Abstract
Novel hydrogel composed of both chondroitin sulfate (CS) and gelatin was developed for better cellular interaction through two step double crosslinking of N-(3-diethylpropyl)-N-ethylcarbodiimide hydrochloride (EDC) chemistries and then click chemistry. EDC chemistry was proceeded during grafting of amino acid dihydrazide (ADH) to carboxylic groups in CS and gelatin network in separate reactions, thus obtaining CS–ADH and gelatin–ADH, respectively. CS–acrylate and gelatin–TCEP was obtained through a second EDC chemistry of the unreacted free amines of CS–ADH and gelatin–ADH with acrylic acid and tri(carboxyethyl)phosphine (TCEP), respectively. In situ CS–gelatin hydrogel was obtained via click chemistry by simple mixing of aqueous solutions of both CS–acrylate and gelatin–TCEP. ATR-FTIR spectroscopy showed formation of the new chemical bonds between CS and gelatin in CS–gelatin hydrogel network. SEM demonstrated microporous structure of the hydrogel. Within serial precursor concentrations of the CS–gelatin hydrogels studied, they showed trends of the reaction rates of gelation, where the higher concentration, the quicker the gelation occurred. In vitro studies, including assessment of cell viability (live and dead assay), cytotoxicity, biocompatibility via direct contacts of the hydrogels with cells, as well as measurement of inflammatory responses, showed their excellent biocompatibility. Eventually, the test results verified a promising potency for further application of CS–gelatin hydrogel in many biomedical fields, including drug delivery and tissue engineering by mimicking extracellular matrix components of tissues such as collagen and CS in cartilage.
Keywords: Chondroitin sulfate, Gelatin, In situ hydrogel, Biocompatibility, Cartilage
Introduction
Functional hydrogels with biocompatibility have become a class of interesting biomaterials and been developed by using biocompatible polymers [1, 2]. Hydrogel has been employed as a source for incorporation of bioactive molecules and their release, as well as diffusion of nutrients for cells and metabolites of entrapped cells [3], when applied in biomedical purposes, especially in tissue engineering. Functional hydrogels in tissue engineering has been explored by many research groups through modulations of their unique characteristics and chemistry. Modifications of polymer’s functional groups, induction of self-assembling [4], modulations of physico-chemical properties [5] and controlled biodegradability, as well as controls of stimulus-responsive sensing and responses [6] were reported. Self-crosslinking by click chemistry has attracted great interesting in tissue engineering by functional hydrogels [7, 8]. In developing functional hydrogels both synthetic and natural polymers have been employed to utilize their unique properties such as biocompatibility, biomimetics and mechanical properties. While natural polymers such as chondroitin sulfate (CS), hyaluronic acid, gelatin and dextran were employed for the purposes of improved biocompatibility and biomimetics, synthetic polymers such as poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), and poly(hydroxyl ethyl methacrylate) were used for controls of physicochemical properties and design of polymer networks. To increase cell interactions of less cell adhesive polymers, combinations of less cell adhesive polymers with cell adhesive natural polymers and domains have been developed in many research groups. For examples, cell adhesion natural polymers, e.g. collagen and gelatin, as well as oligopeptides, e.g. RGD, RGDS, REDV and YIGSR, have been grafted to the cell-nonadhesive synthetic polymers such as PEG and PVA to improve their cell interactions in specific tissues and cells such as cell adhesion, proliferation and cell types [6, 9, 10].
CS, a structural component of extracellular matrix (ECM) in connective tissue, bone, ligaments, tendons and cartilage, consists of a repeating unit of d-glucuronic acid and sulfated N-acetyl-d-galactosamine in C4 or C6 of its repeating unit [11]. CS has been employed as a hydrogel network for tissue engineering of soft tissues such as cartilage and nerves due to its mimetics to patient tissues in physico-chemical and mechanical properties as well as biological ones. As examples, CS showed anti-inflammatory activity [12], stimulations of syntheses of both proteoglycans and hyaluronic acid, reduction of chondrocytes catabolic activity, biodegradation, specific gene expression in chondrocytes, inhibition of proteolytic enzymes synthesis and alleviation of osteoarthritic symptoms by either single or combined with glucosamine administration as well as regeneration of root neurons in brachial plexus injury [13–16].
On the other hand, gelatin, a natural polymer derived from denaturation of collagens has some original characteristics of collagens [17], showing bioactive functions such as cell adhesion and proliferation as well as mechanical properties. It has been transformed into diverse forms for biomedical applications such as hydrogel, films, particles and scaffolds for tissue engineering depending on its processing. As examples, gelatin was converted to thermo-reversible hydrogel with sol–gel transition temperature at around 30 °C, thus showing possibility of its application in a gel shape in body temperature [19, 20]. Micro/nano-spheres of gelatin crosslinked by glutaraldehyde demonstrated delivering fibroblast growth factor (bFGF) for adipogenesis induction in fat tissues regeneration [20]. Porous gelatin hydrogels fabricated by freeze-drying and ice particulates were applied to study cells interaction, where cells attachment, spreading and proliferation were improved by controlling their properties such as pore sizes and mechanical properties [21]. It was also found that low dose Simvastatin-conjugated gelatin hydrogel enabled tendon-bone healing through the mechanisms of angiogenesis and osteogenesis, though could not support biomechanical properties in long-term reconstruction [22].
Even though many researches on CS and gelatin have been reported to utilizing its biological and physicochemical properties, multi-functionalities of CS–gelatin hydrogel have yet to be developed in biomedical fields by combining with cell-adhesive polymers and novel technologies in synthesis. As examples, CS hydrogel fabricated with click chemistry showed promising in vitro cellular biocompatibility, unique biological functions and tissue specific functionality, but had limited properties in induction to specific cell adhesion in tissue engineering [23]. To improve and expand its functions, syntheses of CS-based hydrogels through the mechanisms of click chemistry have been reported in many researchers by using diverse mechanisms such as thiol-ene reaction [24], enzymatic reactions of peroxidase and pHs [25], metal ion removal to increase mechanical strength [17] and others. Various degrees of methacrylate substitutions on CS network have been reported through radical polymerization by grafting CS with methacrylic anhydride, by controlling reaction time and temperature, and the concentrations of methacrylic acid and NaOH [26]. Synthesis of CS–PEG hydrogel was also reported by using versatile pH sensitive mechanism for induction of tissue adhesiveness in wound healing and control of its strength and sealing via amide linkages between CS and PEG [27, 28].
