DOI] [Google Scholar]
- 44.Karimi T, Barati D, Karaman O, Moeinzadeh S, Jabbari E. A developmentally inspired combined mechanical and biochemical signaling approach on zonal lineage commitment of mesenchymal stem cells in articular cartilage regeneration. Integr Biol (Camb) 2015;7:112–127. doi: 10.1039/C4IB00197D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He X, Ma J, Jabbari E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir. 2008;24:12508–12516. doi: 10.1021/la802447v. [DOI] [PubMed] [Google Scholar]
- 46.Choi SH, Jeong WS, Cha JY, Lee JH, Yu HS, Choi EH, et al. Time-dependent effects of ultraviolet and nonthermal atmospheric pressure plasma on the biological activity of titanium. Sci Rep. 2016;6:33421. doi: 10.1038/srep33421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akkan CK, Hür D, Uzun L, Garipcan B. Amino acid conjugated self assembling molecules for enhancing surface wettability of fiber laser treated titanium surfaces. Appl Surf Sci. 2016;366:284–291. doi: 10.1016/j.apsusc.2016.01.083. [DOI] [Google Scholar]
- 48.Guastaldi FP, Yoo D, Marin C, Jimbo R, Tovar N, Zanetta-Barbosa D, et al. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int J Biomater. 2013;2013:354125. doi: 10.1155/2013/354125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz J, Gershman S, Belkind A. Optical emission spectroscopy and contact angle study of plasma cleaning of titanium alloy surfaces: argon plasma. Plasma Med. 2015;5:223–236. doi: 10.1615/PlasmaMed.2016015722. [DOI] [Google Scholar]
- 50.Duske K, Koban I, Kindel E, Schröder K, Nebe B, Holtfreter B, et al. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J Clin Periodontol. 2012;39:400–407. doi: 10.1111/j.1600-051X.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- 51.Lee EJ, Kwon JS, Uhm SH, Kim KM, Kim KN, Kim YH, et al., editors. Effect of non-thermal atmospheric pressure plasma jet on hydrophilicity and cellular activity of SLA-treated titanium surface. In: 2012 Abstracts IEEE international conference on plasma science, 2012 8–13 July; 2012.
- 52.Horbett TA, Waldburger JJ, Ratner BD, Hoffman AS. Cell adhesion to a series of hydrophilic-hydrophobic copolymers studied with a spinning disc apparatus. J Biomed Mater Res. 1988;22:383–404. doi: 10.1002/jbm.820220503. [DOI] [PubMed] [Google Scholar]
- 53.Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied plasma medicine. Plasma Process Polym. 2008;5:503–533. doi: 10.1002/ppap.200700154. [DOI] [Google Scholar]
- 54.Jia S, Zhang Y, Ma T, Chen H, Lin Y. Enhanced hydrophilicity and protein adsorption of titanium surface by sodium bicarbonate solution. J Nanomater. 2015;2015:536801. [Google Scholar]
- 55.Milleret V, Tugulu S, Schlottig F, Hall H. Alkali treatment of microrough titanium surfaces affects macrophage/monocyte adhesion, platelet activation and architecture of blood clot formation. Eur Cell Mater. 2011;21:430–444. doi: 10.22203/eCM.v021a32. [DOI] [PubMed] [Google Scholar]
- 56.Tugulu S, Löwe K, Scharnweber D, Schlottig F. Preparation of superhydrophilic microrough titanium implant surfaces by alkali treatment. J Mater Sci Mater Med. 2010;21:2751–2763. doi: 10.1007/s10856-010-4138-x. [DOI] [PubMed] [Google Scholar]
- 57.Wei J, Igarashi T, Okumori N, Igarashi T, Maetani T, Liu B, et al. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed Mater. 2009;4:045002. doi: 10.1088/1748-6041/4/4/045002. [DOI] [PubMed] [Google Scholar]
