Abstract
Background:
Mass production of exosomes is a prerequisite for their commercial utilization. This study investigated whether three-dimensional (3D) spheroid culture of mesenchymal stem cells (MSCs) could improve the production efficiency of exosomes and if so, what was the mechanism involved.
Methods:
We adopted two models of 3D spheroid culture using the hanging-drop (3D-HD) and poly(2-hydroxyethyl methacrylate) (poly-HEMA) coating methods (3D-PH). The efficiency of exosome production from MSCs in the 3D spheroids was compared with that of monolayer culture in various conditions. We then investigated the mechanism of the 3D spheroid culture-induced increase in exosome production.
Results:
The 3D-HD formed a single larger spheroid, while the 3D-PH formed multiple smaller ones. However, MSCs cultured on both types of spheroids produced significantly more exosomes than those cultured in conventional monolayer culture (2D). We then investigated the cause of the increased exosome production in terms of hypoxia within the 3D spheroids, high cell density, and non-adherent cell morphology. With increasing spheroid size, the efficiency of exosome production was the largest with the least amount of cells in both 3D-HD and 3D-PH. An increase in cell density in 2D culture (2D-H) was less efficient in exosome production than the conventional, lower cell density, 2D culture. Finally, when cells were plated at normal density on the poly-HEMA coated spheroids (3D-N-PH); they formed small aggregates of less than 10 cells and still produced more exosomes than those in the 2D culture when plated at the same density. We also found that the expression of F-actin was markedly reduced in the 3D-N-PH culture.
Conclusion:
These results suggested that 3D spheroid culture produces more exosomes than 2D culture and the non-adherent round cell morphology itself might be a causative factor. The result of the present study could provide useful information to develop an optimal process for the mass production of exosomes.
Electronic supplementary material
The online version of this article (10.1007/s13770-018-0139-5) contains supplementary material, which is available to authorized users.
Keywords: Mesenchymal stem cell, Exosome, Three-dimensional, Spheroid, Poly-HEMA
Introduction
Mesenchymal stem cells (MSCs) have emerged as a good source of cell-based therapies. Extensive studies have revealed the therapeutic efficacy of MSCs in a variety of target diseases and some of them have already obtained market approval in certain countries [1–4]. The therapeutic efficacy of MSCs is attributed mainly to their paracrine secretion of cytokines and growth factors, which exert diverse cellular activities, such as immune modulation, anti-inflammatory effects, cytoprotection, angiogenic effects, and neurogenesis. However, studies have shown that the paracrine effects of MSCs are not only attributable to these trophic factors but also to the secretion of exosomes [5, 6]. Exosomes are secreted vesicles of endocytic origin that are 55–100 nm in size and are secreted by a variety of cells types, including MSCs [6]. Exosomes mediate local or systemic cell-to-cell communications and alter cellular metabolism through the transfer of various proteins, mRNAs, and microRNAs (miRNAs) [7–9]. Recently, studies have shown that exosomes derived from MSCs mediate the therapeutic effects of MSCs [10] and suggested the potential of MSCs exosomes as a cell-free therapy vehicle because of their lower immunogenicity and ease of manipulation or storage compared with cell-based products [11, 12]. The therapeutic activity of MSC exosomes has been reported in myocardial infarction [6], renal fibrosis [13], liver fibrosis [14], skeletal muscle regeneration [15], and osteoarthritis [16, 17].
Recently, several studies suggested that the efficiency of exosome secretion can be improved by preconditioning the cells, such as by genetic manipulation [18], exposure to hypoxia [19], increasing intracellular calcium [20], and treatment with bioactive molecules [21, 22]. These preconditioning methods can increase the yield of exosomes, thereby benefitting their clinical use. However, all the above experiments were performed in a traditional monolayer culture that suffers from limited expansion, phenotypic changes, and loss of cellular therapeutic activity during long-term passaging [23]. Meanwhile, three-dimensional (3D) culture of cells enables mass production of MSCs and could also be advantageous to improve their paracrine function. The large-scale expansion of MSCs has been successfully achieved using dynamic culture of 3D MSCs spheroids in a spinner flask or a rotating wall vessel bioreactor [24]. Interestingly, studies have shown that MSCs cultured in 3D spheroids exhibit more trophic factor section and higher therapeutic potential [25–28]. For example, Potapova et al. [28] showed that MSCs in 3D spheroids secreted more interleukin-11 (IL-11) and proangiogenic cytokines (e.g., vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and angiogenin) than MSCs in monolayer culture. They proposed that the hypoxic microenvironment in the core of the MSCs spheroids could be the driving force for the increased secretion. Furthermore, Bartosh et al. [27] demonstrated that MSC spheroids secreted 60-fold more tumor necrosis factor-inducible gene 6 (TSG-6) than the monolayer culture. In another study, Ylostalo et al. [29] suggested that MSCs in 3D spheroids were self-activated to increase prostaglandin E2 (PGE2) secretion. Therefore, the 3D spheroid culture of MSCs could be a good method for the mass production of therapeutically active MSCs; however, there has been no report of the effect of 3D culture on exosome production by MSCs.