We report here the CS–gelatin hydrogel newly developed via click chemistry of self-crosslinking reaction to enhance potential characteristic of CS hydrogel through grafting of gelatin. Inclusion of gelatin into CS hydrogel was expected to improve its cell adhesion responses than pure CS hydrogel. In this study CS and gelatin were in advance transformed into CS–acrylate and gelatin–tri(carboxyethyl)phosphine (TCEP), respectively, for click chemistry. By simply mixing two separate precursor solutions of CS–acrylate and gelatin–TCEP in distilled water, CS–gelatin hydrogel was then fabricated through click chemistry of Michael type addition reaction between acrylate and phosphine groups. The synthesized CS–gelatin hydrogel was evaluated by various physicochemical and in vitro biological tests to observe its potency for biomedical applications in tissue engineering and drug delivery, potentially targeting on tissues of cartilage and bones.
Experimentals and methods
Materials and reagents
Chondroitin sulfate sodium salt (CS) from shark cartilage, gelatin from porcine skin, N-(3-diethylpropyl)-N-ethylcarbodiimide hydrochloride (EDC), tris(2-carboxyethyl)phosphine) (TCEP), adipic dihydrazide (ADH), acrylic acid, dimethylsulofixde and rhodamine B were purchased from Sigma-Aldrich (St. Louis, MO, USA). While Dulbecco’s Modified Eagle’s Medium (DMEM), fibroblast growth medium-2 (FGM-2) and penicillin–streptomycin were purchased from Lonza Korea (Switzerland), both cell counting kit-8 (CCK-8) solution and live and dead viability/cytotoxicity kit for mammalian cells were bought from Dojindo Laboratories (Japan) and Invitrogen (USA), respectively. Assay kits of both in vitro toxicology and neutral red were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was bought from Gibco BRL (Australia). All chemicals and kits were employed for experiments as received.
Synthesis and chemical analysis of CS–acrylate and gelatin–TCEP
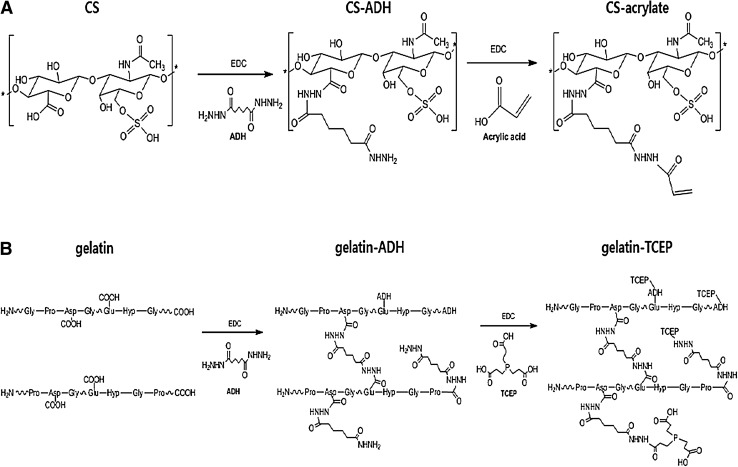
Both CS–acrylate and gelatin–TCEP were synthesized through EDC chemistry by chemical graftings of ADH and TCEP, consecutively, by referencing the detailed methods of our previous reports [24, 29]. In brief CS–acrylate was synthesized by sequential grafting of ADH and acrylic acid to CS. 0.08 g ADH, 0.08 g HOBT and 0.10 mL EDC was separately added into 40 mL CS solution, and then chemical reaction proceeded for 1 h (see overall schematics in Fig. 1). Gelatin–TCEP powder has been newly synthesized in this study by using the same steps of the above CS–acrylate synthesis methods. In specific, after obtaining gelatin solution (0.8 g) in distilled water by stirring at 40–50 °C. 0.66 g ADH and 0.66 mL EDC was sequentially added into the mixture in acidic condition. The reaction proceeded for 3 h and then additions of 0.27 g TCEP and 0.16 mL EDC were added at pH 4.75 for 6 h, thus obtaining gelatin–TCEP solution. After transferring separate solutions of both the obtained CS–acrylate and gelatin–TCEP to regenerated cellulose dialysis tubing (6–8 kDa of molecular weight cut off), sequential dialyses were processed against different solutions of 100 mM NaCl solution (pH 6.8), DW (pH 6.8) and then 20% ethanol. The powder of CS derivative was obtained through lyophilization for 2 d. The powder samples were chemically analyzed by both FTIR (Travel IR; Smiths, USA) and 1H-NMR (Varian, Japan). The absorption of gelatin–TCEP solution was confirmed using UV–Vis (Jasco, Japan) spectroscopy.
Fig. 1.
Schematics of CS–acrylate A and gelatin–TCEP B syntheses via aminodihydrazide (ADH) grafting
Fabrication of CS–gelatin hydrogel
After dissolving separately the powders of both CS–acrylate and gelatin–TCEP in PBS in 20 mL glass vials, in situ CS–CS–gelatin hydrogel was synthesized via click chemistry of Michael type addition reaction by mixing the prepared solutions at predefined ratios and concentrations such as 1:1 and 10%, respectively. Under diverse conditions, formations of CS–gelatin hydrogel were analyzed over time by using a vial tilting method according to our previous reports [24, 30]. Gelation of two precursor solutions was considered as the point where there was no flow for more than 1 min after inverting 100 μL mixed solution in the 1 mL conical vial.
Swelling of CS–gelatin hydrogel
Swelling of CS–gelatin hydrogel (200 μL) was measured under physiological conditions (pH 7.4, 37 °C) as below [24, 30]. After measuring the weight of the fabricated hydrogel with a microbalance, swelling of the CS–gelatin hydrogel was determined by comparing its weights before and after immersing in water. Adherent water was removed by blotting the wet CS–gelatin hydrogels before weighing them on an electronic balance. The percentage of hydrogel swelling was calculated by employing following formula (1).
| 1 |
where Wt = weight of the swelled hydrogel at time t, Wi = weight of the hydrogel at initial gelation time.
Morphologies of the dehydrated CS–gelatin hydrogel
Morphologies of the 10% CS–gelatin hydrogel was observed by scanning electron microscopy (SEM) (Jeol Ltd., Japan) after processing of dehydration and then surface coating of the CS–gelatin hydrogel with gold as follows. It was dehydrated by sequential freezing of the swollen CS–gelatin hydrogel in liquid nitrogen and then at − 55 °C overnight. The dry sample was mounted on an aluminum stub with a double-sided tape, and then gold-coated with a sputter coater for 1 min. Morphologies of both surface and cross section of the dehydrated CS–gelatin hydrogel was analyzed with SEM.