- 58.Secchi AG, Grigoriou V, Shapiro IM, Cavalcanti-Adam EA, Composto RJ, Ducheyne P, et al. RGDS peptides immobilized on titanium alloy stimulate bone cell attachment, differentiation and confer resistance to apoptosis. J Biomed Mater Res A. 2007;83:577–584. doi: 10.1002/jbm.a.31007. [DOI] [PubMed] [Google Scholar]
- 59.Jemat A, Ghazali MJ, Razali M, Otsuka Y. Surface modifications and their effects on titanium dental implants. Biomed Res Int. 2015;2015:2015–791725. doi: 10.1155/2015/791725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang HH, Ho CT, Lee TH, Lee TL, Liao KK, Chen FL. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol Eng. 2004;21:93–97. doi: 10.1016/j.bioeng.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Deligianni DD, Katsala N, Ladas S, Sotiropoulou D, Amedee J, Missirlis Y. Effect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22:1241–1251. doi: 10.1016/S0142-9612(00)00274-X. [DOI] [PubMed] [Google Scholar]
- 62.Khalili AA, Ahmad MR. A review of cell adhesion studies for biomedical and biological applications. Int J Mol Sci. 2015;16:18149–18184. doi: 10.3390/ijms160818149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosales-Leal J, Rodríguez-Valverde M, Mazzaglia G, Ramón-Torregrosa P, Díaz-Rodríguez L, García-Martínez O, et al. Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion. Colloids Surf A Physicochem Eng Asp. 2010;365:222–229. doi: 10.1016/j.colsurfa.2009.12.017. [DOI] [Google Scholar]
- 64.Mustafa K, Wroblewski J, Lopez BS, Wennerberg A, Hultenby K, Arvidson K. Determining optimal surface roughness of TiO2 blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin Oral Implants Res. 2001;12:515–525. doi: 10.1034/j.1600-0501.2001.120513.x. [DOI] [PubMed] [Google Scholar]
- 65.Ponsonnet L, Reybier K, Jaffrezic N, Comte V, Lagneau C, Lissac M, et al. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater Sci Eng C Mater Biol Appl. 2003;23:551–560. doi: 10.1016/S0928-4931(03)00033-X. [DOI] [Google Scholar]
- 66.Mante FK, Little K, Mante MO, Rawle C, Baran GR. Oxidation of titanium, RGD peptide attachment, and matrix mineralization of rat bone marrow stromal cells. J Oral Implantol. 2004;30:343–349. doi: 10.1563/0.667.1. [DOI] [PubMed] [Google Scholar]
- 67.Secchi AG, Grigoriou V, Shapiro IM, Cavalcanti-Adam EA, Composto RJ, Ducheyne P, et al. RGDS peptides immobilized on titanium alloy stimulate bone cell attachment, differentiation and confer resistance to apoptosis. J Biomed Mater Res A. 2007;83:577–584. doi: 10.1002/jbm.a.31007. [DOI] [PubMed] [Google Scholar]
- 68.Puliyalil H, Cvelbar U. Selective plasma etching of polymeric substrates for advanced applications. Nanomaterials (Basel) 2016;6:E108. doi: 10.3390/nano6060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naresh Kumar N, Yap SL, Khan MZ, Pattela Srinivasa RS. Effect of argon plasma treatment on tribological properties of UHMWPE/MWCNT nanocomposites. Polymers. 2016;8:295. doi: 10.3390/polym8080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito Y, Okawa T, Fujii T, Tanaka M. Influence of plasma treatment on surface properties of zirconia. J Osaka Dent Univ. 2016;50:79–84. [Google Scholar]
- 71.Vladescu A, Titorencu I, Dekhtyar Y, Jinga V, Pruna V, Balaceanu M, et al. In vitro biocompatibility of Si alloyed multi-principal element carbide coatings. PLoS one. 2016;11:e0161151. doi: 10.1371/journal.pone.0161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vidal G, Blanchi T, Mieszawska AJ, Calabrese R, Rossi C, Vigneron P, et al. Enhanced cellular adhesion on titanium by silk functionalized with titanium binding and RGD peptides. Acta Biomater. 2013;9:4935–4943. doi: 10.1016/j.actbio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]