In the present study, we tested the hypothesis that the 3D spheroid culture of human bone marrow MSCs (hBM-MSCs) could improve the efficiency of exosomes secretion. We also investigated the critical parameters of 3D MSCs spheroids that affect exosome secretion, such as cell morphology, cell density, and spheroid size.
Materials and methods
Cell culture
Human bone marrow MSCs (hBM-MSCs) were purchased from CEFO Co. Ltd. (Seoul, Korea). Cells were cultured in Minimum Essential Medium Eagle-Alpha Modification (α-MEM; HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Biotechnics Research, Inc., Lake Forest, CA, USA) and 1% penicillin/streptomycin at 37 °C in a 5% CO2 incubator. Cells between passages 6 and 8 were used for all experiments. To measure exosome secretion from cells, FBS was depleted of exosomes by centrifugation at 110,000×g for 18 h before use. hBM-MSCs were cultured in the normal 2D environment (2D-MSC), high-density 2D environment (2D-H), in 3D hanging drop spheroids (3D-HD), in 3D poly(2-hydroxyethyl methacrylate) (poly-HEMA) spheroids (3D-PH), and at normal cell density in 3D poly-HEMA spheroids (3D-N-PH) for 1, 3, and 5 days.
Preparation of experimental groups
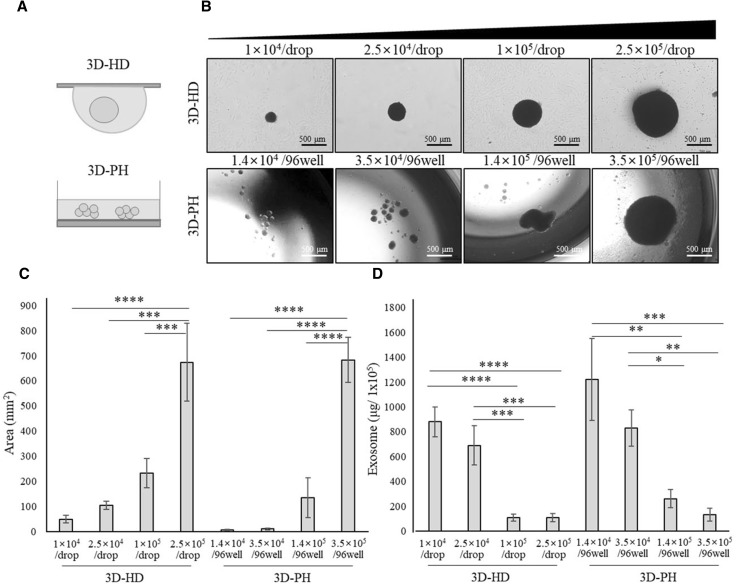
For monolayer culture, hBM-MSCs were seeded in 6-well culture plates either at the conventional seeding density of 1 × 105 cells/6-well (2D) or at a high density of 1.4 × 106 cells/6-well (2D-H) in 2 ml of media. For 3D spheroids culture of hBM-MSCs, the hanging drop and poly-HEMA (Sigma-Aldrich, USA) coating methods were used as described previously [30]. Briefly, a droplet of 2.5 × 104 MSCs in 35 µl medium was placed on a culture plate and incubated upside down for hanging-drop culture (3D-HD), while the same concentration of cells was cultured in a 96-well plate with 50 µl of medium for the poly-HEMA culture (3D-PH). For poly-HEMA culture, 96-well plates were pre-coated with 20 mg/ml poly-HEMA in 95% ethanol and sterilized using UV light after drying. In Fig. 2, the number of cells increased starting from 285 to 714, 2850, and 7140 cells per 1 μl: 3D-HD (1 × 104, 2.5 × 104, 1 × 104, and 2.5 × 104 per drop in 35 μl of medium) or 3D-PH (1.4 × 104, 3.5 × 104, 1.4 × 104, and 3.5 × 104 per 96-well in 50 μl of medium). In Figs. 4 and 5, the poly-HEMA cultures were carried out at the same cell density of 1 × 105 cells as the normal monolayer culture in 6-well plates containing 2 ml of medium (3D-N-PH).
Fig. 2.