In vitro cell viability
After seeding fibroblasts (p = 7) at the densities of both 1 × 104 cells/surface and 1 × 105 cells/inside of the 10% CS–gelatin hydrogel (200 µL), cell culture was performed in an incubator with 5% CO2 at 37 °C for 7 days by feeding fibroblast growth medium-2 (FGM-2) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics of penicillin and streptomycin. Fibroblasts were added in 10% precursor polymer solutions of CS–acrylate in advance and then the cell-containing CS–acrylate solution was mixed with 10% gelatin–TCEP solutions, thus obtaining CS–gelatin hydrogel via Michael type addition reactions, i.e. thio-ene reaction. Cell adhesion and proliferation on the surface of the CS–gelatin hydrogels in a 24 polystyrene culture well plate were quantitatively and qualitatively measured with an assay of cell counting kit-8 (CCK-8, Dojindo, Japan) through a microplate reader (Tecan, Australia) after insertion of 100 µL CCK-8 solution into 900 µL FGM-2 medium. Optical density of the aliquoted 100 µL medium of the samples was measured by the microplate reader at a wavelength of 450 nm.
In vitro cell viability of the CS–gelatin hydrogel was evaluated by evaluating the fibroblasts both on the surface of and encapsulated in the 200 µL 10% CS–gelatin hydrogel. Live and dead viability/cytotoxicity kit for mammalian cells (Invitrogen, USA) was prepared by adding both 1.2 μL ethidium homodimer-1 solution (EthD-1, 2 mM) and 0.3 μL calcein AM solution (4 mM) into 600 μL PBS. After performing the reaction with the prepared kit for 30 min in the in vitro incubator, cell viability was evaluated by a fluorescence microscope (Leica DMLB, Germany).
Cytotoxicity
Cell cytotoxicity of the CS–gelatin hydrogel was evaluated by the assays of methylthiazolydiphenyl-tetrazolium bromide (MTT), bromodeoxyuridine (BrdU) and Neutral Red for its effects on the cell organ damages such as mitochondria, deoxyribonucleic acid (DNA) and lysosome, respectively, according to the protocols suggested by the vendors. The films of both Teflon and Latex, 1 cm × 1 cm, were employed as samples of positive and negative controls, respectively.
MTT assay
Fibroblasts were in vitro cultured in a 96 well plate at a density of 1 × 104 cells for 24 h. Cell culture lasted for another 24 h after addition of the extracts of the 10% CS–gelatin hydrogels (200 μL), Teflon and Latex. After adding up 20 µL MTT assay solution (2 mg/mL in PBS) in the culture medium and then replacing it with 100 µL dimethylsulfoxide, cell culture proceeded for 4 h. The optical density of the final test solution was measured by the microplate reader at the wavelength of 570 nm.
BrdU assay
Fibroblasts at a density of 1 × 104 cells were cultured in the 96 wells for 24 h, after loading 1 mL extract solution of the 10% CS–gelatin hydrogel. 10 µL BrdU labeling solution was added into the culture medium and then cell culture lasted for another 2 h. Subsequent to removal of labeling medium, 200 µL FixDenat solution and 100 µL anti BrdU peroxide–labeled anti-BrdU antibody per well was added according to the manufacturer’s protocol. After washing the wells with PBS, 25 µL 1 M H2SO4 solution was added into each well, and then optical density was measured at the absorbance–wavelength of 450 nm by microplate reader by referring to that of 690 nm.
Neutral red assay
Separate extract solutions from the 10% CS–gelatin hydrogel, Teflon and Latex were prepared after loading them in 1 mL culture medium for 72 h. In vitro cell culture lasted for 24 h after seeding fibroblasts on the sample surface at a density of 1 × 104 cells/well. A second cell culture was performed for 24 h after sequential removal of medium and then addition of the prepared extracts. Cell culture was done for another 2 h after adding up 0.33% neutral red solution. Fixation was proceeded for 10 min after adding up 100 µL solubilization solution per well. The optical density value of the samples was measured at an absorbance–wavelength of 550 nm by the microplate reader by referencing to that of 690 nm.
Cytotoxicity test by direct contact of hydrogel with cells: gel covering
Effects of direct contact of the 10% CS–gelatin hydrogel on fibroblasts have been evaluated as below. For the assay, after seeding fibroblasts at a density of 2 × 104 cells/cm2 on the 24 well plate, the cells were covered with either the CS–gelatin hydrogel (200 μL), Teflon or Latex (Ø = 10 mm). After incubation of the cells covered with the CS–gelatin hydrogel for another 24 h, 200 µL reagent of the live and dead assay was added into cell culture plate. Both viability and morphological changes of the cells were observed by fluorescein microscope (Leica DMLB, Germany).
Inflammatory and immune responses
The amount of nitric oxide (NO) released was in vitro measured using NO detection kit according to the protocol suggested by a vendor (iNtRON Biotechnology, Korea) in supernatants of the RAW264.7 cells for quantification of inflammatory and immune responses. Mouse RAW 264.7 macrophage cell lines (ATCC, USA) were seeded at a density of 5 × 104 cells with 1 mL DMEM supplemented with 10% FBS in a 5% CO2 atmosphere at 37 °C for 24 h. Culture media was replaced with serum-free one with dilute assay solutions. 0.01 and 0.1% 100 μL supernatants of the 10% CS–gelatin hydrogel were collected at the points of 6, 24 and 48 h. They were sequentially added into the buffer solutions of 50 μL sulfanilamide and then 50 μL naphthylethylenediamide at the interval of 10 min. The amount of NO generated was measured with a microplate reader at the wavelength of 540 nm over the medium, a negative control, by applying the obtained values to the standard curve.
Statistical analysis
Data were reported as mean ± standard deviation. Statistical significance was assessed with one-way ANOVA of the SPSS 14.0 program (ver. 14.0, SPSS Inc., Chicago, IL, USA). The values were considered as significantly different when p < 0.05.
Results
Syntheses and chemical analyses of both CS–acrylate and gelatin–TCEP
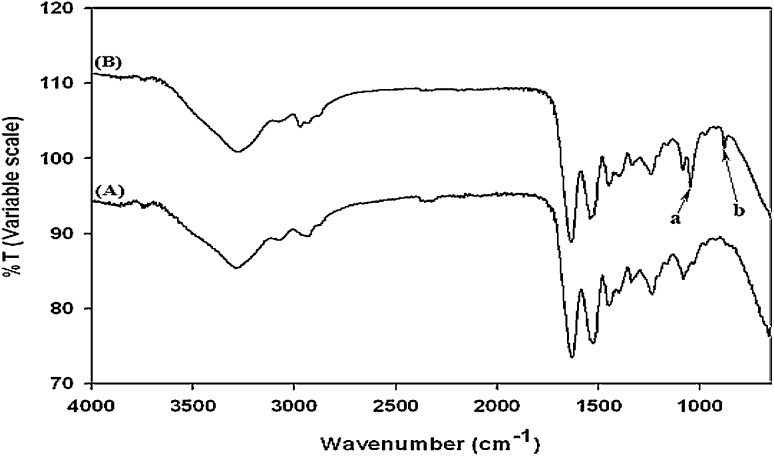
Both CS–acrylate (Fig. 1A) and gelatin–TCEP (Fig. 1B) have been synthesized by sequential grafting of ADH and then either acrylic acid or TCEP to CS and gelatin, respectively, through EDC chemistry. Employment of EDC chemistry for syntheses of other biomedical polymers such as hyaluronic acid, cellulose and CS has been previously reported in our research reports [24, 30]. The gelatin–TCEP was newly synthesized for better cell adhesiveness in this study and chemically characterized by ATR-FTIR spectroscopy. The phosphorus atoms connected to the carbon (P–C) was observed at the characteristic wavelength of both 1010 (peak a in the gelatin–TCEP spectrum) and 760 (peak b in the gelatin–TCEP spectrum) cm−1 in the ATR-FTIR spectrum (Fig. 2). Evaluation of CS–acrylate was described in detail in our previous report [30].
Fig. 2.
Chemical analyses of gelatin A and gelatin–TCEP B by ATR-FTIR
Gelation behaviors
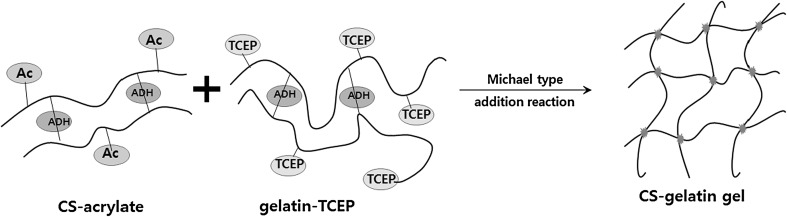
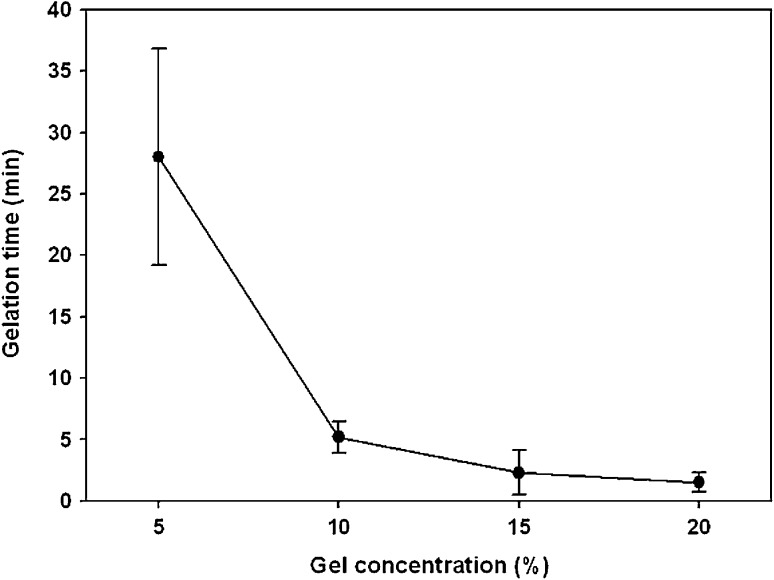
After mixing the precursor solutions of both CS–acrylate and gelatin–TCEP in PBS at a volumetric ratio of 1:1, it was turned into 5% CS–gelatin hydrogel through the click chemistry between their acrylate and phosphine end groups, respectively (Fig. 3). The hydrogel formation was measured by tilting the mixture solution in a centrifuge vial [24, 29]. Both higher concentrations of the precursor solutions induced quick gel formation under physiological condition. In specific, the precursor concentrations of 5, 10, 15 and 20% became hydrogels at the time points of approximately 28, 5.2, 2.3 and 1.5 min, respectively (Fig. 4). The precursor solutions in basic water and higher temperature normally showed quicker hydrogels formation as reported in our previous study (data not shown).
Fig. 3.
Fabrications of CS–gelatin gel through two reaction mechanisms. There was an intra-crosslinking in CS–acrylate and gelatin–TCEP through ADH at the first reaction stage, and click chemistry of Michael type addition reaction has been proceeded as a second reaction
Fig. 4.
Gelation times of CS–gelatin hydrogels in different concentrations in PBS at pH 7.4 and 37 °C
Swelling behaviors of CS–gelatin hydrogel
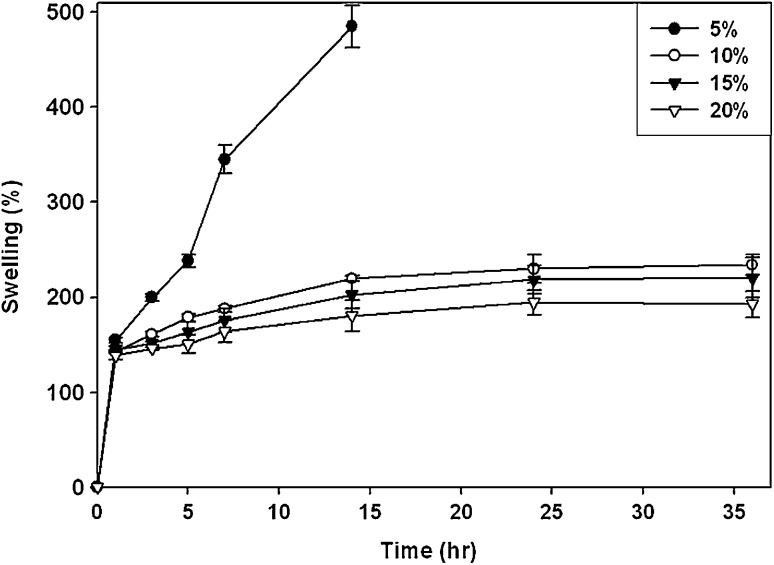
Furthermore, swellings of the CS–gelatin hydrogels with different concentrations were evaluated under physiological condition in PBS during 36 h (Fig. 5). The 10, 15 and 20% CS–gelatin hydrogels swelled to 240, 218 and 195% in 1 day, respectively. Meanwhile the 5% CS–gelatin hydrogel swelled significantly during 7 h with reduction of mechanical strength, possibly due to lower crosslinking density, the hydrogels fabricated with 10, 15 and 20% precursor solutions maintained their shapes for 36 h. However that with 5% lost its shapes in 14 h.
Fig. 5.