The effect of spheroid size on exosome secretion from hBM-MSCs. A Schematic illustration of the experimental groups. hBM-MSCs were cultured at four different densities. 3D-HD (1 × 104, 2.5 × 104, 1 × 104, and 2.5 × 104 per drop) or 3D-PH (1.4 × 104, 3.5 × 104, 1.4 × 104, and 3.5 × 104 per 96-well). B Gross morphology of 3D-HD-MSCs and 3D-PH-MSCs at 3 days after culture. Scale bar: 500 μm. C Size of spheroids was shown for 3D-HD-MSCs and 3D-PH-MSCs at 3 days. The area of the spheroids’ cross-sections was quantified using image J analysis from the bright-field images. D The production efficiency of exosomes derived from 3D-HD and 3D-PH culture. The amounts of exosomes obtained from 1 × 105 cells are presented for each group. Data are presented as the mean and SD from three independent experiments in C and D. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 by one-way ANOVA. ***** 3D: three-dimensional, hBM-MSCs: human bone marrow-mesenchymal stem cells, 3D-HD: 3D spheroids formed by the hanging drop method, 3DPH: 3D spheroids formed by the poly-HEMA coating method, ANOVA: analysis of variance
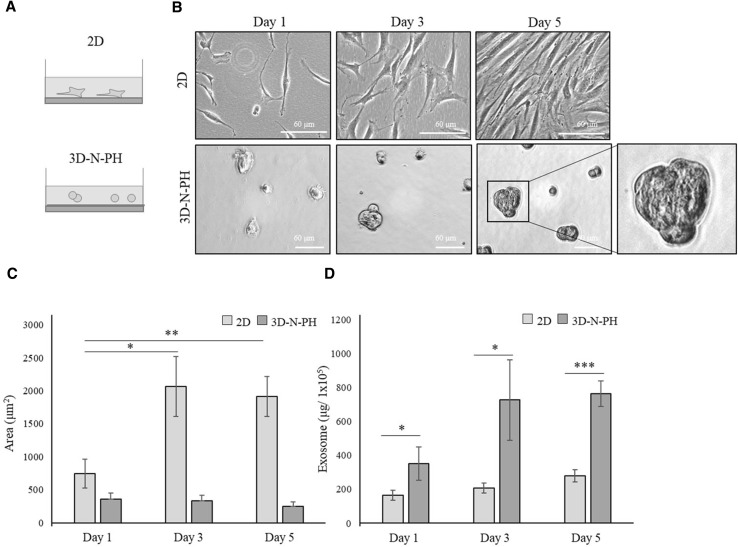
Fig. 4.
The effect of cell morphology on exosome secretion from hBM-MSCs. A Schematic illustration of the experimental groups. hBM-MSCs were cultured in a monolayer at normal density (2D) or in the 3D-PH spheroids with the same normal cell density (3D-N-PH). B Gross morphology of 2D-MSCs and 3D-N-PH MSCs are presented at 1, 3, and 5 days of culture. Scale bar: 60 μm. C Area of 2D-MSCs culture and the size of the 3D-N-PH spheroids are presented at 1, 3, and 5 days of culture. The area of the individual cells was quantified from bright-field images using ImageJ analysis. D The production efficiency of exosomes derived from 2D-MSCs and 3D-N-PH MSCs. The amounts of exosomes obtained from 1 × 105 cells are presented for each group. Data are presented as the mean and SD from three independent experiments in C and D. *p < 0.05, **p < 0.01 and ***p < 0.001 by one-way ANOVA and Student’s t test. **** 3D: three-dimensional, hBM-MSCs: human bone marrow-mesenchymal stem cells, 2D: two-dimensional, 3D-PH: 3D spheroids formed by the poly-HEMA coating method, ANOVA: analysis of variance
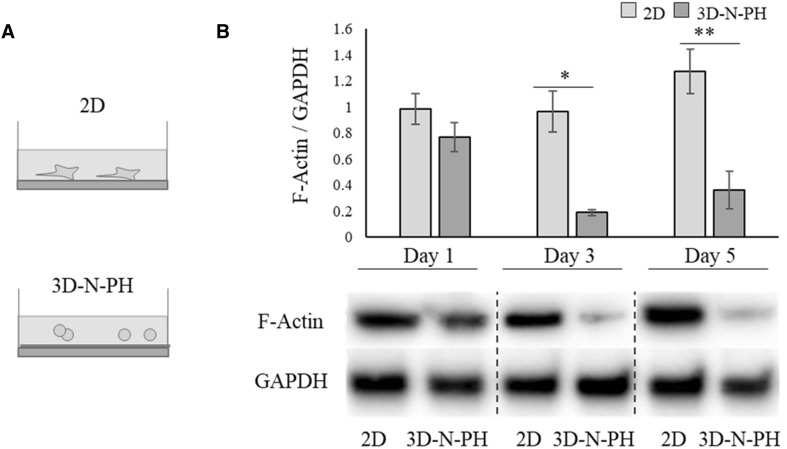
Fig. 5.
The effect of cell morphology on F-actin expression in hBM-MSCs. A Schematic illustration of the experimental groups. hBM-MSCs were cultured in 2D or 3D-N-PH as in Fig. 4. B Protein levels of F-actin were examined in 2D-MSCs or 3D-N-PH-MSCs at 1, 3, and 5 days of culture. The bar graph shows the semi-quantitative analysis of the F-actin protein normalized to the level of GAPDH. Data are presented as the mean and SD from three independent experiments. *p < 0.05 and **p < 0.01 by t test. *** 3D: three-dimensional, hBM-MSCs: human bone marrow-mesenchymal stem cells, 2D: two-dimensional, 3D-N-PH: 3D spheroids formed by the poly-HEMA coating method with a normal cell density, GAPDH: glyceraldehyde-3-phosphate dehydrogenase
Characterization of cell morphology and spheroids size using Image J
MSCs in monolayer and in 3D spheroid culture were characterized morphologically under a microscope (Leica DMi8; Leica, Germany). The sizes of the 3D spheroids were determined by measuring the maximum cross-sectional area using the ImageJ program.
Isolation of exosomes
Culture medium was centrifuged at 3000×g for 15 min to remove cells and debris, and the culture medium containing exosomes were retrieved. Exosomes were isolated using an ExoQuick-TC exosome precipitation solution kit (Systems Biosciences, Palo Alto, CA, USA) according to the manufacturer’s protocol. Briefly, the culture medium was mixed with the ExoQuick-TC solution at a volume ratio of 1:4 (precipitation solution: supernatant) and incubated overnight at 4 °C. Subsequently, the mixture was centrifuged at 1500×g for 30 min and the supernatant was discarded. The exosome pellet was suspended in phosphate-buffered saline (PBS) and the exosome protein concentration was measured using a Nanodrop instrument for subsequent experiments.
Western blot analysis
Western blotting for F-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed [31]. Briefly, total proteins were extracted from cells and exosomes using Radioimmunoprecipitation assay (RIPA) lysis buffer (Rockland, Gilbertsville, PA, USA) and the protein concentrations were quantified using the Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). The protein extracts were subjected to electrophoresis through a 4–20% gradient SDS–polyacrylamide gel (Bio-Rad Laboratories). Subsequently, the proteins were transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories) and the membranes were blocked in 5% non-fat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST). Membranes were incubated for 2 h at 37 °C with primary antibodies against F-actin (ab205, Abcam, Cambridge, MA, USA; dilution of 1:100) and GAPDH (ab9485, Abcam; at a dilution of 1:2000). The membranes were then incubated with horseradish peroxide-conjugated anti-rabbit or anti-mouse secondary antibodies at 37 °C for 1 h.
Statistical analysis
Data for the exosome secretion and spheroids size were expressed as the mean ± standard deviation (SD) from at least three independent experiments. Statistical significance was analyzed using the t test and one-way analysis of variance (ANOVA), followed by a Tukey–Kramer post hoc test. A value of p < 0.05 was considered statistically significant (*p < 0.05, **p <0.01, ***p <0.001, and ****p <0.0001).
Results
hBM-MSCs in 3D spheroid culture secreted more exosomes than those in 2D culture
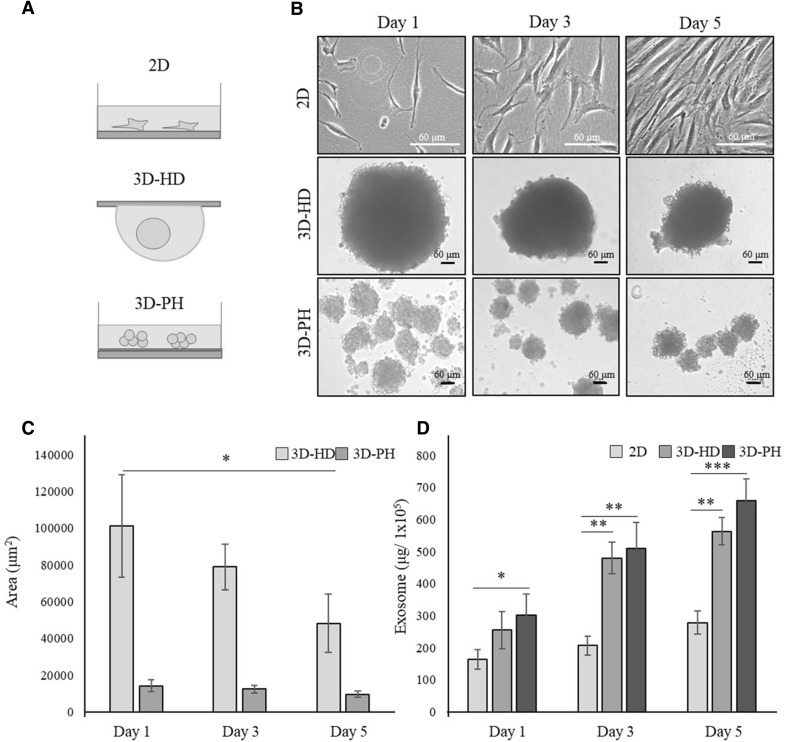