Swelling behaviors of CS–gelatin hydrogels with different concentrations in PBS at pH 7.4 and 37 °C
Morphologies of the surface and cross-sections of CS–gelatin hydrogel
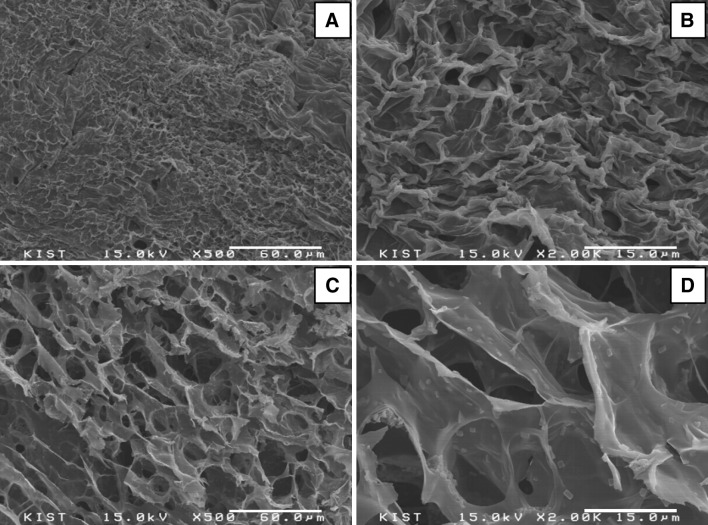
The morphologies of the surface and cross-section of the 10% CS–gelatin hydrogel were observed with SEM (Fig. 6) after dehydration of the fully swollen gel in lyophilizer. While the surface had relatively non-homogeneous and rough morphologies with irregularly shaped pores, the cross-section showed homogeneous morphologies with pore sizes ranging from 10 to 30 μm.
Fig. 6.
Surface A, B and cross-section C, D morphologies of the 10% CS–gelatin hydrogel at the magnifications of ×500 A, C and ×2000 B, D
In vitro cellular behaviors
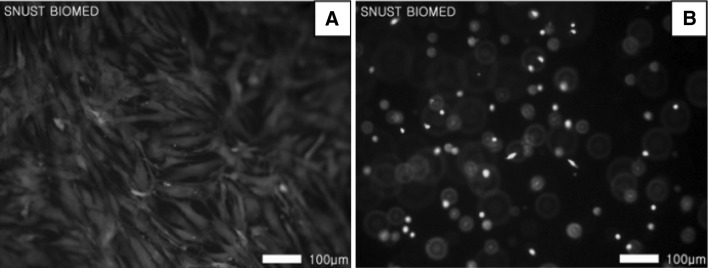
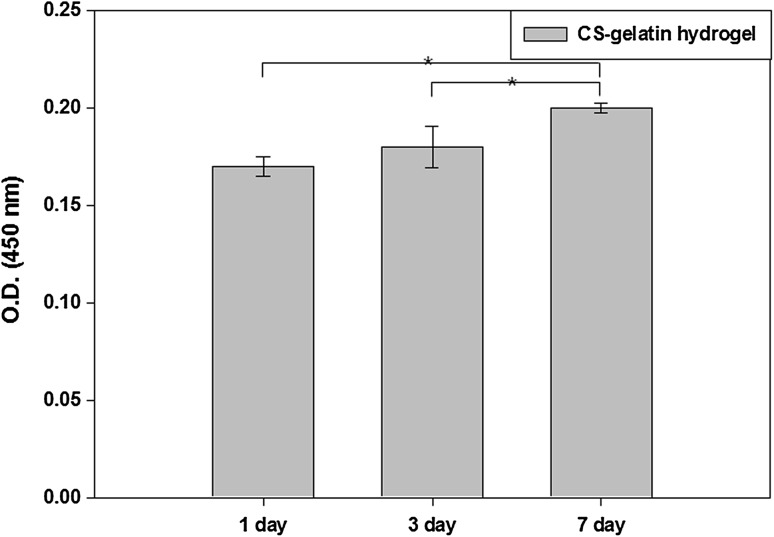
While fibroblasts adhered and spread on the surface of 10% hydrogel for 7 days (Fig. 7A), they adhered on but spread very limitedly inside the hydrogel when encapsulated (Fig. 7B). All the cells were viable for 7 days regardless their locations. The number of adhered cells was very high as measured by CCK-8 (Fig. 8) at day 1, 0.16 in OD value, and then increased to 0.18 and 0.20 at day 3 and 7, respectively.
Fig. 7.
Biocompatibilities of fibroblasts both on the surface of A and inside B the 10% CS–gelatin hydrogel at day 7 by the live and dead assay
Fig. 8.
Proliferation of fibroblasts on the surface of the 10% CS–gelatin hydrogel by the assay of CCK-8, where the cells were seeded at a density of 10,000 cells/cm2
In vitro cytotoxicity of CS–gelatin hydrogel
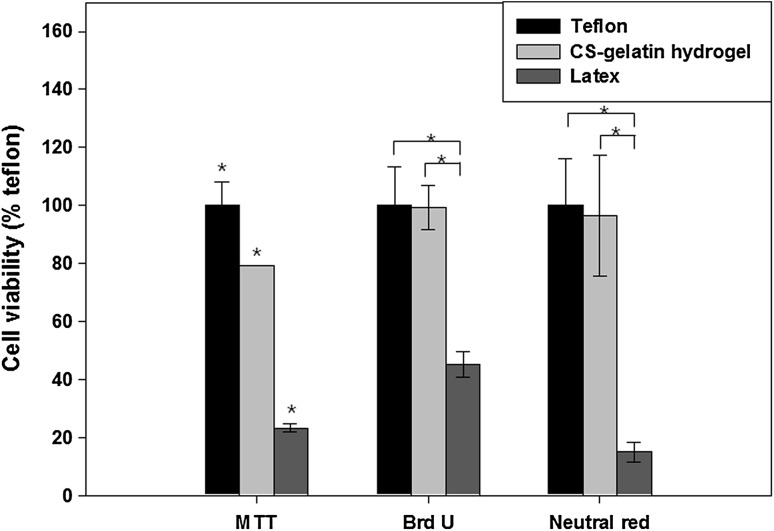
Cytotoxicities by the extracts of the 10% CS–gelatin hydrogel were measured by employing the assays of MTT, BrdU and Neutral Red (Fig. 9) to evaluate its effects on the cell organs such as mitochondria, DNA synthesis and lysosome, respectively. The values were normalized by considering the value of positive control Teflon as 100%. All the hydrogels showed lower cell toxicity similar to those of Teflon, and better cell compatibility compared to those of the negative control Latex. In specific, the 10% CS–gelatin hydrogels showed 79, 98 and 99% cell compatibilities of MTT, BrdU and Neutral Red, but Latex did 23, 15 and 46%, respectively.
Fig. 9.