We first compared exosome secretion from hBM-MSCs cultured in a conventional monolayer (2D) with that from cells cultured in 3D spheroids (3D-HD and 3D-PH) (Fig. 1A). The morphology of the cells and spheroids were observed at 1, 3, and 5 days. The 2D-MSCs grew gradually and reached near confluence at day 5 (Fig. 1B). The 3D-HD formed a single spheroid and showed a gradual decrease in spheroid size, while the 3D-PH formed multiple small spheroids and showed no significant change in size with time (Fig. 1B). The cross-sectional area of the 3D-HD spheroids was approximately 101,244.3 μm2 at day 1, 78,908.6 μm2 at day 3, and 48,203.2 μm2 at day 5, while those of 3D-PH were all within the range of 9665.3–14,169.6 μm2 (Fig. 1C). When the amount of exosomes secreted were normalized using 1 × 105 cells, cells cultured in 3D-HD and 3D-PH spheroids secreted significantly more exosomes than did the 2D-MSCs at day 1 and the difference increased at days 3 and 5 (Fig. 1D). The 3D-PH culture produced slightly more exosomes than the 3D-DH culture, but without statistical significance. Values at days 1, 3, and 5 were approximately 165.7, 210.2, and 270.4 μg/1 × 105 for 2D-MSCs; 255.7, 479.5, and 560.3 μg/1 × 105 for 3D-HD; and 290.7, 468.2, and 640.6 μg/1 × 105 for 3D-PH, respectively. These results indicated that the 3D spheroid hBM-MSC cultures increased exosome production compared with that from 2D culture.
Fig. 1.
The effect of 3D culture on the exosome secretion from hBM-MSCs. A Schematic illustration of the experimental groups. hBM-MSCs were cultured in a monolayer at normal density (2D) or as 3D spheroids (3D-HD) at high cell density using the hanging-drop method or with a poly-HEMA coating (3D-PH), as described in Materials and Methods. B Microscopic morphology of MSCs in 2D, 3D-HD, and 3D-PH cultures after 1, 3, and 5 days. Scale bar: 60 μm. C Sizes of 3D MSCs spheroids cultured in 3D-HD and 3D-PH for 1, 3, and 5 days. The cross-sectional area of the spheroids was quantified from the bright-field images using Image J software. D The production efficiency of exosomes from MSCs cultured in 2D, 3D-HD, and 3D-PH. The amount of exosomes obtained from 1 × 105 cells at 1, 3, and 5 days are shown. Data are presented as the mean and SD from three independent experiments in C and D. *p < 0.05, **p < 0.01 and ***p < 0.001 by one-way ANOVA. **** 3D: three-dimensional, hBM-MSC: human bone marrow-mesenchymal stem cells, 2D: two-dimensional, 3D-HD: 3D spheroids formed by the hanging drop method, 3D-PH: 3D spheroids formed by the poly-HEMA coating method, ANOVA: analysis of variance
The production efficiency of exosomes from hBM-MSCs decreased with increasing 3D spheroid size
Next, we investigated the effect of the size of the 3D spheroids on the production efficiency of exosomes to determine optimal conditions for hBM-MSC exosome secretion (Fig. 2A). 3D-HD and 3D-PH spheroids were produced with increasing numbers of cells and cultured for 3 days before analysis. The size of the 3D-HD spheroids increased with increasing cell number in the morphological analysis (Fig. 2B), and the quantitative analysis showed that the approximate cross-sectional area increased dramatically from 49.4 mm2 at 1 × 104 cells to 673.3 mm2 at 2.5 × 105 cells per drop (Fig. 2C). The approximate size of the 3D-PH spheroids also increased dramatically with increasing cell number, changing from 5.0 mm2 at 1.4 × 104 cells to 683.4 mm2 at 2.5 × 105 cells per 96-well (Fig. 2C). Interestingly, 3D-PH formed multiple very small spheroids at 2.5 × 104 cells, as shown in Fig. 1, but formed a single aggregate at 1 × 105 and 2.5 × 105 cells (Fig. 2B). The amount of exosomes produced from an initial inoculum of 1 × 105 cells decreased as cell density increased in both the 3D-HD and 3D-PH spheroids (Fig. 2D). A rapid decrease in exosome secretion was evident as the spheroid size increased. The mean values at each cell density were approximately 880.4, 690.2, 109.1, and 259.6 μg/1 × 105 for 3D-HD and 1221.1, 831.2, 259.6, and 132.1 μg/1 × 105 for 3D-PH, respectively. This result indicated that the smaller the size of the 3D spheroids, the more exosomes could be produced from a given amount of cells.
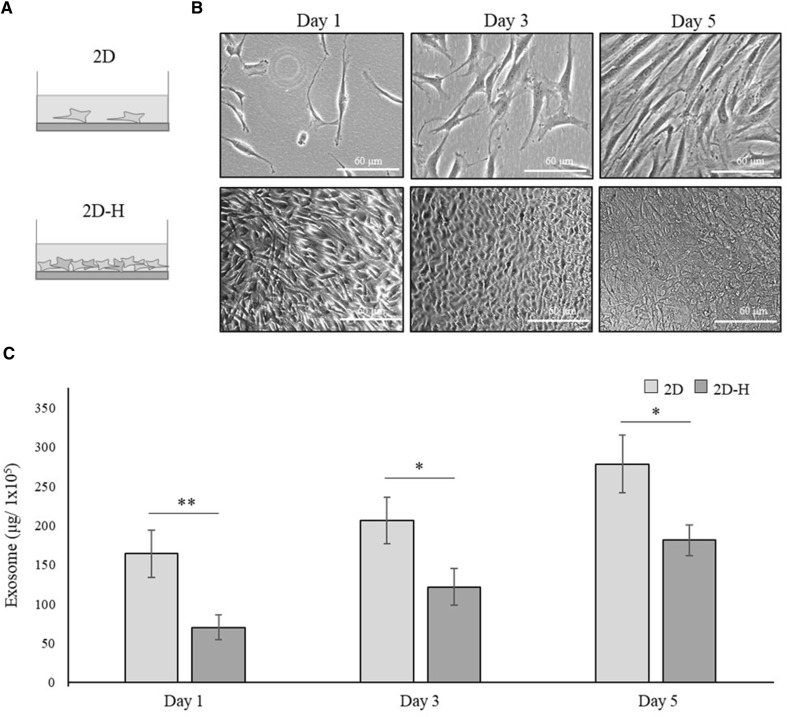
The production efficiency of exosomes decreased with increasing cell density in 2D culture of hBM-MSCs
We then tried to increase the cell seeding density in 2D culture of hBM-MSCs to determine whether it had similar effect to that in the 3D spheroid culture (Fig. 3A). Cells were plated in 6-well plates at 1 × 105 cells/well (2D) as in Fig. 1 or at 1.4 × 106 cells/well (2D-H), which was the same cell density as that used in the 3D-HD and 3D-PH cultures shown in Fig. 1 (Fig. 3B). The amount of exosomes produced from 1 × 105 cells increased gradually over 1, 3 and 5 days; however, the 2D-H culture showed significantly less exosome secretion from day 1 of culture (Fig. 3C). Mean values at 1, 3 and 5 days were approximately 164.3, 207.2, and 278.6 μg/1 × 105 for 2D and 70.2, 121.6, and 181.3 μg/1 × 105 for 2D-H. Taken together with the results shown in Fig. 2, this result suggested that exosome production in hBM-MSCs decreases when the size of culture mass increases, either in 2D or 3D culture.
Fig. 3.
The effect of cell density on exosome secretion from hBM-MSCs. A Schematic illustration of the experimental groups. hBM-MSCs were cultured in monolayer at normal density (2D) or high density (2D-H), as described in Materials and Methods. B Gross morphology of 2D-MSCs and 2D-H-MSCs are shown at 1, 3, and 5 days of culture. Scale bar: 60 μm. c The production efficiency of exosomes derived from the 2D-MSCs and 2D-H-MSCs. The amounts of exosomes obtained from 1 × 105 cells are presented for each group. Data are presented as the mean and SD from three independent experiments. *p < 0.05 and **p < 0.01 by t test. *** hBM-MSCs: human bone marrow-mesenchymal stem cells, 2D: two-dimensional, ANOVA: analysis of variance
The non-adherent state of cell morphology might be important in the enhanced production efficiency of exosomes from hBM-MSCs
From the results above, we hypothesized that the morphology of cells could be associated with the production efficiency of exosomes. Therefore, we plated hBM-MSCs on the poly-HEMA at the same cell density as that of the 2D culture (1 × 105 cells/2 ml) in 6-well plates (3D-N-PH) so that small clumps with few cells were produced instead of spheroids, and then we compared their exosome production efficiency (Fig. 4A). We found that 3D-N-PH formed single cells or small aggregates with several cells having a spherical morphology (Fig. 4B) and their cross-sectional area was slightly decreased but with no statistical significance with increasing time (1, 3, and 5 days) (Fig. 4C). The amount of exosomes produced from 1 × 105 cells was significantly higher in the 3D-N-PH culture than in the 2D-MSCs at all time points (Fig. 4D). It increased rapidly at day 3 and then plateaued at day 5 in 3D-N-PH, while it increased slightly with time in 2D-MSCs. The mean values at 1, 3, and 5 days were approximately 351.3, 727.9, and 765.2 μg/1 × 105 for 3D-N-PH and 164.3, 207.1, and 278.6 μg/1 × 105 for 2D-MSCs, respectively. This result suggested that the spherical morphology of MSCs is important for the increase of exosome production. Western blotting for F-actin revealed that the 3D microsphere culture of hBM-MSCs caused rapid actin depolymerization from day 3 (Fig. 5).