Cytotoxicity of the 10% CS–gelatin hydrogel measured by the assays of MTT, BrdU and Neutral red
Cytotoxicity by direct covering of CS–gelatin hydrogel on cells
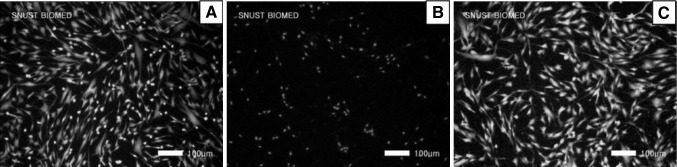
Cell viability and morphologies of the 10% CS–gelatin hydrogel were also evaluated by covering it on the culturing cells on the polystyrene culture plate for 24 h. The positive control Teflon (Fig. 10A) showed no cell damages but the Latex induced death of all the cells as shown in red (Fig. 10B). The CS–gelatin hydrogel (Fig. 10C) did excellent cell viability in green similar to that of positive control. All the cells covered with both Teflon and the CS–gelatin hydrogel remained well spreading, but the cells covered with Latex did not show any spreading with less number of cells remained.
Fig. 10.
Cell compatibility of the 10% CS–gelatin hydrogel measures by the assay of direct sample covering on fibroblasts for 24 h, where A Teflon, B Latex and C 10% CS–gelatin hydrogel
Inflammatory response of CS–gelatin hydrogel
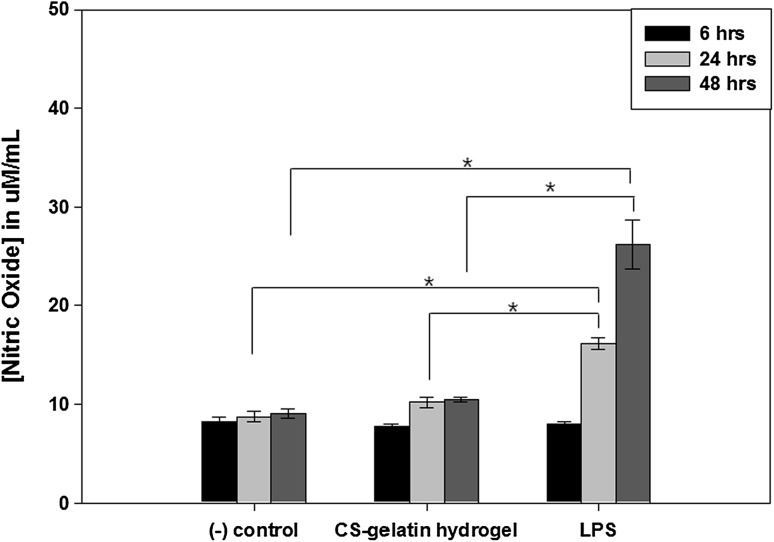
We evaluated the inflammatory and immune responses of the 10% CS–gelatin hydrogel by measuring the amount of nitric oxide (NO) released from the RAW 264.7 macrophage cell lines. The CS–gelatin hydrogel was in vitro exposed to the macrophages for 48 h (Fig. 11). The amount of NO generated by CS–gelatin hydrogel groups was compared with those generated by LPS samples and cell culture medium, which were considered as positive and negative controls, respectively. The results showed that the CS–gelatin hydrogel induced higher amount of NO release over time for up to 48 h, but significantly less amount than the positive control LPS did. In specific when measured at the time points of 6, 24 and 48 h, LPS generated increased amount of NO release but all the CS–gelatin hydrogels did lower amount of NO released, similar to that of the cell culture medium. While the medium induced releases of 8.2, 8.7 and 9.1 μM/mL NO at the time points of 6, 24 and 48 h, the CS–gelatin hydrogel did 7.8, 10.0 and 10.5 μM/mL of NO release at their corresponding time points. However, the positive control LPS induced release of 8.0, 16.0 and 26.0 μM/mL of NO at the corresponding times, which were significantly higher amount of NO release than both the medium solution and the CS–gelatin hydrogels did at the time points of both 24 and 48 h.
Fig. 11.
Inflammatory response of the 10% CS–gelatin hydrogel as measured by the nitric oxide assay
Discussion
In situ hydrogel consisting of both CS and gelatin has been synthesized through chemical modifications and evaluated for regenerative medicine to regenerate the defects of tissues. In tissue engineering applications, it has been well reported that while CS hydrogel exhibited lower but specific cells adhesion with specific interactions in cartilages [24], gelatin hydrogel showed higher cell adhesion in diverse tissues [18]. The hydrogel composed of both CS and gelatin was expected to induce better cell adhesion and at the same time specific cell interaction by controlling its compositions, leading to higher possibility of its applications to biomedical and tissue engineering materials. To achieve these goals, these polymers should be derivertized into functionalization of their chemical structures, and then turned into specific and diverse forms such as hydrogel, scaffolds, films, 3D printing and many others according to their applications. Before applications of in situ CS–gelatin hydrogel, its biocompatibility and functional chemistry should be rigorously verified through diverse methods such cell compatibility, chemical and morphological properties and swelling properties of hydrogel. We here report new synthesis and its mechanism of injectable hydrogel via click chemistry of Michael type addition reaction between acrylate and TCEP and then tests of its biocompatibility with its physico-chemical properties.
Both CS–acrylate and gelatin–TCEP were synthesized as precursor polymers for fabrication of injectable CS–gelatin hydrogel via click chemistry of Michael type addition reaction. While CS–acrylate has been previously proven successfully synthesized [17, 29], gelatin–TCEP has been newly synthesized through sequential grafting of ADH and TCEP to gelatin by EDC chemistry. Successful grafting of TCEP was chemically verified by ATR-FTIR, by observing the emergence of new phosphorus-carbon stretching peaks at the wavelengths of 1010 and 760 cm−1.
In situ CS–gelatin hydrogel was formed by mixing two separate solutions of precursor polymers, i.e. CS–acrylate and gelatin–TCEP. Chemical crosslinking between acrylates and phosphines spontaneously occurred through click chemistry of Michael addition reaction [17]. A series of precursor solution concentrations, 5–20%, were evaluated to observe gelation behaviors over times through a tilting method, where the gelation time responded to their concentrations, i.e. the higher the molecular density in a solution, the higher number of functional group was available for crosslinking reaction. Correspondingly, in situ crosslinkable modified glycosaminoglycan hydrogel also had similar trend where in higher crosslinking density, quicker gelation was achieved [24, 31]. It was also in accordance to swelling profiles where higher crosslinking density led to lower swelling ratio [31], as our previous findings indicated similar trend. CS–gelatin hydrogels with concentrations from 10 to 20% showed relatively uniform trend of gelation and swelling percentage, but at lower concentrations gelation trend was not uniform. Therefore, these results indicated that enough crosslinking density was achieved within approximately 10% hydrogel concentration in this experiment. Besides, by achieving enough crosslinking density, the in situ hydrogels could keep its physical structure for up to 36 h while the low concentration one could only bear to 14 h in handling for swelling tests.