Discussion
The secretion of paracrine factors increases when MSCs are cultured in 3D spheroids [27]. However, there has been no study showing the effect of 3D culture on the efficiency of exosome production in MSCs. The present study showed that the amount of secreted exosomes increased significantly when MSCs were cultured in 3D spheroids compared with that secreted in monolayer culture. We then investigated the mechanism of the 3D spheroid culture-induced increase in exosome production. The central area of a spheroid lacks oxygen supply and is thus in a hypoxic state [28–33]. Hypoxia has been reported to promote the secretion of exosomes in monolayer culture [34]. Therefore, we produced a series of larger spheroids and examined if exosome production increased proportionally with the increase in the hypoxic central area. However, the results showed that the production efficiency of exosomes decreased as the size of spheroids increased.
We then questioned if increase in the local cell density in 3D spheroids was responsible for the increase in exosome production, because it could exert a mechanical stress on the cells, which might promote their trophic function [35]. To test this possibility, we compared the efficiency of exosome production in MSCs at different cell densities in monolayer culture. However, exosome production decreased at higher cell density. These findings led us to speculate that the change in cell morphology itself might be responsible for the increased exosome production. We cultured cells at a normal density on poly-HEMA 3D spheroids to produce non-adherent small aggregates of a few cells and observed increased exosome production compared with that from cells cultured in a monolayer at the same density. This result demonstrated that 3D culture stimulates MSCs to produce more exosomes, probably because the change in cell morphology to a non-adherent, round shape.
MSCs in 3D culture experience an alteration in their actin structure and a relaxation of cytoskeletal tension because of reduced expression of F-actin [36]. Our study also confirmed that the expression of F-actin was significantly reduced in MSCs cultured on non-adherent poly-HEMA spheroids. During regulated exocytosis, the membrane of a secretory vesicle fuses with the plasma membrane and depolymerization of F-actin is essential to allow their appropriate docking at the fusion sites [37]. Therefore, it is likely that the reduced expression of F-actin in MSCs under 3D culture provided a favorable environment for exosome synthesis and secretion in this study. The detailed mechanism of increased exosome production by 3D culture of MSCs remains unclear and requires further investigation in the future.
The 3D spheroids using the hanging-drop and poly-HEMA showed similar performance in the exosome production of MSCs in this study. However, they have different considerations, when developing a manufacturing process for mass production. The hanging-drop produces a single 3D spheroid with a similar size for each droplet, and this phenomenon was observed consistently at different cell densities. However, it is not easy to scale-up the hanging-drop in a manual protocol and the automatic process requires development of a special equipment with additional optimization. In contrast, the poly-HEMA coating provides an easy method to produce 3D spheroids at a large scale. But it suffers from large variations in the spheroids size and the production efficiency of exosomes which makes it difficult to optimize the process. In addition, both methods have a limitation in the mass culture of 3D spheroids. Previously, the suspension culture of MSCs in a single cell or in 3D spheroids has been achieved previously in bioreactors using spinner flasks or rotating wall vessels [24, 38]. It is expected that these technologies could also be a useful tool for the mass production of exosomes but requires further process development and optimization to ensure robustness and consistency for commercial use. Finally, it is also important to understand any changes in the content and biological activity of exosomes in 3D culture before starting commercial development.
In conclusion, this study showed that the secretion efficiency of exosomes increased in MSCs cultured in 3D spheroids, caused by the cells changing to a non-adherent, round morphology. These results will allow the optimization of the manufacturing process of exosomes for mass production.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1 Comparison of the amount of exosomes secreted from hBM-MSCs primed with various stimuli. hBM-MSCs were cultured in monolayer at normal density (2D) and were untreated (Control) or treated respectively with IL-1β, TNF-α, Poly I:C, and 3D-HD. The amounts of exosomes obtained from 1 × 105 cells are presented for each group at 3 days of culture. Data are presented as the mean and SD from three independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001 by one-way ANOVA. 3D, three-dimensional; hBM-MSCs; human bone marrow-mesenchymal stem cells; 2D, two-dimensional; 3D-HD, 3D spheroids formed by the hanging drop method; ANOVA, analysis of variance; IL-1β, interleukin 1 beta; TNF-α, tumor necrosis factor alpha; Poly I:C, polyinosinic-polycytidylic acid. (JPEG 123 kb)
Acknowledgement
This research was supported by a grant from the Korea Health Technology R&D Project funded by the Ministry of Health & Welfare, Republic of Korea (HI17C2191).
Conflict of interest
The authors declare no competing financial interests.
Ethical statement
There are no animal experiments carried out for this article.
Contributor Information
Byung Hyune Choi, Phone: 82-32-890-0939, Email: bryan@inha.ac.kr.
Byoung-Hyun Min, Phone: 82-31-219-4444, Email: dr.bhmin@gmail.com.
References
- 1.Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labusca L, O’Brien M, Mashayekhi K. A clinical perspective to mesenchymal stem cell-based musculoskeletal regeneration. OA Musculoskelet Med. 2013;1:8. doi: 10.13172/2052-9287-1-1-605. [DOI] [Google Scholar]
- 3.Miao Z, Sun H, Xue Y. Isolation and characterization of human chorionic membranes mesenchymal stem cells and their neural differentiation. Tissue Eng Regen Med. 2017;14:143–151. doi: 10.1007/s13770-017-0025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalilpourfarshbafi M, Hajiaghaalipour F, Selvarajan KK, Adam A. Mesenchymal stem cell-based therapies against podocyte damage in diabetic nephropathy. Tissue Eng Regen Med. 2017;14:201–210. doi: 10.1007/s13770-017-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 8.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 11.Yeo RWY, Lai RC, Tan KH, Lim SK. Exosome: a novel and safer therapeutic refinement of mesenchymal stem cell. J Circ Biomark. 2013 [Google Scholar]
- 12.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C, et al. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24:1290–1301. doi: 10.1038/mt.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589:1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Chu W, Lai C, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 19.Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8:e68451. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savina A, Furlán M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 21.Kato T, Miyaki S, Ishitobi H, Nakamura Y, Nakasa T, Lotz MK, et al. Exosomes from IL-1β stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16:R163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKee C, Chaudhry GR. Advances and challenges in stem cell culture. Colloids Surf B Biointerfaces. 2017;159:62–77. doi: 10.1016/j.colsurfb.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–749. doi: 10.1089/ten.tec.2009.0432. [DOI] [PubMed] [Google Scholar]
- 25.Guo L, Ge J, Zhou Y, Wang S, Zhao RC, Wu Y. Three-dimensional spheroid-cultured mesenchymal stem cells devoid of embolism attenuate brain stroke injury after intra-arterial injection. Stem Cells Dev. 2014;23:978–989. doi: 10.1089/scd.2013.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santos JM, Camões SP, Filipe E, Cipriano M, Barcia RN, Filipe M, et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther. 2015;6:90. doi: 10.1186/s13287-015-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, et al. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761–1768. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- 29.Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L, Zhou Y, Wang S, Wu Y. Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) spheroids. J Cell Mol Med. 2014;18:2009–2019. doi: 10.1111/jcmm.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M, Kim J, Park SR, Park DY, Kim YJ, Choi BH, et al. Comparison of fetal cartilage-derived progenitor cells isolated at different developmental stages in a rat model. Dev Growth Differ. 2016;58:167–179. doi: 10.1111/dgd.12267. [DOI] [PubMed] [Google Scholar]
- 32.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 33.Sart S, Tsai AC, Li Y, Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev. 2014;20:365–380. doi: 10.1089/ten.teb.2013.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sart S, Ma T, Li Y. Preconditioning stem cells for in vivo delivery. Biores Open Access. 2014;3:137–149. doi: 10.1089/biores.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Chen H, Li H, Wu Y. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J Cell Mol Med. 2017;21:1073–1084. doi: 10.1111/jcmm.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aunis D, Bader MF. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 1988;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- 38.Alimperti S, Lei P, Wen Y, Tian J, Campbell AM, Andreadis ST. Serum-free spheroid suspension culture maintains mesenchymal stem cell proliferation and differentiation potential. Biotechnol Prog. 2014;30:974–983. doi: 10.1002/btpr.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of the amount of exosomes secreted from hBM-MSCs primed with various stimuli. hBM-MSCs were cultured in monolayer at normal density (2D) and were untreated (Control) or treated respectively with IL-1β, TNF-α, Poly I:C, and 3D-HD. The amounts of exosomes obtained from 1 × 105 cells are presented for each group at 3 days of culture. Data are presented as the mean and SD from three independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001 by one-way ANOVA. 3D, three-dimensional; hBM-MSCs; human bone marrow-mesenchymal stem cells; 2D, two-dimensional; 3D-HD, 3D spheroids formed by the hanging drop method; ANOVA, analysis of variance; IL-1β, interleukin 1 beta; TNF-α, tumor necrosis factor alpha; Poly I:C, polyinosinic-polycytidylic acid. (JPEG 123 kb)