The reason of measurement of hydrogel swelling from the fabricated one at defined concentrations to fully saturated one was to understand the behaviors of the synthesized one when applied directly to the patients. The injectable hydrogel without drying process could show the profiles of its applications to patients, without addition of drying process. The powder of CS–acrylate and gelatin–TCEP was in advance separately sterilized and the fabricated gels was targeted to be applied directly into a patient, thus no reason going back to the processes of drying, swelling and injection of the hydrogel fabricated. Applications of release of bioactive molecules from tissue engineering scaffold were recently reported in bone, cartilage [32] and other tissues by using scaffold and hydrogel with deliveries of in vitro platelet rich plasma and in vivo bone morphogenic protein-2 (BMP-2), respectively [33, 34]. In vitro biological effectiveness of chitosan microparticles cross-linked with sodium triphosphate with activated pure platelet-rich plasma was employed as an injectable composite scaffold for growth factors release, cell proliferation and osteogenic differentiation [33]. Song et al. [34] reported sustained delivery of recombinant human BMP-2 from collagen–chitosan hydrogel with microspheres for tissue regeneration of rabbit mandibular defects in dental surgery.
Microporous morphology of the CS–gelatin hydrogel was observed by SEM, where the pore diameter was ranging from 10 to 30 μm with high interconnectivity. Its pore diameter seemed to be controlled by degrees of gelatin derivatizations since its amino side and end groups for chemical reactions were less than those of chondroitin sulfate. It could be a supportive feature for biocompatible environment which is corresponding to the findings of porous gelatin hydrogel by other research group [20]. As described by cell viability test within 7 days culture, fibroblasts were well attached and proliferated on the surface of the CS–gelatin hydrogel by showing elongated morphology of cells and increasing values of its optical density. Fibroblasts encapsulated in the CS–gelatin hydrogel were also found to be well survival for 7 days in this experiment. Cytotoxicity test towards the CS–gelatin hydrogel also showed superior cell compatibility, concluding that the CS–gelatin hydrogel had a very close trend of cell compatibility to that of the non-toxic positive control, Teflon. It showed much better cell compatibility, as expected, than those of negative control, Latex. These cellular behaviors indicated enhancement of cell adhesion and proliferation by incorporation of gelatin in the CS hydrogel in accordance to other reports [35, 36], where gelatin and collagens in its amino acid sequence have been reported to induce higher cell adhesion and proliferation than those of CS.
Inflammatory responses of the 10% CS–gelatin in situ hydrogel was evaluated by the assay of NO measurement, showing free of inflammatory and immune responses for up to 48 h compared to those of LPS. This method was to detect the concentration of NO by indirectly measuring nitrite (NO2−), the by-product of nitric oxide trans-formation, which is an indication of inflammatory and immune responses of macrophages to the foreign samples. The enzyme nitrate reductase was used to convert nitrate to nitrite in the assay. Nitrite was detected as a colored azo dye product of the Griess reaction that absorbed visible light at 540 nm. Total NO contributed by nitrate and nitrite in the assay system was measured as nitrites after converting all nitrates to nitrites. The results may imply that the CS–gelatin hydrogel remained carrying remarkable privilege of CS’s anti-inflammatory activity, potentially bone and cartilage tissue engineering [37, 38].
In conclusion, a new medically potential CS–gelatin hydrogel with cell adhesiveness was in situ successfully synthesized via click chemistry of Michael type addition reaction after two times of addition EDC chemistry-based intra-crosslinking. From the results of this study, we found a mechanism of two step chemical reactions that initial intra-crosslinking of both CS and gelation was processed via EDC during grafting of functional groups of acrylate and TCEP, thus obtaining separate precursors of CS–acrylate and gelatin–TCEP. Gelation via chemical reaction was achieved in minutes and the swelling of CS–gelatin hydrogel depended on the concentrations of precursor polymer solutions, CS–acrylate and gelatin–TCEP. Microporous morphologies with high interconnectivity could provide hospitable environment for cells adhesion and growth to support its biocompatibility. It was proved by entire in vitro studies which resulted in non-cytotoxic milieu, excellent cells attachment on the CS–gelatin hydrogel surface as well as cells viability on and inside the CS–gelatin hydrogel, not to mention perceptibly free of inflammation-inducing potency. Furthermore, CS–gelatin hydrogel network induced higher cell adhesion and growth by the effects of gelatin network, which is important for its application to tissue engineering of cartilage, as example, which has extracellular matrix of collagen and chondroitin sulfate. All in all, these results provided evidences of a novel potential biomaterial, CS–gelatin hydrogel, for many further biomedical applications, potentially to cartilage and bones.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant (2014K2A2A7060928 and 2015R1A2A1A10054592) and by Grants of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A120822).
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Rahman RA, Radzi MAA, Sukri NM, Nazir NM, Sha’ban M. Tissue engineering of articular cartilage: from bench to bed-side. Tissue Eng Regen Med. 2014;12:1–11. doi: 10.1007/s13770-014-9044-8. [DOI] [Google Scholar]
- 2.Kaushik SN, Kim B, Walma AMC, Choi SC, Wu H, Mao JJ, et al. Biomimetic microenvironments for regenerative endodontics. Biomater Res. 2016;20:14. doi: 10.1186/s40824-016-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gwak GH, Choi AJ, Bae YS, Choi HJ, Oh JM. Electrophoretically prepared hybrid materials for biopolymer hydrogel and layered ceramic nanoparticles. Biomater Res. 2016;20:1. doi: 10.1186/s40824-016-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castelletto V, Hamley IW, Stain C, Connon C. Slow-release RGD-peptide hydrogel monoliths. Langmuir. 2012;28:12575–12580. doi: 10.1021/la302071e. [DOI] [PubMed] [Google Scholar]
- 5.Bang S, Das D, Yu J, Noh I. Evaluation of MC3T3 cells proliferation and drug release study from sodium hyaluronate-BDDGE patterned gel. Nanomaterials (Basel) 2017;7:E328. doi: 10.3390/nano7100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TT, Garcia JR, Paez JI, Singh A, Phelps EA, Weis S, et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14:352–360. doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R. Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules. 2007;8:1844–1850. doi: 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]
- 8.Gan Z, Zhang T, Liu Y, Wu D. Temperature-triggered enzyme immobilization and release based on cross-linked gelatin nanoparticles. PLoS One. 2012;7:e47154. doi: 10.1371/journal.pone.0047154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikh Z, Hamdan N, Ikeda Y, Grynpas M, Ganss B, Glogauer M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: a review. Biomater Res. 2017;21:9. doi: 10.1186/s40824-017-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SC, Chen BH, Wang LF, Chen JS. Characterization of chondroitin sulfate and its interpenetrating polymer network hydrogels for sustained-drug release. Int J Pharm. 2007;329:103–109. doi: 10.1016/j.ijpharm.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Martel-Pelletier J, Kwan Tat S, Pelletier JP. Effects of chondroitin sulfate in the pathophysiology of the osteoarthritic joint: a narrative review. Osteoarthritis Cartilage. 2010;18:S7–S11. doi: 10.1016/j.joca.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Lemaire PA, Huang L, Zhuo Y, Lu J, Bahnck C, Stachel SJ, et al. Chondroitin sulfate promotes activation of cathepsin K. J Biol Chem. 2014;289:21562–21572. doi: 10.1074/jbc.M114.559898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brühl H, Cihak J, Goebel N, Talke Y, Renner K, Hermann F, et al. Chondroitin sulfate activates B cells in vitro, expands CD138+ cells in vivo, and interferes with established humoral immune responses. J Leukoc Biol. 2014;96:65–72. doi: 10.1189/jlb.1A0913-502R. [DOI] [PubMed] [Google Scholar]
- 15.Muzzarelli RA, Greco F, Busilacchi A, Sollazzo V, Gigante A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohydr Polym. 2012;89:723–739. doi: 10.1016/j.carbpol.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 16.Little CJ, Kylyk WM, Chen X. The effect of chondroitin sulphate and hyaluronic acid on chondrocytes cultured within a fibrin–alginate hydrogel. J Funct Biomater. 2014;5:197–210. doi: 10.3390/jfb5030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing Q, Yates K, Vogt C, Qian Z, Frost MC, Zhao F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci Rep. 2014;4:4706. doi: 10.1038/srep04706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benton JA, DeForest CA, Vivekanandan V, Anseth KS. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15:3221–3230. doi: 10.1089/ten.tea.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387–1408. doi: 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- 20.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–2521. doi: 10.1016/S0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Zhang Q, Nakamoto T, Kawazoe N, Chen G. Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue. Tissue Eng Part C Methods. 2016;22:189–198. doi: 10.1089/ten.tec.2015.0281. [DOI] [PubMed] [Google Scholar]
- 22.Oka S, Matsumoto T, Kubo S, Matsushita T, Sasaki H, Nishizawa Y, et al. Local administration of low-dose simvastatin-conjugated gelatin hydrogel for tendon–bone healing in anterior cruciate ligament reconstruction. Tissue Eng Part A. 2013;19:1233–1243. doi: 10.1089/ten.tea.2012.0325. [DOI] [PubMed] [Google Scholar]
- 23.Kwon HJ, Han Y. Chondroitin sulfate-based biomaterials for tissue engineering. Turk J Biol. 2016;40:290–299. doi: 10.3906/biy-1507-16. [DOI] [Google Scholar]
- 24.Jo S, Kim S, Noh I. Synthesis of in situ chondroitin sulfate hydrogel through phosphine-mediated Michael type addition reaction. Macromol Res. 2012;20:968–976. doi: 10.1007/s13233-012-0138-7. [DOI] [Google Scholar]
- 25.Kim MJ, Shin YC, Lee JH, Jun SW, Kim CS, Lee Y, et al. Multiphoton imaging of myogenic differentiation in gelatin-based hydrogels as tissue engineering scaffolds. Biomater Res. 2016;20:2. doi: 10.1186/s40824-016-0050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang LF, Shen SS, Lu SC. Synthesis and characterization of chondroitin sulfate–methacrylate hydrogels. Carbohydr Polym. 2003;52:389–396. doi: 10.1016/S0144-8617(02)00328-4. [DOI] [Google Scholar]
- 27.Jo S, Kim S, Hwang Y, Noh I. Induction and biological evaluations of self cross-linking chondroitin sulfate-poly(ethylene oxide) hydrogel. Macromol Res. 2011;19:1303–1309. doi: 10.1007/s13233-011-1201-5. [DOI] [Google Scholar]
- 28.Kim KS, Kim YS, Bao K, Wada H, Choi HS, Hahn SK. Preparation and investigation of hydrolyzed polyacrylonitrile as a preliminary biomedical hydrogel. Biomater Res. 2017;21:15. doi: 10.1186/s40824-017-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SY, Bang S, Kim S, Jo SY, Kim BC, Hwang Y, et al. Synthesis and in vitro characterizations of porous carboxymethyl cellulose–poly(ethylene oxide) hydrogel film. Biomater Res. 2015;19:12. doi: 10.1186/s40824-015-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo S, Kim D, Woo J, Yoon G, Park YD, Tae G, et al. Development and physicochemical evaluation of chondroitin sulfate–poly(ethylene oxide) hydrogel. Macromol Res. 2011;19:147–155. doi: 10.1007/s13233-011-0205-5. [DOI] [Google Scholar]
- 31.Zheng Shu X, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Li Z, Zheng L, Zhang X, Liu P, Yang T, et al. Electrospun fibrous silk fibroin/poly(L-lactic acid) scaffold for cartilage tissue engineering. Tissue Eng Regen Med. 2016;13:516–526. doi: 10.1007/s13770-016-9099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimojo AAM, Galdames SEM, Perez AGM, Ito TH, Luzo ÂCM, Santana MHA. In vitro performance of injectable chitosan–tripolyphosphate scaffolds combined with platelet-rich plasma. Tissue Eng Regen Med. 2016;13:21–30. doi: 10.1007/s13770-015-9111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song WY, Liu GM, Li J, Luo YG. Bone morphogenetic protein-2 sustained delivery by hydrogels with microspheres repairs rabbit mandibular defects. Tissue Eng Regen Med. 2016;13:750–61. doi: 10.1007/s13770-016-9123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta KC, Haider A, Choi YR, Kang IK. Nanofibrous scaffolds in biomedical applications. Biomater Res. 2014;18:5. doi: 10.1186/2055-7124-18-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subbiah R, Suhaeri M, Hwang MP, Kim W, Park K. Investigation of the changes of biophysical/mechanical characteristics of differentiating preosteoblasts in vitro. Biomater Res. 2015;19:24. doi: 10.1186/s40824-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iovu M, Dumais G, du Souich P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage. 2008;16:S14–S18. doi: 10.1016/j.joca.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Kim MH, Kim BS, Lee D, Cho OH, Kwon WH, Park MH. Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering. Biomater Res. 2017;21:12. doi: 10.1186/s40824-017-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]